Hypoxia and Intestinal Inflammation: Common Molecular Mechanisms and Signaling Pathways
Abstract
1. Introduction
2. Hypoxia and the Gastrointestinal Tract
2.1. Role of Oxygen in the GI and Adaptation to Hypoxia
2.2. Gut Metabolites and HIF Activity
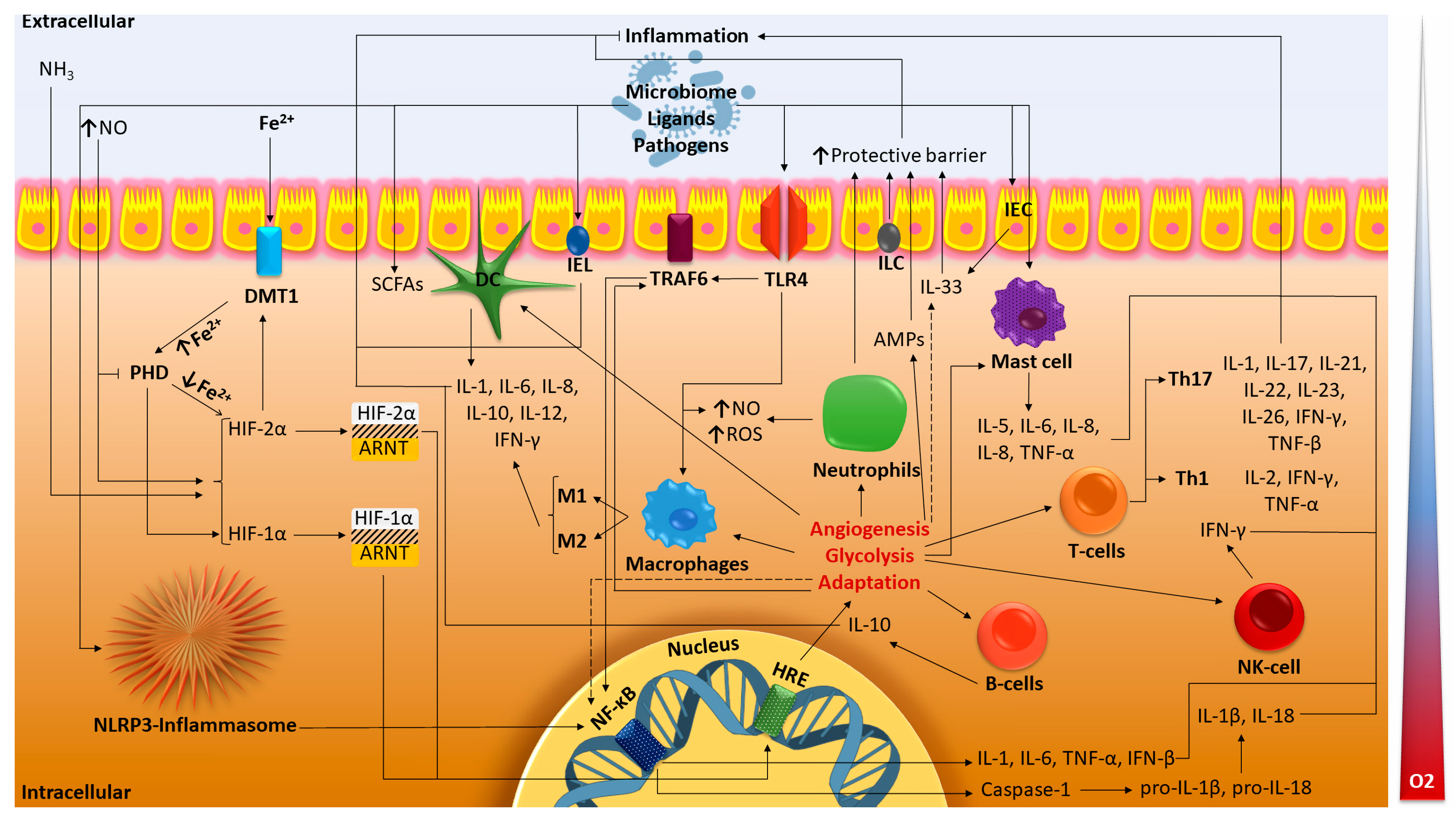
2.3. Gut Immunity and HIF
2.3.1. Innate Immunity of the Intestine and HIF
2.3.2. Adaptive Immunity of the Intestine and HIF
3. Hypoxia and IBD
3.1. General Information on IBD
3.2. Interplay between Hypoxia and Inflammation
3.3. Inflammatory Hypoxia in IBD and Implications for the Gut
3.3.1. Molecular Interactions of HIF-1α in IBD
3.3.2. Molecular Interactions of HIF-2α in IBD
3.3.3. Molecular Interactions of PHD Isoforms in IBD
3.4. Possible IBD Therapy by Targeting HIF
4. Further Perspectives and Conclusive Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IBD | Inflammatory bowel disease |
| CD | Crohn’s disease |
| UC | Ulcerative colitis |
| GI | Gastrointestinal tract |
| pO2 | Partial pressure |
| HIF | Hypoxia-inducible factor |
| ARNT | Aryl hydrocarbon receptor nuclear translocator |
| HRE | Hypoxia response elements |
| PHD | Prolyl hydroxylase domain |
| FIH | Factor inhibiting HIF |
| IDH | Isocitrate dehydrogenase |
| FPN | Basolateral iron transporter ferroportin |
| ROS | Reactive oxygen species |
| IECs | Intestinal epithelial cells |
| IELs | Intestinal intraepithelial lymphocytes |
| ILCs | Innate lymphoid cells |
| DCs | Dendritic cells |
| Th cells | T-helper cells |
| Tregs | Regulatory T-cells |
| NK-cells | Natural killer T-cells |
| IL | Interleukin |
| TNF | Tumor necrosis factor |
| TGF-β | Transforming growth factor beta |
| IFN | Interferon |
| TLRs | Toll-like receptors |
| AMPs | Antimicrobial peptides |
| SCFAs | Short chain fatty acids |
| DSS | Dextran sulfate sodium |
| PRRs | Pattern recognition receptors |
| PAMPs | Pathogen-associated molecular patterns |
| LPS | Lipopolysaccharide |
| FoxP3 | Tregs forkhead box P3 |
| NF-κB | Nuclear factor κB |
| NADPH 1 | Nicotinamide adenine dinucleotide phosphate oxidase complex Nox1 |
| DMOG | Dimethyloxalylglycine |
| PHDi | Prolyl hydroxylase inhibitors |
| SAHA | Suberoylanilide hydroxamic acid |
References
- Konjar, Š.; Pavšič, M.; Veldhoen, M. Regulation of Oxygen Homeostasis at the Intestinal Epithelial Barrier Site. Int. J. Mol. Sci. 2021, 22, 9170. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Prado, E.; Dunn, J.F.; Vasconez, J.; Castillo, D.; Viscor, G. Partial Pressure of Oxygen in the Human Body: A General Review. Am. J. Blood Res. 2019, 9, 1–14. [Google Scholar] [PubMed]
- Reyman, M.; van Houten, M.A.; van Baarle, D.; Bosch, A.A.T.M.; Man, W.H.; Chu, M.L.J.N.; Arp, K.; Watson, R.L.; Sanders, E.A.M.; Fuentes, S.; et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat. Commun. 2019, 10, 4997. [Google Scholar] [CrossRef]
- Lee, J.Y.; Tsolis, R.M.; Bäumler, A.J. The microbiome and gut homeostasis. Science 2022, 377, eabp9960. [Google Scholar] [CrossRef]
- Sommer, F.; Anderson, J.M.; Bharti, R.; Raes, J.; Rosenstiel, P. The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 2017, 15, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Shoenfeld, Y.; Matthias, T. Adverse effects of gluten ingestion and advantages of gluten withdrawal in nonceliac autoimmune disease. Nutr. Rev. 2017, 75, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Chávez, F.; Lopez, C.A.; Bäumler, A.J. Oxygen as a Driver of Gut Dysbiosis. Free. Radic. Biol. Med. 2017, 105, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhong, Z.-F.; Wang, S.-P.; Vong, C.-T.; Yu, B.; Wang, Y.-T. HIF-1: Structure, Biology and Natural Modulators. Chin. J. Nat. Med. 2021, 19, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.K.; Shah, Y.M. Role of Intestinal HIF-2α in Health and Disease. Annu. Rev. Physiol. 2016, 78, 301–325. [Google Scholar] [CrossRef]
- Sun, L.; Li, T.; Tang, H.; Yu, K.; Ma, Y.; Yu, M.; Qiu, Y.; Xu, P.; Xiao, W.; Yang, H. Intestinal Epithelial Cells-Derived Hypoxia-Inducible Factor-1α Is Essential for the Homeostasis of Intestinal Intraepithelial Lymphocytes. Front. Immunol. 2019, 10, 806. [Google Scholar] [CrossRef]
