Abstract
The molecular pathogenesis of endometriosis has been associated with pathological alterations of protein expression via disturbances in homeostatic genes, miRNA expression profiles, and signaling pathways that play an essential role in the epithelial-mesenchymal transition (EMT) process. TGF-β1 has been hypothesized to play a key role in the development and progression of endometriosis, but the activation of a specific mechanism via the TGF-β-SMAD-ILK axis in the formation of endometriotic lesions is poorly understood. The aim of this study was to assess the expression of EMT markers (TGF-β1, SMAD3, ILK) and miR-21 in ectopic endometrium (ECE), in its eutopic (EUE) counterpart, and in the endometrium of healthy women. The expression level of the tested genes and miRNA was also evaluated in peripheral blood mononuclear cells (PBMC) in women with and without endometriosis. Fifty-four patients (n = 54; with endometriosis, n = 29, and without endometriosis, n = 25) were enrolled in the study. The expression levels (RQ) of the studied genes and miRNA were evaluated using qPCR. Endometriosis patients manifested higher TGF-β1, SMAD3, and ILK expression levels in the eutopic endometrium and a decreased expression level in the ectopic lesions in relation to control tissue. Compared to the endometrium of healthy participants, miR-21 expression levels did not change in the eutopic endometrium of women with endometriosis, but the RQ was higher in their endometrial implants. In PBMC, negative correlations were found between the expression level of miR-21 and the studied genes, with the strongest statistically significant correlation observed between miR-21 and TGF-β1. Our results suggest the loss of the endometrial epithelial phenotype defined by the differential expression of the TGF-β1, SMAD3 and ILK genes in the eutopic and ectopic endometrium. We concluded that the TGF-β1-SMAD3-ILK signaling pathway, probably via a mechanism related to the EMT, may be important in the pathogenesis of endometriosis. We also identified miR-21 as a possible inhibitor of this TGF-β1-SMAD3-ILK axis.
1. Introduction
Endometriosis is a debilitating, estrogen-dependent chronic gynecological disease, which is characterized by the presence of endometrial-like tissue and the growth of dysfunctional endometrial glands and stroma outside the uterine cavity [1]. The incidence of endometriosis is difficult to estimate, as some patients with this pathology are asymptomatic; therefore, as much as 11% of cases remain undiagnosed [2,3]. The prevalence rate of clinically symptomatic endometriosis that involves dysmenorrhea, dyspareunia, dysuria, dyschezia, infertility, and cyclic and acyclic pelvic pain [4,5] among women of reproductive age worldwide is estimated at 10–15% [3,6]. Although spontaneous remission is possible, it is suggested that endometriosis with moderate and severe atypia may affect cell architecture and proliferation, resulting in a possible malignant transformation [6,7]. In particular, ovarian endometriosis—one of the most common subtypes occurring in 17–44% of women diagnosed with endometriosis [8] is considered to possess a premalignant potential, which supports a model of endometriosis as a precursor of malignant ovarian cancer. The progression of endometriosis to ovarian cancer has been supported by molecular evidence. The pathogenesis of both diseases consists of cell invasion, unrestrained growth, the development of new blood vessels, and decreased apoptosis and inflammatory responses [7].
At the molecular level, these changes are often associated with pathological protein expression via disturbances in homeostatic genes as well as miRNA expression profiles and signaling pathways involved in ovarian carcinogenesis and, similarly, in endometriosis development. For example, the overexpression of p53, pro-inflammatory cytokines such as: transforming growth factor (TGF)-β1, cyclooxygenase (COX)-2, vascular endothelial growth factor (VEGF) and tumor necrosis factor-alpha (TNF-α), as well as estrogen (ER)-1α and androgen (AR) receptors, or decreased expression levels of the progesterone receptor (PR) and inflammatory interleukin 1 (IL-1), were revealed. All of them presented a similar expression pattern in both ovarian endometriosis tissues and endometriosis-associated ovarian cancer [9,10,11].
Given the reasonable assumptions and also the evidence that endometriosis may pose a risk of malignancy depending on changes in the expression of important genes and regulatory miRNAs, it seems important to provide new information on the molecular origin and development of endometriosis itself. To date, the cause of this disease remains not fully elucidated, and its pathogenesis is only partially understood. Recent studies suggest that mechanisms related to the epithelial-mesenchymal transition (EMT) may play a crucial role in endometriosis. EMT is associated with the disruption of signal transduction pathways and the loss of polarity and intercellular contacts in the epithelial and mesenchymal cells. During this multi-stage process, immotile epithelial cells acquire phenotypes of motile mesenchymal cells and the ability to migrate and invade other regions, which is accompanied by changes in cell morphology and gene expression [12,13].
The transforming growth factor-β1/Smad3 (TGF-β1/Smad3) signaling pathway seems to play an essential role in the EMT mechanism. TGF-β has been shown to be an inhibitor of the proliferation of normal endometrial cells, arresting the cell cycle in the G1 phase. As the major promoter of EMT, it is differentially and abundantly expressed in the endometrium under hormonal control [14,15]. TGF-β up-regulation in the endometriotic tissue, serum, and peritoneal fluid of endometriosis patients may be crucial for the development and/or maintenance of this disease [16,17,18]. Moreover, TGF-β1 is recognized as an important profibrogenic mediator, and probably along with another strong fibrogenesis stimulus, platelet-derived growth factor (PDGF), it can induce increased collagen production, cell contractility, and metaplasia of the endometrial epithelium, which ultimately leads to fibrosis. Such a phenotypic transformation has often been found in ovarian, peritoneal, pleural and extra-organ endometriosis [19,20,21].
The published literature emphasizes the role of activated Smad2 and Smad3 in collagen expression and tissue fibrosis that operate downstream of TGF-β1 ligands [22,23]. Acting as transcription factors, the Smads (Smad2, p-Smad2, Smad3, p-Smad3, Smad2/3, and Smad4) are the key elements of the TGF-β/Smad signaling pathway. Smads are positive in the endometriotic cystic wall and also active in the peritoneum of patients with endometriosis [18,23]. In contrast, the lower number of immunopositive Smad cases shown in ovarian endometriosis than in normal endometrium suggests a suppressive effect on the proliferation of endometrial cells, with the simultaneous disturbance of Smad expression during the formation of endometrial lesions or the involvement of non-Smad signaling pathways in ectopic endometrial cells [24,25]. The Smad protein family ultimately regulates the transcription of genes such as E12/E42, ZEB1, ZEB2, Snail1, Snail2/Slug, and Twist1, resulting in the loss of cell–cell tight and adherens junctions, desmosomes, and the gain of mesenchymal markers characteristic of EMT [16].
Existing evidence has demonstrated the TGFβ-1 regulation of EMT to also be dependent on integrin-linked kinase (ILK) function during the fibrosis process [26,27]. This serine/threonine protein kinase is a focal adhesion adapter, mediating the cell–extracellular matrix (ECM) and cell–cell relationships [28]. Its fundamental role is to regulate cell survival, proliferation and migration by connecting the cytoplasmic domains of β-integrins with the actin cytoskeleton, mediating integrin signaling in various cells [27,29]. Data presented in recent studies broaden this observation by showing that overexpression of ILK increases the ability of human endometrial stromal cells to migrate and invade by facilitating EMT [30]. It is also proposed that the regulation of this process may be associated with ILK-Akt [31] and ILK/GSK-3β/β-catenin/slug signaling pathways [32]. The cyclic switching of integrins is known to take place in endometrial cells throughout the menstrual cycle, and since ILK acts at the central stage of cell-matrix adhesion and can induce focal adhesions, it is reasonable to assume that ILK may also play an important role in the unique pathology of the ectopic spread of the endometrium [33]. While there is agreement that ILK is biologically important, especially in the context of EMT, the expression profile of this gene in endometriotic lesions has yet to be determined. Extensive research into the understanding of endometriosis and its evolution toward ovarian cancer has identified a number of miRNAs with dysregulated expression patterns. Among the range of miRNAs (miR-1, miR-17, miR-30a, miR-34, miR-141, miR-145, miR-155, miR-200a/b/c, miR-205, miR-325 and miR-429) that may contribute to neoplastic transformation, miR-21 is also mentioned [6,12]. It is the first oncogenic miRNA (oncomir) that is commonly expressed in many human cancers, including ovarian, breast, thyroid, colon, rectal, prostate, lung and pancreatic carcinomas [34]. MiR-21 is also dysregulated in endometrial cancer and participates in EMT [35]. It plays an important role in cell proliferation, migration, apoptosis, and invasion and is clearly involved in the key regulatory pathways. Recent studies show that the modulation of its expression results in clear and significant phenotypic changes in endometrial epithelial cells [36,37]. All the above-mentioned processes in which miR-21 participates play an important role in the development and progression of endometriosis; however, the involvement of miR-21 in this disease entity is little studied. miRTarBase suggests that many genes are potential targets of miR-21-5p. This database also points to a miR-21-5p–TGF-B1 interaction, thus speculating a regulatory function of miR-21-5p in modulating TGF-β1 expression (https://mirtarbase.cuhk.edu.cn).
Taken together, the expression and activation of EMT-inducing factors occur in response to a variety of signaling pathways, including those mediated by TGF-β1, SMAD3, and ILK signaling, which are the objects in this work. We assume that some of these signals may be predominant in targeting EMTs at different stages of reprogramming, which may result in differentiated expression of genes depending on the microenvironment, tissue type, and endometriotic lesion location. More importantly, disturbances in intracellular signaling pathways related to the pathological expression of EMT mesenchymal biomarkers (TGF-β1, SMAD3 and ILK) may have clinical significance in the diagnostic and therapeutic aspects of endometriosis. There is still a need to search for early and non-invasive diagnostic markers related to the process of primary changes inside the endometrium itself, changes related to metaplasia and to EMT. Therefore, in order to assess the potential dysregulation of expression in ectopic endometrium, the expression profiles of TGF-β1, SMAD3, ILK and miR-21 were compared not only with their eutopic counterparts but also with the level of expression in the endometrium of healthy women. The present study also determines the expression level of the studied genes and miRNAs in PBMC in women with and without endometriosis. Additionally, the purpose of the study was to analyze the correlation between TGF-β1, SMAD3, ILK and miR-21 expression levels and the clinical features and biochemical parameters of patients with endometriosis.
