Cabozantinib, Vandetanib, Pralsetinib and Selpercatinib as Treatment for Progressed Medullary Thyroid Cancer with a Main Focus on Hypertension as Adverse Effect
Abstract
1. Introduction
1.1. Thyroid Cancer
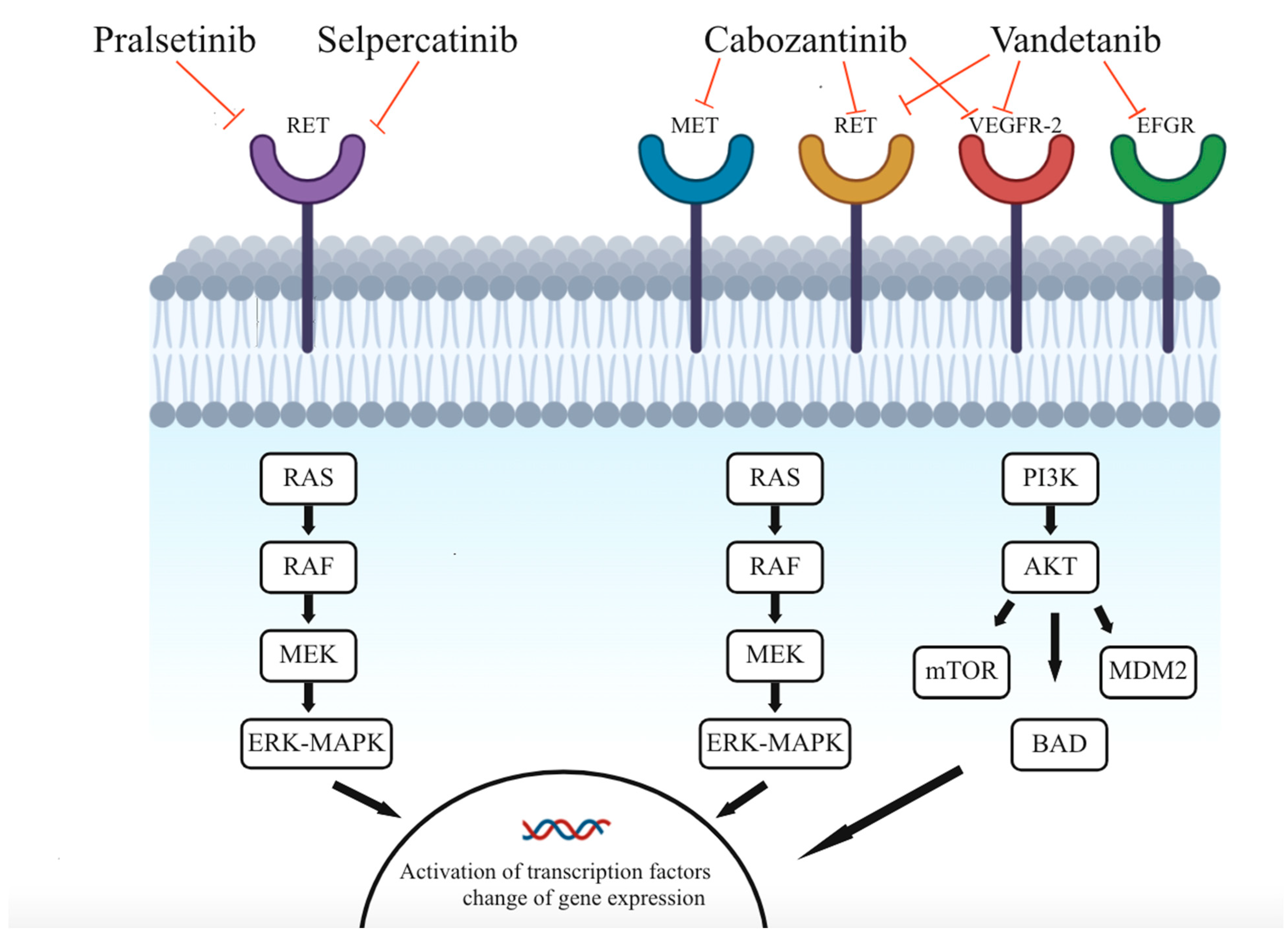
1.2. Tyrosine Kinase Receptors
1.3. Multikinase Inhibitors
1.4. Selective Inhibitors
1.5. Hypertension
1.6. Mechanism of Hypertension as an Adverse Effect
2. Results
2.1. Efficacy of Systemic Treatment with TKIs and Selective Inhibitors
2.2. Clinical Trials
2.3. Therapeutic Management of TKI-induced Hypertension
3. Discussion
4. Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AE | adverse effect |
| ACE | angiotensin-converting enzyme |
| AKT | protein kinase B |
| ARB | angiotensin receptor blocker |
| AXL | tyrosine-protein kinase receptor UFO |
| BAA | beta-adrenoceptor antagonist |
| BAD | BCL2 associated agonist of cell death |
| BP | blood pressure |
| CCB | calcium channel blocker |
| CVD | cardiovascular disease |
| CO | cardiac output |
| EFGR | epidermal growth factor receptor |
| ERK | extracellular regulated kinase |
| ET-1 | endothelin-1 |
| FDA | food and drug administration |
| FLT-3 | fms-like tyrosine kinase 3 |
| GF | growth factor |
| HGF | hepatocyte growth factor |
| HR | hazard ration |
| KIT | tyrosine-protein kinase KIT or CD117 |
| MAP | mean arterial pressure |
| MAPK | mitogen-activated protein kinase |
| MDM2 | mouse double minute 2 |
| MEK | mitogen-activated protein kinase kinase-1 |
| MET | mesenchymal-epithelial transition |
| MET | tyrosine-protein kinase Met or hepatocyte growth factor receptor |
| TKI | multi-kinase inhibitor |
| MTC | medullary thyroid cancer |
| mTOR | mammalian target of rapamycin |
| NO | nitric oxide |
| NTRK-2 | neurotrophic tyrosine receptor kinase 2 |
| OS | overall survival |
| PDGF | platelet-derived growth factor |
| PGI2 | prostaglandins |
| PI3K | phosphoinositide 3 kinases |
| PFS | progression-free survival |
| RAF | rapidly accelerated fibrosarcoma |
| RAS | rat sarcoma |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| RET | rearranged during transfection |
| RTK | receptor tyrosine kinase |
| TC | thyroid cancer |
| TIE-2 | angiopoietin-1 receptor |
| TRKB | tropomyosin receptor kinase B |
| TRP | total peripheral resistance |
| VEGF | vascular endothelial growth factor |
| VEGFR | vascular endothelial growth factor receptor |
| VS | versus |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.; Ouellette, M.; Hunter, M.; Welch, H.G. The increasing incidence of small thyroid cancers: Where are the cases coming from? Laryngoscope 2010, 120, 2446–2451. [Google Scholar] [CrossRef] [PubMed]
- Prete, A.; Borges de Souza, P.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on Fundamental Mechanisms of Thyroid Cancer. Front. Endocrinol (Lausanne) 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Fugazzola, L. Medullary thyroid cancer—An update. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 31, 101655. [Google Scholar] [CrossRef] [PubMed]
- Fagin, J.A.; Wells, S.A., Jr. Biologic and Clinical Perspectives on Thyroid Cancer. N. Engl. J. Med. 2016, 375, 1054–1067. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.X.; Moley, J.F. Surgery for lymph node metastases of medullary thyroid carcinoma: A review. Cancer 2016, 122, 358–366. [Google Scholar] [CrossRef]
- Griebeler, M.L.; Gharib, H.; Thompson, G.B. Medullary thyroid carcinoma. Endocr. Pract. 2013, 19, 703–711. [Google Scholar] [CrossRef]
