What Are the Neurotoxins in Hemotoxic Snake Venoms?
Abstract
:1. Introduction
2. Neurological Signs Produced by Viperid Bites
3. Secretory Phospholipases A2
3.1. Presynaptic Neurotoxicity
3.2. Postsynaptic Neurotoxicity
3.3. sPLA2s in Pain and Analgesia
4. Three-Finger Toxins
5. Other Components of Viperid Venoms with Postsynaptic Neurotoxicity
5.1. Waglerins
5.2. Azemiopsin
5.3. Baptides
5.4. C-Type Lectin-like Proteins (CTLPs)
6. Blockers of Voltage-Dependent Ion Channels
6.1. Cysteine-Rich Secretory Proteins
6.2. Kunitz-Type Protease Inhibitors
6.3. Crotamine
7. Sarafotoxins
8. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lock, B.A.; Wellehan, J. Chapter 8—Ophidia (Snakes). In Fowler’s Zoo and Wild Animal Medicine; Miller, R.E., Fowler, M.E., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2015; Volume 8, pp. 60–74. [Google Scholar]
- Wallach, V.; Williams, K.L.; Boundy, J. Snakes of the World: A Catalogue of Living and Extinct Species, 1st ed.; CRC Press: Boca Raton, FL, USA, 2014; p. IX. [Google Scholar]
- Wikipedia. List of Dangerous Snakes. Available online: https://en.wikipedia.org/wiki/List_of_dangerous_snakes (accessed on 10 January 2023).
- World Health Organization (WHO). Snakebite Information and Data Platform. 2020. Available online: https://www.who.int/teams/control-of-neglected-tropical-diseases/snakebite-envenoming/snakebite-information-and-data-platform/overview#tab=tab_1,2020 (accessed on 10 January 2023).
- Casewell, N.R.; Jackson, T.N.W.; Laustsen, A.H.; Sunagar, K. Causes and consequences of snake venom variation. Trends Pharmacol. Sci. 2020, 41, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Barlow, A.; Pook, C.E.; Harrison, R.A.; Wüster, W. Coevolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proc. Biol. Sci. 2009, 276, 2443–2449. [Google Scholar] [CrossRef] [PubMed]
- Ranawaka, U.K.; Lalloo, D.G.; de Silva, H.J. Neurotoxicity in snakebite—The limits of our knowledge. PLoS Negl. Trop. Dis. 2013, 7, e2302. [Google Scholar] [CrossRef]
- Averin, A.S.; Utkin, Y.N. Cardiovascular effects of snake toxins: Cardiotoxicity and cardioprotection. Acta Nat. 2021, 13, 4–14. [Google Scholar] [CrossRef]
- Modahl, C.M.; Mackessy, S.P. Venoms of rear-fanged snakes: New proteins and novel activities. Front. Ecol. Evol. 2019, 7, 279. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Gutiérrez, J.M.; Lohse, B.; Rasmussen, A.R.; Fernández, J.; Milbo, C.; Lomonte, B. Snake venomics of monocled cobra (Naja kaouthia) and investigation of human IgG response against venom toxins. Toxicon 2015, 99, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Palasuberniam, P.; Chan, Y.W.; Tan, K.Y.; Tan, C.H. Snake venom proteomics of Samar cobra (Naja samarensis) from the Southern Philippines: Short alpha-neurotoxins as the dominant lethal component weakly cross-neutralized by the Philippine cobra antivenom. Front. Pharmacol. 2021, 12, 727756. [Google Scholar] [CrossRef]
- Patra, A.; Chanda, A.; Mukherjee, A.K. Quantitative proteomic analysis of venom from Southern India common krait (Bungarus caeruleus) and identification of poorly immunogenic toxins by immune-profiling against commercial antivenom. Expert Rev. Proteom. 2019, 16, 457–469. [Google Scholar] [CrossRef]
- Ziganshin, R.H.; Kovalchuk, S.I.; Arapidi, G.P.; Starkov, V.G.; Hoang, A.N.; Thi Nguyen, T.T.; Nguyen, K.C.; Shoibonov, B.B.; Tsetlin, V.I.; Utkin, Y.N. Quantitative proteomic analysis of Vietnamese krait venoms: Neurotoxins are the major components in Bungarus multicinctus and phospholipases A2 in Bungarus fasciatus. Toxicon 2015, 107, 197–209. [Google Scholar] [CrossRef]
- Harvey, A.L.; Robertson, B. Dendrotoxins: Structure-activity relationships and effects on potassium ion channels. Curr. Med. Chem. 2004, 11, 3065–3072. [Google Scholar] [CrossRef]
- Nirthanan, S. Snake three-finger α-neurotoxins and nicotinic acetylcholine receptors: Molecules, mechanisms and medicine. Biochem Pharmacol. 2020, 181, 114168. [Google Scholar] [CrossRef]
- Dingwoke, E.J.; Adamude, F.A.; Mohamed, G.; Klein, A.; Salihu, A.; Abubakar, M.S.; Sallau, A.B. Venom proteomic analysis of medically important Nigerian viper Echis ocellatus and Bitis arietans snake species. Biochem. Biophys. Rep. 2021, 28, 101164. [Google Scholar] [CrossRef]