2. Results
2.1. The Expression Profile of TGF-ß1, SMAD3, ILK and miR-21 in Ectopic Lesions (ECE), Eutopic Endometrium (EUE) vs. Control Endometrium (C1)
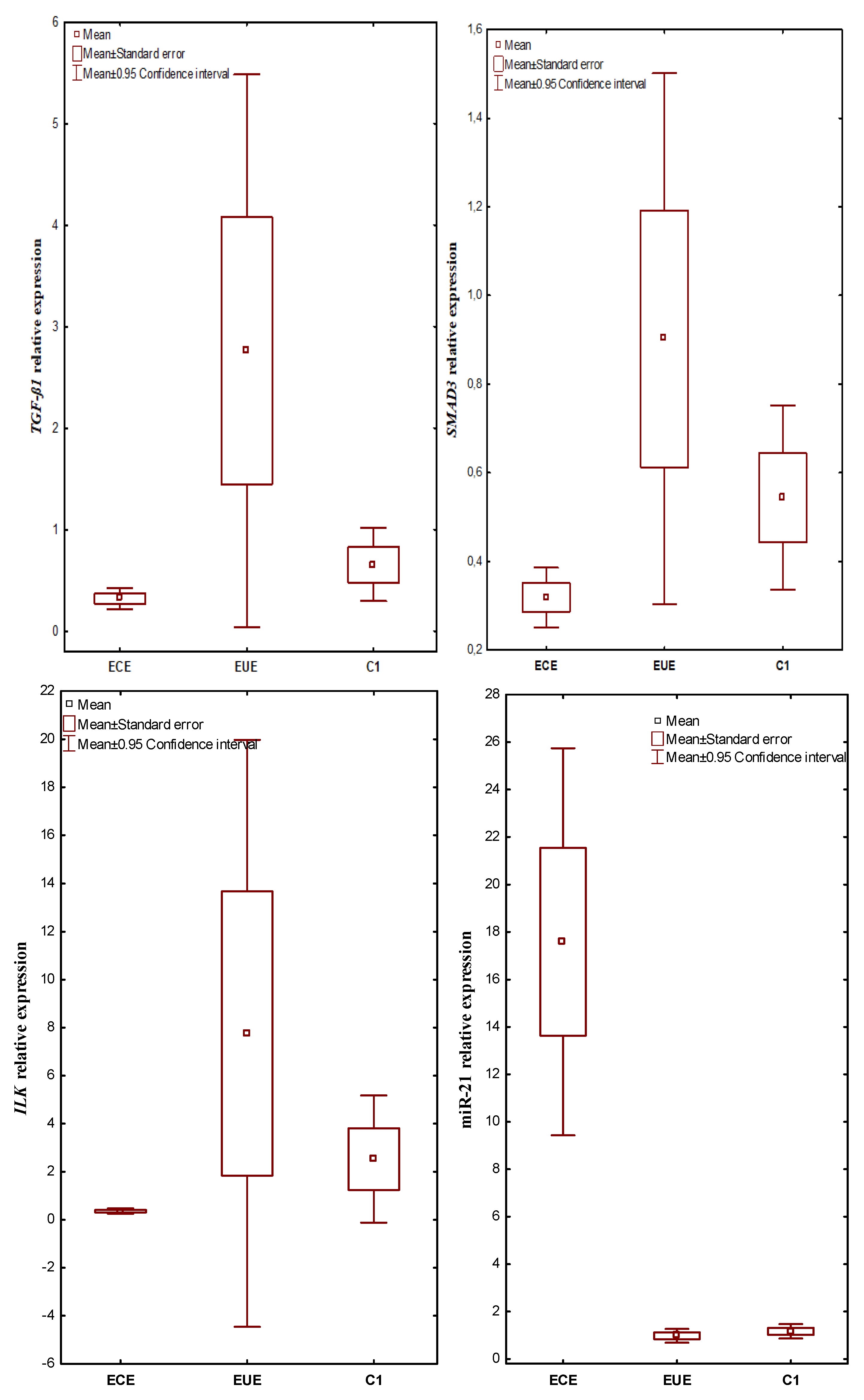
mRNA TGF-ß1, SMAD3, ILK and miR-21 were expressed in all evaluated tissues. The expression level in ectopic lesions and matched eutopic endometrium obtained from the same patient and in control tissues were compared. In patients with endometriosis, the mean TGF-ß1 gene expression level (RQ value) was 0.317748 in the ECE and 2.760536 in the EUE, while in the endometrium of the control women, the mean RQ was 0.654076. In the case of SMAD3, the mean RQ was 0.318956 in the ECE and 0.902240 in the EUE of patients with endometriosis, and 0.543976 in the endometrium of the control group. For ILK, the RQ value was 0.357348 in the ECE and 7.755932 in the EUE of patients with endometriosis, while in the endometrium of women without endometriosis (C1), the mean RQ value was 2.524812. In patients with endometriosis, the mean expression level (RQ value) of miR-21 was 17.58223 in ECE and 0.97790 in EUE, while in the endometrium of control women, the mean RQ was 1.165748.
Kruskal–Wallis rank analysis demonstrated a statistically insignificant difference in the expression levels of the TGF-ß1 (p = 0.0601) and SMAD3 (p = 0.0701) genes between the studied groups. In the case of ILK and miR-21, the Kruskal–Wallis rank analysis showed a statistically significant difference in the level of expression between the studied groups (p = 0.0075 and p = 0.00001, respectively). The Newman-Keuls post hoc test used suggested a negligible trend towards lower expression in ECE than in EUE for TGF-ß1 (p = 0.070437) as well as SMAD3 (p = 0.060457) and higher expression in EUE compared to C1 for TGF-ß1 (p = 0.056815). In contrast, the expression level of miR-21 was significantly higher in ECE than in EUE (p = 0.000116) and in ECE compared to C1 (p = 0.000117; Newman–Keuls test). The above differences for miR-21 were confirmed by an additional Mann–Whitney U test (p = 0.0000001). The additional Mann–Whitney U test also showed statistically significantly lower expression of TGF-ß1, SMAD3 and ILK in ectopic lesions compared to matched eutopic endometrium in women with endometriosis (p = 0.023471, p = 0.026057 and p = 0.006639; respectively). In addition, ILK expression levels were significantly lower in ectopic lesions compared to the control (Mann–Whitney U test, p = 0.006236).
The results of expression levels (mean RQ values) of the studied genes and miR-21 in individual tissue materials (ectopic endometrium—ECE and eutopic endometrium—EUE, obtained from the same patent with endometriosis) and control endometrium (C1) are presented in Figure 1.
Figure 1.
The expression levels (mean RQ values) of the studied genes and miR-21 in individual tissue materials (sources of tissue: ectopic endometrium—ECE and eutopic endometrium—EUE, obtained from the same patient with endometriosis) and control endometrium (C1).
2.2. The Expression Profile of TGF-ß1, SMAD3, ILK and miR-21 in PBMCs: From Patients with Endometriosis vs. from Patients without Endometriosis (C2)
The expression levels of the TGF-ß1, SMAD3, ILK and miR-21 genes were assessed in PBMCs obtained from patients with endometriosis and in PBMCs from patients without endometriosis, who constituted the control group (C2). No statistically significant differences in the level of expression of the genes and miR-21 in PBMCs were observed between the studied groups (p > 0.05, Mann–Whitney U test). The results are presented in Table 1.

Table 1.
The expression levels (mean RQ values) of the studied genes and miR-21 in the PBMCs in the group of patients without endometriosis (C2) vs. the group of patients with endometriosis.
2.3. Correlations between the Expression Level of TGF-ß1, SMAD3, ILK and miR-21 in 3 Different Biological Materials (Ectopic Endometrium–ECE, Eutopic Endometrium–EUE, and PBMCs) Obtained from the Same Patient with Endometriosis
In the ectopic endometrium, a positive correlation was demonstrated between the ILK and SMAD3 genes (Rho = 0.496154, p = 0.011652; Spearman’s rank correlation) as well as ILK and TGF-ß1 (Rho = 0.649231, p = 0.000446; Spearman’s rank correlation).
Similarly, in the eutopic endometrium, a positive correlation was found between the ILK and SMAD3 genes (Rho = 0.904615, p = 0.0000001; Spearman’s rank correlation) as well as ILK and TGF-ß1 (Rho = 0.913077, p = 0.0000001; Spearman’s rank correlation). In addition, a positive correlation was observed between the TGF-ß1 and SMAD3 genes (Rho = 0.830000, p = 0.0000001; Spearman’s rank correlation).
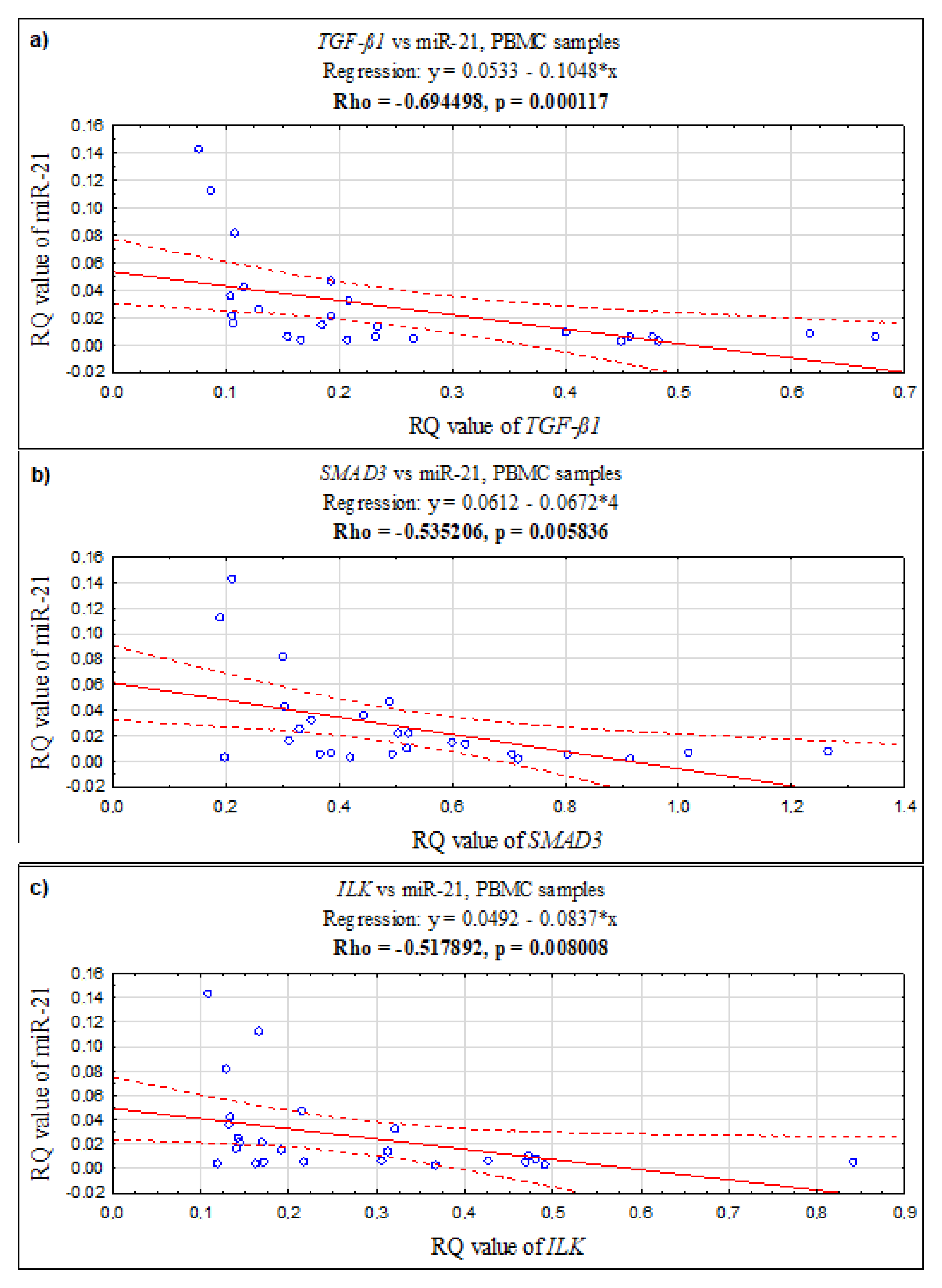
In PBMCs, a positive correlation was found between the ILK and SMAD3 genes (Rho = 0.726923, p = 0.000039; Spearman’s rank correlation), ILK and TGF-ß1 (Rho = 0.870769, p = 0.0000001; Spearman’s rank correlation), and TGF-ß1 and SMAD3 (Rho = 0.683846, p = 0.000164; Spearman’s rank correlation). Additionally, in PBMCs, the expression level of miR-21 negatively correlates with TGF-ß1 (Rho = −0.694498, p = 0.000117; Spearman’s rank correlation), with SMAD3 (Rho = −0.535206, p = 0.005836; Spearman’s rank correlation), and with ILK (Rho = −0.517892, p = 0.008008; Spearman’s rank correlation) (see Figure 2).
