RPLP1 Is Up-Regulated in Human Adenomyosis and Endometrial Adenocarcinoma Epithelial Cells and Is Essential for Cell Survival and Migration In Vitro
Abstract
:1. Introduction
2. Results
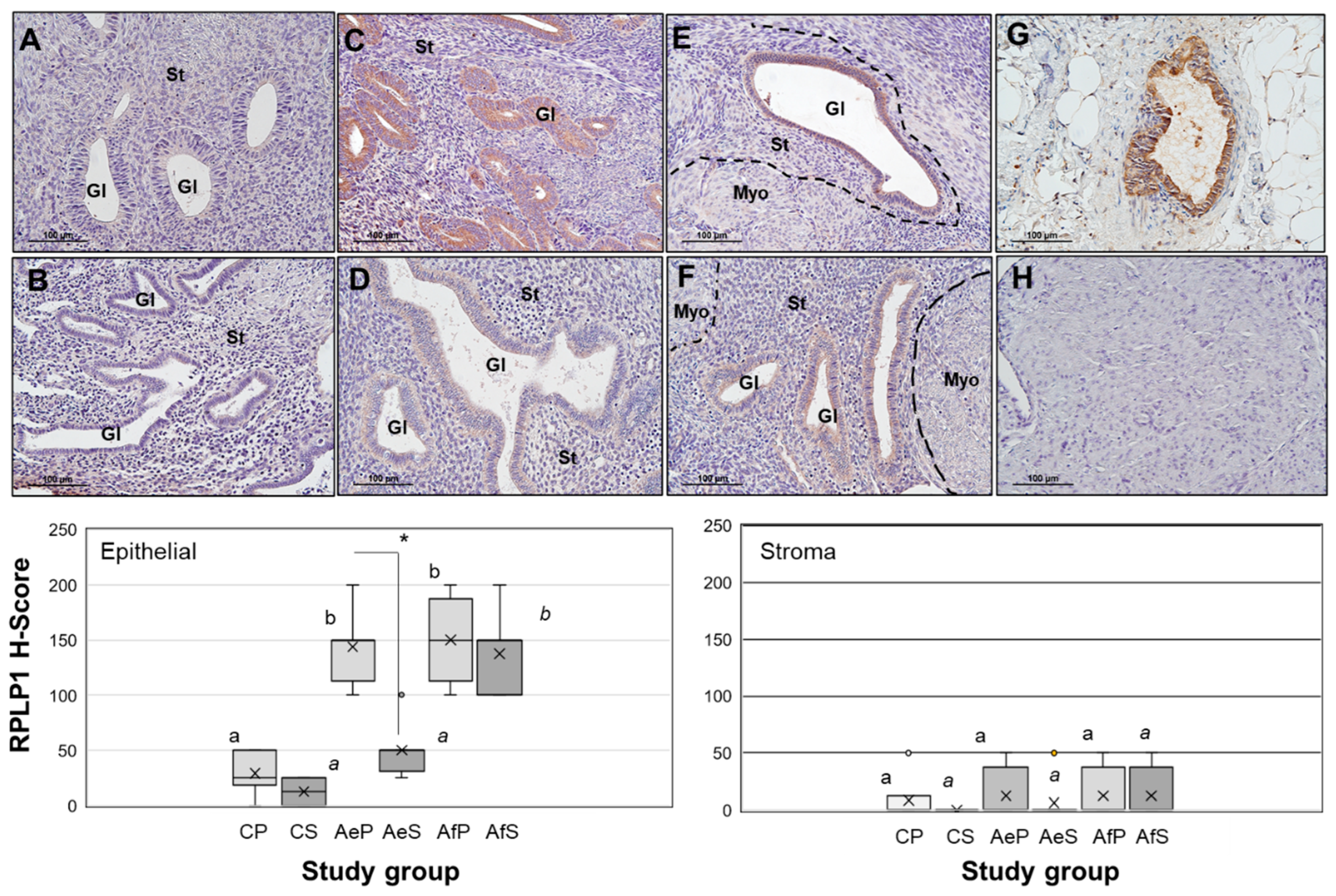
2.1. RPLP1 Protein Localization in Adenomyotic Tissue
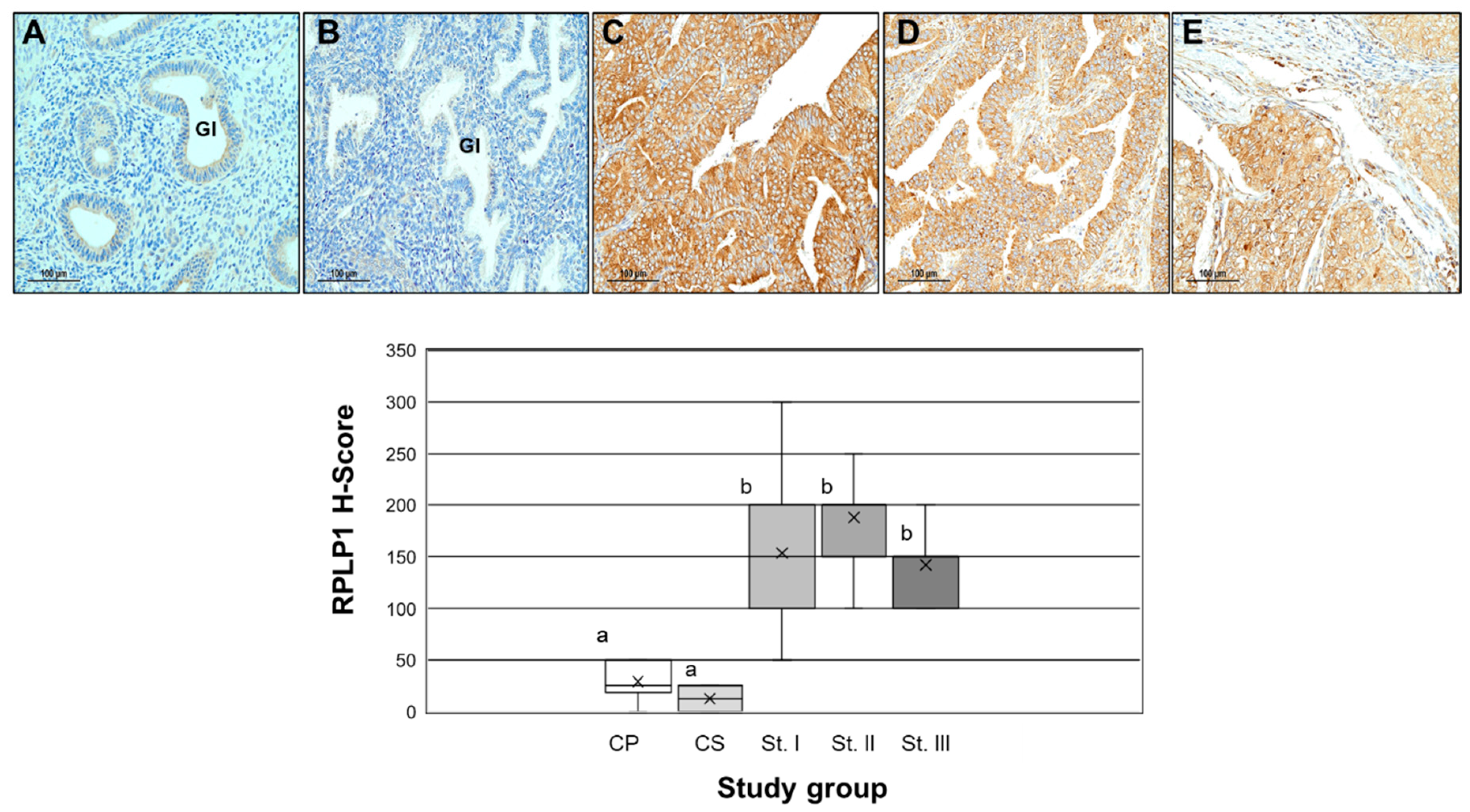
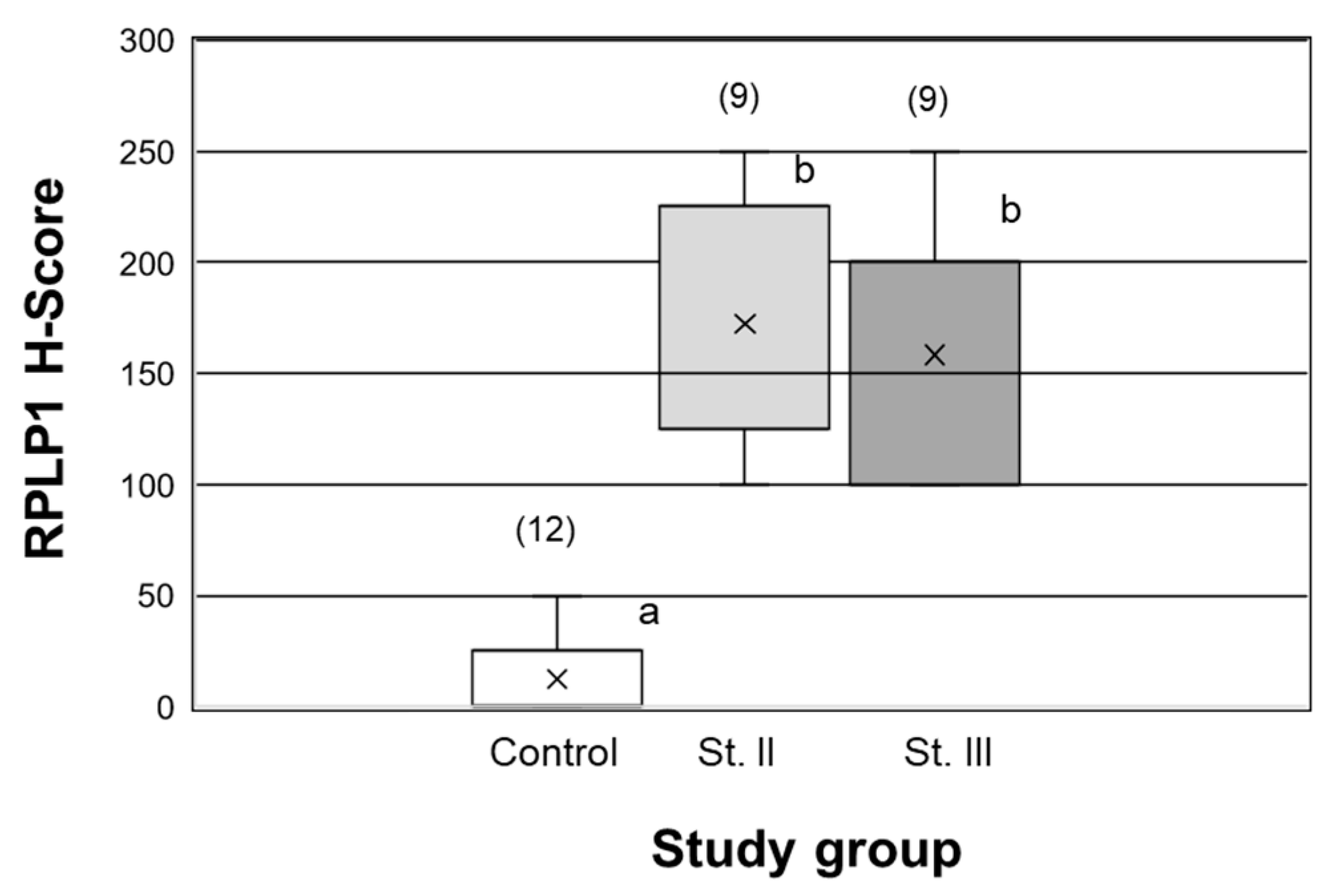
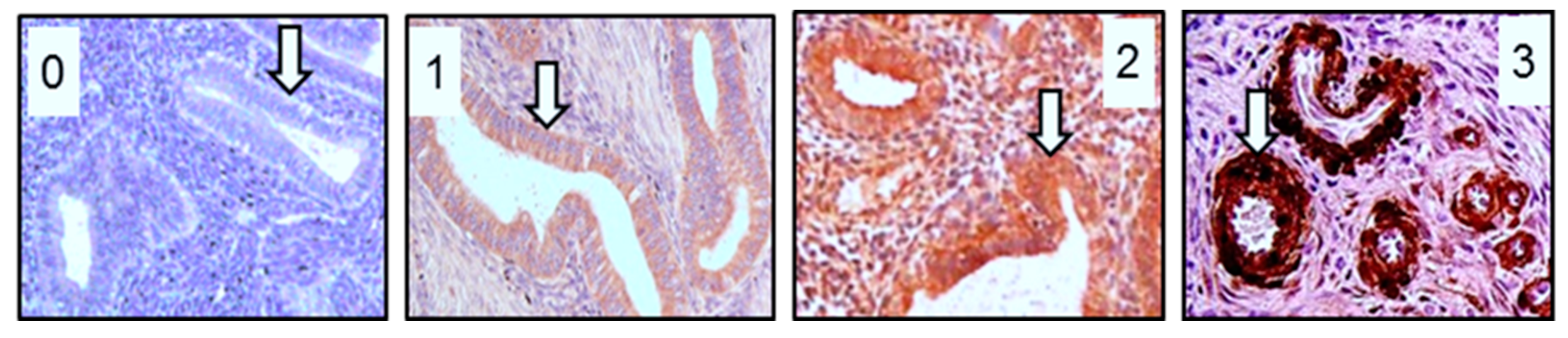
2.2. RPLP1 Protein Localizatoin in Endometrial Carcinoma
2.3. RPLP1 Expression in Endometrial Adenocarcinoma Cell Lines
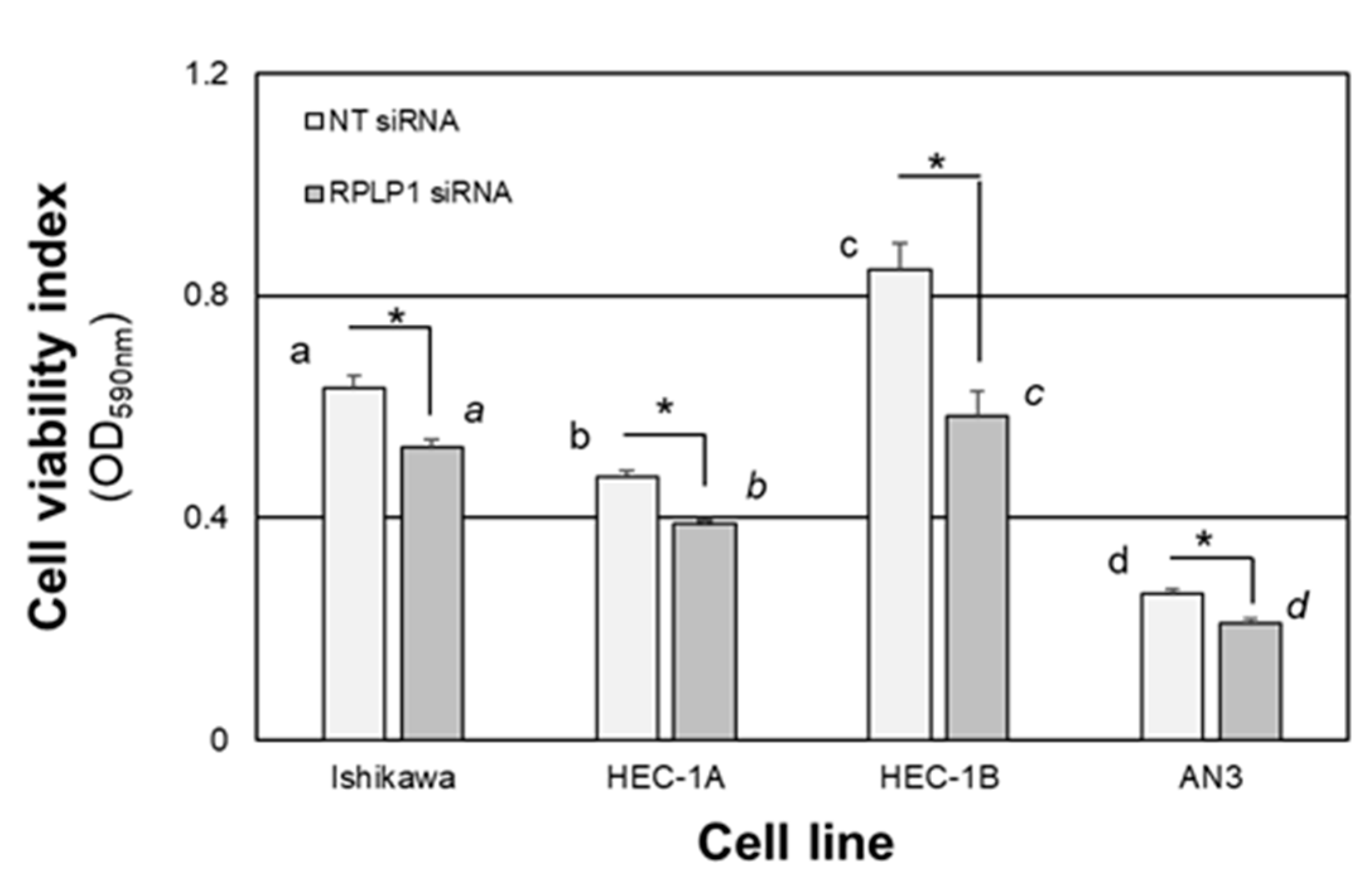
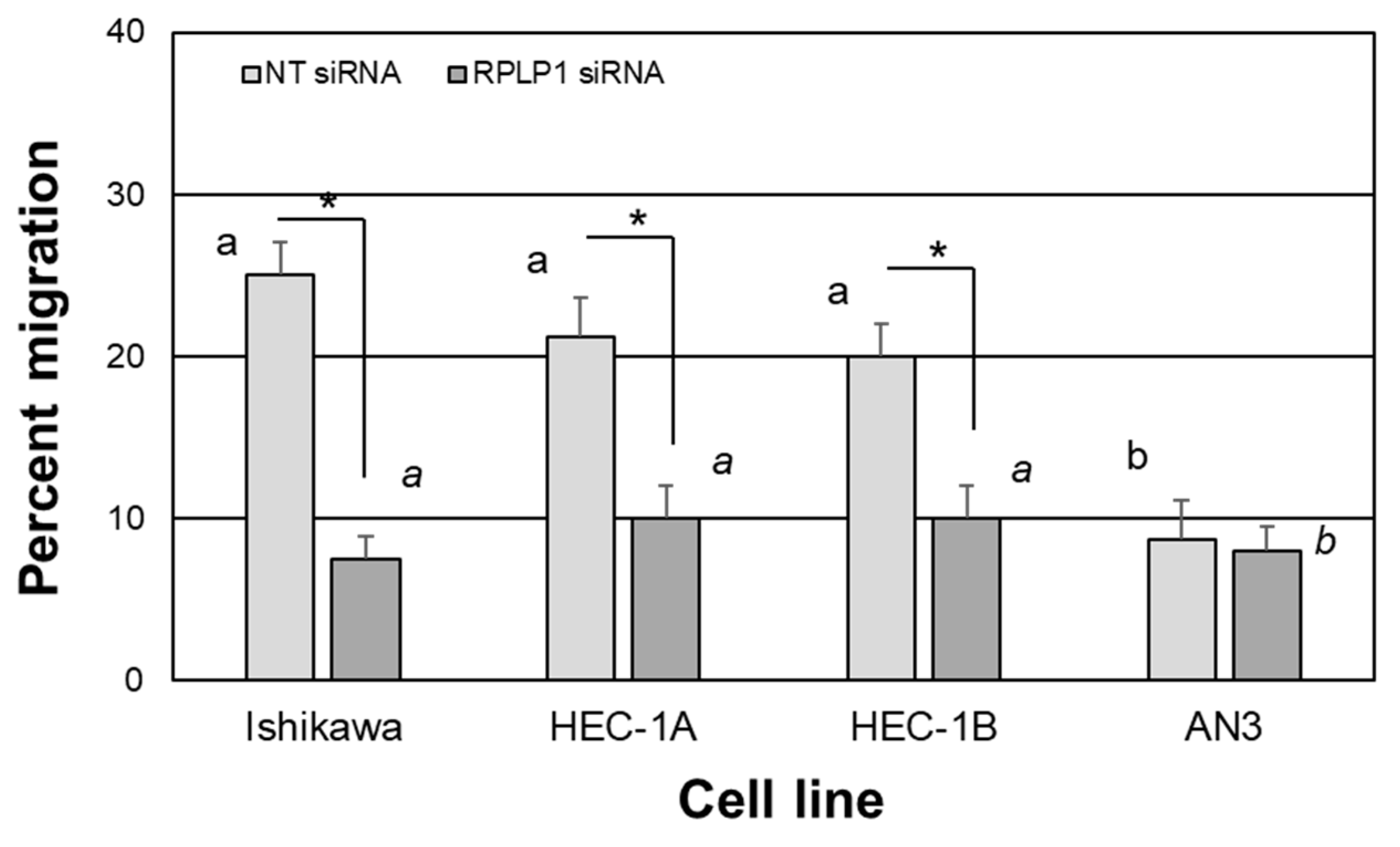
2.4. RPLP1 Regulates Endometrial Adenocarcinoma Cell Survival and Migration
3. Discussion
4. Materials and Methods
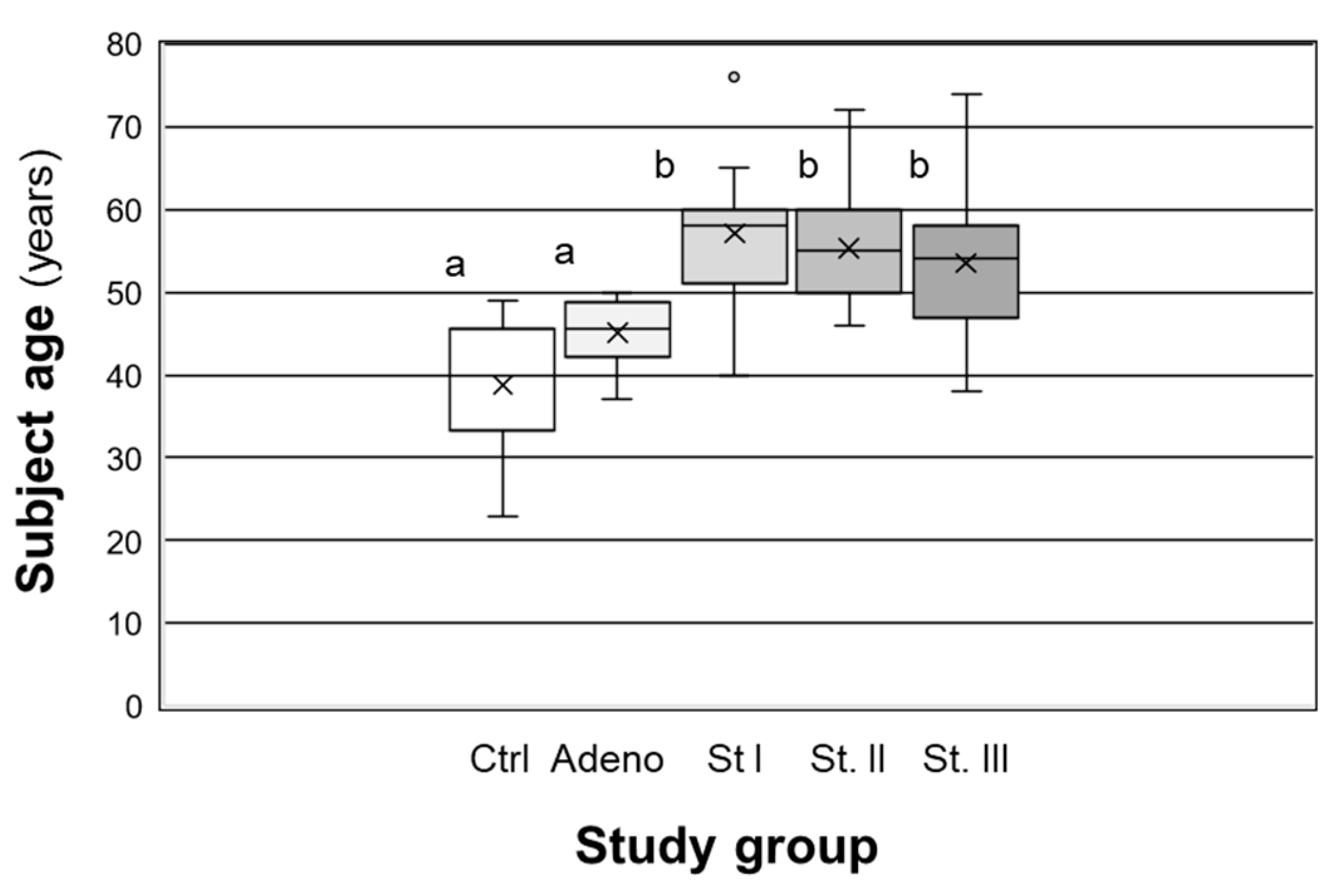
4.1. Human Subjects and Tissue Acquisition
4.2. Immunohistochemistry Staining and Quantitation
4.3. mRNA Isolation and qRT-PCR
4.4. Protein Isolation and Western Blot Analysis
4.5. Cell Culture and Transfection
4.6. Cell Viability/Crystal Violet Staining Assays
4.7. Cell Migration/Scratch Assays
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kobayashi, H.; Matsubara, S.; Imanaka, S. Clinicopathological features of different subtypes in adenomyosis: Focus on early lesions. PLoS ONE 2021, 16, e0254147. [Google Scholar] [CrossRef] [PubMed]
- Vannuccini, S.; Petraglia, F. Recent advances in understanding and managing adenomyosis. F1000Research 2019, 8, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Wang, P.-H.; Yang, S.-T.; Liu, C.-H.; Chang, W.-H.; Lee, F.-K.; Lee, W.-L. Endometrial cancer: Part I. Basic concept. Taiwan J. Obstet. Gynecol. 2022, 61, 951–959. [Google Scholar] [CrossRef]
- Mao, X.; Zheng, W.; Mao, W. Malignant changes in adenomyosis in patients with endometrial adenocarcinoma: A case series. Medicine 2017, 96, e8336. [Google Scholar] [CrossRef]
