Altered Posttranslational Modification of Microtubules Contributes to Disturbed Enterocyte Morphology in Celiac Disease
Abstract
1. Introduction
2. Results
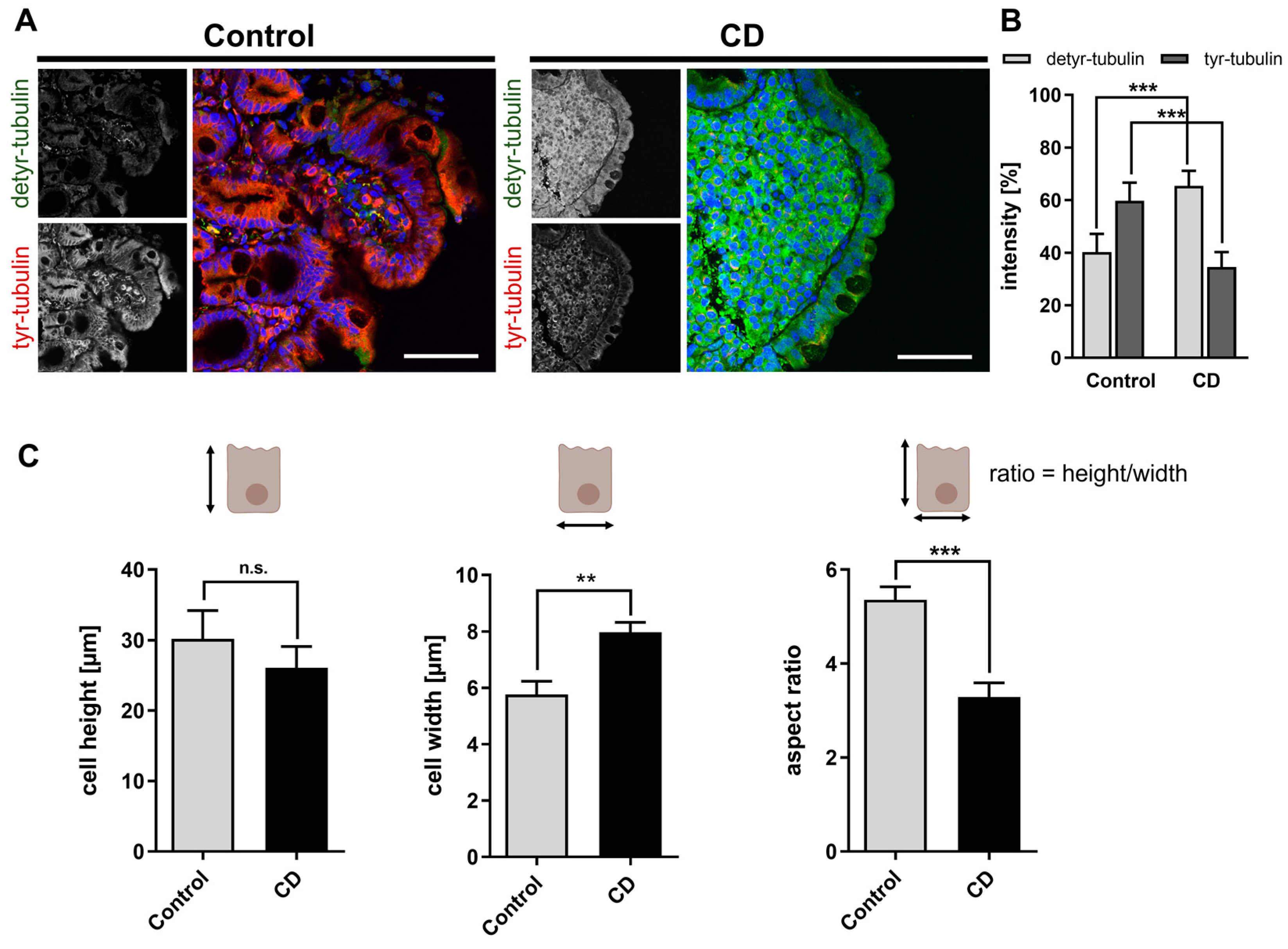
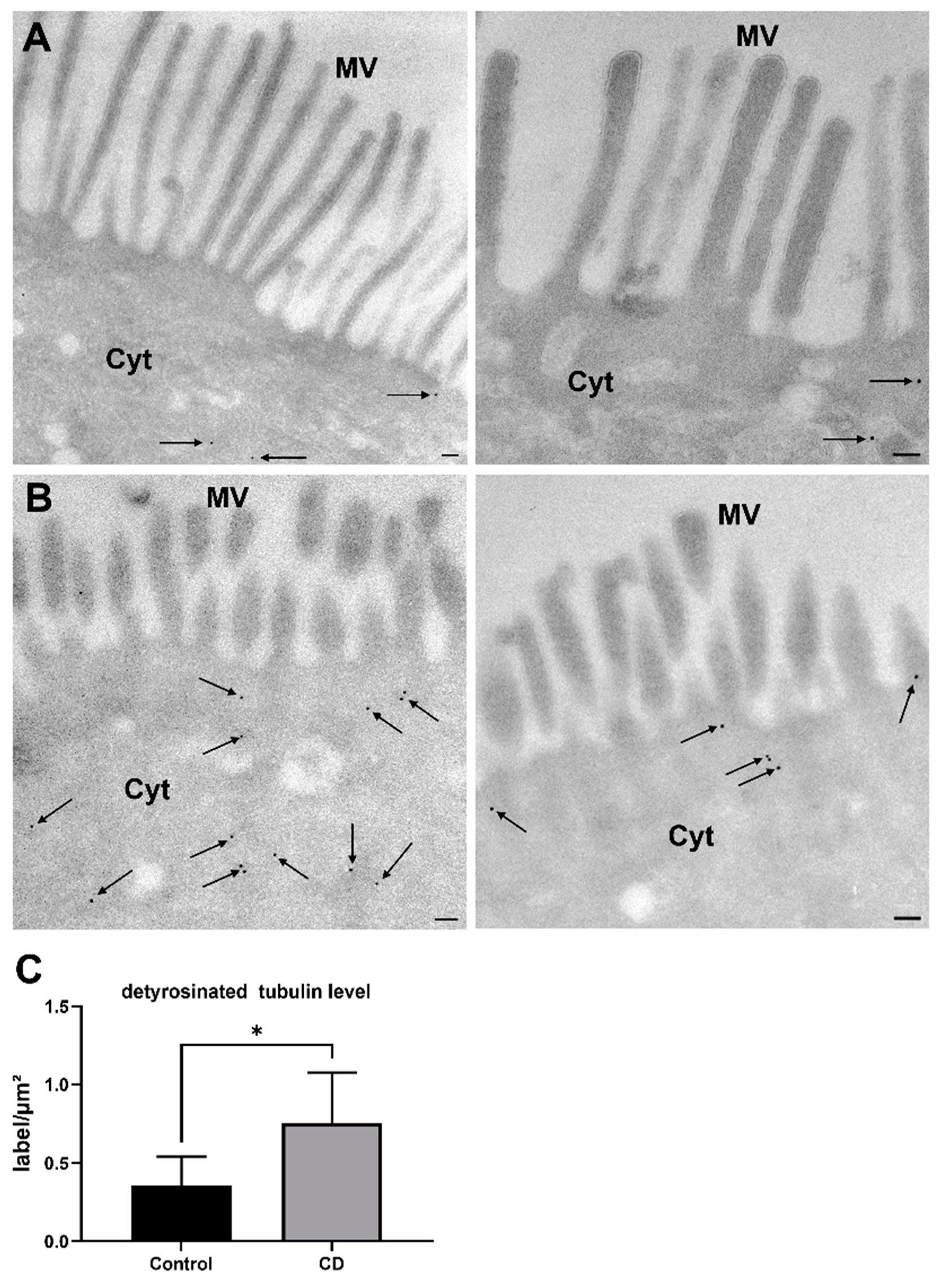
2.1. Increased Levels of Detyr-Tubulin and Disturbed Cellular Morphology in CD Epithelium
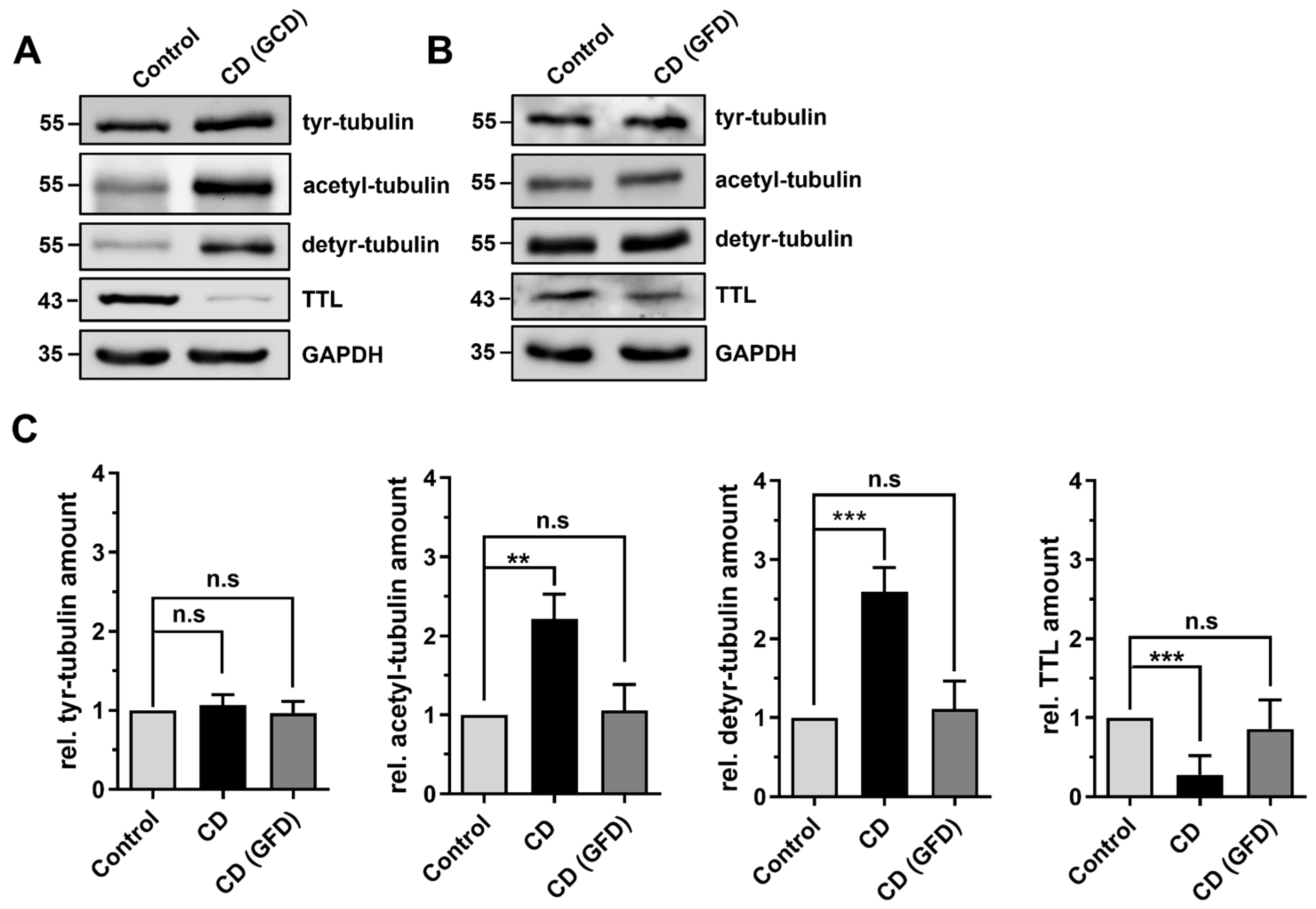
2.2. Differential Posttranslational Modification of Tubulin in CD Patients with Active Disease and in Remission
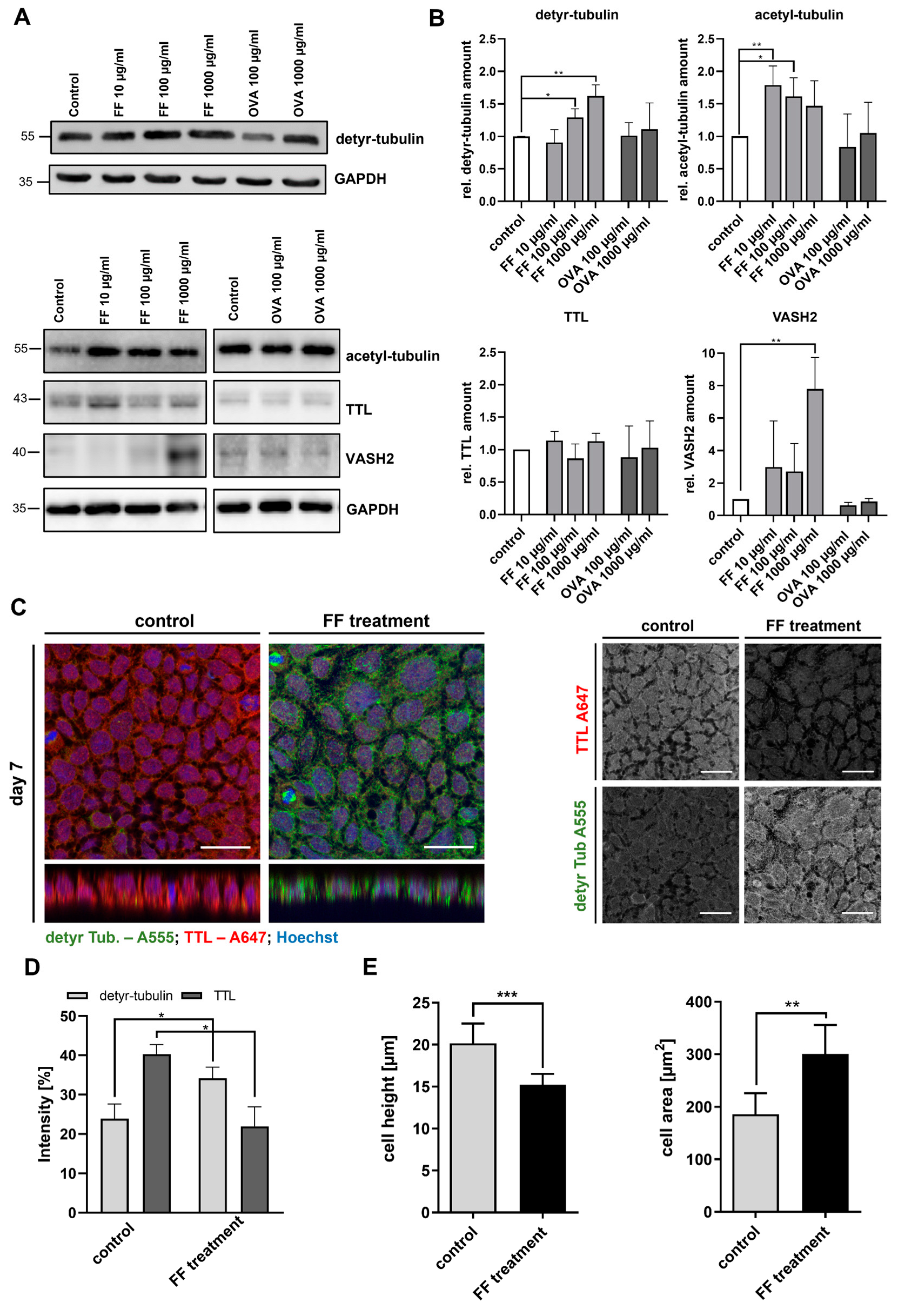
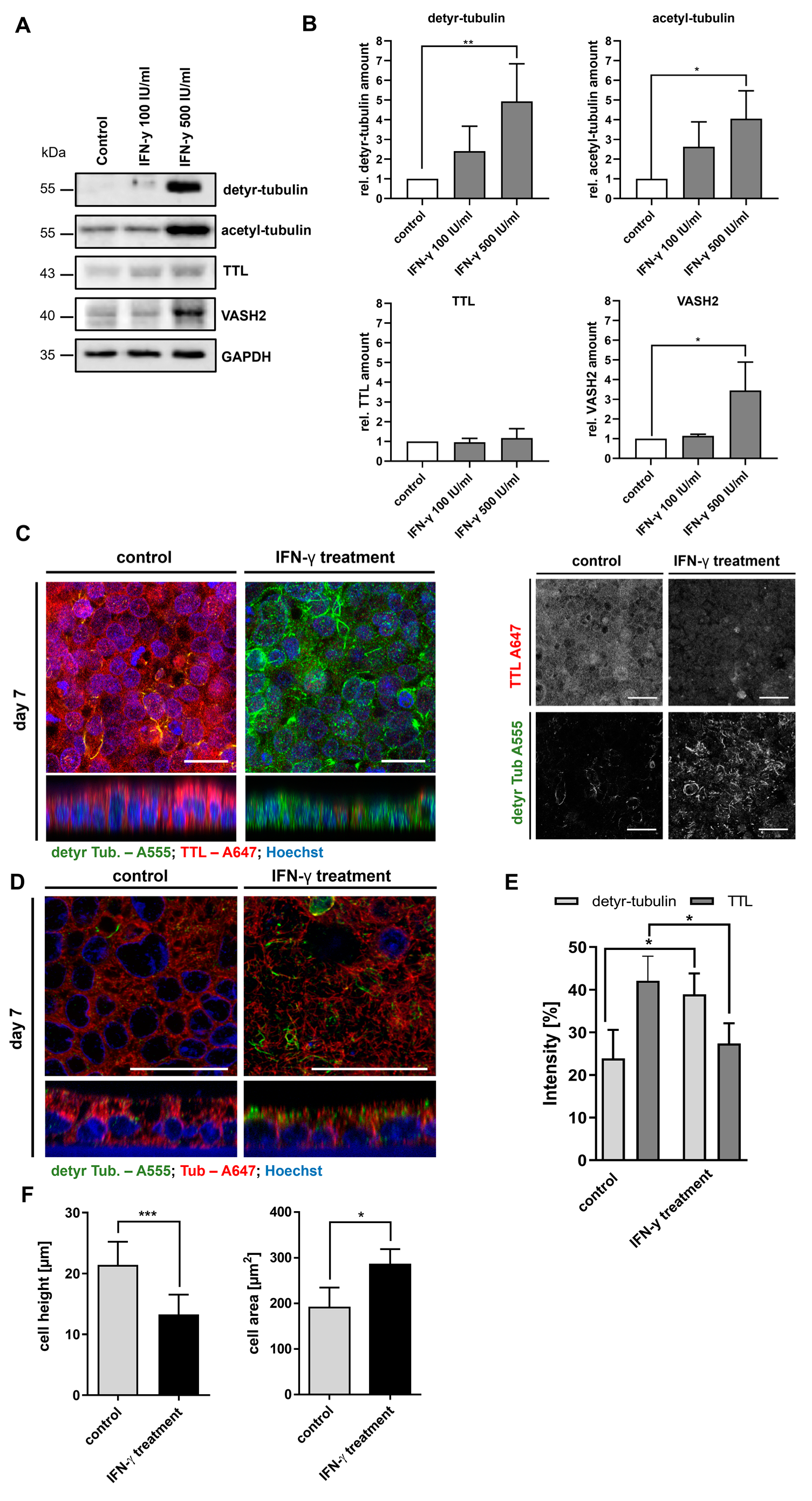
2.3. Stimulation of Caco-2 Cells with Digested Gliadin and IFN-y Alters PTM of Microtubules and Affects Cellular Morphology
2.4. Knockout of TTL Disturbs Brush Border Membrane Architecture in MDCK Cells
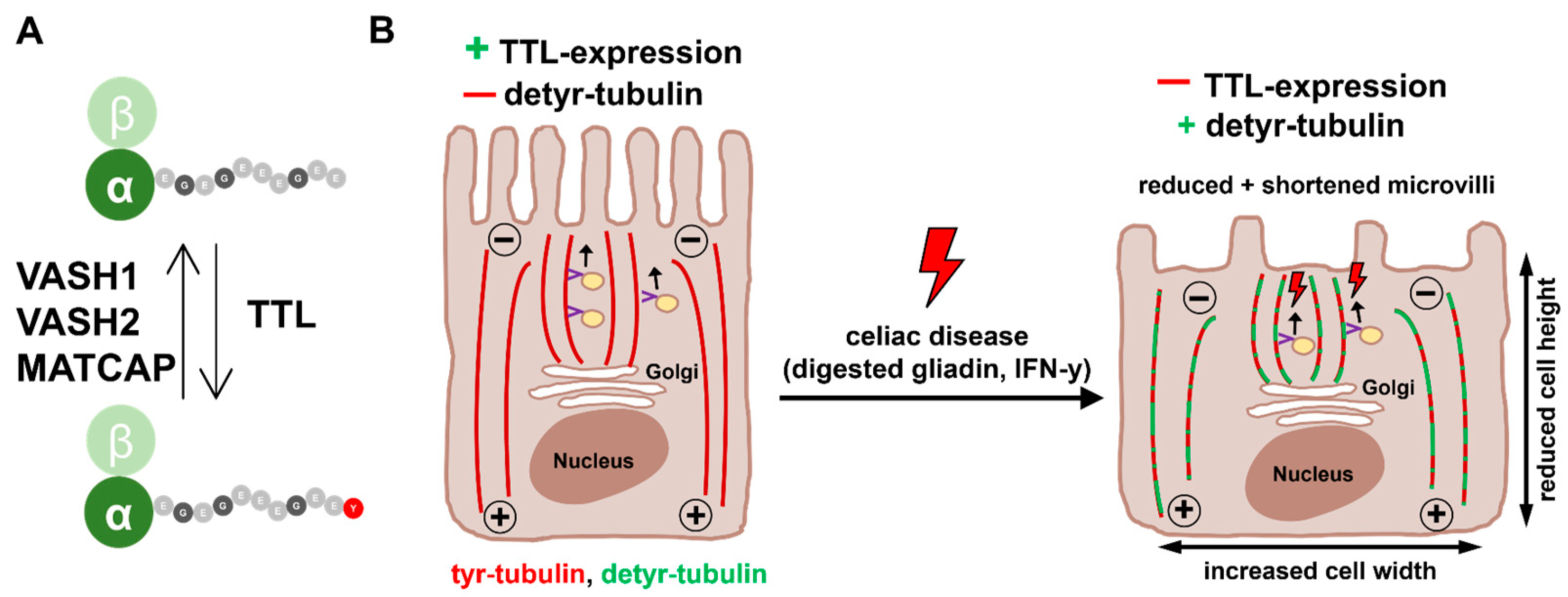
3. Discussion
4. Materials and Methods
4.1. Patients’ Characteristics
4.2. Cell Culture
4.3. Immunoblot
4.4. Immunofluorescence Microscopy
4.5. Electron Microscopy
4.6. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levescot, A.; Malamut, G.; Cerf-Bensussan, N. Immunopathogenesis and environmental triggers in coeliac disease. Gut 2022, 71, 2337–2349. [Google Scholar] [CrossRef] [PubMed]
- Tye-Din, J.A.; Galipeau, H.J.; Agardh, D. Celiac Disease: A Review of Current Concepts in Pathogenesis, Prevention, and Novel Therapies. Front. Pediatr. 2018, 6, 350. [Google Scholar] [CrossRef]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836.e2. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Jeong, J.; Underwood, F.E.; Quan, J.; Panaccione, N.; Windsor, J.W.; Coward, S.; deBruyn, J.; Ronksley, P.E.; Shaheen, A.-A.; et al. Incidence of Celiac Disease Is Increasing Over Time. Am. J. Gastroenterol. 2020, 115, 507–525. [Google Scholar] [CrossRef] [PubMed]
