Identification of Novel Sphydrofuran-Derived Derivatives with Lipid-Lowering Activity from the Active Crude Extracts of Nocardiopsis sp. ZHD001
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of OCE and Its Hypolipidemic Effects In Vivo
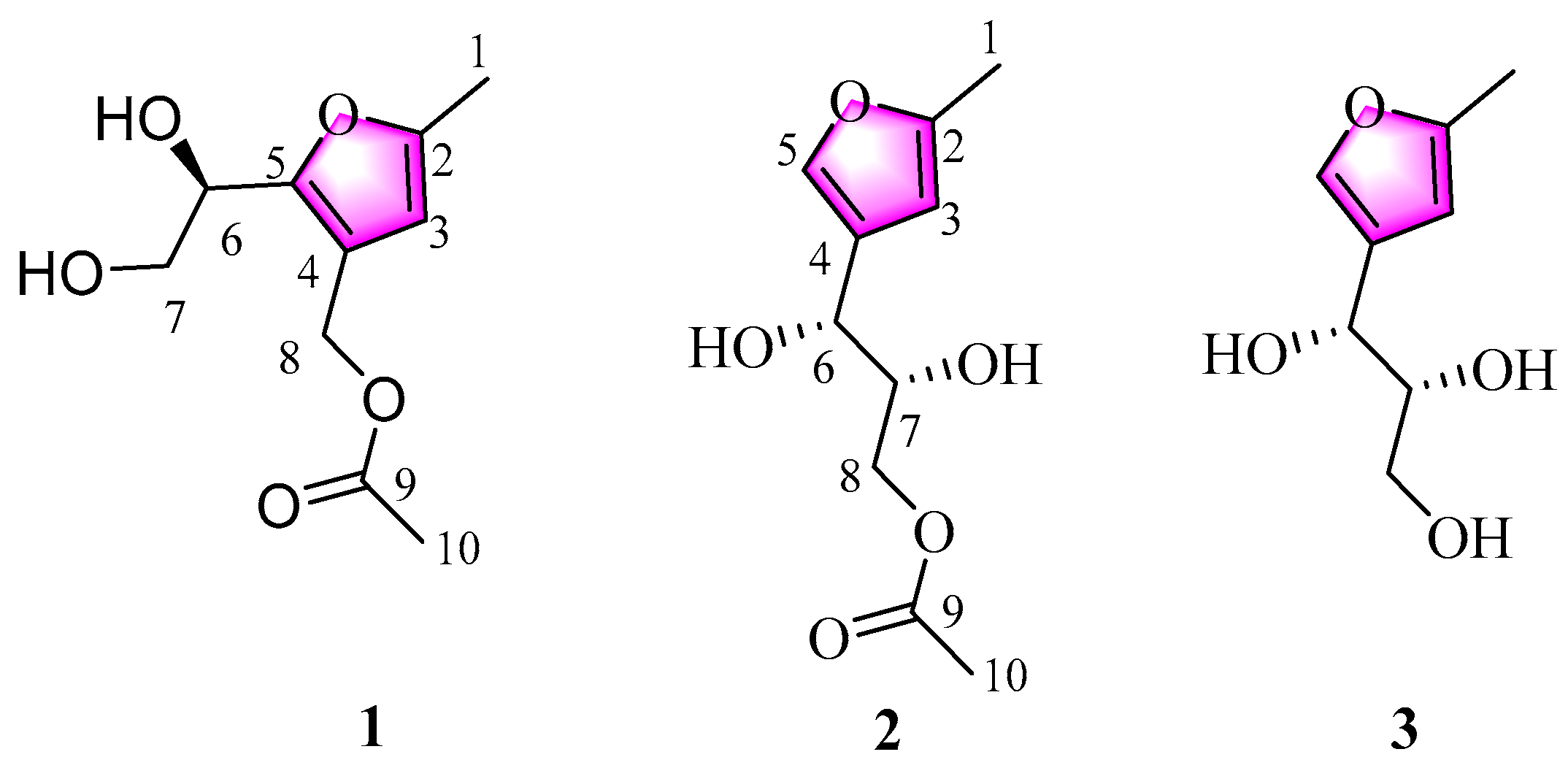
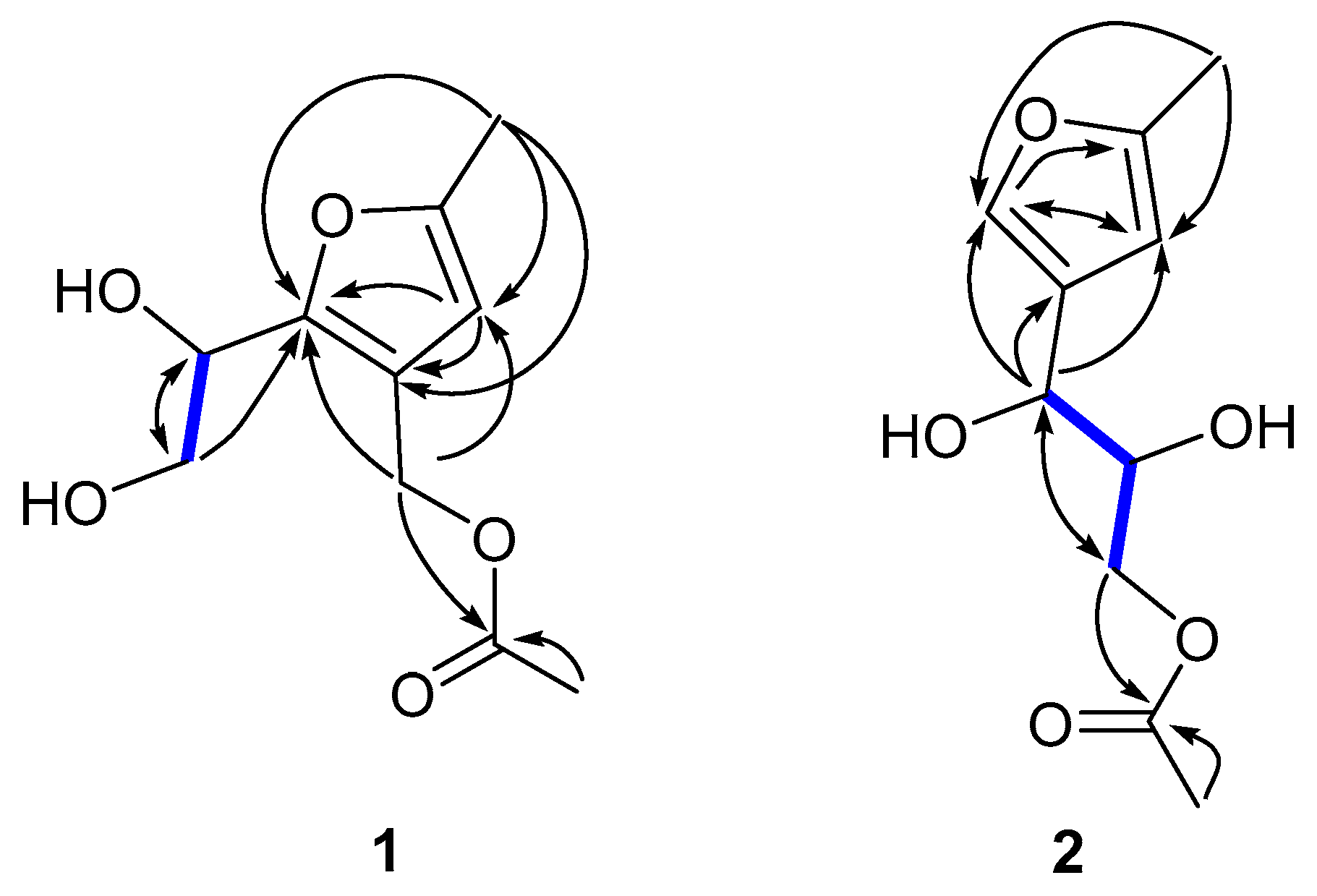
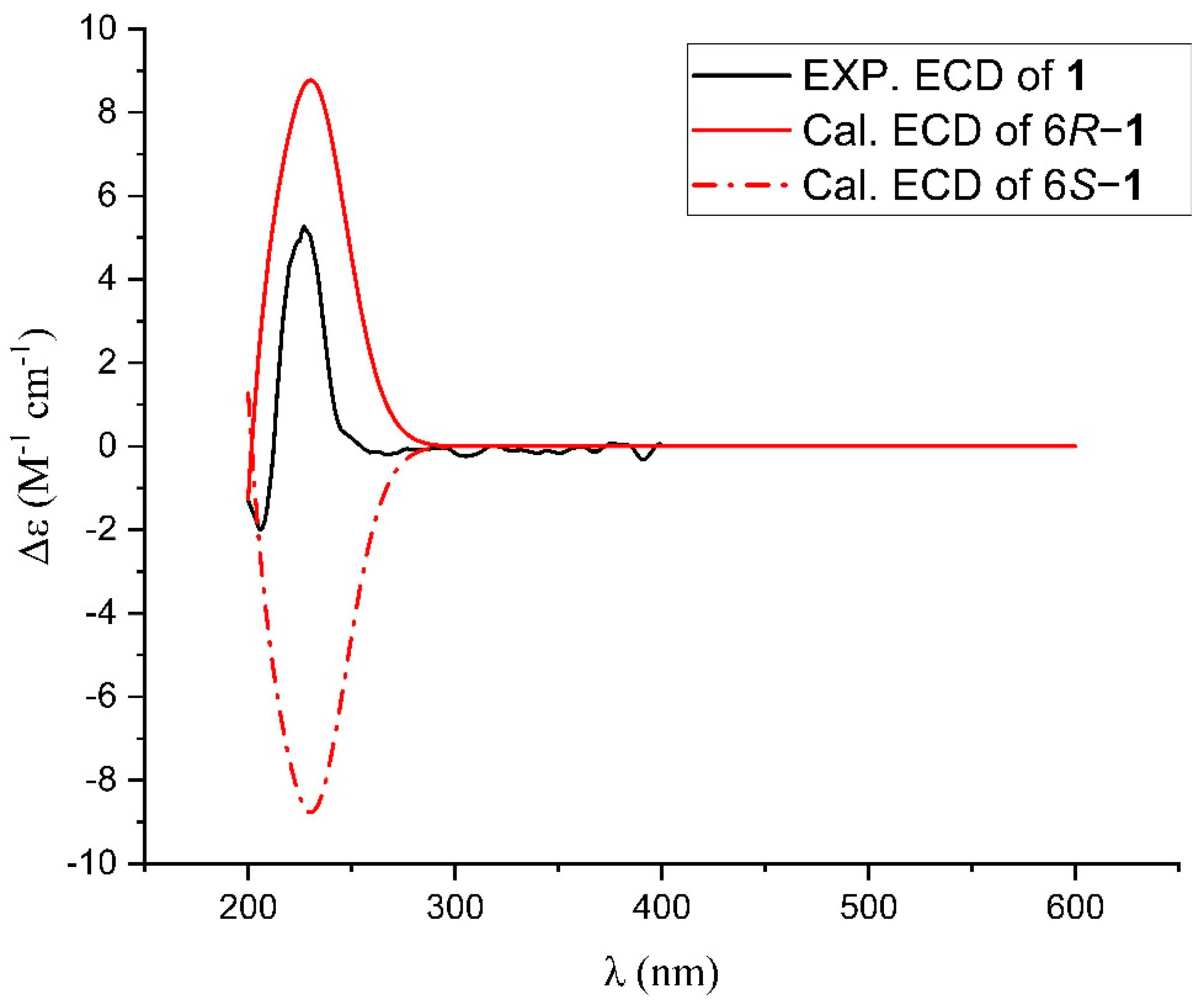
2.2. Isolation and Structure Elucidation of Compounds 1–3
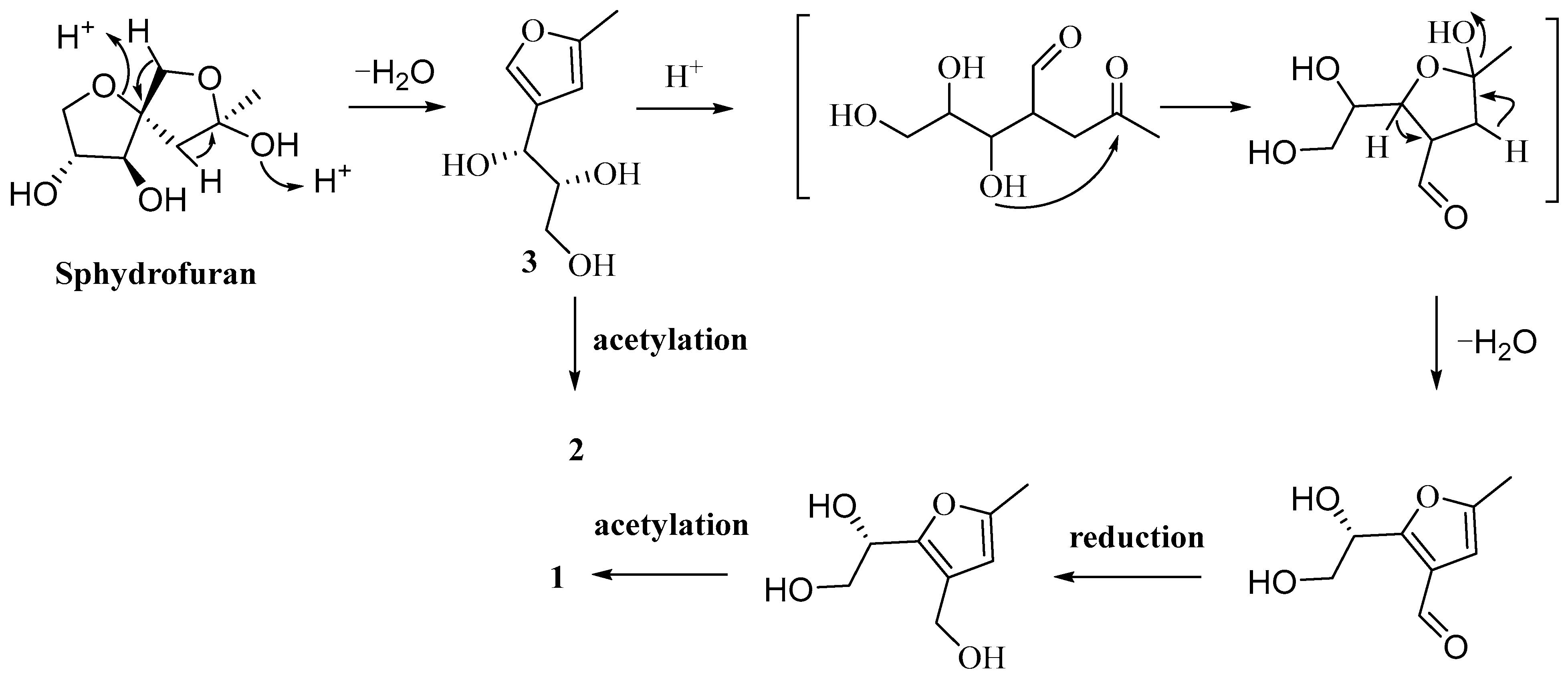
2.3. Proposed Biosynthetic Pathways of Compounds 1–3
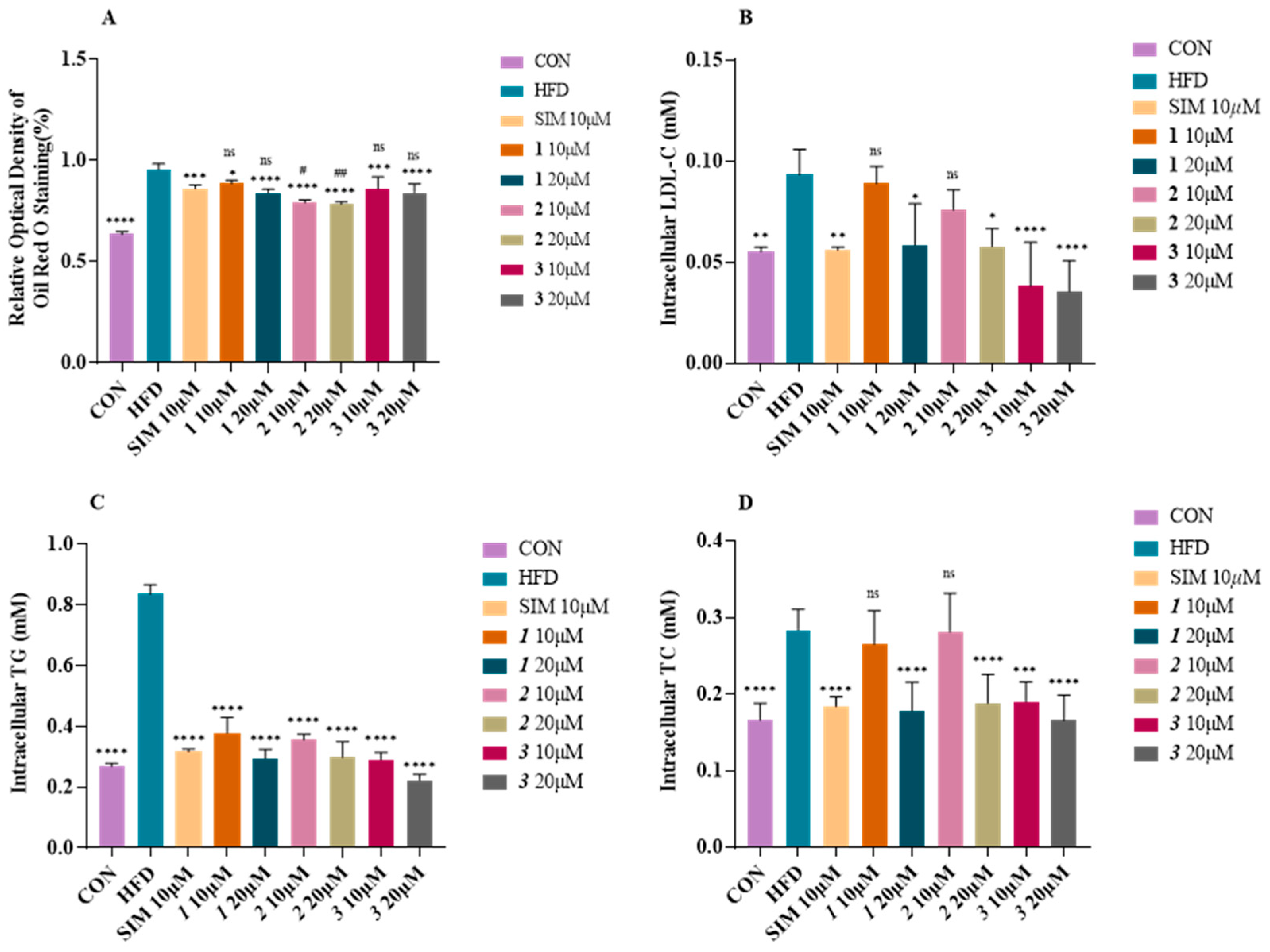
2.4. Effect of Compounds 1–3 on Lipid Accumulation in Fatty-Acid-Elicited HepG2 Cells
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Isolation and Identification of Strain Nocardiopsis sp. ZHD001
3.3. Isolation and Purification of Compounds 1–3
3.4. Cytotoxicity Assay and Lipid-Lowering Activity Assay In Vitro
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, J.; Wang, M.; Zhang, X.G.; Zhou, M.G. Epidemiology of cardiovascular disease in China: Current features and implications. Nat. Rev. Cardiol. 