Fluorescence Imaging of Cell Membrane Potential: From Relative Changes to Absolute Values
Abstract
:1. Introduction
2. Two Most Common Fluorescence Techniques for Membrane Potential Imaging
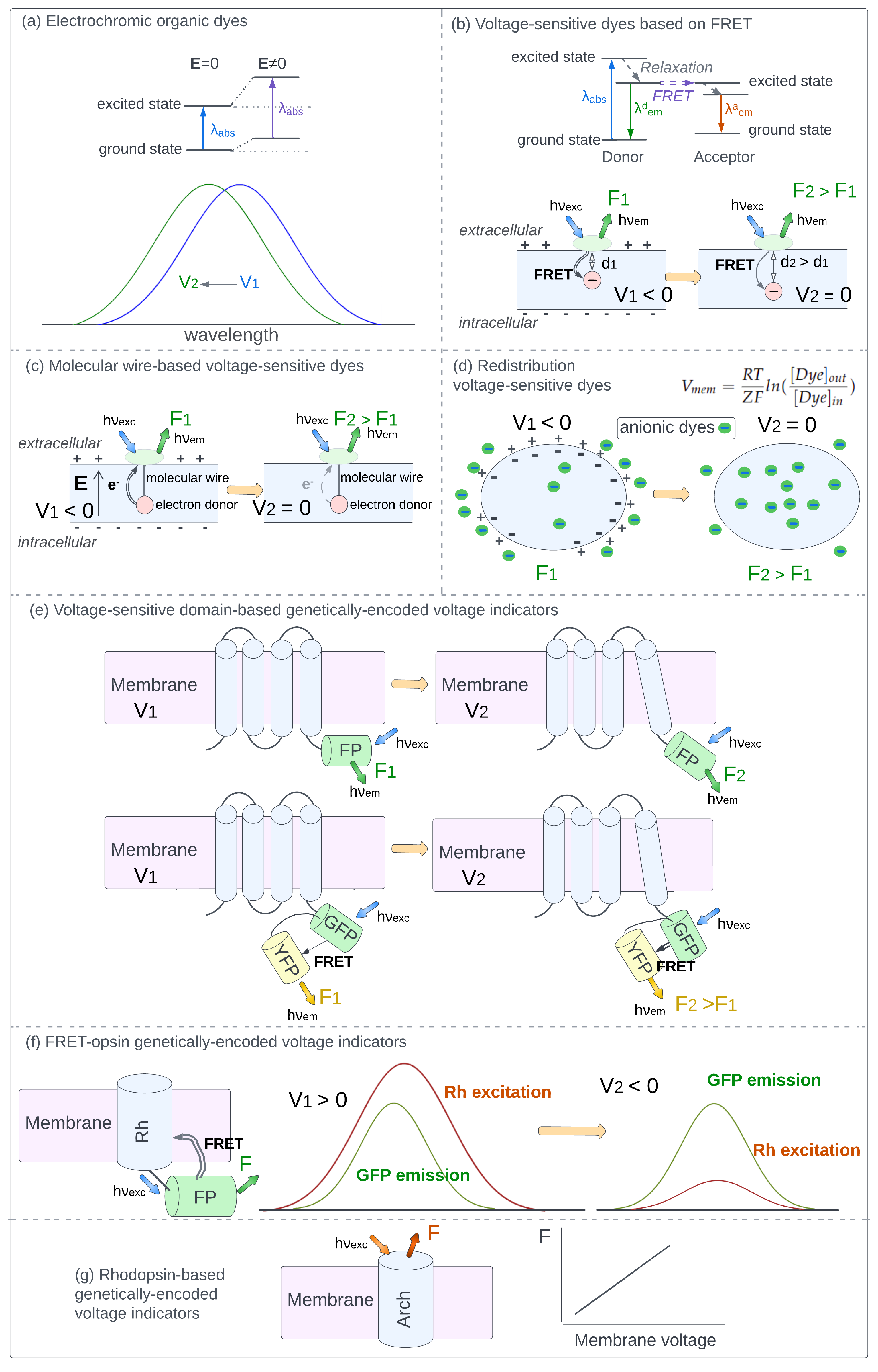
3. Types of Molecular and Biomolecular Potentiometric Sensors
3.1. Electrochromic Organic Dyes
3.2. Voltage-Sensitive Dyes Based on FRET
3.3. Molecular Wire-Based Voltage-Sensitive Dyes
3.4. Redistribution Voltage-Sensitive Dyes
3.5. Voltage-Sensitive Domain-Based Genetically-Encoded Voltage Indicators
3.6. FRET-Opsin Genetically-Encoded Voltage Indicators
3.7. Rhodopsin-Based Genetically-Encoded Voltage Indicators
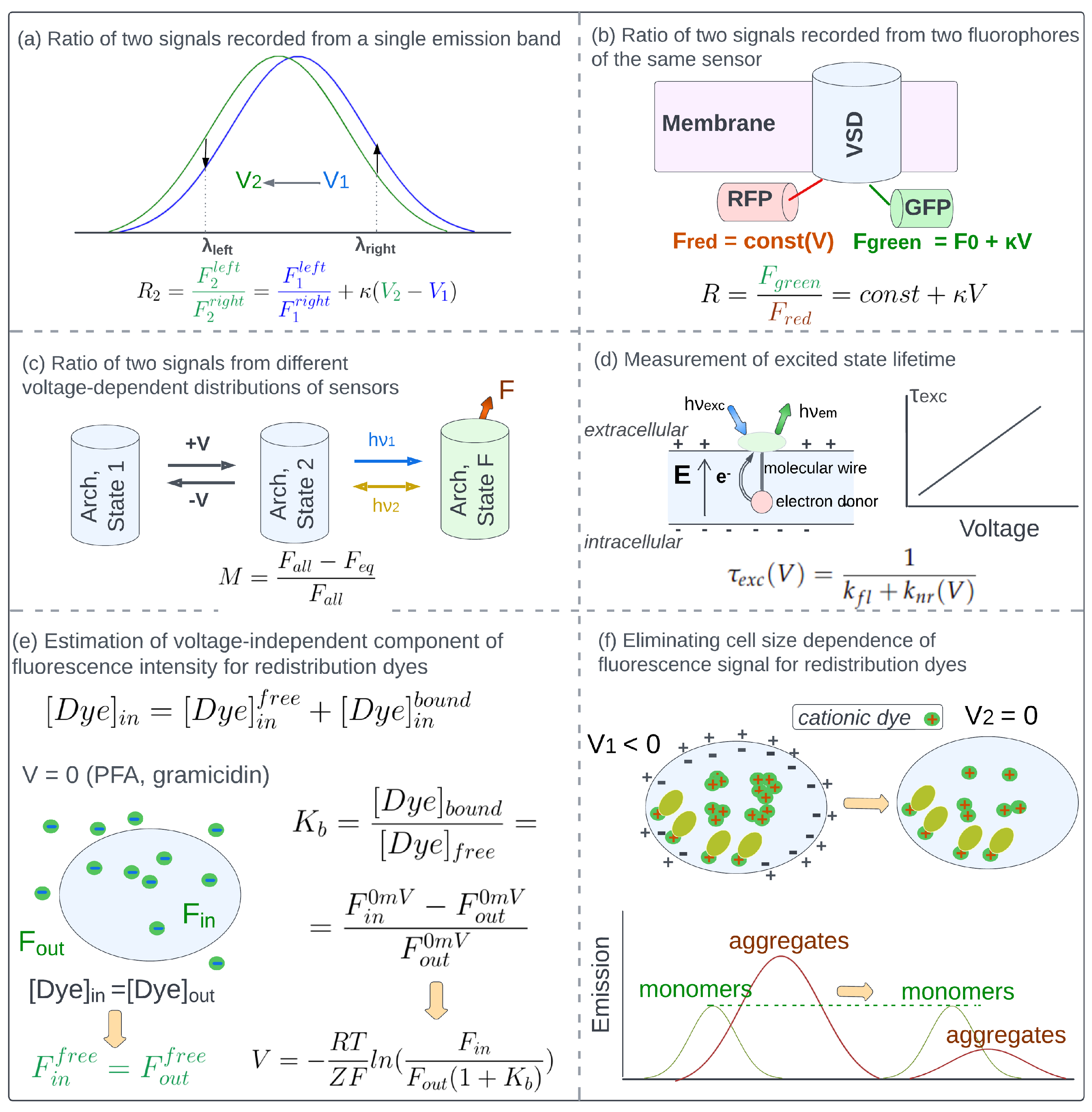
4. Elimination of Fluorescence Changes Caused by Factors Other than Voltage
4.1. Eliminating Concentration-Dependence of Detected Fluorescence with Ratio-Based Approaches
4.2. Measurement of Excited State Lifetime
4.3. Eliminating the Effect of Side Factors Other than Sensors Concentration Changes
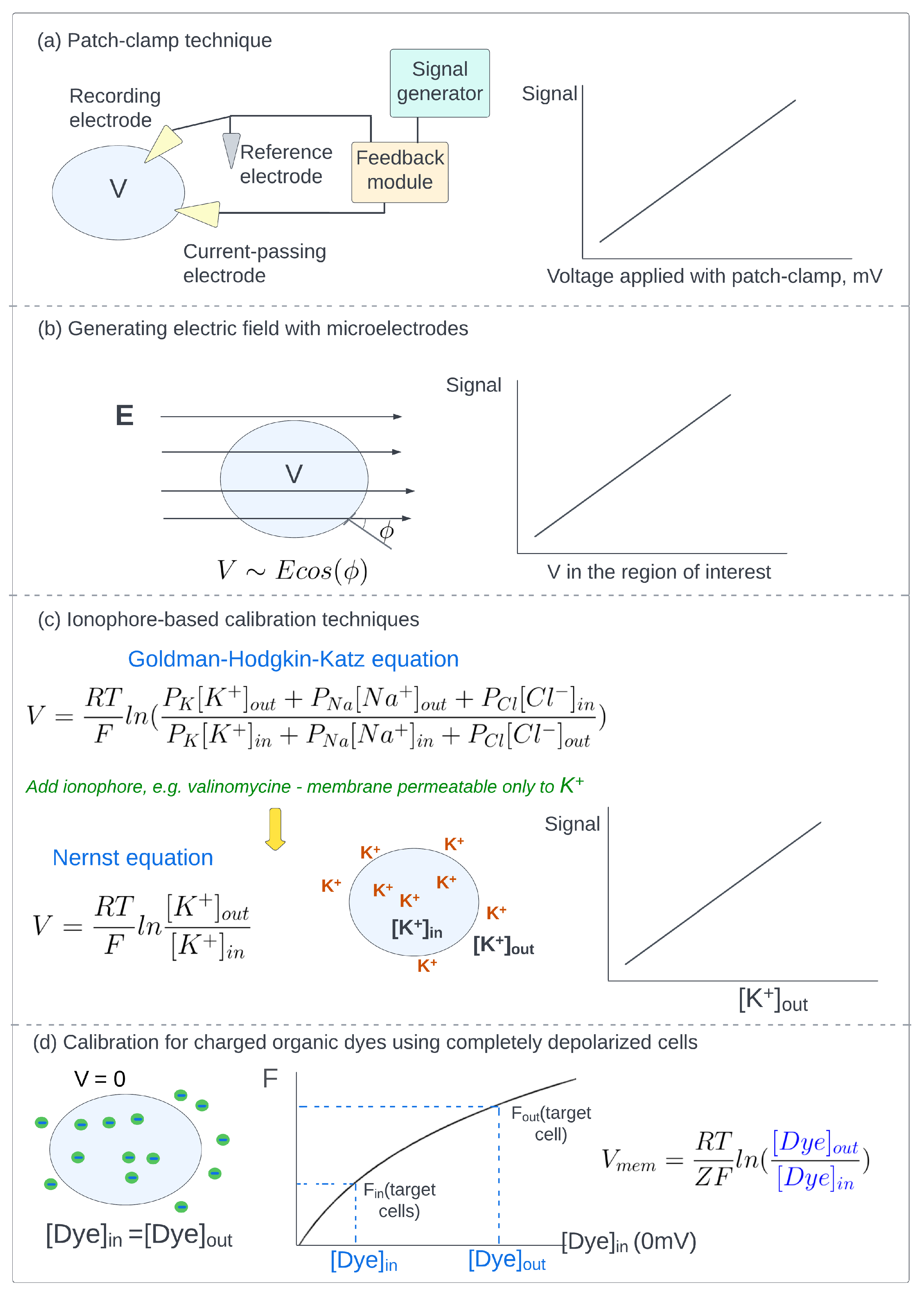
5. Calibration Techniques That Are Applied to Derive the Correspondence between the Detected Optical Signal and Membrane Voltage Values. Measurement of Absolute Values of Cell Membrane Potential
5.1. Patch-Clamp Technique
5.2. Generating Electric Field with Microelectrodes
5.3. Ionophore-Based Calibration Techniques
5.4. Calibration of Charged Organic Dyes Using Completely Depolarized Cells
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Abdul Kadir, L.; Stacey, M.; Barrett-Jolley, R. Emerging roles of the membrane potential: Action beyond the action potential. Front. Physiol. 2018, 9, 1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binggeli, R.; Weinstein, R.C. Membrane potentials and sodium channels: Hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. J. Theor. Biol. 1986, 123, 377–401. [Google Scholar] [CrossRef]
- MacFarlane, S.N.; Sontheimer, H. Changes in ion channel expression accompany cell cycle progression of spinal cord astrocytes. Glia 2000, 30, 39–48. [Google Scholar] [CrossRef]
- Sundelacruz, S.; Levin, M.; Kaplan, D.L. Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS ONE 2008, 3, e3737. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.W.; Yan, F.; Shimamura, S.; Barg, S.; Shyng, S.L. Membrane phosphoinositides control insulin secretion through their effects on ATP-sensitive K+ channel activity. Diabetes 2005, 54, 2852–2858. [Google Scholar] [CrossRef] [Green Version]
- Belle, M.D.; Diekman, C.O.; Forger, D.B.; Piggins, H.D. Daily electrical silencing in the mammalian circadian clock. Science 2009, 326, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Brackenbury, W.J. Membrane potential and cancer progression. Front. Physiol. 2013, 4, 185. [Google Scholar] [CrossRef] [Green Version]
- Lobikin, M.; Chernet, B.; Lobo, D.; Levin, M. Resting potential, oncogene-induced tumorigenesis, and metastasis: The bioelectric basis of cancer in vivo. Phys. Biol. 2012, 9, 065002. [Google Scholar] [CrossRef] [Green Version]
- Jeub, M.; Herbst, M.; Spauschus, A.; Fleischer, H.; Klockgether, T.; Wuellner, U.; Evert, B.O. Potassium channel dysfunction and depolarized resting membrane potential in a cell model of SCA3. Exp. Neurol. 2006, 201, 182–192. [Google Scholar] [CrossRef]
- Brown, S.G.; Publicover, S.J.; Mansell, S.A.; Lishko, P.V.; Williams, H.L.; Ramalingam, M.; Wilson, S.M.; Barratt, C.L.; Sutton, K.A.; Da Silva, S.M. Depolarization of sperm membrane potential is a common feature of men with subfertility and is associated with low fertilization rate at IVF. Hum. Reprod. 2016, 31, 1147–1157. [Google Scholar] [CrossRef] [Green Version]
- Simons, C.; Rash, L.D.; Crawford, J.; Ma, L.; Cristofori-Armstrong, B.; Miller, D.; Ru, K.; Baillie, G.J.; Alanay, Y.; Jacquinet, A.; et al. Mutations in the voltage-gated potassium channel gene KCNH1 cause Temple-Baraitser syndrome and epilepsy. Nat. Genet. 2015, 47, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Van Duijn, B.; Heimovaara-Dijkstra, S. Intracellular microelectrode membrane potential measurements in tobacco cell-suspension protoplasts and barley aleurone protoplasts: Interpretation and artifacts. Biochim. Biophys. Acta (BBA)-Biomembr. 1994, 1193, 77–84. [Google Scholar] [CrossRef]