- Dengler, V.L.; Galbraith, M.; Espinosa, J.M. Transcriptional Regulation by Hypoxia Inducible Factors. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Watts, E.R.; Walmsley, S.R. Inflammation and Hypoxia: HIF and PHD Isoform Selectivity. Trends Mol. Med. 2019, 25, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.; Shah, Y.M. Oxygen Battle in the Gut: Hypoxia and Hypoxia-Inducible Factors in Metabolic and Inflammatory Responses in the Intestine. J. Biol. Chem. 2020, 295, 10493–10505. [Google Scholar] [CrossRef] [PubMed]
- Heir, P.; Ohh, M. Hydroxylation-Dependent Interaction of Substrates to the Von Hippel-Lindau Tumor Suppressor Protein (VHL). In The Tumor Microenvironment: Methods and Protocols; Methods in Molecular Biology; Ursini-Siegel, J., Beauchemin, N., Eds.; Springer: New York, NY, USA, 2016; pp. 87–94. ISBN 978-1-4939-3801-8. [Google Scholar]
- Volkova, Y.L.; Pickel, C.; Jucht, A.E.; Wenger, R.H.; Scholz, C.C. The Asparagine Hydroxylase FIH: A Unique Oxygen Sensor. Antioxid. Redox Signal. 2022, 37, 913–935. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Hu, H. The Roles of 2-Hydroxyglutarate. Front. Cell Dev. Biol. 2021, 9, 651317. [Google Scholar] [CrossRef]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate Is an Inflammatory Signal That Induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiannaki, M.; Matak, P.; Peyssonnaux, C. The Gut in Iron Homeostasis: Role of HIF-2 under Normal and Pathological Conditions. Blood 2013, 122, 885–892. [Google Scholar] [CrossRef]
- Schwartz, A.J.; Das, N.K.; Ramakrishnan, S.K.; Jain, C.; Jurkovic, M.T.; Wu, J.; Nemeth, E.; Lakhal-Littleton, S.; Colacino, J.A.; Shah, Y.M. Hepatic Hepcidin/Intestinal HIF-2α Axis Maintains Iron Absorption during Iron Deficiency and Overload. J. Clin. Investig. 2019, 129, 336–348. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Diebold, L.P.; Kong, H.; Schieber, M.; Huang, H.; Hensley, C.T.; Mehta, M.M.; Wang, T.; Santos, J.H.; Woychik, R.; et al. TCA Cycle and Mitochondrial Membrane Potential Are Necessary for Diverse Biological Functions. Mol. Cell 2016, 61, 199–209. [Google Scholar] [CrossRef]
- Gonzalez, F.J.; Xie, C.; Jiang, C. The Role of Hypoxia-Inducible Factors in Metabolic Diseases. Nat. Rev. Endocrinol. 2019, 15, 21–32. [Google Scholar] [CrossRef]
- Xie, C.; Yagai, T.; Luo, Y.; Liang, X.; Chen, T.; Wang, Q.; Sun, D.; Zhao, J.; Ramakrishnan, S.K.; Sun, L.; et al. Activation of Intestinal Hypoxia-Inducible Factor 2α during Obesity Contributes to Hepatic Steatosis. Nat. Med. 2017, 23, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Berean, K.J.; Burgell, R.E.; Muir, J.G.; Gibson, P.R. Intestinal Gases: Influence on Gut Disorders and the Role of Dietary Manipulations. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, X.; Noviana, M.; Hou, M. Nitric oxide in red blood cell adaptation to hypoxia. Acta Biochim. Biophys. Sin. 2018, 50, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Lercher, A.; Baazim, H.; Bergthaler, A. Systemic Immunometabolism: Challenges and Opportunities. Immunity 2020, 53, 496–509. [Google Scholar] [CrossRef]
- Colgan, S.P.; Furuta, G.T.; Taylor, C.T. Hypoxia and Innate Immunity: Keeping Up with the HIFsters. Annu. Rev. Immunol. 2020, 38, 341–363. [Google Scholar] [CrossRef]
- Taylor, C.T.; Doherty, G.; Fallon, P.G.; Cummins, E.P. Hypoxia-Dependent Regulation of Inflammatory Pathways in Immune Cells. J. Clin. Investig. 2016, 126, 3716–3724. [Google Scholar] [CrossRef]
- Corcoran, S.E.; O’Neill, L.A.J. HIF1α and Metabolic Reprogramming in Inflammation. J. Clin. Investig. 2016, 126, 3699–3707. [Google Scholar] [CrossRef]
- Jasper, H. Intestinal Stem Cell Aging: Origins and Interventions. Annu. Rev. Physiol. 2020, 82, 203–226. [Google Scholar] [CrossRef]
- Steiner, C.A.; Cartwright, I.M.; Taylor, C.T.; Colgan, S.P. Hypoxia-Inducible Factor as a Bridge between Healthy Barrier Function, Wound Healing, and Fibrosis. Am. J. Physiol. Cell Physiol. 2022, 323, C866–C878. [Google Scholar] [CrossRef]
- Olivares-Villagómez, D.; Van Kaer, L. Intestinal Intraepithelial Lymphocytes: Sentinels of the Mucosal Barrier. Trends Immunol. 2018, 39, 264–275. [Google Scholar] [CrossRef]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.-Y.; Ko, H.-J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Birchenough, G.M.H.; Johansson, M.E.V.; Gustafsson, J.K.; Bergström, J.H.; Hansson, G.C. New Developments in Goblet Cell Mucus Secretion and Function. Mucosal Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial Peptides: An Update on Classifications and Databases. Int. J. Mol. Sci. 2021, 22, 11691. [Google Scholar] [CrossRef] [PubMed]
