An Inorganic–Organic Hybrid Framework Composed of Polyoxotungstate and Long-Chained Bolaamphiphile
Abstract
1. Introduction
2. Results
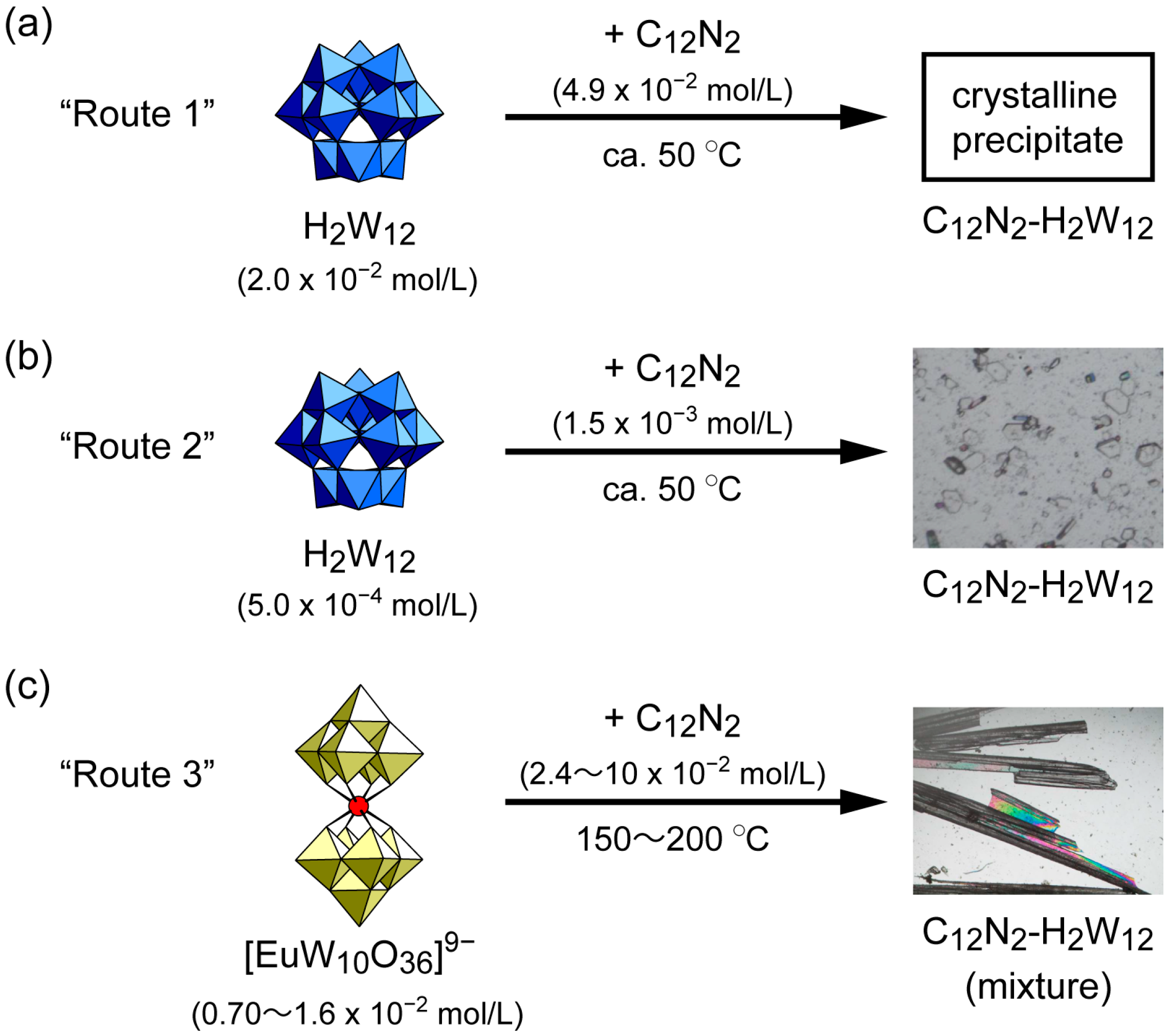
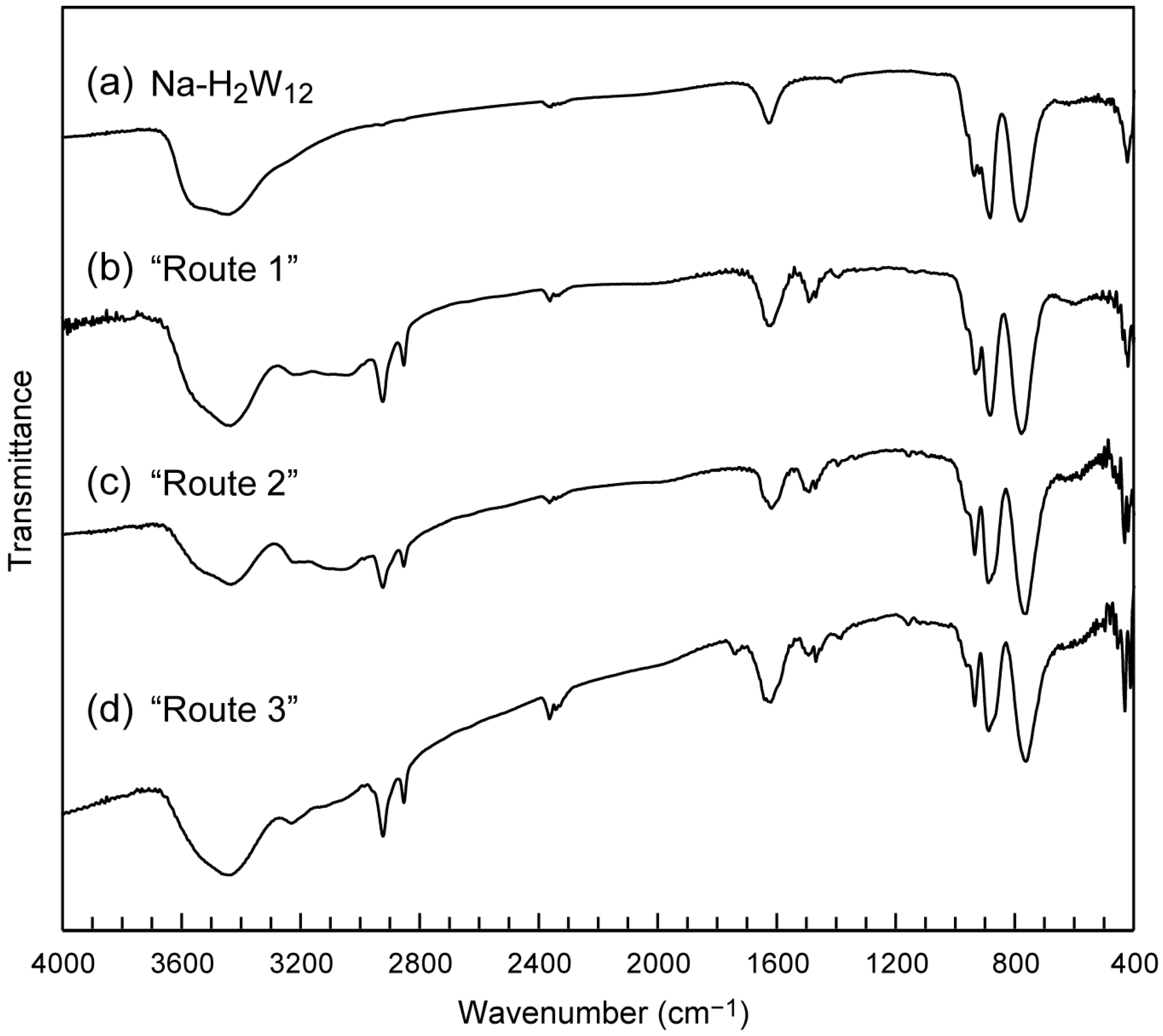
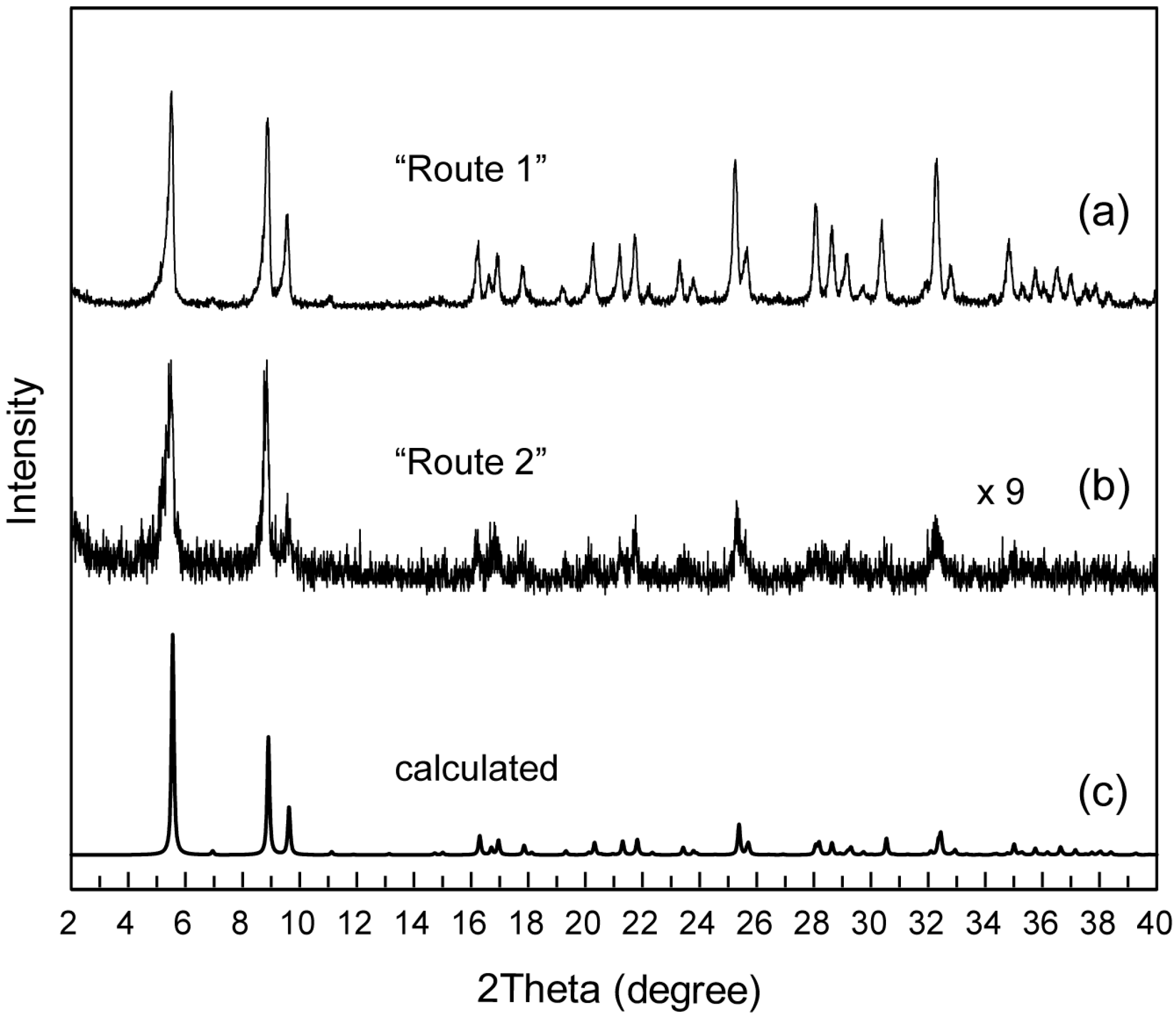
2.1. Syntheses of C12N2-H2W12 Hybrid Crystal
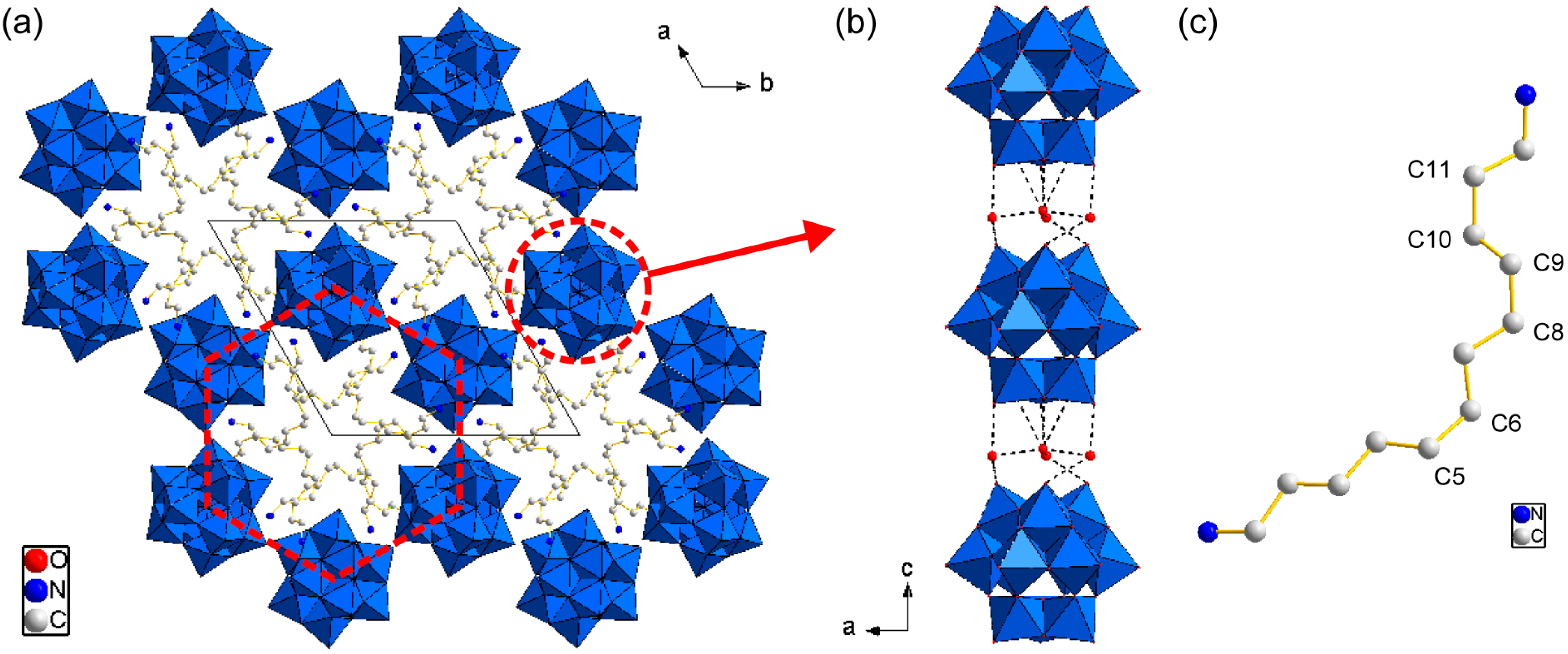
2.2. Crystal Strucure of C12N2-H2W12 Hybrid Crystals
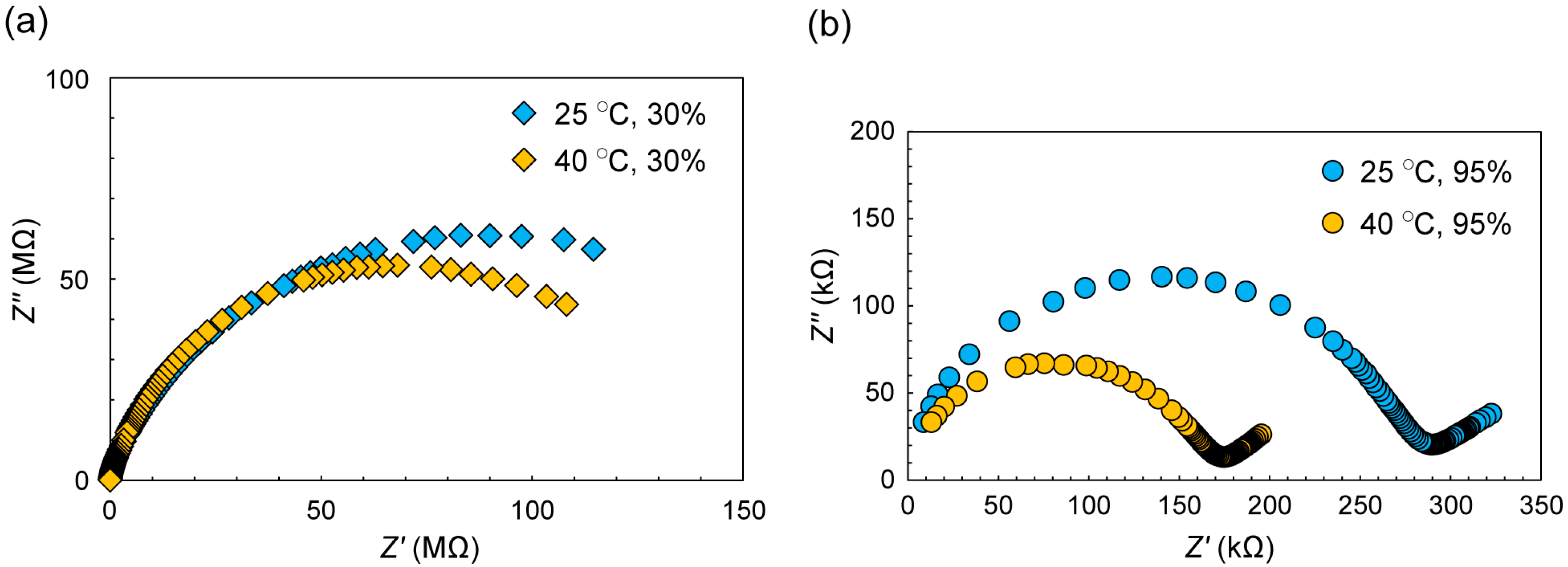
2.3. Conductive Property of C12N2-H2W12 Hybrid Crystal
3. Discussion
4. Materials and Methods
4.1. General Procedures and Instrumental Methods
4.2. Syntheses
4.2.1. Route 1
4.2.2. Route 2
4.2.3. Route 3
4.3. Crystal Structure Determination
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, D.F.; Wennerström, H. The Colloidal Domain, 2nd ed.; Wiley: Hoboken, NJ, USA, 1999. [Google Scholar]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena, 4th ed.; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Kronberg, B.; Holmberg, K.; Lindman, B. Surface Chemistry of Surfactants and Polymers, 1st ed.; Wiley: Chichester, UK, 2014; pp. 1–47. [Google Scholar]
- Bradshaw, D.; El-Hankari, S.; Lupica-Spagnolo, L. Supramolecular templating of hierarchically porous metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 5431–5443. [Google Scholar] [CrossRef] [PubMed]
- Fuhrhop, J.-H.; Wang, T. Bolaamphiphiles. Chem. Rev. 2004, 104, 2901–2937. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Masuda, M.; Minamikawa, H. Supramolecular nanotube architectures based on amphiphilic molecules. Chem. Rev. 2005, 105, 1401–1443. [Google Scholar] [CrossRef] [PubMed]