- Spruston, N.; Jaffe, D.B.; Williams, S.H.; Johnston, D. Voltage-and space-clamp errors associated with the measurement of electrotonically remote synaptic events. J. Neurophysiol. 1993, 70, 781–802. [Google Scholar] [CrossRef]
- Spira, M.E.; Hai, A. Multi-electrode array technologies for neuroscience and cardiology. Nat. Nanotechnol. 2013, 8, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Scanziani, M.; Häusser, M. Electrophysiology in the age of light. Nature 2009, 461, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Fang-Yen, C.; Choi, W.; Yaqoob, Z.; Fu, D.; Park, Y.; Dassari, R.R.; Feld, M.S. Label-free imaging of membrane potential using membrane electromotility. Biophys. J. 2012, 103, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Lee, H.J.; Zhang, D.; Liao, C.S.; Ji, N.; Xia, Y.; Cheng, J.X. Label-free spectroscopic detection of membrane potential using stimulated Raman scattering. Appl. Phys. Lett. 2015, 106, 173704. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Liu, E.; Muller, H.; Cui, B. Optical Electrophysiology: Toward the Goal of Label-Free Voltage Imaging. J. Am. Chem. Soc. 2021, 143, 10482–10499. [Google Scholar] [CrossRef]
- Braubach, O.; Cohen, L.B.; Choi, Y. Historical overview and general methods of membrane potential imaging. In Membrane Potential Imaging in the Nervous System and Heart; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–26. [Google Scholar]
- Wright, B.J.; Jackson, M.B. Voltage imaging in the study of hippocampal circuit function and plasticity. In Membrane Potential Imaging in the Nervous System and Heart; Springer: Berlin/Heidelberg, Germany, 2015; pp. 197–211. [Google Scholar]
- Grinvald, A.; Petersen, C.C. Imaging the dynamics of neocortical population activity in behaving and freely moving mammals. In Membrane Potential Imaging in the Nervous System and Heart; Springer: Berlin/Heidelberg, Germany, 2015; pp. 273–296. [Google Scholar]
- Acker, C.D.; Yan, P.; Loew, L.M. Single-voxel recording of voltage transients in dendritic spines. Biophys. J. 2011, 101, L11–L13. [Google Scholar] [CrossRef] [Green Version]
- Storace, D.A.; Braubach, O.R.; Jin, L.; Cohen, L.B.; Sung, U. Monitoring brain activity with protein voltage and calcium sensors. Sci. Rep. 2015, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Laviv, T.; Scholl, B.; Parra-Bueno, P.; Foote, B.; Zhang, C.; Yan, L.; Hayano, Y.; Chu, J.; Yasuda, R. In vivo imaging of the coupling between neuronal and CREB activity in the mouse brain. Neuron 2020, 105, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Momose-Sato, Y.; Sato, K.; Kamino, K. Monitoring population membrane potential signals during development of the vertebrate nervous system. Membr. Potential Imaging Nerv. Syst. Heart 2015, 213–242. [Google Scholar] [CrossRef]
- Walton, R.D.; Bernus, O. Towards depth-resolved optical imaging of cardiac electrical activity. Membr. Potential Imaging Nerv. Syst. Heart 2015, 405–423. [Google Scholar] [CrossRef]
- Pertsov, A.; Walton, R.D.; Bernus, O. Optical imaging of cardiac action potential. In Membrane Potential Imaging in the Nervous System and Heart; Springer: Berlin/Heidelberg, Germany, 2015; pp. 299–311. [Google Scholar]
- Kim, B.B.; Wu, H.; Hao, Y.A.; Pan, M.; Chavarha, M.; Zhao, Y.; Westberg, M.; St-Pierre, F.; Wu, J.C.; Lin, M.Z. A red fluorescent protein with improved monomericity enables ratiometric voltage imaging with ASAP3. Sci. Rep. 2022, 12, 3678. [Google Scholar] [CrossRef] [PubMed]
- Kisaalita, W.S.; Bowen, J.M. Development of resting membrane potentials in differentiating murine neuroblastoma cells (N1E-115) evaluated by flow cytometry. Cytotechnology 1997, 24, 201–212. [Google Scholar] [CrossRef]
- Leão, R.N.; Reis, A.; Emirandetti, A.; Lewicka, M.; Hermanson, O.; Fisahn, A. A voltage-sensitive dye-based assay for the identification of differentiated neurons derived from embryonic neural stem cell cultures. PLoS ONE 2010, 5, e13833. [Google Scholar] [CrossRef] [Green Version]
- Knisley, S.B.; Justice, R.K.; Kong, W.; Johnson, P.L. Ratiometry of transmembrane voltage-sensitive fluorescent dye emission in hearts. Am. J. Physiol.-Heart Circ. Physiol. 2000, 279, H1421–H1433. [Google Scholar] [CrossRef]
- Chemla, S.; Chavane, F. Voltage-sensitive dye imaging: Technique review and models. J. Physiol.-Paris 2010, 104, 40–50. [Google Scholar] [CrossRef]