- Nguyen, G.T.T.; O’Brien, C.; Wouters, Y.; Seneci, L.; Gallissà-Calzado, A.; Campos-Pinto, I.; Ahmadi, S.; Laustsen, A.H.; Ljungars, A. High-throughput proteomics and in vitro functional characterization of the 26 medically most important elapids and vipers from sub-Saharan Africa. Gigascience 2022, 11, giac121. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Del Brutto, V.J. Neurological complications of venomous snake bites: A review. Acta Neurol. Scand. 2012, 125, 363–372. [Google Scholar] [CrossRef]
- Silva, G.M.; Berto, D.H.; Lima, C.A.; Waitman, K.B.; Lima, C.F.G.; Prezoto, B.C.; Vieira, M.L.; Rocha, M.M.T.; Gonçalves, L.R.C.; Andrade, S.A. Synergistic effect of serine protease inhibitors and a bothropic antivenom in reducing local hemorrhage and coagulopathy caused by Bothrops jararaca venom. Toxicon 2021, 199, 87–93. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Rucavado, A.; Escalante, T.; Díaz, C. Hemorrhage induced by snake venom metalloproteinases: Biochemical and biophysical mechanisms involved in microvessel damage. Toxicon 2005, 45, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.F.; Hsu, C.C.; Kuo, Y.J. Anti-thrombotic agents derived from snake venom proteins. Thromb. J. 2016, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Liu, M.; Li, J.; Xu, R.; Long, C.; Li, H.; Mwangi, J.; Lu, Q.; Lai, R.; Shen, C. Snake C-type lectins potentially contribute to the prey immobilization in Protobothrops mucrosquamatus and Trimeresurus stejnegeri venoms. Toxins 2020, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- Malina, T.; Krecsák, L.; Jelić, D.; Maretić, T.; Tóth, T.; Siško, M.; Pandak, N. First clinical experiences about the neurotoxic envenomings inflicted by lowland populations of the Balkan adder, Vipera berus bosniensis. Neurotoxicology 2011, 32, 68–74. [Google Scholar] [CrossRef]
- Silva, A.; Kuruppu, S.; Othman, I.; Goode, R.J.; Hodgson, W.C.; Isbister, G.K. Neurotoxicity in Sri Lankan Russell’s Viper (Daboia russelii) Envenoming is Primarily due to U1-viperitoxin-Dr1a, a Pre-Synaptic Neurotoxin. Neurotox. Res. 2017, 31, 11–19. [Google Scholar] [CrossRef]
- Silva, A.; Maduwage, K.; Sedgwick, M.; Pilapitiya, S.; Weerawansa, P.; Dahanayaka, N.J.; Buckley, N.A.; Siribaddana, S.; Isbister, G.K. Neurotoxicity in Russell’s viper (Daboia russelii) envenoming in Sri Lanka: A clinical and neurophysiological study. Clin. Toxicol. (Phila) 2016, 54, 411–419. [Google Scholar] [CrossRef]
- Logonder, U.; Krizaj, I.; Rowan, E.G.; Harris, J.B. Neurotoxicity of ammodytoxin a in the envenoming bites of Vipera ammodytes ammodytes. J. Neuropathol. Exp. Neurol. 2008, 67, 1011–1019. [Google Scholar] [CrossRef]
- Varga, C.; Malina, T.; Alföldi, V.; Bilics, G.; Nagy, F.; Oláh, T. Extending knowledge of the clinical picture of Balkan adder (Vipera berus bosniensis) envenoming: The first photographically-documented neurotoxic case from South-Western Hungary. Toxicon 2018, 143, 29–35. [Google Scholar] [CrossRef]
- Lonati, D.; Giampreti, A.; Rossetto, O.; Petrolini, V.M.; Vecchio, S.; Buscaglia, E.; Mazzoleni, M.; Chiara, F.; Aloise, M.; Gentilli, A.; et al. Neurotoxicity of European viperids in Italy: Pavia Poison Control Centre case series 2001–2011. Clin. Toxicol. 2014, 52, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Gessner, T.; Kupferschmidt, H.; Weiler, S. Indigenous venomous snakebites in Switzerland: Analysis of reports to the National Poisons Information Centre over 22 years. Swiss Med. Wkly. 2021, 151, w30085. [Google Scholar] [CrossRef] [PubMed]
- Ferquel, E.; de Haro, L.; Jan, V.; Guillemin, I.; Jourdain, S.; Teynié, A.; d’Alayer, J.; Choumet, V. Reappraisal of Vipera aspis venom neurotoxicity. PLoS ONE 2007, 2, e1194. [Google Scholar] [CrossRef] [PubMed]
- Sahyoun, C.; Krezel, W.; Mattei, C.; Sabatier, J.M.; Legros, C.; Fajloun, Z.; Rima, M. Neuro- and cardiovascular activities of Montivipera bornmuelleri snake venom. Biology 2022, 11, 888. [Google Scholar] [CrossRef]
- Clark, R.F.; Williams, S.R.; Nordt, S.P.; Boyer-Hassen, L.V. Successful treatment of crotalid-induced neurotoxicity with a new polyspecific crotalid Fab antivenom. Ann. Emerg. Med. 1997, 30, 54–57. [Google Scholar] [CrossRef]
- de Sousa-e-Silva, M.C.; Tomy, S.C.; Tavares, F.L.; Navajas, L.; Larsson, M.H.; Lucas, S.R.; Kogika, M.M.; Sano-Martins, I.S. Hematological, hemostatic and clinical chemistry disturbances induced by Crotalus durissus terrificus snake venom in dogs. Hum. Exp. Toxicol. 2003, 22, 491–500. [Google Scholar] [CrossRef]
