Thrombosis Models: An Overview of Common In Vivo and In Vitro Models of Thrombosis
Abstract
1. Introduction
2. Thrombosis Overview
3. Methods and Selection Criteria
4. Models for Thrombosis
4.1. In Vitro Models
4.1.1. Macrofluidic- and Microfluidic-Based Models
Flow Chambers
Thrombosis on a Chip
Other Microfluidic-Based Models
4.2. In Vivo Models
4.2.1. Murine Models
Induction of Endothelial Injury
Promoting Hypercoagulation
Induction of Stasis or Stenosis
4.2.2. Porcine Models
Endothelial Dysfunction
Induction of Stasis or Stenosis
Promoting Coagulation
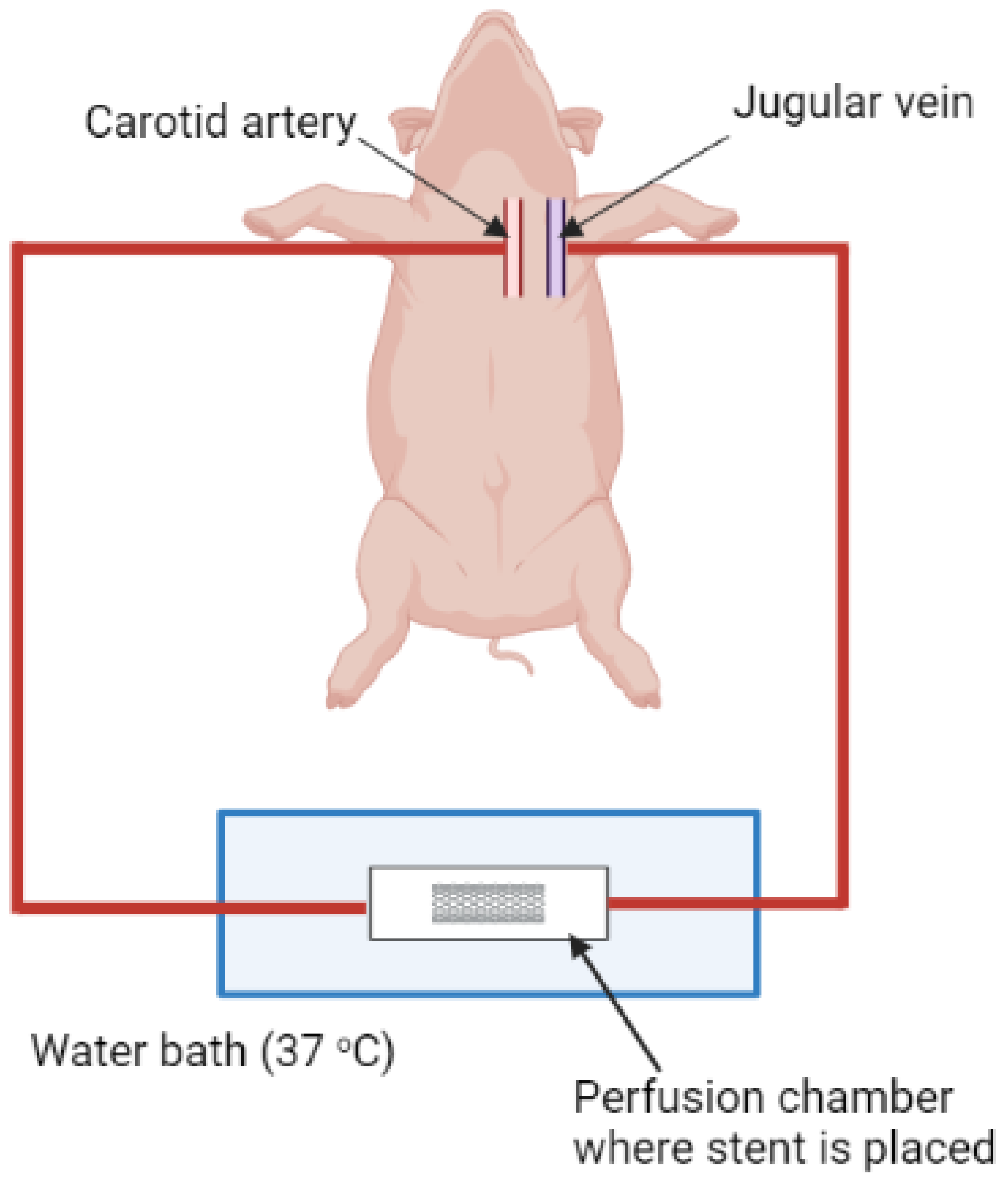
Ex Vivo Arteriovenous (AV) Shunt Model
4.2.3. Zebrafish
Induction of Endothelial Injury
Promoting a Hypercoagulable State
4.3. Advantages and Disadvantages of In Vitro and In Vivo Models
4.3.1. In Vitro Models
4.3.2. In Vivo Models
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AV | Arteriovenous |
| AS | Atherosclerosis |
| CTEPH | Chronic thromboembolic pulmonary hypertension |
| EDTA | Ethylenediaminetetraacetic acid |
| DVT | Deep-vein thrombosis |
| HFD | High-fat diet |
| MI | Myocardial infarction |
| PHZ | Phenylhydrazine |
| PE | Pulmonary embolism |
| PH | Pulmonary hypertension |
| TM | Thrombomodulin |
| TF | Tissue factor |
| vWF | von Willebrand factor |
References
- Wendelboe, A.M.; Raskob, G.E. Global burden of thrombosis: Epidemiologic aspects. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef]
- Mackman, N. Triggers, targets and treatments for thrombosis. Nature 2008, 451, 914–918. [Google Scholar] [PubMed]
- Zipes, D.P. Braunwald’s heart disease: A textbook of cardiovascular medicine. BMH Med. J. 2018, 5, 63. [Google Scholar]
- Klok, F.; Van der Hulle, T.; Exter, P.D.; Lankeit, M.; Huisman, M.; Konstantinides, S. The post-PE syndrome: A new concept for chronic complications of pulmonary embolism. Blood Rev. 2014, 28, 221–226. [Google Scholar] [PubMed]
- Rasche, H. Haemostasis and thrombosis: An overview. Eur. Heart J. Suppl. 2001, 3, Q3–Q7. [Google Scholar] [CrossRef]
- Wu, K.K.; Thiagarajan, M.P. Role of endothelium in thrombosis and hemostasis. Annu. Rev. Med. 1996, 47, 315–331. [Google Scholar] [PubMed]
- Ashorobi, D.; Ameer, M.A.; Fernandez, R. Thrombosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Migliacci, R.; Becattini, C.; Pesavento, R.; Davi, G.; Vedovati, M.C.; Guglielmini, G.; Falcinelli, E.; Ciabattoni, G.; Dalla Valle, F.; Prandoni, P. Endothelial dysfunction in patients with spontaneous venous thromboembolism. Haematologica 2007, 92, 812–818. [Google Scholar] [CrossRef]
- Nieswandt, B.; Pleines, I.; Bender, M. Platelet adhesion and activation mechanisms in arterial thrombosis and ischaemic stroke. J. Thromb. Haemost. 2011, 9, 92–104. [Google Scholar]
- Vilahur, G.; Padro, T.; Badimon, L. Atherosclerosis and thrombosis: Insights from large animal models. J. Biomed. Biotechnol. 2011, 2011, 907575. [Google Scholar]
- Prandoni, P. Venous and arterial thrombosis: Is there a link? Thromb. Embolism Res. Clin. Pract. 2016, 1, 273–283. [Google Scholar]
- Ortel, T.L.; Neumann, I.; Ageno, W.; Beyth, R.; Clark, N.P.; Cuker, A.; Hutten, B.A.; Jaff, M.R.; Manja, V.; Schulman, S. American Society of Hematology 2020 guidelines for management of venous thromboembolism: Treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020, 4, 4693–4738. [Google Scholar]
- Mazzolai, L.; Ageno, W.; Alatri, A.; Bauersachs, R.; Becattini, C.; Brodmann, M.; Emmerich, J.; Konstantinides, S.; Meyer, G.; Middeldorp, S. Second consensus document on diagnosis and management of acute deep vein thrombosis: Updated document elaborated by the ESC Working Group on aorta and peripheral vascular diseases and the ESC Working Group on pulmonary circulation and right ventricular function. Eur. J. Prev. Cardiol. 2022, 29, 1248–1263. [Google Scholar]
- Moster, M.; Bolliger, D. Perioperative Guidelines on Antiplatelet and Anticoagulant Agents: 2022 Update. Curr. Anesthesiol. Rep. 2022, 1–11. [Google Scholar]
- Tsoupras, A.; Zabetakis, I.; Lordan, R. Platelet aggregometry assay for evaluating the effects of platelet agonists and antiplatelet compounds on platelet function in vitro. MethodsX 2019, 6, 63–70. [Google Scholar] [CrossRef]
- Morawitz, P.; Jürgens, R. Gibt es eine Thrombasthenie. Münch. Med. Wschr. 1930, 77, 2001. [Google Scholar]
- Hosseini, V.; Mallone, A.; Nasrollahi, F.; Ostrovidov, S.; Nasiri, R.; Mahmoodi, M.; Haghniaz, R.; Baidya, A.; Salek, M.M.; Darabi, M.A. Healthy and diseased in vitro models of vascular systems. Lab A Chip 2021, 21, 641–659. [Google Scholar]
- Dewey, C., Jr.; Bussolari, S.R.; Gimbrone, M.A., Jr.; Davies, P.F. The dynamic response of vascular endothelial cells to fluid shear stress. J. Biomech. Eng. 1981, 103, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Korolj, A.; Lai, B.F.L.; Radisic, M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018, 3, 257–278. [Google Scholar]
- Lam, W.A. Thrombosis-on-a-Chip: A new way to model a complex process. Blood 2017, 130, SCI-10. [Google Scholar]
- Sebastian, B.; Dittrich, P.S. Microfluidics to mimic blood flow in health and disease. Annu. Rev. Fluid Mech. 2018, 50, 483–504. [Google Scholar]
- Roest, M.; Reininger, A.; Zwaginga, J.; King, M.; Heemskerk, J. Biorheology Subcommittee of the SSC of the ISTH. In Flow Chamber-Based Assays to Measure Thrombus Formation In Vitro: Requirements for Standardization; Wiley Online Library: New York, NY, USA, 2011; 9, pp. 2322–2324. [Google Scholar]
