Obtaining miRNA from Saliva—Comparison of Sampling and Purification Methods
Abstract
:1. Introduction
2. Results
2.1. Saliva Collectors
2.2. miRNA Extraction Kits
2.3. RNA Extraction
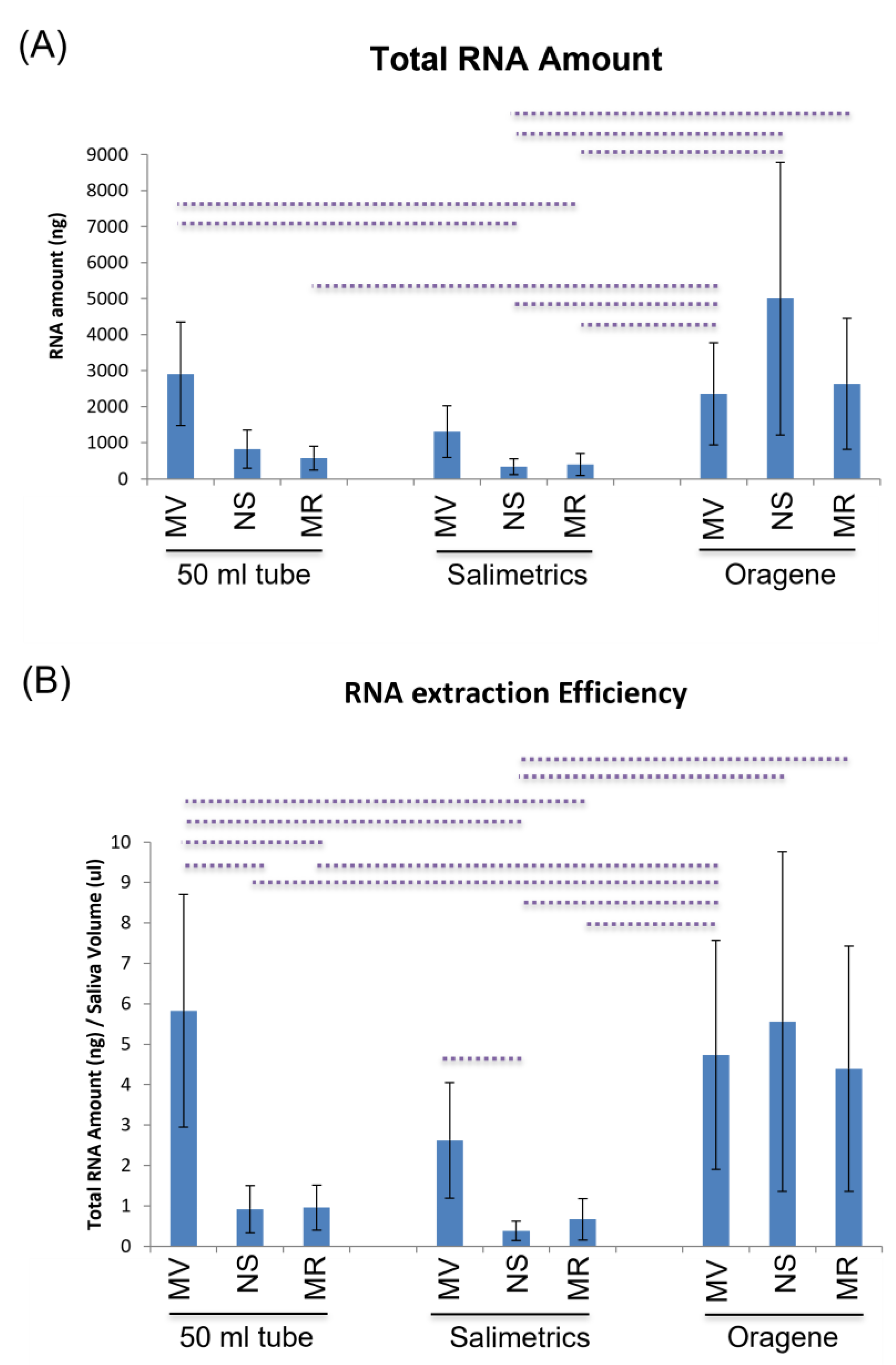
2.3.1. Total RNA Amount
2.3.2. Efficiency
2.3.3. Purity
2.4. DNA Contamination
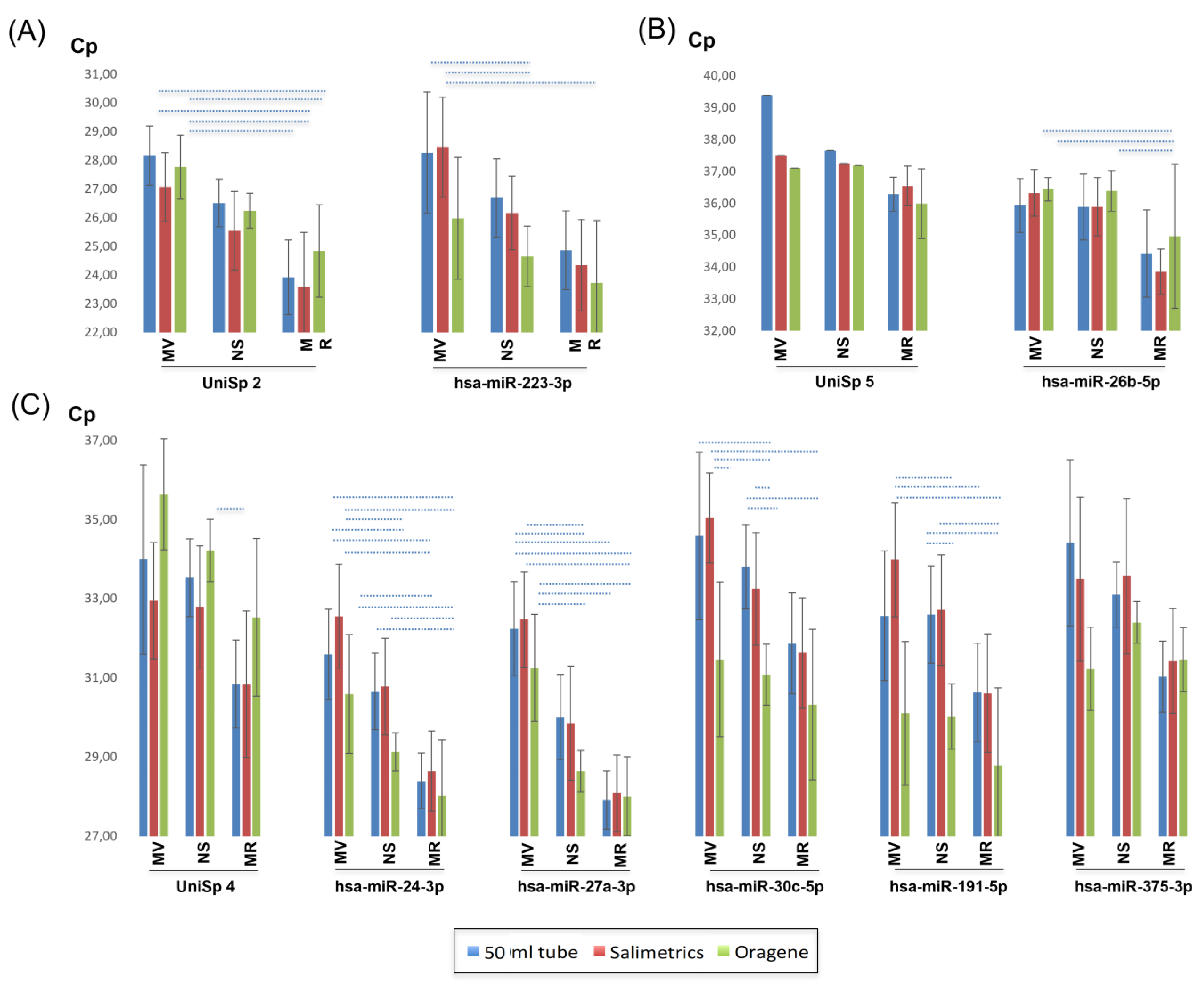
2.5. miRNA Quantification
2.5.1. Synthetic Spike-In Standards
2.5.2. Gender
2.5.3. Quantification of Saliva miRNAs
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Sample Collection
4.3. miRNA Extraction
4.4. RNA Concentration and Purity
4.5. DNA Contamination Check
4.6. Synthetic RNA Spike-In
4.7. miRNA Quantification
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Thaw the frozen samples (if saliva was collected by Oragene collector, incubate the entire sample in the original vial at 50 °C for 2 h in an air incubator).
