Consistency and Variation in Doublecortin and Ki67 Antigen Detection in the Brain Tissue of Different Mammals, including Humans
Abstract
1. Introduction
| Host and Source | References | Animal Species (Common Name) |
|---|---|---|
| Santa Cruz 1 (goat) | Fasemore et al., 2018 [37] | Galago; Lemur; Potto |
| Chawana et al., 2020 [38] | Egyptian fruit bat | |
| La Rosa et al., 2020 [23] | 12 mammals (from mouse to chimpanzee) | |
| Kirby et al., 2012 [39] | Rat | |
| Fudge et al., 2012 [40] | Crab-eating macaque; southern pig-tailed macaque | |
| Zhang et al., 2009 [41] | Rhesus macaque | |
| Flor-Garcìa et al., 2020 [42] | Human | |
| Sorrells et al., 2018 [24] | Human; rhesus macaque | |
| Tobin et al., 2019 [43] | Human | |
| Boekhoorn et al., 2006 [44] | Human | |
| Liu et al., 2020 [45] | Rhesus macaque | |
| Marlatt et al., 2011 [46] | Common marmoset | |
| Parolisi et al., 2017 [32] | Dolphin | |
| La Rosa et al., 2018 [47] | Dolphin; sheep | |
| Moreno-Jiménez et al., 2019 [25] | Human | |
| Jin et al., 2006 [48] | Human | |
| Crews et al., 2010 [49] | Human; mouse | |
| Knoth et al., 2010 [50] | Human | |
| Wang et al., 2011 [51] | Human; rhesus macaque | |
| Gomez-Nicola et al., 2014 [52] | Human; mice | |
| Ekonomou et al., 2015 [53] | Human | |
| Dennis et al., 2016 [54] | Human | |
| Galàn et al., 2017 [55] | Human | |
| Liu et al., 2008 [56] | Human | |
| Ponti et al., 2006 [57] | Rabbit | |
| Kunze et al., 2015 [58] | Mouse | |
| Cai et al., 2009 [59] | Human; rhesus macaque; cat | |
| Verwer et al., 2007 [60] | Human | |
| Bloch et al., 2011 [61] | Human; cynomolgus monkey; African green monkey | |
| Li et al., 2022 [62] | Human | |
| Abcam (rabbit) | Piumatti et al., 2018 [7] | Sheep |
| Jhaveri et al., 2018 [63] | Mouse | |
| Flor-Garcìa et al., 2020 [42] | Human | |
| Sorrells et al., 2018 [24] | Human; rhesus macaque | |
| Cipriani et al., 2018 [64] | Human | |
| Tobin et al., 2019 [43] | Human | |
| Liu et al., 2020 [45] | Rhesus macaque | |
| Parolisi et al., 2017 [32] | Dolphin | |
| La Rosa et al., 2018 [47] | Dolphin; sheep | |
| Wang et al., 2011 [51] | Human; rhesus macaque | |
| Nogueira et al., 2014 [65] | Human | |
| Perry et al., 2012 [66] | Human | |
| Bloch et al., 2011 [61] | Human; cynomolgus monkey; African green monkey | |
| Cai et al., 2009 [59] | Human; rhesus macaque; cat | |
| Millipore (guinea pig) | Akter et al., 2020 [67] | Common marmoset |
| Benedetti et al., 2019 [68] | Mouse | |
| Jhaveri et al., 2018 [63] | Mouse | |
| Flor-Garcìa et al., 2020 [42] | Human | |
| Sorrells et al., 2018 [24] | Human; rhesus macaque | |
| Sorrells et al., 2019 [9] | Human | |
| Cipriani et al., 2018 [64] | Human | |
| Gomez-Nicola et al., 2014 [52] | Human; mice | |
| Paredes et al., 2016 [69] | Human | |
| Kunze et al., 2015 [58] | Mouse | |
| Bloch et al., 2011 [61] | Human; cynomolgus monkey; African green monkey | |
| Alderman et al., 2022 [17] | Human; mouse | |
| Cell Signalling Technology (rabbit) | Sorrells et al., 2018 [24] | Human; rhesus macaque |
| Liu et al., 2008 [56] | Human | |
| Maheu et al., 2015 [70] | Human | |
| Paredes et al., 2016 [69] | Human | |
| Sorrells et al., 2019 [9] | Human | |
| Martì-Mengual et al., 2013 [71] | Human; squirrel monkey; cat | |
| Alderman et al., 2022 [17] | Human; mouse | |
| Coviello et al., 2022 [72] | Human |
| Host and Source | References | Animal Species |
|---|---|---|
| Leica-Novocastra 1 (rabbit) | Fasemore et al., 2018 [37] | Galago; lemur; potto |
| Akter et al., 2020 [67] | Common marmoset | |
| Chawana et al., 2020 [38] | Egyptian fruit bat | |
