Dietary Walnuts Preserve Aspects of Health Span and Alter the Hippocampal Lipidome in Aged High-Fat Diet-Fed Mice
Abstract
:1. Introduction
2. Results
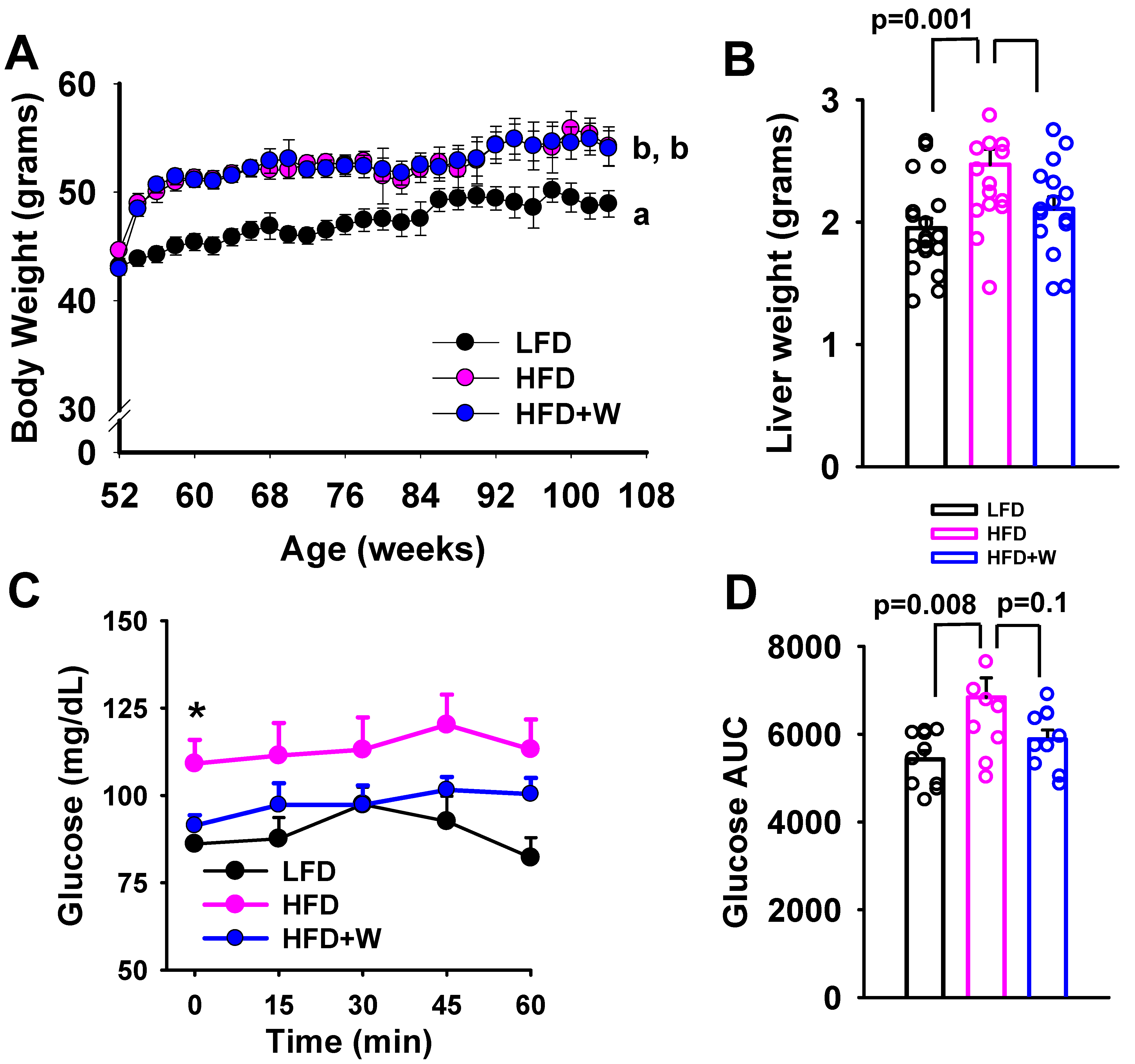
2.1. Walnuts Tend to Preserve Insulin Action and Prevent Increased Liver Weight Accrual on an HFD
2.2. Walnuts Preserve Working Memory with Aging, but No Significant Effect on Frailty or Survival
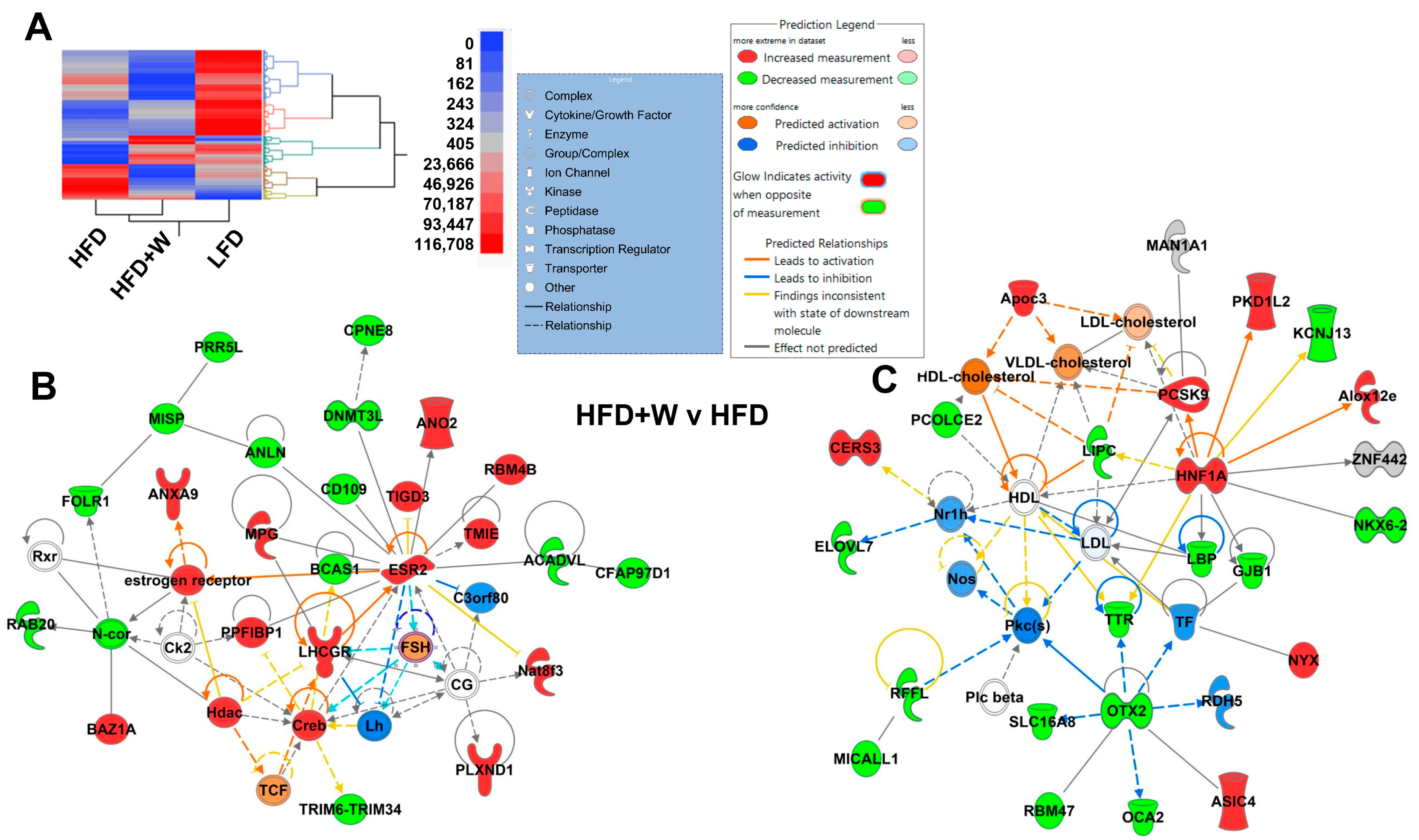
2.3. IPA Identifies Estrogen Receptor β and Lipid Metabolism as Potential Targets of Walnuts in Hippocampus
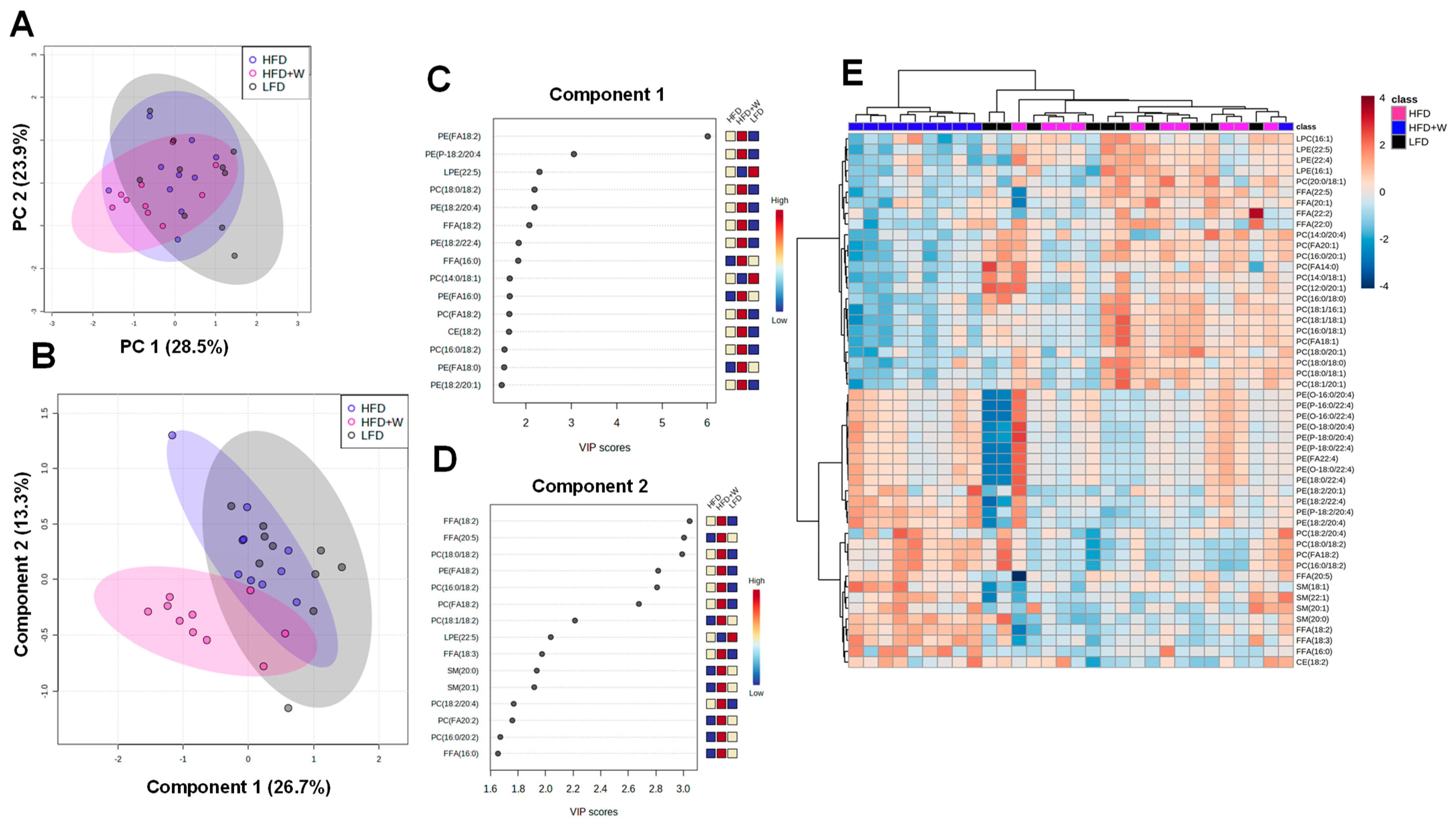
2.4. Walnut Supplementation Alters the Hippocampal Lipidome
3. Discussion
4. Materials and Methods
4.1. Generation of Walnut Diets
4.2. Animals and Design
4.3. Basic Physiology Characteristics
4.4. Physical Performance
4.5. Memory Assessment via Y Maze
4.6. Protein Isolation and Western Blotting
4.7. RNA Isolation and RT-qPCR
4.8. RNAseq and Analysis
4.9. Ingenuity Pathway Analysis
4.10. Antioxidant Capacity and Oxidative Stress Markers
4.11. Hippocampal Lipidomics
4.12. Splenocyte Isolation and Flow Cytometry
4.13. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barzilai, N.; Huffman, D.M.; Muzumdar, R.H.; Bartke, A. The critical role of metabolic pathways in aging. Diabetes 2012, 61, 1315–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, D.E.; Strong, R.; Alavez, S.; Astle, C.M.; DiGiovanni, J.; Fernandez, E.; Flurkey, K.; Garratt, M.; Gelfond, J.A.L.; Javors, M.A.; et al. Acarbose improves health and lifespan in aging HET3 mice. Aging Cell 2019, 18, e12898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadon, N.L.; Strong, R.; Miller, R.A.; Harrison, D.E. NIA Interventions Testing Program: Investigating Putative Aging Intervention Agents in a Genetically Heterogeneous Mouse Model. EBioMedicine 2017, 21, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, K.; Neff, F.; Markert, A.; Rozman, J.; Aguilar-Pimentel, J.A.; Amarie, O.V.; Becker, L.; Brommage, R.; Garrett, L.; Henzel, K.S.; et al. Every-other-day feeding extends lifespan but fails to delay many symptoms of aging in mice. Nat. Commun. 2017, 8, 155. [Google Scholar] [CrossRef] [Green Version]
- Honjoh, S.; Yamamoto, T.; Uno, M.; Nishida, E. Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature 2009, 457, 726–730. [Google Scholar] [CrossRef]
- Newman, J.C.; Covarrubias, A.J.; Zhao, M.; Yu, X.; Gut, P.; Ng, C.P.; Huang, Y.; Haldar, S.; Verdin, E. Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metab. 2017, 26, 547–557.e8. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M.N.; Wallace, M.A.; Tomilov, A.A.; Zhou, Z.; Marcotte, G.R.; Tran, D.; Perez, G.; Gutierrez-Casado, E.; Koike, S.; Knotts, T.A.; et al. A Ketogenic Diet Extends Longevity and Healthspan in Adult Mice. Cell Metab. 2017, 26, 539–546.e5. [Google Scholar] [CrossRef] [Green Version]