2019, 16, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Casula, M.; Olmastroni, E.; Norata, G.D.; Catapano, A.L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 2021, 18, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study (vol 76, pg 2982, 2020). J. Am. Coll. Cardiol. 2021, 77, 1958–1959. [Google Scholar]
- Michos, E.D.; McEvoy, J.W.; Blumenthal, R.S. Lipid Management for the Prevention of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2019, 381, 1557–1567. [Google Scholar] [CrossRef]
- Jose, P.A.; Maharshi, A.; Jha, B. Actinobacteria in natural products research: Progress and prospects. Microbiol. Res. 2021, 246, 126708. [Google Scholar] [CrossRef]
- Banerjee, P.; Mandhare, A.; Bagalkote, V. Marine natural products as source of new drugs: An updated patent review (July 2018–July 2021). Expert Opin. Ther. Pat. 2022, 32, 317–363. [Google Scholar] [CrossRef]
- Demain, A.L.; Sanchez, S. Microbial drug discovery: 80 years of progress. J. Antibiot. 2009, 62, 5–16. [Google Scholar] [CrossRef]
- Mahajan, G.B.; Balachandran, L. Antibacterial agents from actinomycetes—A review. Front. Biosci. (Elite Ed.) 2012, 4, 240–253. [Google Scholar] [CrossRef]