- Louis, N.A.; Hamilton, K.E.; Canny, G.; Shekels, L.L.; Ho, S.B.; Colgan, S.P. Selective Induction of Mucin-3 by Hypoxia in Intestinal Epithelia. J. Cell. Biochem. 2006, 99, 1616–1627. [Google Scholar] [CrossRef] [PubMed]
- Krzywinska, E.; Stockmann, C. Hypoxia, Metabolism and Immune Cell Function. Biomedicines 2018, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, B.J.; Kao, D.J.; Kitzenberg, D.A.; Dobrinskikh, E.; Schwisow, K.D.; Masterson, J.C.; Kendrick, A.A.; Kelly, C.J.; Bayless, A.J.; Kominsky, D.J.; et al. HIF-Dependent Regulation of Claudin-1 Is Central to Intestinal Epithelial Tight Junction Integrity. Mol. Biol. Cell 2015, 26, 2252–2262. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.L.; Sumpter, R.; Levine, B.; Hooper, L.V. Intestinal Epithelial Autophagy Is Essential for Host Defense against Invasive Bacteria. Cell Host Microbe 2013, 13, 723–734. [Google Scholar] [CrossRef]
- Fellows, R.; Denizot, J.; Stellato, C.; Cuomo, A.; Jain, P.; Stoyanova, E.; Balázsi, S.; Hajnády, Z.; Liebert, A.; Kazakevych, J.; et al. Microbiota Derived Short Chain Fatty Acids Promote Histone Crotonylation in the Colon through Histone Deacetylases. Nat. Commun. 2018, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, L.; Li, T.; Sun, L.; Yin, J.; Guan, H.; Wang, L.; Zhu, H.; Xu, P.; Fan, X.; et al. SCFAs Induce Autophagy in Intestinal Epithelial Cells and Relieve Colitis by Stabilizing HIF-1α. J. Mol. Med. 2020, 98, 1189–1202. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- McKernan, D.P. Toll-Like Receptors as Drug Targets in the Intestinal Epithelium. In Toll-Like Receptors in Health and Disease; Handbook of Experimental Pharmacology; Kumar, V., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 291–314. ISBN 978-3-031-06512-5. [Google Scholar]
- Zhang, J.; Han, C.; Dai, H.; Hou, J.; Dong, Y.; Cui, X.; Xu, L.; Zhang, M.; Xia, Q. Hypoxia-Inducible Factor-2α Limits Natural Killer T Cell Cytotoxicity in Renal Ischemia/Reperfusion Injury. J. Am. Soc. Nephrol. 2016, 27, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; O’Neill, L.A. Reprogramming Mitochondrial Metabolism in Macrophages as an Anti-Inflammatory Signal. Eur. J. Immunol. 2016, 46, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Domblides, C.; Lartigue, L.; Faustin, B. Metabolic Stress in the Immune Function of T Cells, Macrophages and Dendritic Cells. Cells 2018, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Palsson-McDermott, E.M.; Curtis, A.M.; Goel, G.; Lauterbach, M.A.R.; Sheedy, F.J.; Gleeson, L.E.; van den Bosch, M.W.M.; Quinn, S.R.; Domingo-Fernandez, R.; Johnston, D.G.W.; et al. Pyruvate Kinase M2 Regulates Hif-1α Activity and IL-1β Induction and Is a Critical Determinant of the Warburg Effect in LPS-Activated Macrophages. Cell Metab. 2015, 21, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Bošnjak, B.; Do, K.T.H.; Förster, R.; Hammerschmidt, S.I. Imaging Dendritic Cell Functions. Immunol. Rev. 2022, 306, 137–163. [Google Scholar] [CrossRef]
- Flück, K.; Breves, G.; Fandrey, J.; Winning, S. Hypoxia-Inducible Factor 1 in Dendritic Cells Is Crucial for the Activation of Protective Regulatory T Cells in Murine Colitis. Mucosal Immunol. 2016, 9, 379–390. [Google Scholar] [CrossRef]
- Wobben, R.; Hüsecken, Y.; Lodewick, C.; Gibbert, K.; Fandrey, J.; Winning, S. Role of Hypoxia Inducible Factor-1α for Interferon Synthesis in Mouse Dendritic Cells. Biol. Chem. 2013, 394, 495–505. [Google Scholar] [CrossRef]
- Walmsley, S.R.; Chilvers, E.R.; Thompson, A.A.; Vaughan, K.; Marriott, H.M.; Parker, L.C.; Shaw, G.; Parmar, S.; Schneider, M.; Sabroe, I.; et al. Prolyl Hydroxylase 3 (PHD3) Is Essential for Hypoxic Regulation of Neutrophilic Inflammation in Humans and Mice. J. Clin. Investig. 2011, 121, 1053–1063. [Google Scholar] [CrossRef]
- Campbell, E.L.; Bruyninckx, W.J.; Kelly, C.J.; Glover, L.E.; McNamee, E.N.; Bowers, B.E.; Bayless, A.J.; Scully, M.; Saeedi, B.J.; Golden-Mason, L.; et al. Transmigrating Neutrophils Shape the Mucosal Microenvironment through Localized Oxygen Depletion to Influence Resolution of Inflammation. Immunity 2014, 40, 66–77. [Google Scholar] [CrossRef]