- Miller, E.W. Small molecule fluorescent voltage indicators for studying membrane potential. Curr. Opin. Chem. Biol. 2016, 33, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Loew, L.M. Design and use of organic voltage sensitive dyes. In Membrane Potential Imaging in the Nervous System and Heart; Springer: Berlin/Heidelberg, Germany, 2015; pp. 27–53. [Google Scholar]
- Kulkarni, R.U.; Miller, E.W. Voltage imaging: Pitfalls and potential. Biochemistry 2017, 56, 5171–5177. [Google Scholar] [CrossRef]
- St-Pierre, F.; Chavarha, M.; Lin, M.Z. Designs and sensing mechanisms of genetically encoded fluorescent voltage indicators. Curr. Opin. Chem. Biol. 2015, 27, 31–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platisa, J.; Pieribone, V.A. Genetically encoded fluorescent voltage indicators: Are we there yet? Curr. Opin. Neurobiol. 2018, 50, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Bando, Y.; Sakamoto, M.; Kim, S.; Ayzenshtat, I.; Yuste, R. Comparative evaluation of genetically encoded voltage indicators. Cell Rep. 2019, 26, 802–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.H.; St-Pierre, F. Genetically encoded voltage indicators: Opportunities and challenges. J. Neurosci. 2016, 36, 9977–9989. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.S.; Levin, M. General principles for measuring resting membrane potential and ion concentration using fluorescent bioelectricity reporters. Cold Spring Harb. Protoc. 2012, 2012, pdb-top067710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazzari-Dean, J.R.; Gest, A.M.; Miller, E.W. Optical estimation of absolute membrane potential using fluorescence lifetime imaging. eLife 2019, 8, e44522. [Google Scholar] [CrossRef]
- Brinks, D.; Klein, A.J.; Cohen, A.E. Two-photon lifetime imaging of voltage indicating proteins as a probe of absolute membrane voltage. Biophys. J. 2015, 109, 914–921. [Google Scholar] [CrossRef] [Green Version]
- Berezin, M.Y.; Achilefu, S. Fluorescence lifetime measurements and biological imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef] [Green Version]
- Fisher, J.A.; Salzberg, B.M. Two-photon excitation of fluorescent voltage-sensitive dyes: Monitoring membrane potential in the infrared. In Membrane Potential Imaging in the Nervous System and Heart; Springer: Berlin/Heidelberg, Germany, 2015; pp. 427–453. [Google Scholar]
- Loew, L.M.; Lewis, A. Second harmonic imaging of membrane potential. In Membrane Potential Imaging in the Nervous System and Heart; Springer: Berlin/Heidelberg, Germany, 2015; pp. 473–492. [Google Scholar]
- Clarke, R.J. Electric field sensitive dyes. In Advanced Fluorescence Reporters in Chemistry and Biology I; Springer: Berlin/Heidelberg, Germany, 2010; pp. 331–344. [Google Scholar]
- Kuhn, B.; Roome, C.J. Primer to voltage imaging with ANNINE dyes and two-photon microscopy. Front. Cell. Neurosci. 2019, 13, 321. [Google Scholar] [CrossRef] [Green Version]
- Roome, C.J.; Kuhn, B. Voltage imaging with ANNINE dyes and two-photon microscopy of Purkinje dendrites in awake mice. Neurosci. Res. 2020, 152, 15–24. [Google Scholar] [CrossRef]
- Fluhler, E.; Burnham, V.G.; Loew, L.M. Spectra, membrane binding, and potentiometric responses of new charge shift probes. Biochemistry 1985, 24, 5749–5755. [Google Scholar] [CrossRef] [PubMed]
- Fairless, R.; Beck, A.; Kravchenko, M.; Williams, S.K.; Wissenbach, U.; Diem, R.; Cavalié, A. Membrane potential measurements of isolated neurons using a voltage-sensitive dye. PLoS ONE 2013, 8, e58260. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, J.E.; Tsien, R.Y. Voltage sensing by fluorescence resonance energy transfer in single cells. Biophys. J. 1995, 69, 1272–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, J.E.; Tsien, R.Y. Improved indicators of cell membrane potential that use fluorescence resonance energy transfer. Chem. Biol. 1997, 4, 269–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matamala, E.; Castillo, C.; Vivar, J.P.; Rojas, P.A.; Brauchi, S.E. Imaging the electrical activity of organelles in living cells. Commun. Biol. 2021, 4, 1–12. [Google Scholar] [CrossRef]
- Chanda, B.; Blunck, R.; Faria, L.C.; Schweizer, F.E.; Mody, I.; Bezanilla, F. A hybrid approach to measuring electrical activity in genetically specified neurons. Nat. Neurosci. 2005, 8, 1619–1626. [Google Scholar] [CrossRef]