- Solon-Biet, S.M.; Cogger, V.C.; Pulpitel, T.; Wahl, D.; Clark, X.; Bagley, E.; Gregoriou, G.C.; Senior, A.M.; Wang, Q.-P.; Brandon, A.E.; et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab. 2019, 1, 532–545. [Google Scholar] [CrossRef]
- Lee, B.C.; Kaya, A.; Ma, S.; Kim, G.; Gerashchenko, M.V.; Yim, S.H.; Hu, Z.; Harshman, L.G.; Gladyshev, V.N. Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino-acid status. Nat. Commun. 2014, 5, 3592. [Google Scholar] [CrossRef] [Green Version]
- Richie, J.P., Jr.; Leutzinger, Y.; Parthasarathy, S.; Malloy, V.; Orentreich, N.; Zimmerman, J.A. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J. 1994, 8, 1302–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orentreich, N.; Matias, J.R.; DeFelice, A.; Zimmerman, J.A. Low methionine ingestion by rats extends life span. J. Nutr. 1993, 123, 269–274. [Google Scholar] [PubMed]
- Sataranatarajan, K.; Pharaoh, G.; Brown, J.L.; Ranjit, R.; Piekarz, K.M.; Street, K.; Wren, J.D.; Georgescu, C.; Kinter, C.; Kinter, M.; et al. Molecular changes in transcription and metabolic pathways underlying muscle atrophy in the CuZnSOD null mouse model of sarcopenia. Geroscience 2020, 42, 1101–1118. [Google Scholar] [CrossRef]
- Perez, V.I.; Van Remmen, H.; Bokov, A.; Epstein, C.J.; Vijg, J.; Richardson, A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell 2009, 8, 73–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massie, H.R.; Aiello, V.R.; Doherty, T.J. Dietary vitamin C improves the survival of mice. Gerontology 1984, 30, 371–375. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Kloting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [Green Version]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Strong, R.; Miller, R.A.; Astle, C.M.; Baur, J.A.; de Cabo, R.; Fernandez, E.; Guo, W.; Javors, M.; Kirkland, J.L.; Nelson, J.F.; et al. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 6–16. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.A.; Harrison, D.E.; Astle, C.M.; Bogue, M.A.; Brind, J.; Fernandez, E.; Flurkey, K.; Javors, M.; Ladiges, W.; Leeuwenburgh, C.; et al. Glycine supplementation extends lifespan of male and female mice. Aging Cell 2019, 18, e12953. [Google Scholar] [CrossRef] [Green Version]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Freedman, N.D.; Park, Y.; Abnet, C.C.; Hollenbeck, A.R.; Sinha, R. Association of coffee drinking with total and cause-specific mortality. N. Engl. J. Med. 2012, 366, 1891–1904. [Google Scholar] [CrossRef]
- Bao, Y.; Han, J.; Hu, F.B.; Giovannucci, E.L.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S. Association of nut consumption with total and cause-specific mortality. N. Engl. J. Med. 2013, 369, 2001–2011. [Google Scholar] [CrossRef] [Green Version]
- Pan, A.; Sun, Q.; Manson, J.E.; Willett, W.C.; Hu, F.B. Walnut consumption is associated with lower risk of type 2 diabetes in women. J. Nutr. 2013, 143, 512–518. [Google Scholar] [CrossRef] [Green Version]
