Natural Inhibitors of Cholinesterases: Chemistry, Structure–Activity and Methods of Their Analysis
Abstract
1. Introduction
2. Chemistry–Structure Activity
2.1. Alkaloids
2.2. Coumarins
2.3. Diarylheptanoids
2.4. Flavonoids
2.5. Phenanthrenes
2.6. Terpenes
2.7. Xanthonoids
| Inhibitors | Source | Activity | Method | Ref. | |||
|---|---|---|---|---|---|---|---|
| Value of Inhibition against AChE | Reference Standard for AChE | Value of Inhibition against BuChE | Reference Standard for BuChE | ||||
| ALKALOIDS | |||||||
| Lindoldhamine isomer | Abuta panurensis Eichler Menispermaceae (branches) | 39.38 ± 0.08 µM a,j | NEO 3.72 ± 0.03 µM a,j | nd | nd | MCE | [48,49,50] |
| 5-N-Methylmaytenine | Abuta panurensis Eichler Menispermaceae (branches) | 19.55 ± 0.09 µM a,j | NEO 3.72 ± 0.03 µM a,j | nd | nd | MCE | |
| N-trans-Feruloyltyramine | Abuta panurensis Eichler Menispermaceae (branches) | na | NEO 3.72 ± 0.03 µM a,j | nd | nd | MCE | |
| Palmatine | Abuta panurensis Eichler Menispermaceae (branches) | 35.25 ± 0.04 µM a,j | NEO 3.72 ± 0.03 µM a,j | nd | nd | MCE | |
| Stepharine | Abuta panurensis Eichler Menispermaceae (branches) | 61.24 ± 0.03 µM a,j | NEO 3.72 ± 0.03 µM a,j | nd | nd | MCE | |
| Aconorine | Aconitum laeve Ranunculaceae (tubers) | 2.51 ± 0.037 µM a,e | GAL 3.26 ± 0.021 µM a,e | 8.72 ± 0.023 µM a,m | GAL 10.13 ± 0.05 µM a,m | MCE | [51,52] |
| Hohenackerine | Aconitum laeve Ranunculaceae (tubers) | 4.53 ± 0.062 µM a,e | GAL 3.26 ± 0.021 µM a,e | 9.94 ± 0.073 µM a,m | GAL 10.13 ± 0.05 µM a,m | MCE | |
| Lappaconotine | Aconitum laeve Ranunculaceae (tubers) | 6.13 ± 0.019 µM a,e | GAL 3.26 ± 0.021 µM a,e | 11.24 ± 0.12 µM a,m | GAL 10.13 ± 0.05 µM a,m | MCE | |
| Swatinine-C | Aconitum laeve Ranunculaceae (tubers) | 3.7 ± 0.085 µM a,e | GAL 3.26 ± 0.021 µM a,e | 12.23 ± 0.014 µM a,m | GAL 10.13 ± 0.05 µM a,m | MCE | |
| 4-Methoxy-1-methyl-2-quinolone | Atractylis cancellata L. Asteraceae (whole plant) | >50 µg mL−1 a,k | GAL 6.27 ± 1.15 µg mL−1 a,k | 37.49 ± 1.61 µg mL−1 a,n | GAL 34.75 ± 1.99 µg mL−1 a,n | MCE | [53] |
| Pyrroloquinolone A | Atractylis cancellata L. Asteraceae (whole plant) | 18.48 ± 0.33 µg mL−1 a,k | GAL 6.27 ± 1.15 µg mL−1 a,k | 9.66 ± 0.16 µg mL−1 a,n | GAL 34.75 ± 1.99 µg mL−1 a,n | MCE | |
| Buthutin A | Buthus martensii Karsch Buthidae (whole body of scorpion) | 7.83 ± 0.06 µM a,e | GAL 1.17 ± 0.01 µM a,e DON 0.049 ± 0.004 µM a,e | 47.44 ± 0.95 µM a,m | GAL 18.78 ± 1.81 µM a,m DON 5.536± 0.018 µM a,m | MCE | [48,54,55] |
| Buthutin B | Buthus martensii Karsch Buthidae (whole body of scorpion) | 61.45 ± 2.34 µM a,e | GAL 1.17 ± 0.01 µM a,e DON 0.049 ± 0.004 µM a,e | 122.64 ± 5.21 µM a,m | GAL 18.78 ± 1.81 µM a,m DON 5.536± 0.018 µM a,i | MCE | |
| Trigonelline | Buthus martensii Karsch Buthidae (whole body of scorpion) | 97.30 ± 4.18 µM a,e | GAL 1.17 ± 0.01 µM a,e DON 0.049 ± 0.004 µM a,e | 441.87 ± 7.99 µM a,m | GAL 18.78 ± 1.81 µM a,m DON 5.536± 0.018 µM a,m | MCE | |
| 17-oxo-3-Benzoylbuxadine | Buxus hyrcana Pojark. Buxaceae (leaves) | 17.6 ± 0.5 µM a,k | GAL 0.53 ± 0.5 µM a,k HUP 1.7 ± 0.3 µM a,k | 186.8 ± 1.0 µM a,n | GAL 8.7 ± 1.0 µM a,n HUP >1000 ± 3.0 µM a,n | MCE | [48,56,57,58] |
| 31-Demethylcyclobuxoviridine | Buxus hyrcana Pojark. Buxaceae (leaves) | 298.3 ± 1.0 µM a,k | GAL 0.53 ± 0.5 µM a,k HUP 1.7 ± 0.3 µM a,k | 15.4 ± 0.5 µM a,n | GAL 8.7 ± 1.0 µM a,n HUP >1000 ± 3.0 µM a,n | MCE | |
| 31-Hydroxybuxamine B | Buxus hyrcana Pojark. Buxaceae (leaves) | 61.3 ± 2.0 µM a,k | GAL 0.53 ± 0.5 µM a,k HUP 1.7 ± 0.3 µM a,k | 112.1 ± 3.0 µM a,n | GAL 8.7 ± 1.0 µM a,n HUP >1000 ± 3.0 µM a,n | MCE | |
| Buxamine A | Buxus hyrcana Pojark. Buxaceae (leaves) | 81.4 ± 2.4 µM a,k | GAL 0.53 ± 0.5 µM a,k HUP 1.7 ± 0.3 µM a,k | 100.2 ± 1.4 µM a,n | GAL 8.7 ± 1.0 µM a,n HUP >1000 ± 3.0 µM a,n | MCE | |
| Buxamine B | Buxus hyrcana Pojark. Buxaceae (leaves) | 79.6 ± 3.0 µM a,k | GAL 0.53 ± 0.5 µM a,k HUP 1.7 ± 0.3 µM a,k | 100.5 ± 2.5 µM a,k | GAL 8.7 ± 1.0 µM a,n HUP >1000 ± 3.0 µM a,n | MCE | |
| Buxhyrcamine | Buxus hyrcana Pojark. Buxaceae (leaves) | 18.2 ± 0.3 µM a,k | GAL 0.53 ± 0.5 µM a,k HUP 1.7 ± 0.3 µM a,k | 209.0 ± 1.0 µM a,n | GAL 8.7 ± 1.0 µM a,n HUP >1000 ± 3.0 µM a,n | MCE | |
| Buxmicrophylline F | Buxus hyrcana Pojark. Buxaceae (leaves) | 22.4 ± 0.7 µM a,k | GAL 0.53 ± 0.5 µM a,k HUP 1.7 ± 0.3 µM a,k | 154.2 ± 1.0 µM a,n | GAL 8.7 ± 1.0 µM a,n HUP >1000 ± 3.0 µM a,n | MCE | |
| Buxrugulosamine | Buxus hyrcana Pojark. Buxaceae (leaves) | 24.8 ± 1.0 µM a,k | GAL 0.53 ± 0.5 µM a,k HUP 1.7 ± 0.3 µM a,k | 160.2 ± 4.0 µM a,n | GAL 8.7 ± 1.0 µM a,n HUP >1000 ± 3.0 µM a,n | MCE | |
| Cyclobuxophylline O | Buxus hyrcana Pojark. Buxaceae (leaves) | 35.4 ± 1.0 µM a,k | GAL 0.53 ± 0.5 µM a,k HUP 1.7 ± 0.3 µM a,k | 45.0 ± 2.0 µM a,n | GAL 8.7 ± 1.0 µM a,n HUP >1000 ± 3.0 µM a,n | MCE | |
| Cyclobuxoviridine | Buxus hyrcana Pojark. Buxaceae (leaves) | 179.7 ± 0.4 µM a,k | GAL 0.53 ± 0.5 µM a,k HUP 1.7 ± 0.3 µM a,k | 304.5 ± 1.0 µM a,n | GAL 8.7 ± 1.0 µM a,n HUP >1000 ± 3.0 µM a,n | MCE | |
| E-Buxenone | Buxus hyrcana Pojark. Buxaceae (leaves) | 71.0 ± 2.5 µM a,k | GAL 0.53 ± 0.5 µM a,k HUP 1.7 ± 0.3 µM a,k | 200.7 ± 2.6 µM a,n | GAL 8.7 ± 1.0 µM a,n HUP >1000 ± 3.0 µM a,n | MCE | |
| Homomoenjodarmine | Buxus hyrcana Pojark. Buxaceae (leaves) | 19.5 ± 1.0 µM a,k | GAL 0.53 ± 0.5 µM a,k HUP 1.7 ± 0.3 µM a,k | 52.2 ± 3.0 µM a,n | GAL 8.7 ± 1.0 µM a,n HUP >1000 ± 3.0 µM a,n | MCE | |
| Moenjodaramine | Buxus hyrcana Pojark. Buxaceae (leaves) | 25.0 ± 2.9 µM a,k | GAL 0.53 ± 0.5 µM a,k HUP 1.7 ± 0.3 µM a,k | 102.4 ± 2.0 µM a,n | GAL 8.7 ± 1.0 µM a,n HUP >1000 ± 3.0 µM a,n | MCE | |
| Nb-Dimethylcyclobuxoviricine | Buxus hyrcana Pojark. Buxaceae (leaves) | 45.5 ± 0.6 µM a,k | GAL 0.53 ± 0.5 µM a,k HUP 1.7 ± 0.3 µM a,k | 133.8 ± 3.4 µM a,n | GAL 8.7 ± 1.0 µM a,n HUP >1000 ± 3.0 µM a,n | MCE | |
| N20-Formylbuxaminol E | Buxus hyrcana Pojark. Buxaceae (leaves) | 25.5 ± 0.8 µM a,k | GAL 0.53 ± 0.5 µM a,k HUP 1.7 ± 0.3 µM a,k | 120.9 ± 2.0 µM a,n | GAL 8.7 ± 1.0 µM a,n HUP >1000 ± 3.0 µM a,n | MCE | |
| Spirofornabuxine | Buxus hyrcana Pojark. Buxaceae (leaves) | 6.3 ± 0.6 µM a,k | GAL 0.53 ± 0.5 µM a,k HUP 1.7 ± 0.3 µM a,k | 125.2 ± 1.0 µM a,n | GAL 8.7 ± 1.0 µM a,n HUP >1000 ± 3.0 µM a,n | MCE | |
| Papillozine C | Buxus hyrcana Pojark. Buxaceae (leaves) | 47.8 ± 1.4 µM a,k | GAL 0.53 ± 0.5 µM a,k HUP 1.7 ± 0.3 µM a,k | 35.2 ± 2.0 µM a,n | GAL 8.7 ± 1.0 µM a,n HUP >1000 ± 3.0 µM µM a,n | MCE | |
| Z-Buxenone | Buxus hyrcana Pojark. Buxaceae (leaves) | 87.4 ± 1.7 µM a,k | GAL 0.53 ± 0.5 µM a,k HUP 1.7 ± 0.3 µM a,k | 155.8 ± 3.8 µM a,n | GAL 8.7 ± 1.0 µM µM a,n HUP >1000 ± 3.0 µM µM a,n | MCE | |
| Dihydroberberine | Coptis chinensis Ranunculaceae (rhizomes) | 1.18 ± 0.03 µM a,e | BER 1.01 ± 0.01 µM a,e TAC 0.22 ± 0.004 µM a,e | 38.82 ± 0.52 µM a,m | TAC 0.014 ± 0.0043 µM a,m | MCE | [48,59,60] |
| 10-Hydroxy-infractopicrin | Cortinarius infractus Berk Cortinariaceae (toadstool) | 12.7 ± 0.16 µM a,d | GAL 8.70 ± 0.05 µM a,d PHY 2.58 ± 0.03 µM a,d | nd < 100 µM a,m | GAL 24.4 ± 2.84 µM a,m PHY 1.34 ± 0.279 µM a,m | MCE | [16,48,61] |
| Infractopicrin | Cortinarius infractus Berk Cortinariaceae (toadstool) | 9.72 ± 0.19 µM a,d | GAL 8.70 ± 0.05 µM a,d PHY 2.58 ± 0.03 µM a,d | nd < 100 µM a,m | GAL 24.4 ± 2.84 µM a,m PHY 1.34 ± 0.279 µM a,m | MCE | |
| (+)-Adlumine | Corydalis mucronifera Maxim. Papaveraceae (whole plants) | >100 µM a,e | GAL 1.34 ± 0.11 µM a,e | >100 µM a,m | GAL 6.81 ± 0.60 µM a,m | MCE | [16,48,62,63] |
| Bicucullinine | Corydalis mucronifera Maxim. Papaveraceae (whole plants) | 85.89 ± 0.92 µM a,e | GAL 1.34 ± 0.11 µM a,e | 59.75 ± 2.40 µM a,m | GAL 6.81 ± 0.60 µM a,m | MCE | |
| (−)-Corydalisol | Corydalis mucronifera Maxim. Papaveraceae (whole plants) | 51.12 ± 0.27 µM a,e | GAL 1.34 ± 0.11 µM a,e | >100 µM a,m | GAL 6.81 ± 0.60 µM a,m | MCE | |
| Demethylcorydalmine | Corydalis mucronifera Maxim. Papaveraceae (whole plants) | 71.43 ± 0.55 µM a,e | GAL 1.34 ± 0.11 µM a,e | >100 µM a,m | GAL 6.81 ± 0.60 µM a,m | MCE | |
| 6,7-Dimethoxy-2-methyl-1,2,3,4-tetrahydroisoquinoline | Corydalis mucronifera Maxim. Papaveraceae (whole plants) | 45.70 ± 0.42 µM a,e | GAL 1.34 ± 0.11 µM a,e | >100 µM a,m | GAL 6.81 ± 0.60 µM a,m | MCE | |
| 1-(1,3-Dioxolo [4,5-g]isoquinolin-5-yl)-ethanone | Corydalis mucronifera Maxim. Papaveraceae (whole plants) | >100 µM a,e | GAL 1.34 ± 0.11 µM a,e | >100 µM a,m | GAL 6.81 ± 0.60 µM a,m | MCE | |
| epi-Coryximine | Corydalis mucronifera Maxim. Papaveraceae (whole plants) | 92.00 ± 0.19 µM a,e | GAL 1.34 ± 0.11 µM a,e | >100 µM a,m | GAL 6.81 ± 0.60 µM a,m | MCE | |
| Hendersine B | Corydalis mucronifera Maxim. Papaveraceae (whole plants) | 14.22 ± 0.34 µM a,e | GAL 1.34 ± 0.11 µM a,e | >100 µM a,m | GAL 6.81 ± 0.60 µM a,m | MCE | |
| Hydrohydrastinine | Corydalis mucronifera Maxim. Papaveraceae (whole plants) | 9.13 ± 0.15 µM a,e | GAL 1.34 ± 0.11 µM a,e | >100 µM a,m | GAL 6.81 ± 0.60 µM a,m | MCE | |
| 9-Methyldecumbenine C | Corydalis mucronifera Maxim. Papaveraceae (whole plants) | >100 µM a,e | GAL 1.34 ± 0.11 µM a,e | >100 µM a,m | GAL 6.81 ± 0.60 µM a,m | MCE | |
| Mucroniferanines H | Corydalis mucronifera Maxim. Papaveraceae (whole plants) | 2.31 ± 0.20 µM a,e | GAL 1.34 ± 0.11 µM a,e | 36.71 ± 1.12 µM a,m | GAL 6.81 ± 0.60 µM a,m | MCE | |
| Mucroniferanines K | Corydalis mucronifera Maxim. Papaveraceae (whole plants) | >100 µM a,e | GAL 1.34 ± 0.11 µM a,e | >100 µM a,m | GAL 6.81 ± 0.60 µM a,m | MCE | |
| Mucroniferanines L | Corydalis mucronifera Maxim. Papaveraceae (whole plants) | >100 µM a,e | GAL 1.34 ± 0.11 µM a,e | >100 µM a,m | GAL 6.81 ± 0.60 µM a,m | MCE | |
| Mucroniferanines M | Corydalis mucronifera Maxim. Papaveraceae (whole plants) | >100 µM a,e | GAL 1.34 ± 0.11 µM a,e | >100 µM a,m | GAL 6.81 ± 0.60 µM a,m | MCE | |
| (+)-Ochotensine | Corydalis mucronifera Maxim. Papaveraceae (whole plants) | >100 µM a,e | GAL 1.34 ± 0.11 µM a,e | >100 µM a,m | GAL 6.81 ± 0.60 µM a,m | MCE | |
| (−)-Ochrobirine | Corydalis mucronifera Maxim. Papaveraceae (whole plants) | >100 µM a,e | GAL 1.34 ± 0.11 µM a,e | >100 µM a,m | GAL 6.81 ± 0.60 µM a,m | MCE | |
| Orientaline | Corydalis mucronifera Maxim. Papaveraceae (whole plants) | 83.96 ± 1.06 µM a,e | GAL 1.34 ± 0.11 µM a,e | >100 µM a,m | GAL 6.81 ± 0.60 µM a,m | MCE | |
| 1R,9S,7′S-Methylegenine | Corydalis mucronifera Maxim. Papaveraceae (whole plants) | >100 µM a,e | GAL 1.34 ± 0.11 µM a,e | >100 µM a,m | GAL 6.81 ± 0.60 µM a,m | MCE | |
| 5,6,7,8-Tetrahydro-1,3-dioxolo [4,5-g]isoquinoline | Corydalis mucronifera Maxim. Papaveraceae (whole plants) | >100 µM a,e | GAL 1.34 ± 0.11 µM a,e | >100 µM a,m | GAL 6.81 ± 0.60 µM a,m | MCE | |
| Pseudocoptisine | Corydalis turtschaninovii Besser forma yanhusuo Papaveraceae (tuber) | 12.8 µM a,i | TAC 0,175 µM a,i | nd | nd | MCE | [64] |
| (−)-Desmethylsecoantofine | Cryptocarya densiflora BI. Lauraceae (leaves) | 201.52 µM a,e | PHY 0.16 µM a,e | 166.69 µM a,m | PHY 0.58 µM a,m | MCE | [48,65,66] |
| (+)-Laurotetanine | Cryptocarya densiflora BI. Lauraceae (leaves) | 100 µg mL−1—17.51 ± 0.68% b,e | nd | 100 µg mL−1—22.58 ± 0.47 µM a,m | PHY 0.58 µM a,m | MCE | |
| (+)-nor-Nantenine | Cryptocarya densiflora BI. Lauraceae (leaves) | 205.55 µM a,e | PHY 0.16 µM a,e | 94.45 µM a,m | PHY 0.58 µM a,m | MCE | |
| (+)-Oridine | Cryptocarya densiflora BI. Lauraceae (leaves) | 100 µg mL−1—27.89 ± 0.64% b,e | nd | 288.34 µM a,m | PHY 0.58 µM a,m | MCE | |
| 2-Methoxyatherosperminine | Cryptocarya griffithiana Wight. Lauraceae (bark) | 100 µg mL−1—31.58 ± 2.87% b,e | nd | 3.95 µM a,m | PHY 0.58 µM a,m | MCE | |
| (+)-Reticuline | Cryptocarya griffithiana Wight. Lauraceae (bark) | 301.01 µM a,e | PHY 0.16 µM a,e | 65.04 µM a,m | PHY 0.58 µM a,m | MCE | |
| Atherosperminine | Cryptocarya infectoria Miq. Lauraceae (bark) | 100 µg mL−1—2.06 ± 1.29% b,e | nd | 19.34 µM a,m | PHY 0.58 µM a,m | MCE | |
| (+)-N-Methylisococlaurine | Cryptocarya infectoria Miq. Lauraceae (bark) | 100 µg mL−1—14.93 ± 0.53% b,e | nd | 100 µg mL−1—37.33 ± 1.56 a,m | PHY 0.58 µM a,m | MCE | |
| (+)-N-Methyllaurotetanine | Cryptocarya infectoria Miq. Lauraceae (bark) | 100 µg mL−1—38.79 ± 2.6% b,e | nd | 218.81 µM a,m | PHY 0.58 µM a,m | MCE | |
| Chitralinine A | Delphinium chitralense H. Riedl in Kew Bull. Ranunculaceae (aerial parts) | 13.86 ± 0.35 µM a,e | GAL 10.12 ± 0.06 µM a,e ALA 8.23 ± 0.01 µM a,e | 28.17 ± 0.92 µM a,m | GAL 20.62 ± 0.08 µM a,m ALA 18 ± 0.06 µM a,m | MCE | [48,67] |
| Chitralinine B | Delphinium chitralense H. Riedl in Kew Bull. Ranunculaceae (aerial parts) | 11.64 ± 0.08 µM a,e | GAL 10.12 ± 0.06 µM a,e ALA 8.23 ± 0.01 µM a,e | 24.31 ± 0.33 µM a,m | GAL 20.62 ± 0.08 µM a,m ALA 18 ± 0.06 µM a,m | MCE | |
| Chitralinine C | Delphinium chitralense H. Riedl in Kew Bull. Ranunculaceae (aerial parts) | 12.11 ± 0.82 µM a,e | GAL 10.12 ± 0.06 µM a,e ALA 8.23 ± 0.01 µM a,e | 26.35 ± 0.06 µM a,m | GAL 20.62 ± 0.08 µM a,m ALA 18 ± 0.06 µM a,m | MCE | |
