Regulation of Cortico-Thalamic JNK1/2 and ERK1/2 MAPKs and Apoptosis-Related Signaling Pathways in PDYN Gene-Deficient Mice Following Acute and Chronic Mild Stress
Abstract
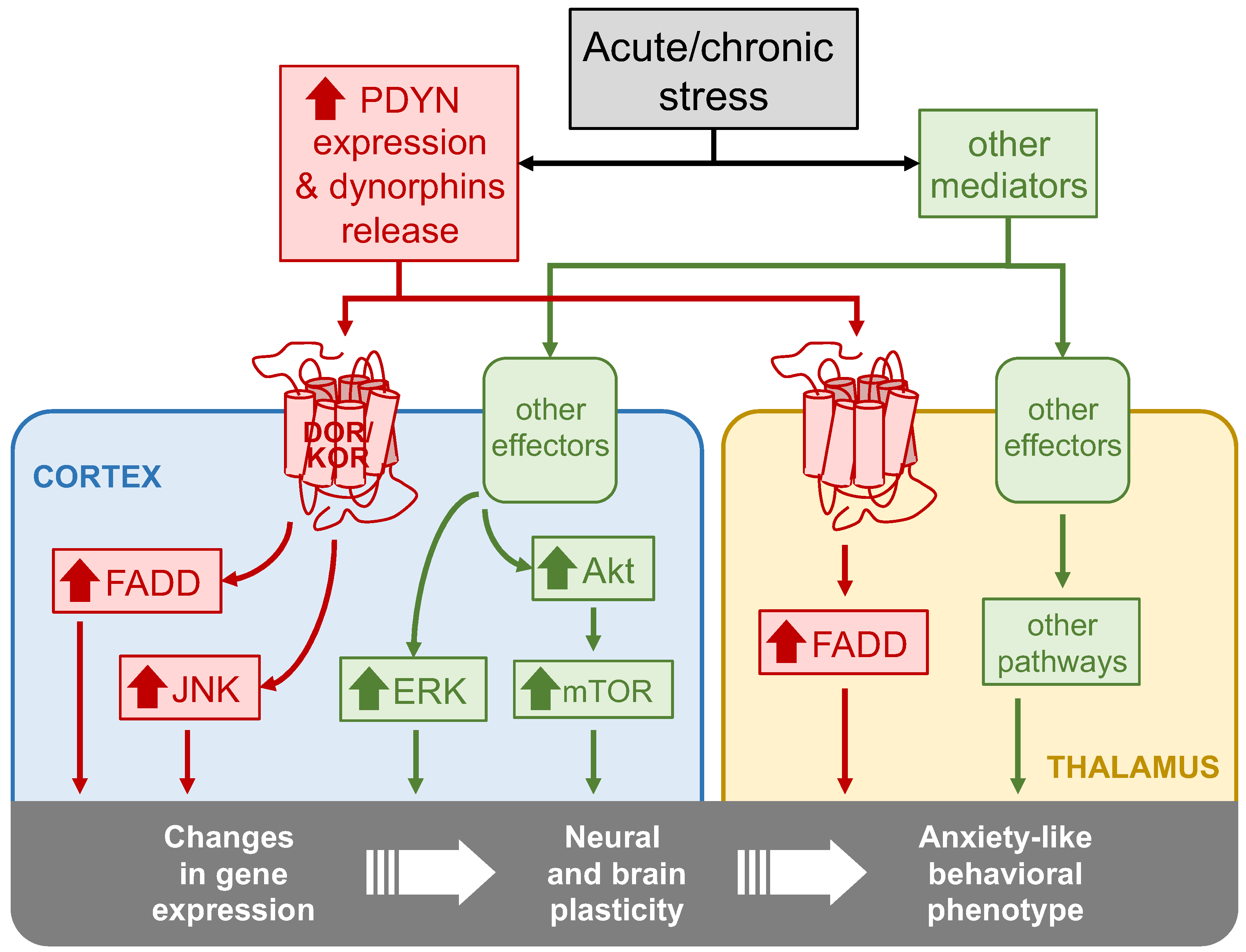
1. Introduction
2. Results
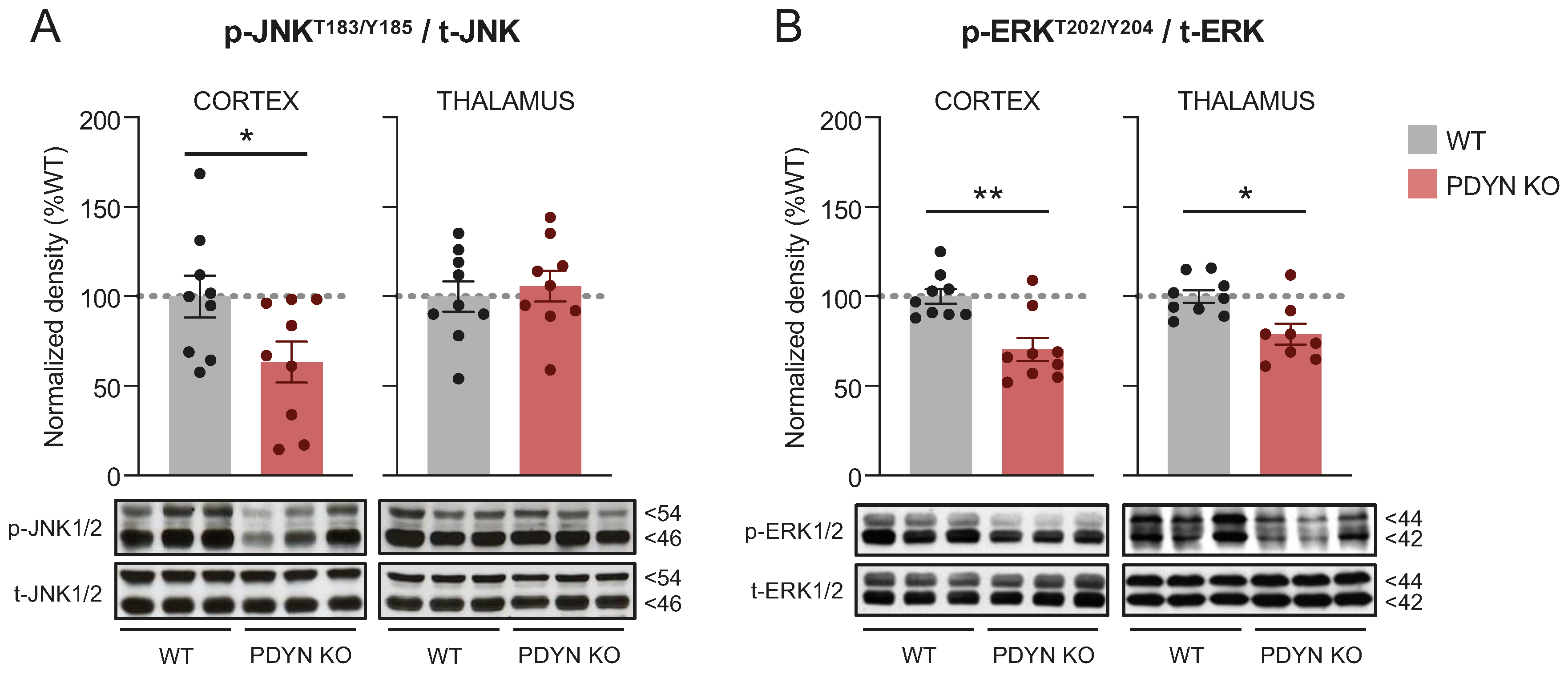
2.1. Effect of PDYN Gene Deletion on MAPK-JNK1/2 and ERK1/2 in Mouse Brain
2.2. Effect of PDYN Gene Deletion on Fas/FADD Immunodensities in Mouse Brain
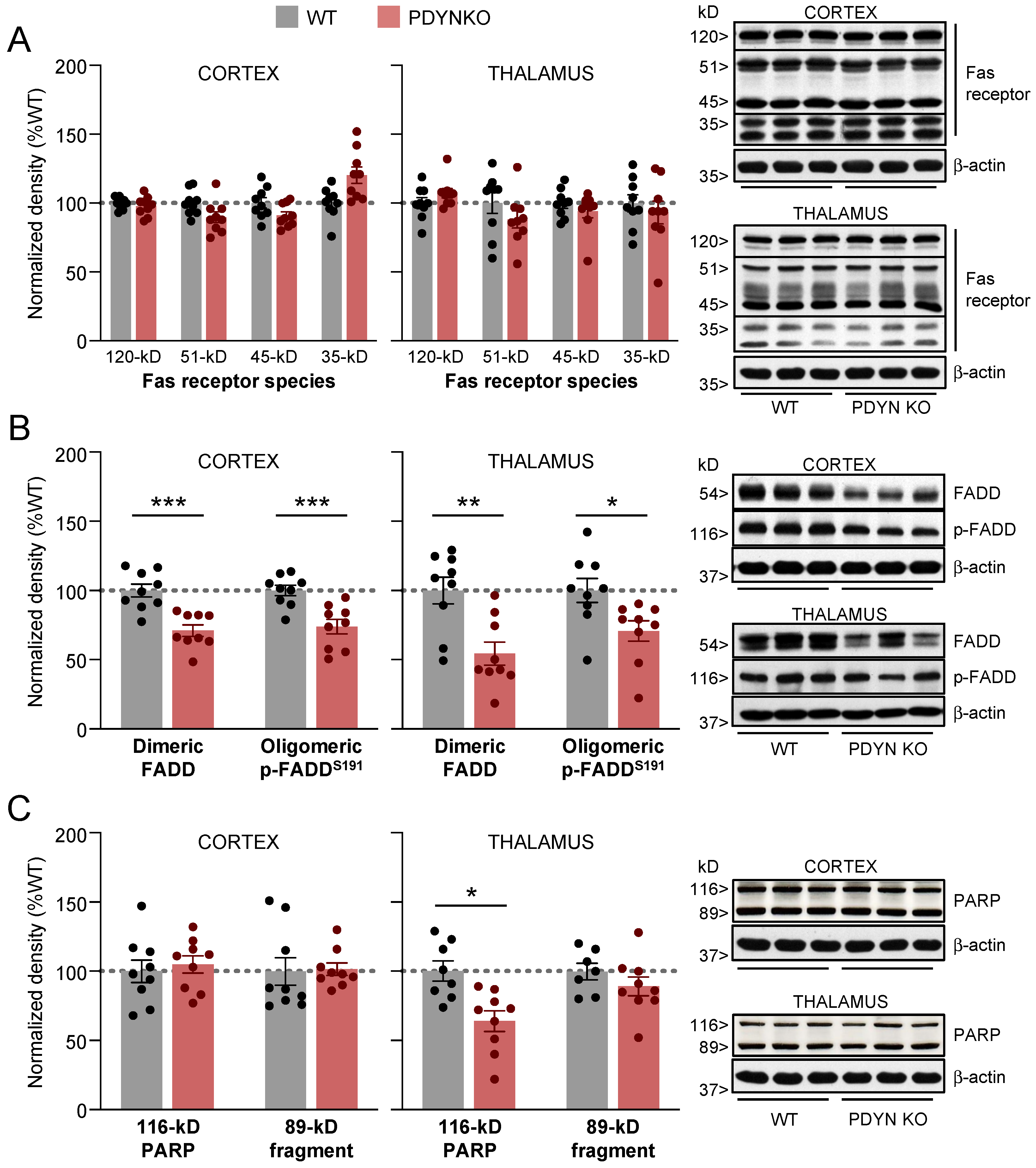
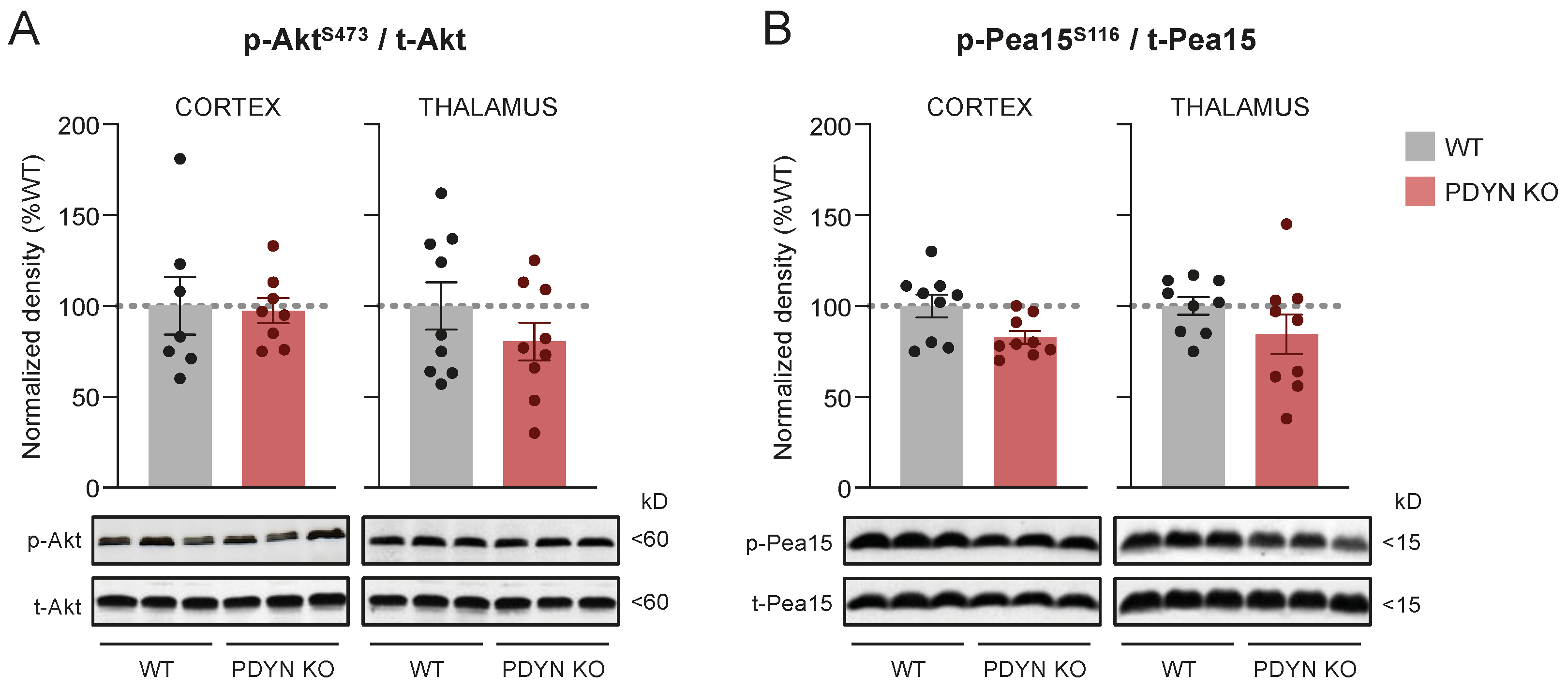
2.3. Effect of PDYN Gene Deletion on the Akt/Pea15/mTOR Pathway
2.4. Effects of Acute and Chronic Stress on Select Signaling Pathways in Cortical and Thalamic Samples from WT and PDYN-KO Mice
2.4.1. ARS and CMS Effects on Cortical Targets in WT and PDYN-KO Mice
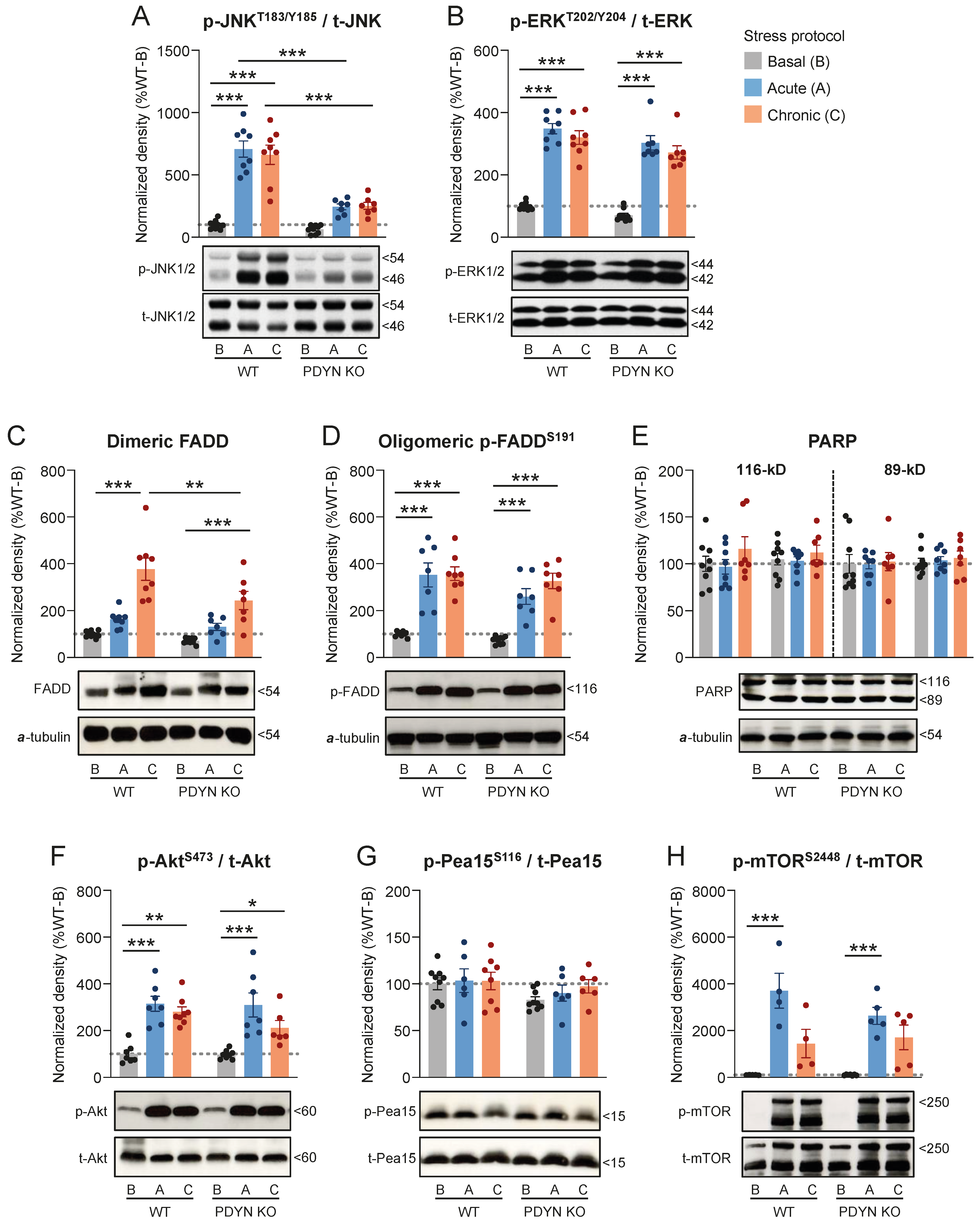
2.4.2. ARS and CMS Effects on Thalamic Targets in WT and PDYN-KO Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Acute and Chronic Stress Procedures
4.3. Brain Tissue Preparation
4.4. Immunoblot Assays and Quantification of Target Proteins
4.5. Data and Statistical Analyses
| Protein | Immunogen | Host | Catalogue (Clone) | Batch | Vendor |
|---|---|---|---|---|---|
| p-JNK1/2 | Human JNK (peptide containing p-Thr183/Tyr185) | Rabbit | 9251 | 10 | Cell Signaling, USA |
| JNK1/2 | Human JNK2 (GST fusion protein) | Rabbit | 9252 | 6 | Cell Signaling, USA |
| p-ERK1/2 | Human p44 MAPK (peptide containing p-Thr202/Tyr204) | Rabbit | 9101 | 20 | Cell Signaling, USA |
| ERK1/2 | Human p42 MAPK (synthetic peptide) | Rabbit | 442704 | D33075 | Calbiochem, FRG |
| Fas receptor | Mouse Fas receptor (C-terminal residues) | Rabbit | sc-716 | F1306 | Santa Cruz, USA |
| FADD | Human FADD (28–208 residues) | Rabbit | sc-5559 | K1407 | Santa Cruz, USA |
| p-FADD | Mouse p-FADD (peptide containing p-Ser191) | Rabbit | 2785 | 1 | Cell Signaling, USA |
| PARP | Human PARP-1 (215–228 residues) | Rabbit | 512729 | KP8501 | Calbiochem, FRG |
| p-Akt | Mouse Akt1 (peptide containing p-Ser473) | Rabbit | 9271 | 10 | Cell Signaling, USA |
| Akt | Human Akt1 (88-100 residues) | Rabbit | 530311 | D35774 | Calbiochem, FRG |
| p-Pea15 | Human PEA-15 (peptide containing p-Ser116) | Rabbit | 44-836G | 0103 | BioSource Inc., USA |
| Pea15 | Human PEA-15 (peptide containing Leu60) | Rabbit | 2780 | 1 | Cell Signaling, USA |
| p-mTOR | Human p-mTOR (p-Ser2448) | Rabbit | 2971 | 14 | Cell Signaling, USA |
| mTOR | Human p-mTOR | Rabbit | 2972 | 7 | Cell Signaling, USA |
| β-actin | Human β-actin (2-16 residues) | Mouse | A1978 (AC-15) | 016K4817 | Sigma-Aldrich, USA |
| α-tubulin | Sea urchin sarkosyl-resistant filaments | Mouse | T6074 (B-5-1-2) | 6 | Sigma-Aldrich, USA |
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTH | adrenocorticotropic hormone |