- Banel, D.K.; Hu, F.B. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: A meta-analysis and systematic review. Am. J. Clin. Nutr. 2009, 90, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Poulose, S.M.; Miller, M.G.; Shukitt-Hale, B. Role of walnuts in maintaining brain health with age. J. Nutr. 2014, 144, 561S–566S. [Google Scholar] [CrossRef] [Green Version]
- Hardman, W.E. Walnuts have potential for cancer prevention and treatment in mice. J. Nutr. 2014, 144, 555S–560S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kris-Etherton, P.M. Walnuts decrease risk of cardiovascular disease: A summary of efficacy and biologic mechanisms. J. Nutr. 2014, 144, 547S–554S. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.J.; Teuber, S.S.; Gobeille, A.; Cremin, P.; Waterhouse, A.L.; Steinberg, F.M. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J. Nutr. 2001, 131, 2837–2842. [Google Scholar] [CrossRef] [Green Version]
- Nagel, J.M.; Brinkoetter, M.; Magkos, F.; Liu, X.; Chamberland, J.P.; Shah, S.; Zhou, J.; Blackburn, G.; Mantzoros, C.S. Dietary walnuts inhibit colorectal cancer growth in mice by suppressing angiogenesis. Nutrition 2012, 28, 67–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, F.; Tabrizian, T.; Novaj, A.; Nakanishi, M.; Rosenberg, D.W.; Huffman, D.M. Dietary Walnuts Protect Against Obesity-Driven Intestinal Stem Cell Decline and Tumorigenesis. Front Nutr. 2018, 5, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, R.A.; Harrison, D.E.; Astle, C.M.; Baur, J.A.; Boyd, A.R.; de Cabo, R.; Fernandez, E.; Flurkey, K.; Javors, M.A.; Nelson, J.F.; et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Quipildor, G.F.; Tabrizian, T.; Novaj, A.; Guan, F.; Walters, R.O.; Delahaye, F.; Hubbard, G.B.; Ikeno, Y.; Ejima, K.; et al. Late-life targeting of the IGF-1 receptor improves healthspan and lifespan in female mice. Nat. Commun. 2018, 9, 2394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farias Quipildor, G.E.; Mao, K.; Hu, Z.; Novaj, A.; Cui, M.H.; Gulinello, M.; Branch, C.A.; Gubbi, S.; Patel, K.; Moellering, D.R.; et al. Central IGF-1 protects against features of cognitive and sensorimotor decline with aging in male mice. Geroscience 2019, 41, 185–208. [Google Scholar] [CrossRef] [PubMed]
- Sabate, J.; Fraser, G.E.; Burke, K.; Knutsen, S.F.; Bennett, H.; Lindsted, K.D. Effects of walnuts on serum lipid levels and blood pressure in normal men. N. Engl. J. Med. 1993, 328, 603–607. [Google Scholar] [CrossRef]
- Zambon, D.; Sabate, J.; Munoz, S.; Campero, B.; Casals, E.; Merlos, M.; Laguna, J.C.; Ros, E. Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women. A randomized crossover trial. Ann. Intern. Med. 2000, 132, 538–546. [Google Scholar] [CrossRef]
- Ros, E.; Nunez, I.; Perez-Heras, A.; Serra, M.; Gilabert, R.; Casals, E.; Deulofeu, R.; Ros, E. A walnut diet improves endothelial function in hypercholesterolemic subjects: A randomized crossover trial. Circulation 2004, 109, 1609–1614. [Google Scholar] [CrossRef] [Green Version]