- Loftus, R.M.; Finlay, D.K. Immunometabolism: Cellular Metabolism Turns Immune Regulator. J. Biol. Chem. 2016, 291, 1–10. [Google Scholar] [CrossRef]
- Bagadia, P.; Huang, X.; Liu, T.-T.; Murphy, K.M. Shared Transcriptional Control of Innate Lymphoid Cell and Dendritic Cell Development. Annu. Rev. Cell Dev. Biol. 2019, 35, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, D.; Zhang, X.; Wan, Q.; Zhang, W.; Zheng, M.; Zou, L.; Elly, C.; Lee, J.H.; Liu, Y.-C. E3 Ligase VHL Promotes Group 2 Innate Lymphoid Cell Maturation and Function via Glycolysis Inhibition and Induction of Interleukin-33 Receptor. Immunity 2018, 48, 258–270.e5. [Google Scholar] [CrossRef] [PubMed]
- Burrows, N.; Maxwell, P.H. Hypoxia and B Cells. Exp. Cell Res. 2017, 356, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Barbi, J.; Pardoll, D.; Pan, F. Metabolic Control of the Treg/Th17 Axis. Immunol. Rev. 2013, 252, 52–77. [Google Scholar] [CrossRef] [PubMed]
- Shehade, H.; Acolty, V.; Moser, M.; Oldenhove, G. Cutting Edge: Hypoxia-Inducible Factor 1 Negatively Regulates Th1 Function. J. Immunol. 2015, 195, 1372–1376. [Google Scholar] [CrossRef]
- Colamatteo, A.; Carbone, F.; Bruzzaniti, S.; Galgani, M.; Fusco, C.; Maniscalco, G.T.; Di Rella, F.; de Candia, P.; De Rosa, V. Molecular Mechanisms Controlling Foxp3 Expression in Health and Autoimmunity: From Epigenetic to Post-Translational Regulation. Front. Immunol. 2019, 10, 3136. [Google Scholar] [CrossRef]
- Lee, J.H.; Elly, C.; Park, Y.; Liu, Y.-C. E3 Ubiquitin Ligase VHL Regulates Hypoxia-Inducible Factor-1α to Maintain Regulatory T Cell Stability and Suppressive Capacity. Immunity 2015, 42, 1062–1074. [Google Scholar] [CrossRef]
- Shi, L.Z.; Wang, R.; Huang, G.; Vogel, P.; Neale, G.; Green, D.R.; Chi, H. HIF1alpha-Dependent Glycolytic Pathway Orchestrates a Metabolic Checkpoint for the Differentiation of TH17 and Treg Cells. J. Exp. Med. 2011, 208, 1367–1376. [Google Scholar] [CrossRef]
- Palazon, A.; Tyrakis, P.A.; Macias, D.; Veliça, P.; Rundqvist, H.; Fitzpatrick, S.; Vojnovic, N.; Phan, A.T.; Loman, N.; Hedenfalk, I.; et al. An HIF-1α/VEGF-A Axis in Cytotoxic T Cells Regulates Tumor Progression. Cancer Cell 2017, 32, 669–683.e5. [Google Scholar] [CrossRef]
- Shin, D.H.; Lin, H.; Zheng, H.; Kim, K.S.; Kim, J.Y.; Chun, Y.S.; Park, J.W.; Nam, J.H.; Kim, W.K.; Zhang, Y.H.; et al. HIF-1α–Mediated Upregulation of TASK-2 K+ Channels Augments Ca2+ Signaling in Mouse B Cells under Hypoxia. J. Immunol. 2014, 193, 4924–4933. [Google Scholar] [CrossRef]
- Cho, S.H.; Raybuck, A.L.; Stengel, K.; Wei, M.; Beck, T.C.; Volanakis, E.; Thomas, J.W.; Hiebert, S.; Haase, V.H.; Boothby, M.R. Germinal Centre Hypoxia and Regulation of Antibody Qualities by a Hypoxia Response System. Nature 2016, 537, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Grötsch, B.; Luo, Y.; Knaup, K.X.; Wiesener, M.S.; Chen, X.-X.; Jantsch, J.; Fillatreau, S.; Schett, G.; Bozec, A. Hypoxia-Inducible Factor-1α Is a Critical Transcription Factor for IL-10-Producing B Cells in Autoimmune Disease. Nat. Commun. 2018, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Hams, E.; Saunders, S.P.; Cummins, E.P.; O’Connor, A.; Tambuwala, M.T.; Gallagher, W.M.; Byrne, A.; Campos-Torres, A.; Moynagh, P.M.; Jobin, C.; et al. The Hydroxylase Inhibitor Dimethyloxallyl Glycine Attenuates Endotoxic Shock via Alternative Activation of Macrophages and IL-10 Production by B1 Cells. Shock 2011, 36, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Simińska, D.; Gąssowska-Dobrowolska, M.; Listos, J.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Chronic and Cycling Hypoxia: Drivers of Cancer Chronic Inflammation through HIF-1 and NF-ΚB Activation: A Review of the Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 10701. [Google Scholar] [CrossRef] [PubMed]
- Scholz, C.C.; Cavadas, M.A.S.; Tambuwala, M.M.; Hams, E.; Rodríguez, J.; von Kriegsheim, A.; Cotter, P.; Bruning, U.; Fallon, P.G.; Cheong, A.; et al. Regulation of IL-1β-Induced NF-ΚB by Hydroxylases Links Key Hypoxic and Inflammatory Signaling Pathways. Proc. Natl. Acad. Sci. USA 2013, 110, 18490–18495. [Google Scholar] [CrossRef]
- D’Ignazio, L.; Shakir, D.; Batie, M.; Muller, H.A.; Rocha, S. HIF-1β Positively Regulates NF-ΚB Activity via Direct Control of TRAF6. Int. J. Mol. Sci. 2020, 21, 3000. [Google Scholar] [CrossRef]