| Akt | no name acronym (also known as protein kinase B or PKB) |
| ANOVA | analysis of variance |
| ARS | acute restraint stress |
| CMS | chronic mild stress |
| df | degrees of freedom |
| DOR | delta opioid receptor |
| ERK1/2 | extracellular signal-regulated kinases 1 and 2 |
| FADD | Fas-associated protein with death domain |
| GABAA | gamma-aminobutyric acid subtype A |
| IOD | integrated optical density |
| JNK1/2 | c-Jun N-terminal kinases 1 and 2 |
| kDa | kiloDalton |
| KO | knockout |
| KOR | kappa opioid receptor |
| MAPK | mitogen-activated protein kinase |
| MAP2K | mitogen-activated protein kinase kinase |
| MAP3K | mitogen-activated protein kinase kinase kinase |
| mTOR | mammalian target of rapamycin |
| p- | phosphorylated- |
| PAGE | polyacrylamide gel electrophoresis |
| PARP | poly (ADP-ribose)-polymerase-1 |
| PBS | phosphate-buffered saline |
| PDYN | prodynorphin |
| Pea-15 | phosphoprotein enriched in astrocytes of 15 kDa |
| ROS | reactive oxygen species |
| SAPK | stress-activated protein kinase |
| SDS | sodium dodecyl sulfate |
| SEM | standard error of the mean |
| t- | total- |
| TBS | Tris-buffered saline |
| TW | two-way |
| WB | Western blotting |
| WT | wildtype |
References
- Schwarzer, C. 30 years of dynorphins. New insights on their functions in neuropsychiatric diseases. Pharmacol. Ther. 2009, 123, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Femenía, T.; Manzanares, J. Increased ethanol intake in prodynorphin knockout mice is associated to changes in opioid receptor function and dopamine transmission. Addict. Biol. 2012, 17, 322–337. [Google Scholar] [CrossRef]
- Wittmann, W.; Schunk, E.; Rosskothen, I.; Gaburro, S.; Singewald, N.; Herzog, H.; Schwarzer, C. Prodynorphin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacology 2009, 34, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Duque-Díaz, E.; Alvarez-Ojeda, O.; Coveñas, R. Enkephalins and ACTH in the mammalian nervous system. Vitam. Horm. 2019, 111, 147–193. [Google Scholar] [CrossRef]
- Kovac, S.; Walker, M.C. Neuropeptides in epilepsy. Neuropeptides 2013, 47, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.J.; Akil, H.; Fischli, W.; Goldstein, A.; Zimmerman, E.; Nilaver, G.; Van Wimersma Griedanus, T.B. Dynorphin and Vasopressin: Common localization in magnocellular neurons. Science 1982, 216, 85–87. [Google Scholar] [CrossRef]
- Weber, E.; Roth, K.A.; Barchas, J.D. Immunohistochemical distribution of alpha-neo-endorphin/dynorphin neuronal systems in rat brain: Evidence for colocalization. Proc. Natl. Acad. Sci. USA 1982, 79, 3062–3066. [Google Scholar] [CrossRef] [PubMed]