- Clement, J.F.; Pietrusko, R.G. Pit viper snakebite in the United States. J. Fam. Pract. 1978, 6, 269–279. [Google Scholar]
- Slotta, K.; Fraenkel-Conrat, H. Schlangengifte III. Mitteilung: Reiningung und Krystallisation des Klapperschlangengiftes. Ber. Dtsch. Chem. Ges. 1938, 71B, 1076–1081. [Google Scholar] [CrossRef]
- Ivanušec, A.; Šribar, J.; Križaj, I. Secreted phospholipases A2—Not just enzymes: Revisited. Int. J. Biol. Sci. 2022, 18, 873–888. [Google Scholar] [CrossRef]
- Murakami, M.; Sato, H.; Taketomi, Y. Updating phospholipase A2 biology. Biomolecules 2020, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Six, D.A.; Dennis, E.A. The expanding superfamily of phospholipase A2 enzymes: Classification and characterization. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2000, 1488, 1–19. [Google Scholar] [CrossRef]
- Faure, G.; Bon, C. Crotoxin, a phospholipase A2 neurotoxin from the South American rattlesnake Crotalus durissus terrificus: Purification of several isoforms and comparison of their molecular structure and of their biological activities. Biochemistry 1988, 27, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Faure, G.; Saul, F. Crystallographic characterization of functional sites of crotoxin and ammodytoxin, potent β-neurotoxins from Viperidae venom. Toxicon 2012, 60, 531–538. [Google Scholar] [CrossRef]
- Pungercar, J.; Krizaj, I. Understanding the molecular mechanism underlying the presynaptic toxicity of secreted phospholipases A2. Toxicon 2007, 50, 871–892. [Google Scholar] [CrossRef]
- Šribar, J.; Oberčkal, J.; Križaj, I. Understanding the molecular mechanism underlying the presynaptic toxicity of secreted phospholipases A(2): An update. Toxicon 2014, 89, 9–16. [Google Scholar] [CrossRef]
- Yagami, T.; Yamamoto, Y.; Kohma, H.; Nakamura, T.; Takasu, N.; Okamura, N. L-type voltage-dependent calcium channel is involved in the snake venom group IA secretory phospholipase A2-induced neuronal apoptosis. Neurotoxicology 2013, 35, 146–153. [Google Scholar] [CrossRef]
- Logonder, U.; Jenko-Praznikar, Z.; Scott-Davey, T.; Pungercar, J.; Krizaj, I.; Harris, J.B. Ultrastructural evidence for the uptake of a neurotoxic snake venom phospholipase A2 into mammalian motor nerve terminals. Exp. Neurol. 2009, 219, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Oberčkal, J.; Kovačič, L.; Šribar, J.; Leonardi, A.; Dolinar, K.; Pucer Janež, A.; Križaj, I. On the role of protein disulfide isomerase in the retrograde cell transport of secreted phospholipases A2. PLoS ONE 2015, 10, e0120692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šribar, J.; Kovačič, L.; Oberčkal, J.; Ivanušec, A.; Petan, T.; Fox, J.W.; Križaj, I. The neurotoxic secreted phospholipase A2 from the Vipera a. ammodytes venom targets cytochrome c oxidase in neuronal mitochondria. Sci. Rep. 2019, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Ivanušec, A.; Šribar, J.; Veranič, P.; Križaj, I. The phospholipase activity of ammodytoxin, a prototype snake venom β-neurotoxin, is not obligatory for cell internalisation and translocation to mitochondria. Toxins 2022, 14, 375. [Google Scholar] [CrossRef]
- Gallacci, M.; Cavalcante, W.L. Understanding the in vitro neuromuscular activity of snake venom Lys49 phospholipase A2 homologues. Toxicon 2010, 55, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Oshima-Franco, Y.; Leite, G.B.; Belo, C.A.; Hyslop, S.; Prado-Franceschi, J.; Cintra, A.C.; Giglio, J.R.; da Cruz-Höfling, M.A.; Rodrigues-Simioni, L. The presynaptic activity of bothropstoxin-I, a myotoxin from Bothrops jararacussu snake venom. Basic Clin. Pharmacol. Toxicol. 2004, 95, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhou, Y.C.; Lin, Z.J. Crystal structure of agkistrodotoxin, a phospholipase A2-type presynaptic neurotoxin from Agkistrodon halys pallas. J. Mol. Biol. 1998, 282, 1–11. [Google Scholar] [CrossRef]
- Prijatelj, P.; Jenko Praznikar, Z.; Petan, T.; Krizaj, I.; Pungercar, J. Mapping the structural determinants of presynaptic neurotoxicity of snake venom phospholipases A2. Toxicon 2008, 51, 1520–1529. [Google Scholar] [CrossRef]
- Renetseder, R.; Brunie, S.; Dijkstra, B.W.; Drenth, J.; Sigler, P.B. A comparison of the crystal structures of phospholipase A2 from bovine pancreas and Crotalus atrox venom. J. Biol. Chem. 1985, 260, 11627–11634. [Google Scholar] [CrossRef]