| La Rosa et al., 2020 [23] | 12 mammals (from mouse to chimpanzee) | |
| Jhaveri et al., 2018 [63] | Mouse | |
| Sorrells et al., 2018 [24] | Human; rhesus macaque | |
| Sorrells et al., 2019 [9] | Human | |
| Tobin et al., 2019 [43] | Human | |
| Boekhoorn et al., 2006 [44] | Human | |
| Quiñones-Hinojosa et al., 2006 [73] | Human | |
| Fahrner et al., 2007 [74] | Human | |
| Parolisi et al., 2017 [32] | Dolphin | |
| La Rosa et al., 2018 [47] | Dolphin; sheep | |
| Martì-Mengual et al., 2013 [71] | Human; squirrel monkey; cat | |
| BD Pharmingen (mouse) | La Rosa et al., 2020 [23] | 12 mammals (from mouse to chimpanzee) |
| Sorrells et al., 2018 [24] | Human; rhesus macaque | |
| Sorrells et al., 2019 [9] | Human | |
| La Rosa et al., 2018 [47] | Dolphin; sheep | |
| Abcam (rabbit) | Gomez-Nicola et al., 2014 [52] | Human; mice |
| Allen et al., 2016 [75] | Human | |
| Cipriani et al., 2018 [64] | Human |
2. Results
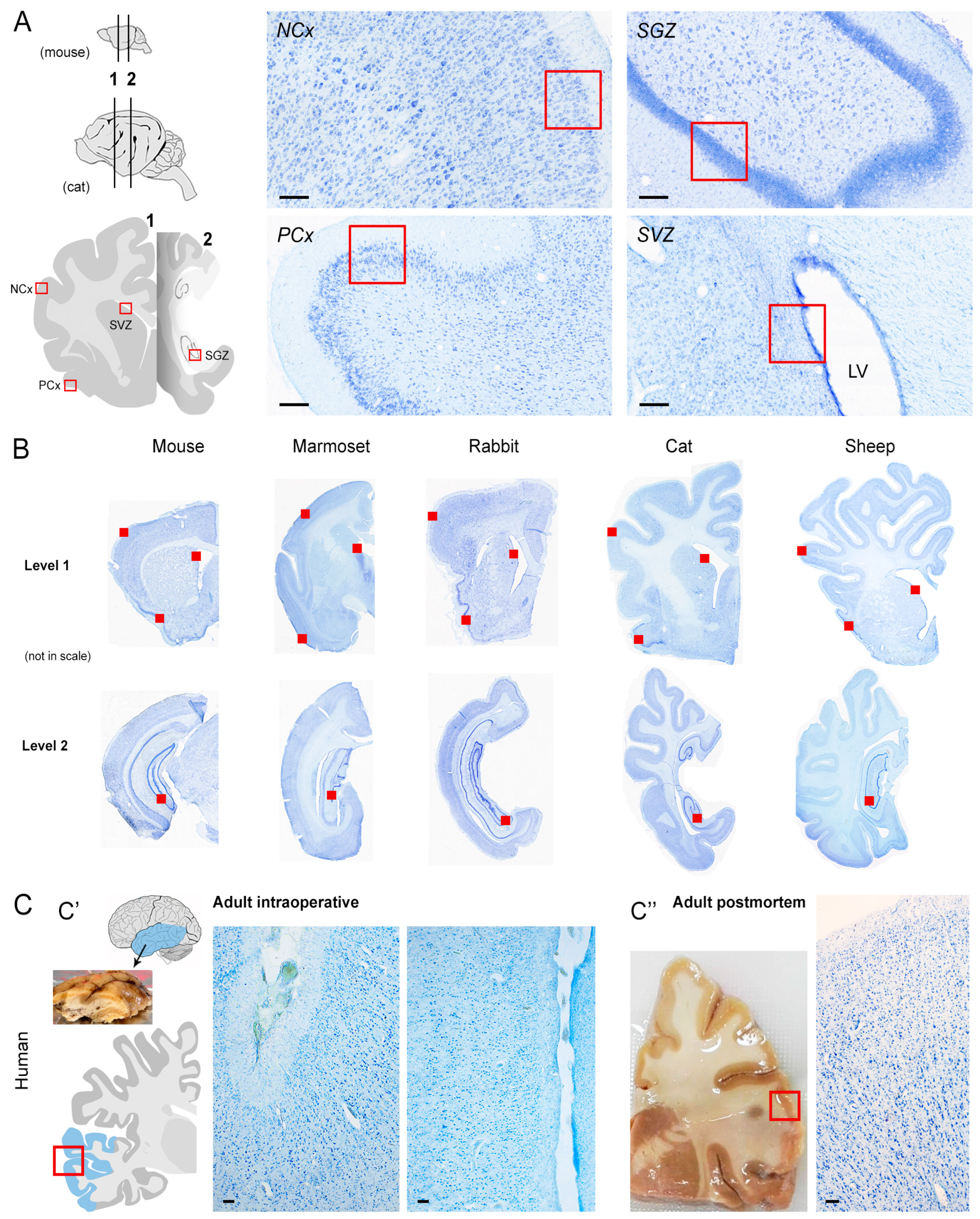
2.1. Comparative Immunostaining in the Brain of Five Mammals
| (a) | |||||||
| Citrate Treatment (5 min) | |||||||
| Antibodies | Regions | Mouse | Marmoset | Rabbit | Cat | Sheep (perfusion) | Sheep (immersion) |
| Santa Cruz (goat) | pc | ● | |||||
| nc | ● | ||||||
| svz | ● | ||||||
| sgz | * | ● | |||||
| Cell Signalling (rabbit) | pc | * | * | * | * | ||
| nc | * | * | * | * | |||
| svz | * | ||||||
| sgz | * | * | * | * | * | ||
| Abcam (rabbit) | pc | * | ● | * | * | * | * |
| nc | ● | * | * | * | |||
| svz | ● | ||||||
| sgz | * | ● | * | * | * | ||
| Santa Cruz (mouse) | pc | * | * | * | ● | ||
| nc | * | * | * | ● | |||
| svz | * | ● | |||||
| sgz | * | * | ● | ||||
| Millipore (guinea pig) | pc | * | * | * | * | ||
| nc | * | * | * | ||||
| svz | * | * | |||||
| sgz | * | * | * | * | * | ||
| No Citrate | |||||||
| Antibodies | Regions | Mouse | Marmoset | Rabbit | Cat | Sheep (perfusion) | Sheep (immersion) |
| Santa Cruz (goat) | pc | * | * | ● | |||
| nc | * | * | * | ● | |||
| svz | * | ● | |||||
| sgz | * | * | * | ● | |||
| Cell Signalling (rabbit) | pc | * | * | * | ● | ||
| nc | * | * | * | * | ● | ||
| svz | * | * | ● | ||||
| sgz | * | * | * | * | ● | ||
| Abcam (rabbit) | pc | * | ● | * | * | * | |
| nc | ● | * | * | * | |||
| svz | ● | ||||||
| sgz | ● | * | * | * | * | ||
| Santa Cruz (mouse) | pc | * | * | * | * | ||
| nc | * | * | * | ● | |||
| svz | * | * | * | * | |||
| sgz | * | * | * | * | |||
| Millipore (guinea pig) | pc | * | * | * | * | * | |
| nc | * | * | * | * | |||
| svz | * | * | * | * | |||
| sgz | * | ● | ● | * | * | ||
| (b) | |||||||
| Antibodies | Regions | Mouse | Marmoset | Rabbit | Cat | Sheep (perfusion) | Sheep (immersion) |
| Santa Cruz (goat) | pc | (5′) | (5′) | ||||
| nc | (5′) | (5′) | (5′) | ||||
| svz | (5′) | ||||||
| sgz | (5′) | (5′) | |||||
| Cell Signalling (rabbit) | pc | only 5′ C | |||||
| nc | only 5′ C | ||||||
| svz | (5′) | (5′) | only 5′ C | ||||
| sgz | (5′) | only 5′ C | |||||
| Abcam (rabbit) | pc | without C | without C | ||||
| nc | without C | ||||||
| svz | |||||||
| sgz | without C | (5′) | |||||
| Santa Cruz (mouse) | pc | only 5′ C | only 5′ C | only 5′ C | only 5′ C | ||
| nc | only 5′ C | only 5′ C | |||||
| svz | only 5′ C | (5′) | (5′) | only 5′ C | |||
| sgz | (5′) | only 5′ C | only 5′ C | ||||
| Millipore (guinea pig) | pc | (5′) | |||||
| nc | |||||||
| svz | (5′) | (5′) | without C | ||||
| sgz | without C | ||||||
 Absence of staining due to real interspecies difference (e.g., no cell population containg DCX in the mouse neocortex);