- Vargas, B.; Rodríguez-López, G.; Solis-Ibarra, D. The emergence of halide layered double perovskites. ACS Energy Lett. 2020, 5, 3591–3608. [Google Scholar] [CrossRef]
- Coronado, E.; Gómez-García, C.J. Polyoxometalate-based molecular materials. Chem. Rev. 1998, 98, 273–296. [Google Scholar] [CrossRef]
- Proust, A.; Matt, B.; Villanneau, R.; Guillemot, G.; Gouzerh, P.; Izzet, G. Functionalization and post-functionalization: A step towards polyoxometalate-based materials. Chem. Soc. Rev. 2012, 41, 7605–7622. [Google Scholar] [CrossRef]
- Misra, A.; Kozma, K.; Streb, C.; Nyman, M. Beyond charge balance: Counter-cations in polyoxometalate chemistry. Angew. Chem. Int. Ed. 2020, 59, 596–612. [Google Scholar] [CrossRef]
- Sadakane, M.; Steckhan, E. Electrochemical properties of polyoxometalates as electrocatalysts. Chem. Rev. 1998, 98, 219–237. [Google Scholar] [CrossRef]
- Song, Y.-F.; Long, D.-L.; Ritchie, C.; Cronin, L. Nanoscale Polyoxometalate-Based Inorganic/Organic Hybrids. Chem. Rec. 2011, 11, 158–171. [Google Scholar] [CrossRef]
- Yin, P.; Li, D.; Liu, T. Solution behaviors and self-assembly of polyoxometalates as models of macroions and amphiphilic polyoxometalate-organic hybrids as novel surfactants. Chem. Soc. Rev. 2012, 41, 7368–7383. [Google Scholar] [CrossRef]