- Tsai, I.H.; Wang, Y.M.; Hseu, M.J. Mutagenesis analyses explore residues responsible for the neurotoxic and anticoagulant activities of Trimucrotoxin, a pit-viper venom Asn6-phospholipase A2. Biochimie 2011, 93, 277–285. [Google Scholar] [CrossRef]
- Nemecz, D.; Ostrowski, M.; Ravatin, M.; Saul, F.; Faure, G. Crystal structure of isoform CBd of the basic phospholipase A2 subunit of Crotoxin: Description of the structural framework of CB for interaction with protein targets. Molecules 2020, 25, 5290. [Google Scholar] [CrossRef]
- Lomeo Rda, S.; Gonçalves, A.P.; da Silva, C.N.; de Paula, A.T.; Costa Santos, D.O.; Fortes-Dias, C.L.; Gomes, D.A.; de Lima, M.E. Crotoxin from Crotalus durissus terrificus snake venom induces the release of glutamate from cerebrocortical synaptosomes via N and P/Q calcium channels. Toxicon 2014, 85, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Moreira, E.G.; Nascimento, N.; Rosa, G.J.; Rogero, J.R.; Vassilieff, V.S. Crotoxin-induced behavioral effects in rats. Braz. J. Med. Biol. Res. 1996, 29, 629–632. [Google Scholar] [PubMed]
- Zhang, Y.Q.; Zhou, Y.C.; Shen, G.G. beta-Agkistrodotoxin inhibition of Ca2+-dependent release of glutamate, aspartate, glycine and gamma-aminobutyric acid from cerebrocortical synaptosomes following its binding to synaptic membrane. Neuroreport 2002, 13, 2313–2317. [Google Scholar] [CrossRef]
- Stoyanova, V.; Aleksandrov, R.; Lukarska, M.; Duhalov, D.; Atanasov, V.; Petrova, S. Recognition of Vipera ammodytes meridionalis neurotoxin vipoxin and its components using phage-displayed scFv and polyclonal antivenom sera. Toxicon 2012, 60, 802–809. [Google Scholar] [CrossRef]
- Vulfius, C.A.; Gorbacheva, E.V.; Starkov, V.G.; Osipov, A.V.; Kasheverov, I.E.; Andreeva, T.V.; Astashev, M.E.; Tsetlin, V.I.; Utkin, Y.N. An unusual phospholipase A2 from puff adder Bitis arietans venom—A novel blocker of nicotinic acetylcholine receptors. Toxicon 2011, 57, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Vulfius, C.A.; Kasheverov, I.E.; Starkov, V.G.; Osipov, A.V.; Andreeva, T.V.; Filkin, S.Y.; Gorbacheva, E.V.; Astashev, M.E.; Tsetlin, V.I.; Utkin, Y.N. Inhibition of nicotinic acetylcholine receptors, a novel facet in the pleiotropic activities of snake venom phospholipases A2. PLoS ONE 2014, 9, e115428. [Google Scholar] [CrossRef]
- Vulfius, C.A.; Kasheverov, I.E.; Kryukova, E.V.; Spirova, E.N.; Shelukhina, I.V.; Starkov, V.G.; Andreeva, T.V.; Faure, G.; Zouridakis, M.; Tsetlin, V.I.; et al. Pancreatic and snake venom presynaptically active phospholipases A2 inhibit nicotinic acetylcholine receptors. PLoS ONE 2017, 12, e0186206. [Google Scholar] [CrossRef]
- Kumar, J.R.; Basavarajappa, B.S.; Vishwanath, B.S.; Gowda, T.V. Biochemical and pharmacological characterization of three toxic phospholipase A2s from Daboia russelii snake venom. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 168, 28–38. [Google Scholar] [CrossRef]
- Cavalcante, W.L.G.; Noronha-Matos, J.B.; Timóteo, M.A.; Fontes, M.R.M.; Gallacci, M.; Correia-de-Sá, P. Neuromuscular paralysis by the basic phospholipase A2 subunit of crotoxin from Crotalus durissus terrificus snake venom needs its acid chaperone to concurrently inhibit acetylcholine release and produce muscle blockage. Toxicol. Appl. Pharmacol. 2017, 334, 8–17. [Google Scholar] [CrossRef]
- Chacur, M.; Longo, I.; Picolo, G.; Gutiérrez, J.M.; Lomonte, B.; Guerra, J.L.; Teixeira, C.F.; Cury, Y. Hyperalgesia induced by Asp49 and Lys49 phospholipases A2 from Bothrops asper snake venom: Pharmacological mediation and molecular determinants. Toxicon 2003, 41, 667–678. [Google Scholar] [CrossRef]
- Zambelli, V.O.; Picolo, G.; Fernandes, C.A.H.; Fontes, M.R.M.; Cury, Y. Secreted phospholipases A2 from animal venoms in pain and analgesia. Toxins 2017, 9, 406. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.L.; Han, R.; Chen, Z.X.; Chen, B.W.; Gu, Z.L.; Reid, P.F.; Raymond, L.N.; Qin, Z.H. Opiate and acetylcholine-independent analgesic actions of crotoxin isolated from Crotalus durissus terrificus venom. Toxicon 2006, 48, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Nogueira-Neto, F.d.S.; Amorim, R.L.; Brigatte, P.; Picolo, G.; Ferreira, W.A.; Gutierrez, V.P.; Conceição, I.M.; Della-Casa, M.S.; Takahira, R.K.; Nicoletti, J.L.M.; et al. The analgesic effect of crotoxin on neuropathic pain is mediated by central muscarinic receptors and 5-lipoxygenase-derived mediators. Pharmacol. Biochem. Behav. 2008, 91, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Wu, D.C.; Zhou, X.P.; Gong, S.; Cheng, B.C.; Qin, Z.H.; Reid, P.F.; Yin, Q.Z.; Jiang, X.H. Inhibitory effect of crotoxin on the pain-evoked discharge of neurons in thalamic parafascicular nucleus in rats. Toxicon 2008, 51, 102–111. [Google Scholar] [CrossRef]