Absence of staining due to real interspecies difference (e.g., no cell population containg DCX in the mouse neocortex);  Clear and clean immunoreaction;
Clear and clean immunoreaction;  Immunoreaction with background;
Immunoreaction with background;  Immunoreaction with non-specific staining;
Immunoreaction with non-specific staining;  No signal with background;
No signal with background;  No signal with non-specific staining;
No signal with non-specific staining;  No signal; Subtable (b): MERGE of results obtained with and without 5′ Citrate:
No signal; Subtable (b): MERGE of results obtained with and without 5′ Citrate:  Same results;
Same results;  Different results; (5′) better with Citrate; only (5′) C signal only with Citrate; without C signal only without Citrate.
Different results; (5′) better with Citrate; only (5′) C signal only with Citrate; without C signal only without Citrate.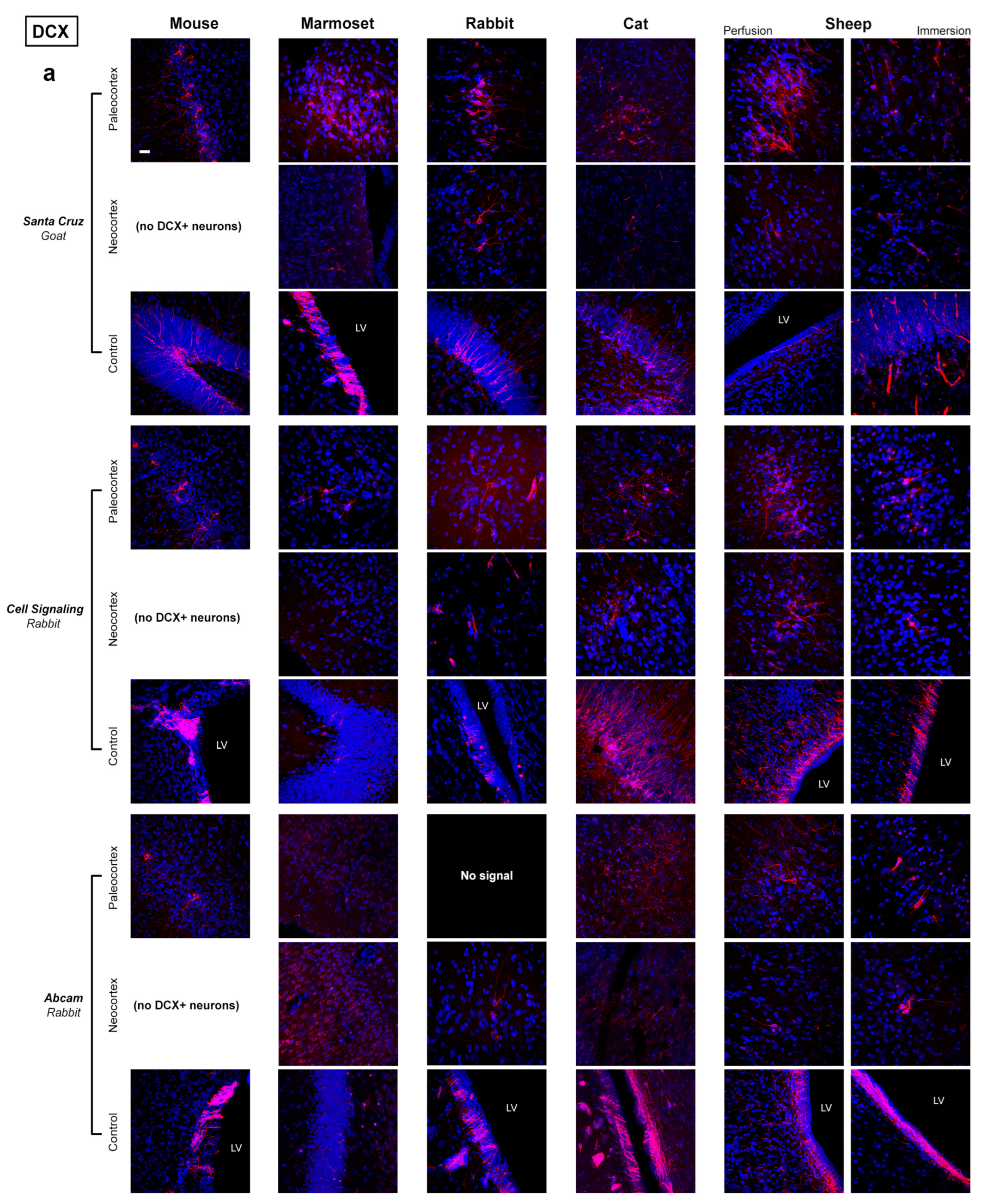

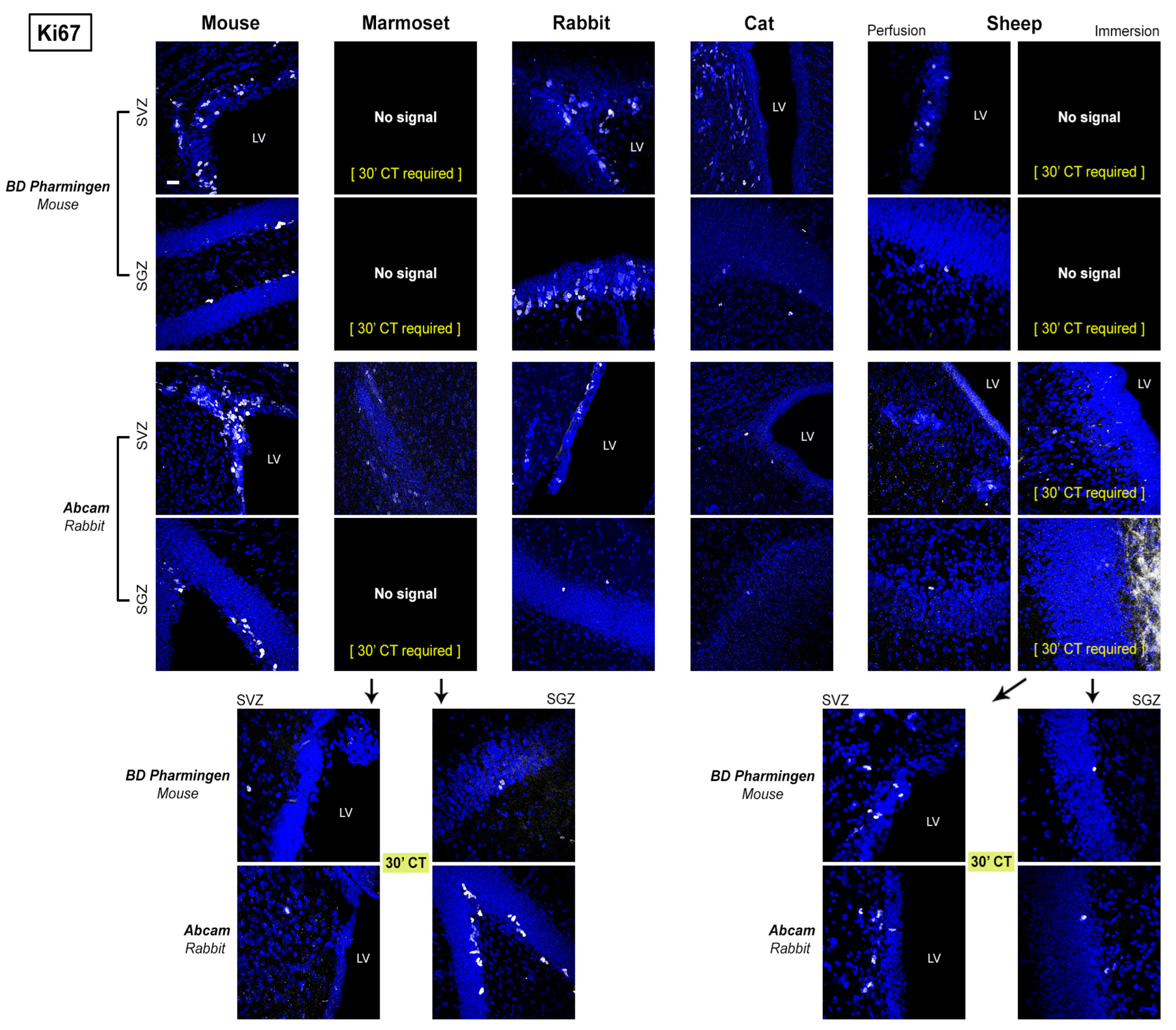
2.2. Regional Differences
| (a) | |||||||
| Citrate Treatment (5 min) | |||||||
| Antibodies | Regions | Mouse | Marmoset | Rabbit | Cat | Sheep (perfusion) | Sheep (immersion) |
| BD Pharmingen (mouse) | svz | * | * | ||||
| sgz | * | ||||||
| Abcam (rabbit) | svz | * | * | * | * | ||
| sgz | * | * | * | ● | |||
| No Citrate | |||||||
| Antibodies | Regions | Mouse | Marmoset | Rabbit | Cat | (perfusion) | (immersion) |
| BD Pharmingen (mouse) | svz | * | * | * | * | ||
| sgz | ● | * | * | ● | |||
| Abcam (rabbit) | svz | * | ● | * | * | * | |
| sgz | * | * | * | ||||
| (b) | |||||||
| Antibodies | Regions | Mouse | Marmoset | Rabbit | Cat | Sheep (perfusion) | Sheep (immersion) |
| BD Pharmingen (mouse) | svz | (5′) | (5′) | only 5′ C | |||
| sgz | only 5′ C | only 5′ C | only 5′ C | ||||
| Abcam (rabbit) | svz | only 5′ C | (5′) | only 5′ C | |||
| sgz | only 5′ C | only 5′ C | only 5′ C | ||||
 Absence of staining due to real interspecies difference (e.g., no cell population containg DCX in the mouse neocortex);
Absence of staining due to real interspecies difference (e.g., no cell population containg DCX in the mouse neocortex);  Clear and clean immunoreaction;
Clear and clean immunoreaction;  Immunoreaction with background;
Immunoreaction with background;  Immunoreaction with non-specific staining;
Immunoreaction with non-specific staining;  No signal with background;
No signal with background;  No signal with non-specific staining;
No signal with non-specific staining;  No signal; Subtable (b): MERGE of results obtained with and without 5′ Citrate:
No signal; Subtable (b): MERGE of results obtained with and without 5′ Citrate:  Same results;
Same results;  Different results; (5′) better with Citrate; only (5′) C signal only with Citrate; without C signal only without Citrate.
Different results; (5′) better with Citrate; only (5′) C signal only with Citrate; without C signal only without Citrate.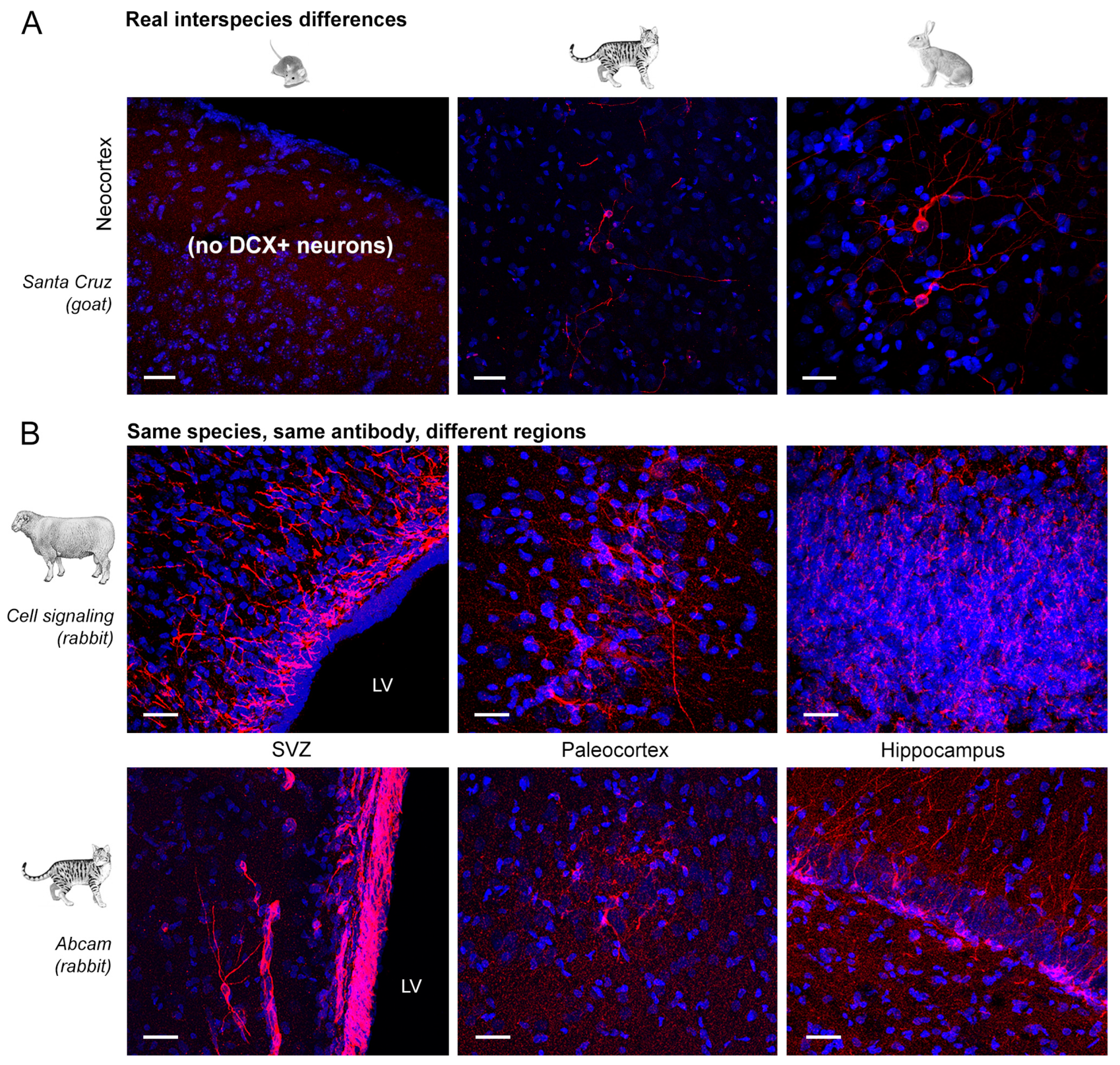
2.3. DCX Detection in the Human Cerebral Cortex
| Antibody | Region | Immuno | RNAscope |
|---|---|---|---|
| Santa Cruz (goat) | Cerebral cortex (temporal) | ● | - |
| Cell Signalling (rabbit) | ● | - | |
| Abcam (rabbit) | * | Co-expression in some DCX+ neurons in layer II-III | |
| Santa Cruz (mouse) | ● | No co-expression in DCX+ neurons (non-specific staining) | |
| Millipore (guinea pig) | ● | No co-expression in DCX+ neurons (non-specific staining) |
 Immunoreaction with background;
Immunoreaction with background;  Immunoreaction with non-specific staining;
Immunoreaction with non-specific staining;  No signal with non-specific staining.