- Nabeshima, T.; Katoh, A.; Wada, M.; Kameyama, T. Stress-induced changes in brain met-enkephalin, leu-enkephalin and dynorphin concentrations. Life Sci. 1992, 51, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Weera, M.M.; Gilpin, N.W. Biobehavioral Interactions Between Stress and Alcohol. Alcohol Res. 2019, 40, 04. [Google Scholar] [CrossRef]
- Leconte, C.; Mongeau, R.; Noble, F. Traumatic Stress-Induced Vulnerability to Addiction: Critical Role of the Dynorphin/Kappa Opioid Receptor System. Front. Pharmacol. 2022, 13, 856672. [Google Scholar] [CrossRef]
- Bilkei-Gorzo, A.; Racz, I.; Michel, K.; Mauer, D.; Zimmer, A.; Klingmüller, D.; Zimmer, A. Control of hormonal stress reactivity by the endogenous opioid system. Psychoneuroendocrinology 2008, 33, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Femenía, T.; Pérez-Rial, S.; Urigüen, L.; Manzanares, J. Prodynorphin gene deletion increased anxiety-like behaviours, impaired the anxiolytic effect of bromazepam and altered GABAA receptor subunits gene expression in the amygdale. J. Psychopharmacol. 2011, 25, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Ménard, C.; Tse, Y.C.; Cavanagh, C.; Chabot, J.G.; Herzog, H.; Schwarzer, C.; Wong, T.P.; Quirion, R. Knockdown of prodynorphin gene prevents cognitive decline, reduces anxiety, and rescues loss of group 1 metabotropic glutamate receptor function in aging. J. Neurosci. 2013, 33, 12792–12804. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.J. Signal transduction by the JNK group of MAP kinases. Cell 2000, 103, 239–252. [Google Scholar] [CrossRef]
- Keshet, Y.; Seger, R. The MAP Kinase signaling cascades: A system of hundreds of components regulates a diverse array of physiological functions. Methods Mol. Bio. 2010, 661, 3–38. [Google Scholar] [CrossRef]
- Plotnikov, A.; Zehorai, E.; Procaccia, S.; Seger, R. The MAPK cascades: Signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 1619–1633. [Google Scholar] [CrossRef]
- Sabio, G.; Davis, R.J. TNF and MAP kinase signalling pathways. Semin. Immunol. 2014, 26, 237–245. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK signaling pathways in apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Seger, R.; Krebs, E.G. The MAPK signaling cascade. FASEB J. 1995, 9, 726–735. [Google Scholar] [CrossRef]
- Gutstein, H.B.; Rubie, E.A.; Mansour, A.; Akil, H.; Woodgett, J.R. Opioid effects on mitogen-activated protein kinase signaling cascades. Anesthesiology 1997, 87, 1118–1126. [Google Scholar] [CrossRef]