- Wolz-Richter, S.; Esser, K.-H.; Hess, A. Antinociceptive activity of crotoxin in the central nervous system: A functional Magnetic Resonance Imaging study. Toxicon 2013, 74, 44–55. [Google Scholar] [CrossRef]
- Sant’Anna, M.B.; Lopes, F.S.R.; Kimura, L.F.; Giardini, A.C.; Sant’Anna, O.A.; Picolo, G. Crotoxin conjugated to SBA-15 nanostructured mesoporous silica induces long-last analgesic effect in the neuropathic pain model in mice. Toxins 2019, 11, 679. [Google Scholar] [CrossRef]
- Dyachenko, I.A.; Murashev, A.N.; Andreeva, T.V.; Tsetlin, V.I.; Utkin, Y.N. Analysis of nociceptive effects of neurotoxic phospholipase A2 from Vipera nikolskii venom in mice. J. Venom Res. 2013, 4, 1–4. [Google Scholar]
- Li, D.; Kim, W.; Shin, D.; Jung, Y.; Bae, H.; Kim, S.K. Preventive effects of bee venom derived phospholipase A2 on oxaliplatin-induced neuropathic pain in mice. Toxins 2016, 8, 27. [Google Scholar] [CrossRef]
- Cintra-Francischinelli, M.; Caccin, P.; Chiavegato, A.; Pizzo, P.; Carmignoto, G.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M.; Montecucco, C. Bothrops snake myotoxins induce a large efflux of ATP and potassium with spreading of cell damage and pain. Proc. Natl. Acad. Sci. USA 2010, 107, 14140–14145. [Google Scholar] [CrossRef]
- Zhang, C.; Medzihradszky, K.F.; Sánchez, E.E.; Basbaum, A.I.; Julius, D. Lys49 myotoxin from the Brazilian lancehead pit viper elicits pain through regulated ATP release. Proc. Natl. Acad. Sci. USA 2017, 114, E2524–E2532. [Google Scholar] [CrossRef] [PubMed]
- Utkin, Y.N. Last decade update for three-finger toxins: Newly emerging structures and biological activities. World J. Biol. Chem. 2019, 10, 17–27. [Google Scholar] [CrossRef]
- Jiang, M.; Häggblad, J.; Heilbronn, E. Isolation and pharmacological characterization of a new alpha-neurotoxin (alpha-AgTx) from venom of the viper Agkistrodon halys (Pallas). Toxicon 1987, 25, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Shelke, R.R.; Sathish, S.; Gowda, T.V. Isolation and characterization of a novel postsynaptic/cytotoxic neurotoxin from Daboia russelli russelli venom. J. Pept. Res. 2002, 59, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, M.; Prasad, N.; Sing, T.; Gowda, V. Purification, characterization, and chemical modification of neurotoxic peptide from Daboia russelii snake venom of India. J. Biochem. Mol. Toxicol. 2013, 27, 295–304. [Google Scholar] [CrossRef]
- Junqueira-de-Azevedo, I.L.; Ching, A.T.; Carvalho, E.; Faria, F.; Nishiyama, M.Y., Jr.; Ho, P.L.; Diniz, M.R. Lachesis muta (Viperidae) cDNAs reveal diverging pit viper molecules and scaffolds typical of cobra (Elapidae) venoms: Implications for snake toxin repertoire evolution. Genetics 2006, 173, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Lopez, I.; Kowalski, P. Toxin transcripts in Crotalus atrox venom and in silico structures of toxins. J. Venom Res. 2020, 10, 18–22. [Google Scholar]
- Doley, R.; Pahari, S.; Mackessy, S.P.; Kini, R.M. Accelerated exchange of exon segments in Viperid three-finger toxin genes (Sistrurus catenatus edwardsii; Desert Massasauga). BMC Evol. Biol. 2008, 8, 196. [Google Scholar] [CrossRef]
- Babenko, V.V.; Ziganshin, R.H.; Weise, C.; Dyachenko, I.; Shaykhutdinova, E.; Murashev, A.N.; Zhmak, M.; Starkov, V.; Hoang, A.N.; Tsetlin, V.; et al. Novel bradykinin-potentiating peptides and three-finger toxins from viper venom: Combined NGS venom gland transcriptomics and quantitative venom proteomics of the Azemiops feae viper. Biomedicines 2020, 8, 249. [Google Scholar] [CrossRef]
- Makarova, Y.V.; Kryukova, E.V.; Shelukhina, I.V.; Lebedev, D.S.; Andreeva, T.V.; Ryazantsev, D.Y.; Balandin, S.V.; Ovchinnikova, T.V.; Tsetlin, V.I.; Utkin, Y.N. The first recombinant viper three-finger toxins: Inhibition of muscle and neuronal nicotinic acetylcholine receptors. Dokl. Biochem. Biophys. 2018, 479, 127–130. [Google Scholar] [CrossRef]
