Unlocking Kuhn Verdazyls: New Synthetic Approach and Useful Mechanistic Insights
Abstract
1. Introduction
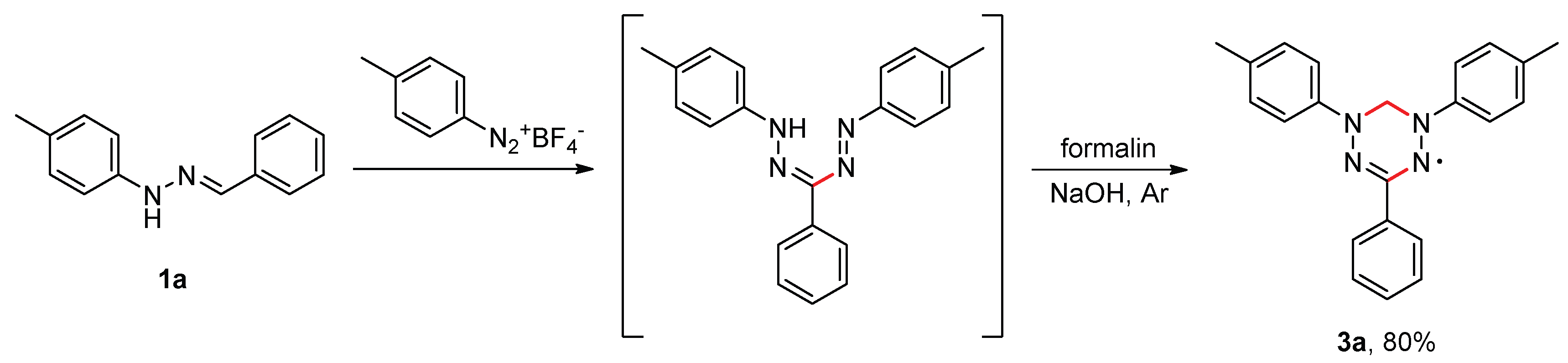
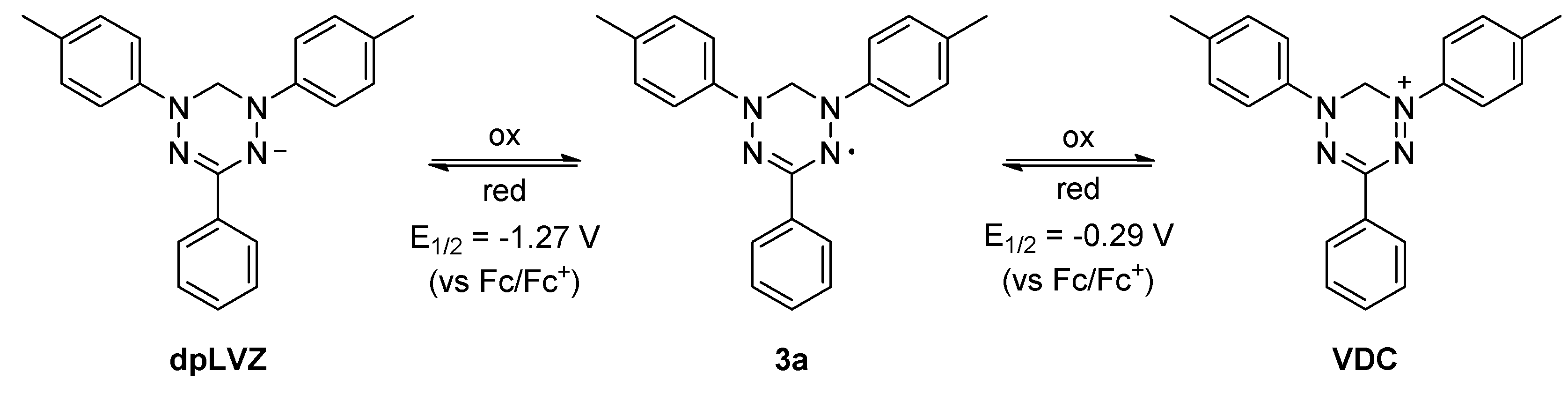
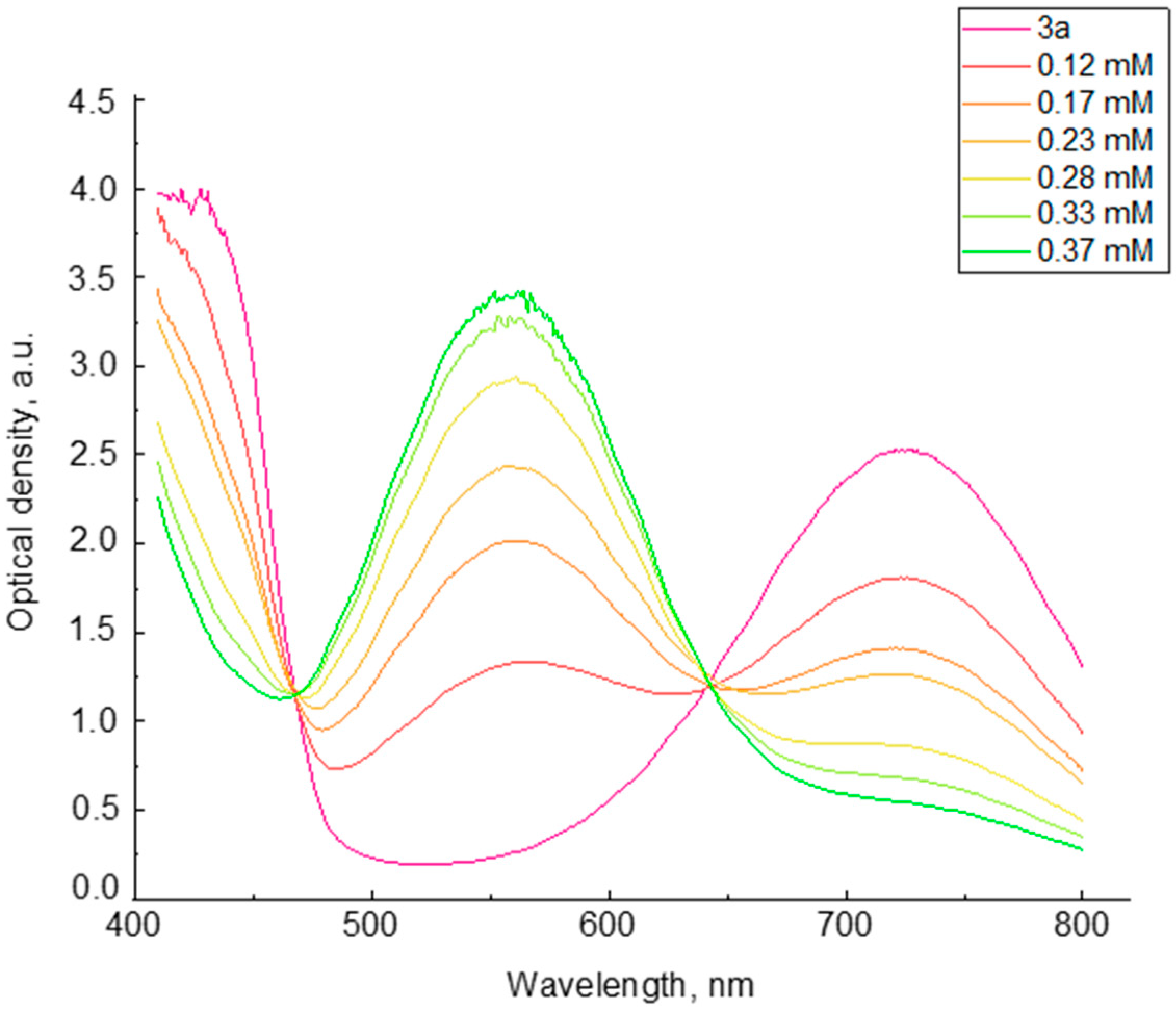
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. X-ray Crystallographic Data and Refinement Details
3.3. Synthesis of Verdazyls 3 (General Procedure)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, D.; Yu, G. Innovation of Materials, Devices, and Functionalized Interfaces in Organic Spintronics. Adv. Funct. Mater. 2021, 31, 2100550. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Lin, P.-H.; Lee, K.-M. Development of Step-Saving Alternative Synthetic Pathways for Functional π-Conjugated Materials. Chem. Rec. 2021, 21, 3498–3508. [Google Scholar] [CrossRef] [PubMed]
- Sathiyan, G.; Wang, H.; Chen, C.; Miao, Y.; Zhai, M.; Cheng, M. Impact of fluorine substitution in organic functional materials for perovskite solar cell. Dyes Pigm. 2022, 198, 110029. [Google Scholar] [CrossRef]
- Yang, X.-D.; Tan, L.; Sun, J.-K. Encapsulation of Metal Clusters within Porous Organic Materials: From Synthesis to Catalysis Applications. Chem. Asian J. 2022, 17, e202101289. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Das, S.K.; Khatua, H.; Das, S.; Chattopadhyay, B. Road Map for the Construction of High-Valued N-Heterocycles via Denitrogenative Annulation. Acc. Chem. Res. 2021, 54, 4395–4409. [Google Scholar] [CrossRef]
- Odom, A.L.; McDaniel, T.J. Titanium-Catalyzed Multicomponent Couplings: Efficient One-Pot Syntheses of Nitrogen Heterocycles. Acc. Chem. Res. 2015, 48, 2822–2833. [Google Scholar] [CrossRef]
- Makhova, N.N.; Belen’kii, L.I.; Gazieva, G.A.; Dalinger, I.L.; Konstantinova, L.S.; Kuznetsov, V.V.; Kravchenko, A.N.; Krayushkin, M.M.; Rakitin, O.A.; Starosotnikov, A.M.; et al. Progress in the chemistry of nitrogen-, oxygen- and sulfur-containing heterocyclic systems. Russ. Chem. Rev. 2020, 89, 55–124. [Google Scholar] [CrossRef]