- Dong, S.; Liang, S.; Cheng, Z.; Zhang, X.; Luo, L.; Li, L.; Zhang, W.; Li, S.; Xu, Q.; Zhong, M.; et al. ROS/PI3K/Akt and Wnt/β-Catenin Signalings Activate HIF-1α-Induced Metabolic Reprogramming to Impart 5-Fluorouracil Resistance in Colorectal Cancer. J. Exp. Clin. Cancer Res. 2022, 41, 15. [Google Scholar] [CrossRef]
- Agrawal, M.; Allin, K.H.; Petralia, F.; Colombel, J.-F.; Jess, T. Multiomics to Elucidate Inflammatory Bowel Disease Risk Factors and Pathways. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 399–409. [Google Scholar] [CrossRef]
- Burke, K.E.; D’Amato, M.; Ng, S.C.; Pardi, D.S.; Ludvigsson, J.F.; Khalili, H. Microscopic colitis. Nat. Rev. Dis. Prim. 2021, 7, 39. [Google Scholar] [CrossRef]
- Kofla-Dłubacz, A.; Pytrus, T.; Akutko, K.; Sputa-Grzegrzółka, P.; Piotrowska, A.; Dzięgiel, P. Etiology of IBD-Is It Still a Mystery? Int. J. Mol. Sci. 2022, 23, 12445. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Turner, J.R. The Intestinal Epithelial Barrier: A Therapeutic Target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Chang, E.B. Inflammatory Bowel Diseases (IBD) and the Microbiome—Searching the Crime Scene for Clues. Gastroenterology 2021, 160, 524–537. [Google Scholar] [CrossRef]
- Binion, D.G. Clostridium Difficile Infection in Patients with Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2012, 8, 615–617. [Google Scholar]
- Wah-Suárez, M.I.; Vázquez, M.A.M.; Bosques-Padilla, F.J. Inflammatory Bowel Disease: The Role of Commensal Microbiome in Immune Regulation. Gastroenterol. Hepatol. 2022, 45, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Mirsepasi-Lauridsen, H.C.; Du, Z.; Struve, C.; Charbon, G.; Karczewski, J.; Krogfelt, K.A.; Wells, J.M. Secretion of Alpha-Hemolysin by Escherichia coli Disrupts Tight Junctions in Ulcerative Colitis Patients. Clin. Transl. Gastroenterol. 2016, 7, e149. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Skinner, N.A.; Catto-Smith, A.G.; Cameron, D.J.; Michalski, W.P.; Visvanathan, K.; Kirkwood, C.D. TLR4, IL10RA, and NOD2 mutation in paediatric Crohn’s disease patients: An association with Mycobacterium avium subspecies paratuberculosis and TLR4 and IL10RA expression. Med. Microbiol. Immunol. 2013, 202, 267–276. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Xavier, R.J. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Mirsepasi-Lauridsen, H.C.; Vrankx, K.; Engberg, J.; Friis-Møller, A.; Brynskov, J.; Nordgaard-Lassen, I.; Krogfelt, K.A. Disease-Specific Enteric Microbiome Dysbiosis in Inflammatory Bowel Disease. Front. Med. 2018, 5, 304. [Google Scholar] [CrossRef]
- Turner, D.; Bishai, J.; Reshef, L.; Abitbol, G.; Focht, G.; Marcus, D.; Ledder, O.; Lev-Tzion, R.; Orlanski-Meyer, E.; Yerushalmi, B.; et al. Antibiotic Cocktail for Pediatric Acute Severe Colitis and the Microbiome: The PRASCO Randomized Controlled Trial. Inflamm. Bowel Dis. 2020, 26, 1733–1742. [Google Scholar] [CrossRef]
- Pigneur, B.; Lepage, P.; Mondot, S.; Schmitz, J.; Goulet, O.; Doré, J.; Ruemmele, F.M. Mucosal Healing and Bacterial Composition in Response to Enteral Nutrition Vs Steroid-Based Induction Therapy-A Randomised Prospective Clinical Trial in Children with Crohn’s Disease. J. Crohn’s Colitis 2019, 13, 846–855. [Google Scholar] [CrossRef]
- Milajerdi, A.; Sadeghi, O.; Siadat, S.D.; Keshavarz, S.A.; Sima, A.; Vahedi, H.; Adibi, P.; Esmaillzadeh, A. A Randomized Controlled Trial Investigating the Effect of a Diet Low in Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols on the Intestinal Microbiome and Inflammation in Patients with Ulcerative Colitis: Study Protocol for a Randomized Controlled Trial. Trials 2020, 21, 201. [Google Scholar] [CrossRef] [PubMed]
- Nissilä, E.; Korpela, K.; Lokki, A.I.; Paakkanen, R.; Jokiranta, S.; de Vos, W.M.; Lokki, M.-L.; Kolho, K.-L.; Meri, S. C4B Gene Influences Intestinal Microbiota through Complement Activation in Patients with Paediatric-Onset Inflammatory Bowel Disease. Clin. Exp. Immunol. 2017, 190, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Schwerd, T.; Bryant, R.V.; Pandey, S.; Capitani, M.; Meran, L.; Cazier, J.-B.; Jung, J.; Mondal, K.; Parkes, M.; Mathew, C.G.; et al. NOX1 Loss-of-Function Genetic Variants in Patients with Inflammatory Bowel Disease. Mucosal Immunol. 2018, 11, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Meir, M.; Flemming, S.; Burkard, N.; Bergauer, L.; Metzger, M.; Germer, C.-T.; Schlegel, N. Glial Cell Line-Derived Neurotrophic Factor Promotes Barrier Maturation and Wound Healing in Intestinal Epithelial Cells in Vitro. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G613–G624. [Google Scholar] [CrossRef] [PubMed]