- Niederle, M.B.; Riss, P.; Selberherr, A.; Koperek, O.; Kaserer, K.; Niederle, B.; Scheuba, C. Omission of lateral lymph node dissection in medullary thyroid cancer without a desmoplastic stromal reaction. Br. J. Surg. 2021, 108, 174–181. [Google Scholar] [CrossRef]
- Tirrò, E.; Martorana, F.; Romano, C.; Vitale, S.R.; Motta, G.; Di Gregorio, S.; Massimino, M.; Pennisi, M.S.; Stella, S.; Puma, A.; et al. Molecular Alterations in Thyroid Cancer: From Bench to Clinical Practice. Genes (Basel) 2019, 10, 709. [Google Scholar] [CrossRef]
- Meijer, J.A.; Bakker, L.E.; Valk, G.D.; de Herder, W.W.; de Wilt, J.H.; Netea-Maier, R.T.; Schaper, N.; Fliers, E.; Lips, P.; Plukker, J.T.; et al. Radioactive iodine in the treatment of medullary thyroid carcinoma: A controlled multicenter study. Eur. J. Endocrinol. 2013, 168, 779–786. [Google Scholar] [CrossRef]
- Wells, S.A., Jr.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Ratajczak, M.; Gaweł, D.; Godlewska, M. Novel Inhibitor-Based Therapies for Thyroid Cancer-An Update. Int. J. Mol. Sci. 2021, 22, 11829. [Google Scholar] [CrossRef] [PubMed]
- Araque, K.A.; Gubbi, S.; Klubo-Gwiezdzinska, J. Updates on the Management of Thyroid Cancer. Horm. Metab. Res. 2020, 52, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Kiesewetter, B.; Riss, P.; Scheuba, C.; Raderer, M. How I treat medullary thyroid cancer. ESMO Open 2021, 6, 100183. [Google Scholar] [CrossRef] [PubMed]
- Milling, R.V.; Grimm, D.; Krüger, M.; Grosse, J.; Kopp, S.; Bauer, J.; Infanger, M.; Wehland, M. Pazopanib, Cabozantinib, and Vandetanib in the Treatment of Progressive Medullary Thyroid Cancer with a Special Focus on the Adverse Effects on Hypertension. Int. J. Mol. Sci. 2018, 19, 3258. [Google Scholar] [CrossRef]
- Fallahi, P.; Ferrari, S.M.; Galdiero, M.R.; Varricchi, G.; Elia, G.; Ragusa, F.; Paparo, S.R.; Benvenga, S.; Antonelli, A. Molecular targets of tyrosine kinase inhibitors in thyroid cancer. Semin. Cancer Biol. 2022, 79, 180–196. [Google Scholar] [CrossRef]
- Eder, J.P.; Vande Woude, G.F.; Boerner, S.A.; LoRusso, P.M. Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin. Cancer Res. 2009, 15, 2207–2214. [Google Scholar] [CrossRef]
- Mohamad Pakarul Razy, N.H.; Wan Abdul Rahman, W.F.; Win, T.T. Expression of Vascular Endothelial Growth Factor and Its Receptors in Thyroid Nodular Hyperplasia and Papillary Thyroid Carcinoma: A Tertiary Health Care Centre Based Study. Asian Pac. J. Cancer Prev. 2019, 20, 277–282. [Google Scholar] [CrossRef]
- Goel, H.L.; Mercurio, A.M. VEGF targets the tumour cell. Nat. Rev. Cancer 2013, 13, 871–882. [Google Scholar] [CrossRef]
- Thein, K.Z.; Velcheti, V.; Mooers, B.H.M.; Wu, J.; Subbiah, V. Precision therapy for RET-altered cancers with RET inhibitors. Trends Cancer 2021, 7, 1074–1088. [Google Scholar] [CrossRef]
- Available online: https://go.drugbank.com/drugs/DB08875 (accessed on 25 May 2022).
- Schmidinger, M.; Danesi, R. Management of Adverse Events Associated with Cabozantinib Therapy in Renal Cell Carcinoma. Oncologist 2018, 23, 306–315. [Google Scholar] [CrossRef]