- Zwaginga, J.; Nash, G.; King, M.; Heemskerk, J.; Frojmovic, M.; Hoylaerts, M.; Sakariassen, K. Biorheology Subcommittee of the SSC of the ISTH. Flow-based assays for global assessment of hemostasis. Part 1: Biorheologic considerations 1. J. Thromb. Haemost. 2006, 4, 2486–2487. [Google Scholar] [PubMed]
- Slack, S.M.; Turitto, V.T. Flow chambers and their standardization for use in studies of thrombosis. Thromb. Haemost. 1994, 72, 777–781. [Google Scholar] [CrossRef]
- Van Kruchten, R.; Cosemans, J.M.; Heemskerk, J.W. Measurement of whole blood thrombus formation using parallel-plate flow chambers–a practical guide. Platelets 2012, 23, 229–242. [Google Scholar] [PubMed]
- Jen, C.; Lin, J. Direct observation of platelet adhesion to fibrinogen-and fibrin-coated surfaces. Am. J. Physiol. Heart Circ. Physiol. 1991, 261, H1457–H1463. [Google Scholar] [CrossRef] [PubMed]
- Savage, B.; Saldívar, E.; Ruggeri, Z.M. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell 1996, 84, 289–297. [Google Scholar] [CrossRef]
- Kuijpers, M.J.; Schulte, V.; Bergmeier, W.; Lindhout, T.; Brakebusch, C.; Offermanns, S.; Fässler, R.; Heemskerk, J.W.; Nieswandt, B. Complementary roles of platelet glycoprotein VI and integrin α2β1 in collagen-induced thrombus formation in flowing whole blood ex vivo. FASEB J. 2003, 17, 685–687. [Google Scholar] [CrossRef]
- Siljander, P.R.-M.; Munnix, I.C.; Smethurst, P.A.; Deckmyn, H.; Lindhout, T.; Ouwehand, W.H.; Farndale, R.W.; Heemskerk, J.W. Platelet receptor interplay regulates collagen-induced thrombus formation in flowing human blood. Blood 2004, 103, 1333–1341. [Google Scholar] [CrossRef]
- Cosemans, J.M.; Kuijpers, M.J.; Lecut, C.; Loubele, S.T.; Heeneman, S.; Jandrot-Perrus, M.; Heemskerk, J.W. Contribution of platelet glycoprotein VI to the thrombogenic effect of collagens in fibrous atherosclerotic lesions. Atherosclerosis 2005, 181, 19–27. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Oklu, R.; Albadawi, H. Bioengineered in vitro models of thrombosis: Methods and techniques. Cardiovasc. Diagn. Ther. 2017, 7 (Suppl. 3), S329. [Google Scholar] [CrossRef]
- Tsai, M.; Kita, A.; Leach, J.; Rounsevell, R.; Huang, J.N.; Moake, J.; Ware, R.E.; Fletcher, D.A.; Lam, W.A. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J. Clin. Investig. 2011, 122, 408–418. [Google Scholar]
- Berry, J.; Peaudecerf, F.J.; Masters, N.A.; Neeves, K.B.; Goldstein, R.E.; Harper, M.T. An “occlusive thrombosis-on-a-chip” microfluidic device for investigating the effect of anti-thrombotic drugs. Lab A Chip 2021, 21, 4104–4117. [Google Scholar] [CrossRef]
- Jain, A.; Graveline, A.; Waterhouse, A.; Vernet, A.; Flaumenhaft, R.; Ingber, D.E. A shear gradient-activated microfluidic device for automated monitoring of whole blood haemostasis and platelet function. Nat. Commun. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Yeom, E.; Park, J.H.; Kang, Y.J.; Lee, S.J. Microfluidics for simultaneous quantification of platelet adhesion and blood viscosity. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Herbig, B.A.; Yu, X.; Diamond, S.L. Using microfluidic devices to study thrombosis in pathological blood flows. Biomicrofluidics 2018, 12, 42201. [Google Scholar] [CrossRef] [PubMed]
- Lurie, F.; Kistner, R.L.; Eklof, B.; Kessler, D. Mechanism of venous valve closure and role of the valve in circulation: A new concept. J. Vasc. Surg. 2003, 38, 955–961. [Google Scholar] [CrossRef]
- Karino, T.; Goldsmith, H.L.; Motomiya, M.; Mabuchi, S.; Sohara, Y. Flow Patterns in Vessels of Simple and Complex Geometries a. Ann. N. Y. Acad. Sci. 1987, 516, 422–441. [Google Scholar] [CrossRef]
- Lehmann, M.; Schoeman, R.M.; Krohl, P.J.; Wallbank, A.M.; Samaniuk, J.R.; Jandrot-Perrus, M.; Neeves, K.B. Platelets drive thrombus propagation in a hematocrit and glycoprotein VI–dependent manner in an in vitro venous thrombosis model. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1052–1062. [Google Scholar] [CrossRef]
- Chen, Z.; Mondal, N.K.; Ding, J.; Gao, J.; Griffith, B.P.; Wu, Z.J. Shear-induced platelet receptor shedding by non-physiological high shear stress with short exposure time: Glycoprotein Ibα and glycoprotein VI. Thromb. Res. 2015, 135, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Mondal, N.K.; Ding, J.; Koenig, S.C.; Slaughter, M.S.; Griffith, B.P.; Wu, Z.J. Activation and shedding of platelet glycoprotein IIb/IIIa under non-physiological shear stress. Mol. Cell. Biochem. 2015, 409, 93–101. [Google Scholar] [CrossRef]
- Chen, Z.; Mondal, N.K.; Ding, J.; Koenig, S.C.; Slaughter, M.S.; Wu, Z.J. Paradoxical Effect of Nonphysiological Shear Stress on Platelets and v on W illebrand Factor. Artif. Organs. 2016, 40, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Lozano, M.; Bos, A.; de Groot, P.G.; Van Willigen, G.; Meuleman, D.; Ordinas, A.; Sixma, J. Suitability of low-molecular-weight heparin (oid) s and a pentasaccharide for an in vitro human thrombosis model. Arterioscler. Thromb. A J. Vasc. Biol. 1994, 14, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.-P.; Han, J.-J.; Zhang, M.-Q.; Yang, C.-X.; Jiao, P.; Tian, H.; Zhu, C.; Qin, S.-C.; Sun, X.-J. Inhibitory effects of hydrogen on in vitro platelet activation and in vivo prevention of thrombosis formation. Life Sci. 2019, 233, 116700. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Barrile, R.; van der Meer, A.D.; Mammoto, A.; Mammoto, T.; De Ceunynck, K.; Aisiku, O.; Otieno, M.A.; Louden, C.S.; Hamilton, G.A. Primary human lung alveolus-on-a-chip model of intravascular thrombosis for assessment of therapeutics. Clin. Pharmacol. Ther. 2018, 103, 332–340. [Google Scholar] [CrossRef]