- Nardecchia, S.; Auricchio, R.; Discepolo, V.; Troncone, R. Extra-Intestinal Manifestations of Coeliac Disease in Children: Clinical Features and Mechanisms. Front. Pediatr. 2019, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Arentz-Hansen, H.; Korner, R.; Molberg, O.; Quarsten, H.; Vader, L.W.; Kooy, Y.M.; Lundin, K.E.A.; Koning, F.; Roepstorff, P.; Sollid, L.M.; et al. The Intestinal T Cell Response to alpha-Gliadin in Adult Celiac Disease is Focused on a Single Deamidated Glutamine Targeted by Tissue Transglutaminase. J. Exp. Med. 2000, 191, 603–612. [Google Scholar] [CrossRef]
- Shan, L.; Molberg, O.; Parrot, I.; Hausch, F.; Filiz, F.; Gray, G.M.; Sollid, L.M.; Khosla, C. Structural basis for gluten intolerance in Celiac sprue. Science 2002, 297, 2275–2279. [Google Scholar] [CrossRef]
- Barone, M.V.; Troncone, R.; Auricchio, S. Gliadin peptides as triggers of the proliferative and stress/innate immune response of the celiac small intestinal mucosa. Int. J. Mol. Sci. 2014, 15, 20518–20537. [Google Scholar] [CrossRef]
- Dickson, B.C.; Streutker, C.J.; Chetty, R. Coeliac disease: An update for pathologists. J. Clin. Pathol. 2006, 59, 1008–1016. [Google Scholar] [CrossRef]
- Sowińska, A.; Morsy, Y.; Czarnowska, E.; Oralewska, B.; Konopka, E.; Woynarowski, M.; Szymańska, S.; Ejmont, M.; Scharl, M.; Bierła, J.B.; et al. Transcriptional and Ultrastructural Analyses Suggest Novel Insights into Epithelial Barrier Impairment in Celiac Disease. Cells 2020, 9, 516. [Google Scholar] [CrossRef]