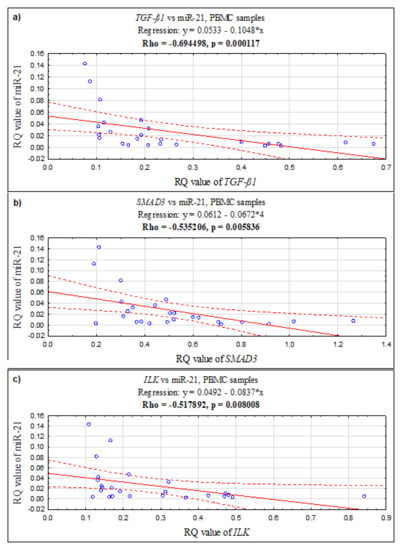
Figure 2.
The scatter plots showing correlations between expression levels (RQ values) of miR-21 and genes: (a) TGF-ß1, (b) SMAD3, (c) ILK in PBMC samples.
2.4. Correlations between the Expression Level of TGF-ß1, SMAD3, ILK, and miR-21 in Relation to Clinical Characteristics and Biochemical Parameters of Patients with Endometriosis
The RQ values for the TGF-ß1, SMAD3, and ILK genes, as well as miR-21, in ectopic tissue were analyzed in relation to the clinical features of patients with endometriosis: age at the time of diagnosis, stage of endometriosis (according to rASRM classifications), pelvic pain symptoms (according to the numerical rating scale–NRS), phase of the menstrual cycle, and concentrations of the biochemical parameters of CA-125 and HE4. The results are shown in Table 2.

Table 2.
Expression level (mean RQ value) of tested genes and miRNA in ectopic tissue in relation to clinical features and biochemical parameters of patients with endometriosis.
Analysis of the level of expression of the tested genes and miRNA-21 in the group of patients with endometriosis according to age showed that the expression of all the studied genes was higher in patients aged ≤40 years compared to older patients, whereas miR-21 expression was higher in patients >40 years of age, although the observed differences were not statistically significant (p > 0.05).
In tissue material collected from patients in the proliferative phase of the cycle, the level of expression of the tested genes was higher compared to tissue samples from patients in their secretory phase. MiR-21 expression was higher in tissue samples from patients in the secretory phase of the cycle, but the changes in expression were not statistically significant (p > 0.05, Mann–Whitney U test). There were also no statistically significant differences in the level of expression of the studied genes and miR-21 between the proliferative and the early, middle and late secretory phases of the menstrual cycle (p > 0.05, Kruskal–Wallis test).
Analysis of the level of gene and miR-21 expression according to the stage of endometriosis demonstrated a statistically insignificant increase in the level of expression of TGF-ß1 and miR-21 in the group of patients with stage IV compared to stage III of endometriosis (p >0.05, Mann–Whitney U test). An inverse correlation was observed between the level of expression of the SMAD3 and ILK genes and the stage of the disease; however, in the case of the ILK gene, only a negligible downward trend in ILK expression was observed in the group of patients with stage IV vs. stage III of endometriosis (p = 0.086857, Mann–Whitney U test).
Analysis of the level of gene and miR-21 expression according to the severity of pain showed a decrease in the level of expression of TGF-ß1 and miR-21 in the group of patients with very strong painful symptoms occurring in the course of endometriosis, but the changes in expression were not statistically significant (p > 0.05, Mann–Whitney U test). In contrast, the level of expression of the SMAD3 and ILK genes correlated positively with the degree of pain severity, and for the ILK gene, a statistically significant increase in expression was confirmed in the group of patients with very strong painful symptoms (p = 0.044271, Mann–Whitney U test), while for the SMAD3 gene, only a negligible upward trend in expression was observed in that group of patients (p = 0.071229, Mann–Whitney U test).
By dividing the group of patients with endometriosis according to the concentration of Ca 125 into the ≤65 U/mL vs. >65 U/mL subgroups, a statistically insignificant increase in the level of expression of the tested genes in the group of patients with a higher concentration of Ca 125 was confirmed, whereas the expression of miR-21 was higher in the group of patients with Ca 125 ≤65 U/mL (p > 0.05, Mann–Whitney U test). Likewise, a statistically insignificant increase in the gene expression correlated with higher HE4 concentrations, while the level of miRNA expression was lower in that group of patients (p >0.05, Mann–Whitney U test).
3. Discussion
The pathophysiology responsible for the onset, development, and course of endometriosis progression remains unsolved. The lack of clinically relevant biomarkers with requisite sensitive and specificity means that the average lag phase between the onset of symptoms of endometriosis and its diagnosis has been estimated to be up to 8–12 years [38,39]. Therefore, the major priority of endometriosis research is the discovery of a non-invasive biomarker or a panel of such biomarkers that would hasten the diagnosis of this disease in women who have pelvic pain and/or subfertility with normal ultrasound results [40]. This is essential due to the fact that endometriosis in a significant number of women has long-term progress without clear symptoms of the disease; hence, the diagnostic process is very long [38,39].
Accumulating evidence on the pathogenesis of endometriosis indicates a polygenetic cause of this disease, but none of the candidate genes have yet been approved as a molecular marker useful for the clinical diagnosis of gynecologic tumors, including endometriosis [1,3,41]. Moreover, despite intensive research on the role of miRNAs which showed changes in expression patterns in endometrial stromal cells, the data on its role in the regulation of pathogenic processes related to the formation of endometrial lesions are still inconsistent [6,11,42].
In connection with the above, in our study, we evaluated the expression of EMT markers (TGF-β1, SMAD3, ILK) and miR-21 in ectopic endometrium (ECE), its eutopic portion (EUE), and in the endometrium of healthy women. Peripheral blood mononuclear cells (PBMCs) from endometriosis patients and non-patients were also investigated.
We suggest that the endometrial epithelial phenotype is defined by the differential expression of TGF-β1, SMAD3, and ILK genes between ectopic and eutopic endometrium. Furthermore, we conclude that the TGF-β1-SMAD3-ILK signaling pathway may be important in the etiology of endometriosis, possibly via EMT-related mechanisms, and we identify miR-21 as an inhibitor of this TGF-β1-SMAD3-ILK axis.
TGF-β1, as a multifunctional cytokine, is thought to play an important role in the development and progression of endometriosis. It was confirmed that at the mRNA level, TGF-β regulates in the endometrium the expression of the extracellular matrix, adhesive molecules, and proteases. Its altered expression is associated with the manifestation of pathological uterine functions, including endometriosis [43]. Activated TGF-β receptors induce the recruitment of multiple specific intracellular signals, including SMADs, which can accumulate in the nucleus and possess the function of transcription factors to modulate the expression of genes and miR-21 after phosphorylation [44,45]. At the same time, many elements of ILK signaling are induced by TGF-β in a SMAD-dependent manner [46]. The specific mechanism of the TGF-β-SMAD-ILK axis in the pathology of endometriosis has been poorly studied. Research evidence suggests that the establishment of endometriosis may be facilitated by endometrial dysfunction involving both eutopic and ectopic endometrium [47].
In our study, changes in the expression levels of miR-21, TGF-β1, SMAD3 and ILK were assessed in both tissues. In addition, we examined the mRNA levels of the genes and miRNA considered in peripheral blood mononuclear cells (PBMC) of patients with and without endometriosis in order to search for informative diagnostic biomarkers. As one of the findings of our study, we detected significantly reduced TGF-β1 expression in the ectopic endometrium compared to eutopic tissue in women with endometriosis. Interestingly, most research focuses on understanding the physiological role of this gene in the human endometrium, emphasizing that TGF-βs are synthesized in the endometrium in large quantities and promote its adhesion to ovarian tissue [23,48,49]. Moreover, TGF-β expression is under hormonal control, and its expression is specific to the endometrial stage [15]. The highest level of TGF-β1 expression is observed during menstruation [50]. It has been suggested that TGF-β1 expression may play a role in the mechanisms regulating the transition from proliferation through invasion and motility to the differentiation of endometrial cells [51]. Thus, this pleiotropic cytokine, by participating in a number of important processes during endometrial tissue remodeling in the perimenstrual period, may promote endometrial tissue repair and protect from extensive fibrosis and scarring [52]. In view of the above data, our observation of high TGF-β1 expression in the eutopic endometrium confirms its protective role and participation in endometrial tissue remodeling. Our observations and conclusions are consistent with the results of Goteri et al. [53]. Another main finding of our study was the observation that the TGF-β1 expression level in the ectopic endometrium was low. We conclude that it may be one of the molecular changes contributing to the progression of already implanted endometrial foci. In contrast, our results differ from those obtained by a team of other authors [54,55], where upregulation of TGF-β1 in ectopic endometrium was demonstrated. Taking our result into consideration, the decreased expression of TGF-β1 in ectopic endometrium may suggest the occurrence of possible post-transcriptional modulations of TGF-β1 depending on the diverse microenvironment of the endometrium. The discrepancy in the results should not be surprising because the mechanisms involved in endometriosis in eutopic and ectopic locations can be different and conditioned by the tissue environment, which is likely to affect TGF-β1 expression. Interestingly, other researchers have proven that the sites adjacent to peritoneal lesions have higher levels of TGF-β1 mRNA transcripts than the distal sites [18], and the activity of TGF-β1 is elevated at the sites affected by the disease [56].
In the presented study, we compared the level of TGF-β1 expression in the eutopic endometrium of women with endometriosis and the control endometrium. The available evidence indicates the existence of physiological differences in normal endometrium compared to the eutopic endometrium of women with endometriosis. In the latter, there was a significant increase in endometrial cell survival as well as changes in DNA fragmentation and expression of TGF-β1, a factor involved in cell proliferation, differentiation and induction of programmed cell death [57]. We have also observed a change in TGF-β1 expression in our study, where the level of TGF-β1 mRNA was higher in the endometriosis-affected patients compared to the endometrium of patients without endometriosis, although that difference did not reach statistical significance. It should be pointed out that our result has been confirmed by other authors, who observed overexpression of TGF-β1 mRNA in the eutopic endometrium compared to control tissue [53,58].
Interestingly, it has been noted that TGF-β1 expression is cyclical in normal endometrium and observed mainly during the middle and late secretory phases, while the cyclic effect was not present in eutopic endometrium in women with endometriosis [57]. On the contrary, in our study, the level of expression of TGF-β1 did not change throughout the menstrual cycle in the eutopic tissue of women without and with endometriosis, nor in endometrial foci. We conclude that such controversial research results may be due to the number of study groups of patients in the individual phases of the menstrual cycle and the heterogeneity of the cellular/tissue material used in the experiment because TGF-β1 is known to be localized in stromal cells, glandular cells and endometrial macrophages [43]. However, the important result of our research is similar to other authors’ [43,57] unequivocal confirmation of the local changes in TGF-β1 synthesis in patients with endometriosis, which may favor cell proliferation and survival in the pathological endometrial tissue.
