Dunaliella salina Alga Protects against Myocardial Ischemia/Reperfusion Injury by Attenuating TLR4 Signaling
Abstract
1. Introduction
2. Results
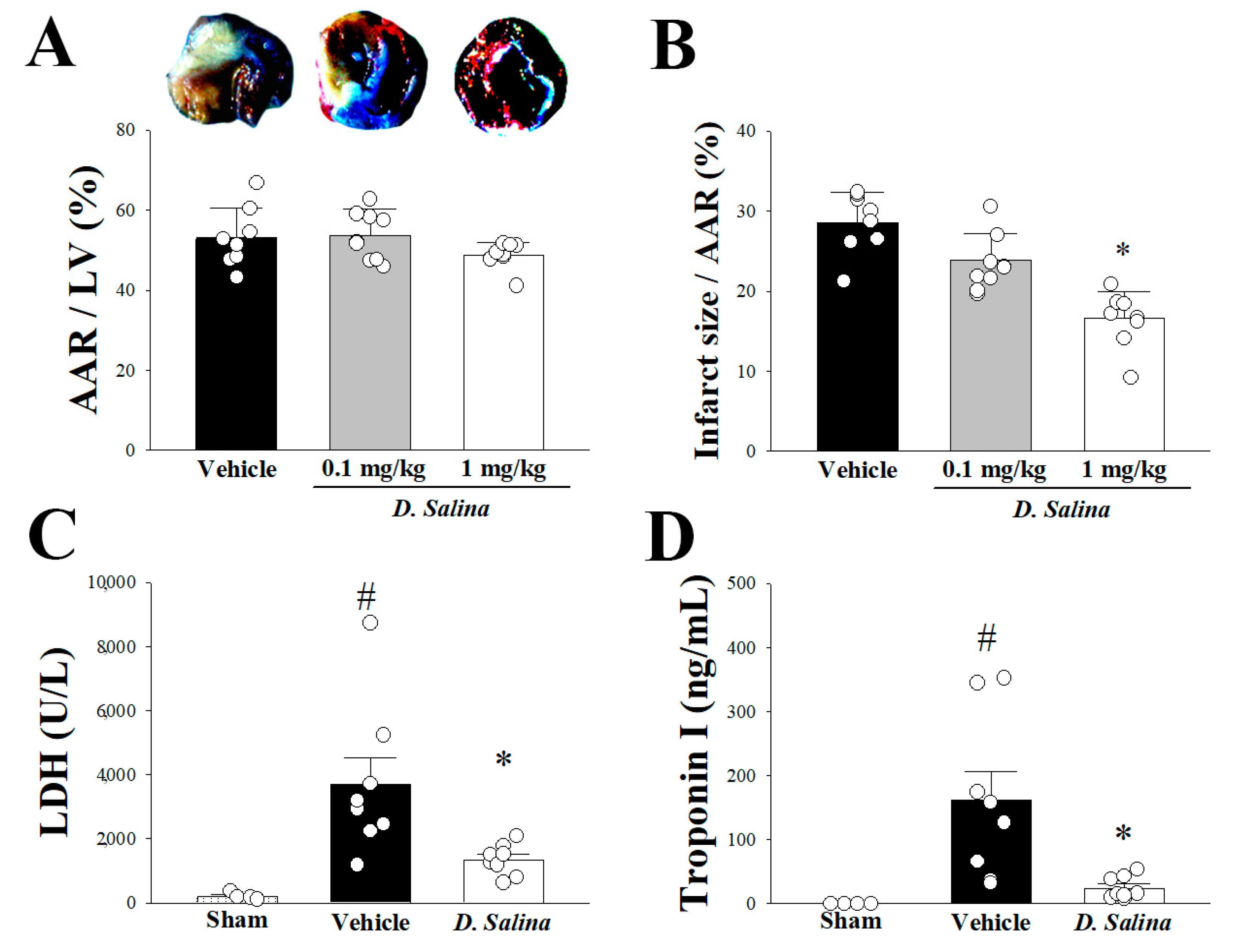
2.1. Myocardial Damage
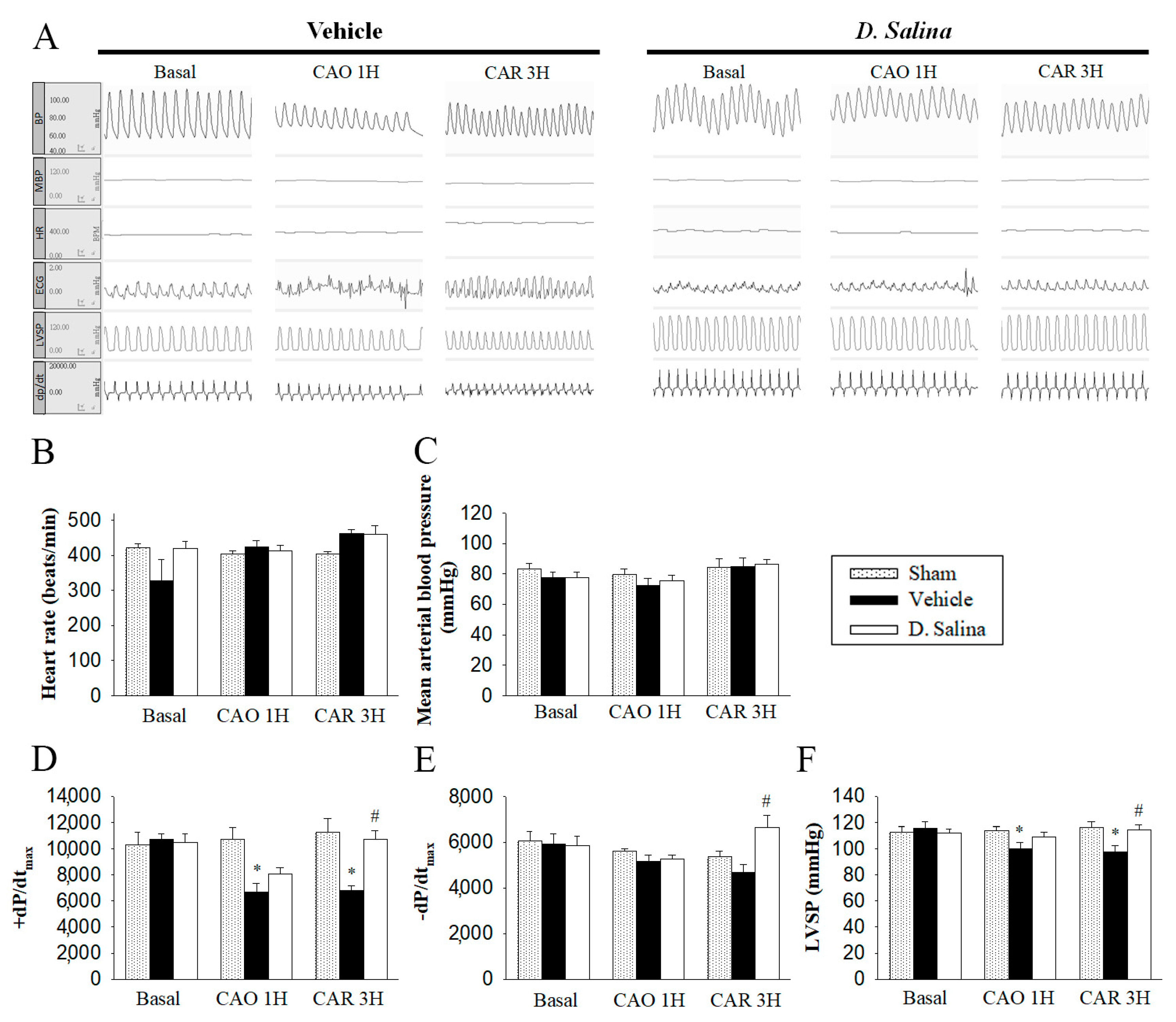
2.2. Hemodynamic Changes during Myocardial I/R Injury
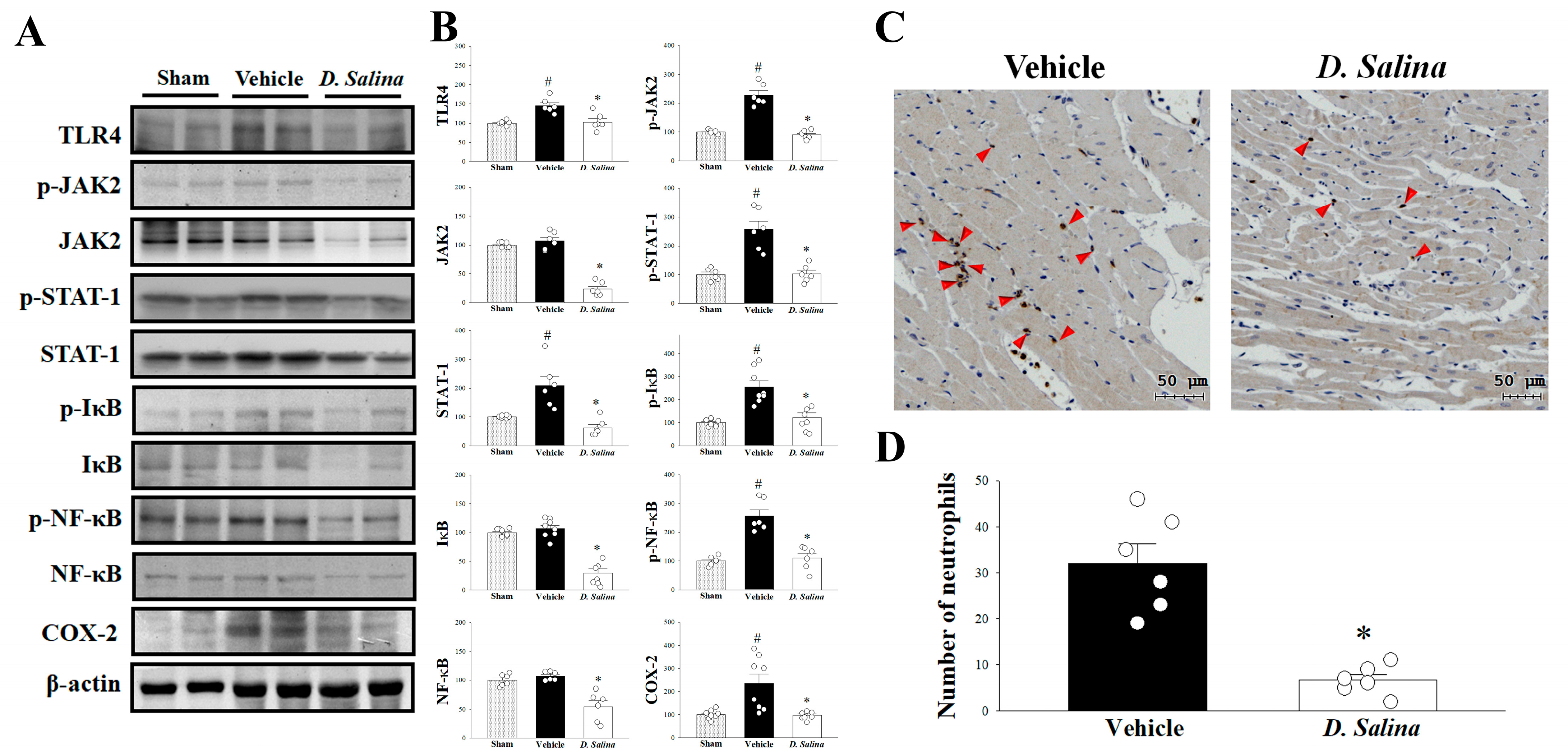
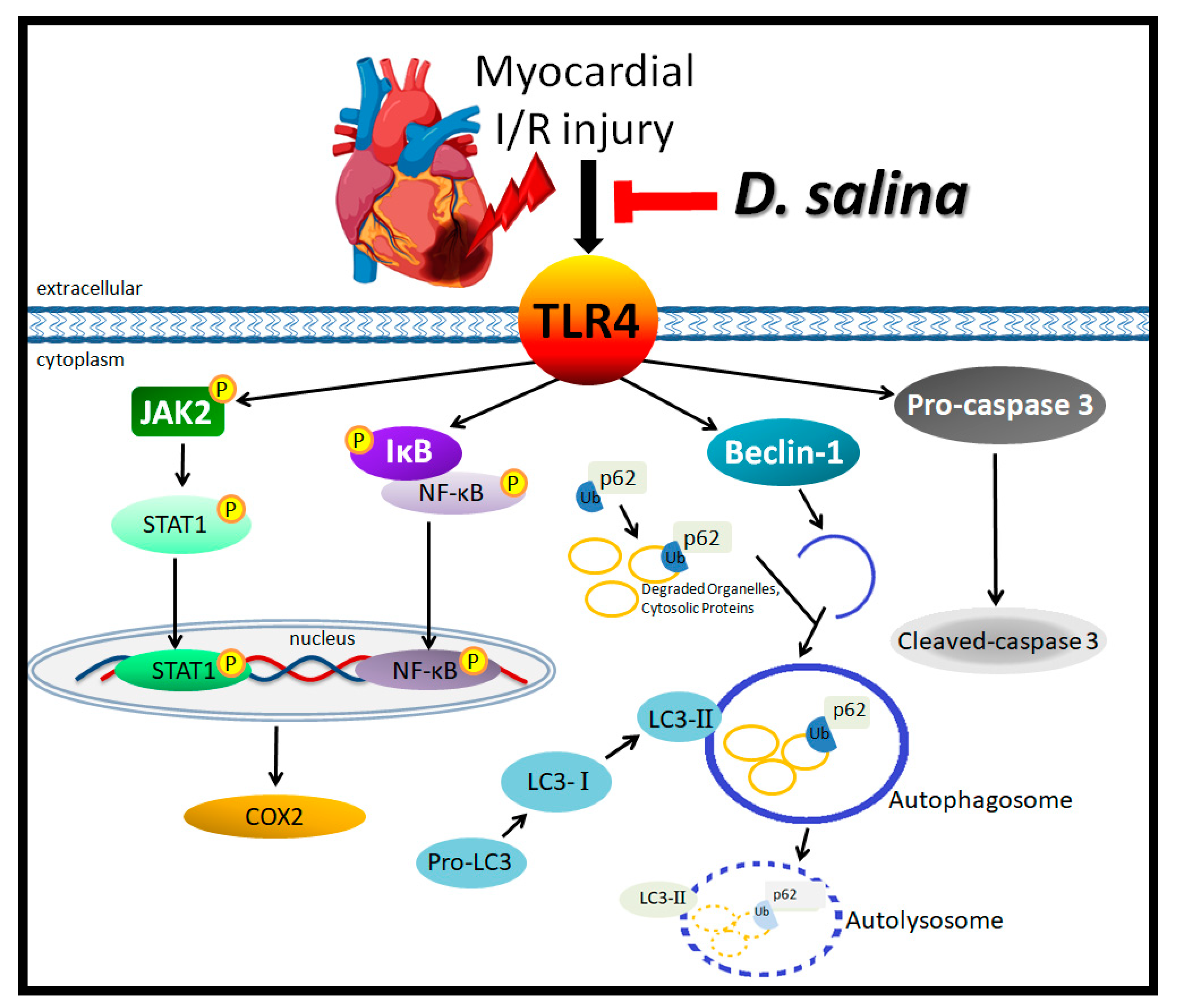
2.3. Effects of D. salina on the Inflammatory Signaling Pathway
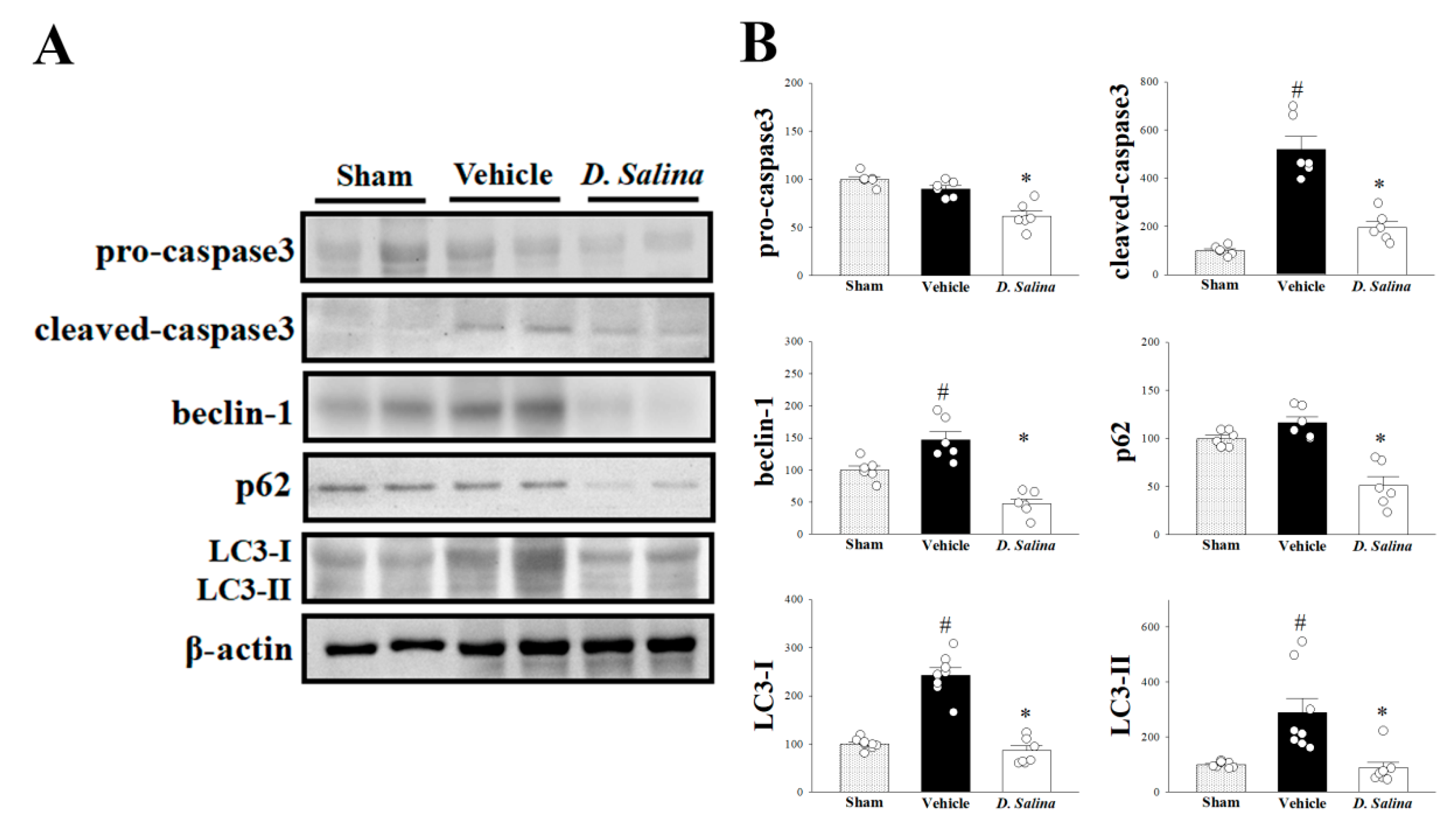
2.4. Effects of D. salina on Apoptosis and Autophagy
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Preparation of D. salina Extract
4.3. D. salina Administration
4.4. Surgical Protocol
4.5. Evaluation of Cardiac Function
4.6. Measurement of Myocardial Injury and Collection of Myocardial Samples
4.7. Protein Extraction and Western Blot Analysis
4.8. Immunohistochemical Staining
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAR | area at risk |
| BP | arterial blood pressure |
| I/R | ischemia/reperfusion |
| HR | heart rate |
| LDH | lactate dehydrogenase |
| LAD | left anterior descending coronary artery |
| LVSP | left ventricular systolic pressure |
| LC3 | light chain 3 |
| STAT1 | signal transducer and activator of transcription-1 |
| ±dp/dtmax | the maximal rate of left ventricular pressure increase and decrease |
| TLR4 | toll-like receptor 4 |
| VT | ventricular tachycardia |
| VF | ventricular fibrillation |
References
- Folino, A.; Losano, G.; Rastaldo, R. Balance of Nitric Oxide and Reactive Oxygen Species in Myocardial Reperfusion Injury and Protection. J. Cardiovasc. Pharmacol. 2013, 62, 567–575. [Google Scholar] [CrossRef]