No signal with non-specific staining.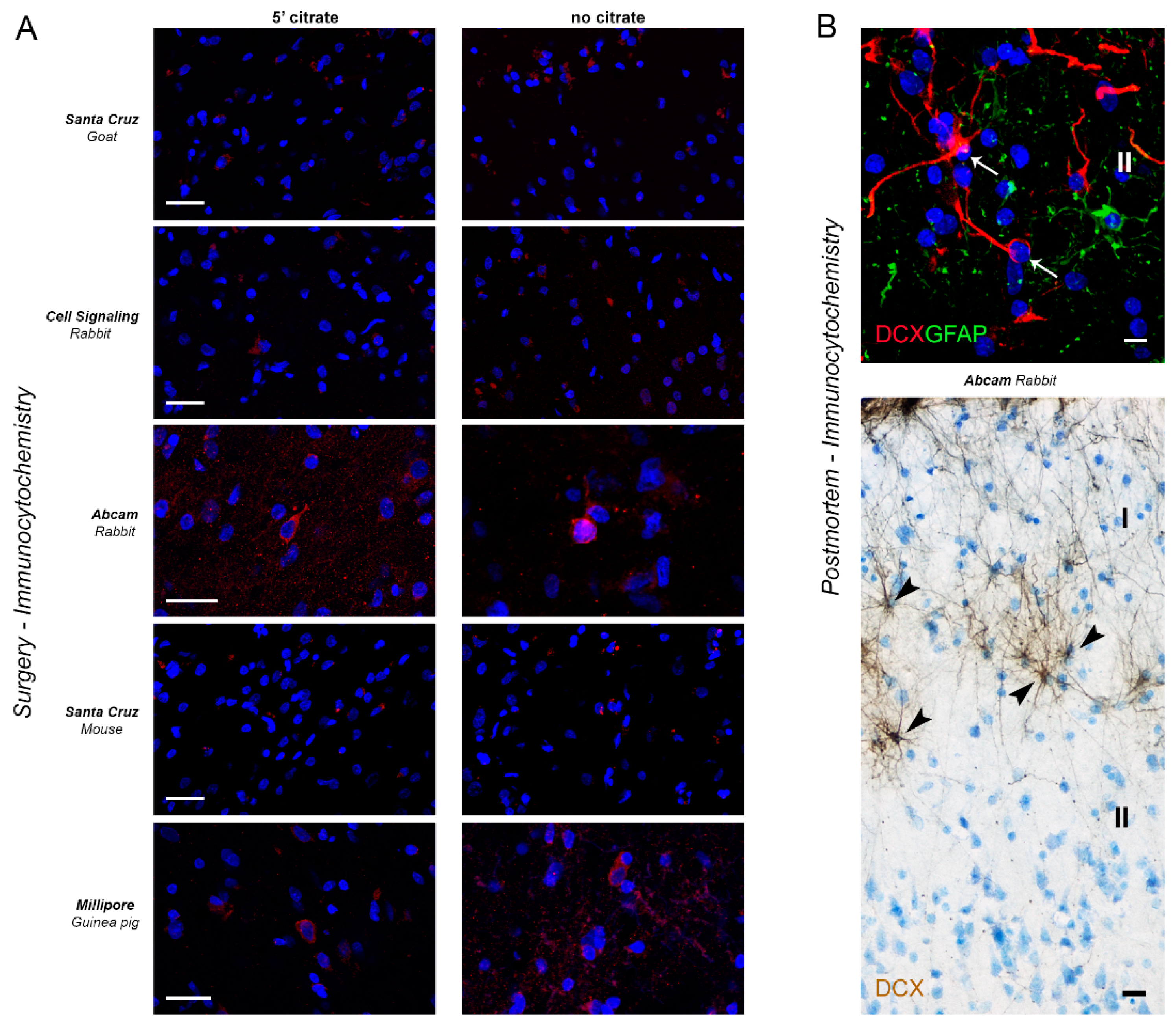
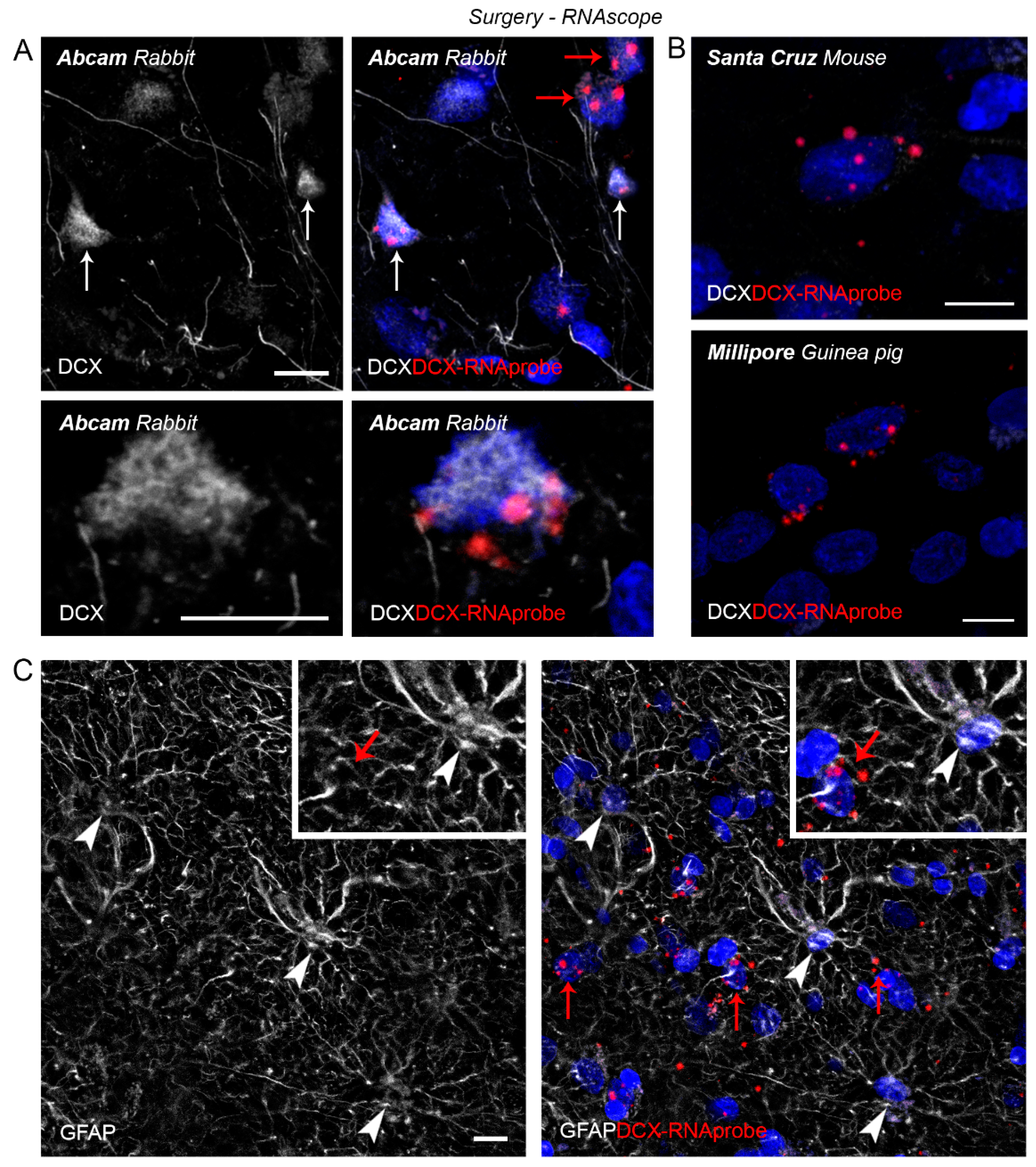
3. Discussion
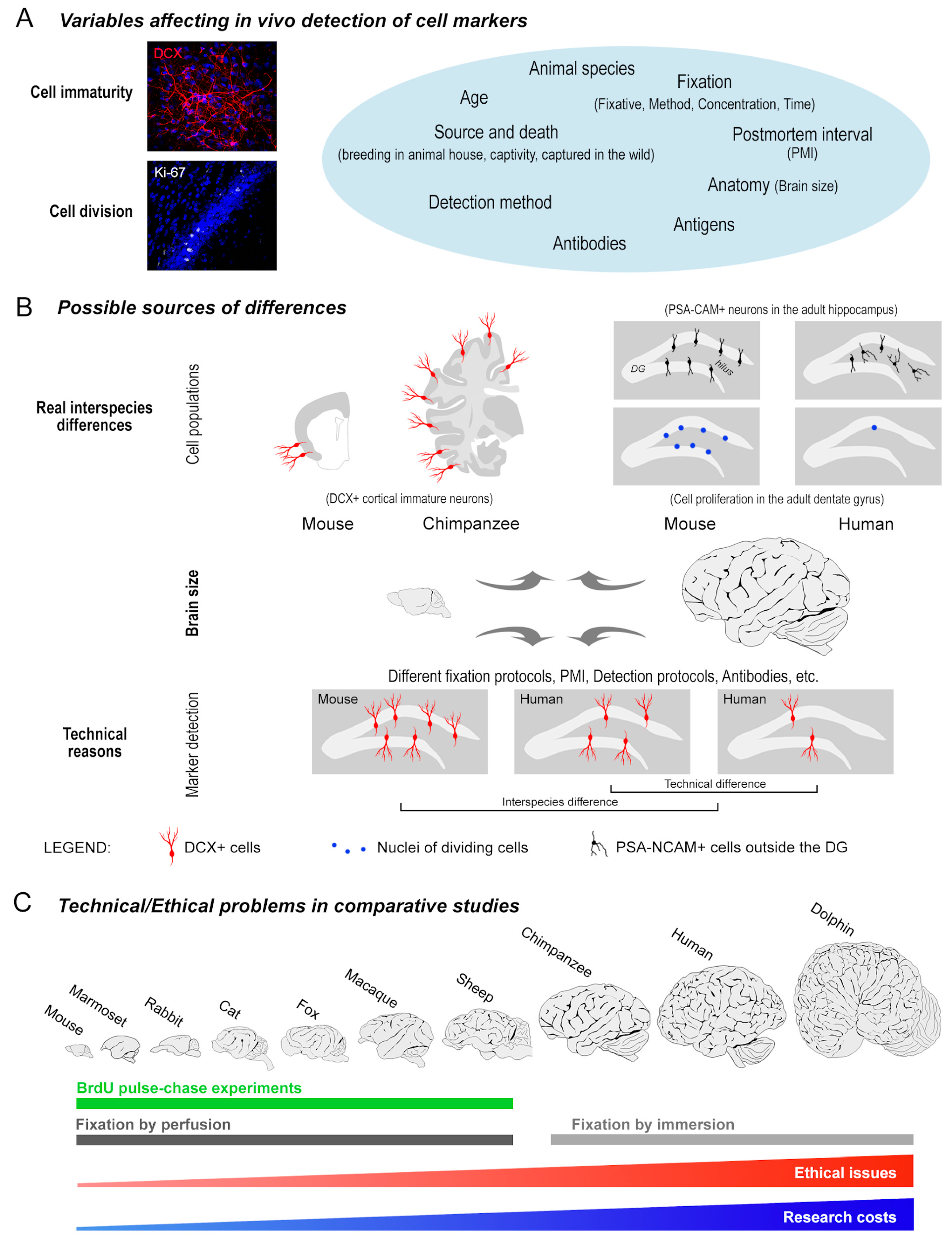
3.1. Variables Affecting the Occurrence/Quality of Staining
3.2. Detecting DCX in the Human Cerebral Cortex
3.3. Comparative Antibody Performance
3.4. The Importance of Investigating the Cerebral Cortex as a Place for Immature Neurons
4. Materials and Methods
4.1. Brain Sample
| Brain Tissues Analysed | ||||||
|---|---|---|---|---|---|---|
| Species | Life Stage | Fixation | Fixative | PMI | Sample processing | |
| Mouse | 3 months | Perfusion | 4% PFA | none |
| |
| Marmoset | Adult | Immersion | 4% PFA (15% picric acid) | 1 h | ||
| Rabbit | 3.5 years | Perfusion | 4% PFA | none | ||
| Cat | 1.5 years | Immersion | 4% buffered formalin | 1 h | ||
| Sheep | 2 years | Perfusion | 4% PFA | none | ||
| 5 years | Immersion | 4% buffered formalin | 20 min | |||
| Human (see Table 7) | Adult (post-mortem) | Immersion | 4% buffered formalin | 16–23 h | ||
| Adult (intraoperative) | 4% PFA | none |
| |||
| Primary Antibodies Tested | ||||||
| Antigen | Host | Type | Code | Raised against (source: data sheet and company information) | Dilution | Source |
| DCX | Goat | Polyclonal | SC8066 | Epitope within the last 50 c-terminal amino acids | 1:1000 | Santa Cruz Biotechnology, Dallas, Texas, USA |
| Mouse | Monoclonal | SC271390 | Amino acids 81-365 mapping at the C-terminus of Doublecortin of human origin | |||
| Rabbit | Polyclonal | ab18723 | Synthetic peptide conjugated to KLH derived from within residues 300 to the C-terminus of human doublecortin | Abcam, Cambridge, UK | ||
| 4604 | Antigenic sequence surrounds amino acid 350 tyrosine of human doublecortin | Cell Signalling, Danvers, Massachusetts, USA | ||||
| Guinea pig | ab2253 | Epitope aminoacidic sequence: YLPLSLDDSDSLGDSM | Merck Millipore, Burlington, Massachusetts, USA | |||
| Ki67 | Rabbit | ab15580 | Synthetic peptide | 1:500 | Abcam, Cambridge, UK | |
| Mouse | Monoclonal | 550609 | Human Ki67 | BD Pharmingen, San Diego, California, USA | ||
| Intraoperative Tissues | |||||
| ID | Fixation | Age | Sex | PSI | Cause of Surgery |
| 226012 | 4% PFA | 81 | Male | <1 h | Diffuse high-grade glioma |
| 201032 | 4% PFA | 67 | Female | <2 h | Gliosarcoma grade IV |
| 130079 | 4% PFA | 79 | Male | <2 h | Glioblastoma grade IV |
| Post-Mortem Tissues | |||||
| ID | Fixation | Age | Sex | PMI | Cause of Death |
| 1271 | 4% buffered formalin | 46.6 | Male | 16 h | Thrombosis |
| 1164 | 4% buffered formalin | 48.1 | Female | 16 h | Homicide |
| 1122 | 4% buffered formalin | 48.6 | Male | 23 h | Cardiomegaly |
4.2. Tissue Processing for Histology
4.3. Comparable Neuroanatomy
4.4. Immunofluorescence Protocol
4.5. RNAscope
4.6. Image Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghibaudi, M.; Bonfanti, L. How widespread are the “young” neurons of the mammalian brain? Front. Neurosci. 2022, 16, 918616. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, L.; Peretto, P. Adult neurogenesis in mammals: A theme with many variations. Eur. J. Neurosci. 2011, 34, 930–950. [Google Scholar] [CrossRef]
- Bond, A.M.; Ming, G.; Song, H. Adult mammalian neural stem cells and neurogenesis: Five decades later. Cell Stem Cell 2015, 17, 385–395. [Google Scholar] [CrossRef]
- Feliciano, D.M.; Bordey, A.; Bonfanti, L. Noncanonical sites of adult neurogenesis in the mammalian brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a018846. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Song, H.; Gage, F.H. Neurogenesis in the adult hippocampus. Cold Spring Harb. Perspect. Biol. 2015, 7, a018812. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.A.; Alvarez-Buylla, A. The adult ventricular-subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8, a018820. [Google Scholar] [CrossRef]
- Piumatti, M.; Palazzo, O.; La Rosa, C.; Crociara, P.; Parolisi, R.; Luzzati, F.; Levy, F.; Bonfanti, L. Non-newly generated, “immature” neurons in the sheep brain are not restricted to cerebral cortex. J. Neurosci. 2018, 38, 826–842. [Google Scholar] [CrossRef]
- Rotheneichner, P.; Belles, M.; Benedetti, B.; König, R.; Dannehl, D.; Kreutzer, C.; Zaunmair, P.; Engelhardt, M.; Aigner, L.; Nacher, J.; et al. Cellular plasticity in the adult murine piriform cortex: Continuous maturation of dormant precursors into excitatory neurons. Cereb. Cortex 2018, 28, 2610–2621. [Google Scholar] [CrossRef]
- Sorrells, S.F.; Paredes, M.F.; Velmeshev, D.; Herranz-Pérez, V.; Sandoval, K.; Mayer, S.; Chang, E.F.; Insausti, R.; Kriegstein, A.R.; Rubenstein, J.L.; et al. Immature excitatory neurons develop during adolescence in the human amygdala. Nat. Commun. 2019, 10, 2748. [Google Scholar] [CrossRef]
- La Rosa, C.; Parolisi, R.; Bonfanti, L. Brain structural plasticity: From adult neurogenesis to immature neurons. Front. Neurosci. 2020, 14, 75. [Google Scholar] [CrossRef]
- Bonfanti, L.; Seki, T. The PSA-NCAM-positive “immature” neurons: An old discovery providingnew vistas on brain structural plasticity. Cells 2021, 10, 2542. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, B.; Couillard-Després, S. Why would the brain need dormant neuronal precursors? Front. Neurosci. 2022, 16, 877167. [Google Scholar] [CrossRef] [PubMed]