| Dihydropentagynine | Delphinium denudatum Ranunculaceae (aerial parts) | 11.2 ± 0.23 µM a,e | GAL 10.1 ± 0.06 µM a,e | 22.2 ± 0.33 µM a,m | GAL 20.6 ± 0.08 µM a,m | MCE | [51,68] |
| Isotalatizidine hydrate | Delphinium denudatum Ranunculaceae (aerial parts) | 12.1 ± 0.43 µM a,e | GAL 10.1 ± 0.06 µM a,e | 21.4 ± 0.23 µM a,m | GAL 20.6 ± 0.08 µM a,m | MCE | |
| Jadwarine-A | Delphinium denudatum Ranunculaceae (aerial parts) | 9.2 ± 0.12 µM a,e | GAL 10.1 ± 0.06 µM a,e | 19.6 ± 0.72 µM a,m | GAL 20.6 ± 0.08 µM a,m | MCE | |
| Coronaridine | Ervatamia hainanensis Tsiang Apocynaceae (stems) | 8.6 µM a,e | GAL 3.2 µM a,e | nd | nd | CE | [25,48] |
| Voacangine | Ervatamia hainanensis Tsiang Apocynaceae (stems) | 4.4 µM a,e | GAL 3.2 µM a,e | nd | nd | CE | |
| 1-O-Acetyl-9-O-methylpseudolycorine | Galanthus woronowii Losinsk Amaryllidaceae (aerial parts and bulbs) | 78.7 µM a,f | GAL 0.15 µM a,f | nd | nd | MCE | [21,48,69] |
| Galanthine | Galanthus woronowii Losinsk Amaryllidaceae (aerial parts and bulbs) | 7.75 µM a,f | GAL 0.15 µM a,f | nd | nd | MCE | |
| Lycorine | Galanthus woronowii Losinsk Amaryllidaceae (aerial parts and bulbs) | na | GAL 0.15 µM a,f | nd | nd | MCE | |
| Narwedine | Galanthus woronowii Losinsk Amaryllidaceae (aerial parts and bulbs) | 11,79 µM a,f | GAL 0.15 µM a,f | nd | nd | MCE | |
| O-Methylleucotamine | Galanthus woronowii Losinsk Amaryllidaceae (aerial parts and bulbs) | 16.42 µM a,f | GAL 0.15 µM a,f | nd | nd | MCE | |
| Salsoline | Galanthus woronowii Losinsk Amaryllidaceae (aerial parts and bulbs) | na | GAL 0.15 µM a,f | nd | nd | MCE | |
| Sanguinine | Galanthus woronowii Losinsk Amaryllidaceae (aerial parts and bulbs) | 0.007 µM a,f | GAL 0.15 µM a,f | nd | nd | MCE | |
| Sternbergine | Galanthus woronowii Losinsk Amaryllidaceae (aerial parts and bulbs) | 0.99 µM a,f | GAL 0.15 µM a,f | nd | nd | MCE | |
| Chlidanthine | Hieronymiella marginata Hunz Amaryllidaceae (bulbs) | 23.50 ± 0.65 µM a,e | GAL 1 ± 0.05 µM a,e | 196.79 ± 2.67 µM a,m | GAL 14 ± 0.03 µM a,m | MCE | [22,48,70] |
| Lycorine | Hieronymiella marginata Hunz Amaryllidaceae (bulbs) | >200 µM a,e | GAL 1 ± 0.05 µM a,e | >200 µM a,m | GAL 14 ± 0.03 µM a,m | MCE | |
| Sanguinine | Hieronymiella marginata Hunz Amaryllidaceae (bulbs) | 0.10 ± 0.03 µM a,e | GAL 1 ± 0.05 µM a,e | 21.50 ± 0.04 µM a,m | GAL 14 ± 0.03 µM a,m | MCE | |
| Tazettine | Hieronymiella marginata Hunz Amaryllidaceae (bulbs) | >200 µM a,e | GAL 1 ± 0.05 µM a,e | >200 µM a,m | GAL 14 ± 0.03 µM a,m | MCE | |
| Hamayne | Hippeastrum argentinum Pax Amaryllidaceae (bulbs) | >200 µM a,e | GAL 0.48 ± 0.03 µM a,e | >200 µM a,m | GAL 22.39 ± 0.09 µM a,m | MCE | [48,69,70] |
| 7-Hydroxyclivonine | Hippeastrum argentinum Pax Amaryllidaceae (bulbs) | 114.07 ± 0.08 µM a,e | GAL 0.48 ± 0.03 µM a,e | 67.3 ± 0.09 µM a,m | GAL 22.39 ± 0.09 µM a,m | MCE | |
| Lycorine | Hippeastrum argentinum Pax Amaryllidaceae (bulbs) | >200 µM a,e | GAL 0.48 ± 0.03 µM a,e | >200 µM a,m | GAL 22.39 ± 0.09 µM a,m | MCE | |
| 4-O-Methylnangustine | Hippeastrum argentinum Pax Amaryllidaceae (bulbs) | >200 µM a,e | GAL 0.48 ± 0.03 µM a,e | >200 µM a,m | GAL 22.39 ± 0.09 µM a,m | MCE | |
| Montanine | Hippeastrum argentinum Pax Amaryllidaceae (bulbs) | >200 µM a,e | GAL 0.48 ± 0.03 µM a,e | >200 µM a,m | GAL 22.39 ± 0.09 µM a,m | MCE | |
| Pancracine | Hippeastrum argentinum Pax Amaryllidaceae (bulbs) | >200 µM a,e | GAL 0.48 ± 0.03 µM a,e | >200 µM a,m | GAL 22.39 ± 0.09 µM a,m | MCE | |
| Discorhabdin C | Latrunculia biformis Latrunculiidae (sponge) | 14.5 ± 1.5 µM a,e 152 ± 12 µM a,f | PHY 3.0 ± 0.3 µM a,e PHY 14.5 ± 2.0 µM a,f | 15.8 ± 3.5 µM a,m | PHY 28.5 ± 3.0 µM a,m | MCE | [48,71] |
| Discorhabdin G | Latrunculia biformis Latrunculiidae (sponge) | 1.3 ± 0.2 µM a,e 116 ± 9 µM a,f | PHY 3.0 ± 0.3 µM a,e PHY 14.5 ± 2.0 µM a,f | 7.0 ± 1.0 µM a,m | PHY 28.5 ± 3.0 µM a,m | MCE | |
| Discorhabdin B | Latrunculia bocagei Latrunculiidae (sponge) | 5.7 ± 0.8 µM a,e 49.4 ± 7.5 µM a,f | PHY 3.0 ± 0.3 µM a,e PHY 14.5 ± 2.0 µM a,f | 137 ± 14.5 µM a,m | PHY 28.5 ± 3.0 µM a,m | MCE | |
| Discorhabdin L | Latrunculia bocagei Latrunculiidae (sponge) | 25.7 ± 3.0 µM a,e 158 ± 15 µM a,f | PHY 3.0 ± 0.3 µM a,e PHY 14.5 ± 2.0 µM a,f | 531 ± 45.0 µM a,m | PHY 28.5 ± 3.0 µM a,m | MCE | |
| Lupanine | Leontice leontopetalum L. subsp. ewersmannii. Berberidaceae (tubers) | 200 µg/µL—35.41 ± 3.55% b,k | GAL 200 µg/µL—89.98 ± 0.61% b,k | 200 µg/µL—81.77 ± 2.41% b,n | GAL 200 µg/µL—92.47 ± 0.63% b,n | CE | [48,72] |
| N-(14-Methylallyl)-nor-galanthamine | Leucojum aestivum L. Amaryllidaceae (aerial parts) | 0.16 ± 0.01 µM a,e | GAL 1.82 ± 0.40 µM a,e | nd | nd | MCE | [20,69] |
| N-Allyl-nor-galanthamine | Leucojum aestivum L. Amaryllidaceae (aerial parts) | 0.18 ± 0.01 µM a,e | GAL 1.82 ± 0.40 µM a,e | nd | nd | MCE | |
| Casuarinine C | Lycopodiastrum casuarinoides Spring Lycopodiaceae (whole plant) | 20.9 µM a,i | HUP 0.125 µM a,i | nd | nd | MCE | [48,73] |
| Casuarinine I | Lycopodiastrum casuarinoides Spring Lycopodiaceae (whole plant) | 12.1 µM a,i | HUP 0.125 µM a,i | nd | nd | MCE | |
| N-Demethylhuperzinine | Lycopodiastrum casuarinoides Spring Lycopodiaceae (whole plant) | 15.0 µM a,i | HUP 0.125 µM a,i | nd | nd | MCE | |
| Huperzine C | Lycopodiastrum casuarinoides Spring Lycopodiaceae (whole plant) | 0.489 µM a,i | HUP 0.125 µM a,i | nd | nd | MCE | |
| Lycoparin C | Lycopodium casuarinoides Spring Lycopodiaceae (whole plant) | 25 µM a,k | nd | nd | nd | CE | [24,48] |
| Serratezomine D | Lycopodium serratum Thunb. var. serratum Lycopodiaceae (whole plant) | 0.6 mM a,e | GAL 6.4 µM a,e | nd | nd | CE | [48,74] |
| Berberine | Mahonia bealei Carrière, Mahonia fortunei Fedde Berberidaceae (root, stem, leaf) | 0.52 ± 0.06 µM a,k | GAL 0.81 ± 0.08 µM a,k | nd | nd | MCE | [23,48,75] |
| Coptisine | Mahonia bealei Carrière, Mahonia fortunei Fedde Berberidaceae (root, stem, leaf) | 0.53 ± 0.04 µM a,k | GAL 0.81 ± 0.08 µM a,k | nd | nd | MCE | |
| Corypalmine | Mahonia bealei Carrière, Mahonia fortunei Fedde Berberidaceae (root, stem, leaf) | 130.10 ± 9.81 µM a,k | GAL 0.81 ± 0.08 µM a,k | nd | nd | MCE | |
| Dihydroberberine | Mahonia bealei Carrière, Mahonia fortunei Fedde Berberidaceae (root, stem, leaf) | 7.33 ± 0.47 µM a,k | GAL 0.81 ± 0.08 µM a,k | nd | nd | MCE | [23,48,75] |
| Epiberberine | Mahonia bealei Carrière, Mahonia fortunei Fedde Berberidaceae (root, stem, leaf) | 0.80 ± 0.15 µM a,k | GAL 0.81 ± 0.08 µM a,k | nd | nd | MCE | |
| Jatrorrhizine | Mahonia bealei Carrière, Mahonia fortunei Fedde Berberidaceae (root, stem, leaf) | 0.51 ± 0.04 µM a,k | GAL 0.81 ± 0.08 µM a,k | nd | nd | MCE | |
| Palmatine | Mahonia bealei Carrière, Mahonia fortunei Fedde Berberidaceae (root, stem, leaf) | 0.74 ± 0.13 µM a,k | GAL 0.81 ± 0.08 µM a,k | nd | nd | MCE | |
| Stylopine | Mahonia bealei Carrière, Mahonia fortunei Fedde Berberidaceae (root, stem, leaf) | 5.07 ± 0.16 µM a,k | GAL 0.81 ± 0.08 µM a,k | nd | nd | MCE | |
| Tetrahydroberberine | Mahonia bealei Carrière, Mahonia fortunei Fedde Berberidaceae (root, stem, leaf) | 13.13 ± 0.4 µM a,k | GAL 0.81 ± 0.08 µM a,k | nd | nd | MCE | |
| Tetrahydropalmatine | Mahonia bealei Carrière, Mahonia fortunei Fedde Berberidaceae (root, stem, leaf) | 47.56 ± 1.46 µM a,k | GAL 0.81 ± 0.08 µM a,k | nd | nd | MCE | |
| Mahanimbine | Murraya koenigii L. Rutaceae (leaves) | 0.03 ± 0.09 mg mL−1 a,d | GAL 0.006 ± 0.001 mg mL−1 a,d | nd | nd | MCE | [48,76] |
| 1,2-Dihydrogalanthamine | Narcissus jonquilla ‘Pipit’ Amaryllidaceae (bulbs) | 0.19 µM a,e | GAL 0.27 µM a,e | nd | nd | BTLC by Mroczek | [77] |
| Haemanthamine | Narcissus poeticus ‘Pink Parasol’ Amaryllidaceae (bulbs) | >500 µM a,f | GAL 1.7 ± 0.1 µM a,f HUP 0.033 ± 0.001 µM a,f | >500 µM a,l | GAL 42.3 ± 1.3 µM a,l HUP >500 µM a,l | MCE | [48,78] |
| Hippeastrine | Narcissus poeticus ‘Pink Parasol’ Amaryllidaceae (bulbs) | >500 µM a,f | GAL 1.7 ± 0.1 µM a,f HUP 0.033 ± 0.001 µM a,f | >500 µM a,l | GAL 42.3 ± 1.3 µM a,l HUP >500 µM a,l | MCE | |
| Homolycorine | Narcissus poeticus ‘Pink Parasol’ Amaryllidaceae (bulbs) | 64 ± 4 µM a,f | GAL 1.7 ± 0.1µM a,f HUP 0.033 ± 0.001µM a,f | 151 ± 19 µM a,l | GAL 42.3 ± 1.3 µM a,l HUP >500 µM a,l | MCE | |
| Incartine | Narcissus poeticus ‘Pink Parasol’ Amaryllidaceae (bulbs) | 208 ± 14 µM a,f | GAL 1.7 ± 0.1µM a,f HUP 0.033 ± 0.001 µM a,f | >500 µM a,l | GAL 42.3 ± 1.3 µM a,l HUP >500 µM a,l | MCE | |
| Lycoramine | Narcissus poeticus ‘Pink Parasol’ Amaryllidaceae (bulbs) | 456 ± 57 µM a,f | GAL 1.7 ± 0.1 µM a,f HUP 0.033 ± 0.001 µM a,f | >500 µM a,l | GAL 42.3 ± 1.3 µM a,l HUP >500 µM a,l | MCE | |
| Masonine | Narcissus poeticus ‘Pink Parasol’ Amaryllidaceae (bulbs) | 304 ± 34 µM a,f | GAL 1.7 ± 0.1 µM a,f HUP 0.033 ± 0.001 µM a,f | 229 ± 24 µM a,l | GAL 42.3 ± 1.3 µM a,l HUP >500 µM a,l | MCE | |
| Narcipavline | Narcissus poeticus ‘Pink Parasol’ Amaryllidaceae (bulbs) | 208 ± 37 µM a,f | GAL 1.7 ± 0.1 µM a,f HUP 0.033 ± 0.001 µM a,f | 24.4 ± 1.2 µM a,l | GAL 42.3 ± 1.3 µM a,l HUP >500 µM a,l | MCE | |
| Narwedine | Narcissus poeticus ‘Pink Parasol’ Amaryllidaceae (bulbs) | 281 ± 33 µM a,f | GAL 1.7 ± 0.1 µM a,f HUP 0.033 ± 0.001 µM a,f | >500 µM a,l | GAL 42.3 ± 1.3 µM a,l HUP >500 µM a,l | MCE | |
| nor-Lycoramine | Narcissus poeticus ‘Pink Parasol’ Amaryllidaceae (bulbs) | >500 µM a,f | GAL 1.7 ± 0.1 µM a,f HUP 0.033 ± 0.001 µM a,f | >500 µM a,l | GAL 42.3 ± 1.3 µM a,l HUP >500 µM a,l | MCE | |
| Oduline | Narcissus poeticus ‘Pink Parasol’ Amaryllidaceae (bulbs) | >500 µM a,f | GAL 1.7 ± 0.1 µM a,f HUP 0.033 ± 0.001 µM a,f | >500 µM a,l | GAL 42.3 ± 1.3 µM a,l HUP >500 µM a,l | MCE | |
| seco-Isopowellaminone | Narcissus poeticus ‘Pink Parasol’ Amaryllidaceae (bulbs) | 293 ± 33 µM a,f | GAL 1.7 ± 0.1 µM a,f HUP 0.033 ± 0.001 µM a,f | >500 µM a,l | GAL 42.3 ± 1.3 µM a,l HUP >500 µM a,l | MCE | |
| Incartine | Narcissus jonquila var. henriquesii Samp. Amaryllidaceae (bulbs) | 208.2 ± 14.3 µM a,f | GAL 1.7 ± 0.06 µM a,f HUP 0.03 ± 0.0 µM a,f PHY 0.06 ± 0.0 µM a,f | 943.4 ± 140.7 µM a,l | GAL 42.3 ± 1.3 µM a,l HUP >1000 µM a,l PHY 0.13 ± 0.0 µM a,l | MCE | [48,79] |
| Narwedine | Narcissus poeticus ’Brackenhurst’ Amaryllidaceae (bulbs) | 281.2 ± 33.9 µM a,f | GAL 1.7 ± 0.06 µM a,f HUP 0.03 ± 0.0 µM a,f PHY 0.06 ± 0.0 µM a,f | 911.3 ± 68.7 µM a,l | GAL 42.3 ± 1.3 µM a,l HUP >1000 µM a,l PHY 0.13 ± 0.0 µM a,l | MCE | |
| 11-Hydroxygalanthine | Narcissus tazetta subsp. tazetta L Amaryllidaceae (bulbs and leaves) | 0.67 µM a,e | GAL 0.15 µM a,e | 18.17 µM a,m | GAL 2.47µM a,m | MCE | [48,80] |
| 9-O-Demetil-2-α-hydroxyhomolycorine | Narcissus tazetta subsp. tazetta L Amaryllidaceae (bulbs and leaves) | 19.84 µM a,e | GAL 0.15 µM a,e | na | GAL 2.47 µM a,m | MCE | |
| Narcissidine | Narcissus tazetta subsp. tazetta L Amaryllidaceae (bulbs and leaves) | 1.85 µM a,e | GAL 0.15 µM a,e | na | GAL 2.47 µM a,m | MCE | |
| Pancratinine-C | Narcissus tazetta subsp. tazetta L Amaryllidaceae (bulbs and leaves) | na | GAL 0.15 µM a,e | 32.04 µM a,m | GAL 2.47 µM a,m | MCE | |
| Pseudolycorine | Narcissus tazetta subsp. tazetta L Amaryllidaceae (bulbs and leaves) | 32.51 µM a,e | GAL 0.15 µM a,e | 21.64 µM a,m | GAL 2.47 µM a,m | MCE | |
| Angustidine | Nauclea officinalis Merr. & Chun. Rubiaceae (bark) | 21.72 µM a,e | GAL 0.94 µM a,e | 1.03 µM a,m | GAL 28.29 µM a,m | CE | [19,48,81] |
| Angustine | Nauclea officinalis Merr. & Chun. Rubiaceae (bark) | 100 μg mL−1—40.19 ± 0.65% b,e | GAL 0.94 µM a,e | 4.98 µM a,m | GAL 28.29 µM a,m | CE | |
| Angustoline | Nauclea officinalis Merr. & Chun. Rubiaceae (bark) | 261.89 µM a,e | GAL 0.94 µM a,e | 25.10 µM a,m | GAL 28.29 µM a,m | CE | |
| Harmane | Nauclea officinalis Merr. & Chun. Rubiaceae (bark) | 300.68 µM a,e | GAL 0.94 µM a,e | 13.18 µM a,m | GAL 28.29 µM a,m | CE | |
| Nauclefine | Nauclea officinalis Merr. & Chun. Rubiaceae (bark) | 100 μg mL−1—34.61 ± 4.84% b,e | GAL 0.94 µM a,e | 7.70 µM a,m | GAL 28.29 µM a,m | CE | |
| 7,8,13,14-Dehydroorientalidine | Papaver setiferum Goldblatt Papaveraceae (capsules) | nd | NEO 6.0 ± 1.1 µM a,e | nd | NEO 92.7 ± 2.2 µM a,m | MCE | [48,82,83] |
| 7,8-Didehydromecambridine TFA salt | Papaver setiferum Goldblatt Papaveraceae (capsules) | 10.3 ± 1.1 µM a,e | NEO 6.0 ± 1.1 µM a,e | 100 ± 5 µM a,m | NEO 92.7 ± 2.2 µM a,m | MCE | |
| 7,8- Didehydroorientalidine TFA salt | Papaver setiferum Goldblatt Papaveraceae (capsules) | 3.4 ± 4.7 µM a,e | NEO 6.0 ± 1.1 µM a,e | 98.5 ± 0.6 µM a,m | NEO 92.7 ± 2.2 µM a,m | MCE | |
| Alborine | Papaver setiferum Goldblatt Papaveraceae (capsules) | 6.8 ± 4.5 µM a,e | NEO 6.0 ± 1.1 µM a,e | 63.1 ± 0.5 µM a,m | NEO 92.7 ± 2.2 µM a,m | MCE | |
| Isothebaine | Papaver setiferum Goldblatt Papaveraceae (capsules) | 260 ± 1 µM a,e | NEO 6.0 ± 1.1 µM a,e | 2.8 ± 3.0 µM a,m | NEO 92.7 ± 2.2 µM a,m | MCE | |
| N-Methylcodamine | Papaver setiferum Goldblatt Papaveraceae (capsules) | nd | NEO 6.0 ± 1.1 µM a,e | 221 ± 1 µM a,m | NEO 92.7 ± 2.2 µM a,m | MCE | |
| N-Methylisothebainium | Papaver setiferum Goldblatt Papaveraceae (capsules) | nd | NEO 6.0 ± 1.1 µM a,e | 7.1 ± 2.7 µM a,m | NEO 92.7 ± 2.2 µM a,m | MCE | |