- Pollacco, J.; Sacco, K.; Portelli, M.; Schembri-Wismayer, P.; Calleja-Agius, J. Molecular links between endometriosis and cancer. Gynecol. Endocrinol. 2012, 28, 577–581. [Google Scholar] [CrossRef]
- Stephens, V.R.; Rumph, J.T.; Ameli, S.; Bruner-Tran, K.L.; Osteen, K.G. The potential relationship between environmental endocrine disruptor exposure and the development of endometriosis and adenomyosis. Front. Physiol. 2022, 12, 807685. [Google Scholar] [CrossRef]
- Hermens, M.; van Altena, A.M.; Velthuis, I.; van de Laar, D.C.M.; Bulten, J.; van Vliet, H.A.A.M.; Siebers, A.G.; Bekkers, R.L.M. Endometrial cancer incidence in endometriosis and adenomyosis. Cancers 2021, 13, 4592. [Google Scholar] [CrossRef]
- Alali, Z.; Graham, A.; Swan, K.; Flyck, R.; Falcone, T.; Cui, W.; Yang, X.; Christianson, J.; Nothnick, W.B. 60S acidic ribosomal protein P1 (RPLP1) is elevated in human endometriotic tissue and in a murine model of endometriosis and is essential for endometriotic epithelial cell survival in vitro. Mol. Hum. Reprod. 2020, 26, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Artero-Castro, A.; Castellvi, J.; García, A.; Hernández, J.; Ramón y Cajal, S.; Lleonart, M.E. Expression of the ribosomal proteins Rplp0, Rplp1, and Rplp2 in gynecologic tumors. Hum. Pathol. 2011, 42, 194–203. [Google Scholar] [CrossRef]
- Bargis-Surgey, P.; Lavergne, J.P.; Gonzalo, P.; Vard, C.; Filhol-Cochet, O.; Reboud, J.P. Interaction of elongation factor eEF-2 with ribosomal P proteins. Eur. J. Biochem. 1999, 262, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Cao, K.; Peng, D.; Qin, L. RPLP1 is highly expressed in hepatocellular carcinoma tissues and promotes proliferation, invasion and migration of human hepatocellular carcinoma Hep3b cells. Exp. Ther. Med. 2021, 752, 10184. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Yue, Y.; Li, M.; Zhang, Y.-N.; Zhao, L.; Lu, W.; Wang, X.; Xie, X. CNN3 acts as a potential oncogene in cervical cancer by affecting RPLP1 mRNA expression. Sci. Rep. 2020, 10, 2427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; Xu, Q.; Wang, X.; Wang, J.; Mu, X.; Cai, Y.; Qian, Y.; Shao, W.; Shao, Z. RPLP1 promotes tumor metastasis and is associated with a poor prognosis in triple-negative breast cancer patients. Cancer Cell Int. 2018, 18, 170. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.J.; Hookway, E.; Athanasou, N.; Kashima, T.; Oppermann, U.; Hughes, S.; Swan, D.; Lueerssen, D.; Anson, J.; Hassan, A.B. A germline mutation of CDKN2A and a novel RPLP1-C19MC fusion detected in a rare melanotic neuroectodermal tumor of infancy: A case report. BMC Cancer 2016, 16, 629. [Google Scholar] [CrossRef] [Green Version]
- Kozak, J.; Wdowiak, P.; Maciejewski, R.; Torres, A. A guide for endometrial cancer cell lines functional assays using the measures of electronic impedence. Cytotechnology 2018, 70, 339–350. [Google Scholar] [CrossRef] [Green Version]
- Artero-Castro, A.; Kondoh, H.; Fernández-Marcos, P.J.; Serrano, M.; Ramón y Cajal, S.; Lleonart, M. E Rplp1 bypasses replicative senescence and contributes to transformation. Exp. Cell Res. 2009, 315, 1372–1383. [Google Scholar] [CrossRef]
- Perucho, L.; Artero-Castro, A.; Guerrero, S.; Ramón y Cajal, S.; Lleonart, M.E.; Wang, Z.Q. RPLP1, a crucial ribosomal protein for embryonic development of the nervous system. PLoS ONE 2014, 9, e99956. [Google Scholar] [CrossRef] [Green Version]
