Insights into Cell-Specific Functions of Microtubules in Skeletal Muscle Development and Homeostasis
Abstract
:1. Introduction
2. Microtubule Arrangement on Skeletal Muscle Cells
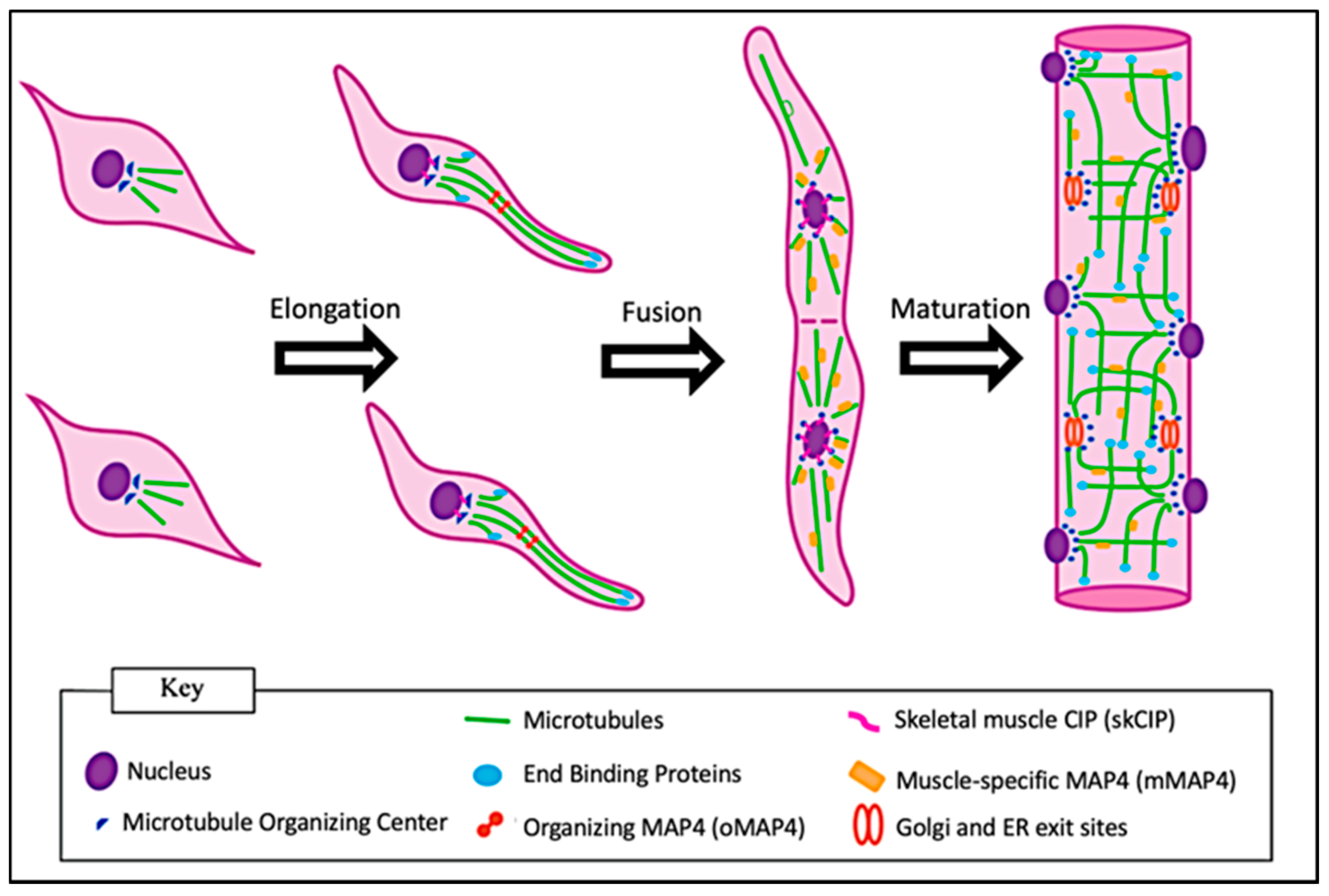
3. Microtubule Functions during Myoblast Differentiation
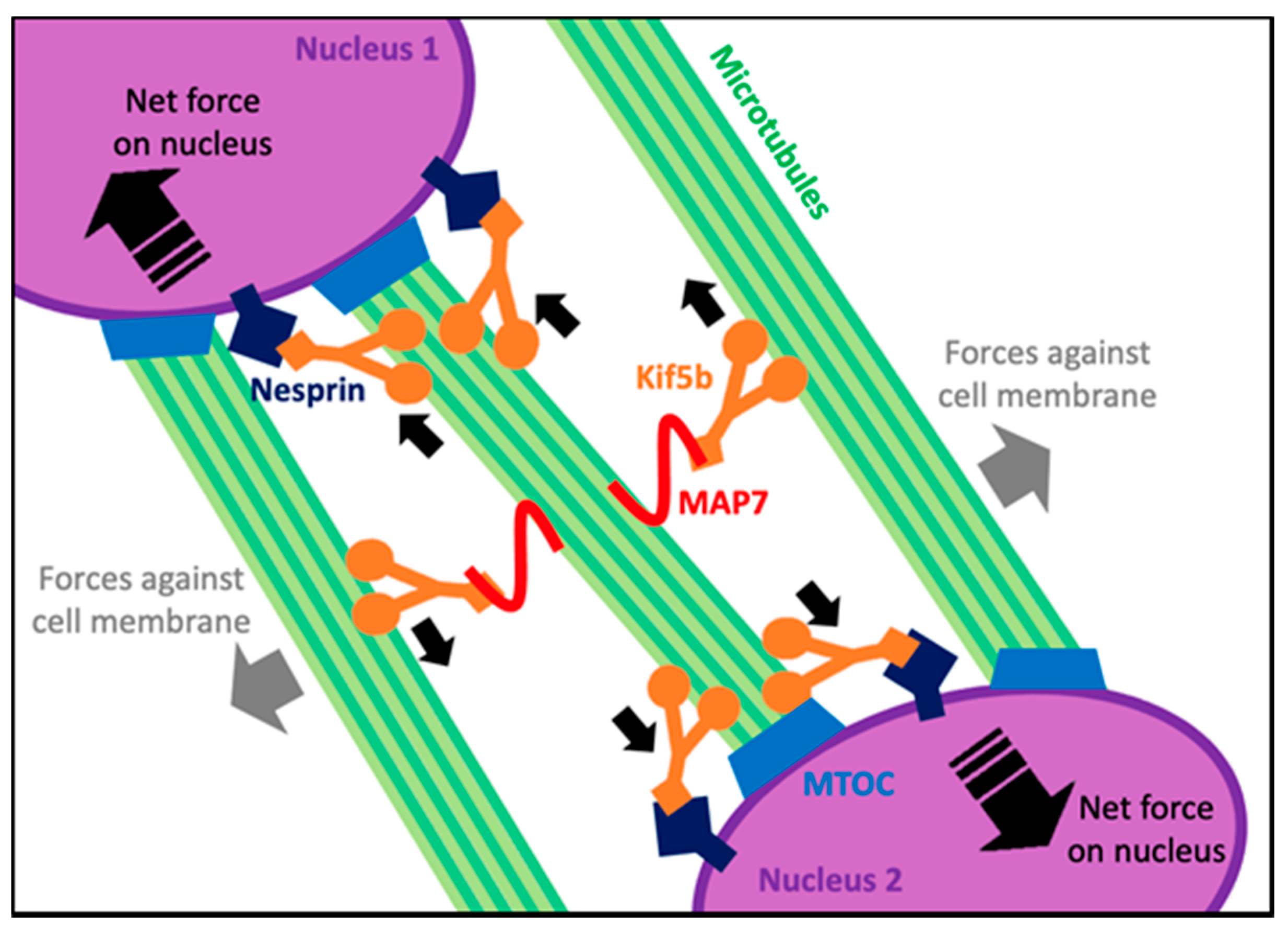
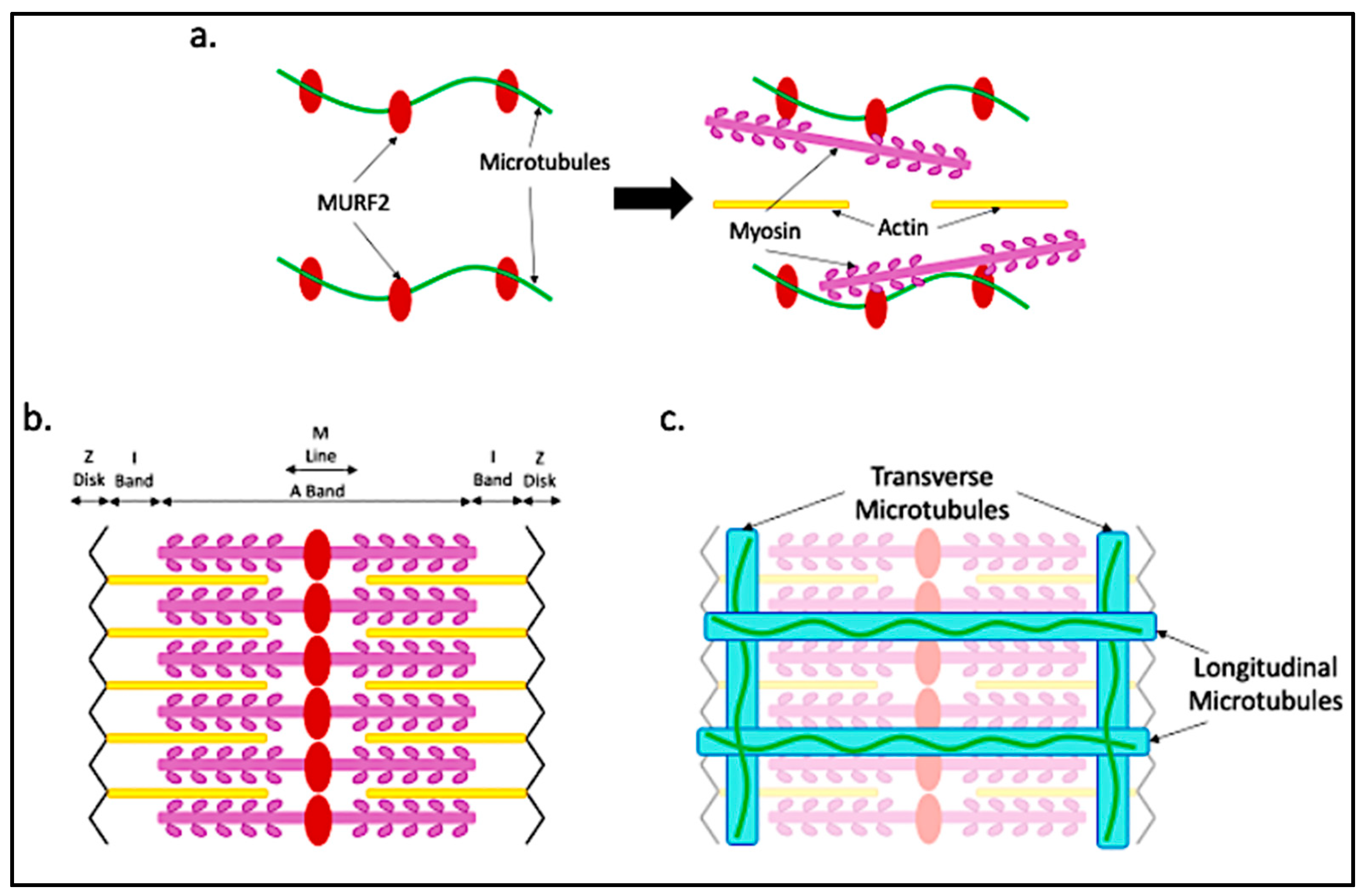
4. Microtubule Functions in Maturing Myofibers
5. Microtubule Form and Function in Adult Myofibers
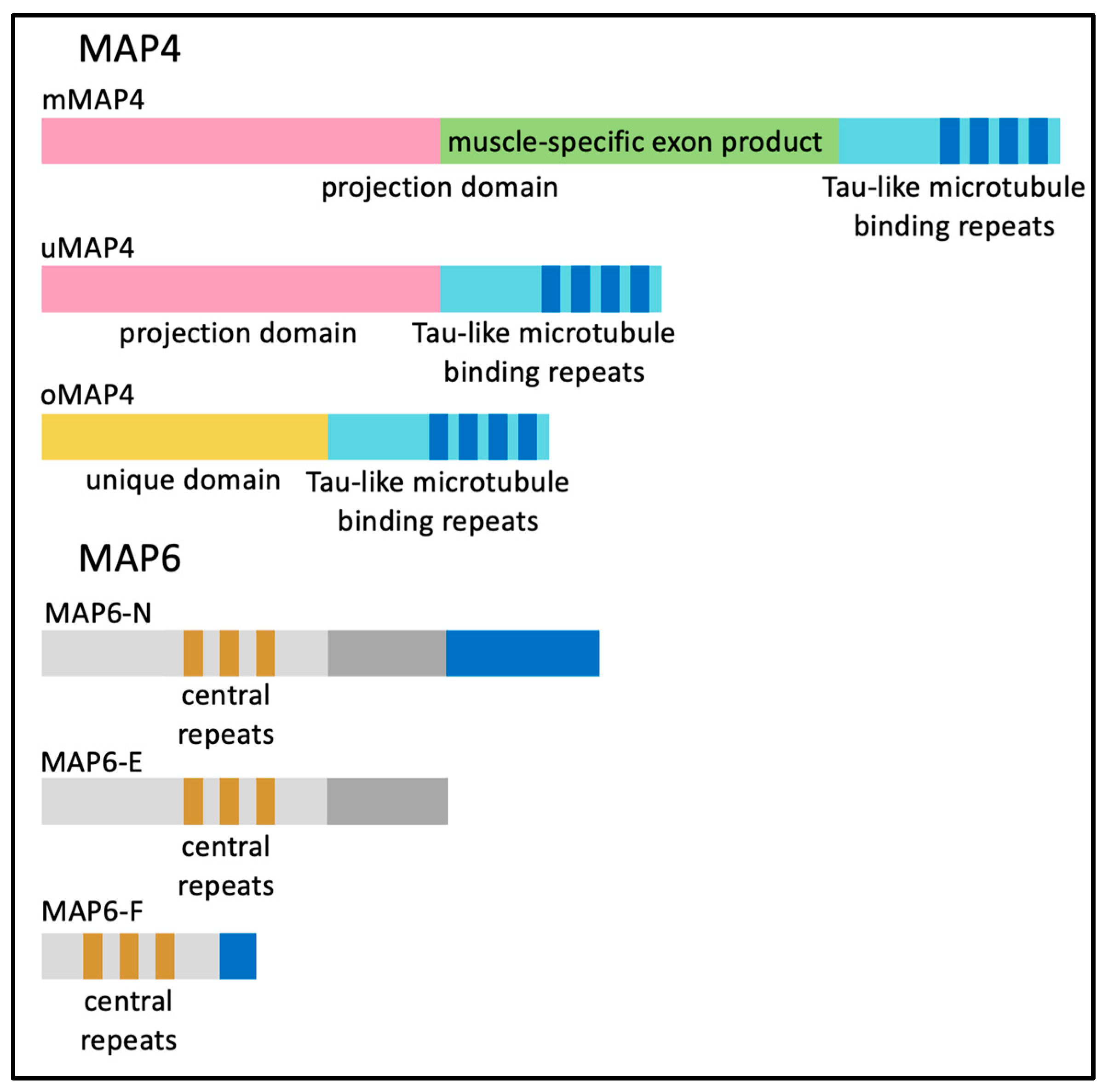
6. Microtubule-Associated Proteins (MAPs) and End-Binding (EB) Proteins in Skeletal Muscle Myofibers
7. Diseases Related to Skeletal Muscle Microtubule Abnormalities
| Gene Name | Protein Name | Disease Defective in: | Role in Relation to Microtubules | Possible Disease Pathogenesis * |
|---|---|---|---|---|
| DNM2 | dynamin 2 | Centronuclear Myopathy (CNM) | Coordinates centrosome localization [70] | Improper microtubule function in myoblasts and/or myofibers |
| DMD | dystrophin | Duchenne Muscular Dystrophy (DMD) | Organizes microtubules within myofibers [46] | Improper alignment of microtubules weakens and subjects myofibers to damage |
| RAC1 | Ras-related C3 botulinum toxin substrate 1 | Duchenne Muscular Dystrophy (DMD) | Activates X-ROS signaling pathway during microtubule stretching [73] | Increased ROS production as a result of dense microtubule structure damages myofibers |
| TUBB6 | Tubulin Beta Class V | Duchenne Muscular Dystrophy (DMD) | Β-tubulin isoform typically expressed during myoblast differentiation [75] | Tubb6 upregulation in DMD myofibers results in microtubule disorganization |
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Description |
| ATP | adenosine triphosphate |
| C2C12 | mouse myoblast cell line |
| CNM | centronuclear myopathies |
| COPII | coat protein complex II |
| Cryo-EM | cryo-electron microscopy |
| DAPC | dystrophin-associated protein complex |
| DMD | Duchenne muscular dystrophy |
| DNM2 | dynamin 2 |
| Drp1 | dynamin-related protein 1 |
| EB | end-binding protein |
| EB1 | end-binding protein 1 |
| EB2 | end-binding protein 2 |
| EB3 | end-binding protein 3 |
| ER | endoplasmic reticulum |
| FCM | fusion competent myoblast |
| FDB | flexor digitorum brevis |
| GFP-EB3 | green fluorecent protein—end-binding protein 3 |
| Kif5b | kinesin-1 heavy chain |
| KO | knock-out |
| L6 | rat myoblast cell line |
| LEWD | lysine, glutamic acid, an acidic amino acid, aspartic acid |
| LKB1 | liver kinase B1 |
| MAP4 | microtubule-associated protein 4 |
| MAP6 | microtubule-associated protein 6 |
| MAP6-E | MAP6—embryonic |