- Jordan, B.A.; Cvejic, S.; Devi, L.A. Kappa opioid receptor endocytosis by dynorphin peptides. DNA Cell Biol. 2000, 19, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.G.; Pesarico, A.P.; Nogueira, C.W. m-Trifluoromethyl-diphenyl diselenide promotes resilience to social avoidance induced by social defeat stress in mice: Contribution of opioid receptors and MAPKs. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 82, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Hollos, P.; Marchisella, F.; Coffey, E.T. JNK regulation of depression and anxiety. Brain Plast. 2018, 3, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Hauger, R.L.; Risbrough, V.; Oakley, R.H.; Olivares-Reyes, J.A.; Dautzenberg, F.M. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann. N. Y. Acad. Sci. 2009, 1179, 120–143. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.P.; Tsimberg, Y.; Salvadore, C.; Meller, E. Activation of ERK and JNK MAPK pathways by acute swim stress in rat brain regions. BMC Neurosci. 2004, 20, 36. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Davis, R.J. Cell signaling and stress responses. Cold Spring Harb. Perspect. Biol. 2016, 8, a006072. [Google Scholar] [CrossRef]
- Bruchas, M.R.; Xu, M.; Chavkin, C. Repeated swim-stress induces kappa opioid-mediated activation of ERK1/2 MAPK. Neuroreport 2008, 19, 1417–1422. [Google Scholar] [CrossRef]
- Mohammad, H.; Marchisella, F.; Ortega-Martínez, S.; Hollos, P.; Eerola, K.; Komulainen, E.; Kulesskaya, N.; Freemantle, E.; Fagerholm, V.; Savontous, E.; et al. JNK1 controls adult hippocampal neurogenesis and imposes cell-autonomous control of anxiety behaviour from the neurogenic niche. Mol. Psychiatry 2018, 23, 362–374. [Google Scholar] [CrossRef]
- Coimbra-Costa, D.; Alva, N.; Duran, M.; Carbonell, T.; Rama, R. Oxidative stress and apoptosis after acute respiratory hypoxia and reoxygenation in rat brain. Redox Biol. 2017, 12, 216–225. [Google Scholar] [CrossRef]
- Patel, M.Y.; Stovall, K.; Franklin, J.L. The intrinsic apoptotic pathway lies upstream of oxidative stress in multiple organs. Free Radic. Biol. Med. 2020, 158, 13–19. [Google Scholar] [CrossRef]
- Álvaro-Bartolomé, M.; Salort, G.; García-Sevilla, J.A. Disruption of brain MEK-ERK sequential phosphorylation and activation during midazolam-induced hypnosis in mice: Roles of GABAA receptor, MEK1 inactivation, and phosphatase MKP-3. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 75, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Salort, G.; Álvaro-Bartolomé, M.; García-Sevilla, J.A. Ketamine-induced hypnosis and neuroplasticity in mice is associated with disrupted p-MEK/p-ERK sequential activation and sustained upregulation of survival p-FADD in brain cortex: Involvement of GABAA receptor. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 88, 121–131. [Google Scholar] [CrossRef] [PubMed]