- Wagner, W.; Hubbell, J. Local thrombin synthesis and fibrin formation in an in vitro thrombosis model result in platelet recruitment and thrombus stabilization on collagen in heparinized blood. J. Lab. Clin. Med. 1990, 116, 636–650. [Google Scholar]
- Kim, D.A.; Ku, D.N. Structure of shear-induced platelet aggregated clot formed in an in vitro arterial thrombosis model. Blood Adv. 2022, 6, 2872–2883. [Google Scholar] [CrossRef] [PubMed]
- Ciciliano, J.C.; Sakurai, Y.; Myers, D.R.; Fay, M.E.; Hechler, B.; Meeks, S.; Li, R.; Dixon, J.B.; Lyon, L.A.; Gachet, C. Resolving the multifaceted mechanisms of the ferric chloride thrombosis model using an interdisciplinary microfluidic approach. Blood J. Am. Soc. Hematol. 2015, 126, 817–824. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, Y.; Liu, Y.; Tian, P.; Yu, L.; Bai, L.; Zhou, F.; Yang, Y.; Cheng, Y.; Wang, F. Microfluidic-based in vitro thrombosis model for studying microplastics toxicity. Lab. Chip 2022, 22, 1344–1353. [Google Scholar] [CrossRef]
- Sperzel, M.; Huetter, J. Evaluation of aprotinin and tranexamic acid in different in vitro and in vivo models of fibrinolysis, coagulation and thrombus formation. J. Thromb. Haemost. 2007, 5, 2113–2118. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Davoudi, F.; Walch, P.; Manbachi, A.; Luo, X.; Dell’Erba, V.; Miri, A.K.; Albadawi, H.; Arneri, A.; Li, X. Bioprinted thrombosis-on-a-chip. Lab. Chip 2016, 16, 4097–4105. [Google Scholar] [CrossRef]
- Costa, P.F.; Albers, H.J.; Linssen, J.E.; Middelkamp, H.H.; Van Der Hout, L.; Passier, R.; Van Den Berg, A.; Malda, J.; Van Der Meer, A.D. Mimicking arterial thrombosis in a 3D-printed microfluidic in vitro vascular model based on computed tomography angiography data. Lab. Chip 2017, 17, 2785–2792. [Google Scholar] [CrossRef]
- Zwaginga, J.; Sixma, J.; de Groot, P.G. Activation of endothelial cells induces platelet thrombus formation on their matrix. Studies of new in vitro thrombosis model with low molecular weight heparin as anticoagulant. Arterioscler. Off. J. Am. Heart Assoc. Inc. 1990, 10, 49–61. [Google Scholar] [CrossRef]
- Kim, C.-W.; Yun, J.-W.; Bae, I.-H.; Park, Y.-H.; Jeong, Y.S.; Park, J.W.; Chung, J.-H.; Park, Y.-H.; Lim, K.-M. Evaluation of anti-platelet and anti-thrombotic effects of cilostazol with PFA-100® and Multiplate® whole blood aggregometer in vitro, ex vivo and FeCl3-induced thrombosis models in vivo. Thromb. Res. 2011, 127, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Kushner, A.; West, D.; Pillarisetty, L.S. Virchow triad. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Albadawi, H.; Witting, A.A.; Pershad, Y.; Wallace, A.; Fleck, A.R.; Hoang, P.; Khademhosseini, A.; Oklu, R. Animal models of venous thrombosis. Cardiovasc. Diagn. Ther. 2017, 7 (Suppl. 3), S197. [Google Scholar] [CrossRef] [PubMed]
- De Curtis, A.; D’Adamo, M.C.; Amore, C.; Polishchuck, R.; Di Castelnuovo, A.; Donati, M.B.; Iacoviello, L. Experimental Arterial Thrombosis in Genetically or Diet Induced Hyperlipidemia in Rats. Thromb. Haemost. 2001, 86, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Raymond, S.; Zollman, A.; Noria, F.; Sandoval-Cooper, M.; Shulman, A.; Merz, J.L.; Castellino, F.J. Laser-induced noninvasive vascular injury models in mice generate platelet-and coagulation-dependent thrombi. Am. J. Pathol. 2001, 158, 1613–1622. [Google Scholar] [CrossRef]
- Kamocka, M.; Mu, J.; Liu, X.; Chen, N.; Zollman, A.; Sturonas-Brown, B.; Dunn, K.; Xu, Z.; Chen, D.Z.; Alber, M.S. Two-photon intravital imaging of thrombus development. J. Biomed. Opt. 2010, 15, 16020. [Google Scholar] [CrossRef]
- Pierangeli, S.S.; Barker, J.H.; Stikovac, D.; Ackerman, D.; Anderson, G.; Barquinero, J.; Acland, R.; Harris, E.N. Effect of human IgG antiphospholipid antibodies on an in vivo thrombosis model in mice. Thromb. Haemost. 1994, 71, 670–674. [Google Scholar] [CrossRef]
- Sawyer, P.N.; Pate, J.W.; Weldon, C.S. Relations of abnormal and injury electric potential differences to intravascular thrombosis. Am. J. Physiol. Leg. Content 1953, 175, 108–112. [Google Scholar] [CrossRef]
- Kurz, K.; Main, B.; Sandusky, G. Rat model of arterial thrombosis induced by ferric chloride. Thromb. Res. 1990, 60, 269–280. [Google Scholar] [CrossRef]
- Wang, X.; Xu, L. An optimized murine model of ferric chloride-induced arterial thrombosis for thrombosis research. Thromb. Res. 2005, 115, 95–100. [Google Scholar] [CrossRef]
- Shang, J.; Chen, Z.; Wang, M.; Li, Q.; Feng, W.; Wu, Y.; Wu, W.; Graziano, M.P.; Chintala, M. Zucker Diabetic Fatty rats exhibit hypercoagulability and accelerated thrombus formation in the Arterio-Venous shunt model of thrombosis. Thromb. Res. 2014, 134, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Millet, J.; Vaillot, M.; Theveniaux, J.; Brown, N.L. Experimental venous thrombosis induced by homologous serum in the rat. Thromb. Res. 1996, 81, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Deykin, D.; Wessler, S. Activation product, factor IX, serum thrombotic accelerator activity, and serum-induced thrombosis. J. Clin. Investig. 1964, 43, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Furugohri, T.; Fukuda, T.; Tsuji, N.; Kita, A.; Morishima, Y.; Shibano, T. Melagatran, a direct thrombin inhibitor, but not edoxaban, a direct factor Xa inhibitor, nor heparin aggravates tissue factor-induced hypercoagulation in rats. Eur. J. Pharmacol. 2012, 686, 74–80. [Google Scholar] [CrossRef]