- Abas, M.; Bahadur, A.; Ashraf, Z.; Iqbal, S.; Rajoka, M.S.R.; Rashid, S.G.; Jabeen, E.; Iqbal, Z.; Abbas, Q.; Bais, A.; et al. Designing novel anticancer sulfonamide based 2,5-disubstituted-1,3,4-thiadiazole derivatives as potential carbonic anhydrase inhibitor. J. Mol. Struct. 2021, 1246, 131145. [Google Scholar] [CrossRef]
- Hussain, R.; Shah, M.; Iqbal, S.; Rehman, W.; Khan, S.; Rasheed, L.; Naz, H.; Al-ghulikah, H.A.; Elkaeed, E.B.; Pashameah, R.A.; et al. Molecular iodine-promoted oxidative cyclization for the synthesis of 1,3,4-thiadiazole-fused-[1,2,4]-thiadiazole incorporating 1,4-benzodioxine moiety as potent inhibitors of α-amylase and α-glucosidase: In vitro and in silico study. Front. Chem. 2022, 10, 1023316. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Zhilin, E.S. Recent Advances in the Synthesis and Biomedical Applications of Heterocyclic NO-Donors. Molecules 2021, 26, 5705. [Google Scholar] [CrossRef]
- Fershtat, L.L. Recent advances in the synthesis and performance of 1,2,4,5-tetrazine-based energetic materials. FirePhysChem 2023, in press. [Google Scholar] [CrossRef]
- Bystrov, D.M.; Pivkina, A.N.; Fershtat, L.L. An Alliance of Polynitrogen Heterocycles: Novel Energetic Tetrazinedioxide-Hydroxytetrazole-Based Materials. Molecules 2022, 27, 5891. [Google Scholar] [CrossRef]
- Rudakov, G.F.; Sinditskii, V.P.; Andreeva, I.A.; Botnikova, A.I.; Veselkina, P.R.; Kostanyan, S.K.; Yudin, N.V.; Serushkin, V.V.; Cherkaev, G.V.; Dorofeeva, O.V. Energetic compounds based on a new fused Bis [1,2,4]Triazolo [1,5-b;5′,1′-f]-1,2,4,5-Tetrazine. Chem. Eng. J. 2022, 450 Pt 3, 138073. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Wang, B.; Qiu, L.; Xu, R.; Sheremetev, A.B. Recent synthetic efforts towards high energy density materials: How to design high-performance energetic structures? FirePhysChem 2022, 2, 83–139. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Chen, Y.; Li, Y.; Nan, H.; Ma, H. Synthesis and properties of a promising high energy and low impact sensitivity explosive: Hydroxylammonium 3-hydrazino-6-(1H-1,2,3,4-tetrazol-5-ylimino)-s-tetrazine. FirePhysChem 2023, in press. [Google Scholar] [CrossRef]
- Mezzina, E.; Manoni, R.; Romano, F.; Lucarini, M. Spin-Labelling of Host-Guest Assemblies with Nitroxide Radicals. Asian J. Org. Chem. 2015, 4, 296–310. [Google Scholar] [CrossRef]
- Haugland, M.M.; Anderson, E.A.; Lovett, J.E. Tuning the properties of nitroxide spin labels for use in electron paramagnetic resonance spectroscopy through chemical modification of the nitroxide framework. Electron Paramagn. Reson. 2017, 25, 1–34. [Google Scholar] [CrossRef]
- Hu, X.; Wang, W.; Wang, D.; Zheng, Y. The electronic applications of stable diradicaloids: Present and future. J. Mater. Chem. C 2018, 6, 11232–11242. [Google Scholar] [CrossRef]
- Constantinides, C.P.; Koutentis, P.A. Stable N- and N/S-Rich Heterocyclic Radicals: Synthesis and Applications. Adv. Heterocycl. Chem. 2016, 119, 173–207. [Google Scholar] [CrossRef]
- Fershtat, L.L. Synthesis of 1,4-dihydro-1,2,4-benzotriazin-4-yl radicals (microreview). Chem. Heterocycl. Compd. 2022, 58, 196–198. [Google Scholar] [CrossRef]
- Epishina, M.A.; Kulikov, A.S.; Fershtat, L.L. Revisiting the Synthesis of Functionally Substituted 1,4-Dihydrobenzo[e][1,2,4]triazines. Molecules 2022, 27, 2575. [Google Scholar] [CrossRef] [PubMed]
- Ovcharenko, V.I.; Terent’ev, A.O.; Tretyakov, E.V.; Krylov, I.B. From the chemistry of radicals to molecular spin devices. Russ. Chem. Rev. 2022, 91, RCR5043. [Google Scholar] [CrossRef]