- Bradley, J.; Luo, R.; Otis, T.S.; DiGregorio, D.A. Submillisecond optical reporting of membrane potential in situ using a neuronal tracer dye. J. Neurosci. 2009, 29, 9197–9209. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, J.E.; Maher, M.P. Cellular fluorescent indicators and voltage/ion probe reader (VIPR TM): Tools for ion channel and receptor drug discovery. Recept. Channels 2002, 8, 283–295. [Google Scholar] [CrossRef]
- Briggman, K.L.; Kristan, W.B.; González, J.E.; Kleinfeld, D.; Tsien, R.Y. Monitoring integrated activity of individual neurons using FRET-based voltage-sensitive dyes. In Membrane Potential Imaging in the Nervous System and Heart; Springer: Berlin/Heidelberg, Germany, 2015; pp. 149–169. [Google Scholar]
- Rowland, C.E.; Brown, C.W.; Medintz, I.L.; Delehanty, J.B. Intracellular FRET-based probes: A review. Methods Appl. Fluoresc. 2015, 3, 042006. [Google Scholar] [CrossRef]
- Khadria, A. Tools to measure membrane potential of neurons. Biomed. J. 2022, 45, 749–762. [Google Scholar] [CrossRef]
- Kikuchi, K.; Takakusa, H.; Nagano, T. Recent advances in the design of small molecule-based FRET sensors for cell biology. TrAC Trends Anal. Chem. 2004, 23, 407–415. [Google Scholar] [CrossRef]
- Li, L.s. Fluorescence probes for membrane potentials based on mesoscopic electron transfer. Nano Lett. 2007, 7, 2981–2986. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.W.; Lin, J.Y.; Frady, E.P.; Steinbach, P.A.; Kristan Jr, W.B.; Tsien, R.Y. Optically monitoring voltage in neurons by photo-induced electron transfer through molecular wires. Proc. Natl. Acad. Sci. USA 2012, 109, 2114–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Miller, E.W. Electrophysiology, unplugged: Imaging membrane potential with fluorescent indicators. Accounts Chem. Res. 2019, 53, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Deal, P.E.; Liu, P.; Al-Abdullatif, S.H.; Muller, V.R.; Shamardani, K.; Adesnik, H.; Miller, E.W. Covalently tethered rhodamine voltage reporters for high speed functional imaging in brain tissue. J. Am. Chem. Soc. 2019, 142, 614–622. [Google Scholar] [CrossRef]
- Woodford, C.R.; Frady, E.P.; Smith, R.S.; Morey, B.; Canzi, G.; Palida, S.F.; Araneda, R.C.; Kristan, W.B., Jr.; Kubiak, C.P.; Miller, E.W.; et al. Improved PeT molecules for optically sensing voltage in neurons. J. Am. Chem. Soc. 2015, 137, 1817–1824. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.L.; Walker, A.S.; Miller, E.W. A photostable silicon rhodamine platform for optical voltage sensing. J. Am. Chem. Soc. 2015, 137, 10767–10776. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, R.U.; Yin, H.; Pourmandi, N.; James, F.; Adil, M.M.; Schaffer, D.V.; Wang, Y.; Miller, E.W. A rationally designed, general strategy for membrane orientation of photoinduced electron transfer-based voltage-sensitive dyes. ACS Chem. Biol. 2017, 12, 407–413. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Wang, X.; Nie, L. Optical recording of brain functions based on voltage-sensitive dyes. Chin. Chem. Lett. 2021, 32, 1879–1887. [Google Scholar] [CrossRef]
- Klapperstück, T.; Glanz, D.; Klapperstück, M.; Wohlrab, J. Methodological aspects of measuring absolute values of membrane potential in human cells by flow cytometry. Cytom. Part A J. Int. Soc. Adv. Cytom. 2009, 75, 593–608. [Google Scholar] [CrossRef]
- Krasznai, Z.; Márián, T.; Izumi, H.; Damjanovich, S.; Balkay, L.; Trón, L.; Morisawa, M. Membrane hyperpolarization removes inactivation of Ca2+ channels, leading to Ca2+ influx and subsequent initiation of sperm motility in the common carp. Proc. Natl. Acad. Sci. USA 2000, 97, 2052–2057. [Google Scholar] [CrossRef] [Green Version]
- Meunier, F. Relationship between presynaptic membrane potential and acetylcholine release in synaptosomes from Torpedo electric organ. J. Physiol. 1984, 354, 121–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasznai, Z.; Márián, T.; Balkay, L.; Emri, M.; Trón, L. Flow cytometric determination of absolute membrane potential of cells. J. Photochem. Photobiol. B Biol. 1995, 28, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Plášek, J.; Sigler, K. Slow fluorescent indicators of membrane potential: A survey of different approaches to probe response analysis. J. Photochem. Photobiol. B: Biol. 1996, 33, 101–124. [Google Scholar] [CrossRef]