- Halvorsen, B.L.; Carlsen, M.H.; Phillips, K.M.; Bohn, S.K.; Holte, K.; Jacobs, D.R., Jr.; Blomhoff, R. Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. Am. J. Clin. Nutr. 2006, 84, 95–135. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, M.; Chen, Y.; Qendro, V.; Miyamoto, S.; Weinstock, E.; Weinstock, G.M.; Rosenberg, D.W. Effects of Walnut Consumption on Colon Carcinogenesis and Microbial Community Structure. Cancer Prev. Res. (Phila) 2016, 9, 692–703. [Google Scholar] [CrossRef] [Green Version]
- Tsoukas, M.A.; Ko, B.J.; Witte, T.R.; Dincer, F.; Hardman, W.E.; Mantzoros, C.S. Dietary walnut suppression of colorectal cancer in mice: Mediation by miRNA patterns and fatty acid incorporation. J. Nutr. Biochem. 2015, 26, 776–783. [Google Scholar] [CrossRef]
- Hardman, W.E.; Ion, G.; Akinsete, J.A.; Witte, T.R. Dietary walnut suppressed mammary gland tumorigenesis in the C(3)1 TAg mouse. Nutr. Cancer 2011, 63, 960–970. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, M.; Matz, A.; Klemashevich, C.; Rosenberg, D.W. Dietary Walnut Supplementation Alters Mucosal Metabolite Profiles During DSS-Induced Colonic Ulceration. Nutrients 2019, 11, 1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandareesh, M.D.; Chauhan, V.; Chauhan, A. Walnut Supplementation in the Diet Reduces Oxidative Damage and Improves Antioxidant Status in Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2018, 64, 1295–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthaiyah, B.; Essa, M.M.; Lee, M.; Chauhan, V.; Kaur, K.; Chauhan, A. Dietary supplementation of walnuts improves memory deficits and learning skills in transgenic mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2014, 42, 1397–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sala-Vila, A.; Valls-Pedret, C.; Rajaram, S.; Coll-Padros, N.; Cofan, M.; Serra-Mir, M.; Pérez-Heras, A.M.; Roth, I.; Freitas-Simoes, T.M.; Doménech, M.; et al. Effect of a 2-year diet intervention with walnuts on cognitive decline. The Walnuts And Healthy Aging (WAHA) study: A randomized controlled trial. Am. J. Clin. Nutr. 2020, 111, 590–600. [Google Scholar] [CrossRef]
- Pribis, P.; Bailey, R.N.; Russell, A.A.; Kilsby, M.A.; Hernandez, M.; Craig, W.J.; Grajales, T.; Shavlik, D.J.; Sabatè, J. Effects of walnut consumption on cognitive performance in young adults. Br. J. Nutr. 2012, 107, 1393–1401. [Google Scholar] [CrossRef] [Green Version]
- Bishop, N.J.; Zuniga, K.E. Investigating walnut consumption and cognitive trajectories in a representative sample of older US adults. Public Health Nutr. 2020, 24, 1741–1752. [Google Scholar] [CrossRef]
- Choi, Y.; Abdelmegeed, M.A.; Song, B.J. Preventive effects of dietary walnuts on high-fat-induced hepatic fat accumulation, oxidative stress and apoptosis in mice. J. Nutr. Biochem. 2016, 38, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.L., Jr.; Yang, Y.; Nagy, T.R.; Patki, A.; Vasselli, J.R.; Zhang, Y.; Dickinson, S.L.; Allison, D.B. Weight Cycling Increases Longevity Compared with Sustained Obesity in Mice. Obesity (Silver Spring) 2018, 26, 1733–1739. [Google Scholar] [CrossRef] [Green Version]