- Dyduch, A.; Karczewska, K.; Grzybek, H.; Kaminski, M. Transmission Electron Microscopy of Microvilli of Intestinal Epithelial Cells in Celiac Disease in Remission and Transient Gluten Enteropathy in Children After a Gluten-Free Diet. J. Pediatr. Gastroenterol. Nutr. 1993, 16, 269–272. [Google Scholar] [CrossRef]
- Shiner, M.; Birbeck, M. The microvilli of the small intestinal surface epithelium in coeliac disease and in idiopathic steatorrhoea. Gut 1961, 2, 277–284. [Google Scholar] [CrossRef]
- Zink, S.; Jacob, R. Tubulin Detyrosination in Epithelial Cells. In The Cytoskeleton in Health and Disease; Schatten, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 187–194. [Google Scholar]
- Janke, C.; Magiera, M.M. The tubulin code and its role in controlling microtubule properties and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 307–326. [Google Scholar] [CrossRef]
- Sirajuddin, M.; Rice, L.M.; Vale, R.D. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat. Cell Biol. 2014, 16, 335–344. [Google Scholar] [CrossRef]
- Xu, Z.; Schaedel, L.; Portran, D.; Aguilar, A.; Gaillard, J.; Marinkovich, M.P.; Théry, M.; Nachury, M.V. Microtubules acquire resistance from mechanical breakage through intralumenal acetylation. Science 2017, 356, 328–332. [Google Scholar] [CrossRef]
- Quinones, G.B.; Danowski, B.A.; Devaraj, A.; Singh, V.; Ligon, L.A. The posttranslational modification of tubulin undergoes a switch from detyrosination to acetylation as epithelial cells become polarized. MBoC 2011, 22, 1045–1057. [Google Scholar] [CrossRef]