- Meir, M.; Burkard, N.; Ungewiß, H.; Diefenbacher, M.; Flemming, S.; Kannapin, F.; Germer, C.-T.; Schweinlin, M.; Metzger, M.; Waschke, J.; et al. Neurotrophic Factor GDNF Regulates Intestinal Barrier Function in Inflammatory Bowel Disease. J. Clin. Investig. 2019, 129, 2824–2840. [Google Scholar] [CrossRef]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.H.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.L.; et al. The Mucus and Mucins of the Goblet Cells and Enterocytes Provide the First Defense Line of the Gastrointestinal Tract and Interact with the Immune System. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef]
- Engevik, M.A.; Luk, B.; Chang-Graham, A.L.; Hall, A.; Herrmann, B.; Ruan, W.; Endres, B.T.; Shi, Z.; Garey, K.W.; Hyser, J.M.; et al. Bifidobacterium Dentium Fortifies the Intestinal Mucus Layer via Autophagy and Calcium Signaling Pathways. mBio 2019, 10, e01087-19. [Google Scholar] [CrossRef]
- Mukherjee, T.; Hovingh, E.S.; Foerster, E.G.; Abdel-Nour, M.; Philpott, D.J.; Girardin, S.E. NOD1 and NOD2 in Inflammation, Immunity and Disease. Arch. Biochem. Biophys. 2019, 670, 69–81. [Google Scholar] [CrossRef]
- Strober, W.; Watanabe, T. NOD2, an Intracellular Innate Immune Sensor Involved in Host Defense and Crohn’s Disease. Mucosal Immunol. 2011, 4, 484–495. [Google Scholar] [CrossRef]
- Roda, G.; Chien Ng, S.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin-Biroulet, L.; Danese, S. Crohn’s Disease. Nat. Rev. Dis. Prim. 2020, 6, 22. [Google Scholar] [CrossRef]
- Yan, J.-B.; Luo, M.-M.; Chen, Z.-Y.; He, B.-H. The Function and Role of the Th17/Treg Cell Balance in Inflammatory Bowel Disease. J. Immunol. Res. 2020, 2020, 8813558. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Kitani, A.; Strober, W.; Fuss, I.J. The Role of NLRP3 and IL-1β in the Pathogenesis of Inflammatory Bowel Disease. Front. Immunol. 2018, 9, 2566. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Long, L.; Wang, X.; Li, S.; Xu, H. Phytochemicals Targeting Toll-like Receptors 4 (TLR4) in Inflammatory Bowel Disease. Chin. Med. 2022, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Y.; Lin, R.; Meng, X.; Zhao, W.; Shen, W.; Fan, H. Cronobacter Sakazakii Induces Necrotizing Enterocolitis by Regulating NLRP3 Inflammasome Expression via TLR4. J. Med. Microbiol. 2020, 69, 748–758. [Google Scholar] [CrossRef] [PubMed]
- McKernan, D.P.; Finn, D.P. An ApPEAling New Therapeutic for Ulcerative Colitis? Gut 2014, 63, 1207–1208. [Google Scholar] [CrossRef]
- Taylor, C.T. Hypoxia in the Gut. Cell. Mol. Gastroenterol. Hepatol. 2017, 5, 61–62. [Google Scholar] [CrossRef]
- Colgan, S.P.; Campbell, E.L.; Kominsky, D.J. Hypoxia and Mucosal Inflammation. Annu. Rev. Pathol. 2016, 11, 77–100. [Google Scholar] [CrossRef]
- Brown, E.; Taylor, C.T. Hypoxia-sensitive Pathways in Intestinal Inflammation. J. Physiol. 2018, 596, 2985–2989. [Google Scholar] [CrossRef]
- Kerber, E.L.; Padberg, C.; Koll, N.; Schuetzhold, V.; Fandrey, J.; Winning, S. The Importance of Hypoxia-Inducible Factors (HIF-1 and HIF-2) for the Pathophysiology of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2020, 21, 8551. [Google Scholar] [CrossRef]
- Yin, J.; Ren, Y.; Yang, K.; Wang, W.; Wang, T.; Xiao, W.; Yang, H. The Role of Hypoxia-Inducible Factor 1-Alpha in Inflammatory Bowel Disease. Cell Biol. Int. 2022, 46, 46–51. [Google Scholar] [CrossRef]