- Belli, C.; Penault-Llorca, F.; Ladanyi, M.; Normanno, N.; Scoazec, J.Y.; Lacroix, L.; Reis-Filho, J.S.; Subbiah, V.; Gainor, J.F.; Endris, V.; et al. ESMO recommendations on the standard methods to detect RET fusions and mutations in daily practice and clinical research. Ann. Oncol. 2021, 32, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Wirth, L.J.; Sherman, E.; Robinson, B.; Solomon, B.; Kang, H.; Lorch, J.; Worden, F.; Brose, M.; Patel, J.; Leboulleux, S.; et al. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N. Engl. J. Med. 2020, 383, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Selpercatinib: First Approval. Drugs 2020, 80, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Hu, M.I.; Wirth, L.J.; Schuler, M.; Mansfield, A.S.; Curigliano, G.; Brose, M.S.; Zhu, V.W.; Leboulleux, S.; Bowles, D.W.; et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): A multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 2021, 9, 491–501. [Google Scholar] [CrossRef]
- Subbiah, V.; Gainor, J.F.; Rahal, R.; Brubaker, J.D.; Kim, J.L.; Maynard, M.; Hu, W.; Cao, Q.; Sheets, M.P.; Wilson, D.; et al. Precision Targeted Therapy with BLU-667 for RET-Driven Cancers. Cancer Discov. 2018, 8, 836–849. [Google Scholar] [CrossRef]
- Elisei, R.; Schlumberger, M.J.; Müller, S.P.; Schöffski, P.; Brose, M.S.; Shah, M.H.; Licitra, L.; Jarzab, B.; Medvedev, V.; Kreissl, M.C.; et al. Cabozantinib in progressive medullary thyroid cancer. J. Clin. Oncol. 2013, 31, 3639–3646. [Google Scholar] [CrossRef]
- Fallahi, P.; Ferrari, S.M.; Di Bari, F.; Materazzi, G.; Benvenga, S.; Miccoli, P.; Antonelli, A. Cabozantinib in Thyroid Cancer. Recent Pat. Anti-Cancer Drug Discov. 2015, 10, 259–269. [Google Scholar] [CrossRef]
- Wells, S.A., Jr.; Robinson, B.G.; Gagel, R.F.; Dralle, H.; Fagin, J.A.; Santoro, M.; Baudin, E.; Elisei, R.; Jarzab, B.; Vasselli, J.R.; et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: A randomized, double-blind phase III trial. J. Clin. Oncol. 2012, 30, 134–141. [Google Scholar] [CrossRef]
- Thornton, K.; Kim, G.; Maher, V.E.; Chattopadhyay, S.; Tang, S.; Moon, Y.J.; Song, P.; Marathe, A.; Balakrishnan, S.; Zhu, H.; et al. Vandetanib for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease: U.S. Food and Drug Administration drug approval summary. Clin. Cancer Res. 2012, 18, 3722–3730. [Google Scholar] [CrossRef]
- Bradford, D.; Larkins, E.; Mushti, S.L.; Rodriguez, L.; Skinner, A.M.; Helms, W.S.; Price, L.S.L.; Zirkelbach, J.F.; Li, Y.; Liu, J.; et al. FDA Approval Summary: Selpercatinib for the Treatment of Lung and Thyroid Cancers with RET Gene Mutations or Fusions. Clin. Cancer Res. 2021, 27, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- Lamirault, G.; Artifoni, M.; Daniel, M.; Barber-Chamoux, N. Nantes University Hospital Working Group On, H. Resistant Hypertension: Novel Insights. Curr. Hypertens Rev. 2020, 16, 61–72. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Taddei, S.; Bruno, R.M.; Masi, S.; Solini, A. ESC CardioMed. In Epidemiology and Pathophysiology of Hypertension; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Oliveros, E.; Patel, H.; Kyung, S.; Fugar, S.; Goldberg, A.; Madan, N.; Williams, K.A. Hypertension in older adults: Assessment, management, and challenges. Clin. Cardiol. 2020, 43, 99–107. [Google Scholar] [CrossRef] [PubMed]
- National Guideline Centre (UK). NICE Evidence Reviews Collection. In Evidence Summary for Pharmacological Treatment in CVD: Hypertension in Adults (Update): Evidence Review K; National Institute for Health and Care Excellence (NICE): London, UK, 2022. [Google Scholar]
- Hong, Z.; Wu, T.; Zhou, S.; Huang, B.; Wang, J.; Jin, D.; Geng, D. Effects of anti-hypertensive treatment on major cardiovascular events in populations within prehypertensive levels: A systematic review and meta-analysis. J. Hum. Hypertens 2018, 32, 94–104. [Google Scholar] [CrossRef]