- Artero-Castro, A.; Pere-Alea, M.; Feliciano, A.; Leal, J.A.; Genestar, M.; Castellvi, J.; Peg, V.; Ramón y Cajal, S.; Lleonart, M.E. Disruption of the ribosomal P complex leads to stress-induced autophagy. Autophagy 2015, 11, 1499–1519. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.K.; Wong, B.; Xie, X.; Lu, Y.F.; Shi, P.Y.; Pompon, J.; Garcia-Blanco, A.; Bradrick, S.S. RPLP1 and RPLP2 are essential flavivirus host proteins that promote early viral protein accumulation. J. Virol. 2017, 91, e01706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, K.; Washio, T.; Uechi, T.; Yoshihama, M.; Kenmochi, N.; Tomita, M. Characteristics and clustering of human ribosomal protein genes. BMC Genom. 2006, 7, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aursnes, I.A.; Rishovd, A.L.; Karlsen, H.E.; Gjøen, T. Validation of reference genes for quantitative RT-qPCR studies of gene expression in Atlantic cod (Gadus morhua l.) during temperature stress. BMC Res. Notes 2011, 4, 104. [Google Scholar] [CrossRef] [Green Version]
- Marques, T.E.; de Mendonça, L.R.; Pereira, M.G.; de Andrade, T.G.; Garcia-Cairasco, N.; Paçó-Larson, M.L.; Gitaí, D.L.G. Validation of suitable reference genes for expression studies in different pilocarpine-induced models of mesial temporal lobe epilepsy. PLoS ONE 2013, 8, e71892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barragán, M.; Martínez, A.; Llonch, S.; Pujol, A.; Vernaeve, V.; Vassena, R. Effect of ribonucleic acid (RNA) isolation methods on putative reference genes messenger RNA abundance in human spermatozoa. Andrology 2015, 3, 797–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, A.H.; Curi, R.; Salazar, L.A. Selection of reference genes for expression analyses in liver of rats with impaired glucose metabolism. Int. J. Clin. Exp. Pathol. 2015, 8, 3946–3954. [Google Scholar]