- Wessler, S.; Ward, K.; Ho, C. Studies in intravascular coagulation. III. The pathogenesis of serum-induced venous thrombosis. J. Clin. Investig. 1955, 34, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Du, Z.; Wen, Z.; Jiang, B.; He, B. Dynamic changes of urinary proteins in a rat model of acute hypercoagulable state induced by tranexamic acid. J. Cell. Physiol. 2019, 234, 10809–10818. [Google Scholar] [CrossRef]
- Weiler, H.; Lindner, V.; Kerlin, B.; Isermann, B.H.; Hendrickson, S.B.; Cooley, B.C.; Meh, D.A.; Mosesson, M.W.; Shworak, N.W.; Post, M.J. Characterization of a mouse model for thrombomodulin deficiency. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1531–1537. [Google Scholar] [CrossRef]
- Cleuren, A.C.; van Vlijmen, B.J.; Reitsma, P.H. Transgenic mouse models of venous thrombosis: Fulfilling the expectations? In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers, Inc.: New York, NY, USA, 2007; pp. 610–616. [Google Scholar]
- Folts, J.D.; Crowell, E.B., Jr.; Rowe, G.G. Platelet aggregation in partially obstructed vessels and its elimination with aspirin. Circulation 1976, 54, 365–370. [Google Scholar] [CrossRef]
- McDonald, A.P.; Meier, T.R.; Hawley, A.E.; Thibert, J.N.; Farris, D.M.; Wrobleski, S.K.; Henke, P.K.; Wakefield, T.W.; Myers, D.D., Jr. Aging is associated with impaired thrombus resolution in a mouse model of stasis induced thrombosis. Thromb. Res. 2010, 125, 72–78. [Google Scholar] [CrossRef]
- Wernersson, R.; Schierup, M.H.; Jørgensen, F.G.; Gorodkin, J.; Panitz, F.; Stærfeldt, H.-H.; Christensen, O.F.; Mailund, T.; Hornshøj, H.; Klein, A. Pigs in sequence space: A 0.66 X coverage pig genome survey based on shotgun sequencing. BMC Genom. 2005, 6, 1–7. [Google Scholar] [CrossRef]
- Folts, J.; Rowe, G. Acute thrombus formation in stenosed pig coronary-arteries, causing sudden-death by ventricular-fibrillation. In Circulation; American Heart Association: Dallas, TX, USA, 1983; p. 264. [Google Scholar]
- Leach, C.M.; Thorburn, G.D. A comparative study of collagen induced thromboxane release from platelets of different species: Implications for human athero sclerosis models. Prostaglandins 1982, 24, 47–59. [Google Scholar] [CrossRef]
- Topaz, O. Cardiovascular Thrombus: From Pathology and Clinical Presentations to imaging, Pharmacotherapy and Interventions; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Steele, P.M.; Chesebro, J.H.; Stanson, A.W.; Holmes, D.R., Jr.; Dewanjee, M.K.; Badimon, L.; Fuster, V. Balloon angioplasty. Natural history of the pathophysiological response to injury in a pig model. Circ. Res. 1985, 57, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.S.; Huber, K.C.; Murphy, J.G.; Edwards, W.D.; Camrud, A.R.; Vlietstra, R.E.; Holmes, D.R. Restenosis and the proportional neointimal response to coronary artery injury: Results in a porcine model. J. Am. Coll. Cardiol. 1992, 19, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, K.; Kasai, T.; Yokayama, T.; Aihara, K.; Kurata, T.; Kajimoto, K.; Okazaki, S.; Ishiyama, H.; Daida, H. Effectiveness of statin-eluting stent on early inflammatory response and neointimal thickness in a porcine coronary model. Circ. J. 2008, 72, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Kohda, N.; Tani, T.; Nakayama, S.; Adachi, T.; Marukawa, K.; Ito, R.; Ishida, K.; Matsumoto, Y.; Kimura, Y. Effect of cilostazol, a phosphodiesterase III inhibitor, on experimental thrombosis in the porcine carotid artery. Thromb. Res. 1999, 96, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.A.; Zitnay, K.M.; Haudenschild, C.C.; Cunningham, L.D. Loss of selective endothelial cell vasoactive functions caused by hypercholesterolemia in pig coronary arteries. Circ. Res. 1988, 63, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Hasdai, D.; Mathew, V.; Schwartz, R.S.; Holmes, D.R., Jr.; Lerman, A. The effect of basic fibroblast growth factor on coronary vascular tone in experimental hypercholesterolemia in vivo and in vitro. Coron. Artery Dis. 1997, 8, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Schwein, A.; Magnus, L.; Markovits, J.; Chinnadurai, P.; Autry, K.; Jenkins, L.; Barnes, R.; Vekilov, D.P.; Shah, D.; Chakfé, N. Endovascular porcine model of iliocaval venous thrombosis. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 623–630. [Google Scholar] [CrossRef]
- Omary, R.A.; Frayne, R.; Unal, O.; Warner, T.; Korosec, F.R.; Mistretta, C.A.; Strother, C.M.; Grist, T.M. MR-guided angioplasty of renal artery stenosis in a pig model: A feasibility study. J. Vasc. Interv. Radiol. 2000, 11, 373–381. [Google Scholar] [CrossRef]
- Prasad, P.V.; Kim, D.; Kaiser, A.M.; Chavez, D.; Gladstone, S.; Li, W.; Buxton, R.B.; Edelman, R.R. Noninvasive comprehensive characterization of renal artery stenosis by combination of STAR angiography and EPISTAR perfusion imaging. Magn. Reson. Med. 1997, 38, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Gromadziński, L.; Skowrońska, A.; Holak, P.; Smoliński, M.; Lepiarczyk, E.; Żurada, A.; Majewski, M.K.; Skowroński, M.T.; Majewska, M. A New Experimental Porcine Model of Venous Thromboembolism. J. Clin. Med. 2021, 10, 1862. [Google Scholar] [CrossRef]