| MAP6-F | MAP6—fibroblast |
| MAP6-N | MAP6—neuronal |
| MAP7 | microtubule-associated protein 7 |
| MAPs | microtubule-associated proteins |
| mdx | dystrophin-depleted mouse model of DMD |
| mMAP4 | muscle-specific MAP4 isoform |
| mRNA | messenger ribose nucleic acid |
| MTOC | microtubule-organizing center |
| MURF2 | Muscle RING-finger 2 |
| NOX-2 | NADPH oxidase-2 |
| oMAP4 | organizing MAP4 isoform |
| Par6β | partitioning defective 6 homolog beta |
| PCM | pericentriolar matrix |
| PCM1 | pericentriolar material 1 |
| Rac1 | ras-related C3 botulinum toxin substrate 1 |
| ROS | reactive oxygen species |
| skCIP | skeletal muscle-specific cardiac islet-1 interaction protein |
| SUN | Sad1 and UNC-84 |
| Tubb6 | beta-tubulin beta 6 class V |
| uMAP4 | ubiquitously expressed MAP4 isoform |
References
- Oddoux, S.; Zaal, K.; Tate, V.; Kenea, A.; Nandkeolyar, S.; Reid, E.; Liu, W.; Ralston, E. Microtubules that form the stationary lattice of muscle fibers are dynamic and nucleated at Golgi elements. J. Cell Biol. 2013, 203, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Starr, D.A. Muscle Development: Nucleating Microtubules at the Nuclear Envelope. Curr. Biol. 2017, 27, R1071–R1073. [Google Scholar] [CrossRef] [PubMed]
- Kollman, J.M.; Merdes, A.; Mourey, L.; Agard, D.A. Microtubule nucleation by γ-tubulin complexes. Nat. Rev. Mol. Cell Biol. 2011, 12, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Teixido-Travesa, N.; Roig, J.; Luders, J. The where, when and how of microtubule nucleation—One ring to rule them all. J. Cell Sci. 2012, 125, 4445–4456. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.C.; Neuner, A.; Schiebel, E. Targeting of γ-tubulin complexes to microtubule organizing centers: Conservation and divergence. Trends Cell Biol. 2015, 25, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.D.; Feldman, J.L. Microtubule-organizing centers: From the centrosome to non-centrosomal sites. Curr. Opin. Cell Biol. 2017, 44, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, J.B.; Wueseke, O.; Hyman, A. Pericentriolar material structure and dynamics. Phil. Trans. R. Soc. B 2014, 369, 20130459. [Google Scholar] [CrossRef]
- Bannykh, S.I.; Rowe, T.; Balch, W. The organization of endoplasmic reticulum export complexes. J. Cell Biol. 1996, 135, 19–35. [Google Scholar] [CrossRef]
- Lu, Z.; Joseph, D.; Bugnard, E.; Zaal, K.J.M.; Ralston, E. Golgi Complex Reorganization during Muscle Differentiation: Visualization in Living Cells and Mechanism. mBoC 2001, 12, 795–808. [Google Scholar] [CrossRef]