- Page, C.E.; Biagiotti, S.W.; Alderman, P.J.; Sorrells, S.F. Immature excitatory neurons in the amygdala come of age during puberty. Dev. Cogn. Neurosci. 2022, 56, 101133. [Google Scholar] [CrossRef]
- Gomez-Climent, M.A.; Castillo-Gomez, E.; Varea, E.; Guirado, R.; Blasco-Ibanez, J.M.; Crespo, C.; Martinez-Guijarro, F.J.; Nacher, J. A population of prenatally generated cells in the rat paleocortex maintains an immature neuronal phenotype into adulthood. Cereb. Cortex 2008, 18, 2229–2240. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, L.; Nacher, J. New scenarios for neuronal structural plasticity in non-neurogenic brain parenchyma: The case of cortical layer II immature neurons. Prog. Neurobiol. 2012, 98, 1–15. [Google Scholar] [CrossRef]
- König, R.; Benedetti, B.; Rotheneichner, P.; O’Sullivan, A.; Kreutzer, C.; Belles, M.; Nacher, J.; Weiger, T.M.; Aigner, L.; Couillard-Després, S. Distribution and fate of DCX/PSA-NCAM expressing cells in the adult mammalian cortex: A local reservoir for adult cortical neuroplasticity? Front. Biol. 2016, 11, 193–213. [Google Scholar] [CrossRef]
- Alderman, P.J.; Saxon, D.; Torrijos-Saiz, L.I.; Sharief, M.; Biagiotti, S.W.; Page, C.E.; Melamed, A.; Kuo, C.T.; Garcia-Verdugo, J.-M.; Herranz-Perez, V.; et al. Mouse paralaminar amygdala excitatory neurons migrate and mature during adolescence. bioRxiv 2022. 2022.09.23.509244. [Google Scholar] [CrossRef]
- Kuhn, H.G.; Cooper-Kuhn, C.M. Bromodeoxyuridine and the detection of neurogenesis. Curr. Pharm. Biotech. 2007, 8, 127–131. [Google Scholar] [CrossRef]
- Amrein, I. Adult hippocampal neurogenesis in natural populations of mammals. Cold Spring Harb. Perspect. Biol. 2015, 7, a021295. [Google Scholar] [CrossRef]
- Paredes, M.F.; Sorrells, S.F.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Brain size and limits to adult neurogenesis. J. Comp. Neurol. 2016, 524, 646–664. [Google Scholar] [CrossRef] [PubMed]
- Lipp, H.P.; Bonfanti, L. Adult neurogenesis in mammals: Variations and confusions. Brain Behav. Evol. 2016, 87, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, O.; La Rosa, C.; Piumatti, M.; Bonfanti, L. Do large brains of long-living mammals prefer non-newly generated, immature neurons? Neural Regen. Res. 2018, 13, 633–634. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, C.; Cavallo, F.; Pecora, A.; Chincarini, M.; Ala, U.; Faulkes, C.; Nacher, J.; Sherwood, C.; Amrein, I.; Bonfanti, L. Phylogenetic variation in cortical layer II immature neuron reservoir of mammals. eLife 2020, 9, e55456. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef]
- Moreno-Jimenéz, E.P.; Flor-Garcia, M.; Terreros-Roncal, J.; Rabano, A.; Cafini, F.; Pallas-Bazarra, N.; Avila, J.; Llorens-Martin, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Zhou, Y.; Su, Y.; Li, S.; Kennedy, B.C.; Zhang, D.Y.; Bond, A.M.; Sun, Y.; Jacob, F.; Lu, L.; Hu, P.; et al. Molecular landscapes of human hippocampal immature neurons across lifespan. Nature 2022, 607, 527–533. [Google Scholar] [CrossRef]
- Sorrells, S.F.; Paredes, M.F.; Zhang, Z.; Kang, G.; Pastor-Alonso, O.; Biagiotti, S.; Page, C.E.; Sandoval, K.; Knox, A.; Connolly, A.; et al. Positive controls in adults and children support that very few, if any, new neurons are born in the adult human hippocampus. J. Neurosci. 2021, 41, 2554–2565. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.P.; Terreros-Roncal, J.; Flor-García, M.; Rábano, A.; Llorens-Martín, M. Evidences for adult hippocampal neurogenesis in humans. J. Neurosci. 2021, 41, 2541–2553. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, D.W.; Cooper, J.A.; Gordon, J.A.; Heemskerk, J.; Hodes, R.; Koob, G.F.; Koroshetz, W.J.; Shurtleff, D.; Sieving, P.A.; Volkow, N.D.; et al. Neuroethics for the national institutes of health BRAIN initiative. J. Neurosci. 2018, 38, 10583–10585. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, B.; Bonfanti, L.; Canali, E.; Minero, M. Brain waste: The neglect of animal brains. Front. Neuroanat. 2020, 14, 573934. [Google Scholar] [CrossRef]
- Kee, N.; Sivalingam, S.; Boonstra, R.; Wojtowicz, J.M. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J. Neurosci. Meth. 2002, 115, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Parolisi, R.; Cozzi, B.; Bonfanti, L. Non-neurogenic SVZ-like niche in dolphins, mammals devoid of olfaction. Brain Struct. Funct. 2017, 222, 2625–2639. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Nguyen, T.; Ihrie, R.A.; Mirzadeh, Z.; Tsai, H.-H.; Wong, M.; Gupta, N.; Berger, M.S.; Huang, E.; Garcia-Verdugo, J.M.; et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 2011, 478, 382–386. [Google Scholar] [CrossRef]
- Franjic, D.; Skarica, M.; Ma, S.; Arellano, J.I.; Tebbenkamp, A.T.N.; Choi, J.; Xu, C.; Li, Q.; Morozov, Y.M.; Andrijevic, D.; et al. Transcriptomic taxonomy and neurogenic trajectories of adult human, macaque, and pig hippocampal and entorhinal cells. Neuron 2022, 110, 452–469. [Google Scholar] [CrossRef] [PubMed]
- Seki, T. Understanding the real state of human adult hippocampal neurogenesis from studies of rodents and non-human primates. Front. Neurosci. 2020, 14, 839. [Google Scholar] [CrossRef] [PubMed]
- Duque, A.; Arellano, J.I.; Rakic, P. An assessment of the existence of adult neurogenesis in humans and value of its rodent models for neuropsychiatric diseases. Mol. Psychiatry 2021, 27, 377–382. [Google Scholar] [CrossRef]