- Peris, L.; Wagenbach, M.; Lafanechère, L.; Brocard, J.; Moore, A.T.; Kozielski, F.; Job, D.; Wordeman, L.; Andrieux, A. Motor-dependent microtubule disassembly driven by tubulin tyrosination. J. Cell Biol. 2009, 185, 1159–1166. [Google Scholar] [CrossRef]
- Kreis, T.E. Microtubules containing detyrosinated tubulin are less dynamic. EMBO J. 1987, 6, 2597–2606. [Google Scholar] [CrossRef]
- Niewenhuis, J.; Adamopoulos, A.; Bleijerveld, O.B.; Mazouzi, A.; Stickel, E.; Celie, P.; Altelaar, M.; Knipscheer, P.; Perrakis, A.; Blomen, V.A.; et al. Vasohibins encode tubulin detyrosinating activity. Science 2017, 358, 1453–1456. [Google Scholar] [CrossRef]
- Landskron, L.; Bak, J.; Adamopoulos, A.; Kaplani, K.; Moraiti, M.; van den Hengel, L.G.; Song, J.-Y.; Bleijerveld, O.B.; Nieuwenhuis, J.; Heidebrecht, T.; et al. Posttranslational modification of microtubules by the MATCAP detyrosinase. Science 2022, 376, eabn6020. [Google Scholar] [CrossRef]
- Magiera, M.M.; Singh, P.; Gadadhar, S.; Janke, C. Tubulin Posttranslational Modifications and Emerging Links to Human Disease. Cell 2018, 173, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Barisic, M.; Maiato, H. The Tubulin Code: A Navigation System for Chromosomes during Mitosis. Trends Cell Biol. 2016, 26, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Ringer, K.; Hub, F.; Kamm, N.; Worzfeld, T.; Jacob, R. TTL-Expression Modulates Epithelial Morphogenesis. Front. Cell Dev. Biol. 2021, 9, 635723. [Google Scholar] [CrossRef]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the Human Colon Carcinoma Cell Line (Caco-2) as a Model System for Intestinal Epithelial Parmeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef]