- Daud, M.Y.; Awan, M.S.; Khan, M.; Hayat, U.; Tuyyab, F. Procedural Outcomes Of Primary Percutaneous Coronary Intervention In Elderly Patients With Stemi. JAMC 2019, 30, 585–588. [Google Scholar]
- Yang, Q.-H.; Yang, M.; Zhang, L.-L.; Xiao, M.-C.; Zhao, Y.; Yan, D.-X. The mechanism of miR-23a in regulating myocardial cell apoptosis through targeting FoxO3. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5789–5797. [Google Scholar]
- Zhou, T.; Prather, E.; Garrison, D.; Zuo, L. Interplay between ROS and Antioxidants during Ischemia-Reperfusion Injuries in Cardiac and Skeletal Muscle. Int. J. Mol. Sci. 2018, 19, 417. [Google Scholar] [CrossRef]
- Jalali, Z.; Khademalhosseini, M.; Soltani, N.; Nadimi, A.E. Smoking, alcohol and opioids effect on coronary microcirculation: An update overview. BMC Cardiovasc. Disord. 2021, 21, 1–17. [Google Scholar] [CrossRef]
- Obad, A.; Peeran, A.; Little, J.I.; Haddad, G.E.; Tarzami, S.T. Alcohol-Mediated Organ Damages: Heart and Brain. Front. Pharmacol. 2018, 9, 81. [Google Scholar] [CrossRef]
- Zhao, H.; Mayhan, W.G.; Arrick, D.M.; Xiong, W.; Sun, H. Dose-Related Influence of Chronic Alcohol Consumption on Cerebral Ischemia/Reperfusion Injury. Alcohol. Clin. Exp. Res. 2011, 35, 1265–1269. [Google Scholar] [CrossRef]
- Yao, L.; Chen, H.; Wu, Q.; Xie, K. Hydrogen-rich saline alleviates inflammation and apoptosis in myocardial I/R injury via PINK-mediated autophagy. Int. J. Mol. Med. 2019, 44, 1048–1062. [Google Scholar] [CrossRef]
- Oyama, J.-I.; Blais, C.; Liu, X.; Pu, M.; Kobzik, L.; Kelly, R.A.; Bourcier, T. Reduced Myocardial Ischemia-Reperfusion Injury in Toll-Like Receptor 4-Deficient Mice. Circulation 2004, 109, 784–789. [Google Scholar] [CrossRef]