- Fasemore, T.M.; Patzke, N.; Kaswera-Kyamakya, C.; Gilissen, E.; Manger, P.R.; Ihunwo, A.O. The distribution of Ki-67 and doublecortin-immunopositive cells in the brains of three strepsirrhine primates: Galago demidoff, perodicticus potto, and lemur catta. Neuroscience 2018, 372, 46–57. [Google Scholar] [CrossRef]
- Chawana, R.; Patzke, N.; Bhagwandin, A.; Kaswera-Kyamakya, C.; Glissen, E.; Bertelsen, M.F.; Hemingway, J.; Manger, P.R. Adult hippocampal neurogenesis in Egyptian fruit bats from three different environments: Are interpretational variations due to the environment or methodology? J. Comp. Neurol. 2020, 528, 2994–3007. [Google Scholar] [CrossRef] [PubMed]
- Kirby, E.D.; Friedman, A.A.; Covarrubias, D.; Ying, C.; Sun, W.G.; Goosens, K.A.; Sapolsky, R.M.; Kaufer, D. Basolateral amygdala regulation of adult hippocampal neurogenesis and fear-related activation of newborn neurons. Mol. Psychiatry 2012, 17, 527–536. [Google Scholar] [CrossRef]
- Fudge, J.L.; deCampo, D.M.; Becoats, K.T. Revisiting the hippocampal-amygdala pathway in primates: Association with immature-appearing neurons. Neuroscience 2012, 212, 104–119. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, Y.; Chu, Y.; Chen, E.; Feng, J.; Luo, X.; Xiong, K.; Struble, R.; Clough, R.; Patrylo, P.R.; et al. Doublecortin-expressing cells persist in the associative cerebral cortex and amygdala in aged nonhuman primates. Front. Neuroanat. 2009, 3, 17. [Google Scholar] [CrossRef]
- Flor-Garcìa, M.; Terreros-Roncal, J.; Moreno-Jiménez, E.P.; Avila, J.; Ràbano, A.; Llorens-Martìn, M. Unraveling human adult hippocampal neurogenesis. Nat. Protoc. 2020, 15, 668–693. [Google Scholar] [CrossRef]
- Tobin, M.K.; Musaraca, K.; Disouky, A.; Shetti, A.; Bheri, A.; Honer, W.G.; Kim, N.; Dawe, R.J.; Bennett, D.A.; Arfanakis, K.; et al. Human hippocampal neurogenesis persists in aged adults and Alzheimer’s disease patients. Cell Stem Cell 2019, 24, 974–982.e3. [Google Scholar] [CrossRef]
- Boekhoorn, K.; Joels, M.; Lucassen, P.J. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol. Dis. 2006, 24, 1–14. [Google Scholar] [CrossRef]
- Liu, R.X.; Ma, J.; Wang, B.; Tian, T.; Guo, N.; Liu, S.J. No DCX-positive neurogenesis in the cerebral cortex of the adult primate. Neural Regen. Res. 2020, 15, 1290–1299. [Google Scholar] [CrossRef]
- Marlatt, M.W.; Philippen, I.; Manders, E.; Czéh, B.; Joels, M.; Krugers, H.; Lucassen, P.J. Distinct structural plasticity in the hippocampus and amygdala of the middle-aged common marmoset (Callithrix jacchus). Exp. Neurol. 2011, 230, 291–301. [Google Scholar] [CrossRef]
- La Rosa, C.; Parolisi, R.; Palazzo, O.; Lévy, F.; Meurisse, M.; Bonfanti, L. Clusters of DCX+ cells “trapped” in the subcortical white matter of early postnatal Cetartiodactyla (Tursiops truncatus, Stenella coeruloalba and Ovis aries). Brain Struct. Funct. 2018, 223, 3613–3632. [Google Scholar] [CrossRef]
- Jin, K.; Wang, X.; Xie, L.; Mao, X.; Zhu, W.; Wang, Y.; Shen, J.; Mao, Y.; Banwait, S.; Greenberg, D.A. Evidence for stroke-induced neurogenesis in the human brain. Proc. Natl. Acad. Sci. USA 2006, 103, 13198–13202. [Google Scholar] [CrossRef]
- Crews, L.; Adame, A.; Patrick, C.; DeLaney, A.; Pham, E.; Rockenstein, E.; Hansen, L.; Masliah, E. Increased BMP6 levels in the brains of Alzheimer’s disease patients and APP transgenic mice are accompanied by impaired neurogenesis. J. Neurosci. 2010, 30, 12252–12262. [Google Scholar] [CrossRef] [PubMed]
- Knoth, R.; Singec, I.; Ditter, M.; Pantazis, G.; Capetian, P.; Meyer, R.P.; Horvat, V.; Volk, B.; Kempermann, G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE 2010, 5, e8809. [Google Scholar] [CrossRef]
- Wang, C.; Liu, F.; Liu, Y.; Zhao, C.; You, Y.; Wang, L.; Zhang, J.; Wei, B.; Ma, T.; Zhang, Q.; et al. Identification and characterization of neuroblasts in the subventricular zone and rostral migratory stream of the adult human brain. Cell Res. 2011, 21, 1534–1550. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Nicola, D.; Suzzi, S.; Vargas-Caballero, M.; Fransen, N.L.; Al-Malki, H.; Cebrian-Silla, A.; Garcia-Verdugo, J.M.; Riecken, K.; Fehse, B.; Perry, V.H. Temporal dynamics of hippocampal neurogenesis in chronic neurodegeneration. Brain 2014, 137 (Pt 8), 2312–2328. [Google Scholar] [CrossRef]
- Ekonomou, A.; Savva, G.M.; Brayne, C.; Forster, G.; Francis, P.T.; Johnson, M.; Perry, E.K.; Attems, J.; Somani, A.; Minger, S.L.; et al. Stage-specific changes in neurogenic and glial markers in Alzheimer’s disease. Biol. Psychiatry 2015, 77, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Dennis, C.V.; Suh, L.S.; Rodriguez, M.L.; Kril, J.J.; Sutherland, G.T. Human adult neurogenesis across the ages: An immunohistochemical study. Neuropathol. Appl. Neurobiol. 2016, 42, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Galàn, L.; Gòmez-Pinedo, U.; Guerrero, A.; Garcìa-Verdugo, J.M.; Matìas-Guiu, J. Amyotrophic lateral sclerosis modifies progenitor neural proliferation in adult classic neurogenic brain niches. BMC Neurol. 2017, 17, 173. [Google Scholar] [CrossRef]
- Liu, Y.W.J.; Curtis, M.A.; Gibbons, H.M.; Mee, E.W.; Bergin, P.S.; Teoh, H.H.; Connor, B.; Dragunow, M.; Faull, R.L.M. Doublecortin expression in the normal and epileptic adult human brain. Eur. J. Neurosci. 2008, 11, 2254–2265. [Google Scholar] [CrossRef]