- Worm, B.; Barbier, E.B.; Beaumont, N.; Duffy, J.E.; Folke, C.; Halpern, B.S.; Jackson, J.B.; Lotze, H.K.; Micheli, F.; Palumbi, S.R.; et al. Impacts of biodiversity loss on ocean ecosystem services. Science 2006, 314, 787–790. [Google Scholar] [CrossRef]
- Fenical, W.; Jensen, P.R. Developing a new resource for drug discovery: Marine actinomycete bacteria. Nat. Chem. Biol. 2006, 2, 666–673. [Google Scholar] [CrossRef]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.K. Pharmaceutically active secondary metabolites of marine actinobacteria. Microbiol. Res. 2014, 169, 262–278. [Google Scholar] [CrossRef]
- Manivasagan, P.; Kang, K.H.; Sivakumar, K.; Li-Chan, E.C.; Oh, H.M.; Kim, S.K. Marine actinobacteria: An important source of bioactive natural products. Environ. Toxicol. Pharmacol. 2014, 38, 172–188. [Google Scholar] [CrossRef]
- Fenical, W.; Jensen, P.R.; Palladino, M.A.; Lam, K.S.; Lloyd, G.K.; Potts, B.C. Discovery and development of the anticancer agent salinosporamide A (NPI-0052). Bioorg. Med. Chem. 2009, 17, 2175–2180. [Google Scholar] [CrossRef]