- Wu, H.; Yang, H.-K.; Wang, W. Covalently-linked polyoxometalate–polymer hybrids: Optimizing synthesis, appealing structures and prospective applications. New J. Chem. 2016, 40, 886–897. [Google Scholar] [CrossRef]
- Li, B.; Li, W.; Li, H.; Wu, L. Ionic complexes of metal oxide clusters for versatile self-assemblies. Acc. Chem. Res. 2017, 50, 1391–1399. [Google Scholar] [CrossRef]
- Nisar, A.; Wang, X. Surfactant-encapsulated polyoxometalate building blocks: Controlled assembly and their catalytic properties. Dalton Trans. 2012, 41, 9832–9845. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; Soriano-Portillo, A.; Mingotaud, C.; Dominguez-Vera, J.M. Langmuir–Blodgett films based on inorganic molecular complexes with magnetic or optical properties. Adv. Colloid Interface Sci. 2005, 116, 193–203. [Google Scholar] [CrossRef]
- Zhang, G.; Ke, H.; He, T.; Xiao, D.; Chen, Z.; Yang, W.; Yao, J. Synthesis and characterization of new layered polyoxometallates-1,10-decanediamine intercalative nanocomposites. J. Mater. Res. 2004, 19, 496–500. [Google Scholar] [CrossRef]
- Janauer, G.G.; Dobley, A.D.; Zavalij, P.Y.; Whittingham, M.S. Evidence for decavanadate clusters in the lamellar surfactant ion phase. Chem. Mater. 1997, 9, 647–649. [Google Scholar] [CrossRef]
- Spahr, M.E.; Nesper, R. Anhydrous octamolybdate with trimethyl hexadecyl ammonium cations. Z. Anorg. Allg. Chem. 2001, 627, 2133–2138. [Google Scholar] [CrossRef]
- Ito, T.; Sawada, K.; Yamase, T. Crystal structure of bis(dimethyldioctadecylammonium) hexamolybdate: A molecular model of Langmuir-Blodgett films. Chem. Lett. 2003, 32, 938–939. [Google Scholar] [CrossRef]