- Brunström, M.; Carlberg, B. Association of Blood Pressure Lowering With Mortality and Cardiovascular Disease Across Blood Pressure Levels: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2018, 178, 28–36. [Google Scholar] [CrossRef]
- Sundhed.dk Sundhedsstyrelsen. Available online: https://www.sundhed.dk/sundhedsfaglig/laegehaandbogen/hjerte-kar/tilstande-og-sygdomme/oevrige-sygdomme/hypertension/ (accessed on 25 May 2022).
- Wu, C.Y.; Hu, H.Y.; Chou, Y.J.; Huang, N.; Chou, Y.C.; Li, C.P. High Blood Pressure and All-Cause and Cardiovascular Disease Mortalities in Community-Dwelling Older Adults. Medicine (Baltimore) 2015, 94, e2160. [Google Scholar] [CrossRef]
- Dienstmann, R.; Braña, I.; Rodon, J.; Tabernero, J. Toxicity as a biomarker of efficacy of molecular targeted therapies: Focus on EGFR and VEGF inhibiting anticancer drugs. Oncologist 2011, 16, 1729–1740. [Google Scholar] [CrossRef]
- Kruzliak, P.; Novák, J.; Novák, M. Vascular endothelial growth factor inhibitor-induced hypertension: From pathophysiology to prevention and treatment based on long-acting nitric oxide donors. Am. J. Hypertens. 2014, 27, 3–13. [Google Scholar] [CrossRef]
- Resteghini, C.; Cavalieri, S.; Galbiati, D.; Granata, R.; Alfieri, S.; Bergamini, C.; Bossi, P.; Licitra, L.; Locati, L.D. Management of tyrosine kinase inhibitors (TKI) side effects in differentiated and medullary thyroid cancer patients. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 349–361. [Google Scholar] [CrossRef]
- Facemire, C.S.; Nixon, A.B.; Griffiths, R.; Hurwitz, H.; Coffman, T.M. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension 2009, 54, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.R.; Rivard, A.; van der Zee, R.; Hariawala, M.; Sheriff, D.D.; Esakof, D.D.; Chaudhry, G.M.; Symes, J.F.; Isner, J.M. Vascular endothelial growth factor/vascular permeability factor produces nitric oxide-dependent hypotension. Evidence for a maintenance role in quiescent adult endothelium. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2793–2800. [Google Scholar] [CrossRef]
- Safar, M.E.; Levy, B.I.; Struijker-Boudier, H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation 2003, 107, 2864–2869. [Google Scholar] [CrossRef] [PubMed]
- van Dorst, D.C.H.; Dobbin, S.J.H.; Neves, K.B.; Herrmann, J.; Herrmann, S.M.; Versmissen, J.; Mathijssen, R.H.J.; Danser, A.H.J.; Lang, N.N. Hypertension and Prohypertensive Antineoplastic Therapies in Cancer Patients. Circ. Res. 2021, 128, 1040–1061. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Qin, Y.; Maxhimer, J.B.; Sipes, J.M.; Despres, D.; Schnermann, J.; Frazier, W.A.; Roberts, D.D. Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol. 2009, 28, 110–119. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Ridnour, L.A.; Dimitry, J.; Frazier, W.A.; Wink, D.A.; Roberts, D.D. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J. Biol. Chem. 2006, 281, 26069–26080. [Google Scholar] [CrossRef] [PubMed]
- Bronte, E.; Bronte, G.; Novo, G.; Rinaldi, G.; Bronte, F.; Passiglia, F.; Russo, A. Cardiotoxicity mechanisms of the combination of BRAF-inhibitors and MEK-inhibitors. Pharmacol. Ther. 2018, 192, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Jiang, C.C.; Yan, X.G.; Tseng, H.Y.; Wang, C.Y.; Zhang, Y.Y.; Yari, H.; La, T.; Farrelly, M.; Guo, S.T.; et al. BRAF/MEK inhibitors promote CD47 expression that is reversible by ERK inhibition in melanoma. Oncotarget 2017, 8, 69477–69492. [Google Scholar] [CrossRef]