- Lin, P.H.; Chen, C.; Surowiec, S.M.; Conklin, B.; Bush, R.L.; Lumsden, A.B. Evaluation of thrombolysis in a porcine model of chronic deep venous thrombosis: An endovascular model. J. Vasc. Surg. 2001, 33, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Choi, D.; Jang, Y.; Nam, C.M.; Hur, S.-H.; Hong, M.-K. Effect of intentional restriction of venous return on tissue oxygenation in a porcine model of acute limb ischemia. PloS ONE 2020, 15, e0243033. [Google Scholar] [CrossRef]
- Al-Amer, O.M. The role of thrombin in haemostasis. Blood Coagul. Fibrinolysis 2022, 33, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M.; Haage, P.; Spuentrup, E.; Günther, R.W.; Tacke, J. A porcine deep vein thrombosis model for magnetic resonance-guided monitoring of different thrombectomy procedures. Investig. Radiol. 2007, 42, 727–731. [Google Scholar] [CrossRef]
- Geier, B.; Muth-Werthmann, D.; Barbera, L.; Bolle, I.; Militzer, K.; Philippou, S.; Mumme, A. Laparoscopic ligation of the infrarenal vena cava in combination with transfemoral thrombin infusion: A new animal model of chronic deep venous thrombosis. Eur. J. Vasc. Endovasc. Surg. 2005, 29, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Aguero, J.; Ishikawa, K.; Fish, K.M.; Hammoudi, N.; Hadri, L.; Garcia-Alvarez, A.; Ibanez, B.; Fuster, V.; Hajjar, R.J.; Leopold, J.A. Combination proximal pulmonary artery coiling and distal embolization induces chronic elevations in pulmonary artery pressure in Swine. PLoS ONE 2015, 10, e0124526. [Google Scholar] [CrossRef]
- Niewiarowski, S.; Rao, A.K. Contribution of thrombogenic factors to the pathogenesis of atherosclerosis. Prog. Cardiovasc. Dis. 1983, 26, 197–222. [Google Scholar] [CrossRef] [PubMed]
- Tandon, N.N.; Hoeg, J.M.; Jamieson, G. Perfusion studies on the formation of mural thrombi with cholesterol-modified and hypercholesterolemic platelets. J. Lab. Clin. Med. 1985, 105, 146–156. [Google Scholar]
- Shattil, S.; Anaya-Galindo, R.; Bennett, J.; Colman, R.W.; Cooper, R. Platelet hypersensitivity induced by cholesterol incorporation. J. Clin. Investig. 1975, 55, 636–643. [Google Scholar] [CrossRef]
- Xi, S.; Yin, W.; Wang, Z.; Kusunoki, M.; Lian, X.; Koike, T.; Fan, J.; Zhang, Q. A minipig model of high-fat/high-sucrose diet-induced diabetes and atherosclerosis. Int. J. Exp. Pathol. 2004, 85, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Reitman, J.; Mahley, R.; Fry, D. Yucatan miniature swine as a model for diet-induced atherosclerosis. Atherosclerosis 1982, 43, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.; Eigler, N.; Kaul, S.; Frimerman, A.; Nakamura, M.; Shah, P.; Forrester, J.; Herbert, J.-M.; Litvack, F. Effects of clopidogrel, aspirin and combined therapy in a porcine ex vivo model of high-shear induced stent thrombosis. Eur. Heart J. 1998, 19, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K. In vivo externalization of phosphatidylserine and phosphatidylethanolamine in the membrane bilayer and hypercoagulability by the lipid peroxidation of erythrocytes in rats. J. Clin. Investig. 1985, 76, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Sakairi, T.; Fujimura, H.; Sugimoto, J.; Kume, E.; Kitamura, K.; Takahashi, K. Hematological and morphological investigation of thrombogenic mechanisms in the lungs of phenylhydrazine-treated rats. Exp. Toxicol. Pathol. 2013, 65, 457–462. [Google Scholar] [CrossRef]
- Zhu, X.-Y.; Liu, H.-C.; Guo, S.-Y.; Xia, B.; Song, R.-S.; Lao, Q.-C.; Xuan, Y.-X.; Li, C.-Q. A zebrafish thrombosis model for assessing antithrombotic drugs. Zebrafish 2016, 13, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.; Hanumanthaiah, R.; Jagadeeswaran, P. Genetic analysis of hemostasis and thrombosis using vascular occlusion. Blood Cells Mol. Dis. 2002, 29, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, R.; Zhang, X.; Dong, J.; Chen, M.; Pan, Y.; Liao, Z.; Zhong, M.; He, J.; Wang, F. Zebrafish Model for Screening Antiatherosclerosis Drugs. Oxidative Med. Cell. Longev. 2021, 2021, 9995401. [Google Scholar] [CrossRef]
- Liu, C.; Gates, K.P.; Fang, L.; Amar, M.J.; Schneider, D.A.; Geng, H.; Huang, W.; Kim, J.; Pattison, J.; Zhang, J. Apoc2 loss-of-function zebrafish mutant as a genetic model of hyperlipidemia. Dis. Model. Mech. 2015, 8, 989–998. [Google Scholar] [CrossRef]
- Cruz-Garcia, L.; Schlegel, A. Lxr-driven enterocyte lipid droplet formation delays transport of ingested lipids. J. Lipid Res. 2014, 55, 1944–1958. [Google Scholar] [CrossRef]
- Gromadziński, L.; Paukszto, Ł.; Skowrońska, A.; Holak, P.; Smoliński, M.; Łopieńska-Biernat, E.; Lepiarczyk, E.; Lipka, A.; Jastrzębski, J.P.; Majewska, M. Transcriptomic profiling of femoral veins in deep vein thrombosis in a porcine model. Cells 2021, 10, 1576. [Google Scholar] [CrossRef]
- Jeong, M.H.; Owen, W.G.; Staab, M.E.; Srivatsa, S.S.; Sangiorgi, G.; Stewart, M.; Holmes, D.R., Jr.; Schwartz, R.S. Porcine model of stent thrombosis: Platelets are the primary component of acute stent closure. Catheter. Cardiovasc. Diagn. 1996, 38, 38–43. [Google Scholar] [CrossRef]
- Maxwell, A.D.; Owens, G.; Gurm, H.S.; Ives, K.; Myers, D.D., Jr.; Xu, Z. Noninvasive treatment of deep venous thrombosis using pulsed ultrasound cavitation therapy (histotripsy) in a porcine model. J. Vasc. Interv. Radiol. 2011, 22, 369–377. [Google Scholar] [CrossRef]