- Add 1 μL Spike-in to 600 μL Lysis Buffer and mix by pipetting.
- Add the mix of Lysis Buffer with Spike-in to 500 μL of saliva sample.
- Add 50 μL of miRNA Homogenate Additive (1/10 volume), mix well by inverting the tubes several times.
- Leave the mixture on ice for 10 min. During this time, prepare two new 1.5 mL tubes.
- Split the sample to the new tubes (around 550 μL in each tube) and add 500 μL (250 μL in each tube) of Acid-Phenol:Chloroform (a volume that is equal to the volume before addition of the Homogenate Additive).
- Vortex for 1 min and centrifuge for 10 min at max speed.
- Transfer the supernatant to new fresh tubes. At this point put in DEPC-treated water at 95 °C for use in eluting the RNA from the filter at the end of procedure.
- Add 1/3 volume room temperature 100% EtOH to the aqueous phase, invert tubes several times to mix.
- Place a filter cartridge into collection tube, pipet the mixture from the previous step onto the filter cartridge (up to 700 μL).
- Centrifuge for approx. 15 s at min. 10,000× g, collect the filtrate (contains the miRNA).
- Add 2/3 volume room temperature 100% EtOH to the filtrate, mix throughly.
- Place a new cartridge into collection tube and pipette the filtrate/EtOH mixture onto a second filter cartridge.
- Centrifuge for around 15 s at equal or less than 10,000× g, discard the flow-through, and reuse the collection tube for the washing steps.
- Add 700 μL of Washing Solution 1 to the filter cartridge and centrifuge for around 5–10 s at equal or less than 10,000× g. Discard the through-flow, reuse the collection tube.
- Add 500 μL of Washing Solution 2/3, centrifuge for 5–10 s at equal or less than 10,000× g.
- Repeat with a second 500 μL of Washing Solution 2/3.
- After discarding the flow-through, replace the filter in the same collection tube and spin for 1 min to remove residual liquid from the filter.
- Transfer the filter into a new collection tube and apply 20 μL of pre-heated (60 °C) water to the center of the filter. Spin 1 min at max speed.
- Store collected miRNA at −20 °C.
- Thaw the frozen samples (if saliva was collected by Oragene collector, incubate the entire sample in the original vial at 50 °C for 2 h in an air incubator).
- Add 180 μL Buffer RPL and 1 μL Spike-in into a 1.5-mL microcentrifuge tube
- Transfer 600 μL saliva into the 1.5 mL microcentrifuge tube with the buffer and spike-in. Close the tube caps and vortex for > 5 s. Leave at room temperature for 3 min.
- Add 60 μL Buffer RPP. Close the tube caps and mix vigorously by vortexing for >20 s. Incubate at room temperature for 3 min.
- Centrifuge at 1200× g for 3 min at room temperature to pellet the precipitate. (Supernatant should be clear and colorless).
- Transfer supernatant to a new microcentrifuge tube. Add 1 volume of isopropanol. Mix well by vortexing.
- Transfer the entire sample to an RNeasy UCP MinElute column. Close the lid, and centrifuge for 15 s at ≥8000× g. Discard the flow-through.
- Add 350 μL Buffer RWT (prepared with isopropanol) to the RNeasy UCP MinElute spin column. Close the lid gently, and centrifuge for 15 s at ≥8000× g to wash the membrane. Discard the flow-through. Reuse the collection tube in the next step.
- Add 10 μL DNase I stock solution to 70 μL Buffer RDD. Mix by gently inverting the tube, and centrifuge briefly to collect residual liquid form the sides of the tube.
- Add 80 μL of the DNase I incubation mix directly to the RNeasy UCP MinElute spin column membrane, and place on the benchtop (20–30 °C) for 15 min.
- Add 500 μL Buffer RWT (prepared with isopropanol) to the RNeasy UCP MinElute spin column. Close the lid gently, and centrifuge for 15 s at ≥8000× g. Save the flow-through for use in step the next step.
- Place the spin column in a new 2-mL collection tube. Apply the flow-through saved in the previous step to the spin column. Centrifuge for 15 s at ≥8000× g. Discard the flow-through. Reuse the collection tube in the next step.