- Rahkila, P.; Väänänen, K.; Saraste, J.; Metsikkö, K. Endoplasmic Reticulum to Golgi Trafficking in Multinucleated Skeletal Muscle Fibers. Exp. Cell Res. 1997, 234, 452–464. [Google Scholar] [CrossRef]
- Srsen, V.; Fant, X.; Heald, R.; Rabouille, C.; Merdes, A. Centrosome proteins form an insoluble perinuclear matrix during muscle cell differentiation. BMC Cell Biol. 2009, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Tassin, A.M.; Maro, B.; Bornens, M. Fate of microtubule-organizing centers during myogenesis in vitro. J. Cell BioL. 1985, 100, 35–46. [Google Scholar] [CrossRef]
- Becker, R.; Leone, M.; Engel, F.B. Microtubule Organization in Striated Muscle Cells. MDPI Cells 2020, 9, 1395. [Google Scholar] [CrossRef] [PubMed]
- Bugnard, E.; Zaal, K.J.M.; Ralston, E. Reorganization of microtubule nucleation during muscle differentiation. Cell Motil. Cytoskelet. 2005, 60, 1–13. [Google Scholar] [CrossRef]
- Nadkarni, A.V.; Heald, R. Reconstitution of muscle cell microtubule organization in vitro. Cytoskeleton (Hoboken) 2021, 78, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, Z.; Nie, M.; Wang, G.; Silva, W.; Yang, O.; Freire, P.; Hu, X.; Chen, H.; Deng, Z.; et al. Regulation of myonuclear positioning and muscle function by the skeletal muscle-specific CIP protein. Proc. Natl. Acad. Sci. USA 2020, 117, 19254–19265. [Google Scholar] [CrossRef]
- Mian, I.; Pierre-Louis, W.S.; Dole, N.; Gilberti, R.M.; Dodge-Kafka, K. LKB1 Destabilizes Microtubules in Myoblasts and Contributes to Myoblast Differentiation. PLoS ONE 2012, 7, e31583. [Google Scholar] [CrossRef] [PubMed]
- Warren, R.H. Microtubular organization in elongating myogenic cells. J. Cell Biol. 1974, 63, 550–566. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.; Dunn, G.; Knibbs, A.; Peckham, M. Alignment of myoblasts on ultrafine gratings inhibits fusion in vitro. Int. J. Biochem. Cell Biol. 2002, 34, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jin, P.; Duan, R.; Chen, E. Mechanisms of myoblast fusion during muscle development. 2015 Curr. Opin. Genet. Dev. 2015, 32, 162–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brukman, N.; Uygur, B.; Podbilewicz, B.; Chernomordik, L. How cells fuse. J. Cell Biol. 2019, 218, 1436–1451. [Google Scholar] [CrossRef] [PubMed]
- Geuens, G.; Gundersen, G.; Nuydens, R.; Cornelissen, F.; Bulinski, J.; DeBrabander, M. Ultrastructural colocalization of tyrosinated and detyrosinated alpha-tubulin in interphase and mitotic cells. J. Cell Biol. 1986, 103, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, G.; Bulinski, J. Microtubule arrays in differentiated cells contain elevated levels of a post-translationally modified form of tubulin. Eur. J. Cell Biol. 1986, 42, 288–294. [Google Scholar]