| N-Methylorientaline | Papaver setiferum Goldblatt Papaveraceae (capsules) | nd | NEO 6.0 ± 1.1 µM a,e | 342 ± 3 µM a,m | NEO 92.7 ± 2.2 µM a,m | MCE | |
| Orientalidine | Papaver setiferum Goldblatt Papaveraceae (capsules) | 5.0 ± 1.0 µM a,e | NEO 6.0 ± 1.1 µM a,e | 104 ± 4 µM a,m | NEO 92.7 ± 2.2 µM a,m | MCE | |
| Salutaridine | Papaver setiferum Goldblatt Papaveraceae (capsules) | nd | NEO 6.0 ± 1.1 µM a,e | 335 ± 4 µM a,m | NEO 92.7 ± 2.2 µM a,m | MCE | |
| 19(S)-Hydroxyibogamine | Tabernaemontana bufalina Lour. (Apocynaceae) | nd | nd | 20.1 µM a,m | TAC 0.025 µM a,m | MCE | [48,84,85] |
| 3α-Dihydrocadambine | Uncaria rhynchophylla Miq. ex Havil Rubiaceae (stems) | 37.01 ± 1.57 µM a,e | TAC 4.39 ± 0.80 µM a,e | 33.34 ± 0.51 µM a,m | TAC 3.25 ± 1.86 µM a,m | MCE | [48,86] |
| 7-epi-Javaniside | Uncaria rhynchophylla Miq. ex Havil Rubiaceae (stems) | 2.85 ± 0.50 µM a,e | TAC 4.39 ± 0.80 µM a,e | 2.13 ± 0.10 µM a,m | TAC 3.25 ± 1.86 µM a,m | MCE | |
| Cadambine | Uncaria rhynchophylla Miq. ex Havil Rubiaceae (stems) | 26.12 ± 2.12 µM a,e | TAC 4.39 ± 0.80 µM a,e | 30.69 ± 0.69 µM a,m | TAC 3.25 ± 1.86 µM a,m | MCE | |
| Strictosamide | Uncaria rhynchophylla Miq. ex Havil Rubiaceae (stems) | 46.57 ± 0.58µM a,e | TAC 4.39 ± 0.80 µM a,e | 6.47 ± 0.72 µM a,m | TAC 3.25 ± 1.86 µM a,m | MCE | |
| Vincosamide | Uncaria rhynchophylla Miq. ex Havil Rubiaceae (stems) | 12.4 ± 0.86 µM a,e | TAC 4.39 ± 0.80 µM a,e | 23.18 ± 0.14 µM a,m | TAC 3.25 ± 1.86 µM a,m | MCE | |
| Deoxyvobtusine lactone | Voacanga globosa Merr. Apocynaceae (leaves) | 10−4.3 M—91% b,e | GAL 0.64 µM a,e | 20.2 µM a,m | GAL 8.40 µM a,m | MCE | [87,88,89] |
| Deoxyvobtusine | Voacanga globosa Merr. Apocynaceae (leaves) | 10−4.3 M—87% b,e | GAL 0.64 µM a,e | 6.2 µM a,m | GAL 8.40 µM a,m | MCE | |
| Globospiramine | Voacanga globosa Merr. Apocynaceae (leaves) | 10−4.3 M—94% b,e | GAL 0.64 µM a,e | 16.4 µM a,m | GAL 8.40 µM a,m | MCE | |
| Vobtusine lactone | Voacanga globosa Merr. Apocynaceae (leaves) | 10−4.3 M—90% b,e | GAL 0.64 µM a,e | 18.0 µM a,m | GAL 8.40 µM a,m | MCE | |
| ANTHRANOIDS | |||||||
| 2-Geranylemodin | Psorospermum glaberrimum Hochr. Hypericaceae (stem bark) | 0.1 mM—12.9% b,e | GAL 0.50 ± 0.001 µM a,e | 11.30 ± 0.23 µM a,m | GAL 8.50 ± 0.001 µM a,m | MCE | [48,90] |
| 3-Prenyloxyemodin | Psorospermum glaberrimum Hochr. Hypericaceae (stem bark) | 0.1 mM—35.0% b,e | GAL 0.50 ± 0.001 µM a,e | 13.3 ± 1.10 µM a,m | GAL 8.50 ± 0.001 µM a,m | MCE | |
| Acetylvismione D | Psorospermum glaberrimum Hochr. Hypericaceae (stem bark) | 0.1 mM—45.70% b,e | GAL 0.50 ± 0.001 µM a,e e | 10.1 ± 0.20 µM a,m | GAL 8.50 ± 0.001 µM a,m | MCE | |
| Bianthrone 1a | Psorospermum glaberrimum Hochr. Hypericaceae (stem bark) | 63.0 ± 0.46 µM a,e | GAL 0.50 ± 0.001 µM a,e a,e | 9.25 ± 0.25 µM a,m | GAL 8.50 ± 0.001 µM a,m | MCE | |
| 3-Geranyloxyemodin anthrone | Psorospermum glaberrimum Hochr. Hypericaceae (stem bark) | 100 µM—5.4% b,e | GAL 0.50 ± 0.001 µM a,e e | 11.60 ± 0,20 µM a,m | GAL 8.50 ± 0.001 µM a,m | MCE | |
| 3-Prenyloxyemodin anthrone | Psorospermum glaberrimum Hochr. Hypericaceae (stem bark) | 100 µM—13.8% b,e | GAL 0.50 ± 0.001 µM a,e | 10.1 ± 0.5 µM a,m | GAL 8.50 ± 0.001 µM a,m | MCE | |
| Emodin | Talaromyces aurantiacus FL 15 (strain from leave Huperzia serrata) | >100 µM a,e | RIV 1.82 ± 0.13 µM a,e HUP 0.045 ± 0.01 µM a,e | >100 µM a,m | nd | MCE | [48,91,92] |
| Physcion | Talaromyces aurantiacus FL 15 (strain from leave Huperzia serrata) | >100 µM a,e | RIV 1.82 ± 0.13 µM a,e HUP 0.045 ± 0.01 µM a,e | >100 µM a,m | nd | MCE | |
| Chrysophanol | Talaromyces aurantiacus FL 15 (strain from leave Huperzia serrata) | >100 µM a,e | RIV 1.82 ± 0.13 µM a,e HUP 0.045 ± 0.01 µM a,e, | >100 µM a,m | nd | MCE | |
| BIBENZYLS | |||||||
| 3,3′-Dihydroxy-4-(4-hydroxybenzyl)-5-methoxybibenzyl | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1—2.6 ± 2.8% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—22.6 ± 2.1% b,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | [37,48] |
| 3′,5-Dihydroxy-2-(4-hydroxybenzyl)-3-methoxybibenzyl | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1—5.0 ± 1.5% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—51.3 ± 2.0% b,m 80.3 ± 5.2 µM a,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | |
| Bulbocol | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1—16.3 ± 3.8% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—67.7 ± 0.3% b,m 33.5 ± 3.7 µM a,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | |
| Gymconopin D | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1—48.1 ± 6.3% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—66.2 ± 3.4% b,m 40.5 ± 5.6 µM a,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | |
| COUMARINS | |||||||
| Scopoletin | Scopolia carniolica Jaqc. Solanaceae (roots) | 168.6 µM a,e | GAL 3.2 µM a,e | nd | nd | MCE | [16,48,93,94,95] |
| Decursinol | Angelica gigas Nakai Apiaceae (underground parts) | 28 μM a,k | nd | nd | nd | MCE | [48,96,97,98] |
| Isoimperatorin | Angelica gigas Nakai Apiaceae (underground parts) | 69 μM a,k | nd | nd | nd | MCE | |
| Marmesin | Angelica gigas Nakai Apiaceae (underground parts) | 67 μM a,k | nd | nd | nd | MCE | |
| Nodakenin | Angelica gigas Nakai Apiaceae (underground parts) | 68 μM a,k | nd | nd | nd | MCE | |
| Xanthotoxin | Angelica gigas Nakai Apiaceae (underground parts) | 54 μM a,k | nd | nd | nd | MCE | |
| Bergapten | Angelica officinalis L. Apiaceae (fruits) | 25 µg mL−1— 32.65 ± 6.10% b,e 100 µg mL−1—nd | GAL 100 µg mL−1—98.97 ± 0.24% b,e | 25 µg mL−1—86.69 ± 2.56% b,m 100 µg mL−1- nd | GAL 100 µg mL−1—80.31 ± 1.14% b,m | MCE | [48,99,100] |
| Imperatorin | Angelica officinalis L. Apiaceae (fruits) | 25 µg mL−1— 18.76 ± 1.07% b,e 100 µg mL−1—46.11 ± 0.92% b,e | GAL 100 µg mL−1—98.97 ± 0.24% b,e | 25 µg mL−1— 37.46 ± 1.09% b,m 100 µg mL−1— 83.98 ± 0.99% b,m | GAL 100 µg mL−1—80.31 ± 1.14% b,m | MCE | |
| Xanthotoxin | Angelica officinalis L. Apiaceae (fruits) | 25 µg mL−1— 38.23 ± 0.06% b,e 100 µg mL−1—66.08 ± 2.88% b,e | GAL 100 µg mL−1—98.97 ± 0.24% b,e | 25 µg mL−1—63.60 ± 1.78% b,m 100 µg mL−1—88.04 ± 0.83% b,m | GAL 100 µg mL−1—80.31 ± 1.14% b,m | MCE | |
| Heraclenol-2′-O-angelate | Archangelicae officinalis L. Apiaceae (roots) | >1000 μM a,e | GAL 0.37 ± 1.1 μM a,e | 7.5 ± 1.8 μM a,m | GAL 8.3 ± 2.6 μM a,m | BTLC by Marston et al. (2002) | [28,48,101] |
| Imperatorin | Archangelicae officinalis L. Apiaceae (fruits) | 156 ± 15 μM a,e | GAL 0.37 ± 1.1 μM a,e | 14.4 ± 3.2 μM a,m | GAL 8.3 ± 2.6 μM a,m | BTLC by Marston et al. (2002) | |
| Isoimperatorin | Citrus hystrix DC. Rutaceae (peels of fruits) | nd | nd | 23 ± 0.2 µM a,m | GAL 3.2 ± 0.2 µM a,m | MCE | [27,48] |
| 6′,7′- Dihydroxybergamottin | Citrus hystrix DC Rutaceae (peels of fruits) | nd | nd | 15.4 ± 0.3 µM a,m | GAL 3.2 ± 0.2 µM a,m | MCE | |
| 6′-Hydroxy-7′-methoxybergamottin | Citrus hystrix DC. Rutaceae (peels of fruits) | nd | nd | 11.2 ± 0.1 µM a,m | GAL 3.2 ± 0.2 µM a,m | MCE | |
| 5,7-Dihydroxy-8-(3-methylbutanoyl)- 6-[(E)-3,7-dimethylocta-2,6-dienyl]-4-phenyl- 2H-chromen-2-one | Mesua elegans Kosterm. Clusiaceae (bark) | 3.06 ± 0.04 µM a,e | TAC 0.074 ± 0.012 µM a,e | nd | nd | CE | [29,48] |
| Mesuagenin A | Mesua elegans Kosterm. Clusiaceae (bark) | 1.06 ± 0.04 µM a,e | TAC 0.074 ± 0.012 µM a,e | nd | nd | CE | |
| Mesuagenin B | Mesua elegans Kosterm. Clusiaceae (bark) | 0.70 ± 0.10 µM a,e | TAC 0.074 ± 0.012 µM a,e | nd | nd | CE | |
| Mesuagenin D | Mesua elegans Kosterm. Clusiaceae (bark) | 8.73 ± 0.25 µM a,e | TAC 0.074 ± 0.012 µM a,e | nd | nd | CE | |
| Lucidafuranocoumarin A | Peucedanum alsaticum L. Apiaceae (fruits) | na | GAL 100 µg mL−1—92.14 ± 2.49% b,k 1.82 ± 0.22 µg mL−1 a,k | 100 µg mL−1—40.66 ± 1.25% b,n | GAL 100 µg mL−1—81.93 ± 2.52% b,n 22.16 ± 0.91 µg mL−1 a,n | MCE | [102] |
| Bergamottin | Peucedanum alsaticum L. Apiaceae (fruits) | 100 µg mL−1—4.00 ± 0.82% b | GAL 100 µg mL−1—92.14 ± 2.49% b,k 1.82 ± 0.22 µg mL−1 a,k | 100 µg mL−1—17.65 ± 1.50% b | GAL 100 µg mL−1—81.93 ± 2.52% b,n 22.16 ± 0.91 µg mL−1 a,n | MCE | |
| CHROMONES | |||||||
| Sargachromanol G | Sargassum siliquastrum Sargassaceae (strains) | 1.81 ± 0.020 µM a,e | BER 1.01 ± 0.01 µM a,e TAC 0.22 ± 0.004 µM a,e | 10.79 ± 0.65 µM a,m | TAC 0.014 ± 0.0043 µM a,m | MCE | [48,59,60] |
| Sargachromanol I | Sargassum siliquastrum Sargassaceae (strains) | 0.79 ± 0.07 µM a,e | BER 1.01 ± 0.01 µM a,e TAC 0.22 ± 0.004 µM a,e | 13.69 ± 5.07 µM a,m | TAC 0.014 ± 0.0043 µM a,m | MCE | |
| DIARYLHEPTANOIDS | |||||||
| (−)-Alpininoid B | Alpinia officinarum Hance Zingiberaceae (rhizomes) | 100 µM—87.6 ± 0.1% b,e 2.6 ± 4.2 µM a,e | TAC 111.8 ± 4.6 µM a,e | 100 µM—64.7 ± 1.4% b,m 35.2 ± 0.7 µM a,m | TAC 8.9 ± 2.4 µM a,m | MCE | [31,66] |
| (4E)−1,7-Diphenyl-4-hepten-3-one | Alpinia officinarum Hance Zingiberaceae (rhizomes) | 100 µM—98.0 ± 0.9% b,e 23.9 ± 2.6 µM a,e | TAC 111.8 ± 4.6 µM a,e | 100 µM—62.3 ± 3.5% b,m 70.7 ± 2.5 µM a,m | TAC 8.9 ± 2.4 µM a,m | MCE | |
| Dihydroyashsbushiketol | Alpinia officinarum Hance Zingiberaceae (rhizomes) | 100 µM—36.2 ± 1.9% b,e | TAC 111.8 ± 4.6 µM a,e | 100 µM—15.7 ± 2.1% b,m | TAC 8.9 ± 2.4 µM a,m | MCE | |
| (4E)-7-(4-Hydroxyphenyl)-1-phenyl-4-hepten-3-one | Alpinia officinarum Hance Zingiberaceae (rhizomes) | 100 µM –57.9 ± 3.2% b,e 87.3 ± 3.4 µM a,e | TAC 111.8 ± 4.6 µM a,e | 100 µM—41.1 ± 0.1% b,m | TAC 8.9 ± 2.4 µM a,m | MCE | |
| (4E)-7-(4-Hydroxy-3-methoxyphenyl)-1-phenyl-hept-4-en-3-one | Alpinia officinarum Hance Zingiberaceae (rhizomes) | 100 µM—76.6 ± 0.3% b,e 39.1 ± 2.3 µM a,e | TAC 111.8 ± 4.6 µM a,e | 100 µM—43.7 ± 1.4% b,m | TAC 8.9 ± 2.4 µM a,m | MCE | |
| (5R)-7-(4-Hydroxy-3-methoxyphenyl)-5-methoxy-1-phenyl-3-heptanone | Alpinia officinarum Hance Zingiberaceae (rhizomes) | 100 µM—35.3 ± 1.0% b,e | TAC 111.8 ± 4.6 µM a,e | 100 µM—21.5 ± 0.6% b,m | TAC 8.9 ± 2.4 µM a,m | MCE | |
| Kaempferide | Alpinia officinarum Hance Zingiberaceae (rhizomes) | 100 µM—67.2 ± 1.8% b,e 31.9 ± 2.0 µM a,e | TAC 111.8 ± 4.6 µM a,e | 100 µM –47.6 ± 1.6% b,m | TAC 8.9 ± 2.4 µM a,m | MCE | |
| Galangin | Alpinia officinarum Hance Zingiberaceae (rhizomes) | 100 µM—65.4 ± 4.5% b,e 70.1 ± 1.5 µM a,e | TAC 111.8 ± 4.6 µM a,e | 100 µM—63.6 ± 3.1% b,m 61.4 ± 1.4 µM a,m | TAC 8.9 ± 2.4 µM a,m | MCE | |
| DITERPENES | |||||||
| Dihydrotanshinone | Salvia miltiorhiza Bunge Lamiaceae (roots) | 1 μM a,d | PHY 0.25 µM a,d | nd | nd | MCE | [38,103] |
| Cryptotanshinone | Salvia miltiorhiza Bunge Lamiaceae (roots) | 7 μM a,d | PHY 0.25 µM a,d | nd | nd | MCE | |
| Tanshinone I | Salvia miltiorhiza Bunge Lamiaceae (roots) | >50 μM a,d | PHY 0.25 µM a,d | nd | nd | MCE | |
| Tanshionone IIA | Salvia miltiorhiza Bunge Lamiaceae (roots) | >140 μM a,d | PHY 0.25 µM a,d | nd | nd | MCE | |
| Scapaundulin C | Scapania undulate L. Scapaniaceae | >250 ng c,e | GAL >10 ng c,e | nd | nd | BTLC by Marston et al. (2002) | [104,105] |
| Scapaundulin A | Scapania undulate L. Scapaniaceae | >250 ng c,e | GAL >10 ng c,e | nd | nd | BTLC by Marston et al. (2002) | |
| 5α, 8α, 9α-Trihydroxy-13E-labden-12-one | Scapania undulate L. Scapaniaceae | >250 ng c,e | GAL >10 ng c,e | nd | nd | BTLC by Marston et al. (2002) | |
| 5α, 8α- Dihydroxy-13E-labden-12-one | Scapania undulate L. Scapaniaceae | >250 ng c,e | GAL >10 ng c,e | nd | nd | BTLC by Marston et al. (2002) | |
| (13S)-15-Hydroxylabd-8 (17)-en-19-oic acid | Scapania undulate L. Scapaniaceae | >500 ng c,e | GAL >10 ng c,e | nd | nd | BTLC by Marston et al. (2002) | |
| FATTY ACID | |||||||
| (2E,4E,6R)-6-Hydroxydeca- 2,4-dienoic acid. | Lycopodiella cernua L. Lycopodiaceae (whole plants) | 0.22 ± 0.03 µM a,k | BER 0.10 ± 0.01 µM a,k | >30 µM a,n | BER 1.09 ± 0.17 µM a,n | MCE | [48,106] |
| FLAVONOIDS | |||||||
| 3-Methoxy quercetin | Agrimonia pilosa Ledeb. Rosaceae (leaves) | 37.9 μM a,e | DEH 37.8 μM a,e | nd | nd | MCE | [48,107] |
| Quercetin | Agrimonia pilosa Ledeb. Rosaceae (leaves) | 19.8 μM a,e | DEH 37.8 μM a,e | nd | nd | MCE | |
| Quercitrin | Agrimonia pilosa Ledeb. Rosaceae (leaves) | 66.9 μM a,e | DEH 37.8 μM a,e | nd | nd | MCE | |
| Tiliroside | Agrimonia pilosa Ledeb. Rosaceae (leaves) | 23.5 μM a,e | DEH 37.8 μM a,e | nd | nd | MCE | |
| Linarin | Buddleja davidii Franch. Buddlejaceae (leaves) | >10 ng c,e | HUP >1 ng c,e | nd | nd | BTLC by Marston et al. (2002) | [101,104] |
| Garcineflavonol A | Garcinia atroviridis Griff. ex T. Anderson Clusiaceae (stem bark) | 100 μg mL−1—68.45 ± 0.97% b,e 14.04 ± 0.77 μg mL−1 a,e | PHY 0.05 ± 0.01 μg mL−1 a,e | 14.50 ± 0.47 μg mL−1 a,m | PHY 0.14 ± 0.015 μg mL−1 a,m | MCE | [48,108,109] |
| Quercetin | Ginkgo biloba L. Ginkgoaceae (leaves) | 95.8 μg mL−1 a,h | CHL 12.4 μg mL−1 a,h | nd | nd | MCE | [48,110,111] |
| Quercetin- 3-O-𝛼-L-rhamnopyranosyl- (1 → 6)-𝛽-D-glucopyranoside | Ginkgo biloba L. Ginkgoaceae (leaves) | 73.1 μg mL−1 a,h | CHL 12.4 μg mL−1 a,h | nd | nd | MCE | |
| Quercetin-3-O- 𝛽-D-glucopyranoside | Ginkgo biloba L. Ginkgoaceae (leaves) | 57.8 μg mL−1 a,h | CHL 12.4 μg mL−1 a,h | nd | nd | MCE | |