- Guo, X.; Jiang, H.; Yang, J.; Chen, J.; Yang, J.; Ding, J.W.; Li, S.; Wu, H.; Ding, H.S. Radioprotective 105 kDa protein attenuates ischemia/reperfusion-induced myocardial apoptosis and autophagy by inhibiting the activation of the TLR4/NF-kappaB signaling pathway in rats. Int. J. Mol. Med. 2016, 38, 885–893. [Google Scholar] [CrossRef]
- Yang, J.; Yang, J.; Ding, J.-W.; Chen, L.-H.; Wang, Y.-L.; Li, S.; Wu, H. Sequential Expression of TLR4 and its Effects on the Myocardium of Rats with Myocardial Ischemia-Reperfusion Injury. Inflammation 2008, 31, 304–312. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, G.; Yao, L.; Xue, P.; Yu, D.; Xu, R.; Shi, W.; Yao, X.; Yan, Z.; Duan, J.A. Protective effect of Ginkgo biloba leaves extract, EGb761, on myocardium injury in ischemia reperfusion rats via regulation of TLR-4/NF-kappaB signaling pathway. Oncotarget 2017, 8, 86671–86680. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, F.; Sun, Z.; Sun, P.; Chen, T.; Chen, F. Microalgal carotenoids: Beneficial effects and potential in human health. Food Funct. 2014, 5, 413–425. [Google Scholar] [CrossRef]
- Hu, C.-C.; Lin, J.-T.; Lu, F.-J.; Chou, F.-P.; Yang, D.-J. Determination of carotenoids in Dunaliella salina cultivated in Taiwan and antioxidant capacity of the algal carotenoid extract. Food Chem. 2008, 109, 439–446. [Google Scholar] [CrossRef]
- Levy, Y.; Zaltsberg, H.; Ben-Amotz, A.; Kanter, Y.; Aviram, M. Dietary Supplementation of a Natural Isomer Mixture of Beta-Carotene Inhibits Oxidation of LDL Derived from Patients with Diabetes mellitus. Ann. Nutr. Metab. 2000, 44, 54–60. [Google Scholar] [CrossRef]