- Gundersen, G.G.; Khawaja, S.; Bulinski, J. Generation of a stable, posttranslationally modified microtubule array is an early event in myogenic differentiation. J. Cell Biol. 1989, 109, 2275–2288. [Google Scholar] [CrossRef]
- Mangan, M.E.; Olmsted, J.B. A muscle-specific variant of microtubule-associated protein 4 (MAP4) is required in myogenesis. Development 1996, 122, 771–781. [Google Scholar] [CrossRef]
- Saitoh, O.; Arai, T.; Obinata, T. Distribution of microtubules and other cytoskeletal filaments during myotube elongation as revealed by fluorescence microscopy. Cell Tissue Res. 1988, 252, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Cadot, B.; Gache, V.; Vasyutina, E.; Falcone, S.; Birchmeier, C.; Gomes, E. Nuclear movement during myotube formation is microtubule and dynein dependent and is regulated by Cdc42, Par6 and Par3. EMBO 2012, 13, 741–749. [Google Scholar] [CrossRef]
- Burakov, A.; Nadezhdina, E.; Slepchenko, B.; Rodionov, V. Centrosome positioning in interphase cells. J. Cell Biol. 2003, 162, 963–969. [Google Scholar] [CrossRef]
- Gönczy, P.; Pichler, S.; Kirkham, M.; Hyman, A. Cytoplasmic Dynein Is Required for Distinct Aspects of Mtoc Positioning, Including Centrosome Separation, in the One Cell Stage Caenorhabditis elegans Embryo. J. Cell Biol. 1999, 147, 135–150. [Google Scholar] [CrossRef]
- Metzger, T.; Gache, V.; Xu, M.; Cadot, B.; Folker, E.; Richardson, B.; Gomes, E.; Baylies, M. MAP and kinesin-dependent nuclear positioning is required for skeletal muscle function. Nature 2012, 484, 120–124. [Google Scholar] [CrossRef]
- Wilson, M.H.; Holzbaur, E.L.F. Opposing microtubule motors drive robust nuclear dynamics in developing muscle cells. J. Cell Sci. 2012, 125, 4158–4169. [Google Scholar] [CrossRef] [Green Version]
- Wilson, M.H.; Holzbaur, E.L.F. Nesprins anchor kinesin-1 motors to the nucleus to drive nuclear distribution in muscle cells. Development 2015, 142, 218–228. [Google Scholar] [CrossRef]
- Manhart, A.; Windner, S.; Baylies, M.; Mogilner, A. Mechanical positioning of multiple nuclei in muscle cells. PLoS Comput. Biol. 2018, 14, e1006208. [Google Scholar] [CrossRef] [PubMed]