- Ponti, G.; Aimar, P.; Bonfanti, L. Cellular composition and cytoarchitecture of the rabbit subventricular zone and its extensions in the forebrain. J. Comp. Neurol. 2006, 498, 491–507. [Google Scholar] [CrossRef]
- Kunze, A.; Achilles, A.; Keiner, S.; Witte, O.W.; Redecker, C. Two distinct populations of doublecortin-positive cells in the perilesional zone of cortical infarcts. BMC Neurosci. 2015, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Xiong, K.; Chu, Y.; Luo, D.; Luo, X.; Yan, X.; Struble, R.G.; Clough, R.W.; Spencer, D.D.; Williamson, A.; et al. Doublecortin expression in adult cat and primate cerebral cortex relates to immature neurons that develop into GABAergic subgroups. Exp. Neurol. 2009, 216, 342–356. [Google Scholar] [CrossRef]
- Verwer, R.W.H.; Sluiter, A.A.; Balesar, R.A.; Baayen, J.C.; Noske, D.P.; Dirven, C.M.F.; Wouda, J.; van Dam, A.M.; Lucassen, P.J.; Swaab, D.F. Mature astrocytes in the adult human neocortex express the early neuronal marker doublecortin. Brain 2007, 130, 3321–3335. [Google Scholar] [CrossRef]
- Bloch, J.; Kaeser, M.; Sadeghi, Y.; Rouiller, E.M.; Redmond, D.E., Jr.; Brunet, J. Doublecortin-positive cells in the adult primate cerebral cortex and possible role in brain plasticity and development. J. Comp. Neurol. 2011, 519, 775–789. [Google Scholar] [CrossRef]
- Li, Y.-N.; Hu, D.-D.; Cai, X.-L.; Wang, Y.; Yang, C.; Jiang, J.; Zhang, Q.-L.; Tu, T.; Wang, X.-S.; Wang, H.; et al. Doublecortin-expressing neurons in human cerebral cortex layer II and amygdala from infancy to 100 year-old. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Jhaveri, D.J.; Tedoldi, A.; Hunt, S.; Sullivan, R.; Watts, N.R.; Power, J.M.; Bartlett, P.F.; Sah, P. Evidence for newly generated interneurons in the basolateral amygdala of adult mice. Mol. Psychiatry 2018, 23, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, S.; Ferrer, I.; Aronica, E.; Kovacs, G.G.; Verney, C.; Nardelli, J.; Khung, S.; Delezoide, A.; Milenkovic, I.; Rasika, S.; et al. Hippocampal radial glial subtypes and their neurogenic potential in human fetuses and healthy and Alzheimer’s disease adults. Cereb. Cortex 2018, 28, 2458–2478. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.B.; Sogayar, M.C.; Colquhoun, A.; Siqueira, S.A.; Nogueira, A.B.; Marchiori, P.E.; Teixeira, M.J. Existence of a potential neurogenic system in the adult human brain. J. Transl. Med. 2014, 12, 75. [Google Scholar] [CrossRef]
- Perry, E.K.; Johnson, M.; Ekonomou, A.; Perry, R.H.; Ballard, C.; Attems, J. Neurogenic abnormalities in Alzheimer’s disease differ between stages of neurogenesis and are partly related to cholinergic pathology. Neurobiol. Dis. 2014, 47, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Kaneko, N.; Herranz-Pérez, V.; Nakamura, S.; Oishi, H.; Garcìa-Verdugo, J.M.; Sawamoto, K. Dynamic changes in the neurogenic potential in the ventricular-subventricular zone of common marmoset during postnatal brain development. Cereb. Cortex 2020, 30, 4092–4109. [Google Scholar] [CrossRef]
- Benedetti, B.; Dannehl, D.; Konig, R.; Coviello, S.; Kreutzer, C.; Zaunmair, P.; Jakubecova, D.; Weiger, T.M.; Aigner, L.; Nacher, J.; et al. Functional Integration of Neuronal Precursors in the Adult Murine Piriform Cortex. Cereb. Cortex 2019, 30, 1499–1515. [Google Scholar] [CrossRef]
- Paredes, M.F.; James, D.; Gil-Perotin, S.; Kim, H.; Cotter, J.A.; Ng, C.; Sandoval, K.; Rowitch, D.H.; Xu, D.; McQuillen, P.S.; et al. Extensive migration of young neurons into the infant human frontal lobe. Science 2016, 354, aaf7073. [Google Scholar] [CrossRef]
- Maheu, M.E.; Devorak, J.; Freibauer, A.; Davoli, M.A.; Turecki, G.; Mechawar, N. Increased doublecortin (DCX) expression and incidence of DCX-immunoreactive multipolar cells in the subventricular zone-olfactory bulb system of suicides. Front. Neuroanat. 2015, 9, 74. [Google Scholar] [CrossRef]
- Martì-Mengual, U.; Varea, E.; Crespo, C.; Blasco-Ibànez, J.M.; Nacher, J. Cells expressing markers of immature neurons in the amygdala of adult humans. Eur. J. Neurosci. 2013, 37, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Coviello, S.; Gramuntell, Y.; Klimczak, P.; Varea, E.; Blasco-Ibañez, J.M.; Crespo, C.; Gutierrez, A.; Nacher, J. Phenotype and distribution of immature neurons in the human cerebral cortex layer II. Front. Neuroanat. 2022, 16, 851432. [Google Scholar] [CrossRef] [PubMed]
- Quinones-Hinojosa, A.; Sanai, N.; Soriano-Navarro, M.; Gonzalez-Perez, O.; Mirzadeh, Z.; Gil-Perotin, S.; Romero-Rodriguez, R.; Berger, M.S.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Cellular composition and cytoarchitecture of the adult human subventricular zone: A niche of neural stem cells. J. Comp. Neurol. 2006, 494, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Fahrner, A.; Kann, G.; Flubacher, A.; Heinrich, C.; Freiman, T.M.; Zentner, J.; Frotscher, M.; Haas, C.A. Granule cell dispersion is not accompanied by enhanced neurogenesis in temporal lobe epilepsy patients. Exp. Neurol. 2007, 203, 320–332. [Google Scholar] [CrossRef]
- Allen, K.M.; Fung, S.J.; Weickert, C.S. Cell proliferation is reduced in the hippocampus in schizophrenia. Aust. N. Z. J. Psychiatry 2016, 50, 473–480. [Google Scholar] [CrossRef]