- Pazmandi, J.; Kalinichenko, A.; Ardy, R.C.; Boztug, K. Early-onset Inflammatory Bowel Disease as a Model Disease to Identify Key Regulators of Immune Homeostasis Mechanisms. Immunol. Rev. 2019, 287, 162–185. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shi, T.; Zhong, C.; Wang, Y.; Chang, M.; Liu, X. IL-10 and IL-10 Receptor Mutations in Very Early Onset Inflammatory Bowel Disease. Gastroenterol. Res. 2017, 10, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, M.; Liu, J.; Ji, Y.; Subramanian, M.; Crompton, J.G.; Yu, Z.; Roychoudhuri, R.; Palmer, D.C.; Muranski, P.; Karoly, E.D.; et al. Inhibiting Glycolytic Metabolism Enhances CD8+ T Cell Memory and Antitumor Function. J. Clin. Investig. 2013, 123, 4479–4488. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; He, C.; Wu, W.; Zhou, G.; Liu, F.; Cong, Y.; Liu, Z. Hypoxia Inducible Factor-1α-Induced Interleukin-33 Expression in Intestinal Epithelia Contributes to Mucosal Homeostasis in Inflammatory Bowel Disease. Clin. Exp. Immunol. 2017, 187, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Bäcker, V.; Cheung, F.-Y.; Siveke, J.T.; Fandrey, J.; Winning, S. Knockdown of Myeloid Cell Hypoxia-Inducible Factor-1α Ameliorates the Acute Pathology in DSS-Induced Colitis. PLoS ONE 2017, 12, e0190074. [Google Scholar] [CrossRef]
- Choudhry, H.; Harris, A.L. Advances in Hypoxia-Inducible Factor Biology. Cell Metab. 2018, 27, 281–298. [Google Scholar] [CrossRef]
- Endo, K.; Kito, H.; Tanaka, R.; Kajikuri, J.; Tanaka, S.; Elboray, E.E.; Suzuki, T.; Ohya, S. Possible Contribution of Inflammation-Associated Hypoxia to Increased K2P5.1 K+ Channel Expression in CD4+ T Cells of the Mouse Model for Inflammatory Bowel Disease. Int. J. Mol. Sci. 2019, 21, 38. [Google Scholar] [CrossRef]
- Triner, D.; Xue, X.; Schwartz, A.J.; Jung, I.; Colacino, J.A.; Shah, Y.M. Epithelial Hypoxia-Inducible Factor 2α Facilitates the Progression of Colon Tumors through Recruiting Neutrophils. Mol. Cell. Biol. 2017, 37, e00481-16. [Google Scholar] [CrossRef]
- Thompson, A.A.R.; Elks, P.M.; Marriott, H.M.; Eamsamarng, S.; Higgins, K.R.; Lewis, A.; Williams, L.; Parmar, S.; Shaw, G.; McGrath, E.E.; et al. Hypoxia-Inducible Factor 2α Regulates Key Neutrophil Functions in Humans, Mice, and Zebrafish. Blood 2014, 123, 366–376. [Google Scholar] [CrossRef]
- Xue, X.; Ramakrishnan, S.; Anderson, E.; Taylor, M.; Zimmermann, E.M.; Spence, J.R.; Huang, S.; Greenson, J.K.; Shah, Y.M. Endothelial PAS Domain Protein 1 Activates the Inflammatory Response in the Intestinal Epithelium to Promote Colitis in Mice. Gastroenterology 2013, 145, 831–841. [Google Scholar] [CrossRef]
- Xie, L.; Xue, X.; Taylor, M.; Ramakrishnan, S.K.; Nagaoka, K.; Hao, C.; Gonzalez, F.J.; Shah, Y.M. Hypoxia-Inducible Factor/MAZ-Dependent Induction of Caveolin-1 Regulates Colon Permeability through Suppression of Occludin, Leading to Hypoxia-Induced Inflammation. Mol. Cell. Biol. 2014, 34, 3013–3023. [Google Scholar] [CrossRef] [PubMed]
- Van Welden, S.; Laukens, D.; Ferdinande, L.; De Vos, M.; Hindryckx, P. Differential Expression of Prolyl Hydroxylase 1 in Patients with Ulcerative Colitis versus Patients with Crohn’s Disease/Infectious Colitis and Healthy Controls. J. Inflamm. 2013, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Tambuwala, M.M.; Cummins, E.P.; Lenihan, C.R.; Kiss, J.; Stauch, M.; Scholz, C.C.; Fraisl, P.; Lasitschka, F.; Mollenhauer, M.; Saunders, S.P.; et al. Loss of Prolyl Hydroxylase-1 Protects against Colitis through Reduced Epithelial Cell Apoptosis and Increased Barrier Function. Gastroenterology 2010, 139, 2093–2101. [Google Scholar] [CrossRef]
- Van Welden, S.; De Vos, M.; Wielockx, B.; Tavernier, S.J.; Dullaers, M.; Neyt, S.; Descamps, B.; Devisscher, L.; Devriese, S.; Van den Bossche, L.; et al. Haematopoietic Prolyl Hydroxylase-1 Deficiency Promotes M2 Macrophage Polarization and Is Both Necessary and Sufficient to Protect against Experimental Colitis. J. Pathol. 2017, 241, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.-S.; Fong, G.-H.; Xi, Q.-L.; Wu, G.-H.; Bai, C.-G.; Ling, Z.-Q.; Fan, L.; Xu, Y.-M.; Qin, Y.-Q.; et al. PHD3 Stabilizes the Tight Junction Protein Occludin and Protects Intestinal Epithelial Barrier Function. J. Biol. Chem. 2015, 290, 20580–20589. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Miao, Y.R.; Diep, A.N.; Wu, C.; Rankin, E.B.; Atwood, T.F.; Xing, L.; Giaccia, A.J. PHD Inhibition Mitigates and Protects against Radiation-Induced Gastrointestinal Toxicity via HIF2. Sci. Transl. Med. 2014, 6, 236ra64. [Google Scholar] [CrossRef] [PubMed]
- Tavernier, S.J.; Vanlangenakker, N.; Vetters, J.; Carmeliet, P.; Janssens, S.; Lambrecht, B.N. Opposing Regulation and Roles for PHD3 in Lung Dendritic Cells and Alveolar Macrophages. J. Leukoc. Biol. 2017, 102, 1115–1126. [Google Scholar] [CrossRef]