- Pipet 500 μL Buffer RPE onto the RNeasy UCP MinElute spin column. Close the lid, and centrifuge for 15 s at ≥8000× g. Discard the flow-through. Reuse the collection tube in the next step.
- Add 500 μL of 80% ethanol to the RNeasy UCP MinElute spin column. Close the lid gently, and centrifuge for 2 min at ≥8000× g to wash the spin column membrane. Discard the flow-through. After centrifuge, remove the RNeasy UCP MinElute spin column from the collection tube so that the column does not contact the flow-through.
- Place the RNeasy UCP MinElute spin column in a new 2-mL collection tube. Open the lid of the spin column and centrifuge at full speed for 5 min to dry the membrane. Discard the flow-through and the collection tube.
- Place the RNeasy UCP MinElute spin column in a new 1.5 mL collection tube. Add 20 μL of pre-heated (60 °C) RNase-free water directly to the center of the spin column membrane and incubate 1min. Close the lid, and centrifuge for 1 min at full speed to elute the RNA.
- Store collected miRNA at −20 °C.
- Thaw the frozen samples (if saliva was collected by Oragene collector, incubate the entire sample in the original vial at 50 °C for 2 h in an air incubator).
- Add 270 μL Buffer MLP and 1 μL Spike-in into a 1.5 mL microcentrifuge tube.
- Add 900 μL saliva sample and vortex for 5 s. Incubate for 3 min at room temperature.
- Add 90 μL Buffer MPP and vortex for 5 s. Incubate for 1 min at room temperature. Centrifuge for 3 min at 11,000× g to pellet the protein.
- Transfer the clear supernatant to two new collection tubes (approx. 600 μL per each tube).
- Add 1200 μL isopropanol (600 μL per each tube) and vortex for 5 s.
- Place a NucleoSpin®® miRNA Column into a collection tube and load the sample onto the column.
- Incubate for 2 min at room temperature and centrifuge for 30 s at 11,000× g. Discard the flow-through and place the column back into the collection tube. Repeat this step until all sample is loaded onto the column.
- Add 700 μL Buffer MW2 to the NucleSpin®® miRNA Column. Centrifuge for 30 s at 11,000× g. Discard flow-through and place the column back into the collection tube.
- Add 250 μL Buffer MW2 to the NucleSpin®® miRNA Column. Centrifuge for 2 min at 11,000× g. It is not necessary to discard the flow-through.
- Add 50 μL rDNase (previously dissolved in Reaction Buffer) directly onto the silica membrane of the NucleSpin®® miRNA Column. Close the lid and incubate at room temperature for 15 min.
- Add 100 μL Buffer MW1 to the NucleSpin®® miRNA Column. Centrifuge for 30 s at 11,000× g. Discard flow-through and place the column back into the collection tube.
- Add 700 μL Buffer MW2 to the NucleSpin®® miRNA Column. Centrifuge for 30 s at 11,000× g. Discard flow-through and place the column back into the collection tube.
- Add 250 μL Buffer MW2 to the NucleSpin®® miRNA Column. Centrifuge for 2 min at 11,000× g to dry the membrane completely.
- Place the NucleSpin®® miRNA Column in a new collection tube. Add 20 μL of pre-heated (60 °C) RNase-free H2O directly onto the silica membrane of the column. Incubate for 1 min at room temperature. Close the lid and centrifuge for 1 min at 11,000× g.
- Store collected miRNA at −20 °C.