- Bechor, S.; Relevy, N.Z.; Harari, A.; Almog, T.; Kamari, Y.; Ben-Amotz, A.; Harats, D.; Shaish, A. 9-cis β-Carotene Increased Cholesterol Efflux to HDL in Macrophages. Nutrients 2016, 8, 435. [Google Scholar] [CrossRef]
- Rotenstreich, Y.; Belkin, M.; Sadetzki, S.; Chetrit, A.; Ferman-Attar, G.; Sher, I.; Harari, A.; Shaish, A.; Harats, D. Treatment with 9-cis β-carotene-rich powder in patients with retinitis pigmentosa: A randomized crossover trial. JAMA Ophthalmol. 2013, 131, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.S.; Melis, A. Microalgal biotechnology: Carotenoid production by the green algae Dunaliella salina. Biotechnol. Bioprocess Eng. 2003, 8, 331–337. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Aly, H.F.; Salama, A.A. Toxicity assessment of the green Dunaliella salina microalgae. Toxicol. Rep. 2019, 6, 850–861. [Google Scholar] [CrossRef]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Effect of Natural Food Antioxidants against LDL and DNA Oxidative Changes. Antioxidants 2018, 7, 133. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Aly, H.F.; Abd-Alla, H.I. The ameliorating effect of carotenoid rich fraction extracted from Dunaliella salina microalga against inflammation- associated cardiac dysfunction in obese rats. Toxicol. Rep. 2019, 7, 118–124. [Google Scholar] [CrossRef] [PubMed]
- El-Baz, F.K.; Hussein, R.A.; Saleh, D.O.; Jaleel, G.A.R.A. Zeaxanthin Isolated from Dunaliella salina Microalgae Ameliorates Age Associated Cardiac Dysfunction in Rats through Stimulation of Retinoid Receptors. Mar. Drugs 2019, 17, 290. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.W.; Chen, Y.C.; Liu, C.W.; Yang, D.J.; Chen, S.Y.; Chang, T.J.; Chang, Y.Y. Regulation of virus-induced inflammatory response by Dunaliella salina alga extract in macrophages. Food Chem. Toxicol. 2014, 71, 159–165. [Google Scholar] [CrossRef]
- Chitranjali, T.; Anoop Chandran, P.; Muraleedhara Kurup, G. Omega-3 fatty acid concentrate from Dunaliella salina possesses anti-inflammatory properties including blockade of NF-kappaB nuclear translocation. Immunopharmacol. Immunotoxicol. 2015, 37, 81–89. [Google Scholar] [PubMed]
- Lin, H.W.; Liu, C.W.; Yang, D.J.; Chen, C.C.; Chen, S.Y.; Tseng, J.K.; Chang, T.J.; Chang, Y.Y. Dunaliella salina alga extract inhibits the production of interleukin-6, nitric oxide, and reactive oxygen species by regulating nuclear factor-κB/Janus kinase/signal transducer and activator of transcription in virus-infected RAW264.7 cells. J. Food Drug Anal. 2017, 25, 908–918. [Google Scholar] [CrossRef]
- Cebova, M.; Pechanova, O. Protective Effects of Polyphenols against Ischemia/Reperfusion Injury. Molecules 2020, 25, 3469. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar]
- Blomhoff, R. Vitamin A and Carotenoid Toxicity. Food Nutr. Bull. 2001, 22, 320–334. [Google Scholar] [CrossRef]
- Csepanyi, E.; Czompa, A.; Haines, D.; Lekli, I.; Bakondi, E.; Balla, G.; Tosaki, A.; Bak, I. Cardiovascular effects of low versus high-dose beta-carotene in a rat model. Pharmacol. Res. 2015, 100, 148–156. [Google Scholar] [CrossRef]
- Csepanyi, E.; Czompa, A.; Szabados-Furjesi, P.; Lekli, I.; Balla, J.; Balla, G.; Tosaki, A.; Bak, I. The Effects of Long-Term, Low- and High-Dose Beta-Carotene Treatment in Zucker Diabetic Fatty Rats: The Role of HO-1. Int. J. Mol. Sci. 2018, 19, 1132. [Google Scholar] [CrossRef]