- Kuhn, R.; Trischmann, H. Über Verdazyle, eine neue Klasse cyclischer N-haltiger Radikale. Monat. Chem. 1964, 95, 457–479. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Petunin, P.V.; Zhivetyeva, S.I.; Gorbunov, D.E.; Gritsan, N.P.; Fedin, M.V.; Stass, D.V.; Samoilova, R.I.; Bagryanskaya, I.Y.; Shundrina, I.K.; et al. Platform for High-Spin Molecules: A Verdazyl-Nitronyl Nitroxide Triradical with Quartet Ground State. J. Am. Chem. Soc. 2021, 143, 8164–8176. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R.O.; Taylor, M.R.; Duggin, M.; Bissember, A.C.; Canty, A.J.; Judd, M.M.; Cox, N.; Moggach, S.A.; Turner, G.F. Enhanced synthesis of oxo-verdazyl radicals bearing sterically-and electronically-diverse C3-substituents. Org. Biomol. Chem. 2021, 19, 10120–10138. [Google Scholar] [CrossRef] [PubMed]
- Fleming, C.; Chung, D.; Ponce, S.; Brook, D.J.R.; DaRos, J.; Das, R.; Ozarowski, A.; Stoian, S.A. Valence tautomerism in a cobalt-verdazyl coordination compound. Chem. Commun. 2020, 56, 4400–4403. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Zhivetyeva, S.I.; Petunin, P.V.; Gorbunov, D.E.; Gritsan, N.P.; Bagryanskaya, I.Y.; Bogomyakov, A.S.; Postnikov, P.S.; Kazantsev, M.S.; Trusova, M.E.; et al. Ferromagnetically Coupled S = 1 Chains in Crystals of Verdazyl-Nitronyl Nitroxide Diradicals. Angew. Chem. Int. Ed. 2020, 59, 20704–20710. [Google Scholar] [CrossRef]
- Johnston, C.W.; McKinnon, S.D.J.; Patrick, B.O.; Hicks, R.G. The first “Kuhn verdazyl” ligand and comparative studies of its PdCl2 complex with analogous 6-oxoverdazyl ligands. Dalton Trans. 2013, 42, 16829–16836. [Google Scholar] [CrossRef]
- Rogers, F.J.M.; Coote, M.L. Computational Assessment of Verdazyl Derivatives for Electrochemical Generation of Carbon-Centered Radicals. J. Phys. Chem. C 2019, 123, 20174–20180. [Google Scholar] [CrossRef]
- Valiev, R.R.; Drozdova, A.K.; Petunin, P.V.; Postnikov, P.S.; Trusova, M.E.; Cherepanov, V.N.; Sundholm, D. The aromaticity of verdazyl radicals and their closed-shell charged species. New J. Chem. 2018, 42, 19987–19994. [Google Scholar] [CrossRef]