References
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Lei, Z.; Sun, T. The role of microRNAs in neurodegenerative diseases: A review. Cell Biol. Toxicol. 2022, 31, 1–31, ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kinser, H.E.; Pincus, Z. MicroRNAs as modulators of longevity and the aging process. Hum. Genet. 2020, 139, 291–308. [Google Scholar] [CrossRef]
- Lehallier, B.; Gate, D.; Schaum, N.; Nanasi, T.; Lee, S.E.; Yousef, H.; Moran Losada, P.; Berdnik, D.; Keller, A.; Verghese, J.; et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat. Med. 2019, 25, 1843–1850. [Google Scholar] [CrossRef]
- Noren Hooten, N.; Abdelmohsen, K.; Gorospe, M.; Ejiogu, N.; Zonderman, A.B.; Evans, M.K. microRNA expression patterns reveal differential expression of target genes with age. PLoS ONE 2010, 5, e10724. [Google Scholar] [CrossRef] [Green Version]
- Heegaard, N.H.; Carlsen, A.L.; Lilje, B.; Ng, K.L.; Rønne, M.E.; Jørgensen, H.L.; Sennels, H.; Fahrenkrug, J. Diurnal Variations of Human Circulating Cell-Free Micro-RNA. PLoS ONE 2016, 11, e0160577. [Google Scholar] [CrossRef] [Green Version]
- Hicks, S.D.; Khurana, N.; Williams, J.; Dowd Greene, C.; Uhlig, R.; Middleton, F.A. Diurnal oscillations in human salivary microRNA and microbial transcription: Implications for human health and disease. PLoS ONE 2018, 13, e0198288. [Google Scholar] [CrossRef]
- Figueredo Dde, S.; Barbosa, M.R.; Gitaí, D.L.; de Andrade, T.G. Predicted microRNAs for mammalian circadian rhythms. J. Biol. Rhythm. 2013, 28, 107–116. [Google Scholar] [CrossRef]
- Li, F.; Bai, M.; Xu, J.; Zhu, L.; Liu, C.; Duan, R. Long-Term Exercise Alters the Profiles of Circulating Micro-RNAs in the Plasma of Young Women. Front. Physiol. 2020, 11, 372. [Google Scholar] [CrossRef]
- Silva, F.C.D.; Iop, R.D.R.; Andrade, A.; Costa, V.P.; Gutierres Filho, P.J.B.; Silva, R.D. Effects of Physical Exercise on the Expression of MicroRNAs: A Systematic Review. J. Strength Cond. Res. 2020, 34, 270–280. [Google Scholar] [CrossRef]
- Barrio, E.; Quirós, A.; Lerma-Puertas, D.; Labarta, J.I.; Gascón-Catalán, A. Identification of miRNAs Involved in Foetal Growth Restriction Due to Maternal Smoking during Pregnancy. J. Clin. Med. 2022, 11, 5808. [Google Scholar] [CrossRef]
- Ostheim, P.; Tichý, A.; Sirak, I.; Davidkova, M.; Stastna, M.M.; Kultova, G.; Paunesku, T.; Woloschak, G.; Majewski, M.; Port, M.; et al. Overcoming challenges in human saliva gene expression measurements. Sci. Rep. 2020, 10, 11147. [Google Scholar] [CrossRef]
- Ostheim, P.; Alemu, S.W.; Tichý, A.; Sirak, I.; Davidkova, M.; Stastna, M.M.; Kultova, G.; Schuele, S.; Paunesku, T.; Woloschak, G.; et al. Examining potential confounding factors in gene expression analysis of human saliva and identifying potential housekeeping genes. Sci. Rep. 2022, 12, 2312. [Google Scholar] [CrossRef]
- Wang, J.; Schipper, H.M.; Velly, A.M.; Mohit, S.; Gornitsky, M. Salivary biomarkers of oxidative stress: A critical review. Free Radic. Biol. Med. 2015, 85, 95–104. [Google Scholar] [CrossRef]
- Roi, A.; Rusu, L.C.; Roi, C.I.; Luca, R.E.; Boia, S.; Munteanu, R.I. A New Approach for the Diagnosis of Systemic and Oral Diseases Based on Salivary Biomolecules. Dis. Mrk. 2019, 2019, 8761860. [Google Scholar] [CrossRef] [Green Version]