- García-Fuster, M.J.; Miralles, A.; García-Sevilla, J.A. Effects of Opiate Drugs on Fas-Associated Protein with Death Domain (FADD) and Effector Caspases in the Rat Brain: Regulation by the ERK1/2 MAP Kinase Pathway. Neuropsychopharmacology 2007, 32, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Boronat, M.A.; García-Fuster, M.J.; García-Sevilla, J.A. Chronic morphine induces up-regulation of the pro-apoptotic Fas receptor and down-regulation of the anti-apoptotic Bcl-2 oncoprotein in rat brain. Br. J. Pharmacol. 2001, 134, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- García-Fuster, M.J.; Ramos-Miguel, A.; Rivero, G.; La Harpe, R.; Meana, J.J.; García-Sevilla, J.A. Regulation of the extrinsic and intrinsic apoptotic pathways in the prefrontal cortex of short- and long-term human opiate abusers. Neuroscience 2008, 157, 105–119. [Google Scholar] [CrossRef]
- García-Fuster, M.J.; Ramos-Miguel, A.; Miralles, A.; García-Sevilla, J.A. Opioid receptor agonists enhance the phosphorylation state of Fas-associated death domain (FADD) protein in the rat brain: Functional interactions with casein kinase Iα, Gαi proteins, and ERK1/2 signaling. Neuropharmacology 2008, 55, 886–899. [Google Scholar] [CrossRef]
- Park, S.M.; Schickel, R.; Peter, M.E. Nonapoptotic functions of FADD-binding death receptors and their signaling molecules. Curr. Opin. Cell Biol. 2005, 17, 610–616. [Google Scholar] [CrossRef]
- Ramos-Miguel, A.; Miralles, A.; García-Sevilla, J.A. Correlation of rat cortical Fas-associated death domain (FADD) protein phosphorylation with the severity of spontaneous morphine abstinence syndrome: Role of α2-adrenoceptors and extracellular signal-regulated kinases. J. Psychopharmacol. 2011, 25, 1691–1702. [Google Scholar] [CrossRef]
- Formstecher, E.; Ramos, J.W.; Fauquet, M.; Calderwood, D.A.; Hsieh, J.C.; Canton, B.; Nguyen, X.T.; Barnier, J.V.; Camonis, J.; Ginsberg, H.M. PEA-15 mediates cytoplasmic sequestration of ERK MAP Kinase. Dev. Cell 2001, 1, 239–250. [Google Scholar] [CrossRef]
- Renganathan, H.; Vaidyanathan, H.; Knapinska, A.; Ramos, J.W. Phosphorylation of PEA-15 switches its binding specificity from ERK/MAPK to FADD. Biochem. J. 2005, 390, 729–735. [Google Scholar] [CrossRef]
- Ramos-Miguel, A.; Sánchez-Blázquez, P.; García-Sevilla, J.A. Effects of Gαi2 and Gαz protein knockdown on alpha2A-adrenergic and cannabinoid CB1 receptor regulation of MEK-ERK and FADD pathways in mouse cerebral cortex. Pharmacol. Rep. 2021, 73, 1122–1135. [Google Scholar] [CrossRef]
- Gao, F.; Wang, J.; Yang, S.; Ji, M.; Zhu, G. Fear extinction induced by activation of PKA ameliorates anxiety-like behavior in PTSD mice. Neuropharmacology 2022, 222, 109306. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Cui, K.; Xing, F.; Liu, X. Akt dependent adult hippocampal neurogenesis regulates the behavioral improvement of treadmill running to mice model of post-traumatic stress disorder. Behav. Brain Res. 2020, 379, 112375. [Google Scholar] [CrossRef] [PubMed]