- Müller, M.; Gorek, L.; Kamm, N.; Jacob, R. Manipulation of the Tubulin Code Alters Directional Cell Migration and Ciliogenesis. Front. Cell Dev. Biol. 2022, 10, 901999. [Google Scholar] [CrossRef]
- Mohamed, B.; Feighery, C.; Williams, Y.; Davies, A.; Kelleher, D.; Volkov, Y.; Kelly, J.; Abuzakouk, M. The use of Cellomics to study enterocyte cytoskeletal proteins in coeliac disease patients. Open Life Sci. 2008, 3, 258–267. [Google Scholar] [CrossRef]
- Frisoni, M.; Corazza, G.R.; Lafiandra, D.; de Ambrogio, E.; Filipponi, C.; Bonvicini, F.; Borasio, E.; Porceddu, E.; Gasbarrini, G. Wheat deficient in gliadins: Promising tool for treatment of coeliac disease. Gut 1995, 36, 375–378. [Google Scholar] [CrossRef][Green Version]
- Zink, S.; Grosse, L.; Freikamp, A.; Bänfer, S.; Müksch, F.; Jacob, R. Tubulin detyrosination promotes monolayer formation and apical trafficking in epithelial cells. J. Cell Sci. 2012, 125, 5998–6008. [Google Scholar] [CrossRef]
- Porpora, M.; Conte, M.; Lania, G.; Bellomo, C.; Rapacciuolo, L.; Chirdo, F.G.; Auricchio, R.; Troncone, R.; Auricchio, S.; Barone, M.V.; et al. Inflammation Is Present, Persistent and More Sensitive to Proinflammatory Triggers in Celiac Disease Enterocytes. Int. J. Mol. Sci. 2022, 23, 1973. [Google Scholar] [CrossRef]
- Achler, C.; Filmer, D.; Merte, C.; Drenckhahn, D. Role of Microtubules in Polarized Delivery of Apical Membrane Proteins to the Brush Border of the Intestinal Epithelium. J. Cell Biol. 1989, 109, 179–189. [Google Scholar] [CrossRef]