- Mao, C.; Kisaalita, W.S. Determination of resting membrane potential of individual neuroblastoma cells (IMR-32) using a potentiometric dye (TMRM) and confocal microscopy. J. Fluoresc. 2004, 14, 739–743. [Google Scholar] [CrossRef]
- Gross, D.; Loew, L.M. Fluorescent indicators of membrane potential: Microspectrofluorometry and imaging. Methods Cell Biol. 1989, 30, 193–218. [Google Scholar] [PubMed]
- Shapiro, H.M. Estimation of membrane potential by flow cytometry. Curr. Protoc. Cytom. 2004, 28, 9–6. [Google Scholar] [CrossRef]
- Villette, V.; Chavarha, M.; Dimov, I.K.; Bradley, J.; Pradhan, L.; Mathieu, B.; Evans, S.W.; Chamberland, S.; Shi, D.; Yang, R.; et al. Ultrafast two-photon imaging of a high-gain voltage indicator in awake behaving mice. Cell 2019, 179, 1590–1608. [Google Scholar] [CrossRef]
- Abdelfattah, A.S.; Farhi, S.L.; Zhao, Y.; Brinks, D.; Zou, P.; Ruangkittisakul, A.; Platisa, J.; Pieribone, V.A.; Ballanyi, K.; Cohen, A.E.; et al. A bright and fast red fluorescent protein voltage indicator that reports neuronal activity in organotypic brain slices. J. Neurosci. 2016, 36, 2458–2472. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Han, Z.; Platisa, J.; Wooltorton, J.R.; Cohen, L.B.; Pieribone, V.A. Single action potentials and subthreshold electrical events imaged in neurons with a fluorescent protein voltage probe. Neuron 2012, 75, 779–785. [Google Scholar] [CrossRef] [Green Version]
- St-Pierre, F.; Marshall, J.D.; Yang, Y.; Gong, Y.; Schnitzer, M.J.; Lin, M.Z. High-fidelity optical reporting of neuronal electrical activity with an ultrafast fluorescent voltage sensor. Nat. Neurosci. 2014, 17, 884–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutoh, H.; Perron, A.; Dimitrov, D.; Iwamoto, Y.; Akemann, W.; Chudakov, D.M.; Knöpfel, T. Spectrally-resolved response properties of the three most advanced FRET based fluorescent protein voltage probes. PLoS ONE 2009, 4, e4555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsutsui, H.; Jinno, Y.; Tomita, A.; Niino, Y.; Yamada, Y.; Mikoshiba, K.; Miyawaki, A.; Okamura, Y. Improved detection of electrical activity with a voltage probe based on a voltage-sensing phosphatase. J. Physiol. 2013, 591, 4427–4437. [Google Scholar] [CrossRef] [PubMed]
- Mollinedo-Gajate, I.; Song, C.; Knöpfel, T. Genetically Encoded Voltage Indicators. In Optogenetics; Springer: Singapore, 2021; pp. 209–224. [Google Scholar]
- Shen, Y.; Nasu, Y.; Shkolnikov, I.; Kim, A.; Campbell, R.E. Engineering genetically encoded fluorescent indicators for imaging of neuronal activity: Progress and prospects. Neurosci. Res. 2020, 152, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Kannan, M.; Vasan, G.; Pieribone, V.A. Optimizing strategies for developing genetically encoded voltage indicators. Front. Cell. Neurosci. 2019, 13, 53. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, S.; Nagai, T. Current progress in genetically encoded voltage indicators for neural activity recording. Curr. Opin. Chem. Biol. 2016, 33, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zou, P.; Cohen, A.E. Voltage imaging with genetically encoded indicators. Curr. Opin. Chem. Biol. 2017, 39, 1–10. [Google Scholar] [CrossRef]
- Storace, D.; Rad, M.S.; Kang, B.; Cohen, L.B.; Hughes, T.; Baker, B.J. Toward better genetically encoded sensors of membrane potential. Trends Neurosci. 2016, 39, 277–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storace, D.; Rad, M.S.; Han, Z.; Jin, L.; Cohen, L.B.; Hughes, T.; Baker, B.J.; Sung, U. Genetically encoded protein sensors of membrane potential. In Membrane Potential Imaging in the Nervous System and Heart; Springer: Berlin/Heidelberg, Germany, 2015; pp. 493–509. [Google Scholar]
- Gong, Y.; Wagner, M.J.; Zhong Li, J.; Schnitzer, M.J. Imaging neural spiking in brain tissue using FRET-opsin protein voltage sensors. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zou, P.; Zhao, Y.; Douglass, A.D.; Hochbaum, D.R.; Brinks, D.; Werley, C.A.; Harrison, D.J.; Campbell, R.E.; Cohen, A.E. Bright and fast multicoloured voltage reporters via electrochromic FRET. Nat. Commun. 2014, 5, 4625. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Huang, C.; Li, J.Z.; Grewe, B.F.; Zhang, Y.; Eismann, S.; Schnitzer, M.J. High-speed recording of neural spikes in awake mice and flies with a fluorescent voltage sensor. Science 2015, 350, 1361–1366. [Google Scholar] [CrossRef] [Green Version]