- Zhai, Y.; Ao, L.; Cleveland, J.C.; Zeng, Q.; Reece, T.B.; Fullerton, D.A.; Meng, X. Toll-like Receptor 4 Mediates the Inflammatory Responses and Matrix Protein Remodeling in Remote Non-Ischemic Myocardium in a Mouse Model of Myocardial Ischemia and Reperfusion. PLsS ONE 2015, 10, e0121853. [Google Scholar] [CrossRef]
- De Prati, A.C.; Ciampa, A.R.; Cavalieri, E.; Zaffini, R.; Darra, E.; Menegazzi, M.; Suzuki, H.; Mariotto, S. STAT1 as a New Molecular Target of Anti-Inflammatory Treatment. Curr. Med. Chem. 2005, 12, 1819–1828. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Surh, Y.J.; Chun, K.S.; Cha, H.H.; Han, S.S.; Keum, Y.S.; Park, K.K.; Lee, S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat. Res. 2001, 480–481, 243–268. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, T.; Zhu, H.; Zheng, X.; Zhang, X.; Jiang, N.; Cheng, X.; Lai, X.; Shunnar, A.; Singh, M.; et al. Prevention of hyperglycemia-induced myocardial apoptosis by gene silencing of Toll-like receptor-4. J. Transl. Med. 2010, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Hamacher-Brady, A.; Brady, N.; Gottlieb, R.A. The Interplay between Pro-Death and Pro-Survival Signaling Pathways in Myocardial Ischemia/Reperfusion Injury: Apoptosis Meets Autophagy. Cardiovasc. Drugs Ther. 2006, 20, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Sheu, M.-J.; Huang, G.-J.; Wu, C.-H.; Chen, J.-S.; Chang, H.-Y.; Chang, S.-J.; Chung, J.-G. Ethanol extract of Dunaliella salina induces cell cycle arrest and apoptosis in A549 human non-small cell lung cancer cells. In Vivo 2008, 22, 369–378. [Google Scholar] [PubMed]