| Quercetin-3-O-𝛼-L-rhamnopyranoside | Ginkgo biloba L. Ginkgoaceae (leaves) | 110.9 μg mL−1 a,h | CHL 12.4 μg mL−1 a,h | nd | nd | MCE | |
| Quercetin-3-O-𝛼-L-rhamnopyranosyl- (1 → 4)-O-𝛼-L-rhamnopyranosyl- (1 → 2)-𝛽-D-glucopyranoside | Ginkgo biloba L. Ginkgoaceae (leaves) | 112.6 μg mL−1 a,h | CHL 12.4 μg mL−1 a,h | nd | nd | MCE | |
| Taxifolin | Ginkgo biloba L. Ginkgoaceae (leaves) | 133.1 μg mL−1 a,h | CHL 12.4 μg mL−1 a,h | nd | nd | MCE | |
| Quercetin-3-O-neohesperidoside | Lysimachia clethroides Duby Primulaceae (whole plant) | 6.98 ± 0.47 µM a,e | BER 1.01 ± 0.01 µM a,e TAC 0.22 ± 0.004 µM a,e | >40 µM a,m | TAC 0.014 ± 0.0043 µM a,m | MCE | [48,59,60] |
| Diplacone | Paulownia tomentosa Steud. Paulowniaceae (fruits) | 7.2 ± 0.6 µM a,f | PHY 0.15 ± 0.03 µM a,f | 1.4 ± 0.3 µM a,m | PHY 3.7 ± 0.6 µM a,m | MCEF | [34,48,112] |
| 3′-O-Methyldiplacol | Paulownia tomentosa Steud. Paulowniaceae (fruits) | 48.5 ± 2.1 µM a,f | PHY 0.15 ± 0.03 µM a,f | 11.2 ± 2.1 µM a,m | PHY 3.7 ± 0.6 µM a,m | MCEF | |
| 3′-O-Methyldiplacone | Paulownia tomentosa Steud. Paulowniaceae (fruits) | 109.2 ±8.4 µM a,f | PHY 0.15 ± 0.03 µM a,f | 24.5 ± 1.2 µM a,m | PHY 3.7 ± 0.6 µM a,m | MCEF | |
| 4′-O-Methyldiplacone | Paulownia tomentosa Steud. Paulowniaceae (fruits) | 92.4 ± 4.1 µM a,f | PHY 0.15 ± 0.03 µM a,f | 25.6 ± 1.6 µM a,m | PHY 3.7 ± 0.6 µM a,m | MCEF | |
| 4′-O-Methyldiplacol | Paulownia tomentosa Steud. Paulowniaceae (fruits) | 31.9 ± 1.2 µM a,f | PHY 0.15 ± 0.03 µM a,f | 12.7 ± 1.3 µM a,m | PHY 3.7 ± 0.6 µM a,m | MCEF | |
| 6-Geranyl-3,3′,5,5′,7-pentahydroxy- 4′-methoxyflavane | Paulownia tomentosa Steud. Paulowniaceae (fruits) | 15.6 ± 0.8 µM a,f | PHY 0.15 ± 0.03 µM a,f | 3.8 ± 0.8 µM a,m | PHY 3.7 ± 0.6 µM a,m | MCEF | |
| 6-Geranyl-3′,5,5′,7-tetrahydroxy- 4′-methoxyflavanone | Paulownia tomentosa Steud. Paulowniaceae (fruits) | 22.9 ± 1.6 µM a,f | PHY 0.15 ± 0.03 µM a,f | 6.4 ± 0.9 µM a,m | PHY 3.7 ± 0.6 µM a,m | MCEF | |
| 6-Geranyl-4′,5,7-trihydroxy-3′,5′-dimethoxyflavanone | Paulownia tomentosa Steud. Paulowniaceae (fruits) | 316.3 ± 12.5 µM a,f | PHY 0.15 ± 0.03 µM a,f | 80.00 ± 2.6 µM a,m | PHY 3.7 ± 0.6 µM a,m | MCEF | |
| Mimulone | Paulownia tomentosa Steud. Paulowniaceae (fruits) | 91.5 ± 5.3 µM a,f | PHY 0.15 ± 0.03 µM a,f | 20.6 ± 1.1 µM a,m | PHY 3.7 ± 0.6 µM a,m | MCEF | |
| Dihydrowogonin | Prunus padus var. seoulensis Nakai Rosaceae (leaves) | 21.53 ± 0.32 µM a,e | TAC 0.22 ± 0.001 µM a,e | nd | nd | MCE | [48,59] |
| Dihydrowogonin 7-O-glucoside | Prunus padus var. seoulensis Nakai Rosaceae (leaves) | 15.49 ± 0.11 µM a,e | TAC 0.22 ± 0.001 µM a,e | nd | nd | MCE | |
| Genkwanin | Prunus padus var. seoulensis Nakai Rosaceae (leaves) | 17.03 ± 0.77 µM a,e | TAC 0.22 ± 0.001 µM a,e | nd | nd | MCE | |
| Rhamnocitrin | Prunus padus var. seoulensis Nakai Rosaceae (leaves) | 18.26 ± 0.075 µM a,e | TAC 0.22 ± 0.001 µM a,e | nd | nd | MCE | |
| 3,5,7-Trihydroxy-8-methoxyflavanone | Prunus padus var. seoulensis Nakai Rosaceae (leaves) | 17.92 ± 0.63 µM a,e | TAC 0.22 ± 0.001 µM a,e | nd | nd | MCE | |
| Amentoflavone | Selaginella doederleinii Hieron Selaginellaceae (whole plant) | 0.73 ± 0.009 µM a,e | TAC 1.26 ± 0.017 µM a,e | nd | nd | MCE | [48,113] |
| Bilobetin | Selaginella doederleinii Hieron Selaginellaceae (whole plant) | 5.76 ± 0.021 µM a,e | TAC 1.26 ± 0.017 µM a,e | nd | nd | MCE | |
| Isoginkgetin | Selaginella doederleinii Hieron Selaginellaceae (whole plant) | 4.11 ± 0.019 µM a,e | TAC 1.26 ± 0.017 µM a,e | nd | nd | MCE | |
| Robustaflavone | Selaginella doederleinii Hieron Selaginellaceae (whole plant) | 6.16 ± 0.032 µM a,e | TAC 1.26 ± 0.017 µM a,e | nd | nd | MCE | |
| Kaempferol | Spiranthes sinensis Ames Orchidaceae (whole plant) | 12.64 ± 0.31 a,k | GAL 0.19 ± 0.02 µg/mL a,k | nd | nd | MCE | [48,114] |
| Quercetin | Spiranthes sinensis Ames Orchidaceae (whole plant) | 8.63 ± 0.37 a,k | GAL 0.19 ± 0.02 µg/mL a,k | nd | nd | MCE | |
| LANOSTANE TRITERPENES | |||||||
| Methyl lucidenate E2 | Ganoderma lucidum Karst. Ganodermataceae (fruiting bodies) | 17.14 ± 2.88 µM a,k | BERCl 0.04 ± 0.01 µM a,k | >200 µM a,n | BERCl 18.97 ± 0.41 µM a,n | MCE | [48,115] |
| n-Butyl lucidenate A | Ganoderma lucidum Karst. Ganodermataceae (fruiting bodies) | 12.26 ± 0.68 µM a,k | BERCl 0.04 ± 0.01 µM a,k | >200 µM a,n | BERCl 18.97 ± 0.41 µM a,n | MCE | |
| Ganoderic acid E | Ganoderma lucidum Karst. Ganodermataceae (fruiting bodies) | 18.35 ± 2.95 µM a,k | BERCl 0.04 ± 0.01 µM a,k | >200 µM a,n | BERCl 18.97 ± 0.41 µM a,n | MCE | |
| N-Butyl ganoderate H | Ganoderma lucidum Karst. Ganodermataceae (fruiting bodies) | 9.40 ± 0.88 µM a,k | BERCl 0.04 ± 0.01 µM a,k | >200 µM a,n | BERCl 18.97 ± 0.41 µM a,n | MCE | |
| Lucidadiol | Ganoderma lucidum Karst. Ganodermataceae (fruiting bodies) | 31.03 ± 1.69 µM a,k | BERCl 0.04 ± 0.01 µM a,k | 156.27 ± 6.12 µM a,n | BERCl 18.97 ± 0.41 µM a,n | MCE | |
| Lucidenic acid N | Ganoderma lucidum Karst. Ganodermataceae (fruiting bodies) | 25.91 ± 0.89 µM a,k | BERCl 0.04 ± 0.01 µM a,k | 188.36 ± 3.05 µM a,n | BERCl 18.97 ± 0.41 µM a,n | MCE | |
| Lucidumol B | Ganoderma lucidum Karst. Ganodermataceae (fruiting bodies) | 16.27 ± 0.51 µM a,k | BERCl 0.04 ± 0.01 µM a,k | >200 µM a,n | BERCl 18.97 ± 0.41 µM a,n | MCE | |
| n-Butyl lucidenate N | Ganoderma lucidum Karst. Ganodermataceae (fruiting bodies) | 11.58 ± 0.36 µM a,k | BERCl 0.04 ± 0.01 µM a,k | >200 µM a,n | BERCl 18.97 ± 0.41 µM a,n | MCE | |
| LIGNANS | |||||||
| Macelignan | Myristica fragrans Houtt. Myristicaceae (seeds) | 4.16 ± 0.070 µM a,e | BER 1.01 ± 0.01 µM a,e TAC 0.22 ± 0.004 µM a,e | 9.69 ± 0.98 µM a,m | TAC 0.014 ± 0.0043 µM a,m | MCE | [48,59,60] |
| (+)-(7R,8S)-Erythro-4,7,9′-trihydroxy-8-O-4′-neolignan-9-O-β-D-glucopyranoside | Camelia sinensis var. sinensis Theaceae (leaves and buds) | 0.75 ± 0.04 µM a,e | HUP 0.29 ± 0.05 µM a,e | nd | nd | MCE | [48,116,117] |
| (7S,8S)-Threo-4,9,9′-trihydroxy-8-O-4′-neolignan-7-O-β-D-glucopyranoside | Camelia sinensis var. sinensis Theaceae (leaves and buds) | 0.19 ± 0.02 µM a,e | HUP 0.29 ± 0.05 µM a,e | nd | nd | MCE | |
| STILBENOID | |||||||
| Isoarundinin II | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1—0.9 ± 0.8% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—39.3 ± 2.3% b,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | [37,48] |
| PHENANTHRENES | |||||||
| 1-[(4-Hydroxyphenyl)methyl]-4-methoxy-2,7-phenanthrenediol | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1—19.1 ± 3.8% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—96.6 ± 1.2% b,m 2.1 ± 0.3 µM a,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | [37,48] |
| 1,8-bis(4-Hydroxybenzyl)-4-methoxyphenanthrene-2,7-diol | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1—16.1 ± 5.0% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—95.4 ± 0.3% b,m 2.3 ± 0.4 µM a,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | |
| 2,7-Dihydroxy-1,3-bi(p-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1—20.1 ± 3.5% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—53.1 ± 1.2% b,m 44.6 ± 4.1 µM a,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | |
| 1-(p-Hydroxybenzyl)-4, 7-dimethoxyphenanthrene-2,8-diol | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1—20.4 ± 4.5% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—85.2 ± 2.9% b,m 6.4 ± 0.2 µM a,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | |
| 3-(4-Hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1—9.6 ± 2.6% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—65.7 ± 0.7% b,m 34.0 ± 1.4 µM a,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | |
| 9-(4′-Hydroxy-3′- methoxyphenyl)-10-(hydroxymethyl)-11-methoxy-5,6,9, 10-tetrahydrophenanthro [2,3-b] furan-3-ol | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1—3.3 ± 1.8% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—61.2 ± 1.3% b,m 35.8 ± 9.2 µM a,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | |
| Bleformin A | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1—18.5 ± 1.7% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—70.0 ± 1.0% b,m 5.2 ± 0.4 µM a,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | |
| Bleformin B | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1—9.9 ± 4.7% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—75.7 ± 1.1% b,m 16.7 ± 2.4 µM a,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | |
| Blestrin D | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1—6.8 ± 1.6% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—69.0 ± 2.5% b,m 8.1 ± 0.5 µM a,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | |
| Blestrin A | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1—8.4 ± 3.1% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—64.0 ± 2.6% b,m 17.9 ± 4.7 µM a,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | |
| Blestrin C | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1—4.9 ± 3.2% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—64.3 ± 2.4% b,m 12.1 ± 3.4 µM a,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | |
| Bletilol D | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1—5.7 ± 2.8% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—31.6 ± 2.8% b,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | |
| Bletilol E | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1—5.1 ± 4.0% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—8.0 ± 2.4% b,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | |
| Favanthrin | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1—13.3 ± 2.9% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—56.7 ± 2.0% b,m 42.2 ± 5.1 µM a,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | |
| Pholidotol | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1 –5.2 ± 3.2% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—29.1 ± 1.3% b,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | |
| Shancidin | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1—15.2 ± 3.6% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—72.8 ± 3.4% b,m 16.7 ± 2.0 µM a,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | |
| Shanciol F | Bletilla striata Reichb. f. Orchidaceae (tuber) | 25 μg mL−1—5.5 ± 1.8% b,e | GAL 25 μg mL−1—94.8 ± 0.9% b,e | 25 μg mL−1—21.8 ± 3.1% b,m | GAL 25 μg mL−1—64.2 ± 0.6% b,m 46.3 ± 5.8 µM a,m TAC 0.0101 ± 0.0005 µM a,m | MCE | |
| Cremaphenanthrene F | Cremastra appendiculata Makino Orchidaceae (tubers) | >200 µM a,e | GAL 0.39 ± 0.04 µM a,e | 14.62 ± 2.15 µM a,m | GAL 1.12 ± 0.67 µM a,m | MCE | [44,48] |
| Cremaphenanthrene G | Cremastra appendiculata Makino Orchidaceae (tubers) | >200 µM a,e | GAL 0.39 ± 0.04 µM a,e | 79.56 ± 0.78 µM a,m | GAL 1.12 ± 0.67 µM a,m | MCE | |
| PHENYLPROPANOIDS | |||||||
| Lapathoside A | Fallopia dentatoalata Holub Polygonaceae (aerial part) | 30.6 ± 4.7 µM a,e | TAC 0.1267 ± 0.0011 µM a,e | 2.7 ± 1.7 µM a,m | TAC 0.0055 ± 0.0017 µM a,m | MCE | [48,118,119] |
| Lapathoside B | Fallopia dentatoalata Holub Polygonaceae (aerial part) | >100 µM a,e | TAC 0.1267 ± 0.0011 µM a,e | 10.9 ± 4.9 µM a,m | TAC 0.0055 ± 0.0017 µM a,m | MCE | |
| Smilaside G | Fallopia dentatoalata Holub Polygonaceae (aerial part) | >100 µM a,e | TAC 0.1267 ± 0.0011 µM a,e | 17.1 ± 3.4 µM a,m | TAC 0.0055 ± 0.0017 µM a,m | MCE | |
| Smilaside J | Fallopia dentatoalata Holub Polygonaceae (aerial part) | 56.0 ± 2.4 µM a,e | TAC 0.1267 ± 0.0011 µM a,e | 10.1 ± 4.6 µM a,m | TAC 0.0055 ± 0.0017 µM a,m | MCE | |
| Vanicoside B | Fallopia dentatoalata Holub Polygonaceae (aerial part) | 32.3 ± 4.7µM a,e | TAC 0.1267 ± 0.0011 µM a,e | 7.5 ± 4.1 µM a,m | TAC 0.0055 ± 0.0017 µM a,m | MCE | |
| PHLOROTANNINS | |||||||
| 974-B | Eisenia bicyclis (Kjellman) Stechell Laminariaceae (leafy thalli) | 1.95 ± 0.01 μM a,e | BER 0.22 ± 0.03 µM a,e | 3.26 ± 0.08 µM a,m | BER 11.74 ± 0.85 µM a,m | CE | [48,120] |
| PHTHALATES | |||||||
| bis (7-Acetoxy-2-ethyl- 5-methylheptyl) phthalate | Lonicera quinquelocularis Hard. Caprifoliaceae (whole plant) | 1.65 ± 0.03 µM a,k | GAL 1.79 ± 0.061 µM a,k | 5.98 ± 0.079 µM a,m | GAL 7.98 ± 0.01 µM a,m | MCE | [48,51,121] |
| Neopentyl-4-hydroxy-3,5-bis (3-methyl-2-butenyl) benzoate | Lonicera quinquelocularis Hard. Caprifoliaceae (whole plant) | 3.43 ± 0.02 µM a,k | GAL 1.79 ± 0.061 µM a,k | 9.84 ± 0.037 µM a,m | GAL 7.98 ± 0.01 µM a,m | MCE | |
| PHENOLIC ACIDS | |||||||
| 4-Hydroxybenzoic acid methyl ester | Spiranthes sinensis Ames Orchidaceae (whole plant) | 42.89 ± 1.21 a,k | GAL 0.19 ± 0.02 µg/mL a,k | nd | nd | MCE | [48,114] |
| Ethyl ferulate | Spiranthes sinensis Ames Orchidaceae (whole plant) | 19.97 ± 1.05 a,k | GAL 0.19 ± 0.02 µg/mL a,k | nd | nd | MCE | |
| 3-(4-Tolyloxy)-propanoic acid | Spiranthes sinensis Ames Orchidaceae (whole plant) | 15.31 ± 0.64 a,k | GAL 0.19 ± 0.02 µg/mL a,k | nd | nd | MCE | |
| POLYKETIDES | |||||||
| Aspilactonol G | Phaeospaeria sp. LF5 (strain from Huperzia serrata) | >100 µM a,k | RIV 1.82 ± 0.13 µM a,k HUP 0.045 ± 0.01 µM a,k | nd | nd | MCE | [48,122,123] |