- Thorwest, M.; Balling, E.; Kristensen, S.D.; Aagaard, S.; Hakami, A.; Husted, S.E.; Marqversen, J.; Hjortdal, V.E. Dietary fish oil reduces microvascular thrombosis in a porcine experimental model. Thromb. Res. 2000, 99, 203–208. [Google Scholar] [CrossRef]
- Thierry, B.; Merhi, Y.; Bilodeau, L.; Trepanier, C.; Tabrizian, M. Nitinol versus stainless steel stents: Acute thrombogenicity study in an ex vivo porcine model. Biomaterials 2002, 23, 2997–3005. [Google Scholar] [CrossRef]
- Salartash, K.; Lepore, M.; Gonze, M.D.; Leone-Bay, A.; Baughman, R.; Sternbergh III, W.C.; Bowen, J.C.; Money, S.R. Treatment of experimentally induced caval thrombosis with oral low molecular weight heparin and delivery agent in a porcine model of deep venous thrombosis. Ann. Surg. 2000, 231, 789. [Google Scholar] [CrossRef]
- Vodovotz, Y.; Waksman, R.; Kim, W.-H.; Bhargava, B.; Chan, R.C.; Leon, M. Effects of intracoronary radiation on thrombosis after balloon overstretch injury in the porcine model. Circulation 1999, 100, 2527–2533. [Google Scholar] [CrossRef]
- Screaton, N.J.; Coxson, H.O.; Kalloger, S.E.; Baile, E.M.; Nakano, Y.; Hiorns, M.; Mayo, J.R. Detection of lung perfusion abnormalities using computed tomography in a porcine model of pulmonary embolism. J. Thorac. Imaging 2003, 18, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Macoskey, J.J.; Ives, K.; Owens, G.E.; Gurm, H.S.; Shi, J.; Pizzuto, M.; Cain, C.A.; Xu, Z. Non-invasive thrombolysis using microtripsy in a porcine deep vein thrombosis model. Ultrasound Med. Biol. 2017, 43, 1378–1390. [Google Scholar] [CrossRef]
- Becker, E.; Perzborn, E.; Klipp, A.; Lücker, C.; Bütehorn, U.; Kast, R.; Badimon, J.; Laux, V. Effects of rivaroxaban, acetylsalicylic acid and clopidogrel as monotherapy and in combination in a porcine model of stent thrombosis. J. Thromb. Haemost. 2012, 10, 2470–2480. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Reheman, A.; Hou, Y.; Zhou, H.; Wang, Y.; Marshall, A.H.; Liang, C.; Dai, X.; Li, B.X.; Vanhoorelbeke, K. Anfibatide, a novel GPIb complex antagonist, inhibits platelet adhesion and thrombus formation in vitro and in vivo in murine models of thrombosis. Thromb. Haemost. 2014, 112, 279–289. [Google Scholar]
- Lawson, C.A.; Yan, S.; Yan, S.F.; Liao, H.; Zhou, Y.S.; Sobel, J.; Kisiel, W.; Stern, D.M.; Pinsky, D.J. Monocytes and tissue factor promote thrombosis in a murine model of oxygen deprivation. J. Clin. Investig. 1997, 99, 1729–1738. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.L.; Jiang, Q.; Raman, S.B.; Cantwell, L.; Chopp, M. A new rat model of thrombotic focal cerebral ischemia. J. Cereb. Blood Flow Metab. 1997, 17, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Hennan, J.; Morgan, G.; Swillo, R.; Antrilli, T.; Mugford, C.; Vlasuk, G.; Gardell, S.; Crandall, D. Effect of tiplaxtinin (PAI-039), an orally bioavailable PAI-1 antagonist, in a rat model of thrombosis. J. Thromb. Haemost. 2008, 6, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, H.; Wang, W.; Ke, Z.; Xu, P.; Zhong, Z.; Li, X.; Wang, S. Antithrombotic effect of grape seed proanthocyanidins extract in a rat model of deep vein thrombosis. J. Vasc. Surg. 2011, 53, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, P.-D.; Bao, B.-H.; Shan, M.-Q.; Zhang, K.-C.; Cheng, F.-F.; Cao, Y.-D.; Zhang, L.; Ding, A.-W. Anti-thrombotic and pro-angiogenic effects of Rubia cordifolia extract in zebrafish. J. Ethnopharmacol. 2018, 219, 152–160. [Google Scholar] [CrossRef]
- Qi, Y.; Zhao, X.; Liu, H.; Wang, Y.; Zhao, C.; Zhao, T.; Zhao, B.; Wang, Y. Identification of a quality marker (Q-Marker) of danhong injection by the zebrafish thrombosis model. Molecules 2017, 22, 1443. [Google Scholar] [CrossRef]
- Zhu, H.; Lan, C.; Zhao, D.; Wang, N.; Du, D.; Luo, H.; Lu, H.; Peng, Z.; Wang, Y.; Qiao, Z. Wuliangye Baijiu but not ethanol reduces cardiovascular disease risks in a zebrafish thrombosis model. NPJ Sci. Food 2022, 6, 1–8. [Google Scholar] [CrossRef]
- Lu, S.; Hu, M.; Wang, Z.; Liu, H.; Kou, Y.; Lyu, Z.; Tian, J. Generation and application of the zebrafish heg1 mutant as a cardiovascular disease model. Biomolecules 2020, 10, 1542. [Google Scholar] [CrossRef]
- Mangin, P.H.; Neeves, K.B.; Lam, W.A.; Cosemans, J.M.; Korin, N.; Kerrigan, S.W.; Panteleev, M.A.; Biorheology, S.O. In vitro flow-based assay: From simple toward more sophisticated models for mimicking hemostasis and thrombosis. J. Thromb. Haemost. 2021, 19, 582–587. [Google Scholar] [CrossRef]
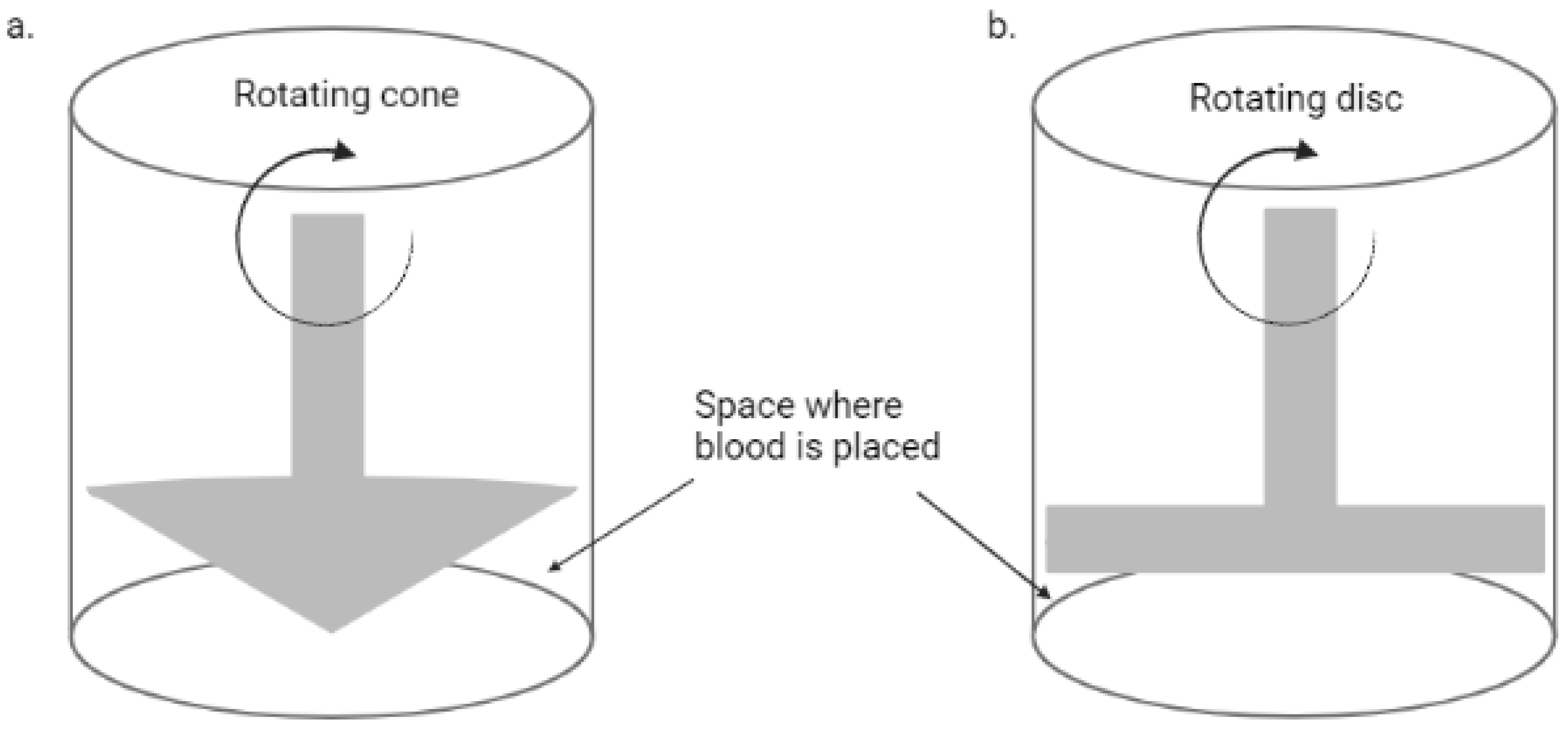
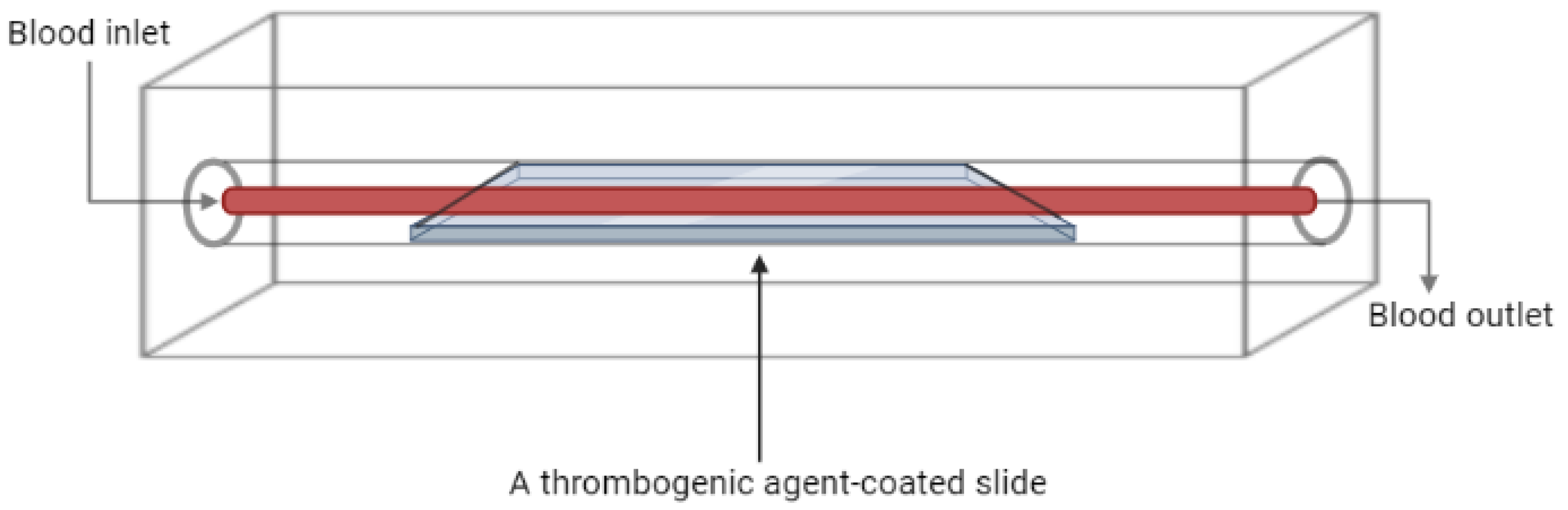
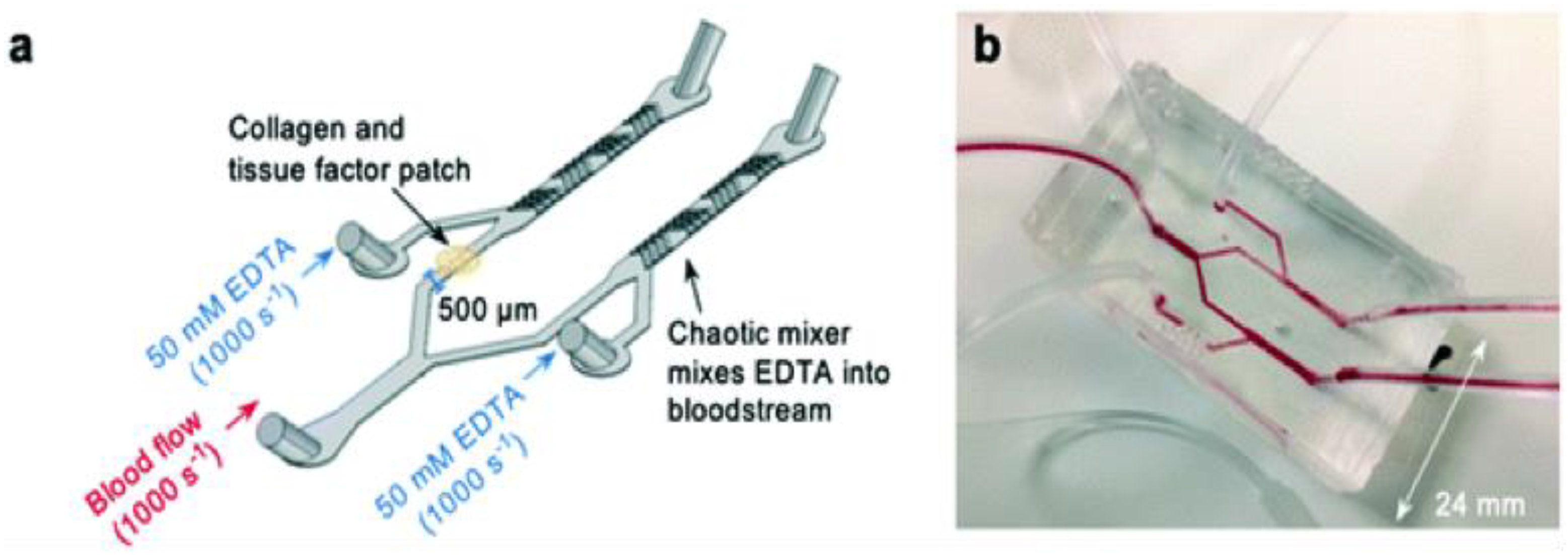
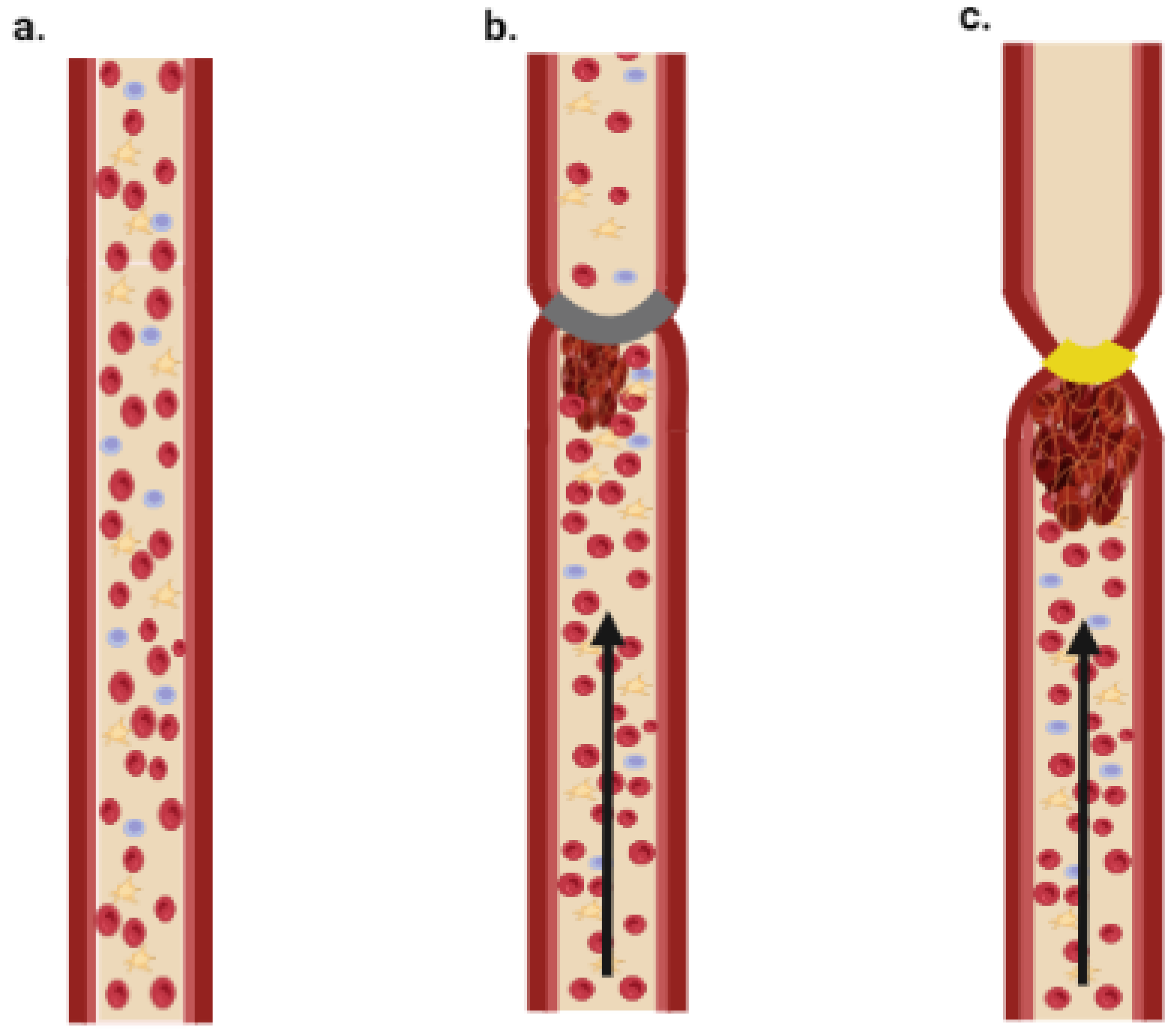

| Type of In Vitro Model | Application | Reference |
|---|---|---|
| Parallel-plate flow chamber with endothelial cells matrix-covered surface | Compare various low-molecular-weight heparin and a pentasaccharide for suitability in the in vitro thrombosis model | [43] |
| Parallel-plate flow chamber-based model with fibrin- or fibrinogen-coated surface | Compare and characterize platelet adhesion to fibrin- and fibrinogen-coated surfaces under controlled flow | [26] |
| Parallel-plate flow chamber-based model with collagen- or plaque-coated surface | Compare the thrombogenic effect of different collagen fibers to atherosclerotic plaque | [30] |
| Flow chamber-based model with fibrinogen- or vWF-coated surface | Identify the mechanism of platelet adhesion to fibrinogen and vWF | [27] |