- Jobelius, H.; Wagner, N.; Schnakenburg, G.; Meyer, A. Verdazyls as Possible Building Blocks for Multifunctional Molecular Materials: A Case Study on 1,5-Diphenyl-3-(p-iodophenyl)-verdazyl Focusing on Magnetism, Electron Transfer and the Applicability of the Sonogashira-Hagihara Reaction. Molecules 2018, 23, 1758. [Google Scholar] [CrossRef] [PubMed]
- Steen, J.S.; de Vries, F.; Hjelm, J.; Otten, E. Bipolar Verdazyl Radicals for Symmetrical Batteries: Properties and Stability in All States of Charge. ChemPhysChem 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Petunin, P.V.; Martynko, E.A.; Trusova, M.E.; Kazantsev, M.S.; Rybalova, T.V.; Valiev, R.R.; Uvarov, M.N.; Mostovich, E.A.; Postnikov, P.S. Verdazyl Radical Building Blocks: Synthesis, Structure, and Sonogashira Cross-Coupling Reactions. Eur. J. Org. Chem. 2018, 2018, 4802–4811. [Google Scholar] [CrossRef]
- Votkina, D.E.; Petunin, P.V.; Zhivetyeva, S.I.; Bagryanskaya, I.Y.; Uvarov, M.N.; Kazantsev, M.S.; Trusova, M.E.; Tretyakov, E.V.; Postnikov, P.S. Preparation of Multi-Spin Systems: A Case Study of Tolane-Bridged Verdazyl-Based Hetero-Diradicals. Eur. J. Org. Chem. 2020, 2020, 1996–2004. [Google Scholar] [CrossRef]
- Fedorchenko, T.G.; Lipunova, G.N.; Shchepochkin, A.V.; Tsmokalyuk, A.N.; Slepukhin, P.A.; Chupakhin, O.N. Synthesis and properties of 1,3-diphenyl-5-(benzothiazol-2-yl)-6-R-verdazyls. Mendeleev Commun. 2018, 28, 297–299. [Google Scholar] [CrossRef]
- Gilroy, G.B.; McKinnon, S.D.J.; Koivisto, B.D.; Hicks, R.G. Electrochemical Studies of Verdazyl Radicals. Org. Lett. 2007, 9, 4837–4840. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPro. Version 1.171.41.106a. In Rigaku Oxford Diffraction; Rigaku Corporation: Oxford, UK, 2021. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 229–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and Analysis of Crystal Structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]