| Aspilactonol H | Phaeospaeria sp. LF5 (strain from Huperzia serrata) | >100 µM a,k | RIV 1.82 ± 0.13 µM a,k HUP 0.045 ± 0.01 µM a,k | nd | nd | MCE | |
| Aspilactonol I | Phaeospaeria sp. LF5 (strain from Huperzia serrata) | 6.26 ± 0.15 µM a,k | RIV 1.82 ± 0.13 µM a,k HUP 0.045 ± 0.01 µM a,k | nd | nd | MCE | |
| de-O-Methyldiaporthin | Phaeospaeria sp. LF5 (strain from Huperzia serrata) | 21.18 ± 1.53 µM a,k | RIV 1.82 ± 0.13 µM a,k HUP 0.045 ± 0.01 µM a,k | nd | nd | MCE | |
| 6,8-Dihydroxy-3-(10R, 20R-dihydroxypropyl)-isocoumarin | Phaeospaeria sp. LF5 (strain from Huperzia serrata) | >100 µM a,k | RIV 1.82 ± 0.13 µM a,k HUP 0.045 ± 0.01 µM a,k | nd | nd | MCE | |
| E-Δ2-Anhydromevalonic acid | Phaeospaeria sp. LF5 (strain from Huperzia serrata) | >100 µM a,k | RIV 1.82 ± 0.13 µM a,k HUP 0.045 ± 0.01 µM a,k | nd | nd | MCE | |
| 2-(1-Hydroxyethyl)-6- methylisonicotinic acid | Phaeospaeria sp. LF5 (strain from Huperzia serrata) | >100 µM a,k | RIV 1.82 ± 0.13 µM a,k HUP 0.045 ± 0.01 µM a,k | nd | nd | MCE | |
| 6-Hydroxy-8-methoxy-3- methylisocoumarin | Phaeospaeria sp. LF5 (strain from Huperzia serrata) | >100 µM a,k | RIV 1.82 ± 0.13 µM a,k HUP 0.045 ± 0.01 µM a,k | nd | nd | MCE | |
| 3-(Hydroxymethyl)-5-methylfuran-2(5H)-one | Phaeospaeria sp. LF5 (strain from Huperzia serrata) | >100 µM a,k | RIV 1.82 ± 0.13 µM a,k HUP 0.045 ± 0.01 µM a,k | nd | nd | MCE | |
| 4-Methyl-5,6-dihydropyren-2-one | Phaeospaeria sp. LF5 (strain from Huperzia serrata) | >100 µM a,k | RIV 1.82 ± 0.13 µM a,k HUP 0.045 ± 0.01 µM a,k | nd | nd | MCE | |
| (R)-6-Hydroxymellein | Phaeospaeria sp. LF5 (strain from Huperzia serrata) | >100 µM a,k | RIV 1.82 ± 0.13 µM a,k HUP 0.045 ± 0.01 µM a,k | nd | nd | MCE | |
| Asterric acid | Talaromyces aurantiacus FL 15 (strain from leave Huperzia serrata) | 66.7 ± 1.7 µM a,e | RIV 1.82 ± 0.13 µM a,e HUP 0.045 ± 0.01 µM a,e | >100 µM a,m | ns | MCE | [48,91,92] |
| Ethyl asterrate | Talaromyces aurantiacus FL 15 (strain from leave Huperzia serrata) | 20.1 ± 0.9 µM a,e | RIV 1.82 ± 0.13 µM a,e HUP 0.045 ± 0.01 µM a,e | >100 µM a,m | ns | MCE | |
| Methyl asterrate | Talaromyces aurantiacus FL 15 (strain from leave Huperzia serrata) | 23.3 ± 1.2 µM a,e | RIV 1.82 ± 0.13 µM a,e HUP 0.045 ± 0.01 µM a,e | >100 µM a,m | ns | MCE | |
| Sulochrin | Talaromyces aurantiacus FL 15 (strain from leave Huperzia serrata) | >100 µM a,e | RIV 1.82 ± 0.13 µM a,e HUP 0.045 ± 0.01 µM a,e | >100 µM a,m | ns | MCE | |
| POLYPHENOLS | |||||||
| Broussonin A | Anemarrhena asphodeloides Bunge Asparagaceae (roots) | 15.88 ± 1.02 µM a,e | BER 1.01 ± 0.01 µM a,e TAC 0.22 ± 0.004 µM a,e | 7.50 ± 0.07 µM a,m | TAC 0.014 ± 0.0043 µM a,m | MCE | [48,59,60] |
| Mangiferin | Anemarrhena asphodeloides Bunge Asparagaceae (whole plant) | 62.8 µM a,g | TAC nd a,g | nd | nd | MCE | [48,124] |
| Caffeoylated catechin | Camellia sinensis var. assamica Theaceae (leaves) | 2.49 ± 0.43 µM a,e | HUP 0.088 ± 0.004 µM a,e | nd | d | MCE | [48,116] |
| Epigallocatechin 3-O-p-coumaroate | Camellia sinensis var. assamica Theaceae (leaves) | 11.41 ± 2.00 µM a,e | HUP 0.088 ± 0.004 µM a,e | nd | nd | MCE | |
| Epigallocatechin-3-O-ferulate | Camellia sinensis var. assamica Theaceae (leaves) | 62.26 ± 10.18 µM a,e | HUP 0.088 ± 0.004 µM a,e | nd | nd | MCE | |
| Creoside IV | Codonopsis pilosula Nannf Campanulaceae (roots) | 7.30 ± 0.49 µM a,e | BER 1.01 ± 0.01 µM a,e TAC 0.22 ± 0.004 µM a,e | >40 a,m | TAC 0.014 ± 0.0043 µM a,m | MCE | [48,59,60] |
| Heyneanol A | Vitis amurensis Rupr. Vitaceae (roots) | 1.66 ± 0.09 µM a,f | GAL 0.93 ± 0.07 µM a,f | 1.75 ± 0.09 µM a,l | GAL 9.24 ± 1.32 µM a,l | MCE | [48,125] |
| Vitisin A | Vitis amurensis Rupr. Vitaceae (roots) | 1.04 ± 0.05 µM a,f | GAL 0.93 ± 0.07 µM a,f | 4.41 ± 0.39 µM a,l | GAL 9.24 ± 1.32 µM a,l | MCE | |
| SESQUITERPENE LACTONES | |||||||
| Britannin | Inula aucheriana DC. Asteraceae (aerial parts) | 300 μg mL−1—25.2% b,k | DON | nd | nd | MCE | [48,126] |
| Gaillardin | Inula oculus-christi L. Asteraceae (aerial parts) | 300 μg mL−1—67% b,k | DON | nd | nd | MCE | |
| Pulchellin C | Inula oculus-christi L. Asteraceae (aerial parts) | 300 μg mL−1—10.9% b,k | DON | nd | nd | MCE | |
| Amberin | Amberboa ramosa Jafri. Asteraceae (whole plant) | 17.5 ± 0.01 μM a,e | GAL 0.5 ± 0.01 μM a,e PHY 0.04 ± 0.0001 μM a,e | 2.7 ± 0.02 μM a,m | GAL 8.2 ± 0.02 μM a,m PHY 0.82 ± 0.001 μM a,m | MCE | [48,127] |
| Amberbin A | Amberboa ramosa Jafri. Asteraceae (whole plant) | 8.6 ± 0.15 μM a,e | GAL 0.5 ± 0.01 μM a,e PHY 0.04 ± 0.0001 μM a,e | 4.8 ± 0.15 μM a,m | GAL 8.2 ± 0.02 μM a,m PHY 0.82 ± 0.001 μM a,m | MCE | |
| Amberbin B | Amberboa ramosa Jafri. Asteraceae (whole plant)) | 0.91 ± 0.015 μM a,e | GAL 0.5 ± 0.01 μM a,e PHY 0.04 ± 0.0001 μM a,e | 2.5 ± 0.15 μM a,m | GAL 8.2 ± 0.02 μM a,m PHY 0.82 ± 0.001 μM a,m | MCE | |
| Amberbin C | Amberboa ramosa Jafri. Asteraceae (whole plant) | 1.1 ± 0.08 μM a,e | GAL 0.5 ± 0.01 μM a,e PHY 0.04 ± 0.0001 μM a,e | 17.9 ± 0.05 μM a,m | GAL 8.2 ± 0.02 μM a,m PHY 0.82 ± 0.001 μM a,m | MCE | |
| Zerumbone | Zingiber zerumbet L. Zingiberaceae (whole plant) | 1 mg mL−1 c,k | TAC 10 mM c,k | nd | nd | BTLC by Rhee et al. (2001) | [16,128] |
| Silphiperfolene acetate | Leontopodium alpinum Cass. Asteraceae (sub-aerial parts) | 200 μM—40.64 ± 7.09% b,k | GAL 3.2 µM a,k GAL 100 μM—89.30 ± 2.29% b,k | nd | nd | MCE | [93,95,129] |
| STEROIDS | |||||||
| Leucisterol | Leucas urticifolia Vahl. Lamiaceae (whole plant) | 83.6 ± 0.59 µM a,k | PHY 0.04 µM a,k | 3.2 ± 0.85 µM a,n | PHY 0.93 ± 0.3 µM a,n | CE | [48,130] |
| STEROLS | |||||||
| Haloxylon A | Haloxylon recurvum Bunge ex Boiss Chenopodiaceae (whole plant) | 8.3 ± 0.02 µM a,e | GAL 0.5 ± 0.001 µM a,e | 4.7 ± 0.01 µM a,m | GAL 8.5 ± 0.00 µM a,m | MCE | [48,131] |
| Haloxylon B | Haloxylon recurvum Bunge ex Boiss Chenopodiaceae (whole plant) | 0.89 ± 0.002 µM a,e | GAL 0.5 ± 0.001 µM a,e | 2.3 ± 0.001 µM a,m | GAL 8.5 ± 0.00 µM a,m | MCE | |
| TRIFLAVANONES | |||||||
| Garcineflavanone A | Garcinia atroviridis Griff. ex T. Anders. Clusiaceae (stem bark) | 100 μg mL−1—80.15 ± 6.65% b,e 28.52 ± 5.23 μg mL−1 a,e | PHY 0.05 ± 0.01 μg mL−1 a,e | ns | PHY 0.14 ± 0.015 μg mL−1 a,m | MCE | [48,108,109] |
| TRITERPENOIDS | |||||||
| Arbora- 1,9(11)-dien-3-one | Buxus hyrcana Pojark. Buxaceae (leaves) | 47.9 ± 1.2 µM a,k | GAL 0.53 ± 0.5 µM a,k HUP 1.7 ± 0.3 µM a,k | 220.1 ± 1.0 µM a,n | GAL 8.7 ± 1.0 µM a,n HUP >1000 ± 3.0 µM a,n | MCE | [48,56,57,58] |
| Asiatic acid | Centella asiatica Urb Apiaceae (whole plant) | 15.05 ± 0.05 µM a,e | PHY 0.05 ± 0.12 µM a,e | nd | nd | MCE | [48,132,133] |
| Asiaticoside | Centella asiatica Urb Apiaceae (whole plant) | 59.13 ± 0.18 µM a,e | PHY 0.05 ± 0.12 µM a,e | nd | nd | MCE | |
| Madecassic acid | Centella asiatica Urb Apiaceae (whole plant) | 17.83 ± 0.06 µM a,e | PHY 0.05 ± 0.12 µM a,e | nd | nd | MCE | |
| Madecassoside | Centella asiatica Urb Apiaceae (whole plant) | 37.14 ± 0.04 µM a,e | PHY 0.05 ± 0.12 µM a,e | nd | nd | MCE | |
| Betulin | Garcinia hombroniana Pierre Clusiaceae (bark) | 28.5 ± 0.78 µM a,e | PHY 0.04 ± 0.004 µM a,e | nd | PHY 0.09 ± 0.003 µM a,m | MCE | [48,81] |
| Betulinic acid | Garcinia hombroniana Pierre Clusiaceae (bark) | 24.2 ± 0.99 µM a,e | PHY 0.04 ± 0.004 µM a,e | 19.1 ± 1.33 µM a,m | PHY 0.09 ± 0.003 µM a,m | MCE | |
| 2β-Hydroxy-3α-O-caffeoyltaraxar-14-en-28- oic acid | Garcinia hombroniana Pierre Clusiaceae (bark) | 13.5 ± 0.95 µM a,e | PHY 0.04 ± 0.004 µM a,e | 10.6 ± 0.54 µM a,m | PHY 0.09 ± 0.003 µM a,m | MCE | |
| Taraxerol | Garcinia hombroniana Pierre Clusiaceae (bark) | nd | PHY 0.04 ± 0.004 µM a,e | 17.8 ± 1.73 µM a,m | PHY 0.09 ± 0.003 µM a,m | MCE | |
| 21β-Hydroxyserrat- 14-en-3,16-dione | Lycopodiella cernua L. Lycopodiaceae) (whole plants) | 10.67 ± 0.66 µM a,k | BER 0.10 ± 0.01 µM a,k | >30 µM a,n | BER 1.09 ± 0.17 µM a,n | MCE | [48,106] |
| 3β,21α-Diacetoxyserratan- 14β-ol | Lycopodiella cernua L. Lycopodiaceae (whole plants) | 0.91 ± 0.01 µM a,k | BER 0.10 ± 0.01 µM a,k | >30 µM a,n | BER 1.09 ± 0.17 µM a,n | MCE | |
| 3β,21β,29-Trihydroxyserrat- 14-en-3β-yl p-dihydrocoumarate | Lycopodiella cernua L. Lycopodiaceae (whole plants) | 1.69 ± 0.10 µM a,k | BER 0.10 ± 0.01 µM a,k | 0.42 ± 0.01 µM a,n | BER 1.09 ± 0.17 µM a,n | MCE | |
| SESQUITERPENES | |||||||
| 1α-Acetoxy-6β,9β-difuroyloxy-4β-hydroxydihydro-β-agarofuran | Maytenus disticha Urb. Celastraceae (seeds) | 738.0 ± 0.007 µM a,e | GAL 10.0 ± 0.015 µM a,e CAR 45.0 ± 0.031 µM a,e | ns a,m | ns a,m | MCE | [48,134] |
| 6β-Acetoxy-9β-benzyloxy-1α,8α-dihydroxydihydro-β-agarofuran | Maytenus disticha Urb. Celastraceae (seeds) | 500.0 ± 0.03 µM a,e | GAL 10.0 ± 0.015 µM a,e CAR 45.0 ± 0.031 µM a,e | ns a,m | ns a,m | MCE | |
| 6β,8α-Diacetoxy-9β-furoyloxy-1α-hydroxydihydro-β-agarofuran | Maytenus disticha Urb. Celastraceae (seeds) | 740.0 ± 0.045 µM a,e | GAL 10.0 ± 0.015 µM a,e CAR 45.0 ± 0.031 µM a,e | ns a,m | ns a,m | MCE | |
| 1α,6β,14-Triacetoxy-9β-benzyloxydihydro-β-agarofuran | Maytenus magellanica Hook.f. Celastraceae (seeds) | 695.0 ± 0.001 µM a,e | GAL 10.0 ± 0.015 µM a,e CAR 45.0 ± 0.031 µM a,e | ns a,m | ns a,m | MCE | |
| 2α,3β,6β-Triacetoxy-1α,9β-dibenzyloxy-4β-hydroxydihydro-β-agarofuran | Maytenus magellanica Hook.f. Celastraceae (seeds) | 30.0 ± 0.06 µM a,e | GAL 10.0 ± 0.015 µM a,e CAR 45.0 ± 0.031 µM a,e | ns a,m | ns a,m | MCE | |
| XANTHONES | |||||||
| Bellidin | Gentianella amarella ssp. acuta J.M.Gillett Gentianaceae (whole plants) | 10 μM—17.5 ± 5.7% b,e | GAL 10 μM—96.82 ± 0.04% b,e | nd | nd | MCE BTLC by Marston et al. (2002) | [42,48,101,135] |
| Bellidifolin | Gentianella amarella ssp. acuta J.M.Gillett Gentianaceae (whole plants) | 10 μM—21.9 ± 6.2% b,e | GAL 10 μM—96.82 ± 0.04% b,e | nd | nd | MCE BTLC by Marston et al. (2002) | |
| Corymbiferin 1-O-glucoside | Gentianella amarella ssp. acuta J.M.Gillett Gentianaceae (whole plants) | 10 μM—1.5 ± 1.2% b,e | GAL 10 μM—96.82 ± 0.04% b,e | nd | nd | MCE BTLC by Marston et al. (2002) | |
| Corymbiferin 3-O-β-D-glucopyranoside | Gentianella amarella ssp. acuta J.M.Gillett Gentianaceae (whole plants) | 10 μM—17.6 ± 1.8% b,e | GAL 10 μM—96.82 ± 0.04% b,e | nd | nd | MCE BTLC by Marston et al. (2002) | |
| nor-Swertianolin | Gentianella amarella ssp. acuta J.M.Gillett Gentianaceae (whole plants) | 10 μM—4.4 ± 4.4% b,e | GAL 10 μM—96.82 ± 0.04% b,e | nd | nd | MCE BTLC by Marston et al. (2002) | |
| Swertianolin | Gentianella amarella ssp. acuta J.M.Gillett Gentianaceae (whole plants) | 10 μM—9.8 ± 3.9% b,e | GAL 10 μM—96.82 ± 0.04% b,e | nd | nd | MCE BTLC by Marston et al. (2002) | |
| Swertiabisxanthone-I | Gentianella amarella ssp. acuta J.M.Gillett Gentianaceae (whole plants) | 10 μM—20.9 ± 3.3% b,e | GAL 10 μM—96.82 ± 0.04% b,e | nd | nd | MCE BTLC by Marston et al. (2002) | |
| Swertiabisxanthone-I 8′-O-β-D-glucopyranoside | Gentianella amarella ssp. acuta J.M.Gillett Gentianaceae (whole plants) | 10 μM—12.3 ± 2.9% b,e | GAL 10 μM—96.82 ± 0.04% b,e | nd | nd | MCE BTLC by Marston et al. (2002) | |
| Triptexanthoside C | Gentianella amarella ssp. acuta J.M.Gillett Gentianaceae (whole plants) | 10 μM—43.7 ± 3.3% b,e 13.8 ± 1.6 µM a,e | GAL 10 μM—96.82 ± 0.04% b,e GAL 0.35 ± 0.02 µM a,e | nd | nd | MCE BTLC by Marston et al. (2002) | |
| Veratriloside | Gentianella amarella ssp. acuta J.M.Gillett Gentianaceae (whole plants) | 10 μM—28.2 ± 2.5% b,e | GAL 10 μM—96.82 ± 0.04% b,e | nd | nd | MCE BTLC by Marston et al. (2002) | |
| XANTHONOIDS | |||||||
| Allanxanthone E | Garcinia mangostana L. Clusiaceas (seedcases) | 15.0 ± 1.2 µM a,f 67.4 ± 0.3 µM a,e | PHY 0.043 ± 0.002 µM a,f 0.049 ± 0.003 µM a,e | 11.0 ± 0.4 µM a,m | PHY 0.073 ± 0.006 µM a,m | MCEF | [48,112,136] |
| α-Mangostin | Garcinia mangostana L. Clusiaceas (seedcases) | 8.0 ± 0.5 µM a,f 6.3 ± 0.6 µM a,e | PHY 0.043 ± 0.002 µM a,f 0.049 ± 0.003 µM a,e | 2.9 ± 0.7 µM a,m | PHY 0.073 ± 0.006 µM a,m | MCEF | |
| 8-Deoxygartanin | Garcinia mangostana L. Clusiaceas (seedcases) | 6.2 ± 0.3 µM a,f 11.0 ± 0.6 µM e | PHY 0.043 ± 0.002 µM a,f 0.049 ± 0.003 µM a,e | 9.2 ± 0.5 µM a,m | PHY 0.073 ± 0.006 µM a,m | MCEF | |
| γ-Mangostin | Garcinia mangostana L. Clusiaceas (seedcases) | 5.4 ± 0.3 µM a,f 2.5 ± 3.3 µM a,e | PHY 0.043 ± 0.002 µM a,f 0.049 ± 0.003 µM a,e | 0.7 ± 0.03 µM a,m | PHY 0.073 ± 0.006 µM a,m | MCEF | |
| Gudraxanthone | Garcinia mangostana L. Clusiaceas (seedcases) | 11.7 ± 0.7 µM a,f 18.9 ± 1.7 µM a,e | PHY 0.043 ± 0.002 µM a,f 0.049 ± 0.003 µM a,e | 9.0 ± 1.2 µM a,m | PHY 0.073 ± 0.006 µM a,m | MCEF | |
| 9-Hydroxy-calabaxanthone | Garcinia mangostana L. Clusiaceas (seedcases) | >100 µM a,f >100 µM a,e | PHY 0.043 ± 0.002 µM a,f 0.049 ± 0.003 µM a,e | 86.3 ± 2.4 µM a,m | PHY 0.073 ± 0.006 µM a,m | MCEF | |
| Mangostanol | Garcinia mangostana L. Clusiaceas (seedcases) | 14.6 ± 0.7 µM a f 6.3 ± 5.4 µM a,e | PHY 0.043 ± 0.002 µM a,f 0.049 ± 0.003 µM a,e | 6.0 ± 0.2 µM a,m | PHY 0.073 ± 0.006 µM a,m | MCEF | |
| MISCELLANOUS | |||||||