- Chan, M.C.; Holt-Martyn, J.P.; Schofield, C.J.; Ratcliffe, P.J. Pharmacological Targeting of the HIF Hydroxylases—A New Field in Medicine Development. Mol. Asp. Med. 2016, 47–48, 54–75. [Google Scholar] [CrossRef]
- Halligan, D.N.; Khan, M.N.; Brown, E.; Rowan, C.R.; Coulter, I.S.; Doherty, G.A.; Tambuwala, M.M.; Taylor, C.T. Hypoxia-Inducible Factor Hydroxylase Inhibition Enhances the Protective Effects of Cyclosporine in Colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G90–G97. [Google Scholar] [CrossRef]
- Tambuwala, M.M.; Manresa, M.C.; Cummins, E.P.; Aversa, V.; Coulter, I.S.; Taylor, C.T. Targeted Delivery of the Hydroxylase Inhibitor DMOG Provides Enhanced Efficacy with Reduced Systemic Exposure in a Murine Model of Colitis. J. Control Release 2015, 217, 221–227. [Google Scholar] [CrossRef]
- Keely, S.; Campbell, E.L.; Baird, A.W.; Hansbro, P.M.; Shalwitz, R.A.; Kotsakis, A.; McNamee, E.N.; Eltzschig, H.K.; Kominsky, D.J.; Colgan, S.P. Contribution of Epithelial Innate Immunity to Systemic Protection Afforded by Prolyl Hydroxylase Inhibition in Murine Colitis. Mucosal Immunol. 2014, 7, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Curtis, V.F.; Ehrentraut, S.F.; Campbell, E.L.; Glover, L.E.; Bayless, A.; Kelly, C.J.; Kominsky, D.J.; Colgan, S.P. Stabilization of HIF through Inhibition of Cullin-2 Neddylation Is Protective in Mucosal Inflammatory Responses. FASEB J. 2015, 29, 208. [Google Scholar] [CrossRef]
- Gupta, R.; Chaudhary, A.R.; Shah, B.N.; Jadhav, A.V.; Zambad, S.P.; Gupta, R.C.; Deshpande, S.; Chauthaiwale, V.; Dutt, C. Therapeutic Treatment with a Novel Hypoxia-Inducible Factor Hydroxylase Inhibitor (TRC160334) Ameliorates Murine Colitis. Clin. Exp. Gastroenterol. 2014, 7, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zou, X.; Chen, Y.; Wang, H.; Duan, Y.; Bruick, R.K. Modulation of HIF-2α PAS-B Domain Contributes to Physiological Responses. Proc. Natl. Acad. Sci. USA 2018, 115, 13240–13245. [Google Scholar] [CrossRef] [PubMed]
- Scheuermann, T.H.; Li, Q.; Ma, H.-W.; Key, J.; Zhang, L.; Chen, R.; Garcia, J.A.; Naidoo, J.; Longgood, J.; Frantz, D.E.; et al. Allosteric Inhibition of Hypoxia Inducible Factor-2 with Small Molecules. Nat. Chem. Biol. 2013, 9, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.N.; Choijookhuu, N.; Takagi, H.; Srisowanna, N.; Nguyen Nhat Huynh, M.; Yamaguchi, Y.; Synn Oo, P.; Tin Htwe Kyaw, M.; Sato, K.; Yamaguchi, R.; et al. The HDAC Inhibitor, SAHA, Prevents Colonic Inflammation by Suppressing Pro-Inflammatory Cytokines and Chemokines in DSS-Induced Colitis. Acta Histochem. Cytochem. 2018, 51, 33–40. [Google Scholar] [CrossRef]
- Cosin-Roger, J.; Simmen, S.; Melhem, H.; Atrott, K.; Frey-Wagner, I.; Hausmann, M.; de Vallière, C.; Spalinger, M.R.; Spielmann, P.; Wenger, R.H.; et al. Hypoxia Ameliorates Intestinal Inflammation through NLRP3/MTOR Downregulation and Autophagy Activation. Nat. Commun. 2017, 8, 98. [Google Scholar] [CrossRef]
- Zhen, Y.; Zhang, H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 276. [Google Scholar] [CrossRef]
- Mao, L.; Kitani, A.; Similuk, M.; Oler, A.J.; Albenberg, L.; Kelsen, J.; Aktay, A.; Quezado, M.; Yao, M.; Montgomery-Recht, K.; et al. Loss-of-Function CARD8 Mutation Causes NLRP3 Inflammasome Activation and Crohn’s Disease. J. Clin. Investig. 2018, 128, 1793–1806. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dvornikova, K.A.; Platonova, O.N.; Bystrova, E.Y. Hypoxia and Intestinal Inflammation: Common Molecular Mechanisms and Signaling Pathways. Int. J. Mol. Sci. 2023, 24, 2425. https://doi.org/10.3390/ijms24032425
Dvornikova KA, Platonova ON, Bystrova EY. Hypoxia and Intestinal Inflammation: Common Molecular Mechanisms and Signaling Pathways. International Journal of Molecular Sciences. 2023; 24(3):2425. https://doi.org/10.3390/ijms24032425
Chicago/Turabian StyleDvornikova, Kristina A., Olga N. Platonova, and Elena Y. Bystrova. 2023. "Hypoxia and Intestinal Inflammation: Common Molecular Mechanisms and Signaling Pathways" International Journal of Molecular Sciences 24, no. 3: 2425. https://doi.org/10.3390/ijms24032425
APA StyleDvornikova, K. A., Platonova, O. N., & Bystrova, E. Y. (2023). Hypoxia and Intestinal Inflammation: Common Molecular Mechanisms and Signaling Pathways. International Journal of Molecular Sciences, 24(3), 2425. https://doi.org/10.3390/ijms24032425