Endometriotic cells have been proven to synthesize TGF-β1, inducing fibrosis around the endometrial foci. In this pathological process, which results in damage to the adjacent ovarian tissues, SMAD transcription factors are involved [23]. In our work, we also investigated the expression of SMAD3 mRNA in the endometrium of women with endometriosis. The expression level of this gene was significantly lower in ECE compared to the matched EUE of women with endometriosis and also lower than in the controls. Similarly to our results, other authors also demonstrated reduced SMAD3 transcript levels in sick women vs. controls, but in the cumulus cells of the antral [59]. In contrast, observations conducted in a group of patients with endometriosis, from whom the peritoneal adhesion biopsies were taken, show results opposite to ours [60]. However, it should be emphasized that our biological material consisted mainly of tissue fragments from endometrial ovarian cysts, which may explain the discrepancy in the results obtained. Nevertheless, as SMAD3 is one of the most important intracellular mediators in TGF-β signaling [23], based on our observations, it can be hypothesized that in the case of a disorder of its expression in endometriosis, the functionality of all proteins in the TGF-β superfamily can be changed.
Contrary to our observations, Cruz et al. [61] showed a significantly reduced expression of SMAD3 mRNA as well as the total SMAD3 protein in the eutopic endometrium (EUE) of women with endometriosis. However, because pSMAD3 levels were similar in patients with and without endometriosis, the authors postulate that the phosphorylation of SMAD3 is precisely regulated by specific mechanisms, mainly by activating TGFβ/activin receptor ligands [61]. Unfortunately, we have not studied these mechanisms. Analyzing the expression of SMAD3 mRNA in the eutopic tissue of women with and without endometriosis, we also did not demonstrate statistically significant differences between the studied groups. The above result suggests that the endometrium of women with endometriosis is not subject to significantly abnormal activation of the SMAD protein cascade, and small changes in SMAD3 mRNA expression are likely to be compensated further down the TGF-β/SMAD signaling pathway, which may, in effect, prevent malignant transformation. On the other hand, the system of TGF-β/SMAD activity regulation in the endometrium is complicated due to a number of receptors with serine-threonine kinases activity and the cooperation of various cytokines interacting with this pathway, which requires deeper investigation in the context of the development of endometriosis.
The TGF-β/SMAD pathway is integrated into the intracellular signaling network through cross-talk with other signaling pathways, such as the PI3K-Akt pathway, an important mediator of TGF-β-induced activation of various EMT responses [62,63]. The integrin-linked kinase (ILK) has also been reported to be involved in Akt activation by TGF-β [33], and by binding to intracellular integrins, it promotes signaling associated with cell adhesion, apoptosis, proliferation, or cell migration [64].
Elevated ILK expression has been detected in various human cancers originating from epithelial cells [65,66,67]. In the course of endometriosis, both eutopic and ectopic endometrial stromal cells exhibit adhesive properties with similarity to cancer cells, among others, through different expressions of peritoneal mesothelial adhesion factors, including loss of close connections with the consequent acquisition of invasive and metastatic properties [68]. As the initial stage of endometriosis is considered to be the adhesion ability of the exfoliated endometrial tissues retreating into the pelvic cavity, research also focuses on the specific adhesive particles, such as ILK, regulating the ability of endometrial stromal cells to adhere [30,31,69,70]. In our study, an increase in the level of ILK expression in the endometrium (EUE) of women with endometriosis compared to the control group indicates the importance of ILK in the pathogenesis of endometriosis. The research of other authors, where increased levels of mRNA ILK expression in endometrial tissues have been demonstrated, both eutopic and ectopic [30,31,69,71], is consistent with ours.
Interestingly, in our study, the ILK gene expression profiles in eutopic and ectopic tissue in endometriosis patients differed, manifesting the upregulation of ILK in the eutopic endometrium and downregulation in endometrial lesions compared to normal endometrium. The above result may suggest a loss of endometrial epithelial phenotype defined by a differential ILK expression in the ectopic and eutopic endometrium, and the likely underlying mechanism may include EMT via the TGF-β1-SMAD-ILK signaling pathway. Thus far, it has been demonstrated that ILK is a critical intracellular mediator of tubular EMT involved in the pathogenesis of chronic renal fibrosis [72]. In view of the fact that fibrosis is a characteristic feature of endometriosis and the expression of ILK in renal tubular epithelial cells is strictly controlled by TGF-β1 and dependent on intracellular SMAD signaling [46,72], we speculate that an analogous molecular mechanism could occur in endometrial epithelial cells and promote their mesenchymal transformation in endometriosis. The strength of our results is supportive of the presence of an ILK connection with the extensive TGFß-SMAD signaling network, also possible in the endometrium. We present the result concerning a strong positive correlation between the expression levels of ILK and SMAD3 as well as ILK and TGF-ß1. Moreover, the evidence of published studies indicates that ILK increases the ability of cells to migrate and invade through EMT induction, which plays a significant part in the initial formation of endometriosis [30,31,69,70]. Therefore, inhibition of the expression and activity of ILK is being increasingly considered as a new target in the prevention of endometriosis and a possible therapeutic alternative to hormone therapy, often effective but carrying numerous side effects [30,31,69,70].
An interesting result of our work is an increase in ILK expression along with an increase in pain severity in women with endometriosis. The mechanisms associated with the pathophysiology of the development of pelvic pain in these women are poorly understood. It has been speculated that the predominance of the inflammatory environment with the reaction of fibrosis and the formation of local scars and adhesions may be involved in this process. It is probable that the increased local release of pro-inflammatory cytokines in the endometrial tissue leads to excessive sensory innervation by impairing the regulation of neurotrophins [73]. Currently, there are no available data on the role of ILK in the development of chronic pelvic pain. TGF-ß1 is known to affect the expression of growth factor (NGF) and neurotrophin-3 (NT-3) mRNA [74], and as ILK remains under TGF-ß1 control [72], the involvement of ILK in the mechanism of pelvic pain in women with endometriosis is therefore possible.
More and more evidence indicates that altered miRNA expression may be involved in the development of endometriosis [11,75,76]. In several studies [77,78,79], including our own research work, the possible involvement of miR-21 in the pathogenesis of this disease has been emphasized. In the ectopic endometrial tissue (ECE) compared to the eutopic endometrium (EUE) in the same patient, we noted a significant increase in the level of miR-21 expression. Other authors have also observed that miR-21 exhibited an overexpression profile in endometriosis (in ECE) compared to paired eutopic endometrium [77,80]. Therefore, it seems that the altered expression of miR-21 may be relevant to the formation of endometrial foci. However, no differences in miR-21 expression between the eutopic endometrium of women with endometriosis and control eutopic endometrium, confirmed in the study by Haikalis et al. [78], suggest that the modification of miR-21 expression may be a secondary molecular change in the pathogenesis of endometrial implants. Nevertheless, the validity of miR-21 identification for the molecular mechanisms involved in the development of endometriosis is emphasized by the fact that miR-21-5p expression occurs at statistically significantly lower levels after endometriotic peritoneal fluid treatment of primary cell cultures from eutopic endometrial tissue obtained from patients, which allows to open up new therapeutic strategies in the treatment of endometriosis through the use of miR-21 [81]. It is also suggested that there are molecular differences in the profile of the miRNA studied by us between endometrial lesions collected from different anatomical sites. A comparison of the level of miR-21 expression between implants from the peritoneal cavity, deeply infiltrating endometriosis, and endometrial lesions of the ovary indicates a significantly higher expression in the last case [78]. Thus it should be pointed out that the miR-21 expression profile may be dependent on the location of an endometrial lesion.
Not without significance for the miR-21 expression pattern is also the degree of development of the endometrial focus and the fact that the miRNA is involved in angiogenesis [81] and controls cell growth, invasion and tissue repair, potentially by regulating gene silencing and epigenetic mechanisms [82]. Interestingly, in our study and in other works [83], the regulation of miR-21 has been noted in severe vs. mild endometriosis. Moreover, there is evidence that miR-21 inhibits the translation of TGFβ-1 mRNA in various diseases, including endometriosis [81,82,84,85], and the post-transcriptional regulation of miR-21 via SMAD3 plays a key role in its upregulation and the development of fibrosis [45].
Our study suggests that both miR-21 and the components of the TGF-β1-SMAD-ILK signaling pathway, to which it is targeted, are expressed differentially between ectopic endometrial lesions and eutopic endometrium in women with endometriosis. In this study, TGF-β1, SMAD3 and ILK mRNA transcripts in endometrial foci were downregulated, and the level of miR-21 expression was significantly high compared to eutopic and control tissue. It has been proven that the inhibition of miR-21-5p effectively activates the apoptotic potential of endometrial cells, and the high level of expression of this miRNA may reflect the characteristics of endometriosis, such as the pathological growth or proliferation of cells [79]. Thus, we hypothesize that excessive suppression of the studied genes occurs through miR-21, especially inhibition of the anti-inflammatory cytokine TGF-β1, also known as the main promoter of EMT [14,15,52]. It is assumed to mediate a cascade of events from the transition from an initially ischemic and inflammatory environment, with the consequent tissue damage and necrosis, to an environment that promotes cellular proliferation, tissue remodeling and fibrosis during the development and progression of endometrial lesions, and the current findings partly confirm our assumptions.
The interesting results of our work are the negative correlations between miR-21 and the studied genes. They indicate that miR-21 mediates post-transcriptional regulation in peripheral blood mononuclear cells (PBMCs) in women with endometriosis and regulates transcripts in the studied molecular network associated with endometriosis, with the strongest correlation detected between miR-21 and TGF-β1, which confirms that this gene is a direct target for miR-21. Now there is a need to develop a non-invasive test for patients with symptomatic endometriosis; however, as of today, despite many biomarkers of endometriosis proposed in peripheral blood, none have been approved for endometriosis.
To the best of our knowledge, we were the first to assess changes in the level of expression of TGF-β1, SMAD3, ILK and miR-21 in the PBMC of patients with and without endometriosis. Such an approach seems very important because the ectopic foci of peritoneal endometrium provoke a local inflammatory response with the recruitment of macrophages, release of cytokines and production of reactive oxygen species [86], which may be reflected systemically. It can be expected that specific genes involved in leukocyte activation participate in the abnormal development of the immune response and are expressed differently in the blood leukocytes of women with endometriosis compared to the control subjects. Bearing in mind the role of an important immune suppressor such as TGF-β1 in the activation of Th17 cells [87], as well as the release of that cytokine by the T-reg lymphocytes [88], the disturbance of the distribution and activation of the Treg and Th17 lymphocyte system in an inflammatory disease such as endometriosis may be related to the deregulation of TGF-β1 signaling in peripheral blood. However, the results of our study show that the systemic gene levels of the TGF-β1-SMAD3-ILK pathway, as well as the miR-21 targeting this axis in women with endometriosis, did not change compared to women without the disease. We conclude that endometriotic cells have not changed the immunophenotype of peripheral lymphocytes, and the extent of the microenvironmental impact of endometrial foci is probably local and affects the peritoneal fluid, in which differentiated expression of anti-inflammatory cytokines, including TGF-β1, is observed in women with endometriosis [89].