- Zheng, X.; Ji, Q.; Sun, Y.; Ge, M.; Zhang, B.; Cheng, Y.; Lei, S.; Shi, F.; Guo, Y.; Li, L.; et al. Efficacy and safety of selpercatinib in Chinese patients with advanced RET-altered thyroid cancers: Results from the phase II LIBRETTO-321 study. Ther. Adv. Med. Oncol. 2022, 14, 17588359221119318. [Google Scholar] [CrossRef]
- Wirth, L.J.; Brose, M.S.; Elisei, R.; Capdevila, J.; Hoff, A.O.; Hu, M.I.; Tahara, M.; Robinson, B.; Gao, M.; Xia, M.; et al. LIBRETTO-531: A phase III study of selpercatinib in multikinase inhibitor-naïve RET-mutant medullary thyroid cancer. Future Oncol. 2022, 18, 3143–3150. [Google Scholar] [CrossRef]
- Kim, J.; Bradford, D.; Larkins, E.; Pai-Scherf, L.H.; Chatterjee, S.; Mishra-Kalyani, P.S.; Wearne, E.; Helms, W.S.; Ayyoub, A.; Bi, Y.; et al. FDA Approval Summary: Pralsetinib for the Treatment of Lung and Thyroid Cancers With RET Gene Mutations or Fusions. Clin. Cancer Res. 2021, 27, 5452–5456. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Elisei, R.; Müller, S.; Schöffski, P.; Brose, M.; Shah, M.; Licitra, L.; Krajewska, J.; Kreissl, M.C.; Niederle, B.; et al. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann. Oncol. 2017, 28, 2813–2819. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, B.H. Current Guidelines for Management of Medullary Thyroid Carcinoma. Endocrinol. Metab. (Seoul) 2021, 36, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Tappenden, P.; Carroll, C.; Hamilton, J.; Kaltenthaler, E.; Wong, R.; Wadsley, J.; Moss, L.; Balasubramanian, S. Cabozantinib and vandetanib for unresectable locally advanced or metastatic medullary thyroid cancer: A systematic review and economic model. Health Technol. Assess. 2019, 23, 1–144. [Google Scholar] [CrossRef]
- Koehler, V.F.; Adam, P.; Frank-Raue, K.; Raue, F.; Berg, E.; Hoster, E.; Allelein, S.; Schott, M.; Kroiss, M.; Spitzweg, C. Real-World Efficacy and Safety of Cabozantinib and Vandetanib in Advanced Medullary Thyroid Cancer. Thyroid 2021, 31, 459–469. [Google Scholar] [CrossRef]
- Ceolin, L.; Duval, M.; Benini, A.F.; Ferreira, C.V.; Maia, A.L. Medullary thyroid carcinoma beyond surgery: Advances, challenges, and perspectives. Endocr. Relat. Cancer 2019, 26, R499–R518. [Google Scholar] [CrossRef]
- Wang, S.X.; Zhang, X.W.; Wang, X.X.; An, C.M.; Zhang, Y.B.; Liu, W.; Zhao, Y.F.; He, X.H.; Li, Z.J.; Niu, L.J.; et al. Efficacy and safety of vandetanib on advanced medullary thyroid carcinoma: Single center result from a phase Ⅲ study. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2019, 54, 439–444. [Google Scholar]
- Kurzrock, R.; Sherman, S.I.; Ball, D.W.; Forastiere, A.A.; Cohen, R.B.; Mehra, R.; Pfister, D.G.; Cohen, E.E.; Janisch, L.; Nauling, F.; et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J. Clin. Oncol. 2011, 29, 2660–2666. [Google Scholar] [CrossRef]
- Kreissl, M.C.; Bastholt, L.; Elisei, R.; Haddad, R.; Hauch, O.; Jarząb, B.; Robinson, B.; Colzani, R.; Foster, M.; Weiss, R.; et al. Efficacy and Safety of Vandetanib in Progressive and Symptomatic Medullary Thyroid Cancer: Post Hoc Analysis From the ZETA Trial. J. Clin. Oncol. 2020, 38, 2773–2781. [Google Scholar] [CrossRef]
- Trimboli, P.; Castellana, M.; Virili, C.; Giorgino, F.; Giovanella, L. Efficacy of Vandetanib in Treating Locally Advanced or Metastatic Medullary Thyroid Carcinoma According to RECIST Criteria: A Systematic Review and Meta-Analysis. Front. Endocrinol. (Lausanne) 2018, 9, 224. [Google Scholar] [CrossRef]