- Bister, B.; Bischoff, D.; Strobele, M.; Riedlinger, J.; Reicke, A.; Wolter, F.; Bull, A.T.; Zahner, H.; Fiedler, H.P.; Sussmuth, R.D. Abyssomicin C-A polycyclic antibiotic from a marine Verrucosispora strain as an inhibitor of the p-aminobenzoic acid/tetrahydrofolate biosynthesis pathway. Angew. Chem. Int. Ed. Engl. 2004, 43, 2574–2576. [Google Scholar] [CrossRef]
- Umezawa, S.; Usui, T.; Umezawa, H.; Tsuchiya, T.; Takeuchi, T. A new microbial metabolite, sphydrofuran. I. Isolation and the structure of a hydrolysis product. J. Antibiot. 1971, 24, 85–92. [Google Scholar] [CrossRef]
- Usui, T.; Umezawa, S.; Tsuchiya, T.; Naganawa, H.; Takeuchi, T. A new microbial metabolite, sphydrofuran. II. The structure of sphydrofuran. J. Antibiot. 1971, 24, 93–106. [Google Scholar] [CrossRef]
- Bindseil, K.U.; Henkel, T.; Zeeck, A.; Bur, D.; Niederer, D.; Sequin, U. Metabolic Products of Microorganisms. 262. The Absolute-Configuration of Sphydrofuran, a Widespread Metabolite from Streptomycetes. Helv. Chim. Acta 1991, 74, 1281–1286. [Google Scholar] [CrossRef]
- Yu, P.; Yang, Y.; Zhang, Z.Y.; Mak, T.C.W.; Wong, H.N.C. Total syntheses of sphydrofuran, secosyrins, and syributins. J. Org. Chem. 1997, 62, 6359–6366. [Google Scholar] [CrossRef]