| Flow chamber-based model with collagen-coated surface | Identify the role of human collagen receptors GPVI and α2β1 in thrombus formation | [29] |
| Fibrinogen-coated flow chambers | Assess platelet adhesion and aggregation following incubation with H2-rich saline | [44] |
| Microfluidic-based device with blood flow under pathophysiological shear rate | Measurement of coagulation and platelet function | [34] |
| Microfluidic-based device with collagen-coated glass substrate | Measurement of platelet adhesion and blood viscosity | [35] |
| Microfluidic lung chip device lined with primary human alveolar epithelium | Monitor pulmonary thrombosis development and evaluate the effect of different pro-thrombotic and anti-thrombotic factors | [45] |
| Microfluidic device mimicking human venous valves | Develop a venous valvular stasis model and study the effect of platelets and red blood cells on thrombus development | [39] |
| Occlusive thrombosis-on-a-chip microfluidic device | Evaluation of anti-thrombotic drugs | [33] |
| Collagen-coated capillary with controlled rheological conditions | Examine the role of thrombin in platelet recruitment and thrombus stabilization | [46] |
| Collagen-coated glass stenosis model | Describe the structure of arterial thrombi | [47] |
| Endothelialized microfluidic device | Study the mechanism of FeCl3-induced thrombosis | [48] |
| Endothelialized microfluidic device | Study the effect of microplastics on thrombus properties | [49] |
| Endothelialized microfluidic device | A bioassay for hematological disorders and evaluating drug efficacy | [32] |
| In vitro human plasma clot formation assay | Compare the effect of aprotinin and tranexamic acid on the coagulation pathway and thrombus formation | [50] |
| 3D-bioprinted thrombosis on a chip model coated with human endothelium embedded in a hydrogel | Develop a highly human biomimetic thrombosis model and study its pathophysiology and potential drug efficacy assessment | [51] |
| 3D-printed microfluidic chip coated with human umbilical vein endothelial cells | Recapitulate the three-dimensional structure of healthy and stenotic coronary arteries and assess platelet aggregation | [52] |
| Annular and rectangular perfusion chambers with steady flow | Study the effect of endothelial cells activation on thrombus formation | [53] |
| Multiplate aggregometer and platelet function analyzer (PFA-100) | Test platelet aggregation to investigate cilostazol’s anti-platelet effect | [54] |
| Blood-shearing device | Study the influence of non-physiological stress on platelets and vWF | [42] |
| Method Employed in the Thrombosis Model | Mechanism of Thrombus Development | Application | Reference |
|---|---|---|---|
| Porcine | |||
| Balloon angioplasty-induced thrombosis | Endothelial injury | Evaluate angioplasty-induced thrombosis | [78] |
| Angioplasty balloon wrapped with a metallic wire coil | Endothelial injury | Determine the relationship between the degree of vascular injury and restenosis magnitude | [79] |
| Surgical ligation and thrombin administration followed by thrombus release to induce PE | Stasis and promoting a hypercoagulable state | Develop a new venous thromboembolism model for possible use in therapeutic testing | [87] |
| Surgical ligation and thrombin administration | Stasis and promoting a hypercoagulable state | Develop a new model of chronic venous thrombosis | [92] |
| Balloon catheter and thrombin administration | Stasis and promoting a hypercoagulable state | Monitor thrombolytic procedures with magnetic resonance imaging | [91] |
| Pulmonary artery embolization with dextran microspheres and surgical coiling of pulmonary branches | Stenosis | Develop a new model of chronic pulmonary hypertension with thrombosis | [93] |
| High-fat/high-sucrose diet-induced atherosclerosis | Promoting a hypercoagulable state | Develop a model of diabetic atherosclerosis | [97] |
| High-fat/high-cholesterol diet-induced atherosclerosis | Promoting a hypercoagulable state | Develop and characterize a diet-induced atherosclerosis model | [98] |
| Surgical ligation of femoral vein and thrombin administration | Stasis and promoting a hypercoagulable state | Develop a DVT model and assess changes in the femoral vein gene expression | [107] |
| Mechanical arterial injury in combination with stent placement followed by total occlusion | Endothelial injury and stasis | Characterize a stent thrombosis model | [108] |
| Balloon catheter and thrombin infusion | Stasis and promoting a hypercoagulable state | Evaluate a high-intensity ultrasound pulse (histotripsy) as a method of thrombolysis | [109] |
| Ischemia-reperfusion injured tissue model | Promoting a hypercoagulable state | Evaluate the role of fish oil in thrombosis development | [110] |
| Balloon catheter and thrombin infusion | Stasis and promoting a hypercoagulable state | Develop a survivable and reproducible iliocaval DVT model for possible use in therapeutic and imaging modalities’ evaluation | [84] |
| Electrical stimulation of the carotid artery endothelium | Endothelial injury | Compare the effect of cilostazol to ticlopidine in inhibiting occlusive thrombus formation | [81] |
| AV shunt model with nitinol stent exposed to arterial blood under high shear rate | Altering blood flow | Evaluate the effect of aspirin, clopidogrel, and combined therapy in inhibiting stent thrombosis development | [99] |