- Roman, W.; Martins, J.; Carvalho, F.; Voituriez, R.; Abella, J.; Santos, N.; Cadot, B.; Way, M.; Gomes, E. Myofibril contraction and crosslinking drive nuclear movement to the periphery of skeletal muscle. Nat. Cell Biol. 2017, 19, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Bruusgaard, J.C.; Liestøl, K.; Gundersen, K. Distribution of myonuclei and microtubules in live muscle fibers of young, middle-aged, and old mice. J. Appl. Physiol. 2006, 100, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- Shaw, N.M.; Rios-Monterrosa, J.L.; Fedorchak, G.R.; Ketterer, M.R.; Coombs, G.S.; Lammerding, J.; Wallrath, L.L. Effects of mutant lamins on nucleo-cytoskeletal coupling in Drosophilamodels of LMNA muscular dystrophy. Front Cell Dev. Biol. 2022, 10, 934586. [Google Scholar] [CrossRef]
- Earle, A.J.; Kirby, T.J.; Fedorchak, G.R.; Isermann, P.; Patel, J.; Iruvanti, S.; Moore, S.A.; Bonne, G.; Wallrath, L.L.; Lammerding, J. Mutant lamins cause nuclear envelope rupture and DNA damage in skeletal muscle cells. Nat. Mater. 2020, 19, 464–473. [Google Scholar] [CrossRef]
- Dhanyasi, N.; VijayRaghavan, K.; Shilo, B.; Schejter, E. Microtubules provide guidance cues for myofibril and sarcomere assembly and growth. Dev. Dyn. 2021, 250, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Trinick, J. Cytoskeleton: Titin as a scaffold and spring. Curr. Biol. 1996, 6, 258–260. [Google Scholar] [CrossRef]
- Pizon, V. Transient association of titin and myosin with microtubules in nascent myofibrils directed by the MURF2 RING-finger protein. J. Cell Sci. 2002, 115, 4469–4482. [Google Scholar] [CrossRef]
- Pizon, V.; Gerbal, F.; Diaz, C.; Karsenti, E. Microtubule-dependent transport and organization of sarcomeric myosin during skeletal muscle differentiation. EMBO J. 2005, 24, 3781–3792. [Google Scholar] [CrossRef] [PubMed]
- Ralston, E.; Lu, Z.; Ploug, T. The Organization of the Golgi Complex and Microtubules in Skeletal Muscle Is Fiber Type-Dependent. J. Neurosci. 1999, 19, 10694–10705. [Google Scholar] [CrossRef]
- Ralston, E.; Ploug, T.; Kalhovde, J.; Lømo, T. Golgi Complex, Endoplasmic Reticulum Exit Sites, and Microtubules in Skeletal Muscle Fibers Are Organized by Patterned Activity. J. Neurosci. 2001, 21, 875–883. [Google Scholar] [CrossRef]
- Barlowe, C. COPII and selective export from the endoplasmic reticulum. Biochim. Et Biophys. Acta (BBA)–-Mol. Cell Res. 1998, 1404, 67–76. [Google Scholar] [CrossRef]
- Demonbreun, A.R.; McNally, E.M. DNA Electroporation, Isolation and Imaging of Myofibers. J. Vis. Exp. 2015, 106, e53551. [Google Scholar]
- Prins, K.W.; Humston, J.; Mehta, A.; Tate, V.; Ralston, E.; Ervasti, J. Dystrophin is a microtubule-associated protein. J. Cell Biol. 2009, 186, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Hood, D.A. Cytoskeletal regulation of mitochondrial movements in myoblasts. Cytoskeleton (Hoboken) 2014, 71, 564–572. [Google Scholar] [CrossRef]