- Nosaka, C.; Kunimoto, S.; Masuda, T.; Ohsono, M.; Iinuma, H.; Tsuchida, T.; Hamada, M.; Takeuchi, T. A new cytotoxic 2H-pyran compound produced by acid treatment of the fermentation broth of Streptomyces sp. Biol. Pharm. Bull. 1999, 22, 546–548. [Google Scholar] [CrossRef]
- Sasaki, K.; Hayashi, K.; Matsuya, Y.; Sugimoto, K.; Lee, J.B.; Kurosaki, F.; Hayashi, T. In vitro and in vivo antiherpetic effects of (1R,2R)-1-(5′-methylful-3′-yl)propane-1,2,3-triol. J. Nat. Med. 2016, 70, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.J.; Li, J.Q.; Zhang, H.J.; Ding, W.J.; Ma, Z.J. Cyclizidine-Type Alkaloids from Streptomyces sp HNA39. J. Nat. Prod. 2018, 81, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.J.; Zhang, D.S.; Zhang, H.J.; Li, J.Q.; Ding, W.J.; Xu, C.D.; Ma, Z.J. Medermycin-Type Naphthoquinones from the Marine-Derived Streptomyces sp XMA39. J. Nat. Prod. 2018, 81, 2120–2124. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.W.; Li, J.Q.; Jiang, Y.J.; Liu, H.Z.; Huo, C. A new indolizinium alkaloid from marine-derived Streptomyces sp. HNA39. J. Asian Nat. Prod. Res. 2021, 23, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.J.; Huang, Y.; Chen, S.; Ji, Y.Y.; Ding, W.J.; Ma, Z.J. Strepolyketides A-C, three novel SEK15-derived polyketides from Streptomyces sp. HN2A53. Tetrahedron Lett. 2020, 61, 151996. [Google Scholar] [CrossRef]