- Nguyen, L.; Holland, J.; Miles, D.; Engel, C.; Benrimoh, N.; O′Reilly, T.; Lacy, S. Pharmacokinetic (PK) drug interaction studies of cabozantinib: Effect of CYP3A inducer rifampin and inhibitor ketoconazole on cabozantinib plasma PK and effect of cabozantinib on CYP2C8 probe substrate rosiglitazone plasma PK. J. Clin. Pharmacol. 2015, 55, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.; Kurschat, C.; Reuter, H. Arterial Hypertension. Dtsch. Arztebl. Int. 2018, 115, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Wiysonge, C.S.; Bradley, H.A.; Volmink, J.; Mayosi, B.M.; Opie, L.H. Beta-blockers for hypertension. Cochrane Database Syst. Rev. 2017, 1, Cd002003. [Google Scholar] [CrossRef]
- Gong, Q.; Davis, M.; Chipitsyna, G.; Yeo, C.J.; Arafat, H.A. Blocking angiotensin II Type 1 receptor triggers apoptotic cell death in human pancreatic cancer cells. Pancreas 2010, 39, 581–594. [Google Scholar] [CrossRef]
- Hashemzehi, M.; Rahmani, F.; Khoshakhlagh, M.; Avan, A.; Asgharzadeh, F.; Barneh, F.; Moradi-Marjaneh, R.; Soleimani, A.; Fiuji, H.; Ferns, G.A.; et al. Angiotensin receptor blocker Losartan inhibits tumor growth of colorectal cancer. EXCLI J. 2021, 20, 506–521. [Google Scholar]
- Wasa, J.; Sugiura, H.; Kozawa, E.; Kohyama, K.; Yamada, K.; Taguchi, O. The tumor suppressive effect of angiotensin II type 1 receptor antagonist in a murine osteosarcoma model. Anticancer. Res. 2011, 31, 123–127. [Google Scholar] [PubMed]
- Kruzliak, P.; Kovacova, G.; Pechanova, O. Therapeutic potential of nitric oxide donors in the prevention and treatment of angiogenesis-inhibitor-induced hypertension. Angiogenesis 2013, 16, 289–295. [Google Scholar] [CrossRef]
- Valerio, L.; Bottici, V.; Matrone, A.; Piaggi, P.; Viola, D.; Cappagli, V.; Agate, L.; Molinaro, E.; Ciampi, R.; Tacito, A.; et al. Medullary thyroid cancer treated with vandetanib: Predictors of a longer and durable response. Endocr. Relat. Cancer 2020, 27, 97–110. [Google Scholar] [CrossRef]
- Viola, D.; Valerio, L.; Molinaro, E.; Agate, L.; Bottici, V.; Biagini, A.; Lorusso, L.; Cappagli, V.; Pieruzzi, L.; Giani, C.; et al. Treatment of advanced thyroid cancer with targeted therapies: Ten years of experience. Endocr. Relat. Cancer 2016, 23, R185–R205. [Google Scholar] [CrossRef]
- Liu, X.; Shen, T.; Mooers, B.H.M.; Hilberg, F.; Wu, J. Drug resistance profiles of mutations in the RET kinase domain. Br. J. Pharmacol. 2018, 175, 3504–3515. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.; Klochikhin, A.; Leboulleux, S.; Isaev, P.; Badiu, C.; Robinson, B.; Hughes, B.G.M.; Keam, B.; Parnis, F.; Elisei, R.; et al. A Randomized, Double-Blind Noninferiority Study to Evaluate the Efficacy of the Cabozantinib Tablet at 60 mg per Day Compared with the Cabozantinib Capsule at 140 mg per Day in Patients With Progressive, Metastatic Medullary Thyroid Cancer. Thyroid 2022, 32, 515–524. [Google Scholar] [CrossRef]
- Du, B.; Wang, F.; Wu, L.; Wang, Z.; Zhang, D.; Huang, Z.; Gao, L.; Li, Y.; Liang, C.; Li, P.; et al. Cause-specific mortality after diagnosis of thyroid cancer: A large population-based study. Endocrine 2021, 72, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Roman, S.; Lin, R.; Sosa, J.A. Prognosis of medullary thyroid carcinoma: Demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer 2006, 107, 2134–2142. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.R. Clinical trial structures. J. Exp. Stroke Transl. Med. 2010, 3, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Vandetanib Study. Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT01496313?cond=Vandetanib+MTC&draw=2&rank=10&view=results (accessed on 27 May 2022).