- Matsui, Y.; Kyoi, S.; Takagi, H.; Hsu, C.-P.; Hariharan, N.; Ago, T.; Vatner, S.F.; Sadoshima, J. Molecular mechanisms and physiological significance of autophagy during myocardial ischemia and reperfusion. Autophagy 2008, 4, 409–415. [Google Scholar] [CrossRef]
- Shi, C.-S.; Kehrl, J.H. Traf6 and A20 differentially regulate TLR4-Induced autophagy by affecting the ubiquitination of Beclin 1. Autophagy 2010, 6, 986–987. [Google Scholar] [CrossRef]
- Kirkin, V.; McEwan, D.G.; Novak, I.; Dikic, I. A Role for Ubiquitin in Selective Autophagy. Mol. Cell 2009, 34, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Panahi, B.; Frahadian, M.; Dums, J.; Hejazi, M.A. Integration of Cross Species RNA-seq Meta-Analysis and Machine-Learning Models Identifies the Most Important Salt Stress–Responsive Pathways in Microalga Dunaliella. Front. Genet. 2019, 10, 752. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Chen, K.-M.; Chiu, P.-S.; Lai, S.-C.; Su, H.-H.; Jan, M.-S.; Lin, C.-W.; Lu, D.-Y.; Fu, Y.-T.; Liao, J.-M.; et al. Lumbrokinase attenuates myocardial ischemia-reperfusion injury by inhibiting TLR4 signaling. J. Mol. Cell. Cardiol. 2016, 99, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Folkerts, H.; Wierenga, A.T.; Heuvel, F.A.V.D.; Woldhuis, R.R.; Kluit, D.S.; Jaques, J.; Schuringa, J.J.; Vellenga, E. Elevated VMP1 expression in acute myeloid leukemia amplifies autophagy and is protective against venetoclax-induced apoptosis. Cell Death Dis. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Su, H.H.; Liao, J.M.; Wang, Y.H.; Chen, K.M.; Lin, C.W.; Lee, I.H.; Li, Y.J.; Huang, J.Y.; Tsai, S.K.; Yen, J.C.; et al. Exogenous GDF11 attenuates non-canonical TGF-beta signaling to protect the heart from acute myocardial ischemia-reperfusion injury. Basic Res. Cardiol. 2019, 114, 20. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-F.; Lin, H.-W.; Liao, J.-M.; Chen, K.-M.; Tsai, J.-W.; Chang, C.-S.; Chou, C.-Y.; Su, H.-H.; Liu, P.-H.; Chu, Y.-C.; et al. Dunaliella salina Alga Protects against Myocardial Ischemia/Reperfusion Injury by Attenuating TLR4 Signaling. Int. J. Mol. Sci. 2023, 24, 3871. https://doi.org/10.3390/ijms24043871
Tsai C-F, Lin H-W, Liao J-M, Chen K-M, Tsai J-W, Chang C-S, Chou C-Y, Su H-H, Liu P-H, Chu Y-C, et al. Dunaliella salina Alga Protects against Myocardial Ischemia/Reperfusion Injury by Attenuating TLR4 Signaling. International Journal of Molecular Sciences. 2023; 24(4):3871. https://doi.org/10.3390/ijms24043871
Chicago/Turabian StyleTsai, Chin-Feng, Hui-Wen Lin, Jiuan-Miaw Liao, Ke-Min Chen, Jen-Wei Tsai, Chia-Sung Chang, Chia-Yu Chou, Hsing-Hui Su, Pei-Hsun Liu, Ya-Chun Chu, and et al. 2023. "Dunaliella salina Alga Protects against Myocardial Ischemia/Reperfusion Injury by Attenuating TLR4 Signaling" International Journal of Molecular Sciences 24, no. 4: 3871. https://doi.org/10.3390/ijms24043871
APA StyleTsai, C.-F., Lin, H.-W., Liao, J.-M., Chen, K.-M., Tsai, J.-W., Chang, C.-S., Chou, C.-Y., Su, H.-H., Liu, P.-H., Chu, Y.-C., Wang, Y.-H., Wang, M., & Huang, S.-S. (2023). Dunaliella salina Alga Protects against Myocardial Ischemia/Reperfusion Injury by Attenuating TLR4 Signaling. International Journal of Molecular Sciences, 24(4), 3871. https://doi.org/10.3390/ijms24043871