- Cressatti, M.; Juwara, L.; Galindez, J.M.; Velly, A.M.; Nkurunziza, E.S.; Marier, S.; Canie, O.; Gornistky, M.; Schipper, H.M. Salivary microR-153 and microR-223 Levels as Potential Diagnostic Biomarkers of Idiopathic Parkinson’s Disease. Mov. Disord. 2020, 35, 468–477. [Google Scholar] [CrossRef]
- Ogawa, Y.; Taketomi, Y.; Murakami, M.; Tsujimoto, M.; Yanoshita, R. Small RNA transcriptomes of two types of exosomes in human whole saliva determined by next generation sequencing. Biol. Pharm. Bull. 2013, 36, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Keller, S.; Ridinger, J.; Rupp, A.K.; Janssen, J.W.; Altevogt, P. Body fluid derived exosomes as a novel template for clinical diagnostics. J. Transl. Med. 2011, 9, 86. [Google Scholar] [CrossRef] [Green Version]
- Luedemann, C.; Reinersmann, J.L.; Klinger, C.; Degener, S.; Dreger, N.M.; Roth, S.; Kaufmann, M.; Savelsbergh, A. Prostate Cancer-Associated miRNAs in Saliva: First Steps to an Easily Accessible and Reliable Screening Tool. Biomolecules 2022, 12, 1366. [Google Scholar] [CrossRef]
- Hicks, S.D.; Carpenter, R.L.; Wagner, K.E.; Pauley, R.; Barros, M.; Tierney-Aves, C.; Barns, S.; Greene, C.D.; Middleton, F.A. Saliva MicroRNA Differentiates Children with Autism From Peers With Typical and Atypical Development. J. Am. Acad. Child Adolesc. Psychiatry 2020, 59, 296–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, R.; Montgomery, A.; Scipioni, A.; Jhaveri, P.; Schmidt, A.T.; Hicks, S.D. Confounding Factors Impacting microRNA Expression in Human Saliva: Methodological and Biological Considerations. Genes 2022, 13, 1874. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.S.; Jakymiw, A.; Yao, B.; Pauley, B.A.; Carcamo, W.C.; Katz, J.; Cheng, J.Q.; Chan, E.K. High resolution of microRNA signatures in human whole saliva. Arch. Oral. Biol. 2011, 56, 1506–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prinz, F.; Schlange, T.; Asadullah, K. Believe it or not: How much can we rely on published data on potential drug targets? Nature reviews. Drug. Discov. 2011, 10, 712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Errington, T.M.; Mathur, M.; Soderberg, C.K.; Denis, A.; Perfito, N.; Iorns, E.; Nosek, B.A. Investigating the replicability of preclinical cancer biology. eLife 2021, 10, e71601. [Google Scholar] [CrossRef]
- Baker, M. 1500 scientists lift the lid on reproducibility. Nature 2016, 533, 452–454. [Google Scholar] [CrossRef] [Green Version]
- Kabitzke, P.; Cheng, K.M.; Altevogt, B. Guidelines and Initiatives for Good Research Practice. In Good Research Practice in Non-Clinical Pharmacology and Biomedicine, 1st ed.; Bespalov, A., Michel, M.C., Steckler, T., Eds.; Springer: Cham, Switzerland; Berlin/Heidelberg, Germany, 2020; Volume 257, pp. 19–34. [Google Scholar]
- Schwab, S.; Janiaud, P.; Dayan, M.; Amrhein, V.; Panczak, R.; Palagi, P.M.; Hemkens, L.G.; Ramon, M.; Rothen, N.; Senn, S. Ten simple rules for good research practice. PLoS Comput. Biol. 2022, 18, e1010139. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, R.; Heavey, S.; Graham, D.G.; Wellman, R.; Khan, S.; Thrumurthy, S.; Simpson, B.S.; Baker, T.; Jevons, S.; Ariza, J.; et al. An zoptimized saliva collection method to produce high-yield, high-quality RNA for translational research. PLoS ONE 2020, 15, e0229791. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, V.; O’Brien, M.H.; Yadav, S. High-Quality and High-Yield RNA Extraction Method from Whole Human Saliva. Biomark. Insights 2020, 15, 1177271920929705. [Google Scholar] [CrossRef]