- Ito, T. Inorganic–organic hybrid surfactant crystals: Structural aspects and functions. Crystals 2016, 6, 24. [Google Scholar] [CrossRef]
- Nyman, M.; Ingersoll, D.; Singh, S.; Bonhomme, F.; Alam, T.M.; Brinker, C.J.; Rodriguez, M.A. Comparative study of inorganic cluster-surfactant arrays. Chem. Mater. 2005, 17, 2885–2895. [Google Scholar] [CrossRef]
- Nyman, M.; Rodriguez, M.A.; Anderson, T.M.; Ingersoll, D. Two structures toward understanding evolution from surfactant-polyoxometalate lamellae to surfactant-encapsulated polyoxometalates. Cryst. Growth Des. 2009, 9, 3590–3597. [Google Scholar] [CrossRef]
- Yin, P.; Wu, P.; Xiao, Z.; Li, D.; Bitterlich, E.; Zhang, J.; Cheng, P.; Vezenov, D.V.; Liu, T.; Wei, Y. A Double-tailed fluorescent surfactant with a hexavanadate cluster as the head group. Angew. Chem. Int. Ed. 2011, 50, 2521–2525. [Google Scholar] [CrossRef] [PubMed]
- Rosnes, M.H.; Musumeci, C.; Yvon, C.; Macdonell, A.; Pradeep, C.P.; Sartorio, C.; Long, D.-L.; Pignataro, B.; Cronin, L. Exploring the interplay between ligand derivatisation and cation type in the assembly of hybrid polyoxometalate Mn-Andersons. Small 2013, 9, 2316–2324. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, X.; Huang, B.; Chen, W.; Xiao, Z.; Wu, P. The crystal packing, morphology and hydrophobicity of polyoxometalate-based amphiphilic materials. CrystEngComm 2020, 22, 2434–2438. [Google Scholar] [CrossRef]