- Anesti, V.; Scorrano, L. The relationship between mitochondrial shape and function and the cytoskeleton. Biochim. Biophys. Acta. 2006, 1757, 692–699. [Google Scholar] [CrossRef]
- Milner, D.J.; Mavroidis, M.; Weisleder, N.; Capetanaki, Y. Desmin cytoskeleton linked to muscle mitochondrial distribution and respiratory function. J. Cell Biol. 2000, 150, 1283–1298. [Google Scholar] [CrossRef] [PubMed]
- Dalpe, G.; Mathieu, M.; Comtois, A.; Zhu, E.; Wasiak, S.; De Repen-tigny, Y. Dystonin-deficient mice exhibit an intrinsic muscle weakness and an instability of skeletal muscle cytoarchitecture. Dev. Biol. 1999, 210, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Winter, L.; Kuznetsov, A.V.; Grimm, M.; Zeold, A.; Fischer, I.; Wiche, G. Plectin isoform P1b and P1d deficiencies differentially affect mitochondrial morphology and function in skeletal muscle. Hum. Mol. Genet. 2015, 24, 4530–4544. [Google Scholar] [CrossRef] [PubMed]
- Giovarelli, M.; Zecchini, S.; Martini, E.; Garrè, M.; Barozzi, S.; Ripolone, M.; Napoli, L.; Coazzoli, M.; Vantaggiato, C.; Roux-Biejat, P.; et al. Drp1 overexpression induces desmin disassembling and drives kinesin-1 activation promoting mitochondrial trafficking in skeletal muscle. Cell Death Differ. 2020, 27, 2383–2401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, H.; Pimentel, M.R.; Sequeira, C.; Oliveira, L.M.; Pezzarossa, A.; Roman, W.; Gomes, E.R. mRNA distribution in skeletal muscle is associated with mRNA size. J. Cell Sci. 2021, 15, jcs256388. [Google Scholar] [CrossRef] [PubMed]
- Denes, L.T.; Kelley, C.P.; Wang, E.T. Microtubule-based transport is essential to distribute RNA and nascent protein in skeletal muscle. Nat, Commun. 2021, 27, 6079. [Google Scholar] [CrossRef]
- Shigematsu, H.; Imasaki, T.; Doki, C.; Sumi, T.; Aoki, M.; Uchikubo-Kamo, T.; Sakamoto, A.; Tokuraku, K.; Shirouzu, M.; Nitta, R. Structural insight into microtubule stabilization and kinesin inhibition by Tau family MAPs. J. Cell Biol. 2018, 217, 4155–4163. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, H.; Emori, Y.; Mori, A.; Murofushi, H.; Sakai, H.; Suzuki, K. Functional analyses of the domain structure of microtubule-associated protein-4 (MAP-U). J. Biol. Chem. 1991, 266, 9841–9846. [Google Scholar] [CrossRef] [PubMed]
- West, R.R.; Tenbarge, K.M.; Olmsted, J.B. A model for microtubule-associated protein 4 structure. Domains defined by comparisons of human, mouse, and bovine sequences. J. Biol. Chem. 1991, 266, 21886–21896. [Google Scholar] [CrossRef]