| Drug | Targets | Dose | Approval for MTC | Structure |
|---|---|---|---|---|
| Cabozantinib [28,29] | MET, RET and VEGFR-2 | 140 mg/day | 2012 (FDA) 2013 (EMA |  |
| Vandetanib [30,31] | RET, VEGFR-2 and EGFR | 300 mg/day | 2012 (FDA) 2012 (EMA) |  |
| Selpercatinib [32] | RET | 160 mg twice a day | 2020 (FDA) 2021 (EMA) |  |
| Pralsetinib [26] | RET | 300mg/day | 2020 (FDA) NA (EMA) |  |
| Category | Systolic (mmHg) | Diastolic (mmHg) |
|---|---|---|
| Optimal | <120 | <80 |
| Normal | 120–129 | 80–84 |
| High normal | 130–139 | 85–89 |
| Grade 1 hypertension | 140–159 | 90–99 |
| Grade 2 hypertension | 160–170 | 100–109 |
| Grade 3 hypertension | ≥180 | ≥110 |
| Study | Deign | Participants | Outcome |
|---|---|---|---|
| Cabozantinib vs. placebo (EXAM) (NCT00704730) [28,56] | Phase III | 219 vs. 111 | PFS of 11.2 months vs. 4.0 months HR: 0.28;95%CI: 0.19–0.40; p < 0.001 |
| Vandetanib vs. placebo (ZETA) (NCT00410761) [30,63] | Phase III | 231 vs. 100 | PFS of 30.5 months vs. 19.3 months HR: 0.46; 95%CI: 0.31–0.69; p < 0.001 |
| Selpercatinib (LIBRETTO-001) (NCT04280081) [25] | Phase I/II | 55 pretreated 88 untreated All RET-mutated | HR: 0.73; 95%CI: 0.62–0.82 |
| Pralsetinib (ARROW) (NCT03037385) [26,55] | Phase I/II | 55 pretreated 29 untreated All RET-mutated | HR: 0.71; 95%CI: 0.48–0.89 |
| Grade 3–4 | Dose Reduction | Interruption | Discontinuation | |
|---|---|---|---|---|
| Selpercatinib [25] | 21% | 31% | 5% | 2% |
| Pralsetinib [26] | 17% | NA | NA | 4% |
| Cabozantinib [28] | 8.4% | 79% | 65% | 16% |
| Vandetanib [30] | 9% | 35% | NA | 12% |
| Selpercatinib [25] | Pralsetinib [55] | Vandetanib [30] | Cabozantinib [28] | |||||
|---|---|---|---|---|---|---|---|---|
| Most common AEs | Dry mouth | 39 | Musculoskeletal pain | 42 | Diarrhea | 56 | Diarrhea | 63 |
| Diarrhea | 37 | Constipation | 41 | Rash | 45 | Palmar-plantar Erythrodysesthesia | 50 | |
| Hypertension | 35 | Hypertension | 40 | Nausea | 33 | Decreased weight | 48 | |
| Fatigue | 35 | Fatigue | 38 | Hypertension | 32 | Decreased appetite | 48 | |
| Oedema | 33 | Diarrhea | 34 | Headache | 26 | Nausea | 43 | |
| Title and NCT Number | Design | Recruitment Status |
|---|---|---|
| A phase III, randomized, open-label study of pralsetinib versus standard of care for treatment of RET-mutated medullary thyroid cancer (NCT04760288) | A phase III, randomized, open-label study of pralsetinib versus standard of care for treatment of RET-mutated medullary thyroid cancer | Not yet recruiting |
| A phase 1/2 study of the highly-selective RET inhibitor, BLU-667, in patients with thyroid cancer, non-small cell lung cancer (NSCLC), and other advanced solid tumors (NCT03037385) | A phase I/II, non- randomized, open-label, first-in-human study | Active, not recruiting |
| A multicenter, randomized, open-label, phase 3 trial comparing selpercatinib to physicians’ choice of cabozantinib or vandetanib in patients with progressive, advanced, kinase inhibitor naive, RET-mutant medullary thyroid cancer (LIBRETTO-531) (NCT04211337) | A phase III, randomized, open-label study | Recruiting |
| A phase 1/2 study of oral selpercatinib (LOXO-292) in patients with advanced solid tumors, including RET fusion-positive solid tumors, medullary thyroid cancer, and other tumors with RET activation (LIBRETTO-001) (NCT03157128) | A phase I/II, open-label study | Recruiting |
| Neoadjuvant treatment with selpercatinib in RET-altered thyroid cancers cancer (NCT04759911) | A phase II, open-label study | Recruiting |
| An international, randomized, double-blind, two-arm study to evaluate the safety and efficacy of vandetanib 150 And 300mg/Day In Patients With Unresectable Locally Advanced Or Metastatic Medullary Thyroid Carcinoma With Progressive Or Symptomatic Disease (NCT01496313) | A phase IV, randomized, double-blind study | Active, not recruiting |
| A randomized, double-blind study to evaluate the efficacy and safety of cabozantinib (XL184) at 60 mg/day compared to a 140 mg/day in progressive, metastatic medullary thyroid cancer patients (NCT01896479) | A phase IV, randomized, double-blind study | Active, not recruiting |