- Criado-Marrero, M.; Gebru, N.T.; Gould, L.A.; Smith, T.M.; Kim, S.; Blackburn, R.J.; Dickey, C.A.; Blair, L.J. Early Life Stress and High FKBP5 Interact to Increase Anxiety-Like Symptoms through Altered AKT Signaling in the Dorsal Hippocampus. Int. J. Mol. Sci. 2019, 20, 2738. [Google Scholar] [CrossRef] [PubMed]
- García-Fuster, M.J.; Ferrer-Alcón, M.; Martin, M.; Kieffer, B.L.; Maldonado, R.; García-Sevilla, J.A. Effects of constitutive deletion of opioid receptors on the basal densities of Fas and Fas-associated protein with death domain (FADD) in the mouse brain: A δ-opioid tone inhibits FADD. Eur. Neuropsychopharmacol. 2007, 17, 366–374. [Google Scholar] [CrossRef]
- Bouvier, E.; Brouillard, F.; Molet, J.; Claverie, D.; Cabungcal, J.H.; Cresto, N.; Doligez, N.; Rivat, C.; Do, K.Q.; Bernard, C.; et al. Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol. Psychiatry. 2017, 22, 1701–1713. [Google Scholar] [CrossRef]
- Wigner, P.; Synowiec, E.; Jóźwiak, P.; Czarny, P.; Bijak, M.; Białek, K.; Szemraj, J.; Gruca, P.; Papp, M.; Śliwiński, T. The Effect of Chronic Mild Stress and Escitalopram on the Expression and Methylation Levels of Genes Involved in the Oxidative and Nitrosative Stresses as Well as Tryptophan Catabolites Pathway in the Blood and Brain Structures. Int. J. Mol. Sci. 2020, 22, 10. [Google Scholar] [CrossRef]
- Baek, J.H.; Jung, S.; Son, H.; Kang, J.S.; Kim, H.J. Glutamine Supplementation Prevents Chronic Stress-Induced Mild Cognitive Impairment. Nutrients 2020, 12, 910. [Google Scholar] [CrossRef]
- Block, M.L.; Li, G.; Qin, L.; Wu, X.; Pei, Z.; Wang, T.; Wilson, B.; Yang, J.; Hong, J.S. Potent regulation of microglia-derived oxidative stress and dopaminergic neuron survival: Substance P vs. dynorphin. FASEB J. 2006, 20, 251–258. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, X.; Fan, J.; Sun, H.; Yao, Q.; Shi, J.; Qu, H.; Du, S.; Cheng, Y.; Ma, S.; et al. Dynorphin A (1-8) inhibits oxidative stress and apoptosis in MCAO rats, affording neuroprotection through NMDA receptor and κ-opioid receptor channels. Neuropeptides 2021, 89, 102182. [Google Scholar] [CrossRef]
- Zan, G.-Y.; Sun, X.; Wang, Y.J.; Liu, R.; Wang, C.Y.; Du, W.J.; Guo, L.B.; Chai, J.R.; Li, Q.L.; Liu, Z.Q.; et al. Amygdala dynorphin/κ opioid receptor system modulates depressive-like behavior in mice following chronic social defeat stress. Acta Pharmacol. Sin. 2022, 43, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Putt, K.S.; Beilman, G.J.; Hergenrother, P.J. Direct quantitation of poly(ADP-ribose) polymerase (PARP) activity as a means to distinguish necrotic and apoptotic death in cell and tissue samples. Chembiochem 2005, 6, 53–55. [Google Scholar] [CrossRef]