In summary, the molecular cascade in the pathogenesis of endometriosis is still not fully understood. Nevertheless, it is generally accepted that variations in gene expression patterns and regulatory miRNAs in endometrial stromal cells are important factors in the development and maintenance of endometrial lesions. Based on our experimental results involving biological material (samples from the ectopic endometrium, its eutopic counterpart, and from the control endometrium), we found higher expression levels of the mRNA TGF-β1, SMAD3, and ILK in the eutopic endometrium and reduced expression levels in ectopic lesions compared to control tissue. In women with endometriosis, the miR-21 expression level in the eutopic endometrium was similar to the control but lower than in endometrial lesions. We also confirmed negative correlations between miR-21 expression levels and the studied genes, identifying this miRNA as a potential negative regulator of the TGF-β1-SMAD3-ILK axis. This finding indicates that genes studied by us and miR-21 may be some of the factors involved in the molecular pathogenesis of endometriosis. However, more research focused on this topic needs to be conducted for the accurate evaluation of the exact mechanism of molecular interactions between miR-21 and the TGF-β1-SMAD3-ILK signaling pathway in endometriosis.
Regarding the strengths and weaknesses of our study, we highlighted the importance of the miR-21-TGF-β1-SMAD3-ILK axis in the pathogenesis of endometriosis. The novelty of our work was the analysis of the expression of miR-21, TGF-β1, SMAD3 and ILK in PBMCs obtained from women with diagnosed endometriosis compared to healthy ones in expectation of changes in the expression level profile of the studied genes/miRNA in connection with changes in the immune response (Treg and Th17 lymphocyte system). Unfortunately, we did not observe differences in the expression levels of miR-21, TGF-β1, SMAD3 and ILK in PBMCs in women with and without the disease, which excluded indications for further immunophenotype assessment.
Our study has some limitations that need to be addressed. First, at this stage of our research, we were unable to perform experiments to verify the mechanism of action of miR-21 in the TGF-β1-SMAD3-ILK signaling pathway and the function of these molecules in EMT; second, the sample size of our study was limited. Therefore, in the future, we plan to analyze cell lines regarding the effect of miR-21, TGF-β1, SMAD3, and ILK expression levels on the biological functions of endometrial cells, which will provide innovative theoretical references in terms of molecular targets for endometriosis treatments.
4. Materials and Methods
4.1. Research Ethics
This study was conducted in accordance with Good Clinical Practice and the principles of the Helsinki Declaration. All methods were carried out in accordance with the relevant guidelines and regulations. The protocols of this study were approved by the Bioethics Committee of the Medical University in Lodz (resolution No. RNN/67/19/KE, 12 February 2019). All participants were fully informed and signed an individual consent form for participation in the study.
4.2. Clinical Groups and Sample Collection
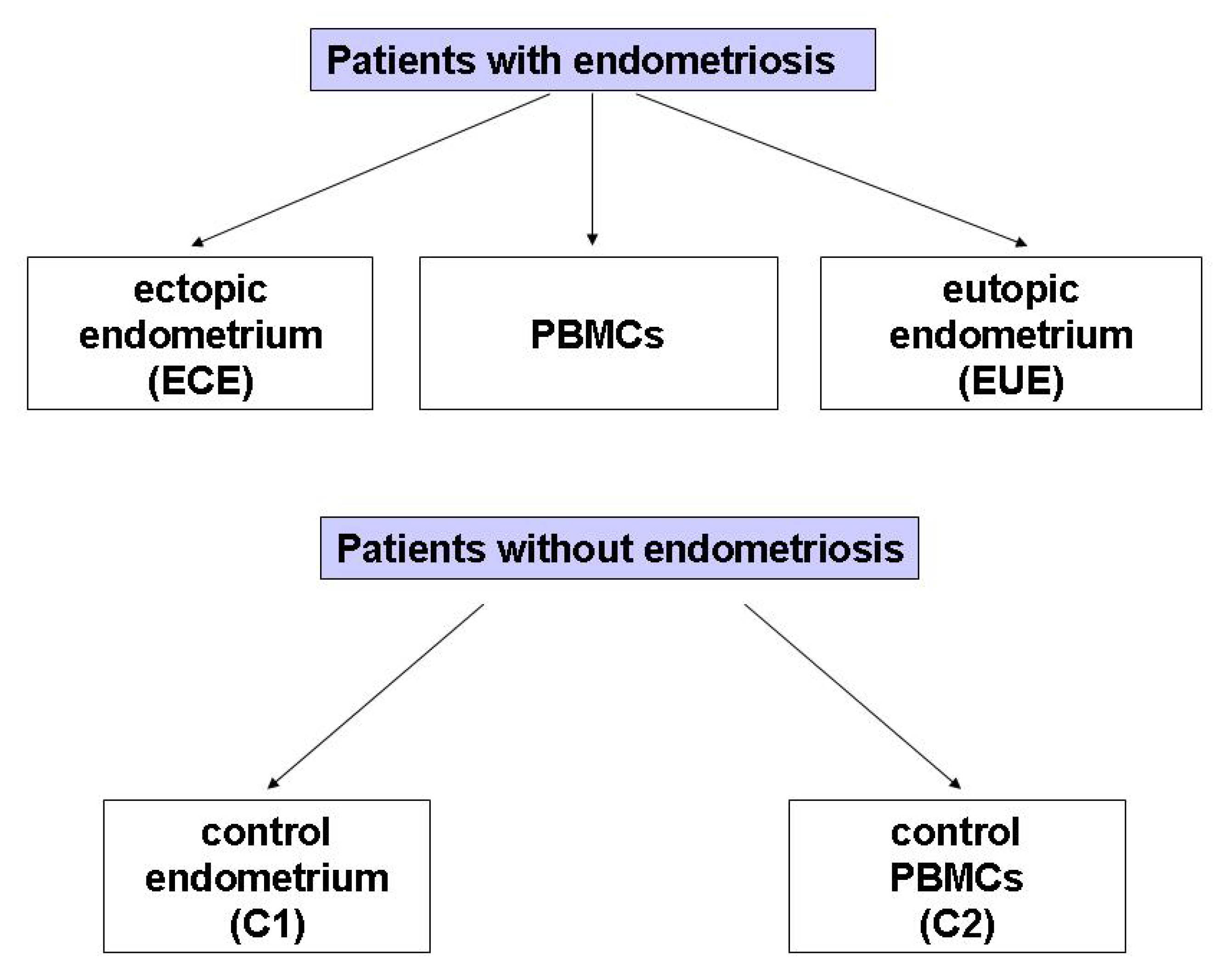
The study included 29 women with endometriosis (mean age: 38.38, SD: 6.83 years; age range: 22–49 years) and 25 (mean age: 44.68, SD: 3.34 years; age range: 38–44 years) without endometriosis at the Operative and Conservative Gynecology Ward, Dr. K. Jonscher Municipal Medical Centre, Lodz, Poland, admitted in the period of March 2019–May 2020. The exclusion criteria for all participants were: pregnancy, breastfeeding, the presence of leiomyoma, adenomyosis, or cancer of the uterine and cervix, as well as of other diseases of the uterus, fallopian tubes and ovaries, and hormone treatment for at least 12 months at the time of surgery. The diagram illustrating the biological material obtained from patients with and without endometriosis selected for the study is presented in Figure 3.
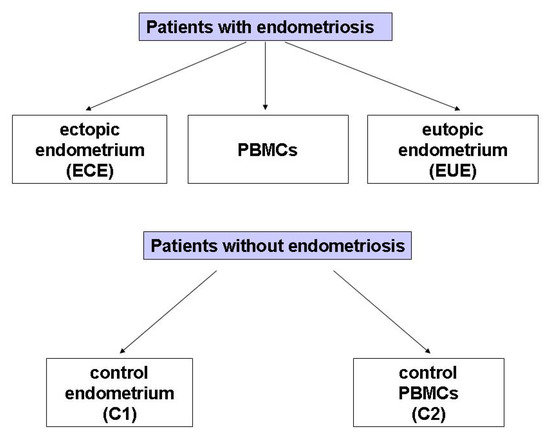
Figure 3.
The groups of biological material obtained from patients with diagnosed endometriosis selected for the study.
Pathological confirmation was necessary for all patients. The inclusion criteria were: diagnosis of endometriosis with simultaneous surgical treatment. During the elective diagnostic or therapeutic laparoscopy for suspected extrauterine endometriosis due to pain symptoms and/or infertility investigation, biological material was obtained from the same patient from 3 locations (paired samples):
(1) A piece of tissue taken from an ovarian endometrial cyst (n = 27) or sections from the peritoneum of the small pelvis (n = 2); ectopic endometrium (ECE),
(2) A piece of the mucosa/endometrium from the uterine cavity obtained by biopsy (n = 25); eutopic endometrium (EUE); no biopsy was obtained from 4 patients with endometriosis,
(3) whole blood from which peripheral blood mononuclear cells (PBMCs) were isolated (n = 25). Whole blood was not obtained from 4 patients with endometriosis.
Paired EUE, ECE tissues and PBMCs were obtained from women who presented with mild to severe endometriosis (n = 25). The diagnosis of endometriosis for all obtained biological samples was confirmed by histopathological examination. According to the criteria laid down by the revised American Fertility Society guidelines [90], the investigated biological samples (n = 29) were divided into 4 groups according to the stage of endometriosis: I (n = 1), and II (n = 2), III (n = 16), and IV (n = 10). Additionally, women with endometriosis reported mild (n = 11), moderate (n = 2), or severe (n = 16) pelvic pain, which was distinguished using the numerical rating scale (NRS). Before surgery, the fertility status and concentration data of cancer antigen (CA)-125 and human epididymis protein 4 (HE4) were collected. The results of CA-125 were not available for 3 patients, and the results of HE4 were not available for 14 patients. The phases of the menstrual cycle were determined on the basis of the histological evaluation of the endometrium and the date of the patient’s last menstruation. Seven (7) patients were in the proliferative phase, and eighteen (18) were in the secretory phase of their cycle, which was divided into early (n = 7), middle (n = 4) and late (n = 7).
Control samples were collected from patients with uterine myomas who underwent hysteroscopy or laparoscopic myomectomy without visual evidence of endometriosis and adenomyosis. During the surgery, the absence of endometriosis and adenomyosis was confirmed by meticulous examination of the pelvic and extra-pelvic peritoneum, ovaries, intestine, and diaphragm. Histopathological evaluations confirmed normal endometrium in all controls. The patients in the control group did not suffer from endometriosis. Six (6) patients were in the proliferative phase, and nineteen (19) were in the secretory phase of the cycle, which was divided into early (n = 5), middle (n = 5) and late (n = 9). Paired samples were obtained from each woman, including:
(1) A piece of the normal endometrial tissue from the uterine cavity obtained by biopsy (n = 25); C1;
(2) Whole blood, from which PBMCs were isolated (n = 25); C2.
4.3. RNA Isolation from Eutopic, Ectopic, Normal Endometrial Tissue and Peripheral Blood Mononuclear Cells (PBMCs)
Patient and control tissue samples were placed in fixRNA buffer (Eurx, Gdańsk, Poland) and stored at 2–8 °C until use. After the fixRNA buffer was removed, the samples were divided into smaller portions with 600 μL of lysis buffer added and homogenized using a Pellet pestle homogenizer (Sigma-Aldrich, Darmstadt, Germany).
A total of 5 mL of whole blood was collected on EDTA from each of the patients and controls. In order to obtain PBMCs, the blood samples were centrifuged in a density gradient using the Histopaque-1077 agent (Sigma-Aldrich, Poznań, Polska) according to the manufacturer’s protocol. The peripheral blood mononuclear cells were placed in fixRNA buffer (Eurx, Gdańsk, Poland) and frozen at −80 °C until use.
Total RNA isolation from tissue homogenates and PBMCs was performed using the mirVana miRNA Isolation Kit (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocol.