- Mogessie, B.; Roth, D.; Rahil, Z.; Straube, A. A novel isoform of MAP4 organises the paraxial microtubule array required for muscle cell differentiation. eLife 2015, 4, e05697. [Google Scholar] [CrossRef]
- Baratier, J.; Peris, L.; Brocard, J.; Gory-Fauré, S.; Dufour, F.; Bosc, C.; Fourest-Lieuvin, A.; Blanchoin, L.; Salin, P.; Job, D.; et al. Phosphorylation of Microtubule-associated Protein STOP by Calmodulin Kinase II. J. Biol. Chem. 2006, 281, 19561–19569. [Google Scholar] [CrossRef]
- Deloulme, J.-C.; Gory-Fauré, S.; Mauconduit, F.; Chauvet, S.; Jonckheere, J.; Boulan, B.; Mire, E.; Xue, J.; Jany, M.; Maucler, C.; et al. Microtubule-associated protein 6 mediates neuronal connectivity through Semaphorin 3E-dependent signalling for axonal growth. Nat. Commun. 2015, 6, 7246. [Google Scholar] [CrossRef]
- Sébastien, M.; Giannesini, B.; Aubin, P.; Brocard, J.; Chivet, M.; Pietrangelo, L.; Boncompagni, S.; Bosc, C.; Brocard, J.; Rendu, J.; et al. Deletion of the microtubule-associated protein 6 (MAP6) results in skeletal muscle dysfunction. Skelet. Muscle 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.; Fitton, B.; Chmel, N.; Wasiluk, N.; Straube, A. Spatial positioning of EB family proteins at microtubule tips involves distinct nucleotide-dependent binding properties. J. Cell Sci. 2019, 132, jcs219550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, L.-K.; Qi, Y. Characterization of Human MAPRE Genes and Their Proteins. Genomics 2001, 71, 142–149. [Google Scholar] [CrossRef]
- Straube, A.; Merdes, A. EB3 Regulates Microtubule Dynamics at the Cell Cortex and Is Required for Myoblast Elongation and Fusion. Curr. Biol. 2007, 17, 1318–1325. [Google Scholar] [CrossRef]
- Zhang, T.; Zaal, K.J.; Sheridan, J.; Mehta, A.; Gundersen, G.G.; Ralston, E. Microtubule plus-end binding protein EB1 is necessary for muscle cell differentiation, elongation and fusion. J. Cell Sci. 2009, 122, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Jungbluth, H.; Wallgren-Pettersson, C.; Laporte, J. Centronuclear (myotubular) myopathy. Orphanet J. Rare Dis. 2008, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Takei, K. Dynamic instability of microtubules requires dynamin 2 and is impaired in a Charcot-Marie-Tooth mutant. J. Cell Biol. 2009, 185, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Warnock, D.E.; Baba, T.; Schmid, S. Ubiquitously Expressed Dynamin-II Has a Higher Intrinsic GTPase Activity and a Greater Propensity for Self-assembly Than Neuronal Dynamin-I. mBoC 1997, 8, 2553–2562. [Google Scholar] [CrossRef]