- Bachis, A.; Cruz, M.I.; Nosheny, R.L.; Mocchetti, I. Chronic Unpredictable Stress Promotes Neuronal Apoptosis in the Cerebral Cortex. Neurosci. Lett. 2008, 442, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhang, Y.; Zhang, J.; Wang, H.; Ren, B. ERK in learning and memory: A review of recent research. Int. J. Mol. Sci. 2010, 11, 222–232. [Google Scholar] [CrossRef]
- Sweatt, J.D. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr. Opin. Neurobiol. 2004, 14, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Knoll, A.T.; Carlezon, W.A., Jr. Dynorphin, stress, and depression. Brain Res. 2010, 1314, 56–73. [Google Scholar] [CrossRef]
- Reul, J.M. Making memories of stressful events: A journey along epigenetic, gene transcription, and signaling pathways. Front. Psychiatry 2014, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Hoeffer, C.A.; Klann, E. mTOR signaling: At the crossroads of plasticity, memory and disease. Trends Neurosci. 2010, 33, 67–75. [Google Scholar] [CrossRef]
- Xia, B.; Chen, C.; Zhang, H.; Xue, W.; Tang, J.; Tao, W.; Wu, R.; Ren, L.; Wang, W.; Chen, G.B. Chronic stress prior to pregnancy potentiated long-lasting postpartum depressive-like behavior, regulated by Akt-mTOR signaling in the hippocampus. Sci. Rep. 2016, 6, 35042. [Google Scholar] [CrossRef]
- Seo, M.K.; Choi, C.M.; McIntyre, R.S.; Cho, H.Y.; Lee, C.H.; Mansur, R.B.; Lee, Y.; Lee, J.H.; Kim, Y.H.; Park, S.W.; et al. Effects of escitalopram and paroxetine on mTORC1 signaling in the rat hippocampus under chronic restraint stress. BMC Neurosci. 2017, 18, 39. [Google Scholar] [CrossRef]
- Sharifi, N.; Diehl, N.; Yaswen, L.; Brennan, M.B.; Hochgeschwender, U. Generation of dynorphin knockout mice. Brain Res. Mol. Mol. Brain Res. 2001, 86, 70–75. [Google Scholar] [CrossRef] [PubMed]
- García-Gutiérrez, M.S.; Pérez-Ortiz, J.M.; Gutiérrez-Adán, A.; Manzanares, J. Depression-resistant endophenotype in mice overexpressing cannabinoid CB2 receptors. Br. J. Pharmacol. 2010, 160, 1773–1784. [Google Scholar] [CrossRef]
- Cannistraro, P.A.; Rauch, S.L. Neural circuitry of anxiety: Evidence from structural and functional neuroimaging studies. Psychopharmacol. Bull. 2003, 37, 8–25. [Google Scholar] [PubMed]
- Maren, S.; Holmes, A. Stress and fear extinction. Neuropsychopharmacology 2016, 41, 58–79. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Khachaturian, H.; Lewis, M.E.; Akil, H.; Watson, S.J. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J. Neurosci. 1987, 7, 2445–2464. [Google Scholar] [PubMed]
- Boyarskikh, U.A.; Bondar, N.P.; Filipenko, M.L.; Kudryavtseva, N.N. Downregulation of serotonergic gene expression in the Raphe nuclei of the midbrain under chronic social defeat stress in male mice. Mol. Neurobiol. 2013, 48, 13–21. [Google Scholar] [CrossRef]