| 3-Methylbuthyl hydrodisulfide | Buthus martensii Karsch Buthidae (whole body of scorpion) | 40.93 ± 3.21 µM a,e | GAL 1.17 ± 0.01 µM a,e DON 0.049 ± 0.004 µM a,e | 152.84 ± 7.22 µM a,m | GAL 18.78 ± 1.81 µM a,m DON 5.536± 0.018 µM a,m | MCE | [48,54,55] |
| 2-Benzothiazolol | Spiranthes sinensis Ames Orchidaceae (whole plant) | 37.67 ± 0.52 a,k | GAL 0.19 ± 0.02 µg/mL a,k | nd | nd | MCE | [48,114] |
3. Activity
4. Analysis Methods
4.1. The Colorimetric Method of Ellman (1961)
4.2. Spectrophotometric Modification of Ellman’s Method
4.3. TLC Modification of Ellman’s Method
4.4. TLC Bioautography by Marston
4.5. TLC Bioautography by Mroczek
4.6. Fluorimetric Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| AChE | Acetylcholinesterase |
| AD | Alzheimer’s disease |
| ATCI | Acetylthiocholine |
| BuChE | Butyrylcholinesterase |
| ChE | Cholinesterase |
| DTNB | 5,5′-Dithiobis-(2-nitrobenzoic acid) |
| eeAChE | Electrophors electricus acetylcholinesterase |
| e.g., (lat. exempli gratia) | For example |
| hAChE | Human erythrocyte acetylcholinesterase |
| IBuChE | Inhibitor of butyrylcholinesterase |
| IC50 | Inhibitory concentration for which enzyme activity is equal to half-maximal |
| IChE | Inhibitor of cholinesterases |
| SAR | Structure–activity relationship |
| TLC/HPLC/DAD/MS | Thin-layer chromatography/high-performance liquid chromatography/electrospray ionization time-of-flight mass spectrometry |
| Tris-HCl | Trizma hydrochloride with bovine serum |
| UPLC-PDA-QTOF-MS | Ultra-performance liquid chromatography coupled with photo-diode array detector and quadrupole time-of-flight mass spectrometry |
| vs. | Versus |
References
- Bukowska, B.; Pieniazek, D.; Hutnik, K.; Duda, W. Acetyl- and Butyrylcholinesterase—Structure, Functions and Their Inhibitors. Curr. Top. Biophys. 2007, 30, 11–23. [Google Scholar]
- Widy-Tyszkiewicz, E. Leki Układu Cholinergicznego. I. Leki Cholinomimetyczne. In Farmakologia. Podstawy Farmakoterapii. Podręcznik dla Studentów Medycyny i Lekarzy; Kostowski, W., Herman, Z., Eds.; Wydawnictwo Lekarskie PZWL: Warszawa, Poland, 2013; Volume 1, pp. 401–414. [Google Scholar]
- Bullock, R.; Lane, R. Executive dyscontrol in dementia, with emphasis on subcortical pathology and the role of butyrylcholinesterase. Curr. Alzheimer Res. 2007, 4, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Rolinski, M.; Fox, C.; Maidment, I.; McShane, R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst. Rev. 2012, 3, CD006504. [Google Scholar] [CrossRef] [PubMed]
- Vetulani, J. Leki Nootropowe i Prokognitywne. Farmakoterapia Choroby Alzheimera. In Farmakologia. Podstawy Farmakoterapii. Podręcznik dla Studentów Medycyny i Lekarzy; Kostowski, W., Herman, Z., Eds.; Wydawnictwo Lekarskie PZWL: Warszawa, Poland, 2013; Volume 2, pp. 154–169. [Google Scholar]
- Sugimoto, H.; Yamanishi, Y.; Iimura, Y.; Kawakami, Y. Donepezil Hydrochloride (E2020) and Other Acetyl-cholinesterase Inhibitors. Curr. Med. Chem. 2000, 7, 303–339. [Google Scholar] [CrossRef]
- Research, C. For D.E. and FDA’s Decision to Approve New Treatment for Alzheimer’s Disease; FDA: Silver Spring, MD, USA, 2021. [Google Scholar]
- Wright, C.I.; Geula, C.; Mesulam, M.-M. Neuroglial cholinesterases in the normal brain and in Alzheimer’s disease: Relationship to plaques, tangles, and patterns of selective vulnerability. Ann. Neurol. 1993, 34, 373–384. [Google Scholar] [CrossRef]
- Perry, E.K.; Perry, R.H.; Blessed, G.; Tomlinson, B.E. Changes in brain cholinesterases in senile dementia of alzheimer type. Neuropathol. Appl. Neurobiol. 1978, 4, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Pepeu, G.; Giovannini, M.G.; Bracco, L. Effect of cholinesterase inhibitors on attention. Chem. Biol. Interact. 2013, 203, 361–364. [Google Scholar] [CrossRef]
- Little, J.T.; Walsh, S.; Aisen, P.S. An update on huperzine A as a treatment for Alzheimer’s disease. Expert Opin. Investig. Drugs 2008, 17, 209–215. [Google Scholar] [CrossRef]
- Macdonald, I.R.; Rockwood, K.; Martin, E.; Darvesh, S. Cholinesterase Inhibition in Alzheimer’s Disease: Is Specificity the Answer? J. Alzheimer’s Dis. 2014, 42, 379–384. [Google Scholar] [CrossRef]
- Pinho, B.R.; Ferreres, F.; Valentão, P.; Andrade, P.B. Nature as a source of metabolites with cholinesterase-inhibitory activity: An approach to Alzheimer’s disease treatment. J. Pharm. Pharmacol. 2013, 65, 1681–1700. [Google Scholar] [CrossRef]
- Matławska, I.; Byłka, W.; Gawron-Gzella, A.; Sikorska, M.; Szaufer-Hajdrych, M.; Wojcińska, M.; Dudek-Makuch, M.; Witkowska-Banaszczak, E. Farmakognozja. Podręcznik dla Studentów Farmacji, 3rd ed.; Wydawnictwo Naukowe Uniwersytetu Medycznego im; Karola Marcinkowskiego w Poznaniu: Warszawa, Poland, 2008; ISBN 978-83-7597-004-3. [Google Scholar]
- Berkov, S.; Ivanov, I.; Georgiev, V.; Codina, C.; Pavlov, A. Galanthamine biosynthesis in plant in vitro systems. Eng. Life Sci. 2014, 14, 643–650. [Google Scholar] [CrossRef]
- Rhee, I.K.; van de Meent, M.; Ingkaninan, K.; Verpoorte, R. Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining. J. Chromatogr. A 2001, 915, 217–223. [Google Scholar] [CrossRef]
- Gulcan, H.O.; Orhan, I.E.; Sener, B. Chemical and Molecular Aspects on Interactions of Galanthamine and Its Derivatives with Cholinesterases. Curr. Pharm. Biotechnol. 2015, 16, 252–258. [Google Scholar] [CrossRef]
- McNulty, J.; Nair, J.J.; Little, J.R.; Brennan, J.D.; Bastida, J. Structure–activity studies on acetylcholinesterase inhibition in the lycorine series of Amaryllidaceae alkaloids. Bioorg. Med. Chem. Lett. 2010, 20, 5290–5294. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.Y.; Khaw, K.Y.; Murugaiyah, V.; Looi, C.Y.; Wong, Y.L.; Mustafa, M.R.; Litaudon, M.; Awang, K. Natural indole butyrylcholinesterase inhibitors from Nauclea officinalis. Phytomedicine 2015, 22, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Berkov, S.; Codina, C.; Viladomat, F.; Bastida, J. N-Alkylated galanthamine derivatives: Potent acetylcholinesterase inhibitors from Leucojum aestivum. Bioorg. Med. Chem. Lett. 2008, 18, 2263–2266. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, B.B.; Kaya, G.I.; Onur, M.A.; Bastida, J.; Somer, N.U. Phytochemical investigation of Galanthus woronowii. Biochem. Syst. Ecol. 2013, 51, 276–279. [Google Scholar] [CrossRef]
- Ortiz, J.E.; Garro, A.; Pigni, N.B.; Agüero, M.B.; Roitman, G.; Slanis, A.; Enriz, R.D.; Feresin, G.E.; Bastida, J.; Tapia, A. Cholinesterase-inhibitory effect and in silico analysis of alkaloids from bulbs of Hieronymiella species. Phytomedicine 2017, 39, 66–74. [Google Scholar] [CrossRef]
- Song, H.-P.; Zhang, H.; Hu, R.; Xiao, H.-H.; Guo, H.; Yuan, W.-H.; Han, X.-T.; Xu, X.-Y.; Zhang, X.; Ding, Z.-X.; et al. A strategy to discover lead chemome from traditional Chinese medicines based on natural chromatogram-effect correlation (NCEC) and natural structure-effect correlation (NSEC): Mahonia bealei and Mahonia fortunei as a case study. J. Chromatogr. B 2021, 1181, 122922. [Google Scholar] [CrossRef]
- Hirasawa, Y.; Kato, E.; Kobayashi, J.; Kawahara, N.; Goda, Y.; Shiro, M.; Morita, H. Lycoparins A–C, new alkaloids from Lycopodium casuarinoides inhibiting acetylcholinesterase. Bioorg. Med. Chem. 2008, 16, 6167–6171. [Google Scholar] [CrossRef]
- Zhan, Z.-J.; Yu, Q.; Wang, Z.-L.; Shan, W.-G. Indole alkaloids from Ervatamia hainanensis with potent acetylcholinesterase inhibition activities. Bioorg. Med. Chem. Lett. 2010, 20, 6185–6187. [Google Scholar] [CrossRef] [PubMed]
- Skalicka-Woźniak, K.; Orhan, I.E.; Cordell, G.A.; Nabavi, S.M.; Budzyńska, B. Implication of coumarins towards central nervous system disorders. Pharmacol. Res. 2016, 103, 188–203. [Google Scholar] [CrossRef] [PubMed]
- Youkwan, J.; Sutthivaiyakit, S.; Sutthivaiyakit, P. Citrusosides A−D and Furanocoumarins with Cholinesterase Inhibitory Activity from the Fruit Peels of Citrus hystrix. J. Nat. Prod. 2010, 73, 1879–1883. [Google Scholar] [CrossRef] [PubMed]
- Wszelaki, N.; Paradowska, K.; Jamróz, M.K.; Granica, S.; Kiss, A.K. Bioactivity-Guided Fractionation for the Butyrylcholinesterase Inhibitory Activity of Furanocoumarins from Angelica archangelica L. Roots and Fruits. J. Agric. Food Chem. 2011, 59, 9186–9193. [Google Scholar] [CrossRef] [PubMed]
- Awang, K.; Chan, G.; Litaudon, M.; Ismail, N.H.; Martin, M.-T.; Gueritte, F. 4-Phenylcoumarins from Mesua elegans with acetylcholinesterase inhibitory activity. Bioorg. Med. Chem. 2010, 18, 7873–7877. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; She, G. Naturally Occurring Diarylheptanoids—A Supplementary Version. Rec. Nat. Prod. 2012, 13, 321–333. [Google Scholar]
- Lee, J.S.; Kim, J.H.; Han, Y.K.; Ma, J.Y.; Kim, Y.H.; Li, W.; Yang, S.Y. Cholinesterases inhibition studies of biological active compounds from the rhizomes of Alpinia officinarum Hance and in silico molecular dynamics. Int. J. Biol. Macromol. 2018, 120, 2442–2447. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Chen, X.; Xiao, J. Inhibition of flavonoids on acetylcholine esterase: Binding and structure–activity relationship. Food Funct. 2014, 5, 2582–2589. [Google Scholar] [CrossRef]
- Ryu, H.W.; Curtis-Long, M.J.; Jung, S.; Jeong, I.Y.; Kim, D.S.; Kang, K.Y.; Park, K.H. Anticholinesterase potential of flavonols from paper mulberry (Broussonetia papyrifera) and their kinetic studies. Food Chem. 2012, 132, 1244–1250. [Google Scholar] [CrossRef]
- Cho, J.K.; Ryu, Y.B.; Curtis-Long, M.J.; Ryu, H.W.; Yuk, H.J.; Kim, D.W.; Kim, H.J.; Lee, W.S.; Park, K.H. Cholinestrase inhibitory effects of geranylated flavonoids from Paulownia tomentosa fruits. Bioorg. Med. Chem. 2012, 20, 2595–2602. [Google Scholar] [CrossRef]
- Katalinić, M.; Rusak, G.; Barović, J.D.; Šinko, G.; Jelić, D.; Antolović, R.; Kovarik, Z. Structural aspects of flavonoids as inhibitors of human butyrylcholinesterase. Eur. J. Med. Chem. 2010, 45, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Vasas, A.; Hohmann, J. Natural phenanthrenes and their biological activity. Phytochemistry 2008, 69, 1084–1110. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tu, Y.; Kang, Y.; Zhu, C.; Wu, C.; Chen, G.; Liu, Z.; Li, Y. Biological evaluation, molecular modeling and dynamics simulation of phenanthrenes isolated from Bletilla striata as butyrylcholinesterase inhibitors. Sci. Rep. 2022, 12, 13649. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Houghton, P.J.; Hider, R.C.; Howes, M.-J.R. Novel Diterpenoid Acetylcholinesterase Inhibitors from Salvia miltiorhiza. Planta Med. 2004, 70, 201–204. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, W.; Xu, L.; Chen, L. In Salvia miltiorrhiza, phenolic acids possess protective properties against amyloid β-induced cytotoxicity, and tanshinones act as acetylcholinesterase inhibitors. Environ. Toxicol. Pharmacol. 2011, 31, 443–452. [Google Scholar] [CrossRef]
- Raut, N.A.; Dhore, P.W.; Saoji, S.D.; Kokare, D.M. Chapter 9—Selected Bioactive Natural Products for Diabetes Mellitus. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 48, pp. 287–322. [Google Scholar]
- Urbain, A.; Marston, A.; Queiroz, E.F.; Ndjoko, K.; Hostettmann, K. Xanthones from Gentiana campestris as New Acetylcholinesterase Inhibitors. Planta Med. 2004, 70, 1011–1014. [Google Scholar] [CrossRef]
- Urbain, A.; Marston, A.; Grilo, L.S.; Bravo, J.; Purev, O.; Purevsuren, B.; Batsuren, D.; Reist, M.; Carrupt, P.-A.; Hostettmann, K. Xanthones from Gentianella amarella ssp. acuta with Acetylcholinesterase and Monoamine Oxidase Inhibitory Activities. J. Nat. Prod. 2008, 71, 895–897. [Google Scholar] [CrossRef]
- Fink, K.; Boratyński, J. Oddziaływania Niekowalencyjne Kation-π—Ich Rola w Przyrodzie. Postepy Hig. Med. Dosw. 2014, 68, 1276–1286. [Google Scholar] [CrossRef]
- Liu, L.; Yin, Q.-M.; Gao, Q.; Li, J.; Jiang, Y.; Tu, P.-F. New biphenanthrenes with butyrylcholinesterase inhibitory activitiy from Cremastra appendiculata. Nat. Prod. Res. 2019, 35, 750–756. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, S.T.; Zargaham, M.K.; Khan, A.U.; Khan, S.; Hussain, A.; Uddin, J.; Khan, A.; Al-Harrasi, A. Potential therapeutic natural products against Alzheimer’s disease with Reference of Acetylcholinesterase. Biomed. Pharmacother. 2021, 139, 111609. [Google Scholar] [CrossRef]
- Santos, T.C.; dos Gomes, T.M.; Pinto, B.A.S.; Camara, A.L.; de Paes, A.M.A. Naturally Occurring Acetylcholinesterase Inhibitors and Their Potential Use for Alzheimer’s Disease Therapy. Front. Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Dar, T.A.; Dar, P.A.; Ganie, S.A.; Kamal, M.A. A Current Perspective on the Inhibition of Cholinesterase by Natural and Synthetic Inhibitors. Curr. Drug Metab. 2017, 18, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Senol, F.S.; Orhan, I.E.; Ustun, O. In vitro cholinesterase inhibitory and antioxidant effect of selected coniferous tree species. Asian Pac. J. Trop. Med. 2015, 8, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, R.D.S.; Kyrylchuk, A.; Cherednichenko, A.; Sá, I.S.C.; Bastos, L.M.; da Silva, F.M.A.; Nunomura, R.D.C.S.; Grafov, A. In Vitro and In Silico Evaluation of Cholinesterase Inhibition by Alkaloids Obtained from Branches of Abuta panurensis Eichler. Molecules 2022, 27, 3138. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.B.; Emanuelli, T.; Pereira, M.E. Effects of Early Undernutrition on Kinetic Parameters of Brain Acetylcholinesterase from Adult Rats. Acta Neurobiol. Exp. 1993, 53, 431–437. [Google Scholar]