- Kang, I.N.; Lee, C.Y.; Tan, S.C. Selection of best reference genes for qRT-PCR analysis of human neural stem cells preconditioned with hypoxia or baicalein-enriched fraction extracted from Oroxylum indicum medicinal plant. Heliyon 2019, 5, e02156. [Google Scholar] [CrossRef] [Green Version]
- Benak, D.; Sotakova-Kasparova, D.; Neckar, J.; Kolar, F.; Hlavackova, M. Selection of optimal reference genes for gene expression studies in chronically hypoxic rat heart. Mol. Cell Biochem. 2019, 461, 15–22. [Google Scholar] [CrossRef]
- Panina, Y.; Germond, A.; Watanabe, T.M. Analysis of the stability of 70 housekeeping genes during iPS reprogramming. Sci. Rep. 2020, 10, 21711. [Google Scholar] [CrossRef]
- Ghule, P.N.; Medina, R.; Lengner, C.J.; Mandeville, M.; Qiao, M.; Dominski, Z.; Lian, J.B.; Stein, J.L.; van Wijnen, A.J.; Stein, G.S. Reprogramming the pluripotent cell cycle: Restoration of an abbreviated G1 phase in human induced pluripotent stem (iPS) cells. J. Cell Physiol. 2011, 226, 1149–1156. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, S.; Panopoulos, A.D.; Herrerías, A.; Bissig, K.-D.; Lutz, M.; Berggren, W.T.; Verma, I.M.; Belmonte, J.C.I. A high proliferation rate is required for cell reprogramming and maintenance of human embryonic stem cell identity. Curr. Biol. 2011, 21, 45–52. [Google Scholar] [CrossRef]
- Kapinas, K.; Grandy, R.; Ghule, P.; Medina, R.; Becker, K.; Pardee, A.; Zaidi, S.K.; Lian, J.; Stein, J.; van Wijnen, A.; et al. The abbreviated pluripotent cell cycle. J. Cell Physiol. 2013, 228, 9–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furlan, M.; de Pretis, S.; Pelizzola, M. Dynamics of transcriptional and post-transcriptional regulation. Brief Bioinform. 2021, 22, bbaa389. [Google Scholar] [CrossRef] [PubMed]