- Augenlicht, L. Hidden effects of mouse chow. Science 2014, 346, 710. [Google Scholar] [CrossRef]
- List, E.O.; Berryman, D.E.; Wright-Piekarski, J.; Jara, A.; Funk, K.; Kopchick, J.J. The effects of weight cycling on lifespan in male C57BL/6J mice. Int. J. Obes. (Lond) 2013, 37, 1088–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surra, J.C.; Barranquero, C.; Torcal, M.P.; Orman, I.; Segovia, J.C.; Guillen, N.; Navarro, M.A.; Arnal, C.; Osada, J. In comparison with palm oil, dietary nut supplementation delays the progression of atherosclerotic lesions in female apoE-deficient mice. Br. J. Nutr. 2013, 109, 202–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Zheng, L.; Zhao, T.; Zhang, Q.; Liu, Y.; Sun, B.; Su, G.; Zhao, M. Inhibitory Effects of Walnut (Juglans regia) Peptides on Neuroinflammation and Oxidative Stress in Lipopolysaccharide-Induced Cognitive Impairment Mice. J. Agric. Food Chem. 2020, 68, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Poulose, S.M.; Bielinski, D.F.; Shukitt-Hale, B. Walnut diet reduces accumulation of polyubiquitinated proteins and inflammation in the brain of aged rats. J. Nutr. Biochem. 2013, 24, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.; Garrido, P.; Alonso, A.; Cabello, E.; Gonzalez, C. 17beta-Estradiol and genistein acute treatments improve some cerebral cortex homeostasis aspects deteriorated by aging in female rats. Exp. Gerontol. 2013, 48, 414–421. [Google Scholar] [CrossRef]
- Sumien, N.; Chaudhari, K.; Sidhu, A.; Forster, M.J. Does phytoestrogen supplementation affect cognition differentially in males and females? Brain Res. 2013, 1514, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Esselun, C.; Dilberger, B.; Silaidos, C.V.; Koch, E.; Schebb, N.H.; Eckert, G.P. A Walnut Diet in Combination with Enriched Environment Improves Cognitive Function and Affects Lipid Metabolites in Brain and Liver of Aged NMRI Mice. Neuromolecular Med. 2020, 23, 140–160. [Google Scholar] [CrossRef]
- Chauhan, A.; Chauhan, V. Beneficial Effects of Walnuts on Cognition and Brain Health. Nutrients 2020, 12, 550. [Google Scholar] [CrossRef] [Green Version]
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Hernandez-Hierro, J.M.; Garcia, R.; Barroso, J.M.; Heredia, F.J.; Rato, A.E. Assessment of Total Fat and Fatty Acids in Walnuts Using Near-Infrared Hyperspectral Imaging. Front. Plant Sci. 2021, 12, 729880. [Google Scholar] [CrossRef]
- Nakamura, M.T.; Nara, T.Y. Essential fatty acid synthesis and its regulation in mammals. Prostaglandins Leukot Essent Fat. Acids 2003, 68, 145–150. [Google Scholar] [CrossRef]
- Brenna, J.T.; Salem, N., Jr.; Sinclair, A.J.; Cunnane, S.C. International Society for the Study of Fatty A, Lipids I. alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fat. Acids 2009, 80, 85–91. [Google Scholar] [CrossRef]