- Gallo, A.; Tandon, M.; Alevizos, I.; Illei, G.G. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE 2012, 7, e30679. [Google Scholar] [CrossRef] [Green Version]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [Green Version]
- Marsh, P.D.; Do, T.; Beighton, D.; Devine, D.A. Influence of saliva on the oral microbiota. Periodontology 2000 2016, 70, 80–92. [Google Scholar] [CrossRef]
- Lim, Y.; Totsika, M.; Morrison, M.; Punyadeera, C. The saliva microbiome profiles are minimally affected by collection method or DNA extraction protocols. Sci. Rep. 2017, 7, 8523. [Google Scholar] [CrossRef] [Green Version]
- Park, N.J.; Zhou, H.; Elashoff, D.; Henson, B.S.; Kastratovic, D.A.; Abemayor, E.; Wong, D.T. Salivary microRNA: Discovery, characterisation, and clinical utility for oral cancer detection. Clin. Cancer Res. 2009, 15, 5473–5477. [Google Scholar] [CrossRef]
- Martinez, B.; Peplow, P.V. MicroRNAs in Parkinson’s disease and emerging therapeutic targets. Neural. Regen. Res. 2017, 12, 1945–1959. [Google Scholar]



| Spike-In | miRNA Extraction Kit | Saliva Collector | |||||
|---|---|---|---|---|---|---|---|
| 50 mL Tube | Salimetrics | Oragene | |||||
| Cq Average | Cq SD | Cq Average | Cq SD | Cq Average | Cq SD | ||
| UniSp2 | MV | 28.17 | 1.11 | 27.07 | 1.29 | 27.77 | 1.18 |
| NS | 26.51 | 0.89 | 25.55 | 1.46 | 26.24 | 0.64 | |
| MR | 23.92 | 1.39 | 23.59 | 2.03 | 24.84 | 1.70 | |
| UniSp4 | MV | 33.99 | 2.58 | 32.95 | 1.57 | 35.63 | 1.54 |
| NS | 33.54 | 1.05 | 32.79 | 1.65 | 34.22 | 0.83 | |
| MR | 30.85 | 1.19 | 30.84 | 1.97 | 32.53 | 2.10 | |
| UniSp5 | MV | 39.40 | - | 37.50 | - | 37.11 | - |
| NS | 37.66 | - | 37.25 | - | 37.19 | - | |
| MR | 36.29 | 0.62 | 36.55 | 0.70 | 35.99 | 1.23 | |
| UniSp2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MV | NS | MR | ||||||||
| 50 mL 1 | Salim. 2 | Orag. 3 | 50 mL | Salim. | Orag. | 50 mL | Salim. | Orag. | ||
| MV | 50 mL | 0.152 | 0.669 | 0.0059 | 0.0022 | 0.0011 | 0.0014 | 0.0003 | 0.0001 | |
| Salim. | 0.360 | 0.344 | 0.0379 | 0.068 | 0.0038 | 0.0011 | 0.0062 | |||
| Orag. | 0.0434 | 0.0044 | 0.0058 | 0.0006 | 0.0002 | 0.0001 | ||||
| NS | 50 mL | 0.382 | 0.450 | 0.0013 | 0.0047 | 0.0117 | ||||
| Salim. | 0.351 | 0.031 | 0.083 | 0.460 | ||||||
| Orag. | 0.0025 | 0.0087 | 0.064 | |||||||
| MR | 50 mL | 0.713 | 0.3066 | |||||||
| Salim. | 0.122 | |||||||||
| Orag. | ||||||||||
| UniSp4 | ||||||||||
| MV | NS | MR | ||||||||
| 50 mL | Salim. | Orag. | 50 mL | Salim. | Orag. | 50 mL | Salim. | Orag. | ||
| MV | 50 mL | 0.418 | 0181 | 0.613 | 0.281 | 0.660 | 0.020 | 0.205 | 0.315 | |
| Salim. | 0.0080 | 0.382 | 0.795 | 0.068 | 0.031 | 0.083 | 0.897 | |||
| Orag. | 0.0080 | 0.0080 | 0.049 | 0.0024 | 0.0013 | 0.0018 | ||||
| NS | 50 mL | 0.328 | 0.144 | 0.0013 | 0.010 | 0.460 | ||||
| Salim. | 0.024 | 0.014 | 0.049 | 0.897 | ||||||
| Orag. | 0.0004 | 0.0016 | 0.028 | |||||||
| MR | 50 mL | 0.958 | 0.068 | |||||||