- Pu, M.; Chen, J.; Tao, Z.; Miao, L.; Qi, X.; Wang, Y.; Ren, J. Regulatory network of miRNA on its target: Coordination between transcriptional and post-transcriptional regulation of gene expression. Cell Mol. Life. Sci. 2019, 76, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, P.; Lu, Y.; Dou, H. Correlation of MACC1/c-Myc expression in endometrial carcinoma with clinical/pathological features or prognosis. Med. Sci. Monit. 2018, 24, 4738–4744. [Google Scholar] [CrossRef]
- Schenken, R.S.; Johnson, J.V.; Riehl, R.M. c-myc protooncogene polypeptide expression in endometriosis. Am. J. Obstet. Gynecol. 1991, 164, 1031–1037. [Google Scholar] [CrossRef]
- Pellegrini, C.; Gori, I.; Achtari, C.; Hornung, D.; Chardonnens, E.; Wunder, D.; Fiche, M.; Canny, G.O. The expression of estrogen receptors as well as GREB1, c-MYC, and cyclin D1, estrogen-regulated genes implicated in proliferation, is increased in peritoneal endometriosis. Fertil. Steril. 2012, 98, 1200–1208. [Google Scholar] [CrossRef]
- Milde-Langosch, K.; Wilckens, C.; Brack, T.; Löning, T. Demonstration and biological significance of viral and cellular oncogenes in uterine carcinomas. Verh. Dtsch. Ges. Pathol. 1991, 75, 363–365. [Google Scholar] [PubMed]
- Jin, K.S.; Park, C.M.; Lee, Y.W. Identification of differentially expressed genes by 2,3,7,8-tetrachlorodibenzo-p-dioxin in human bronchial epithelial cells. Hum. Exp. Toxicol. 2012, 31, 107–112. [Google Scholar] [CrossRef]
- Koninckx, P.R. The pathophysiology of endometriosis: Pollution and dioxin. Gynecol. Obstet. Investig. 1999, 47 (Suppl. S1), 47–50. [Google Scholar] [CrossRef]
- Rier, S.; Foster, W.G. Environmental dioxins and endometriosis. Toxicol. Sci. 2002, 70, 161–170. [Google Scholar] [CrossRef]
- Bruner-Tran, K.L.; Gnecco, J.; Ding, T.; Glore, D.R.; Pensabene, V.; Osteen, K.G. Exposure to the environmental endocrine disruptor TCDD and human reproductive dysfunction: Translating lessons from murine models. Reprod. Toxicol. 2017, 68, 59–71. [Google Scholar] [CrossRef]