- Fu, Z.; Sinclair, A.J. Increased alpha-linolenic acid intake increases tissue alpha-linolenic acid content and apparent oxidation with little effect on tissue docosahexaenoic acid in the guinea pig. Lipids 2000, 35, 395–400. [Google Scholar] [CrossRef]
- Qu, M.H.; Yang, X.; Wang, Y.; Tang, Q.; Han, H.; Wang, J.; Wang, G.-D.; Xue, C.; Gao, Z. Docosahexaenoic Acid-Phosphatidylcholine Improves Cognitive Deficits in an Abeta23-35-Induced Alzheimer’s Disease Rat Model. Curr. Top. Med. Chem. 2016, 16, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.G.; Chan, R.B.; Bravo, F.V.; Miranda, A.; Silva, R.R.; Zhou, B.; Marques, F.; Pinto, V.; Cerqueira, J.J.; Di Paolo, G.; et al. The impact of chronic stress on the rat brain lipidome. Mol. Psychiatry 2016, 21, 80–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, D.E.; Strong, R.; Reifsnyder, P.; Kumar, N.; Fernandez, E.; Flurkey, K.; Javors, M.A.; Lopez-Cruzan, M.; Macchiarini, F.; Nelson, J.F.; et al. 17-a-estradiol late in life extends lifespan in aging UM-HET3 male mice; nicotinamide riboside and three other drugs do not affect lifespan in either sex. Aging Cell 2021, 20, e13328. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Harrison, D.E.; Allison, D.B.; Bogue, M.; Debarba, L.; Diaz, V.; Fernandez, E.; Galecki, A.; Garvey, W.T.; Jayarathne, H.; et al. Canagliflozin extends life span in genetically heterogeneous male but not female mice. JCI Insight 2020, 5, e140019. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Fernandez, E.; Liu, Y.; Strong, R.; Salmon, A.B. Metformin reduces glucose intolerance caused by rapamycin treatment in genetically heterogeneous female mice. Aging (Albany NY) 2018, 10, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.E.; Strong, R.; Allison, D.B.; Ames, B.N.; Astle, C.M.; Atamna, H.; Fernandez, E.; Flurkey, K.; Javors, M.A.; Nadon, N.L.; et al. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell 2014, 13, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Huffman, D.M.; Augenlicht, L.H.; Zhang, X.; Lofrese, J.J.; Atzmon, G.; Chamberland, J.P.; Mantzoros, C.S. Abdominal obesity, independent from caloric intake, accounts for the development of intestinal tumors in Apc(1638N/+) female mice. Cancer Prev. Res. (Phila) 2013, 6, 177–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walters, R.O.; Arias, E.; Diaz, A.; Burgos, E.S.; Guan, F.; Tiano, S.; Mao, K.; Green, C.L.; Qiu, Y.; Shah, H.; et al. Sarcosine Is Uniquely Modulated by Aging and Dietary Restriction in Rodents and Humans. Cell Rep. 2018, 25, 663–676.e6. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, J.C.; Hildebrand, B.A.; Sun, M.; Rockwood, M.R.; Rose, R.A.; Rockwood, K.; Howlett, S.E. A clinical frailty index in aging mice: Comparisons with frailty index data in humans. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 621–632. [Google Scholar] [CrossRef]