| A multicenter, randomized, open-label, phase 3 trial comparing selpercatinib to physicians’ choice of cabozantinib or vandetanib in patients with progressive, advanced, kinase inhibitor naïve, RET-mutant medullary thyroid cancer (LIBRETTO-531) (NCT04211337) | A phase III, randomized, open-label study | Recruiting |
| Pilot trial of nivolumab plus cabozantinib for advanced solid tumors in patients with HIV infection (NCT04514484) | A phase I, open-label study | Recruiting |
| A Randomized, Int., Open-Label Phase III Study to Assess the Effect of a Patient Outreach Program on the Percentage of Time Patients With Locally Advanced or Metastatic MTC Experience Grade 2 or Higher AEs in the First 12 Months of Treatment With Vandetanib (NCT01298323) | A phase III, open-label study | Active, not recruiting |
| Effects of tyrosine kinase inhibitors on body composition in endocrine tumors—a pilot study (NCT02592356) | Phase is not applicable, open-label study | Active, not recruiting |
| Title and NCT Number | Design | Recruitment Status |
|---|---|---|
| An international, randomized, double-blinded, phase 3 efficacy study of XL184 versus placebo in subjects with unresectable, locally advanced, or metastatic medullary thyroid cancer (NCT00704730) | A phase III, randomized, double-blinded study | Completed |
| Molecular profile of metastatic sporadic medullary thyroid cancer (sMTC) patients and possible correlation with vandetanib therapy (NCT02268734) | Observational study | Completed |
| European, observational, prospective study to evaluate the benefit/risk of vandetanib in RET mutation negative and positive patients with symptomatic, aggressive, sporadic, unresectable, locally advanced/metastatic medullary thyroid cancer (NCT01945762) | Observational study | Completed |
| A phase I/II, open-label study to evaluate the safety and tolerability of vandetanib 300 mg/Day in Japanese patients With unresectable locally advanced or metastatic medullary thyroid carcinoma (NCT01661179) | A phase I/II, open-label study | Completed |
| CAPRELSA®® REGISTRY: a Belgian registry to evaluate the use of vandetanib (Caprelsa®®) in current clinical practice (NCT02109250) | Observational study | Completed |
| Effectiveness of Risk minimization interventions for vandetanib in Canada (NCT01757470) | Observational study | Completed |
| A Phase I, randomized, open-label, single-center study to assess the pharmacokinetics of vandetanib (CAPRELSA) in healthy subjects when a single oral dose of vandetanib 300 mg is administered alone and in combination with omeprazole or ranitidine (NCT01539655) | A phase I, randomized, open-label study | Completed |
| Recommended antihypertensive drugs | ACE inhibitors ARB BAA CCB (but not in combination with cabozantinib) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Højer Wang, L.; Wehland, M.; Wise, P.M.; Infanger, M.; Grimm, D.; Kreissl, M.C. Cabozantinib, Vandetanib, Pralsetinib and Selpercatinib as Treatment for Progressed Medullary Thyroid Cancer with a Main Focus on Hypertension as Adverse Effect. Int. J. Mol. Sci. 2023, 24, 2312. https://doi.org/10.3390/ijms24032312
Højer Wang L, Wehland M, Wise PM, Infanger M, Grimm D, Kreissl MC. Cabozantinib, Vandetanib, Pralsetinib and Selpercatinib as Treatment for Progressed Medullary Thyroid Cancer with a Main Focus on Hypertension as Adverse Effect. International Journal of Molecular Sciences. 2023; 24(3):2312. https://doi.org/10.3390/ijms24032312
Chicago/Turabian StyleHøjer Wang, Linnea, Markus Wehland, Petra M. Wise, Manfred Infanger, Daniela Grimm, and Michael C. Kreissl. 2023. "Cabozantinib, Vandetanib, Pralsetinib and Selpercatinib as Treatment for Progressed Medullary Thyroid Cancer with a Main Focus on Hypertension as Adverse Effect" International Journal of Molecular Sciences 24, no. 3: 2312. https://doi.org/10.3390/ijms24032312
APA StyleHøjer Wang, L., Wehland, M., Wise, P. M., Infanger, M., Grimm, D., & Kreissl, M. C. (2023). Cabozantinib, Vandetanib, Pralsetinib and Selpercatinib as Treatment for Progressed Medullary Thyroid Cancer with a Main Focus on Hypertension as Adverse Effect. International Journal of Molecular Sciences, 24(3), 2312. https://doi.org/10.3390/ijms24032312