- Rakovský, E.; Zúrková, L.; Marek, J. 1,6-Hexanediammonium dihydrogendecavanadate dihydrate, (H3N-(CH2)6-NH3)2H2V10O28·2H2O. Cryst. Res. Technol. 2001, 36, 339–344. [Google Scholar] [CrossRef]
- Rakovský, E.; Žúrková, L.; Marek, J. Synthesis, Crystal structure, and IR spectroscopic characterization of 1,6-hexanediammnoium dihydrogendecavanadate. Monatsh. Chem. 2002, 133, 277–283. [Google Scholar] [CrossRef]
- Hölscher, M.; Englert, U.; Zibrowius, B.; Hölderich, W.F. (H3N(CH2)6NH3)4[W18P2O62]·3H2O, a microporous solid from dawson anions and 1,6-diaminohexane. Angew. Chem. Int. Ed. Engl. 1994, 33, 2491–2493. [Google Scholar] [CrossRef]
- Gabriel, J.-C.P.; Nagarajan, R.; Natarajan, S.; Cheetham, A.K.; Rao, C.N.R. Hydrothermal synthesis and structure of a mixed valent heteropoly-oxometallate Keggin salt: [PMo4.27W7.73O406−][H3N(CH2)6NH32+]3. J. Solid State Chem. 1997, 129, 257–262. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Wang, E.; Qin, C.; Li, Y.; Qi, Y.; Wang, X. Two extended structures constructed from sandwich-type polyoxometalates functionalized by organic amines. Dalton Trans. 2008, 8, 463–468. [Google Scholar] [CrossRef]
- Pan, X.; Wang, X.; Wang, X.; Liu, G.; Lin, H.; Li, Y. Four Octamolybdate-based complexes based on flexible bis-imidazole-bis-amide ligands with different lengths: Structure, electrochemical and photocatalytic properties. Inorg. Chim. Acta 2019, 495, 118998. [Google Scholar] [CrossRef]