- Zhao, X.; van Praag, H. Steps towards standardized quantification of adult neurogenesis. Nat. Commun. 2020, 11, 4275. [Google Scholar] [CrossRef]
- Srikandarajah, N.; Martinian, L.; Sisodiya, S.M.; Squier, W.; Blumcke, I.; Aronica, E.; Thom, M. Doublecortin expression in focal cortical dysplasia in epilepsy. Epilepsia 2009, 50, 2619–2628. [Google Scholar] [CrossRef]
- Falcone, C.; Wolf-Ochoa, M.; Amina, S.; Hong, T.; Vakilzadeh, G.; Hopkins, W.D.; Hof, P.R.; Sherwood, C.C.; Manger, P.R.; Noctor, S.C.; et al. Cortical interlaminar astrocytes across the therian mammal radiation. J. Comp. Neurol. 2019, 527, 1654–1674. [Google Scholar] [CrossRef]
- Patzke, N.; Spocter, M.A.; Karlsson, K.Æ.; Bertelsen, M.F.; Haagensen, M.; Chawana, R.; Streicher, S.; Kaswera, C.; Gilissen, E.; Alagaili, A.N.; et al. In contrast to many other mammals, cetaceans have relatively small hippocampi that appear to lack adult neurogenesis. Brain Struct. Funct. 2015, 220, 361–383. [Google Scholar] [CrossRef]
- van Dijk, R.M.; Huang, S.H.; Slomianka, L.; Amrein, I. Taxonomic separation of hippocampal networks: Principal cell populations and adult neurogenesis. Front. Neuroanat. 2016, 10, 22. [Google Scholar] [CrossRef]
- Parolisi, R.; Cozzi, B.; Bonfanti, L. Humans and dolphins: Decline and fall of adult neurogenesis. Front. Neurosci. 2018, 12, 497. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.P.; Couillard-Després, S.; Cooper-Kuhn, C.M.; Winkler, J.; Aigner, L.; Kuhn, H.G. Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 2003, 467, 1–10. [Google Scholar] [CrossRef]
- La Rosa, C.; Ghibaudi, M.; Bonfanti, L. Newly generated and non-newly generated “immature” neurons in the mammalian brain: A possible reservoir of young cells to prevent brain ageing and disease? J. Clin. Med. 2019, 8, 685. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Luo, D.; Patrylo, P.R.; Struble, R.G.; Clough, R.W.; Yan, X. Doublecortin-expressing cells are present in layer II across the adult guinea pig cerebral cortex: Partial colocalization with mature interneuron markers. Exp. Neurol. 2008, 211, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Faykoo-Martinez, M.; Toor, I.; Holmes, M.M. Solving the neurogenesis puzzle: Looking for pieces outside the traditional box. Front. Neurosci. 2017, 11, 505. [Google Scholar] [CrossRef]
- Roth, G.; Dicke, U. Evolution of the brain and intelligence. Trends Cogn. Sci. 2005, 9, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Vinters, H.V. Emerging concepts in Alzheimer disease. Annu. Rev. Pathol. 2015, 10, 291–319. [Google Scholar] [CrossRef]
- Brenowitz, E.A.; Zakon, H.H. Emerging from the bottleneck: Benefits of the comparative approach to modern neuroscience. Trends Neurosci. 2015, 38, 273–278. [Google Scholar] [CrossRef]
- Deep-Soboslay, A.; Akil, M.; Martin, C.E.; Bigelow, L.B.; Herman, M.M.; Hyde, T.M.; Kleinman, J.E. Reliability of psychiatric diagnosis in postmortem research. Biol. Psychiatry 2005, 57, 96–101. [Google Scholar] [CrossRef]
- Lipska, B.K.; Deep-Soboslay, A.; Weickert, C.S.; Hyde, T.M.; Martin, C.E.; Herman, M.M.; Kleinman, J.E. Critical factors in gene expression in postmortem human brain: Focus on studies in schizophrenia. Biol. Psychiatry 2006, 60, 650–658. [Google Scholar] [CrossRef]
- Highet, B.; Anekal, P.V.; Ryan, B.; Murray, H.; Coppieters, N.; Dieriks, B.V.; Singh-Bains, M.K.; Mehrabi, N.F.; Faull, R.L.M.; Dragunow, M.; et al. fISHing with immunohistochemistry for housekeeping gene changes in Alzheimer’s disease using an automated quantitative analysis workflow. J. Neurochem. 2021, 157, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Ciarpella, F.; Zamfir, R.G.; Campanelli, A.; Ren, E.; Pedrotti, G.; Bottani, E.; Borioli, A.; Caron, D.; Di Chio, M.; Dolci, S.; et al. Murine cerebral organoids develop network of functional neurons and hippocampal brain region identity. iScience 2021, 24, 103438. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghibaudi, M.; Amenta, A.; Agosti, M.; Riva, M.; Graïc, J.-M.; Bifari, F.; Bonfanti, L. Consistency and Variation in Doublecortin and Ki67 Antigen Detection in the Brain Tissue of Different Mammals, including Humans. Int. J. Mol. Sci. 2023, 24, 2514. https://doi.org/10.3390/ijms24032514
Ghibaudi M, Amenta A, Agosti M, Riva M, Graïc J-M, Bifari F, Bonfanti L. Consistency and Variation in Doublecortin and Ki67 Antigen Detection in the Brain Tissue of Different Mammals, including Humans. International Journal of Molecular Sciences. 2023; 24(3):2514. https://doi.org/10.3390/ijms24032514
Chicago/Turabian StyleGhibaudi, Marco, Alessia Amenta, Miriam Agosti, Marco Riva, Jean-Marie Graïc, Francesco Bifari, and Luca Bonfanti. 2023. "Consistency and Variation in Doublecortin and Ki67 Antigen Detection in the Brain Tissue of Different Mammals, including Humans" International Journal of Molecular Sciences 24, no. 3: 2514. https://doi.org/10.3390/ijms24032514
APA StyleGhibaudi, M., Amenta, A., Agosti, M., Riva, M., Graïc, J.-M., Bifari, F., & Bonfanti, L. (2023). Consistency and Variation in Doublecortin and Ki67 Antigen Detection in the Brain Tissue of Different Mammals, including Humans. International Journal of Molecular Sciences, 24(3), 2514. https://doi.org/10.3390/ijms24032514