- Khanal, I.; Elbediwy, A.; La Diaz de Loza, M.D.C.; Fletcher, G.C.; Thompson, B.J. Shot and Patronin polarise microtubules to direct membrane traffic and biogenesis of microvilli in epithelia. J. Cell Sci. 2016, 129, 2651–2659. [Google Scholar] [CrossRef] [PubMed]
- D’Alterio, C.; Tran, D.D.D.; Yeung, M.W.Y.A.; Hwang, M.S.H.; Li, M.A.; Arana, C.J.; Mulligan, V.K.; Kubesh, M.; Sharma, P.; Chase, M.; et al. Drosophila melanogaster Cad99C, the orthologue of human Usher cadherin PCDH15, regulates the length of microvilli. J. Cell Biol. 2005, 171, 549–558. [Google Scholar] [CrossRef]
- Schneider, G.; Nieznanski, K.; Jozwiak, J.; Slomnicki, L.P.; Redowicz, M.J.; Filipek, A. Tubulin binding protein, CacyBP/SIP, induces actin polymerization and may link actin and tubulin cytoskeletons. Biochim. Biophys. Acta 2010, 1803, 1308–1317. [Google Scholar] [CrossRef]
- Grefner, N.M.; Gromova, L.V.; Gruzdkov, A.A.; Komissarchik, Y.Y. Interaction of glucose transporters SGLT1 and GLUT2 with cytoskeleton in enterocytes and Caco2 cells during hexose absorption. Cell Tiss. Biol. 2015, 9, 45–52. [Google Scholar] [CrossRef]
- Reinke, Y.; Zimmer, K.-P.; Naim, H.Y. Toxic peptides in Frazer’s fraction interact with the actin cytoskeleton and affect the targeting and function of intestinal proteins. Exp. Cell Res. 2009, 315, 3442–3452. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabo, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef]
- Frazer, A.C.; Fletcher, R.F.; Ross, C.A.; Shaw, B.; Sammons, H.G.; Schneider, R. Gluten-induced enteropathy: The effect of partially digested gluten. Lancet 1959, 2, 252–255. [Google Scholar] [CrossRef]