- Kiyota, Y.; Kojima, T.; Kawahara, R.; Taira, M.; Naruke, H.; Kawano, M.; Uchida, S.; Ito, T. Porous layered inorganic−organic hybrid frameworks constructed from polyoxovanadate and bolaamphiphiles. Cryst. Growth Des. 2021, 21, 7230–7239. [Google Scholar] [CrossRef]
- Eda, K.; Iriki, Y. Crystal Engineering with [Mo36O112(H2O)16]8− anion as nanosized building block. Chem. Lett. 2005, 34, 612–613. [Google Scholar] [CrossRef]
- Nelson, J.H.; Johnston, A.R.; Narducci Sarjeant, A.; Norquist, A.J. Composition space analysis of templated molybdates. Solid State Sci. 2007, 9, 472–484. [Google Scholar] [CrossRef]
- Niu, Y.-Y.; Wang, L.-F.; Lv, X.R.; Du, H.-J.; Qiao, Y.-Z.; Wang, H.-M.; Song, L.-S.; Wu, B.-L.; Hou, H.-W.; Ng, S.W. Construction and isomeric transformation of polyoxometalates directed by 1,ω-bis(pyridinium)alkane templates. CrystEngComm 2011, 13, 5071–5081. [Google Scholar] [CrossRef]
- Du, H.-J.; Mi, L.-W.; Yue, Z.-C.; Niu, Y.-Y.; Hou, H.-W. Templated fabrication, isomer recognition of series of 1,10-(alkane-1,ω-diyl)-bis(3-methylimidazolium)-induced polyoxometalates (ω= 1–11). Inorg. Chim. Acta 2014, 409, 418–426. [Google Scholar] [CrossRef]
- Sugeta, M.; Yamase, T. Crystal structure and luminescence site of Na9[EuW10O36]·32H2O. Bull. Chem. Soc. Jpn. 1993, 66, 444–449. [Google Scholar] [CrossRef]