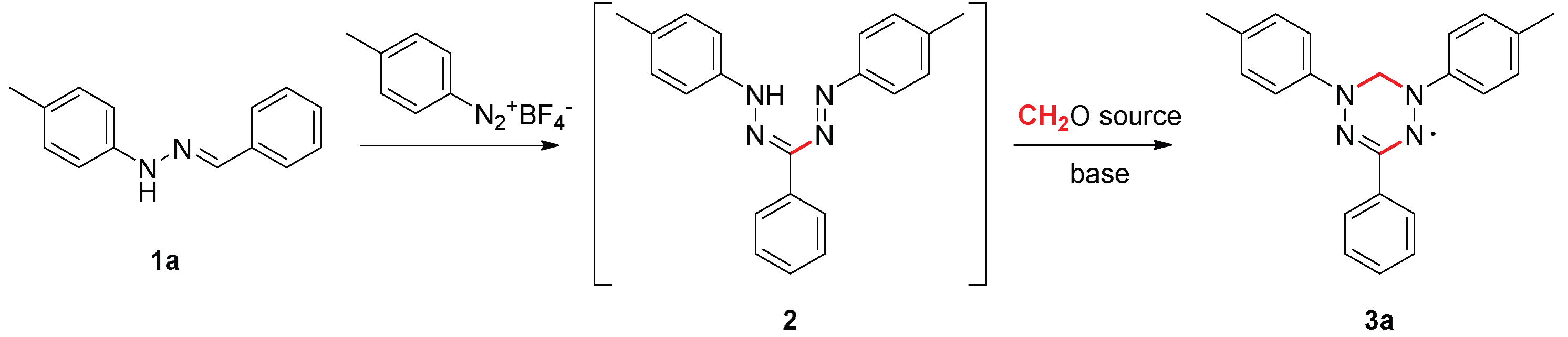 | |||||||
| Entry | Diazonium salt source (equiv) | Solvent | Base | Yield of 2a (%) a | Formaldehyde source (equiv) | Base (equiv) | Yield of 3a (%) a |
| 1 | generated in situ (1.1) | DMF | Py | 24 | paraform (2) | NaOH (2) | ─ |
| 2 | generated in situ (1.1) | DMF | Py | 24 | formalin (2) | NaOH (2) | ─ |
| 3 | generated in situ (1.1) | DMF | NaOH | 12 | formalin (2) | NaOH (2) | ─ |
| 4 | generated in situ (1.1) | DMSO | Py | traces | ─ | ─ | ─ |
| 5 | isolated (1.1) | DMF | ─ | traces | ─ | ─ | ─ |
| 6 | isolated (1.1) | DMF | NaOH | 13 | formalin (11) | NaOH (2) | 5 |
| 7 | isolated (1.1) | DMF | Py | 36 | formalin (11) | NaOH (3.5) | 31 |
| 8 | isolated (1.1) | DMF | Py | 36 | paraform (11) | NaOH (3.5) | ─ |
| 9 | isolated (1.1) | DMF | Py | ─ b | formalin (11) | NaOH (3.5) | 77 |
| 10 | isolated (1.1) | DMF | Py | ─ b | formalin (11) | Py (4) | ─ |
| 11 | generated in situ (1.1) | DMF | Py | ─ b | formalin (11) | NaOH (3.5) | ─ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teslenko, F.E.; Fershtat, L.L. Unlocking Kuhn Verdazyls: New Synthetic Approach and Useful Mechanistic Insights. Int. J. Mol. Sci. 2023, 24, 2693. https://doi.org/10.3390/ijms24032693
Teslenko FE, Fershtat LL. Unlocking Kuhn Verdazyls: New Synthetic Approach and Useful Mechanistic Insights. International Journal of Molecular Sciences. 2023; 24(3):2693. https://doi.org/10.3390/ijms24032693
Chicago/Turabian StyleTeslenko, Fedor E., and Leonid L. Fershtat. 2023. "Unlocking Kuhn Verdazyls: New Synthetic Approach and Useful Mechanistic Insights" International Journal of Molecular Sciences 24, no. 3: 2693. https://doi.org/10.3390/ijms24032693
APA StyleTeslenko, F. E., & Fershtat, L. L. (2023). Unlocking Kuhn Verdazyls: New Synthetic Approach and Useful Mechanistic Insights. International Journal of Molecular Sciences, 24(3), 2693. https://doi.org/10.3390/ijms24032693