- Tokuyasu, K.T. A technique for ultracryotomy of cell suspensions and tissues. J. Cell Biol. 1973, 57, 551–565. [Google Scholar] [CrossRef]
- Griffiths, G. Fine Structure Immunocytochemistry; Springer: Berlin/Heidelberg, Germany, 1993; ISBN 3642770959. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]

| Patient | Patient Group | Age | Sex | Anti-TG2-IgA (IU/mL) | MARSH | Remarks |
|---|---|---|---|---|---|---|
| 1 | CD | 10 | f | 20 | 3a-b | |
| 2 | CD | 16 | f | 200 | 3b | |
| 3 | CD | 14 | m | 11 | 3b-c | IgA deficiency |
| 4 | CD | 13 | f | 200 | 3a-b | |
| 5 | CD | 11 | m | 200 | 3b-c | |
| 6 | CD | 3 | f | 200 | 3b | |
| 7 | CD | 6 | f | 200 | 3a | |
| 8 | CD | 10 | f | 186 | 3b | |
| 9 | CD | 13 | m | 200 | 3a | |
| 10 | CD GFD | 4 | f | 62 | 2 | |
| 11 | CD GFD | 16 | f | 20 | 0 | |
| 12 | CD GFD | 9 | f | 34 | 0 | |
| 13 | CD GFD | 3 | f | 200 | 3a | |
| 14 | Co | 15 | f | <20 | 0 | Functional abdominal pain |
| 15 | Co | 15 | f | <20 | 0 | Familial adenomatous polyposis |
| 16 | Co | 8 | m | <20 | 0 | Reflux esophagitis A |
| 17 | Co | 14 | f | <20 | 0 | Reflux esophagitis A |
| 18 | Co | 13 | m | <20 | 0 | Eosinophilic esophagitis |
| 19 | Co | 11 | f | <20 | 0 | Crohn’s colitis |
| 20 | Co | 14 | f | <20 | 0 | Gastritis type C |
| 21 | Co | 16 | f | <20 | 0 | Gastritis type C |
| 22 | Co | 11 | m | <20 | 0 | Crohn’s colitis |
| 23 | Co | 10 | f | <20 | 0 | Functional abdominal pain |
| Target | Antibody | Host/Clonality | Dilution | Company |
|---|---|---|---|---|
| Acetylated α-tubulin | T6739 | Mouse/monoclonal Clone 6-11B-1 | IB: 1–500 | Sigma-Aldrich, St. Louis, MO, USA |
| Detyrosinated α-tubulin | #AB3201 | Rabbit/polyclonal | IB: 1–5000 IF: 1–200 EM: 1–10 | Merck Millipore, Darmstadt, Germany |