Qualitative and quantitative evaluation of the isolated RNA was performed by the spectrophotometric method, measuring the absorbance with the Eppendorf BioPho-tometerTM Plus (Eppendorf, Hamburg, Germany) at 260/280 nm wavelengths. Only samples fulfilling the following requirements were selected for further use: miRNA concentrations of 1–10 ng/μL and RNA concentration over 50 ng/μL, with a 260/280 nm ratio of 1.8–2.0. Then, RNA was aliquoted and frozen at −80 °C until the real-time polymerase chain reaction (qPCR) was performed.
4.4. Reverse Transcription (RT) and Real-Time Quantitative Polymerase Chain Reactions (Real-Time qPCR)
The reverse transcription reaction for genes was performed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA) in a volume of 20 μL. The reaction mixture contained: 10× RT buffer, 25× dNTP Mix (100 mM), 10× RT Random Primers, MultiScribe Reverse Transcriptase (50 U/µL), RNase Inhibitor and nuclease-free water. A total of 100 ng of total RNA was added to the reaction mixture. The following RT reaction conditions were used: 10 min at 25 °C, 120 min at 37 °C, 5 min at 85 °C, and cooling at 4 °C.
The reverse transcription for miRNA of 5 μL (10 ng) of total RNA in a 15-μL reaction was carried out using a TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA). The RT master mix contained: 25× dNTP Mix (100 mM), MultiScribe Reverse Transcriptase (50 U/µL), 10× RT buffer, RNase Inhibitor (20 U/µL), and nuclease-free water, as well as the specific RT primers (small RNA-specific RT primers) included in individual TaqMan MicroRNA Assays: hsa-miR-21-5p (UAGCUUAUCAGACUGAUGUUGA) and RNU6B (CGCAAGGATGACACGCAAATTCGTGAAGCGTTCCATATTTTT) as endogenous control (Applied Biosystems, Carlsbad, CA, USA). The following RT reaction conditions were used: 30 min at 16 °C, followed by 30 min at 42 °C; then, the samples were heated to 85 °C for 5 min and held at 4 °C.
The negative control was included in each RT reaction using water instead of RNA. The RT reaction was performed in a Personal Thermocycler (Eppendorf, Germany). RT products were stored at −20° C until further analysis.
Relative genes/miRNA expression was assessed by real-time polymerase chain reaction (qPCR) using a 7900HT Fast Real-Time PCR System apparatus (Applied Biosystems, Carlsbad, CA, USA). A total reaction mixture volume of 20 μL contained: cDNA (1–100 ng), KAPA PROBE FAST qPCR Master Mix (2X) ABI Prism (Kapa Biosystems Ltd., London, UK), RNase-free water, and a 20xTaqMan Gene Expression Assay for the following genes: TGF-β1 (Hs00998133_m1), SMAD3 (Hs00969210_m1), ILK (Hs00177914_m1) and GAPDH (Hs99999905_m1), which was selected as the reference gene in the qPCR reaction. Assays for the following miRNA: miR-21-5p and RNU6B (endogenous control) were used in the qPCR reaction.
The relative expression levels of the analyzed gene/miRNA were evaluated by the delta-delta CT method (TaqMan Relative Quantification Assay software, Applied Biosystems) and presented as RQ values relative to the GAPDH/RNU6B reference genes/miRNA, respectively. The following formula was used to determine the ΔΔCT value: ΔΔCT = ΔCT test sample − ΔCT calibrator sample. For the calibrator (RNA isolated from biological material from a healthy patient without endometriosis), the RQ (relative quantification) value was considered equal to 1. In the case of the test samples, increased expression was recognized when the RQ value was more than 1, and decreased expression was recognized when the RQ value was less than 1.
4.5. Statistical Analysis
The statistical analysis was carried out using the Statistica 13.1 program (StatSoft, Cracow, Poland). The Shapiro–Wilk test showed that the data distribution was not normal. In order to search for the statistical significance between the study groups, Mann–Whitney U-test and/or Kruskal–Wallis tests were performed, depending on the size of the groups. Neuman–Keuls’ multiple comparison test was used to identify possible significant differences in RQ values between the individual variables. The Spearman rank correlation coefficient was used to measure the direction and strength of the relationship for individual variables and the potential relations between RQ genes and miRNA. The level of correlation was fixed in the following categories: very strong (Rho ≥ 0.80), moderate (Rho = 0.60–0.79), fair (Rho = 0.30–0.59) and poor (Rho ≤ 0.29) [91]. For all statistical analyses, the level of statistical significance was assumed at p < 0.05. The RQ values for the studied genes/miRNA are presented as mean.
5. Conclusions
The eutopic endometrium of women with endometriosis showed higher expression of the mRNA TGF-β1, SMAD3 and ILK, and the level of miR-21 did not change compared to the endometrium of healthy participants. In contrast, lower mRNA levels of the studied genes and higher miR-21 levels were present in their endometrial implants. Our results may suggest a loss of endometrial epithelial phenotype defined by the differential expression of the TGF-β1, SMAD3 and ILK genes in eutopic and ectopic endometrium, and the likely underlying mechanism may involve EMT via the TGF-β1-SMAD3-ILK signaling pathway. We also identified miR-21 as a possible inhibitor of that signaling (TGF-β1-SMAD3-ILK) in the context of endometriosis. Although functional research on the effects of disturbances in the expression pattern of the studied genes and miRNA in the disease mechanisms, especially in the induction/progression of endometrial EMT, as well as a comprehensive insight into this system, will require analysis at the protein level, these data, including previous evidence, suggest a role of TGF-β1, SMAD3 and ILK, as well as the regulator miR-21, in the pathogenesis of endometriosis.
Author Contributions
Study conception and design, E.B.-L., M.M.-S. and A.Z.; material preparation and data collection, A.Z., M.M.-S. and S.J.; data analysis, M.M.-S.; first draft of the manuscript, A.Z.; revised manuscript, E.B.-L. and M.M.-S. All authors commented on subsequent versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Medical University of Lodz (Statute No. 503/1-013-02/503-11-001-19-00).
Institutional Review Board Statement
The study was approved by the Bioethics Committee of the Medical University of Lodz, Poland (resolution No. RNN/67/19/KE).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saunders, P.T.K.; Horne, A.W. Endometriosis: Etiology, Pathobiology, and Therapeutic Prospects. Cell 2021, 184, 2807–2824. [Google Scholar] [CrossRef] [PubMed]
- Kavoussi, S.K.; Lim, C.S.; Skinner, B.D.; Lebovic, D.I.; As-Sanie, S. New Paradigms in the Diagnosis and Management of Endometriosis. Curr. Opin. Obstet. Gynecol. 2016, 28, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Laganŕ, A.S.; Garzon, S.; Götte, M.; Viganň, P.; Franchi, M.; Ghezzi, F.; Martin, D.C. The Pathogenesis of Endometriosis: Molecular and Cell Biology Insights. Int. J. Mol. Sci. 2019, 20, 5615. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.P.; Hummelshoj, L.; World Endometriosis Society Montpellier Consortium. Consensus on Current Management of Endometriosis. Hum. Reprod. 2013, 28, 1552–1568. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C. Clinical Practice. Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Gaia-Oltean, A.I.; Braicu, C.; Gulei, D.; Ciortea, R.; Mihu, D.; Roman, H.; Irimie, A.; Berindan-Neagoe, I. Ovarian Endometriosis, a Precursor of Ovarian Cancer: Histological Aspects, Gene Expression and MicroRNA Alterations (Review). Exp. Ther. Med. 2021, 21, 243. [Google Scholar] [CrossRef]
- Moga, M.A.; Bălan, A.; Dimienescu, O.G.; Burtea, V.; Dragomir, R.M.; Anastasiu, C.V. Circulating MiRNAs as Biomarkers for Endometriosis and Endometriosis-Related Ovarian Cancer—An Overview. J. Clin. Med. 2019, 8, 735. [Google Scholar] [CrossRef]
- Busacca, M.; Vignali, M. Ovarian Endometriosis: From Pathogenesis to Surgical Treatment. Curr. Opin. Obstet. Gynecol. 2003, 15, 321–326. [Google Scholar] [CrossRef]
- Meng, Q.; Sun, W.; Jiang, J.; Fletcher, N.M.; Diamond, M.P.; Saed, G.M. Identification of Common Mechanisms between Endometriosis and Ovarian Cancer. J. Assist. Reprod. Genet. 2011, 28, 917–923. [Google Scholar] [CrossRef][Green Version]
- Keita, M.; Bessette, P.; Pelmus, M.; Ainmelk, Y.; Aris, A. Expression of Interleukin-1 (IL-1) Ligands System in the Most Common Endometriosis-Associated Ovarian Cancer Subtypes. J. Ovarian Res. 2010, 3, 3. [Google Scholar] [CrossRef]
- Braicu, O.-L.; Budisan, L.; Buiga, R.; Jurj, A.; Achimas-Cadariu, P.; Pop, L.A.; Braicu, C.; Irimie, A.; Berindan-Neagoe, I. MiRNA Expression Profiling in Formalin-Fixed Paraffin-Embedded Endometriosis and Ovarian Cancer Samples. OncoTargets Ther. 2017, 10, 4225–4238. [Google Scholar] [CrossRef]
- Cela, V.; Malacarne, E.; Obino, M.E.R.; Marzi, I.; Papini, F.; Vergine, F.; Pisacreta, E.; Zappelli, E.; Pietrobono, D.; Scarfň, G.; et al. Exploring Epithelial-Mesenchymal Transition Signals in Endometriosis Diagnosis and In Vitro Fertilization Outcomes. Biomedicines 2021, 9, 1681. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, J.; Li, T.; Guo, X.; Ding, S.; Che, X.; Zhu, L.; Peng, Y.; Xu, X.; Zou, G.; et al. Recepteur d’origine Nantais Contributes to the Development of Endometriosis via Promoting Epithelial-Mesenchymal Transition of a Endometrial Epithelial Cells. J. Cell. Mol. Med. 2021, 25, 1601–1612. [Google Scholar] [CrossRef]