- Rocchiccioli-Deltcheff, C.; Fournier, M.; Franck, R.; Thouvenot, R. Vibrational investigations of polyoxometalates. 2. Evidence for anion-anion interactions in molybdenum(VI) and tungsten(VI) compounds related to the Keggin structure. Inorg. Chem. 1983, 22, 207–216. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; Wiley: New York, NY, USA, 2005; Chapter 2. [Google Scholar]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: New York, NY, USA, 1999; pp. 12–16. [Google Scholar]
- Misawa, T.; Kobayashi, J.; Kiyota, Y.; Watanabe, M.; Ono, S.; Okamura, Y.; Koguchi, S.; Higuchi, M.; Nagase, Y.; Ito, T. Dimensional control in polyoxometalate crystals hybridized with amphiphilic polymerizable ionic liquids. Materials 2019, 12, 2283. [Google Scholar] [CrossRef]
- David, W.I.F.; Shankland, K.; van de Streek, J.; Pidcock, E.; Motherwell, W.D.S.; Cole, J.C. DASH: A program for crystal structure determination from powder diffraction data. J. Appl. Crystallogr. 2006, 39, 910–915. [Google Scholar] [CrossRef]
- PROCESS-AUTO; Rigaku Corporation: Tokyo, Japan, 2002.
- CrysAlisPro; Rigaku Oxford Diffraction: Tokyo, Japan, 2015.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]

| Compound | C12N2-H2W12 |
|---|---|
| Chemical formula | C36H92N6W12O44 |
| Formula weight | 3519.35 |
| Crystal system | trigonal |
| Space group | P3 (No. 147) |
| a (Å) | 18.3644 (4) |
| b (Å) | 18.3644 (4) |
| c (Å) | 12.7069 (3) |
| α (°) | 90.000 |
| β (°) | 90.000 |
| γ (°) | 120.000 |
| V (Å3) | 3711.28 (17) |
| Z | 2 |
| ρcalcd (g cm−3) | 3.149 |
| T (K) | 296 (2) |
| Wavelength (Å) | 0.71075 |
| μ (mm−1) | 18.604 |
| No. of reflections measured | 60,648 |