| No. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH, J in Hz | δC, type | δH, J in Hz | δC, type | δH, J in Hz | δC, type | |
| 1 | 2.24, s | 13.3, CH3 | 2.24, s | 13.4, CH3 | 2.24, s | 13.4, CH3 |
| 2 | 152.7, C | 153.9, C | 153.7, C | |||
| 3 | 6.01, s | 108.7, CH | 6.05, s | 106.1, CH | 6.06, s | 106.1, CH |
| 4 | 119.7, C | 127.8, C | 128.2, C | |||
| 5 | 151.6, C | 7.31, s | 139.6, CH | 7.30, s | 139.4, CH | |
| 6 | 4.76, dd (7.2, 6.0) | 67.9, CH | 4.51, d (6.0) | 68.6, CH | 4.52, d (6.0) | 68.3, CH |
| 7 | 3,71, dd (11.4, 5.4) 3.78, dd (10.8, 7.2) | 65.6, CH2 | 3.86, m | 73.8, CH | 3.42, dd (10.8, 6.0) | 76.4, CH |
| 8 | 4.98, s | 58.6, CH2 | 3.92, dd (10.8, 6.6) 4.10, dd (11.4, 3.6) | 66.9, CH2 | 3.56, dd (10.8, 4.2) 3.67, m | 64.1, CH2 |
| 9 | 172.8, C | 172.9, C | ||||
| 10 | 2.02, s | 20.8, CH3 | 2.04, s | 20.7, CH3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Jiang, Y.; Wen, Z.; Guan, L.; Ouyang, X.; Ding, W.; Ma, Z. Identification of Novel Sphydrofuran-Derived Derivatives with Lipid-Lowering Activity from the Active Crude Extracts of Nocardiopsis sp. ZHD001. Int. J. Mol. Sci. 2023, 24, 2822. https://doi.org/10.3390/ijms24032822