- Jana, N.R.; Sarkar, S.; Ishizuka, M.; Yonemoto, J.; Tohyama, C.; Sone, H. Role of estradiol receptor-α in differential expression of 2,3,7,8-tetrachlorodibenzo-p-dioxin-inducible genes in the RL95-2 and KLE human endometrial cancer cell lines. Arch. Biochem. Biophys. 1999, 368, 31–39. [Google Scholar] [CrossRef]
- Kogevinas, M.; Becher, H.; Benn, T.; Bertazzi, P.A.; Boffetta, P.; Bueno-de-Mesquita, H.B.; Coggon, D.; Colin, D.; Flesch-Janys, D.; Fingerhut, M.; et al. Cancer mortality in workers exposed to phenoxy herbicides, chlorophenols, and dioxins. An expanded and updated international cohort study. Am. J. Epidemol. 1997, 145, 1061–1075. [Google Scholar] [CrossRef] [PubMed]
- Bertazzi, P.A.; Pesatori, A.C.; Bernucci, I.; Landi, M.T.; Consonni, D. Dioxin exposure and human leukemias and lymphomas. Lessons from the Seveso accident and studies on industrial workers. Leukemia 1999, 13 (Suppl. S1), S72–S74. [Google Scholar] [CrossRef] [Green Version]
- Kogevinas, M. Human health effects of dioxins: Cancer, reproductive and endocrine system effects. Hum. Reprod. Update 2001, 7, 331–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guidelines: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, T.; Van Schoubroeck, D. Ultrasound diagnosis of endometriosis and adenomyosis: State of the art. Best. Prac. Res. Clin. Obstet. Gynecol. 2018, 51, 16–24. [Google Scholar] [CrossRef]
- Homesley, H.D. Management of endometrial cancer. Am. J. Obstet. Gynecol. 1996, 174, 529–534. [Google Scholar] [CrossRef]
- Apu, E.-H.; Akram, S.U.; Fissanen, J.; Wan, H.; Salo, T. Desmoglein 3-influence on oral carcinoma cell migration and invasion. Exp. Cell Res. 2018, 370, 353–364. [Google Scholar]