| AV shunt model | Altering blood flow | Compare the thrombogenicity of nitinol to stainless steel stents | [111] |
| Balloon catheter occlusion | Stasis | Evaluate oral administration of low-molecular-weight heparin with a carrier compound in DVT treatment | [112] |
| Self-expanding stent-graft device | Altering blood flow through stasis | Evaluate a thrombolytic therapy with urokinase | [88] |
| Balloon catheter injury | Endothelial injury | Study the effect of ionizing radiation on thrombosis development | [113] |
| Balloon catheter occlusion | Stasis | Use computed tomography to identify lung perfusion abnormalities | [114] |
| Balloon catheter occlusion and thrombin administration | Stasis and promoting a hypercoagulable state | Evaluate the safety and efficacy of microtripsy thrombolysis treatment | [115] |
| AV shunt model | Altering blood flow | Study the effect of rivaroxaban alone or in combination with dual antiplatelet therapy | [116] |
| Murine models | |||
| Laser-induced thrombosis in mice | Endothelial injury | Evaluation of anti-thrombotic drugs | [58] |
| Serum-induced thrombosis in rats | Promoting a hypercoagulable state | Compare the thrombogenicity of homologous and heterologous serum | [65] |
| Tissue factor-induced thrombosis in rats | Promoting a hypercoagulable state | Compare the anti-thrombotic effect of thrombin inhibitor and factor Xa inhibitor | [67] |
| Vascular ligation in mice | Stasis | Evaluate the influence of aging on thrombus resolution | [73] |
| FeCl3-induced thrombosis in mice | Endothelial injury | Develop a refined ferric chloride-induced thrombosis model and test it against anticoagulants | [63] |
| FeCl3 and laser-induced thrombosis in mice | Endothelial injury | Evaluate the potency and safety of anfibatide as an antithrombotic agent | [117] |
| Hypoxia-induced thrombosis in mice | Promoting a hypercoagulable state | Develop and study the mechanism of hypoxia-induced thrombosis | [118] |
| Thrombin-induced thrombosis in rats | Promoting a hypercoagulable state | Develop and characterize a thrombotic ischemia model that mimics human thromboembolic stroke | [119] |
| FeCl3-induced thrombosis in rats | Endothelial injury | Characterize the thrombus, evaluate a novel antithrombotic agent, and determine the relationship between vessel temperature and vascular occlusion | [62] |
| FeCl3-induced thrombosis in rats | Endothelial injury | Assessment of tiplaxtinin antithrombotic effect | [120] |
| Vascular ligation in rats | Stasis | Study the antithrombotic effect of grape seed proanthocyanidins extract | [121] |
| Zebrafish | |||
| PHZ-induced thrombosis | Endothelial injury and promoting a hypercoagulable state | Assessment of antithrombotic drugs | [102] |
| PHZ-induced thrombosis | Endothelial injury and promoting a hypercoagulable state | Evaluate the antithrombotic effect of Rubia cordifolia | [122] |
| FeCl3 or laser irradiation-induced thrombosis | Vascular injury | Genetic screening | [103] |
| Arachidonic acid-induced thrombosis | Promoting platelet aggregation | Evaluate the antithrombotic effect of danhong injection | [123] |
| Arachidonic acid-induced thrombosis | Promoting platelet aggregation | Evaluate the antithrombotic effect of Wuliangye Baijiu | [124] |
| Apoc2 mutant zebrafish | Promoting a hypercoagulable state | Characterization of apoc2 mutant zebrafish | [105] |
| Heg1 knockout zebrafish | Damaging the vascular endothelium integrity | Develop a zebrafish model of dilated cardiomyopathy and thrombosis and employ it in drug screening | [125] |
| High cholesterol and lipopolysaccharide diet | Promoting a hypercoagulable state | Drug screening | [104] |
| Category | In Vitro Models | In Vivo Models |
|---|---|---|
| Reproducibility | Possible, especially when using the same device and conditions. | Variable, considering inter-species variations. |
| Ethical concerns | Minimal. | Strict, especially with larger animals. |
| Cost | Relatively cheap. | Relatively expensive. |
| Simplicity | Relatively simple, especially when using pre-designed devices. | Relatively complicated and time consuming. |
| Result translation | Results need to be further confirmed by in vivo studies. | Considering the settings, results can be more closely related to human conditions, with higher possibility of clinical translation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayyoub, S.; Orriols, R.; Oliver, E.; Ceide, O.T. Thrombosis Models: An Overview of Common In Vivo and In Vitro Models of Thrombosis. Int. J. Mol. Sci. 2023, 24, 2569. https://doi.org/10.3390/ijms24032569
Ayyoub S, Orriols R, Oliver E, Ceide OT. Thrombosis Models: An Overview of Common In Vivo and In Vitro Models of Thrombosis. International Journal of Molecular Sciences. 2023; 24(3):2569. https://doi.org/10.3390/ijms24032569
Chicago/Turabian StyleAyyoub, Sana, Ramon Orriols, Eduardo Oliver, and Olga Tura Ceide. 2023. "Thrombosis Models: An Overview of Common In Vivo and In Vitro Models of Thrombosis" International Journal of Molecular Sciences 24, no. 3: 2569. https://doi.org/10.3390/ijms24032569
APA StyleAyyoub, S., Orriols, R., Oliver, E., & Ceide, O. T. (2023). Thrombosis Models: An Overview of Common In Vivo and In Vitro Models of Thrombosis. International Journal of Molecular Sciences, 24(3), 2569. https://doi.org/10.3390/ijms24032569