Tian Y, Jiang Y, Wen Z, Guan L, Ouyang X, Ding W, Ma Z. Identification of Novel Sphydrofuran-Derived Derivatives with Lipid-Lowering Activity from the Active Crude Extracts of Nocardiopsis sp. ZHD001. International Journal of Molecular Sciences. 2023; 24(3):2822. https://doi.org/10.3390/ijms24032822
Chicago/Turabian StyleTian, Yuhong, Yongjun Jiang, Zhengshun Wen, Liping Guan, Xiaokun Ouyang, Wanjing Ding, and Zhongjun Ma. 2023. "Identification of Novel Sphydrofuran-Derived Derivatives with Lipid-Lowering Activity from the Active Crude Extracts of Nocardiopsis sp. ZHD001" International Journal of Molecular Sciences 24, no. 3: 2822. https://doi.org/10.3390/ijms24032822
APA StyleTian, Y., Jiang, Y., Wen, Z., Guan, L., Ouyang, X., Ding, W., & Ma, Z. (2023). Identification of Novel Sphydrofuran-Derived Derivatives with Lipid-Lowering Activity from the Active Crude Extracts of Nocardiopsis sp. ZHD001. International Journal of Molecular Sciences, 24(3), 2822. https://doi.org/10.3390/ijms24032822