| GAPDH | 5G4 | Mouse/monoclonal | IB: 1–500 | HyTest Ltd., Turku, Finland |
| Tubulin tyrosine ligase | 13618-1-AP | Rabbit/polyclonal | IB: 1–500 IF: 1–200 | Proteintech, Rosemont, IL, USA |
| Tyrosinated α-tubulin | Sc-53029 | Rat/monoclonal Clone YL1/2 | IB: 1–5000 IF: 1–200 | Santa Cruz Biotechnology, Dallas, TX, USA |
| VASH2 | ab224723 | Rabbit/polyclonal | IB: 1–2000 | Abcam, Cambridge, UK |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stricker, S.; Müller, M.; Zimmer, K.-P.; Jacob, R. Altered Posttranslational Modification of Microtubules Contributes to Disturbed Enterocyte Morphology in Celiac Disease. Int. J. Mol. Sci. 2023, 24, 2635. https://doi.org/10.3390/ijms24032635
Stricker S, Müller M, Zimmer K-P, Jacob R. Altered Posttranslational Modification of Microtubules Contributes to Disturbed Enterocyte Morphology in Celiac Disease. International Journal of Molecular Sciences. 2023; 24(3):2635. https://doi.org/10.3390/ijms24032635
Chicago/Turabian StyleStricker, Sebastian, Manuel Müller, Klaus-Peter Zimmer, and Ralf Jacob. 2023. "Altered Posttranslational Modification of Microtubules Contributes to Disturbed Enterocyte Morphology in Celiac Disease" International Journal of Molecular Sciences 24, no. 3: 2635. https://doi.org/10.3390/ijms24032635
APA StyleStricker, S., Müller, M., Zimmer, K.-P., & Jacob, R. (2023). Altered Posttranslational Modification of Microtubules Contributes to Disturbed Enterocyte Morphology in Celiac Disease. International Journal of Molecular Sciences, 24(3), 2635. https://doi.org/10.3390/ijms24032635