- Junqueira-de-Azevedo, I.L.; Campos, P.F.; Ching, A.T.; Mackessy, S.P. Colubrid venom composition: An -omics perspective. Toxins 2016, 8, 230. [Google Scholar] [CrossRef]
- Broaders, M.; Faro, C.; Ryan, M.F. Partial purification of acetylcholine receptor binding components from the Duvernoy’s secretions of blanding’s tree snake (Boiga blandingi) and the mangrove snake (Boiga dendrophila). J. Nat. Toxins 1999, 8, 155–166. [Google Scholar]
- Fry, B.G.; Lumsden, N.G.; Wüster, W.; Wickramaratna, J.C.; Hodgson, W.C.; Kini, R.M. Isolation of a neurotoxin (α-colubritoxin) from a nonvenomous Colubrid: Evidence for early origin of venom in snakes. J. Mol. Evol. 2003, 57, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Modahl, C.M.; Frietze, S.; Mackessy, S.P. Transcriptome-facilitated proteomic characterization of rear-fanged snake venoms reveal abundant metalloproteinases with enhanced activity. J. Proteomics 2018, 187, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.A.; Schmidt, J.J.; Bernheimer, A.W.; Smith, L.A. Characterization and amino acid sequences of two lethal peptides isolated from venom of Wagler’s pit viper, Trimeresurus wagleri. Toxicon 1991, 29, 227–236. [Google Scholar] [CrossRef]
- Schmidt, J.J.; Weinstein, S.A.; Smith, L.A. Molecular properties and structure-function relationships of lethal peptides from venom of Wagler’s pit viper, Trimeresurus wagleri. Toxicon 1992, 30, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Debono, J.; Xie, B.; Violette, A.; Fourmy, R.; Jaeger, M.; Fry, B.G. Viper venom Botox: The molecular origin and evolution of the waglerin peptides used in anti-wrinkle skin cream. J. Mol. Evol. 2017, 84, 8–11. [Google Scholar] [CrossRef]
- Lin, W.W.; Smith, L.A.; Lee, C.Y. A study on the cause of death due to waglerin-I, a toxin from Trimeresurus wagleri. Toxicon 1995, 33, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Molles, B.E.; Rezai, P.; Kline, E.F.; McArdle, J.J.; Sine, S.M.; Taylor, P. Identification of residues at the alpha and epsilon subunit interfaces mediating species selectivity of Waglerin-1 for nicotinic acetylcholine receptors. J. Biol. Chem. 2002, 277, 5433–5440. [Google Scholar] [CrossRef] [PubMed]
- McArdle, J.J.; Lentz, T.L.; Witzemann, V.; Schwarz, H.; Weinstein, S.A.; Schmidt, J.J. Waglerin-1 selectively blocks the epsilon form of the muscle nicotinic acetylcholine receptor. J. Pharmacol. Exp. Ther. 1999, 289, 543–550. [Google Scholar]
- Aiken, S.P.; Sellin, L.C.; Schmidt, J.J.; Weinstein, S.A.; McArdle, J.J. A novel peptide toxin from Trimeresurus wagleri acts pre- and post-synaptically to block transmission at the rat neuromuscular junction. Pharmacol. Toxicol. 1992, 70, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.H.; McArdle, J.J. Waglerin-1 modulates gamma-aminobutyric acid activated current of murine hypothalamic neurons. J. Pharmacol. Exp. Ther. 1997, 282, 74–80. [Google Scholar] [PubMed]
- Ye, J.H.; Ren, J.; McArdle, J.J. Waglerin-1 inhibits GABA(A) current of neurons in the nucleus accumbens of neonatal rats. Brain Res. 1999, 837, 29–37. [Google Scholar] [CrossRef]
- Utkin, Y.N.; Weise, C.; Kasheverov, I.E.; Andreeva, T.V.; Kryukova, E.V.; Zhmak, M.N.; Starkov, V.G.; Hoang, N.A.; Bertrand, D.; Ramerstorfer, J.; et al. Azemiopsin from Azemiops feae viper venom, a novel polypeptide ligand of nicotinic acetylcholine receptor. J. Biol. Chem. 2012, 287, 27079–27086. [Google Scholar] [CrossRef] [PubMed]
- Shelukhina, I.V.; Zhmak, M.N.; Lobanov, A.V.; Ivanov, I.A.; Garifulina, A.I.; Kravchenko, I.N.; Rasskazova, E.A.; Salmova, M.A.; Tukhovskaya, E.A.; Rykov, V.A.; et al. Azemiopsin, a selective peptide antagonist of muscle nicotinic acetylcholine receptor: Preclinical evaluation as a local muscle relaxant. Toxins 2018, 10, 34. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Nguyen, T.N.; Nguyen, K.C.; Tran, Q.N.; Hoang, A.N.; Egorova, N.S.; Starkov, V.G.; Tsetlin, V.I.; Utkin, Y.N. Encapsulation of neurotoxins, blockers of nicotinic acetylcholine receptors, in nanomaterials based on sulfated polysaccharides. Dokl. Biochem. Biophys. 2019, 487, 251–255. [Google Scholar] [CrossRef]
- Vulfius, C.A.; Spirova, E.N.; Serebryakova, M.V.; Shelukhina, I.V.; Kudryavtsev, D.S.; Kryukova, E.V.; Starkov, V.G.; Kopylova, N.V.; Zhmak, M.N.; Ivanov, I.A.; et al. Peptides from puff adder Bitis arietans venom, novel inhibitors of nicotinic acetylcholine receptors. Toxicon 2016, 121, 70–76. [Google Scholar] [CrossRef]