- Kralj, J.M.; Hochbaum, D.R.; Douglass, A.D.; Cohen, A.E. Electrical spiking in Escherichia coli probed with a fluorescent voltage-indicating protein. Science 2011, 333, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Kralj, J.M.; Douglass, A.D.; Hochbaum, D.R.; Maclaurin, D.; Cohen, A.E. Optical recording of action potentials in mammalian neurons using a microbial rhodopsin. Nat. Methods 2012, 9, 90–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maclaurin, D.; Venkatachalam, V.; Lee, H.; Cohen, A.E. Mechanism of voltage-sensitive fluorescence in a microbial rhodopsin. Proc. Natl. Acad. Sci. USA 2013, 110, 5939–5944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silapetere, A.; Hwang, S.; Hontani, Y.; Fernandez Lahore, R.G.; Balke, J.; Escobar, F.V.; Tros, M.; Konold, P.E.; Matis, R.; Croce, R.; et al. QuasAr Odyssey: The origin of fluorescence and its voltage sensitivity in microbial rhodopsins. Nat. Commun. 2022, 13, 5501. [Google Scholar] [CrossRef]
- Hochbaum, D.R.; Zhao, Y.; Farhi, S.L.; Klapoetke, N.; Werley, C.A.; Kapoor, V.; Zou, P.; Kralj, J.M.; Maclaurin, D.; Smedemark-Margulies, N.; et al. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat. Methods 2014, 11, 825–833. [Google Scholar] [CrossRef] [Green Version]
- McIsaac, R.S.; Engqvist, M.K.; Wannier, T.; Rosenthal, A.Z.; Herwig, L.; Flytzanis, N.C.; Imasheva, E.S.; Lanyi, J.K.; Balashov, S.P.; Gradinaru, V.; et al. Directed evolution of a far-red fluorescent rhodopsin. Proc. Natl. Acad. Sci. USA 2014, 111, 13034–13039. [Google Scholar] [CrossRef] [Green Version]
- Piatkevich, K.D.; Jung, E.E.; Straub, C.; Linghu, C.; Park, D.; Suk, H.J.; Hochbaum, D.R.; Goodwin, D.; Pnevmatikakis, E.; Pak, N.; et al. A robotic multidimensional directed evolution approach applied to fluorescent voltage reporters. Nat. Chem. Biol. 2018, 14, 352–360. [Google Scholar] [CrossRef]
- Govorunova, E.G.; Sineshchekov, O.A.; Li, H.; Spudich, J.L. Microbial rhodopsins: Diversity, mechanisms, and optogenetic applications. Annu. Rev. Biochem. 2017, 86, 845. [Google Scholar] [CrossRef] [Green Version]
- Shtyrov, A.A.; Nikolaev, D.M.; Mironov, V.N.; Vasin, A.V.; Panov, M.S.; Tveryanovich, Y.S.; Ryazantsev, M.N. Simple models to study spectral properties of microbial and animal rhodopsins: Evaluation of the electrostatic effect of charged and polar residues on the first absorption band maxima. Int. J. Mol. Sci. 2021, 22, 3029. [Google Scholar] [CrossRef]
- Nikolaev, D.M.; Shtyrov, A.A.; Mereshchenko, A.S.; Panov, M.S.; Tveryanovich, Y.S.; Ryazantsev, M.N. An assessment of water placement algorithms in quantum mechanics/molecular mechanics modeling: The case of rhodopsins’ first spectral absorption band maxima. Phys. Chem. Chem. Phys. 2020, 22, 18114–18123. [Google Scholar] [CrossRef] [PubMed]
- Ryazantsev, M.N.; Altun, A.; Morokuma, K. Color tuning in rhodopsins: The origin of the spectral shift between the chloride-bound and anion-free forms of halorhodopsin. J. Am. Chem. Soc. 2012, 134, 5520–5523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryazantsev, M.N.; Nikolaev, D.M.; Struts, A.V.; Brown, M.F. Quantum mechanical and molecular mechanics modeling of membrane-embedded rhodopsins. J. Membr. Biol. 2019, 252, 425–449. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.P.; Brinks, D.; Testa-Silva, G.; Tian, H.; Phil Brooks, F., III; Adam, Y.; Bloxham, B.; Gmeiner, B.; Kheifets, S.; Cohen, A.E. Photoactivated voltage imaging in tissue with an archaerhodopsin-derived reporter. Sci. Adv. 2021, 7, eabe3216. [Google Scholar] [CrossRef] [PubMed]
- Rühl, P.; Langner, J.M.; Reidel, J.; Schönherr, R.; Hoshi, T.; Heinemann, S.H. Monitoring of compound resting membrane potentials of cell cultures with ratiometric genetically encoded voltage indicators. Commun. Biol. 2021, 4, 1164. [Google Scholar] [CrossRef]
- Beach, J.M.; McGAHREN, E.D.; Xia, J.; Duling, B.R. Ratiometric measurement of endothelial depolarization in arterioles with a potential-sensitive dye. Am. J. Physiol.-Heart Circ. Physiol. 1996, 270, H2216–H2227. [Google Scholar] [CrossRef]