| Brain Area | Target | Whole Model | Stress Protocol | Genotype | Interaction | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| df | F-Ratio | p-Val | F-Ratio | p-val | F-Ratio | p-Val | F-Ratio | p-Val | ||
| Cortex | p-JNK1/2 | 5, 42 | 26.6 | <.001 | 35.3 | <.001 | 39.6 | <.001 | 8.38 | <.001 |
| p-ERK1/2 | 5, 42 | 29.8 | <.001 | 139 | <.001 | 9.62 | 0.003 | 0.12 | 0.820 | |
| FADD | 5, 42 | 20.8 | <.001 | 42.7 | <.001 | 9.98 | 0.003 | 2.71 | 0.078 | |
| p-FADD | 5, 41 | 22.5 | <.001 | 53.0 | <.001 | 47.4 | 0.035 | 0.83 | 0.444 | |
| PARP 116 kDa | 5, 42 | 1.08 | 0.384 | 0.41 | 0.668 | 3.33 | 0.075 | 0.84 | 0.440 | |
| PARP 89 kDa | 5, 42 | 0.12 | 0.988 | 0.21 | 0.809 | 0.17 | 0.682 | 0.01 | 0.994 | |
| p-Akt | 5, 38 | 13.0 | <.001 | 30.6 | <.001 | 1.86 | 0.181 | 0.54 | 0.588 | |
| p-Pea15 | 5, 38 | 1.19 | 0.331 | 0.71 | 0.499 | 3.45 | 0.071 | 0.29 | 0.748 | |
| p-mTOR | 5, 27 | 12.7 | <.001 | 29.6 | <.001 | 0.65 | 0.427 | 1.42 | 0.261 | |
| Thalamus | p-JNK1/2 | 5, 42 | 1.20 | 0.324 | 0.38 | 0.686 | 4.68 | 0.036 | 0.15 | 0.861 |
| FADD | 5, 42 | 40.7 | <.001 | 20.8 | <.001 | 140 | <.001 | 11.9 | <.001 | |
| p-FADD | 5, 42 | 6.42 | <.001 | 4.00 | 0.026 | 24.6 | <.001 | 0.23 | 0.796 | |
| p-Akt | 5, 41 | 2.11 | 0.084 | 4.34 | 0.020 | 0.53 | 0.471 | 0.58 | 0.566 | |
| p-Pea15 | 5, 42 | 0.48 | 0.792 | 0.54 | 0.585 | 0.21 | 0.650 | 0.50 | 0.612 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yáñez-Gómez, F.; Ramos-Miguel, A.; García-Sevilla, J.A.; Manzanares, J.; Femenía, T. Regulation of Cortico-Thalamic JNK1/2 and ERK1/2 MAPKs and Apoptosis-Related Signaling Pathways in PDYN Gene-Deficient Mice Following Acute and Chronic Mild Stress. Int. J. Mol. Sci. 2023, 24, 2303. https://doi.org/10.3390/ijms24032303
Yáñez-Gómez F, Ramos-Miguel A, García-Sevilla JA, Manzanares J, Femenía T. Regulation of Cortico-Thalamic JNK1/2 and ERK1/2 MAPKs and Apoptosis-Related Signaling Pathways in PDYN Gene-Deficient Mice Following Acute and Chronic Mild Stress. International Journal of Molecular Sciences. 2023; 24(3):2303. https://doi.org/10.3390/ijms24032303
Chicago/Turabian StyleYáñez-Gómez, Fernando, Alfredo Ramos-Miguel, Jesús A. García-Sevilla, Jorge Manzanares, and Teresa Femenía. 2023. "Regulation of Cortico-Thalamic JNK1/2 and ERK1/2 MAPKs and Apoptosis-Related Signaling Pathways in PDYN Gene-Deficient Mice Following Acute and Chronic Mild Stress" International Journal of Molecular Sciences 24, no. 3: 2303. https://doi.org/10.3390/ijms24032303
APA StyleYáñez-Gómez, F., Ramos-Miguel, A., García-Sevilla, J. A., Manzanares, J., & Femenía, T. (2023). Regulation of Cortico-Thalamic JNK1/2 and ERK1/2 MAPKs and Apoptosis-Related Signaling Pathways in PDYN Gene-Deficient Mice Following Acute and Chronic Mild Stress. International Journal of Molecular Sciences, 24(3), 2303. https://doi.org/10.3390/ijms24032303