- Kryukova, E.V.; Vulfius, C.A.; Ziganshin, R.H.; Andreeva, T.V.; Starkov, V.G.; Tsetlin, V.I.; Utkin, Y.N. Snake C-type lectin-like proteins inhibit nicotinic acetylcholine receptors. J. Venom Res. 2020, 10, 23–29. [Google Scholar]
- Yamazaki, Y.; Morita, T. Structure and function of snake venom cysteine-rich secretory proteins. Toxicon 2004, 44, 227–231. [Google Scholar] [CrossRef]
- Lodovicho, M.E.; Costa, T.R.; Bernardes, C.P.; Menaldo, D.L.; Zoccal, K.F.; Carone, S.E.; Rosa, J.C.; Pucca, M.B.; Cerni, F.A.; Arantes, E.C.; et al. Investigating possible biological targets of Bj-CRP, the first cysteine-rich secretory protein (CRISP) isolated from Bothrops jararaca snake venom. Toxicol. Lett. 2017, 265, 156–169. [Google Scholar] [CrossRef]
- Bernardes, C.P.; Menaldo, D.L.; Zoccal, K.F.; Boldrini-França, J.; Peigneur, S.; Arantes, E.C.; Rosa, J.C.; Faccioli, L.H.; Tytgat, J.; Sampaio, S.V. First report on BaltCRP, a cysteine-rich secretory protein (CRISP) from Bothrops alternatus venom: Effects on potassium channels and inflammatory processes. Int. J. Biol. Macromol. 2019, 140, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Estrella, A.; Sánchez, E.E.; Galán, J.A.; Tao, W.A.; Guerrero, B.; Navarrete, L.F.; Rodríguez-Acosta, A. Characterization of toxins from the broadbanded water snake Helicops angulatus (Linnaeus, 1758): Isolation of a cysteine-rich secretory protein, helicopsin. Arch. Toxicol. 2011, 85, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Koike, H.; Sugiyama, Y.; Motoyoshi, K.; Wada, T.; Hishinuma, S.; Mita, M.; Morita, T. Cloning and characterization of novel snake venom proteins that block smooth muscle contraction. Eur. J. Biochem. 2002, 269, 2708–2715. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Hyodo, F.; Morita, T. Wide distribution of cysteine-rich secretory proteins in snake venoms: Isolation and cloning of novel snake venom cysteine-rich secretory proteins. Arch. Biochem. Biophys. 2003, 412, 133–141. [Google Scholar] [CrossRef]
- Zupunski, V.; Kordis, D.; Gubensek, F. Adaptive evolution in the snake venom Kunitz/BPTI protein family. FEBS Lett. 2003, 547, 131–136. [Google Scholar] [CrossRef]
- Thakur, R.; Mukherjee, A.K. Pathophysiological significance and therapeutic applications of snake venom protease inhibitors. Toxicon 2017, 131, 37–47. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Mackessy, S.P. Pharmacological properties and pathophysiological significance of a Kunitz-type protease inhibitor (Rusvikunin-II) and its protein complex (Rusvikunin complex) purified from Daboia russelii russelii venom. Toxicon 2014, 89, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Drofenik, S.; Leonardi, A.; Žužek, M.C.; Frangež, R.; Križaj, I. The first Kunitz-type proteins from a viperid venom that potentiate neuromuscular transmission. Toxicon 2020, 187, 262–270. [Google Scholar] [CrossRef]
- Mancin, A.C.; Soares, A.M.; Andrião-Escarso, S.H.; Faça, V.M.; Greene, L.J.; Zuccolotto, S.; Pelá, I.R.; Giglio, J.R. The analgesic activity of crotamine, a neurotoxin from Crotalus durissus terrificus (South American rattlesnake) venom: A biochemical and pharmacological study. Toxicon 1998, 36, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Do, B.H.; Lee, J.S.; Yang, H.C.; Nguyen, A.N.; Krupa, M.; Kim, C.J.; Jang, Y.J.; Choe, H. Antinociceptive and anti-inflammatory effects of recombinant crotamine in mouse models of pain. Toxins 2021, 13, 707. [Google Scholar] [CrossRef] [PubMed]
- Matavel, A.C.S.; Ferreira-Alves, D.L.; Beirão, P.S.L.; Cruz, J.S. Tension generation and increase of voltage-activated sodium current by crotamine. Eur. J. Pharmacol. 1998, 348, 167–173. [Google Scholar] [CrossRef]
- Rizzi, C.T.; Carvalho-de-Souza, J.L.; Schiavon, E.; Cassola, A.C.; Wanke, E.; Troncone, L.R. Crotamine inhibits preferentially fast-twitching muscles but is inactive on sodium channels. Toxicon 2007, 50, 553–562. [Google Scholar] [CrossRef]
- Yount, N.Y.; Kupferwasser, D.; Spisni, A.; Dutz, S.M.; Ramjan, Z.H.; Sharma, S.; Waring, A.J.; Yeaman, M.R. Selective reciprocity in antimicrobial activity versus cytotoxicity of hBD-2 and crotamine. Proc. Natl. Acad. Sci. USA 2009, 106, 14972–14977. [Google Scholar] [CrossRef]