| No. of independent reflections | 5667 |
| Rint | 0.0619 |
| No. of parameters | 301 |
| R1 (I > 2σ(I)) | 0.0227 |
| wR2 (all data) | 0.0501 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikuma, H.; Aoki, S.; Kawahara, K.; Ono, S.; Iwamatsu, H.; Kobayashi, J.; Kiyota, Y.; Okamura, Y.; Higuchi, M.; Ito, T. An Inorganic–Organic Hybrid Framework Composed of Polyoxotungstate and Long-Chained Bolaamphiphile. Int. J. Mol. Sci. 2023, 24, 2824. https://doi.org/10.3390/ijms24032824
Ikuma H, Aoki S, Kawahara K, Ono S, Iwamatsu H, Kobayashi J, Kiyota Y, Okamura Y, Higuchi M, Ito T. An Inorganic–Organic Hybrid Framework Composed of Polyoxotungstate and Long-Chained Bolaamphiphile. International Journal of Molecular Sciences. 2023; 24(3):2824. https://doi.org/10.3390/ijms24032824
Chicago/Turabian StyleIkuma, Haruka, Shunsuke Aoki, Kai Kawahara, Seiji Ono, Hironori Iwamatsu, Jun Kobayashi, Yoshiki Kiyota, Yosuke Okamura, Masashi Higuchi, and Takeru Ito. 2023. "An Inorganic–Organic Hybrid Framework Composed of Polyoxotungstate and Long-Chained Bolaamphiphile" International Journal of Molecular Sciences 24, no. 3: 2824. https://doi.org/10.3390/ijms24032824
APA StyleIkuma, H., Aoki, S., Kawahara, K., Ono, S., Iwamatsu, H., Kobayashi, J., Kiyota, Y., Okamura, Y., Higuchi, M., & Ito, T. (2023). An Inorganic–Organic Hybrid Framework Composed of Polyoxotungstate and Long-Chained Bolaamphiphile. International Journal of Molecular Sciences, 24(3), 2824. https://doi.org/10.3390/ijms24032824