- Xu, H.; Chen, K.; Shang, R.; Chen, X.; Zhang, Y.; Song, X.; Evert, M.; Zhong, S.; Li, B.; Calvisi, D.F.; et al. Alpelisib combination treatment as novel targeted therapy against hepatocellular carcinoma. Cell Death Dis. 2021, 12, 920. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Song, X.; Utpatel, K.; Sang, R.; Yang, Y.M.; Xu, M.; Zhang, J.; Che, L.; Gordan, J.; Cigliano, A.; et al. MEK inhibition suppresses K-Ras wild-type cholangiocarcinoma in vitro and in vivo via inhibiting cell proliferation and modulating tumor microenvironment. Cell Death Dis. 2019, 10, 120. [Google Scholar] [CrossRef] [PubMed]

| Cell Line | Fold Change V1 | Fold Change V2 |
|---|---|---|
| HEC-1A | 1 | 1 |
| Ishikawa | 3.23 | 3.21 |
| 12Z | 1.85 | 1.96 |
| HEC-1B | 1.88 | 1.16 |
| AN3 | 3.37 | 2.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peterson, R.; Minchella, P.; Cui, W.; Graham, A.; Nothnick, W.B. RPLP1 Is Up-Regulated in Human Adenomyosis and Endometrial Adenocarcinoma Epithelial Cells and Is Essential for Cell Survival and Migration In Vitro. Int. J. Mol. Sci. 2023, 24, 2690. https://doi.org/10.3390/ijms24032690
Peterson R, Minchella P, Cui W, Graham A, Nothnick WB. RPLP1 Is Up-Regulated in Human Adenomyosis and Endometrial Adenocarcinoma Epithelial Cells and Is Essential for Cell Survival and Migration In Vitro. International Journal of Molecular Sciences. 2023; 24(3):2690. https://doi.org/10.3390/ijms24032690
Chicago/Turabian StylePeterson, Riley, Paige Minchella, Wei Cui, Amanda Graham, and Warren B. Nothnick. 2023. "RPLP1 Is Up-Regulated in Human Adenomyosis and Endometrial Adenocarcinoma Epithelial Cells and Is Essential for Cell Survival and Migration In Vitro" International Journal of Molecular Sciences 24, no. 3: 2690. https://doi.org/10.3390/ijms24032690