- Bitoun, M.; Maugenre, S.; Jeannet, P.-Y.; Lacène, E.; Ferrer, X.; Laforêt, P.; Martin, J.-J.; Laporte, J.; Lochmüller, H.; Beggs, A.H.; et al. Mutations in dynamin 2 cause dominant centronuclear myopathy. Nat. Genet. 2005, 37, 1207–1209. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.M.; Cao, H.; Chen, J.; Euteneuer, U.; McNiven, M.A. Dynamin 2 binds γ-tubulin and participates in centrosome cohesion. Nat. Cell Biol. 2004, 6, 335–342. [Google Scholar] [CrossRef]
- Hoffman, E.P.; Brown, R.H.; Kunkel, L.M. Dystrophin: The protein product of the duchennee muscular dystrophy locus. Cell 1987, 51, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.J.; Weir, A.; Newey, S.; Davies, K.E. Function and Genetics of Dystrophin and Dystrophin-Related Proteins in Muscle. Physiol. Rev. 2002, 82, 291–329. [Google Scholar] [CrossRef] [Green Version]
- Khairallah, R.J.; Shi, G.; Sbrana, F.; Prosser, B.; Borroto, C.; Mazaitis, M.; Hoffman, E.; Mahurkar, A.; Sachs, F.; Sun, Y.; et al. Microtubules Underlie Dysfunction in Duchenne Muscular Dystrophy. Sci. Signal. 2012, 5, ra56. [Google Scholar] [CrossRef] [PubMed]
- Percival, J.M.; Gregorevic, P.; Odom, G.L.; Banks, G.B.; Chamberlain, J.S.; Froehner, S.C. rAAV6-Microdystrophin Rescues Aberrant Golgi Complex Organization in mdx Skeletal Muscles. Traffic 2007, 8, 1424–1439. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, D.; Khalique, U.; Belanto, J.; Kenea, A.; Talsness, D.; Olthoff, J.; Tran, M.; Zaal, K.; Pak, K.; Pinal-Fernandez, I.; et al. Persistent upregulation of the β-tubulin tubb6, linked to muscle regeneration, is a source of microtubule disorganization in dystrophic muscle. Hum. Mol. Genet. 2019, 28, 1117–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucas, L.; Cooper, T.A. Insights into Cell-Specific Functions of Microtubules in Skeletal Muscle Development and Homeostasis. Int. J. Mol. Sci. 2023, 24, 2903. https://doi.org/10.3390/ijms24032903
Lucas L, Cooper TA. Insights into Cell-Specific Functions of Microtubules in Skeletal Muscle Development and Homeostasis. International Journal of Molecular Sciences. 2023; 24(3):2903. https://doi.org/10.3390/ijms24032903
Chicago/Turabian StyleLucas, Lathan, and Thomas A. Cooper. 2023. "Insights into Cell-Specific Functions of Microtubules in Skeletal Muscle Development and Homeostasis" International Journal of Molecular Sciences 24, no. 3: 2903. https://doi.org/10.3390/ijms24032903
APA StyleLucas, L., & Cooper, T. A. (2023). Insights into Cell-Specific Functions of Microtubules in Skeletal Muscle Development and Homeostasis. International Journal of Molecular Sciences, 24(3), 2903. https://doi.org/10.3390/ijms24032903