- Lee, K.Y.; Bae, S.-C. TGF-Beta-Dependent Cell Growth Arrest and Apoptosis. J. Biochem. Mol. Biol. 2002, 35, 47–53. [Google Scholar] [CrossRef]
- Omwandho, C.O.A.; Konrad, L.; Halis, G.; Oehmke, F.; Tinneberg, H.-R. Role of TGF-Betas in Normal Human Endometrium and Endometriosis. Hum. Reprod. 2010, 25, 101–109. [Google Scholar] [CrossRef]
- Yang, Y.-M.; Yang, W.-X. Epithelial-to-Mesenchymal Transition in the Development of Endometriosis. Oncotarget 2017, 8, 41679–41689. [Google Scholar] [CrossRef]
- Liu, Z.; Yi, L.; Du, M.; Gong, G.; Zhu, Y. Overexpression of TGF-β Enhances the Migration and Invasive Ability of Ectopic Endometrial Cells via ERK/MAPK Signaling Pathway. Exp. Ther. Med. 2019, 17, 4457–4464. [Google Scholar] [CrossRef]
- Young, V.J.; Brown, J.K.; Saunders, P.T.K.; Duncan, W.C.; Horne, A.W. The Peritoneum Is Both a Source and Target of TGF-β in Women with Endometriosis. PLoS ONE 2014, 9, e106773. [Google Scholar] [CrossRef]
- Yan, D.; Liu, X.; Xu, H.; Guo, S.-W. Mesothelial Cells Participate in Endometriosis Fibrogenesis Through Platelet-Induced Mesothelial-Mesenchymal Transition. J. Clin. Endocrinol. Metab. 2020, 105, dgaa550. [Google Scholar] [CrossRef]
- Zhang, Q.; Duan, J.; Liu, X.; Guo, S.-W. Platelets Drive Smooth Muscle Metaplasia and Fibrogenesis in Endometriosis through Epithelial-Mesenchymal Transition and Fibroblast-to-Myofibroblast Transdifferentiation. Mol. Cell. Endocrinol. 2016, 428, 1–16. [Google Scholar] [CrossRef]
- Zhang, Q.; Duan, J.; Olson, M.; Fazleabas, A.; Guo, S.-W. Cellular Changes Consistent With Epithelial-Mesenchymal Transition and Fibroblast-to-Myofibroblast Transdifferentiation in the Progression of Experimental Endometriosis in Baboons. Reprod. Sci. 2016, 23, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, C.; Zhou, D.; Zhang, L. TGF-β/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2016, 64, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.B.; Zhou, F.; Zhu, H.Y.; Huang, D.; Jin, X.Y.; Li, C.; Dai, Y.; Pan, Y.B.; Zhang, S.Y. Transforming Growth Factor Beta1 from Endometriomas Promotes Fibrosis in Surrounding Ovarian Tissues via Smad2/3 Signaling. Biol. Reprod. 2017, 97, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, Y.; Yamoto, M.; Minami, S.; Umesaki, N. Immunohistochemical Localization of Inhibin and Activin Subunits, Activin Receptors and Smads in Ovarian Endometriosis. Int. J. Mol. Med. 2010, 25, 17–23. [Google Scholar] [CrossRef]
- Zhang, Y.E. Non-Smad Pathways in TGF-Beta Signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef]
- Li, Y.; Tan, X.; Dai, C.; Stolz, D.B.; Wang, D.; Liu, Y. Inhibition of Integrin-Linked Kinase Attenuates Renal Interstitial Fibrosis. J. Am. Soc. Nephrol. 2009, 20, 1907–1918. [Google Scholar] [CrossRef]
- Serrano, I.; McDonald, P.C.; Lock, F.E.; Dedhar, S. Role of the Integrin-Linked Kinase (ILK)/Rictor Complex in TGFβ-1-Induced Epithelial-Mesenchymal Transition (EMT). Oncogene 2013, 32, 50–60. [Google Scholar] [CrossRef]
- Zheng, C.-C.; Hu, H.-F.; Hong, P.; Zhang, Q.-H.; Xu, W.W.; He, Q.-Y.; Li, B. Significance of Integrin-Linked Kinase (ILK) in Tumorigenesis and Its Potential Implication as a Biomarker and Therapeutic Target for Human Cancer. Am. J. Cancer Res. 2019, 9, 186–197. [Google Scholar]
- McDonald, P.C.; Fielding, A.B.; Dedhar, S. Integrin-Linked Kinase--Essential Roles in Physiology and Cancer Biology. J. Cell Sci. 2008, 121, 3121–3132. [Google Scholar] [CrossRef]
- Zheng, Q.-M.; Chen, X.-Y.; Bao, Q.-F.; Yu, J.; Chen, L.-H. ILK Enhances Migration and Invasion Abilities of Human Endometrial Stromal Cells by Facilitating the Epithelial-Mesenchymal Transition. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2018, 34, 1091–1096. [Google Scholar] [CrossRef]
- Zheng, Q.-M.; Lu, J.-J.; Zhao, J.; Wei, X.; Wang, L.; Liu, P.-S. Periostin Facilitates the Epithelial-Mesenchymal Transition of Endometrial Epithelial Cells through ILK-Akt Signaling Pathway. BioMed Res. Int. 2016, 2016, 9842619. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, J.; Li, W.; Chen, X.; Chen, S.; Chen, L. Emodin Reverses the Epithelial-Mesenchymal Transition of Human Endometrial Stromal Cells by Inhibiting ILK/GSK-3β Pathway. Drug Des. Dev. Ther. 2020, 14, 3663–3672. [Google Scholar] [CrossRef]
- Yen, C.-F.; Wang, H.-S.; Lee, C.-L.; Liao, S.-K. Roles of Integrin-Linked Kinase in Cell Signaling and Its Perspectives as a Therapeutic Target. Gynecol. Minim. Invasive Ther. 2014, 3, 67–72. [Google Scholar] [CrossRef]
- Pfeffer, S.R.; Yang, C.H.; Pfeffer, L.M. The Role of miR-21 in Cancer. Drug Dev. Res. 2015, 76, 270–277. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; He, Y. MicroRNA-21-5p promotes epithelial to mesenchymal transition by targeting SRY-box 17 in endometrial cancer. Oncol. Rep. 2020, 43, 1897–1905. [Google Scholar] [CrossRef]
- Zhang, Q.-C. METTL3 is aberrantly expressed in endometriosis and suppresses proliferation, invasion, and migration of endometrial stromal cells. Kaohsiung J. Med. Sci. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Song, M.; Zhao, G.; Sun, H.; Yao, S.; Zhou, Z.; Jiang, P.; Wu, Q.; Zhu, H.; Wang, H.; Dai, C.; et al. circPTPN12/miR-21-5 p/∆Np63α pathway contributes to human endometrial fibrosis. Elife 2021, 10, e65735. [Google Scholar] [CrossRef]
- Bulletti, C.; Coccia, M.E.; Battistoni, S.; Borini, A. Endometriosis and Infertility. J. Assist. Reprod. Genet. 2010, 27, 441–447. [Google Scholar] [CrossRef]
- Kimber-Trojnar, Ż.; Pilszyk, A.; Niebrzydowska, M.; Pilszyk, Z.; Ruszała, M.; Leszczyńska-Gorzelak, B. The Potential of Non-Invasive Biomarkers for Early Diagnosis of Asymptomatic Patients with Endometriosis. J. Clin. Med. 2021, 10, 2762. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D.C. Pathogenesis of Endometriosis: The Genetic/Epigenetic Theory. Fertil. Steril. 2019, 111, 327–340. [Google Scholar] [CrossRef]
- Giannini, A.; Bogani, G.; Vizza, E.; Chiantera, V.; Laganŕ, A.S.; Muzii, L.; Salerno, M.G.; Caserta, D.; D’Oria, O. Advances on Prevention and Screening of Gynecologic Tumors: Are We Stepping Forward? Healthcare 2022, 10, 1605. [Google Scholar] [CrossRef] [PubMed]
- Nothnick, W.B. MicroRNAs and Endometriosis: Distinguishing Drivers from Passengers in Disease Pathogenesis. Semin. Reprod. Med. 2017, 35, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Young, V.J.; Ahmad, S.F.; Duncan, W.C.; Horne, A.W. The Role of TGF-β in the Pathophysiology of Peritoneal Endometriosis. Hum. Reprod. Update 2017, 23, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Massagué, J. Mechanisms of TGF-Beta Signaling from Cell Membrane to the Nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Loboda, A.; Sobczak, M.; Jozkowicz, A.; Dulak, J. TGF-Β1/Smads and MiR-21 in Renal Fibrosis and Inflammation. Mediat. Inflamm. 2016, 2016, 8319283. [Google Scholar] [CrossRef]
- Qi, F.; Cai, P.; Liu, X.; Peng, M.; Si, G. Adenovirus-Mediated P311 Inhibits TGF-Β1-Induced Epithelial-Mesenchymal Transition in NRK-52E Cells via TGF-Β1-Smad-ILK Pathway. Biosci. Trends 2015, 9, 299–306. [Google Scholar] [CrossRef][Green Version]
- Benagiano, G.; Brosens, I.; Habiba, M. Structural and Molecular Features of the Endomyometrium in Endometriosis and Adenomyosis. Hum. Reprod. Update 2014, 20, 386–402. [Google Scholar] [CrossRef]
- Correa, L.F.; Zheng, Y.; Delaney, A.A.; Khan, Z.; Shenoy, C.C.; Daftary, G.S. TGF-β Induces Endometriotic Progression via a Noncanonical, KLF11-Mediated Mechanism. Endocrinology 2016, 157, 3332–3343. [Google Scholar] [CrossRef]
- Lin, X.; Dai, Y.; Xu, W.; Shi, L.; Jin, X.; Li, C.; Zhou, F.; Pan, Y.; Zhang, Y.; Lin, X.; et al. Hypoxia Promotes Ectopic Adhesion Ability of Endometrial Stromal Cells via TGF-Β1/Smad Signaling in Endometriosis. Endocrinology 2018, 159, 1630–1641. [Google Scholar] [CrossRef]
- Gaide Chevronnay, H.P.; Cornet, P.B.; Delvaux, D.; Lemoine, P.; Courtoy, P.J.; Henriet, P.; Marbaix, E. Opposite Regulation of Transforming Growth Factors-Beta2 and -Beta3 Expression in the Human Endometrium. Endocrinology 2008, 149, 1015–1025. [Google Scholar] [CrossRef]
- Marshburn, P.B.; Arici, A.M.; Casey, M.L. Expression of Transforming Growth Factor-Beta 1 Messenger Ribonucleic Acid and the Modulation of Deoxyribonucleic Acid Synthesis by Transforming Growth Factor-Beta 1 in Human Endometrial Cells. Am. J. Obstet. Gynecol. 1994, 170, 1152–1158. [Google Scholar] [CrossRef]
- Nasu, K.; Nishida, M.; Matsumoto, H.; Bing, S.; Inoue, C.; Kawano, Y.; Miyakawa, I. Regulation of Proliferation, Motility, and Contractivity of Cultured Human Endometrial Stromal Cells by Transforming Growth Factor-Beta Isoforms. Fertil. Steril. 2005, 84 (Suppl. 2), 1114–1123. [Google Scholar] [CrossRef]
- Goteri, G.; Altobelli, E.; Tossetta, G.; Zizzi, A.; Avellini, C.; Licini, C.; Lorenzi, T.; Castellucci, M.; Ciavattini, A.; Marzioni, D. High Temperature Requirement A1, Transforming Growth Factor Beta1, PhosphoSmad2 and Ki67 in Eutopic and Ectopic Endometrium of Women with Endometriosis. Eur. J. Histochem. 2015, 59, 2570. [Google Scholar] [CrossRef]
- Esfandiari, F.; Heidari Khoei, H.; Saber, M.; Favaedi, R.; Piryaei, A.; Moini, A.; Shahhoseini, M.; Ramezanali, F.; Ghaffari, F.; Baharvand, H. Disturbed Progesterone Signalling in an Advanced Preclinical Model of Endometriosis. Reprod. Biomed. Online 2021, 43, 139–147. [Google Scholar] [CrossRef]