- Ahmad, H.; Ahmad, S.; Shah, S.A.A.; Khan, H.U.; Khan, F.A.; Ali, M.; Latif, A.; Shaheen, F.; Ahmad, M. Selective dual cholinesterase inhibitors from Aconitum laeve. J. Asian Nat. Prod. Res. 2017, 20, 172–181. [Google Scholar] [CrossRef]
- Badaoui, M.I.; Magid, A.A.; Benkhaled, M.; Bensouici, C.; Harakat, D.; Voutquenne-Nazabadioko, L.; Haba, H. Pyrroloquinolone A, a new alkaloid and other phytochemicals from Atractylis cancellata L. with antioxidant and anticholinesterase activities. Nat. Prod. Res. 2019, 35, 2997–3003. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Fan, J.-J.; Wang, L.-N. Discovery of Guanidine Derivatives from Buthus martensii Karsch with Metal-Binding and Cholinesterase Inhibition Properties. Molecules 2021, 26, 6737. [Google Scholar] [CrossRef]
- Cai, R.; Wang, L.-N.; Fan, J.-J.; Geng, S.-Q.; Liu, Y.-M. New 4-N-phenylaminoquinoline derivatives as antioxidant, metal chelating and cholinesterase inhibitors for Alzheimer’s disease. Bioorg. Chem. 2019, 93, 103328. [Google Scholar] [CrossRef]
- Ata, A.; Iverson, C.D.; Kalhari, K.S.; Akhter, S.; Betteridge, J.; Meshkatalsadat, M.H.; Orhan, I.; Sener, B. Triterpenoidal alkaloids from Buxushyrcana and their enzyme inhibitory, anti-fungal and anti-leishmanial activities. Phytochemistry 2010, 71, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Babar, Z.U.; Ata, A.; Meshkatalsadat, M.H. New bioactive steroidal alkaloids from Buxus hyrcana. Steroids 2006, 71, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Ata, A.; Conci, L.J.; Orhan, I. Mucoralactone A: An Unusual Steroid from the Liquid Culture of Mucor plumbeus. Heterocycles 2006, 68, 2097. [Google Scholar] [CrossRef]
- Baek, S.C.; Park, M.H.; Ryu, H.W.; Lee, J.P.; Kang, M.-G.; Park, D.; Park, C.M.; Oh, S.-R.; Kim, H. Rhamnocitrin isolated from Prunus padus var. seoulensis: A potent and selective reversible inhibitor of human monoamine oxidase A. Bioorg. Chem. 2018, 83, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.P.; Kang, M.-G.; Lee, J.Y.; Oh, J.M.; Baek, S.C.; Leem, H.H.; Park, D.; Cho, M.-L.; Kim, H. Potent inhibition of acetylcholinesterase by sargachromanol I from Sargassum siliquastrum and by selected natural compounds. Bioorg. Chem. 2019, 89, 103043. [Google Scholar] [CrossRef]
- Geissler, T.; Brandt, W.; Porzel, A.; Schlenzig, D.; Kehlen, A.; Wessjohann, L.A.; Arnold, N. Acetylcholinesterase inhibitors from the toadstool Cortinarius infractus. Bioorg. Med. Chem. 2010, 18, 2173–2177. [Google Scholar] [CrossRef]
- Mehfooz, H.; Saeed, A.; Sharma, A.; Albericio, F.; Larik, F.A.; Jabeen, F.; Channar, P.A.; Flörke, U. Dual Inhibition of AChE and BChE with the C-5 Substituted Derivative of Meldrum’s Acid: Synthesis, Structure Elucidation, and Molecular Docking Studies. Crystals 2017, 7, 211. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Xu, F.-C.; Quesheng; Zhang, Q.-Y.; Tu, P.-F.; Liang, H. Cholinesterase inhibitory isoquinoline alkaloids from Corydalis mucronifera. Phytochemistry 2019, 159, 199–207. [Google Scholar] [CrossRef]
- Hung, T.M.; Ngoc, T.M.; Youn, U.J.; Min, B.S.; Na, M.; Thuong, P.T.; Bae, K. Anti-Amnestic Activity of Pseudocoptisine from Corydalis Tuber. Biol. Pharm. Bull. 2008, 31, 159–162. [Google Scholar] [CrossRef]
- Khaw, K.Y.; Choi, S.B.; Tan, S.C.; Wahab, H.A.; Chan, K.L.; Murugaiyah, V. Prenylated xanthones from mangosteen as promising cholinesterase inhibitors and their molecular docking studies. Phytomedicine 2014, 21, 1303–1309. [Google Scholar] [CrossRef]
- Othman, W.N.N.W.; Liew, S.Y.; Khaw, K.Y.; Murugaiyah, V.; Litaudon, M.; Awang, K. Cholinesterase inhibitory activity of isoquinoline alkaloids from three Cryptocarya species (Lauraceae). Bioorg. Med. Chem. 2016, 24, 4464–4469. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ahmad, M.; Almehmadi, M.; Shah, S.A.A.; Khan, F.A.; Khan, N.M.; Khan, A.; Zainab; Halawi, M.; Ahmad, H. In Vitro and In Silico Investigation of Diterpenoid Alkaloids Isolated from Delphinium chitralense. Molecules 2022, 27, 4348. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Ahmad, S.; Ali, M.; Latif, A.; Shah, S.A.A.; Naz, H.; Rahman, N.U.; Shaheen, F.; Wadood, A.; Khan, H.U.; et al. Norditerpenoid alkaloids of Delphinium denudatum as cholinesterase inhibitors. Bioorg. Chem. 2018, 78, 427–435. [Google Scholar] [CrossRef]
- López, S.; Bastida, J.; Viladomat, F.; Codina, C. Acetylcholinesterase Inhibitory Activity of Some Amaryllidaceae Alkaloids and Narcissus Extracts. Life Sci. 2002, 71, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.E.; Pigni, N.B.; Andujar, S.A.; Roitman, G.; Suvire, F.D.; Enriz, R.D.; Tapia, A.; Bastida, J.; Feresin, G.E. Alkaloids from Hippeastrum argentinum and Their Cholinesterase-Inhibitory Activities: An in Vitro and in Silico Study. J. Nat. Prod. 2016, 79, 1241–1248. [Google Scholar] [CrossRef]
- Botić, T.; Defant, A.; Zanini, P.; Žužek, M.C.; Frangež, R.; Janussen, D.; Kersken, D.; Knez, Ž.; Mancini, I.; Sepčić, K. Discorhabdin alkaloids from Antarctic Latrunculia spp. sponges as a new class of cholinesterase inhibitors. Eur. J. Med. Chem. 2017, 136, 294–304. [Google Scholar] [CrossRef]
- Kolak, U.; Hacibekiroglu, I.; Boga, M.; Ozgokce, F.; Unal, M.; Choudhary, M.I.; Ayhan, U. Phytochemi-cal Investigation of Leontice leontopetalum L. subsp. ewersmannii with Antioxidant and Anticholinesterase Ac-tivities. Rec. Nat. Prod. 2011, 5, 309–313. [Google Scholar]
- Tang, Y.; Fu, Y.; Xiong, J.; Li, M.; Ma, G.-L.; Yang, G.-X.; Wei, B.-G.; Zhao, Y.; Zhang, H.-Y.; Hu, J.-F. Lycodine-Type Alkaloids from Lycopodiastrum casuarinoides. J. Nat. Prod. 2013, 76, 1475–1484. [Google Scholar] [CrossRef]
- Kubota, T.; Yahata, H.; Yamamoto, S.; Hayashi, S.; Shibata, T.; Kobayashi, J. Serratezomines D and E, new Lycopodium alkaloids from Lycopodiumserratum var. serratum. Bioorg. Med. Chem. Lett. 2009, 19, 3577–3580. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Y.-H.; Wang, T.-M.; Kang, T.-G.; Sun, H.-Y.; Pei, W.-H.; Song, H.-P.; Zhang, H. A strategy to discover selective α-glucosidase/acetylcholinesterase inhibitors from five function-similar citrus herbs through LC-Q-TOF-MS, bioassay and virtual screening. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021, 1174, 122722. [Google Scholar] [CrossRef]
- Kumar, N.S.; Mukherjee, P.K.; Bhadra, S.; Saha, B.P.; Pal, B.C. Acetylcholinesterase inhibitory potential of a carbazole alkaloid, mahanimbine, from Murraya koenigii. Phytotherapy Res. 2009, 24, 629–631. [Google Scholar] [CrossRef]
- Mroczek, T. Highly efficient, selective and sensitive molecular screening of acetylcholinesterase inhibitors of natural origin by solid-phase extraction-liquid chromatography/electrospray ionisation-octopole-orthogonal acceleration time-of-flight-mass spectrometry and novel thin-layer chromatography-based bioautography. J. Chromatogr. A 2009, 1216, 2519–2528. [Google Scholar] [CrossRef] [PubMed]
- Šafratová, M.; Hošťálková, A.; Hulcová, D.; Breiterová, K.; Hrabcová, V.; Machado, M.; Fontinha, D.; Prudêncio, M.; Kuneš, J.; Chlebek, J.; et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch. Pharmacal Res. 2017, 41, 208–218. [Google Scholar] [CrossRef]
- Havlasová, J.; Safratova, M.; Siatka, T.; Štěpánková, Š.; Novák, Z.; Ločárek, M.; Opletal, L.; Hrabinova, M.; Jun, D.; Benešová, N.; et al. Chemical Composition of Bioactive Alkaloid Extracts from Some Narcissus Species and Varieties and their Biological Activity. Nat. Prod. Commun. 2014, 9, 1151–1155. [Google Scholar] [CrossRef]
- Karakoyun, Ç.; Bozkurt, B.; Çoban, G.; Masi, M.; Cimmino, A.; Evidente, A.; Somer, N.U. A comprehensive study on narcissus tazetta subsp. tazetta L.: Chemo-profiling, isolation, anticholinesterase activity and molecular docking of amaryllidaceae alkaloids. S. Afr. J. Bot. 2020, 130, 148–154. [Google Scholar] [CrossRef]
- Jamila, N.; Khairuddean, M.; Yeong, K.K.; Osman, H.; Murugaiyah, V. Cholinesterase inhibitory triterpenoids from the bark of Garcinia hombroniana. J. Enzym. Inhib. Med. Chem. 2014, 30, 133–139. [Google Scholar] [CrossRef]
- Safa, N.; Trobec, T.; Holland, D.C.; Slazak, B.; Jacobsson, E.; Hawkes, J.A.; Frangež, R.; Sepčić, K.; Göransson, U.; Moodie, L.W.K.; et al. Spatial Distribution and Stability of Cholinesterase Inhibitory Protoberberine Alkaloids from Papaver setiferum. J. Nat. Prod. 2021, 85, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Ristovski, S.; Uzelac, M.; Kljun, J.; Lipec, T.; Uršič, M.; Jokhadar, Š.Z.; Žužek, M.C.; Trobec, T.; Frangež, R.; Sepcic, K.; et al. Organoruthenium Prodrugs as a New Class of Cholinesterase and Glutathione-S-Transferase Inhibitors. ChemMedChem 2018, 13, 2166–2176. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.-S.; Ge, Y.-X.; Cheng, Z.-Q.; Song, J.-L.; Wang, Y.-Y.; Zhu, K.; Zhang, H. Discovery of new multifunctional selective acetylcholinesterase inhibitors: Structure-based virtual screening and biological evaluation. J. Comput. Mol. Des. 2019, 33, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Q.; Jia, J.; Hu, J.-Y.; Wu, J.; Sun, W.-T.; Zheng, M.; Wang, X.; Zhu, K.-K.; Jiang, C.-S.; Yang, S.-P.; et al. Iboga-type alkaloids with Indolizidino[8,7-b]Indole scaffold and bisindole alkaloids from Tabernaemontana bufalina Lour. Phytochemistry 2022, 196, 113089. [Google Scholar] [CrossRef]
- Yu, P.; Chen, Z.; Liu, Y.; Gu, Z.; Wang, X.; Zhang, Y.; Ma, Y.; Dong, M.; Tian, Z. Bioactivity-Guided Separation of Anti-Cholinesterase Alkaloids from Uncaria rhynchophlly (Miq.) Miq. Ex Havil Based on HSCCC Coupled with Molecular Docking. Molecules 2022, 27, 2013. [Google Scholar] [CrossRef] [PubMed]
- Decker, M. Novel inhibitors of acetyl- and butyrylcholinesterase derived from the alkaloids dehydroevodiamine and rutaecarpine. Eur. J. Med. Chem. 2005, 40, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Decker, M.; Krauth, F.; Lehmann, J. Novel tricyclic quinazolinimines and related tetracyclic nitrogen bridgehead compounds as cholinesterase inhibitors with selectivity towards butyrylcholinesterase. Bioorg. Med. Chem. 2006, 14, 1966–1977. [Google Scholar] [CrossRef] [PubMed]
- Macabeo, A.P.G.; Vidar, W.S.; Chen, X.; Decker, M.; Heilmann, J.; Wan, B.; Franzblau, S.; Galvez, E.V.; Aguinaldo, M.A.M.; Cordell, G.A. Mycobacterium tuberculosis and cholinesterase inhibitors from Voacanga globosa. Eur. J. Med. Chem. 2011, 46, 3118–3123. [Google Scholar] [CrossRef]
- Lenta, B.N.; Devkota, K.P.; Ngouela, S.; Boyom, F.F.; Naz, Q.; Choudhary, M.I.; Tsamo, E.; Rosenthal, P.J.; Sewald, N. Anti-plasmodial and Cholinesterase Inhibiting Activities of some Constituents of Psorospermum glaberrimum. Chem. Pharm. Bull. 2008, 56, 222–226. [Google Scholar] [CrossRef]
- Xiao, Y.; Liang, W.; Liu, D.; Zhang, Z.; Chang, J.; Zhu, D. Isolation and acetylcholinesterase inhibitory activity of asterric acid derivatives produced by Talaromyces aurantiacus FL15, an endophytic fungus from Huperzia serrata. 3 Biotech 2022, 12, 60. [Google Scholar] [CrossRef]
- Devidas, S.B.; Rahmatkar, S.N.; Singh, R.; Sendri, N.; Purohit, R.; Singh, D.; Bhandari, P. Amelioration of cognitive deficit in zebrafish by an undescribed anthraquinone from Juglans regia L.: An in-silico, in-vitro and in-vivo approach. Eur. J. Pharmacol. 2021, 906, 174234. [Google Scholar] [CrossRef]
- Ingkaninan, K.; De Best, C.M.; Van Der Heijden, R.; Hofte, A.J.P.; Karabatak, B.; Irth, H.; Tjaden, U.R.; Van der Greef, J.; Verpoorte, R. High-Performance Liquid Chromatography with on-Line Coupled UV, Mass Spec-trometric and Biochemical Detection for Identification of Acetylcholinesterase Inhibitors from Natural Products. J. Chromatogr. A 2000, 872, 61–73. [Google Scholar] [CrossRef]
- Rhee, I.K.; van Rijn, R.M.; Verpoorte, R. Qualitative determination of false-positive effects in the acetylcholinesterase assay using thin layer chromatography. Phytochem. Anal. 2003, 14, 127–131. [Google Scholar] [CrossRef]
- Rollinger, J.M.; Hornick, A.; Langer, T.; Stuppner, H.; Prast, H. Acetylcholinesterase Inhibitory Activity of Scopolin and Scopoletin Discovered by Virtual Screening of Natural Products. J. Med. Chem. 2004, 47, 6248–6254. [Google Scholar] [CrossRef]
- Se-Young, H.; Young-Pyo, C.; Soon-Jung, B.; Mee-Hee, J.; Young-Choong, K. An Acetylcholinesterase Inhibitor Isolated from Corydalis Tuber and Its Mode of Action. Korean J. Pharmacogn. 1996, 27, 91–95. [Google Scholar]
- Kim, S.R.; Hwang, S.Y.; Jang, Y.P.; Park, M.J.; Markelonis, G.J.; Oh, T.H.; Kim, Y.C. Protopine from Corydalis ternata has Anticholinesterase and Antiamnesic Activities. Planta Med. 1999, 65, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Lee, K.Y.; Sung, S.H.; Park, M.J.; Kim, Y.C. Coumarins Isolated from Angelica gigas Inhibit Acetylcholinesterase: Structure−Activity Relationships. J. Nat. Prod. 2001, 64, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Şenol, F.S.; Orhan, I.; Celep, F.; Kahraman, A.; Doğan, M.; Yilmaz, G.; Şener, B. Survey of 55 Turkish Salvia taxa for their acetylcholinesterase inhibitory and antioxidant activities. Food Chem. 2010, 120, 34–43. [Google Scholar] [CrossRef]