- Engel, M.G.; Smith, J.; Mao, K.; Quipildor, G.F.; Cui, M.H.; Gulinello, M.; Branch, C.A.; Gandy, S.E.; Huffman, D.M. Evidence for preserved insulin responsiveness in the aging rat brain. Geroscience 2022, 44, 2491–2508. [Google Scholar] [CrossRef] [PubMed]
- Farias Quipildor, G.; Mao, K.; Beltran, P.J.; Barzilai, N.; Huffman, D.M. Modulation of Glucose Production by Central Insulin Requires IGF-1 Receptors in AgRP Neurons. Diabetes 2021, 70, 2237–2249. [Google Scholar] [CrossRef] [PubMed]
- Tabrizian, T.; Wang, D.; Guan, F.; Hu, Z.; Beck, A.P.; Delahaye, F.; Huffman, D.M. Apc inactivation, but not obesity, synergizes with Pten deficiency to drive intestinal stem cell-derived tumorigenesis. Endocr. Relat. Cancer 2017, 24, 253–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [Green Version]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. (Lond) 1993, 84, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Rice-Evans, C.A. Measurement of total antioxidant activity as a marker of antioxidant status in vivo: Procedures and limitations. Free Radic. Res. 2000, 33, S59–S66. [Google Scholar]
- Ghiselli, A.; Serafini, M.; Natella, F.; Scaccini, C. Total antioxidant capacity as a tool to assess redox status: Critical view and experimental data. Free Radic. Biol. Med. 2000, 29, 1106–1114. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.J. Total antioxidant status in plasma and body fluids. Methods Enzymol. 1994, 234, 279–293. [Google Scholar] [PubMed]
- Hanson, A.J.; Banks, W.A.; Bettcher, L.F.; Pepin, R.; Raftery, D.; Craft, S. Cerebrospinal fluid lipidomics: Effects of an intravenous triglyceride infusion and apoE status. Metabolomics 2019, 16, 6. [Google Scholar] [CrossRef] [PubMed]

| Species [nmol/g] | LFD | HFD | HFD+Walnut | FDR-Corrected p Value |
|---|---|---|---|---|
| PE(P-18:2/20:4) | 3.84 ± 0.20 a | 5.38 ± 0.56 b | 6.58 ± 0.26 b | <0.001 |
| LPE(22:5) | 2.22 ± 0.18 a | 2.12 ± 0.16 a | 1.29 ± 0.13 b | 0.007 |
| PC(18:1/16:1) | 169.1 ± 9.9 a | 154.6 ± 4.8 a | 119.6 ± 7.8 b | 0.007 |
| PE(18:2/20:4) | 4.06 ± 0.24 a | 5.18 ± 0.48 b | 5.77 ± 0.18 b | 0.007 |
| FFA(18:2) | 50.8 ± 4.2 a | 52.8 ± 2.7 a | 69.4 ± 3.6 b | 0.007 |
| PC(14:0/18:1) | 163.1 ± 12.4 a | 160.2 ± 11.8 a | 107.9 ± 9.0 b | 0.007 |
| SM(20:0) | 184.6 ± 13.1 ab | 171.5 ± 4.0 a | 204.0 ± 8.2 b | 0.008 |
| FFA(18:3) | 3.30 ± 0.20 ab | 3.18 ± 0.12 a | 3.82 ± 0.16 b | 0.008 |
| PC(18:0/18:2) | 101.9 ± 13.1 a | 106.3 ± 8.1 a | 141.2 ± 12.9 b | 0.022 |
| PC(18:1/18:1) | 795.7 ± 46.5 a | 769.1 ± 24.9 a | 603.9 ± 40.2 b | 0.025 |
| FFA(22:5) | 38.6 ± 2.0 a | 35.5 ± 1.63 a | 26.6 ± 1.9 b | 0.025 |
| PC(16:0/18:1) | 13,783 ± 733 a | 13,300 ± 402 a | 10,736 ± 623 b | 0.031 |
| PC(FA18:1) | 20,362 ± 1056 a | 19,891 ± 602 a | 16,098 ± 924 b | 0.041 |
| PC(FA14:0) | 266.9 ± 19.9 a | 272.2 ± 14.8 a | 204.0 ± 8.2 b | 0.041 |
| PC(FA20:1) | 331.0 ± 18.6 a | 322.4 ± 13.9 a | 253.2 ± 17.3 b | 0.041 |