- Yu, Y.-X.; Xiu, Y.-L.; Chen, X.; Li, Y.-L. Transforming Growth Factor-Beta 1 Involved in the Pathogenesis of Endometriosis through Regulating Expression of Vascular Endothelial Growth Factor under Hypoxia. Chin. Med. J. 2017, 130, 950–956. [Google Scholar] [CrossRef]
- Komiyama, S.; Aoki, D.; Komiyama, M.; Nozawa, S. Local Activation of TGF-Beta1 at Endometriosis Sites. J. Reprod. Med. 2007, 52, 306–312. [Google Scholar]
- Johnson, M.C.; Torres, M.; Alves, A.; Bacallao, K.; Fuentes, A.; Vega, M.; Boric, M.A. Augmented Cell Survival in Eutopic Endometrium from Women with Endometriosis: Expression of c-Myc, TGF-Beta1 and Bax Genes. Reprod. Biol. Endocrinol. 2005, 3, 45. [Google Scholar] [CrossRef]
- Ping, S.; Ma, C.; Liu, P.; Yang, L.; Yang, X.; Wu, Q.; Zhao, X.; Gong, B. Molecular Mechanisms Underlying Endometriosis Pathogenesis Revealed by Bioinformatics Analysis of Microarray Data. Arch. Gynecol. Obstet. 2016, 293, 797–804. [Google Scholar] [CrossRef]
- De Conto, E.; Matte, U.; Cunha-Filho, J.S. BMP-6 and SMAD4 Gene Expression Is Altered in Cumulus Cells from Women with Endometriosis-Associated Infertility. Acta Obstet. Gynecol. Scand. 2021, 100, 868–875. [Google Scholar] [CrossRef]
- Li, C.; Leng, J.; Li, M.; Shi, J.; Jia, S.; Lang, J. Expressions and roles of TGFβ/Smad signal pathway in peritoneum of endometriosis. Zhonghua Fu Chan Ke Za Zhi 2011, 46, 826–830. [Google Scholar]
- Cruz, C.D.; Del Puerto, H.L.; Rocha, A.L.L.; Cavallo, I.K.; Clarizia, A.D.; Petraglia, F.; Reis, F.M. Expression of Nodal, Cripto, SMAD3, Phosphorylated SMAD3, and SMAD4 in the Proliferative Endometrium of Women with Endometriosis. Reprod. Sci. 2015, 22, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Eritja, N.; Navaridas, R.; Ruiz-Mitjana, A.; Vidal-Sabanés, M.; Egea, J.; Encinas, M.; Matias-Guiu, X.; Dolcet, X. Endometrial PTEN Deficiency Leads to SMAD2/3 Nuclear Translocation. Cancers 2021, 13, 4990. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-C.; Li, C.-J.; Yiang, G.-T.; Tsai, A.P.-Y.; Wu, M.-Y. Epithelial to Mesenchymal Transition and Cell Biology of Molecular Regulation in Endometrial Carcinogenesis. J. Clin. Med. 2019, 8, 439. [Google Scholar] [CrossRef] [PubMed]
- Martucci, N.; Michalopoulos, G.K.; Mars, W.M. Integrin Linked Kinase (ILK) and Its Role in Liver Pathobiology. Gene Expr. 2021, 20, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yin, H.; Wang, R.; Wu, D.; Sun, W.; Liu, B.; Su, Q. Overexpression of Integrin-Linked Kinase (ILK) Promotes Migration and Invasion of Colorectal Cancer Cells by Inducing Epithelial-Mesenchymal Transition via NF-ΚB Signaling. Acta Histochem. 2014, 116, 527–533. [Google Scholar] [CrossRef]
- Lin, S.-W.; Ke, F.-C.; Hsiao, P.-W.; Lee, P.-P.; Lee, M.-T.; Hwang, J.-J. Critical Involvement of ILK in TGFbeta1-Stimulated Invasion/Migration of Human Ovarian Cancer Cells Is Associated with Urokinase Plasminogen Activator System. Exp. Cell Res. 2007, 313, 602–613. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Zhang, X.; Li, J.; Han, B.; Liu, S.; Wang, L.; Ling, Y.; Mao, S.; Wang, X. Overexpression of Integrin-Linked Kinase Correlates with Malignant Phenotype in Non-Small Cell Lung Cancer and Promotes Lung Cancer Cell Invasion and Migration via Regulating Epithelial-Mesenchymal Transition (EMT)-Related Genes. Acta Histochem. 2013, 115, 128–136. [Google Scholar] [CrossRef]
- Renner, S.P.; Strissel, P.L.; Beckmann, M.W.; Lermann, J.; Burghaus, S.; Hackl, J.; Fasching, P.A.; Strick, R. Inhibition of Adhesion, Proliferation, and Invasion of Primary Endometriosis and Endometrial Stromal and Ovarian Carcinoma Cells by a Nonhyaluronan Adhesion Barrier Gel. BioMed Res. Int. 2015, 2015, 450468. [Google Scholar] [CrossRef]
- Zheng, Q.; Xu, Y.; Lu, J.; Zhao, J.; Wei, X.; Liu, P. Emodin Inhibits Migration and Invasion of Human Endometrial Stromal Cells by Facilitating the Mesenchymal-Epithelial Transition Through Targeting ILK. Reprod. Sci. 2016, 23, 1526–1535. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, Q.; Zhang, Z.; Zhang, X.; Liu, R.; Liu, P. Periostin Enhances Migration, Invasion, and Adhesion of Human Endometrial Stromal Cells Through Integrin-Linked Kinase 1/Akt Signaling Pathway. Reprod. Sci. 2015, 22, 1098–1106. [Google Scholar] [CrossRef]
- Zhou, W.; Peng, Z.; Zhang, C.; Liu, S.; Zhang, Y. ILK-Induced Epithelial-Mesenchymal Transition Promotes the Invasive Phenotype in Adenomyosis. Biochem. Biophys. Res. Commun. 2018, 497, 950–956. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Dai, C.; Wu, C.; Liu, Y. Role for Integrin-Linked Kinase in Mediating Tubular Epithelial to Mesenchymal Transition and Renal Interstitial Fibrogenesis. J. Clin. Investig. 2003, 112, 503–516. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yamada, Y.; Morioka, S.; Niiro, E.; Shigemitsu, A.; Ito, F. Mechanism of Pain Generation for Endometriosis-Associated Pelvic Pain. Arch. Gynecol. Obstet. 2014, 289, 13–21. [Google Scholar] [CrossRef]
- Buchman, V.L.; Sporn, M.; Davies, A.M. Role of Transforming Growth Factor-Beta Isoforms in Regulating the Expression of Nerve Growth Factor and Neurotrophin-3 MRNA Levels in Embryonic Cutaneous Cells at Different Stages of Development. Dev. Camb. Engl. 1994, 120, 1621–1629. [Google Scholar] [CrossRef]
- Bjorkman, S.; Taylor, H.S. MicroRNAs in Endometriosis: Biological Function and Emerging Biomarker Candidates. Biol. Reprod. 2019, 100, 1135–1146. [Google Scholar] [CrossRef]
- Zubrzycka, A.; Migdalska-Sęk, M.; Jędrzejczyk, S.; Brzeziańska-Lasota, E. Circulating MiRNAs Related to Epithelial-Mesenchymal Transitions (EMT) as the New Molecular Markers in Endometriosis. Curr. Issues Mol. Biol. 2021, 43, 64. [Google Scholar] [CrossRef]
- Ramón, L.A.; Braza-Boďls, A.; Gilabert-Estellés, J.; Gilabert, J.; Espańa, F.; Chirivella, M.; Estellés, A. MicroRNAs Expression in Endometriosis and Their Relation to Angiogenic Factors. Hum. Reprod. 2011, 26, 1082–1090. [Google Scholar] [CrossRef]
- Haikalis, M.E.; Wessels, J.M.; Leyland, N.A.; Agarwal, S.K.; Foster, W.G. MicroRNA Expression Pattern Differs Depending on Endometriosis Lesion Type. Biol. Reprod. 2018, 98, 623–633. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.K.; Kim, M.K.; Lee, J.H.; Yun, B.H.; Park, J.H.; Seo, S.K.; Cho, S.; Choi, Y.S. Saponin Extracts Induced Apoptosis of Endometrial Cells From Women With Endometriosis Through Modulation of MiR-21-5p. Reprod. Sci. 2018, 25, 292–301. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Y.; Li, W.; Tian, X.; Cui, H.; Zhang, Q. Identification of Small-Molecule Ligands That Bind to MiR-21 as Potential Therapeutics for Endometriosis by Screening ZINC Database and in-Vitro Assays. Gene 2018, 662, 46–53. [Google Scholar] [CrossRef]
- Braza-Boďls, A.; Salloum-Asfar, S.; Marí-Alexandre, J.; Arroyo, A.B.; González-Conejero, R.; Barceló-Molina, M.; García-Oms, J.; Vicente, V.; Estellés, A.; Gilabert-Estellés, J.; et al. Peritoneal Fluid Modifies the MicroRNA Expression Profile in Endometrial and Endometriotic Cells from Women with Endometriosis. Hum. Reprod. 2015, 30, 2292–2302. [Google Scholar] [CrossRef] [PubMed]
- Teague, E.M.C.O.; Print, C.G.; Hull, M.L. The Role of MicroRNAs in Endometriosis and Associated Reproductive Conditions. Hum. Reprod. Update 2010, 16, 142–165. [Google Scholar] [CrossRef] [PubMed]
- Aghajanova, L.; Giudice, L.C. Molecular Evidence for Differences in Endometrium in Severe versus Mild Endometriosis. Reprod. Sci. 2011, 18, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xiao, J.; Lin, H.; Bai, Y.; Luo, X.; Wang, Z.; Yang, B. A Single Anti-MicroRNA Antisense Oligodeoxyribonucleotide (AMO) Targeting Multiple MicroRNAs Offers an Improved Approach for MicroRNA Interference. Nucleic Acids Res. 2009, 37, e24. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, L.; Shi, C.; Sun, H.; Wang, J.; Li, R.; Zou, Z.; Ran, X.; Su, Y. TGF-β-Induced MiR-21 Negatively Regulates the Antiproliferative Activity but Has No Effect on EMT of TGF-β in HaCaT Cells. Int. J. Biochem. Cell Biol. 2012, 44, 366–376. [Google Scholar] [CrossRef]
- Abramiuk, M.; Grywalska, E.; Małkowska, P.; Sierawska, O.; Hrynkiewicz, R.; Niedźwiedzka-Rystwej, P. The Role of the Immune System in the Development of Endometriosis. Cells 2022, 11, 2028. [Google Scholar] [CrossRef]
- Han, G.; Li, F.; Singh, T.P.; Wolf, P.; Wang, X.-J. The Pro-Inflammatory Role of TGFβ1: A Paradox? Int. J. Biol. Sci. 2012, 8, 228–235. [Google Scholar] [CrossRef]
- Hanada, T.; Tsuji, S.; Nakayama, M.; Wakinoue, S.; Kasahara, K.; Kimura, F.; Mori, T.; Ogasawara, K.; Murakami, T. Suppressive Regulatory T Cells and Latent Transforming Growth Factor-β-Expressing Macrophages Are Altered in the Peritoneal Fluid of Patients with Endometriosis. Reprod. Biol. Endocrinol. 2018, 16, 9. [Google Scholar] [CrossRef]
- Au, H.-K.; Chang, J.-H.; Wu, Y.-C.; Kuo, Y.-C.; Chen, Y.-H.; Lee, W.-C.; Chang, T.-S.; Lan, P.-C.; Kuo, H.-C.; Lee, K.-L.; et al. TGF-ΒI Regulates Cell Migration through Pluripotent Transcription Factor OCT4 in Endometriosis. PLoS ONE 2015, 10, e0145256. [Google Scholar] [CrossRef]
- Canis, M.; Donnez, J.G.; Guzick, D.S.; Halme, J.K.; Rock, J.A.; Schenken, R.S.; Vernon, M.W. Revised American Society for Reproductive Medicine Classification of Endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [Google Scholar] [CrossRef]
- Chan, Y.H. Biostatistics 104: Correlational Analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).