- Gross, E.; Bedlack, R.S., Jr.; Loew, L.M. Dual-wavelength ratiometric fluorescence measurement of the membrane dipole potential. Biophys. J. 1994, 67, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Davidson, R.M.; Loew, L.M. Membrane electric properties by combined patch clamp and fluorescence ratio imaging in single neurons. Biophys. J. 1998, 74, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Ross, W.; Reichardt, L. Species-specific effects on the optical signals of voltage-sensitive dyes. J. Membr. Biol. 1979, 48, 343–356. [Google Scholar] [CrossRef]
- Loew, L.; Cohen, L.; Salzberg, B.; Obaid, A.; Bezanilla, F. Charge-shift probes of membrane potential. Characterization of aminostyrylpyridinium dyes on the squid giant axon. Biophys. J. 1985, 47, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Bashford, C.L.; Chance, B.; Smith, J.C.; Yoshida, T. The behavior of oxonol dyes in phospholipid dispersions. Biophys. J. 1979, 25, 63–85. [Google Scholar] [CrossRef] [Green Version]
- Montana, V.; Farkas, D.L.; Loew, L.M. Dual-wavelength ratiometric fluorescence measurements of membrane potential. Biochemistry 1989, 28, 4536–4539. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.H.; Venkatachalam, V.; Cohen, A.E. Temporal dynamics of microbial rhodopsin fluorescence reports absolute membrane voltage. Biophys. J. 2014, 106, 639–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrenberg, B.; Montana, V.; Wei, M.; Wuskell, J.; Loew, L. Membrane potential can be determined in individual cells from the nernstian distribution of cationic dyes. Biophys. J. 1988, 53, 785–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novo, D.; Perlmutter, N.G.; Hunt, R.H.; Shapiro, H.M. Accurate flow cytometric membrane potential measurement in bacteria using diethyloxacarbocyanine and a ratiometric technique. Cytom. J. Int. Soc. Anal. Cytol. 1999, 35, 55–63. [Google Scholar] [CrossRef]
- Polder, H.R.; Weskamp, M.; Linz, K.; Meyer, R. Voltage-clamp and patch-clamp techniques. In Practical Methods in Cardiovascular Research; Springer: Berlin/Heidelberg, Germany, 2005; pp. 272–323. [Google Scholar]
- Flickinger, B.; Berghöfer, T.; Hohenberger, P.; Eing, C.; Frey, W. Transmembrane potential measurements on plant cells using the voltage-sensitive dye ANNINE-6. Protoplasma 2010, 247, 3–12. [Google Scholar] [CrossRef]
- Gross, D.; Loew, L.M.; Webb, W.W. Optical imaging of cell membrane potential changes induced by applied electric fields. Biophys. J. 1986, 50, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Schwan, H.P. Electrical properties of tissue and cell suspensions. In Advances in Biological and Medical Physics; Elsevier: Amsterdam, The Netherlands, 1957; Volume 5, pp. 147–209. [Google Scholar]
- Kotnik, T.; Miklavčič, D. Analytical description of transmembrane voltage induced by electric fields on spheroidal cells. Biophys. J. 2000, 79, 670–679. [Google Scholar] [CrossRef] [Green Version]
- Hodgkin, A.L.; Katz, B. The effect of sodium ions on the electrical activity of the giant axon of the squid. J. Physiol. 1949, 108, 37. [Google Scholar] [CrossRef]
- Pressman, B.C. Biological applications of ionophores. Annu. Rev. Biochem. 1976, 45, 501–530. [Google Scholar] [CrossRef]
- Shapiro, H.M.; Natale, P.J.; Kamentsky, L.A. Estimation of membrane potentials of individual lymphocytes by flow cytometry. Proc. Natl. Acad. Sci. USA 1979, 76, 5728–5730. [Google Scholar] [CrossRef] [Green Version]
- Rink, T.; Montecucco, C.; Hesketh, T.; Tsien, R. Lymphocyte membrane potential assessed with fluorescent probes. Biochim. Biophys. Acta (BBA)-Biomembr. 1980, 595, 15–30. [Google Scholar] [CrossRef]
- Wilson, H.A.; Chused, T.M. Lymphocyte membrane potential and Ca2+-sensitive potassium channels described by oxonol dye fluorescence measurements. J. Cell. Physiol. 1985, 125, 72–81. [Google Scholar] [CrossRef]
- Defrise-Quertain, F.; Fraser-L’Hostis, C.; Coral, D.; Deshusses, J. Kinetic study of the plasma-membrane potential in procyclic and bloodstream forms of Trypanosoma brucei brucei using the fluorescent probe bisoxonol. Biochem. J. 1996, 314, 595–601. [Google Scholar] [CrossRef] [Green Version]
- Kopper, K.L.; Adorante, J.S. Regulation of intracellular calcium in N1E-115 neuroblastoma cells: The role of Na+/Ca2+ exchange. Am. J.-Physiol.-Cell Physiol. 2002, 285, C1000–C1008. [Google Scholar] [CrossRef] [Green Version]
- Konig, S.; Hinard, V.; Arnaudeau, S.; Holzer, N.; Potter, G.; Bader, C.R.; Bernheim, L. Membrane hyperpolarization triggers myogenin and myocyte enhancer factor-2 expression during human myoblast differentiation. J. Biol. Chem. 2004, 279, 28187–28196. [Google Scholar] [CrossRef] [Green Version]