| Salim. | 0.083 | |||||||||
| Orag. | ||||||||||
| UniSp5 | ||||||||||
| MV | NS | MR | ||||||||
| 50 mL | Salim. | Orag. | 50 mL | Salim. | Orag. | 50 mL | Salim. | Orag. | ||
| MV | 50 mL | 1 | 1 | 1 | 1 | 0.429 | 0.276 | 0.333 | 0.333 | |
| Salim. | 1 | 1 | 1 | 0.479 | 0.276 | 0.333 | 0.333 | |||
| Orag. | 1 | 1 | 0.479 | 0.717 | 0.667 | 1 | ||||
| NS | 50 mL | 1 | 0.479 | 0.276 | 0.333 | 0.333 | ||||
| Salim. | 0.479 | 0.276 | 0.667 | 0.667 | ||||||
| Orag. | 0.095 | 0.329 | 0.845 | |||||||
| MR | 50 mL | 0.539 | 0.712 | |||||||
| Salim. | 0.421 | |||||||||
| Orag. | ||||||||||
| miR-223-3p | miR-24-3p | miR-191-5p | miR-30c-5p | miR-375-3p | miR-27a-3p | miR-26b-5p | ||
|---|---|---|---|---|---|---|---|---|
| Coll. 1 | 50 mL 3 vs. Salim. 4 | 0.992 | 0.377 | 0.728 | 0.958 | 0.744 | 0.808 | 0.601 |
| 50mL vs. Orag. 5 | <0.0001 | 0.015 | <0.0001 | <0.0001 | 0.131 | 0.404 | 0.573 | |
| Salim. vs. Orag. | 0.0002 | 0.0097 | <0.0001 | <0.0001 | 0.893 | 0.608 | 0.866 | |
| Kit 2 | MV vs. NS | 0.116 | 0.0023 | 0.978 | 0.651 | 0.409 | <0.0001 | 0.614 |
| MV vs. MR | <0.0001 | <0.0001 | 0.0028 | 0.0026 | 0.440 | <0.0001 | <0.0001 | |
| NS vs. MR | <0.0001 | <0.0001 | 0.0003 | 0.0008 | 0.0014 | 0.0031 | <0.0001 |
| Primer Name | Primer SEQUENCE (5′->3′) | Length (bp) | Denaturation Temp/Time | Annealing Temp/Time | Extension Temp/Time | Cycles |
|---|---|---|---|---|---|---|
| rs2736990 PrU | TGGCAGTTGAGAGGAGTATTC | 280 | 95 °C/40″ | 62 °C/30″ | 72 °C/50″ | 35 |
| rs2736990 PrL | GTGACTAGCAGATGATGAGCA | |||||
| L2-16485 | GAACTGTATCCGACATCTGG | 560 | 94 °C/60″ | 55 °C/40″ | 72 °C/60″ | 35 |
| H2-481 | GATTAGTAGTATGGGAGTGG |
| Cat. Nº MiRCury Assay | miRNA ID | Target Sequence | |
|---|---|---|---|
| 1 | YP00205986 | hsa-miR-223-3p | UGUCAGUUUGUCAAAUACCCCA |
| 2 | YP00204260 | hsa-miR-24-3p | UGGCUCAGUUCAGCAGGAACAG |
| 3 | YP00204306 | hsa-miR-191-5p | CAACGGAAUCCCAAAAGCAGCUG |
| 4 | YP00204783 | hsa-miR-30c-5p | UGUAAACAUCCUACACUCUCAGC |
| 5 | YP00204362 | hsa-miR-375-3p | UUUGUUCGUUCGGCUCGCGUGA |
| 6 | YP00206038 | hsa-miR-27a-3p | UUCACAGUGGCUAAGUUCCGC |
| 7 | YP00204117 | hsa-miR-26b-5p | CCUGUUCUCCAUUACUUGGCUC |
| 8 | YP00203950 | UniSp2 | |
| 9 | YP00203953 | UniSp4 | |
| 10 | YP00203955 | UniSp5 | |
| 11 | YP02119288 | UniSp3 | |
| 12 | YP00203954 | UniSp6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbizu, A.; Arnaldo, L.; Beyer, K. Obtaining miRNA from Saliva—Comparison of Sampling and Purification Methods. Int. J. Mol. Sci. 2023, 24, 2386. https://doi.org/10.3390/ijms24032386
Urbizu A, Arnaldo L, Beyer K. Obtaining miRNA from Saliva—Comparison of Sampling and Purification Methods. International Journal of Molecular Sciences. 2023; 24(3):2386. https://doi.org/10.3390/ijms24032386
Chicago/Turabian StyleUrbizu, Aintzane, Laura Arnaldo, and Katrin Beyer. 2023. "Obtaining miRNA from Saliva—Comparison of Sampling and Purification Methods" International Journal of Molecular Sciences 24, no. 3: 2386. https://doi.org/10.3390/ijms24032386
APA StyleUrbizu, A., Arnaldo, L., & Beyer, K. (2023). Obtaining miRNA from Saliva—Comparison of Sampling and Purification Methods. International Journal of Molecular Sciences, 24(3), 2386. https://doi.org/10.3390/ijms24032386