| PC(16:0/18:0) | 771.7 ± 42.8 a | 734.7 ± 25.1 a | 594.0 ± 30.0 b | 0.041 |
| Lipid Class [nmol/g] | LFD | HFD | HFD+Walnut | ANOVA p Value |
|---|---|---|---|---|
| Cholesterol ester (CE) | 104.5 ± 7.1 | 108.1 ± 5.8 | 93.3 ± 7.1 | 0.28 |
| Ceramide (CER) | 2386 ± 129 | 2750 ± 219 | 2124 ± 593 | 0.07 |
| Free Fatty Acid (FFA) | 8751 ± 388 | 8662 ± 468 | 9074 ± 371 | 0.76 |
| Lysophosphatidylcholine (LPC) | 840.1 ± 57.5 | 802.0 ± 27.0 | 703.2 ± 64.7 | 0.18 |
| Lysophosphatidylethanolamine (LPE) | 308.2 ± 21.4 | 295.7 ± 17.0 | 247.2 ± 24.1 | 0.11 |
| Phosphatidylcholine (PC) | 29,982 ± 1227 a | 30,171 ± 778 a | 26,105 ± 1421 b | 0.02 |
| Phosphatidylethanolamine (PE) | 11,304 ± 1916 | 10,138 ± 1943 | 13,086 ± 1837 | 0.55 |
| Sphingomyelin (SM) | 3824 ± 213 | 3722 ± 151 | 3438 ± 170 | 0.31 |
| Triacylglycerol (TAG) | 177.3 ± 14.3 | 208.2 ± 18.4 | 176.5 ± 12.6 | 0.27 |
| Component (g/kg) | LFD | HFD | HFD+Walnut |
|---|---|---|---|
| Casein | 210.0 | 245.0 | 231.8 |
| L-Cystine | 3.0 | 3.5 | 3.5 |
| Corn Starch | 465 | 85 | 85 |
| Maltodextrin | 100 | 115 | 102 |
| Sucrose | 90 | 200 | 200 |
| Lard | 20.0 | 195.0 | 145.6 |
| Soybean Oil | 20 | 30 | 30 |
| Cellulose | 37.2 | 58.0 | 58.0 |
| Mineral Mix, AIN-93G-MX (94046) | 35 | 43 | 43 |
| Calcium Phosphate, dibasic | 2.0 | 3.4 | 3.4 |
| Vitamin Mix, AIN-93-VX (94047) | 15 | 19 | 19 |
| Choline Bitartrate | 2.75 | 3.00 | 3.00 |
| Kcal from CHO, % | 69.1 | 36.2 | 36.1 |
| Kcal from PRO, % | 20.5 | 19.0 | 19.0 |
| Kcal from Fat, % | 10.4 | 44.8 | 44.9 |
| Walnuts, ground | 0.0 | 0.0 | 75.7 |
| Kcal/g | 3.6 | 4.6 | 4.6 |
| Catalog number | TD.08806 | TD.06415 | TD.140817 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novaj, A.; Engel, M.G.; Wang, R.; Mao, K.; Xue, X.; Amir, Y.; Atzmon, G.; Huffman, D.M. Dietary Walnuts Preserve Aspects of Health Span and Alter the Hippocampal Lipidome in Aged High-Fat Diet-Fed Mice. Int. J. Mol. Sci. 2023, 24, 2314. https://doi.org/10.3390/ijms24032314
Novaj A, Engel MG, Wang R, Mao K, Xue X, Amir Y, Atzmon G, Huffman DM. Dietary Walnuts Preserve Aspects of Health Span and Alter the Hippocampal Lipidome in Aged High-Fat Diet-Fed Mice. International Journal of Molecular Sciences. 2023; 24(3):2314. https://doi.org/10.3390/ijms24032314
Chicago/Turabian StyleNovaj, Ardijana, Matthew G. Engel, Ruixuan Wang, Kai Mao, Xiaonan Xue, Yam Amir, Gil Atzmon, and Derek M. Huffman. 2023. "Dietary Walnuts Preserve Aspects of Health Span and Alter the Hippocampal Lipidome in Aged High-Fat Diet-Fed Mice" International Journal of Molecular Sciences 24, no. 3: 2314. https://doi.org/10.3390/ijms24032314
APA StyleNovaj, A., Engel, M. G., Wang, R., Mao, K., Xue, X., Amir, Y., Atzmon, G., & Huffman, D. M. (2023). Dietary Walnuts Preserve Aspects of Health Span and Alter the Hippocampal Lipidome in Aged High-Fat Diet-Fed Mice. International Journal of Molecular Sciences, 24(3), 2314. https://doi.org/10.3390/ijms24032314