- Emiliani, V.; Entcheva, E.; Hedrich, R.; Hegemann, P.; Konrad, K.R.; Lüscher, C.; Mahn, M.; Pan, Z.H.; Sims, R.R.; Vierock, J.; et al. Optogenetics for light control of biological systems. Nat. Rev. Methods Prim. 2022, 2, 55. [Google Scholar] [CrossRef]
- Entcheva, E.; Kay, M.W. Cardiac optogenetics: A decade of enlightenment. Nat. Rev. Cardiol. 2021, 18, 349–367. [Google Scholar] [CrossRef]
- Tan, P.; He, L.; Huang, Y.; Zhou, Y. Optophysiology: Illuminating cell physiology with optogenetics. Physiol. Rev. 2022, 102, 1263–1325. [Google Scholar] [CrossRef]
- Nikolaev, D.M.; Panov, M.S.; Shtyrov, A.A.; Boitsov, V.M.; Vyazmin, S.Y.; Chakchir, O.B.; Yakovlev, I.P.; Ryazantsev, M.N. Perspective tools for optogenetics and photopharmacology: From design to implementation. In Progress in Photon Science; Springer: Berlin/Heidelberg, Germany, 2019; pp. 139–172. [Google Scholar]
- Bansal, A.; Shikha, S.; Zhang, Y. Towards translational optogenetics. Nat. Biomed. Eng. 2022, 1–21. [Google Scholar] [CrossRef]
- Hull, K.; Morstein, J.; Trauner, D. In vivo photopharmacology. Chem. Rev. 2018, 118, 10710–10747. [Google Scholar] [CrossRef] [PubMed]
- Fuchter, M.J. On the promise of photopharmacology using photoswitches: A medicinal chemist’s perspective. J. Med. Chem. 2020, 63, 11436–11447. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, M.V.; Strashkov, D.M.; Ryazantsev, M.N.; Tikhonov, D.B. Optical Control of N-Methyl-d-aspartate Receptors by Azobenzene Quaternary Ammonium Compounds. ACS Chem. Neurosci. 2021, 12, 3347–3357. [Google Scholar] [CrossRef]
- Strashkov, D.M.; Mironov, V.N.; Nikolaev, D.M.; Panov, M.S.; Linnik, S.A.; Mereshchenko, A.S.; Kochemirovsky, V.A.; Vasin, A.V.; Ryazantsev, M.N. Azobenzene/Tetraethyl Ammonium Photochromic Potassium Channel Blockers: Scope and Limitations for Design of Para-Substituted Derivatives with Specific Absorption Band Maxima and Thermal Isomerization Rate. Int. J. Mol. Sci. 2021, 22, 13171. [Google Scholar] [CrossRef] [PubMed]
- Ryazantsev, M.N.; Strashkov, D.M.; Nikolaev, D.M.; Shtyrov, A.A.; Panov, M.S. Photopharmacological compounds based on azobenzenes and azoheteroarenes: Principles of molecular design, molecular modelling, and synthesis. Russ. Chem. Rev. 2021, 90, 868. [Google Scholar] [CrossRef]
- Nikolaev, M.V.; Strashkov, D.M.; Ryazantsev, M.N.; Tikhonov, D.B. Development of a quaternary ammonium photoswitchable antagonist of NMDA receptors. Eur. J. Pharmacol. 2022, 938, 175448. [Google Scholar] [CrossRef]
- Häfner, S.; Sandoz, G. Photopharmacological approaches for dissecting potassium channel physiology. Curr. Opin. Pharmacol. 2022, 63, 102178. [Google Scholar] [CrossRef]
- Castagna, R.; Kolarski, D.; Durand-de Cuttoli, R.; Maleeva, G. Orthogonal Control of Neuronal Circuits and Behavior Using Photopharmacology. J. Mol. Neurosci. 2022, 72, 1433–1442. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaev, D.M.; Mironov, V.N.; Shtyrov, A.A.; Kvashnin, I.D.; Mereshchenko, A.S.; Vasin, A.V.; Panov, M.S.; Ryazantsev, M.N. Fluorescence Imaging of Cell Membrane Potential: From Relative Changes to Absolute Values. Int. J. Mol. Sci. 2023, 24, 2435. https://doi.org/10.3390/ijms24032435
Nikolaev DM, Mironov VN, Shtyrov AA, Kvashnin ID, Mereshchenko AS, Vasin AV, Panov MS, Ryazantsev MN. Fluorescence Imaging of Cell Membrane Potential: From Relative Changes to Absolute Values. International Journal of Molecular Sciences. 2023; 24(3):2435. https://doi.org/10.3390/ijms24032435
Chicago/Turabian StyleNikolaev, Dmitrii M., Vladimir N. Mironov, Andrey A. Shtyrov, Iaroslav D. Kvashnin, Andrey S. Mereshchenko, Andrey V. Vasin, Maxim S. Panov, and Mikhail N. Ryazantsev. 2023. "Fluorescence Imaging of Cell Membrane Potential: From Relative Changes to Absolute Values" International Journal of Molecular Sciences 24, no. 3: 2435. https://doi.org/10.3390/ijms24032435
APA StyleNikolaev, D. M., Mironov, V. N., Shtyrov, A. A., Kvashnin, I. D., Mereshchenko, A. S., Vasin, A. V., Panov, M. S., & Ryazantsev, M. N. (2023). Fluorescence Imaging of Cell Membrane Potential: From Relative Changes to Absolute Values. International Journal of Molecular Sciences, 24(3), 2435. https://doi.org/10.3390/ijms24032435