- Senol, F.S.; Skalicka-Woźniak, K.; Khan, M.T.H.; Orhan, I.E.; Sener, B.; Głowniak, K. An in vitro and in silico approach to cholinesterase inhibitory and antioxidant effects of the methanol extract, furanocoumarin fraction, and major coumarins of Angelica officinalis L. fruits. Phytochem. Lett. 2011, 4, 462–467. [Google Scholar] [CrossRef]
- Marston, A.; Kissling, J.; Hostettmann, K. A rapid TLC bioautographic method for the detection of acetylcholinesterase and butyrylcholinesterase inhibitors in plants. Phytochem. Anal. 2002, 13, 51–54. [Google Scholar] [CrossRef]
- Kozioł, E.; Sezor Desni, F.D.; Orhan, I.E.; Marcourt, L.; Budzynska, B.; Wolfender, J.-L.; Crawford, A.D.; Skalicka-Woźniak, K. High-performance counter-current chromatography isolation and initial neuroactivity characterization of furanocoumarin derivatives from Peucedanum alsaticum L. (Apiaceae). Phytomedicine 2019, 15, 259–264. [Google Scholar] [CrossRef]
- Perry, N.S.L.; Houghton, P.J.; Jenner, P.; Keith, A.; Perry, E.K. Salvia lavandulaefolia essential oil inhibits cholinesterase in vivo. Phytomedicine 2002, 9, 48–51. [Google Scholar] [CrossRef]
- Fan, P.; Hay, A.-E.; Marston, A.; Hostettmann, K. Acetylcholinesterase-Inhibitory Activity of Linarin from Buddleja davidii, Structure-Activity Relationships of Related Flavonoids, and Chemical Investigation of Buddleja nitida. Pharm. Biol. 2008, 46, 596–601. [Google Scholar] [CrossRef]
- Kang, Y.-Q.; Zhou, J.-C.; Fan, P.-H.; Wang, S.-Q.; Lou, H.-X. Scapaundulin C, a novel labdane diterpenoid isolated from Chinese liverwort Scapania undulate, inhibits acetylcholinesterase activity. Chin. J. Nat. Med. 2015, 13, 933–936. [Google Scholar] [CrossRef]
- Nguyen, V.T.; To, D.C.; Tran, M.H.; Oh, S.H.; Kim, J.A.; Ali, Y.; Woo, M.-H.; Choi, J.S.; Min, B.S. Isolation of cholinesterase and β-secretase 1 inhibiting compounds from Lycopodiella cernua. Bioorg. Med. Chem. 2015, 23, 3126–3134. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Park, M. Acetylcholinesterase Inhibition by Flavonoids from Agrimonia pilosa. Molecules 2007, 12, 2130–2139. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Gilani, A.-H. Inhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia may explain medicinal use of turmeric in Alzheimer’s disease. Pharmacol. Biochem. Behav. 2009, 91, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.-N.; Khairuddean, M.; Wong, K.-C.; Khaw, K.-Y.; Vikneswaran, M. New cholinesterase inhibitors from Garcinia atroviridis. Fitoterapia 2014, 97, 261–267. [Google Scholar] [CrossRef]
- Park, H.M.; Choi, S.Y. Changes in Esterase Activity and Acetylcholinesterase Sensitivity of Insecti-cide-Selected Strains of the Brown Planthopper (Nilaparvata lugens Stal). Korean J. Appl. Entomol. 1991, 1, 19–37. [Google Scholar]
- Ding, X.; Ouyang, M.-A.; Liu, X.; Wang, R.-Z. Acetylcholinesterase Inhibitory Activities of Flavonoids from the Leaves of Ginkgo biloba against Brown Planthopper. J. Chem. 2013, 2013, 645086. [Google Scholar] [CrossRef]
- Radić, Z.; Kalisiak, J.; Fokin, V.V.; Sharpless, K.B.; Taylor, P. Interaction kinetics of oximes with native, phosphylated and aged human acetylcholinesterase. Chem. Interact. 2010, 187, 163–166. [Google Scholar] [CrossRef]
- Zhang, F.; Li, S.; Liu, C.; Fang, K.; Jiang, Y.; Zhang, J.; Lan, J.; Zhu, L.; Pang, H.-Q.; Wang, G. Rapid screening for acetylcholinesterase inhibitors in Selaginella doederleinii Hieron by using functionalized magnetic Fe3O4 nanoparticles. Talanta 2022, 243, 123284. [Google Scholar] [CrossRef]
- Zou, M.; Wang, R.; Yin, Q.; Liu, L. Bioassay-guided isolation and identification of anti-Alzheimer’s active compounds from Spiranthes sinensis (Pers.) Ames. Med. Chem. Res. 2021, 30, 1849–1855. [Google Scholar] [CrossRef]
- Lee, I.; Ahn, B.; Choi, J.; Hattori, M.; Min, B.; Bae, K. Selective cholinesterase inhibition by lanostane triterpenes from fruiting bodies of Ganoderma lucidum. Bioorg. Med. Chem. Lett. 2011, 21, 6603–6607. [Google Scholar] [CrossRef]
- Wang, W.; Fu, X.-W.; Dai, X.-L.; Hua, F.; Chu, G.-X.; Chu, M.-J.; Hu, F.-L.; Ling, T.-J.; Gao, L.-P.; Xie, Z.-W.; et al. Novel acetylcholinesterase inhibitors from Zijuan tea and biosynthetic pathway of caffeoylated catechin in tea plant. Food Chem. 2017, 237, 1172–1178. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Ke, J.-P.; Wang, W.; Kong, Y.-S.; Zhang, P.; Ling, T.-J.; Bao, G.-H. Discovery of Neolignan Glycosides with Acetylcolinesterase Inhibitory Activity from Huangjinya Green Tea Guided by Ultra Performance Liquid Chromatography–Tandem Mass Spectrometry Data and Global Natural Product Social Molecular Networking. J. Agric. Food Chem. 2019, 67, 11986–11993. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Su, X.; Lu, J.; Wu, M.; Yang, S.Y.; Mai, Y.; Deng, W.; Xue, Y. In Vitro and in Silico Analysis of Phytochemicals from Fallopia dentatoalata as Dual Functional Cholinesterase Inhibitors for the Treatment of Alzheimer’s Disease. Front. Pharmacol. 2022, 13, 905708. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.-H.; Lee, H.W.; Sun, Y.N.; Jang, W.-H.; Yang, S.-Y.; Jang, H.-D.; Kim, Y.H. (−)-Epicatechin derivate from Orostachys japonicus as potential inhibitor of the human butyrylcholinesterase. Int. J. Biol. Macromol. 2016, 91, 1033–1039. [Google Scholar] [CrossRef]
- Choi, J.S.; Haulader, S.; Karki, S.; Jung, H.J.; Kim, H.R.; Jung, H.A. Acetyl- and butyryl-cholinesterase inhibitory activities of the edible brown alga Eisenia bicyclis. Arch. Pharmacal. Res. 2014, 38, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Dilfaraz, K.; Wang, Z.; Saeed, A.; Shafiullah, K. New antioxidant and cholinesterase inhibitory constituents from Lonicera quinquelocularis. J. Med. Plants Res. 2014, 8, 313–317. [Google Scholar] [CrossRef]
- Ortiz, J.E.; Berkov, S.; Pigni, N.B.; Theoduloz, C.; Roitman, G.; Tapia, A.; Bastida, J.; Feresin, G.E. Wild Argentinian Amaryllidaceae, a New Renewable Source of the Acetylcholinesterase Inhibitor Galanthamine and Other Alkaloids. Molecules 2012, 17, 13473–13482. [Google Scholar] [CrossRef]
- Xiao, Y.; Liang, W.; Zhang, Z.; Wang, Y.; Zhang, S.; Liu, J.; Chang, J.; Ji, C.; Zhu, D. Polyketide Derivatives from the Endophytic Fungus Phaeosphaeria sp. LF5 Isolated from Huperzia serrata and Their Acetylcholinesterase Inhibitory Activities. J. Fungi 2022, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Lee, B.; Han, S.J.; Ryu, J.H.; Kim, D.-H. Mangiferin Ameliorates Scopolamine-Induced Learning Deficits in Mice. Biol. Pharm. Bull. 2009, 32, 242–246. [Google Scholar] [CrossRef]
- Jang, M.H.; Piao, X.L.; Kim, J.M.; Kwon, S.W.; Park, J.H. Inhibition of cholinesterase and amyloid-β aggregation by resveratrol oligomers from Vitis amurensis. Phytotherapy Res. 2008, 22, 544–549. [Google Scholar] [CrossRef]
- Hajimehdipoor, H.; Mosaddegh, M.; Naghibi, F.; Haeri, A.; Hamzeloo-Moghadam, M. Natural sesquiterpen lactones as acetylcholinesterase inhibitors. An. Acad. Bras. Cienc. 2014, 86, 801–806. [Google Scholar] [CrossRef]
- Ibrahim, M.; Farooq, T.; Hussain, N.; Hussain, A.; Gulzar, T.; Hussain, I.; Akash, M.S.H.; Rehmani, F.S. Acetyl and butyryl cholinesterase inhibitory sesquiterpene lactones from Amberboa ramosa. Chem. Central J. 2013, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Bustamam, A.; Ibrahim, S.; Al-Zubairi, A.S.; Met, M.; Syam, M.M. Zerumbone: A Natural Compound with Anti-Cholinesterase Activity. Am. J. Pharmacol. Toxicol. 2008, 3, 209–211. [Google Scholar] [CrossRef]
- Hornick, A.; Schwaiger, S.; Rollinger, J.M.; Vo, N.P.; Prast, H.; Stuppner, H. Extracts and constituents of Leontopodium alpinum enhance cholinergic transmission: Brain ACh increasing and memory improving properties. Biochem. Pharmacol. 2008, 76, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Fatima, I.; Ahmad, I.; Anis, I.; Malik, A.; Afza, N.; Iqbal, L.; Latif, M. New butyrylcholinesterase inhibitory steroid and peroxy acid from Leucas urticifolia. Arch. Pharmacal Res. 2008, 31, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Nawaz, S.A.; Malik, A.; Choudhary, M.I. Isolation and Cholinesterase-Inhibition Studies of Sterols from Haloxylon recurvum. Cheminform 2006, 37, 573–580. [Google Scholar] [CrossRef]
- Jusril, N.; Juhari, A.M.; Abu Bakar, S.; Saad, W.M.; Adenan, M. Combining In Silico and In Vitro Studies to Evaluate the Acetylcholinesterase Inhibitory Profile of Different Accessions and the Biomarker Triterpenes of Centella asiatica. Molecules 2020, 25, 3353. [Google Scholar] [CrossRef]
- Tallini, L.R.; Osorio, E.H.; dos Santos, V.D.; Borges, W.D.S.; Kaiser, M.; Viladomat, F.; Zuanazzi, J.A.S.; Bastida, J. Hippeastrum reticulatum (Amaryllidaceae): Alkaloid Profiling, Biological Activities and Molecular Docking. Molecules 2017, 22, 2191. [Google Scholar] [CrossRef]
- Alarcón-Enos, J.; Muñoz-Núñez, E.; Gutiérrez, M.; Quiroz-Carreño, S.; Pastene-Navarrete, E.; Acuña, C.C. Dyhidro-β-agarofurans natural and synthetic as acetylcholinesterase and COX inhibitors: Interaction with the peripheral anionic site (AChE-PAS), and anti-inflammatory potentials. J. Enzym. Inhib. Med. Chem. 2022, 37, 1845–1856. [Google Scholar] [CrossRef]
- Di Giovanni, S.; Borloz, A.; Urbain, A.; Marston, A.; Hostettmann, K.; Carrupt, P.-A.; Reist, M. In vitro screening assays to identify natural or synthetic acetylcholinesterase inhibitors: Thin layer chromatography versus microplate methods. Eur. J. Pharm. Sci. 2008, 33, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.W.; Oh, S.-R.; Curtis-Long, M.J.; Lee, J.H.; Song, H.-H.; Park, K.H. Rapid Identification of Cholinesterase Inhibitors from the Seedcases of Mangosteen Using an Enzyme Affinity Assay. J. Agric. Food Chem. 2014, 62, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Hostettmann, K.; Borloz, A.; Urbain, A.; Marston, A. Natural Product Inhibitors of Acetylcholinesterase. Curr. Org. Chem. 2006, 10, 825–847. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Mroczek, T. Application of hydrostatic CCC–TLC–HPLC–ESI-TOF-MS for the bioguided fractionation of anticholinesterase alkaloids from Argemone mexicana L. roots. Anal. Bioanal. Chem. 2015, 407, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Mroczek, T. Qualitative and quantitative two-dimensional thin-layer chromatography/high performance liquid chromatography/diode-array/electrospray-ionization-time-of-flight mass spectrometry of cholinesterase inhibitors. J. Pharm. Biomed. Anal. 2016, 129, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Smyrska-Wieleba, N. Analiza Fitochemiczna oraz Ocena Aktywności Biologicznej Alkaloidów w Wybranych Gatunkach Rodzaju Narcissus spp.; Medical University of Lublin: Lublin, Poland, 2021. [Google Scholar]
- Mroczek, T.; Dymek, A.; Widelski, J.; Wojtanowski, K.K. The Bioassay-Guided Fractionation and Identification of Potent Acetylcholinesterase Inhibitors from Narcissus c.v. ‘Hawera’ Using Optimized Vacuum Liquid Chromatography, High Resolution Mass Spectrometry and Bioautography. Metabolites 2020, 10, 395. [Google Scholar] [CrossRef]
- Maciejewska-Turska, M.; Zgórka, G. In-depth phytochemical and biological studies on potential AChE inhibitors in red and zigzag clover dry extracts using reversed–phase liquid chromatography (RP-LC) coupled with photodiode array (PDA) and electron spray ionization-quadrupole/time of flight-mass spectrometric (ESI-QToF/MS-MS) detection and thin-layer chromatography-bioautography. Food Chem. 2021, 375, 131846. [Google Scholar] [CrossRef] [PubMed]

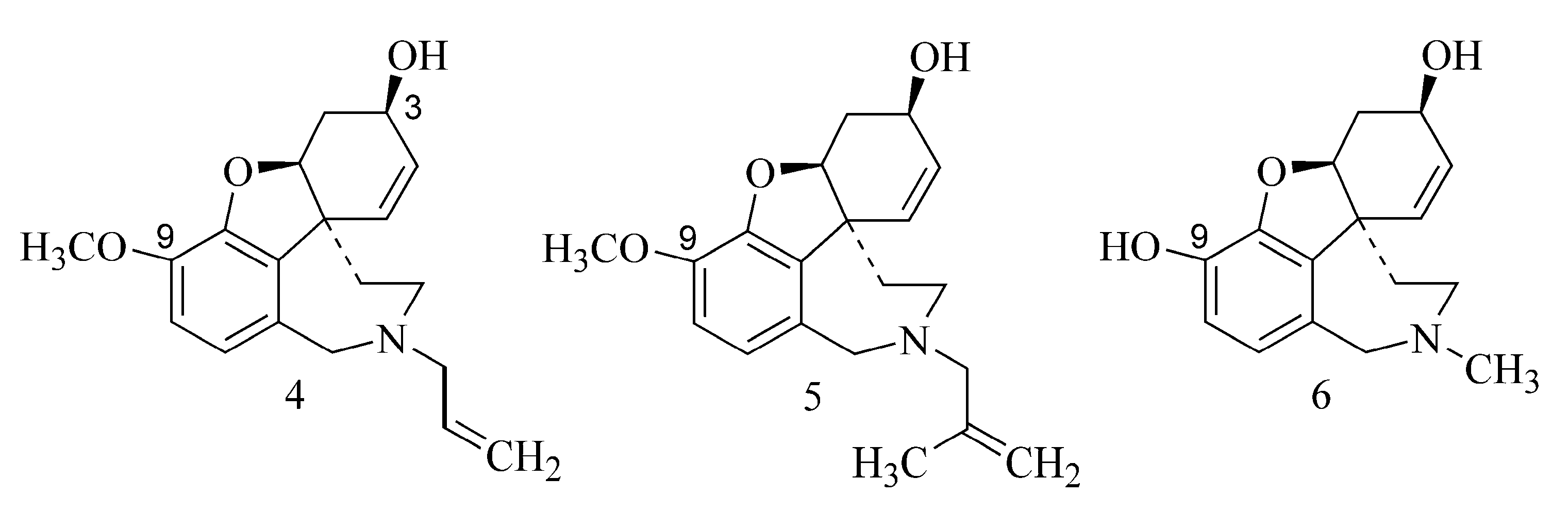
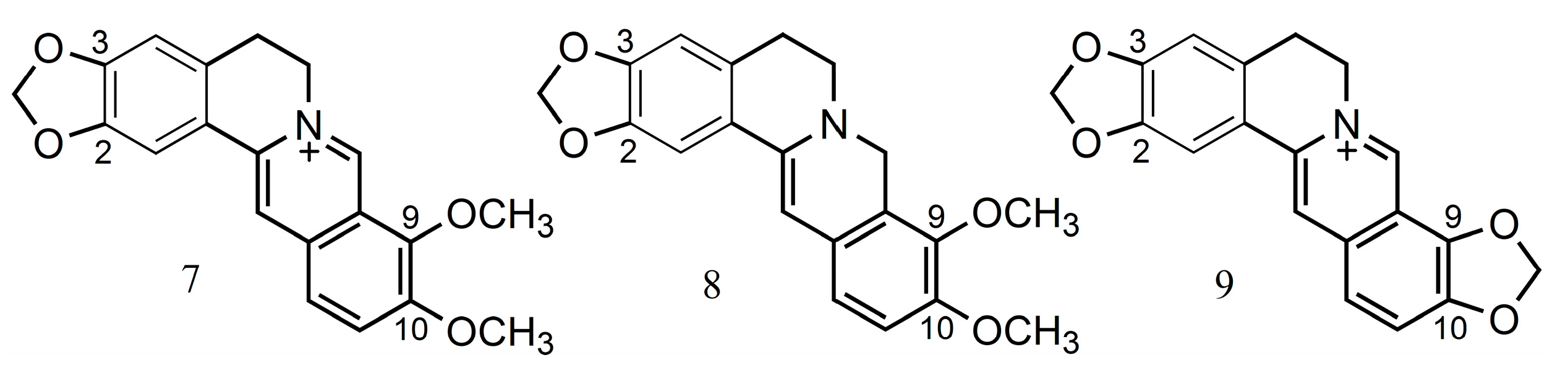

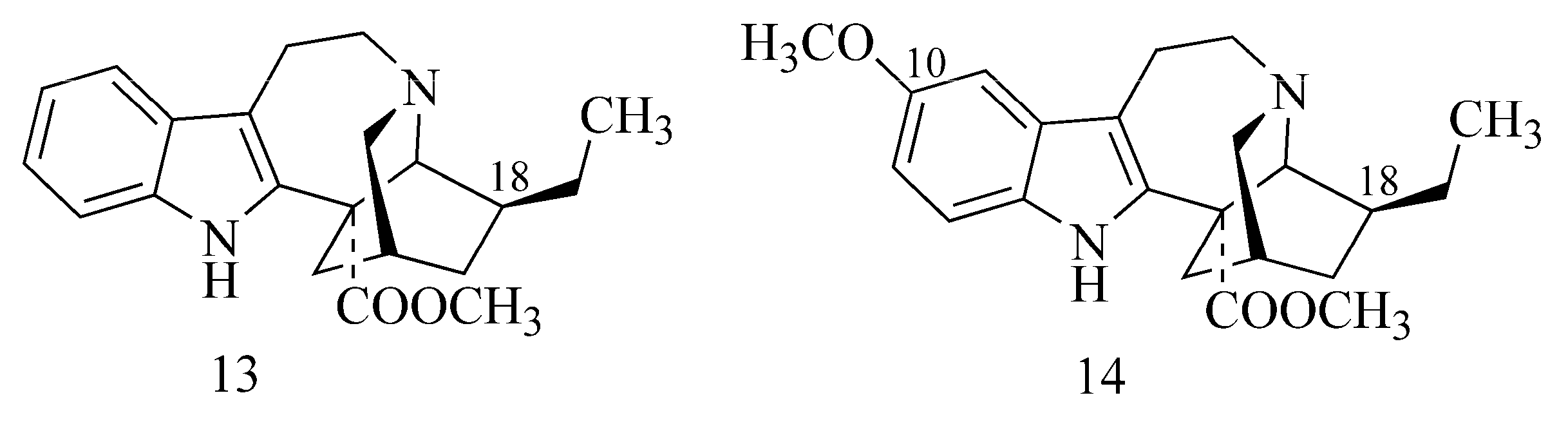
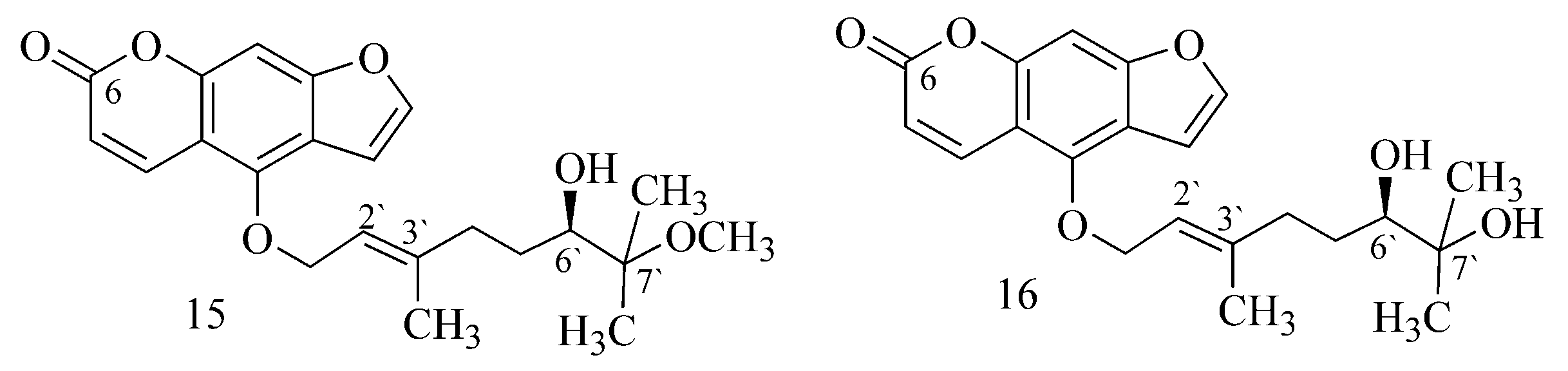
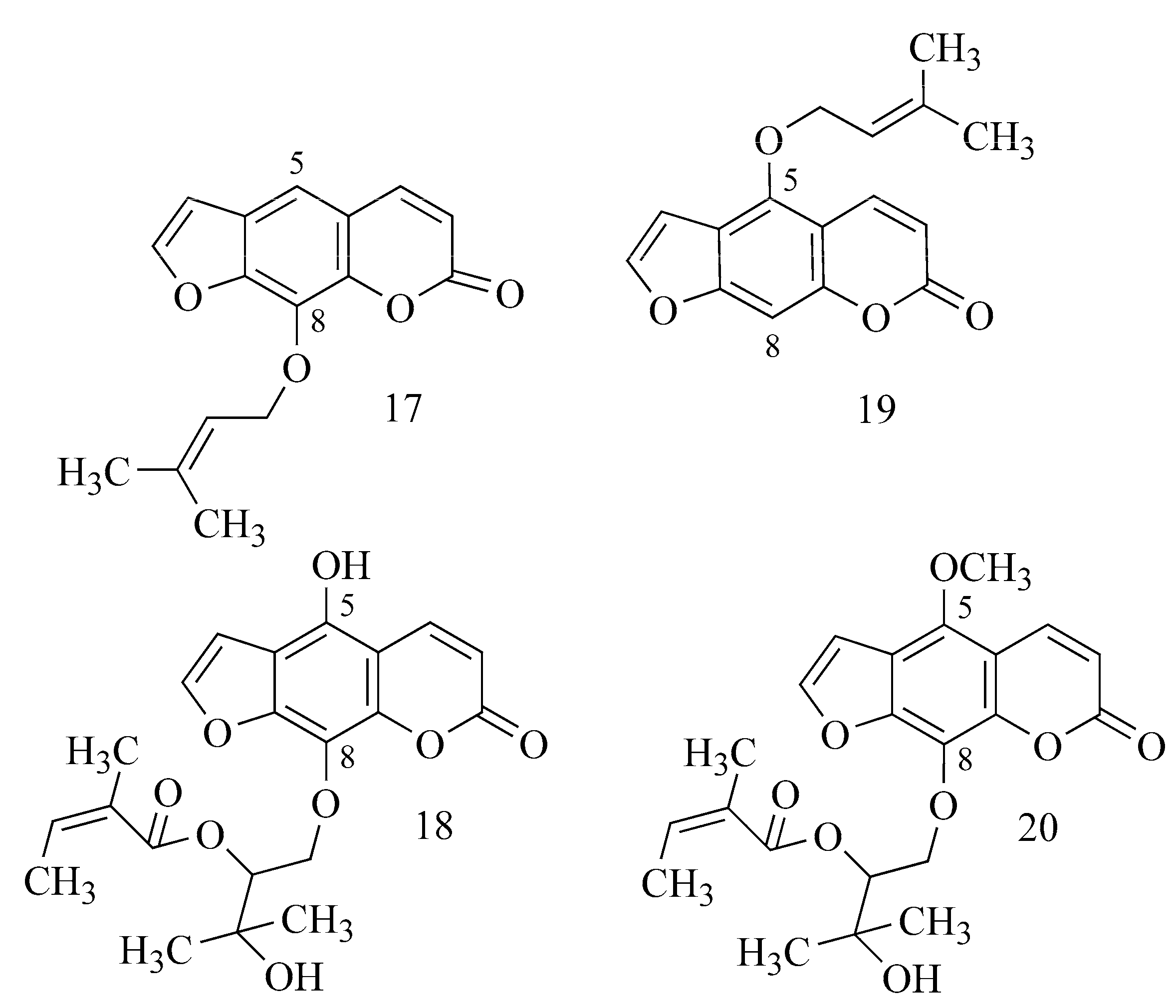

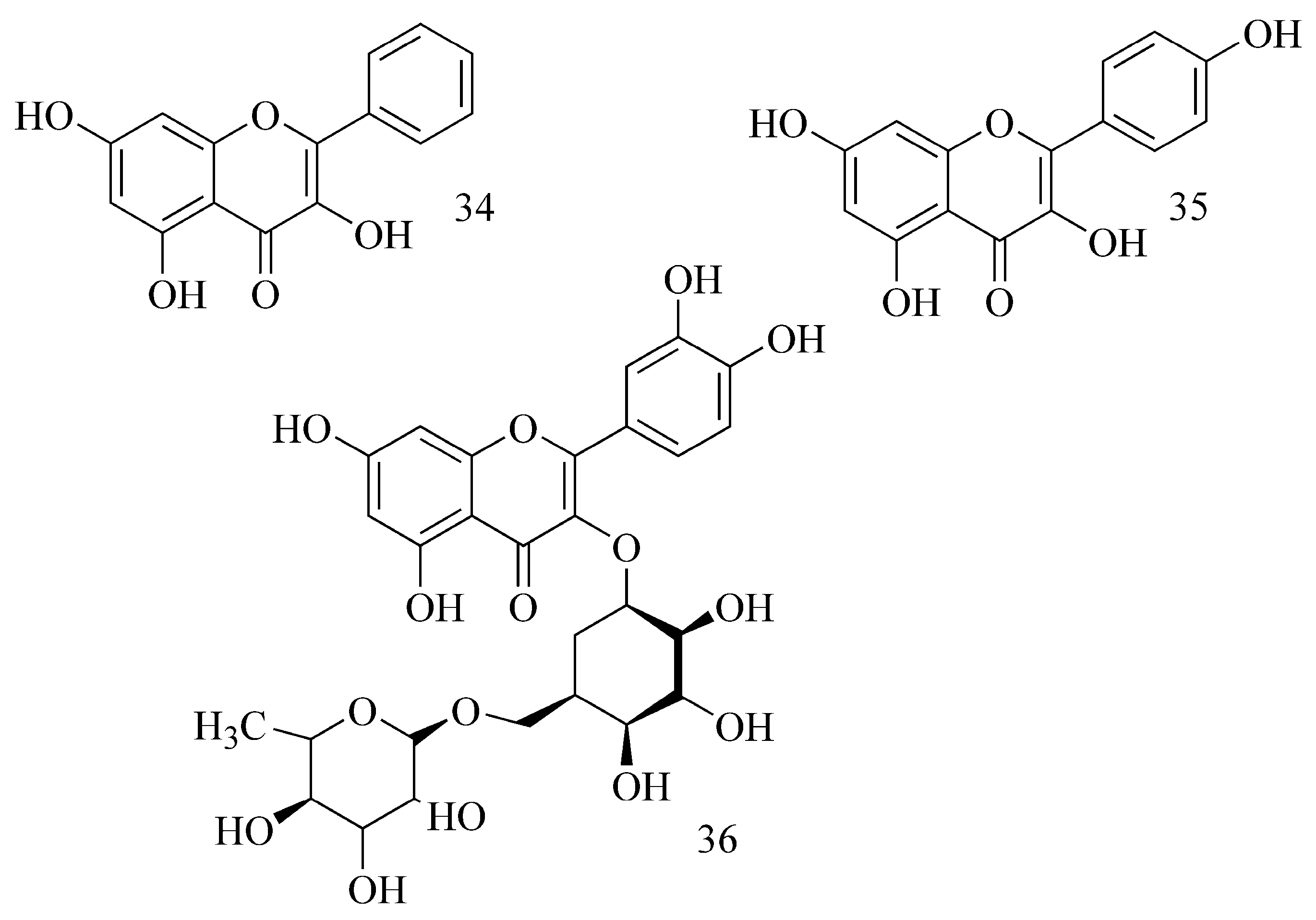
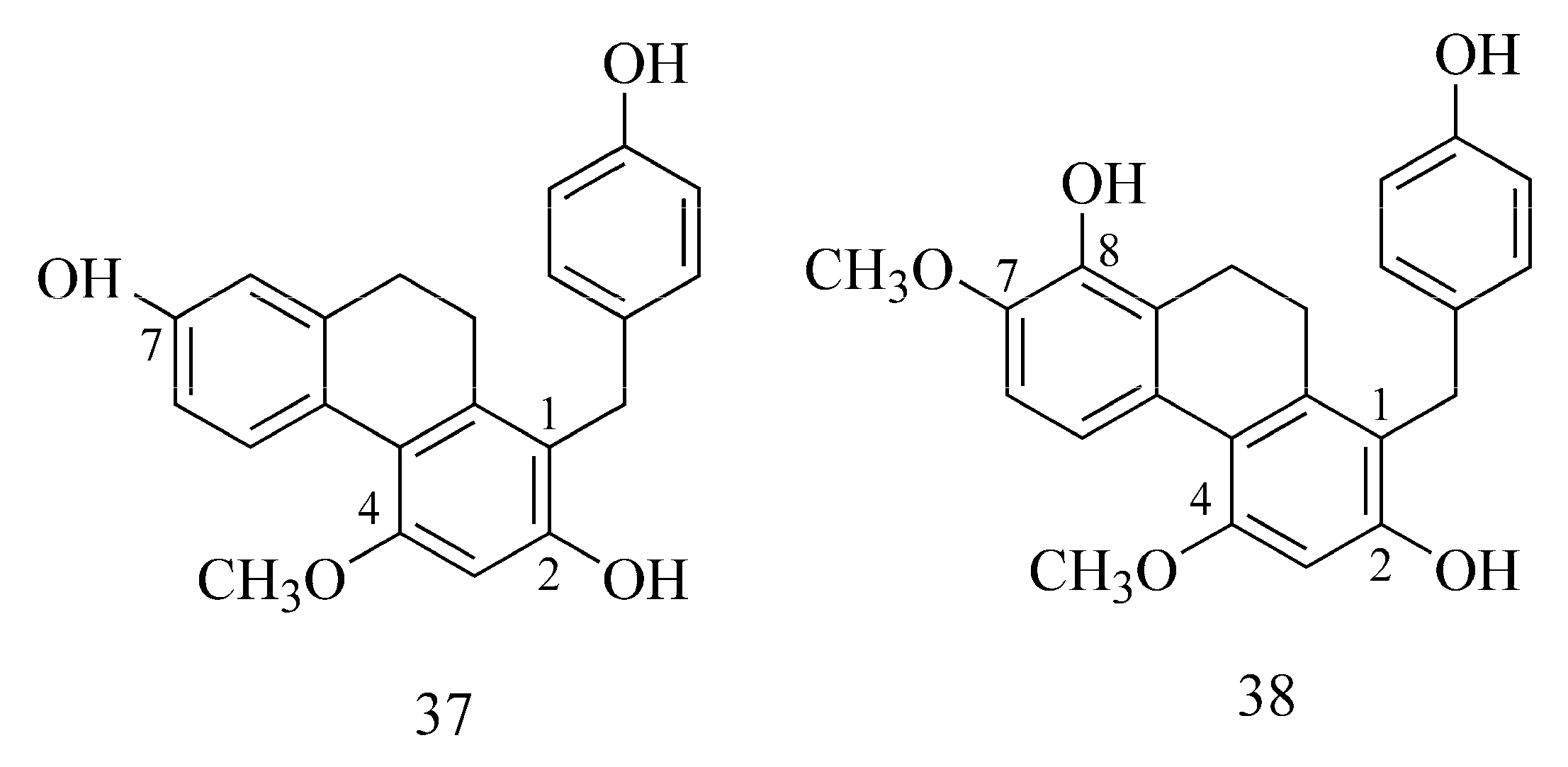


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smyrska-Wieleba, N.; Mroczek, T. Natural Inhibitors of Cholinesterases: Chemistry, Structure–Activity and Methods of Their Analysis. Int. J. Mol. Sci. 2023, 24, 2722. https://doi.org/10.3390/ijms24032722
Smyrska-Wieleba N, Mroczek T. Natural Inhibitors of Cholinesterases: Chemistry, Structure–Activity and Methods of Their Analysis. International Journal of Molecular Sciences. 2023; 24(3):2722. https://doi.org/10.3390/ijms24032722
Chicago/Turabian StyleSmyrska-Wieleba, Natalia, and Tomasz Mroczek. 2023. "Natural Inhibitors of Cholinesterases: Chemistry, Structure–Activity and Methods of Their Analysis" International Journal of Molecular Sciences 24, no. 3: 2722. https://doi.org/10.3390/ijms24032722
APA StyleSmyrska-Wieleba, N., & Mroczek, T. (2023). Natural Inhibitors of Cholinesterases: Chemistry, Structure–Activity and Methods of Their Analysis. International Journal of Molecular Sciences, 24(3), 2722. https://doi.org/10.3390/ijms24032722