- Peigneur, S.; Orts, D.J.; da Silva, A.R.P.; Oguiura, N.; Boni-Mitake, M.; de Oliveira, E.B.; Zaharenko, A.J.; de Freitas, J.C.; Tytgat, J. Crotamine pharmacology revisited: Novel insights based on the inhibition of KV channels. Mol. Pharmacol. 2012, 82, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.C.; Porta, L.C.; Lima, Á.D.C.; Campeiro, J.D.; Meurer, Y.; Teixeira, N.B.; Duarte, T.; Oliveira, E.B.; Picolo, G.; Godinho, R.O.; et al. Pharmacological characterization of crotamine effects on mice hind limb paralysis employing both ex vivo and in vivo assays: Insights into the involvement of voltage-gated ion channels in the crotamine action on skeletal muscles. PLoS Negl. Trop. Dis. 2018, 12, e0006700. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Oliveira e Silva, S.; Rostelato-Ferreira, S.; Rocha-e-Silva, T.A.; Randazzo-Moura, P.; Dal-Belo, C.A.; Sanchez, E.F.; Borja-Oliveira, C.R.; Rodrigues-Simioni, L. Beneficial effect of crotamine in the treatment of myasthenic rats. Muscle Nerve 2013, 47, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Porta, L.C.; Campeiro, J.D.; Hayashi, M.A.F. A Native CPP from rattlesnake with therapeutic and theranostic properties. Methods Mol. Biol. 2022, 2383, 91–104. [Google Scholar] [CrossRef]
- Porta, L.C.; Campeiro, J.D.; Papa, G.B.; Oliveira, E.B.; Godinho, R.O.; Rodrigues, T.; Hayashi, M.A.F. In vivo effects of the association of the psychoactive phenotiazine thioridazine on antitumor activity and hind limb paralysis induced by the native polypeptide crotamine. Toxicon 2020, 185, 64–71. [Google Scholar] [CrossRef]
- Ducancel, F. Endothelin-like peptides. Cell. Mol. Life Sci. 2005, 62, 2828–2839. [Google Scholar] [CrossRef]
- Becker, B.K.; Speed, J.S.; Powell, M.; Pollock, D.M. Activation of neuronal endothelin B receptors mediates pressor response through alpha-1 adrenergic receptors. Physiol. Rep. 2017, 5, e13077. [Google Scholar] [CrossRef]
- Ambar, I.; Kloog, Y.; Kochva, E.; Wollberg, Z.; Oron, U.; Sokolovski, M. Characterization and localization of a novel neuroreceptor for the peptide sarafotoxin. Biochem. Biophys. Res. Commun. 1988, 157, 1104–1110. [Google Scholar] [CrossRef]
- Zigdon-Arad, T.; Bdolah, A.; Kochva, E.; Wollberg, Z. Activity of sarafotoxin/endothelin peptides in the heart and brain of lower vertebrates. Toxicon 1992, 30, 439–448. [Google Scholar] [CrossRef]
- Holzwarth, J.A.; Glaum, S.R.; Miller, R.J. Activation of endothelin receptors by sarafotoxin regulates Ca2+ homeostasis in cerebellar astrocytes. Glia 1992, 5, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Izume, T.; Miyauchi, H.; Shihoya, W.; Nureki, O. Crystal structure of human endothelin ETB receptor in complex with sarafotoxin S6b. Biochem. Biophys. Res. Commun. 2020, 528, 383–388. [Google Scholar] [CrossRef]
- Cura, J.E.; Blanzaco, D.P.; Brisson, C.; Cura, M.A.; Cabrol, R.; Larrateguy, L.; Mendez, C.; Sechi, J.C.; Silveira, J.S.; Theiller, E.; et al. Phase I and pharmacokinetics study of crotoxin (cytotoxic PLA(2), NSC-624244) in patients with advanced cancer. Clin. Cancer Res. 2002, 8, 1033–1041. [Google Scholar] [PubMed]
- Moreira, L.A.; Oliveira, L.P.; Magalhães, M.R.; Oliveira, S.A.M.; Oliveira-Neto, J.R.; Carvalho, P.M.G.; Carvalho, A.A.V.; Fajemiroye, J.O.; Cruz, A.C.; Cunha, L.C. Acute toxicity, antinociceptive, and anti-inflammatory activities of the orally administered crotamine in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 1703–1711. [Google Scholar] [CrossRef]
- Utkin, Y.N.; Andreeva, T.V.; Osipov, A.V.; Shelukhina, I.V.; Kudryavtsev, D.S.; Tukhovskaya, E.A.; Ivanov, I.A.; Tsetlin, V.I.; Egorova, N.S.; Slashcheva, G.A.; et al. Method for the treatment of muscular dystonia. Russian Patent RU 2 704 815; filed 11 November 2017, and issued 31 October 2019,
- Cousin, X.; Bon, C. Acetylcholinesterase from snake venom as a model for its nerve and muscle counterpart. J. Nat. Toxins 1999, 8, 285–294. [Google Scholar] [PubMed]
- Geron, M.; Kumar, R.; Matzner, H.; Lahiani, A.; Gincberg, G.; Cohen, G.; Lazarovici, P.; Priel, A. Protein toxins of the Echis coloratus viper venom directly activate TRPV1. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 615–623. [Google Scholar] [CrossRef]
- Koua, D.; Ebou, A.; Habbouche, Z.; Ballouard, J.M.; Caron, S.; Bonnet, X.; Dutertre, S. Proteomic insight into the venom composition of the largest European rear-fanged snake, Malpolon monspessulanus monspessulanus. Toxicon X 2022, 15, 100130. [Google Scholar] [CrossRef]

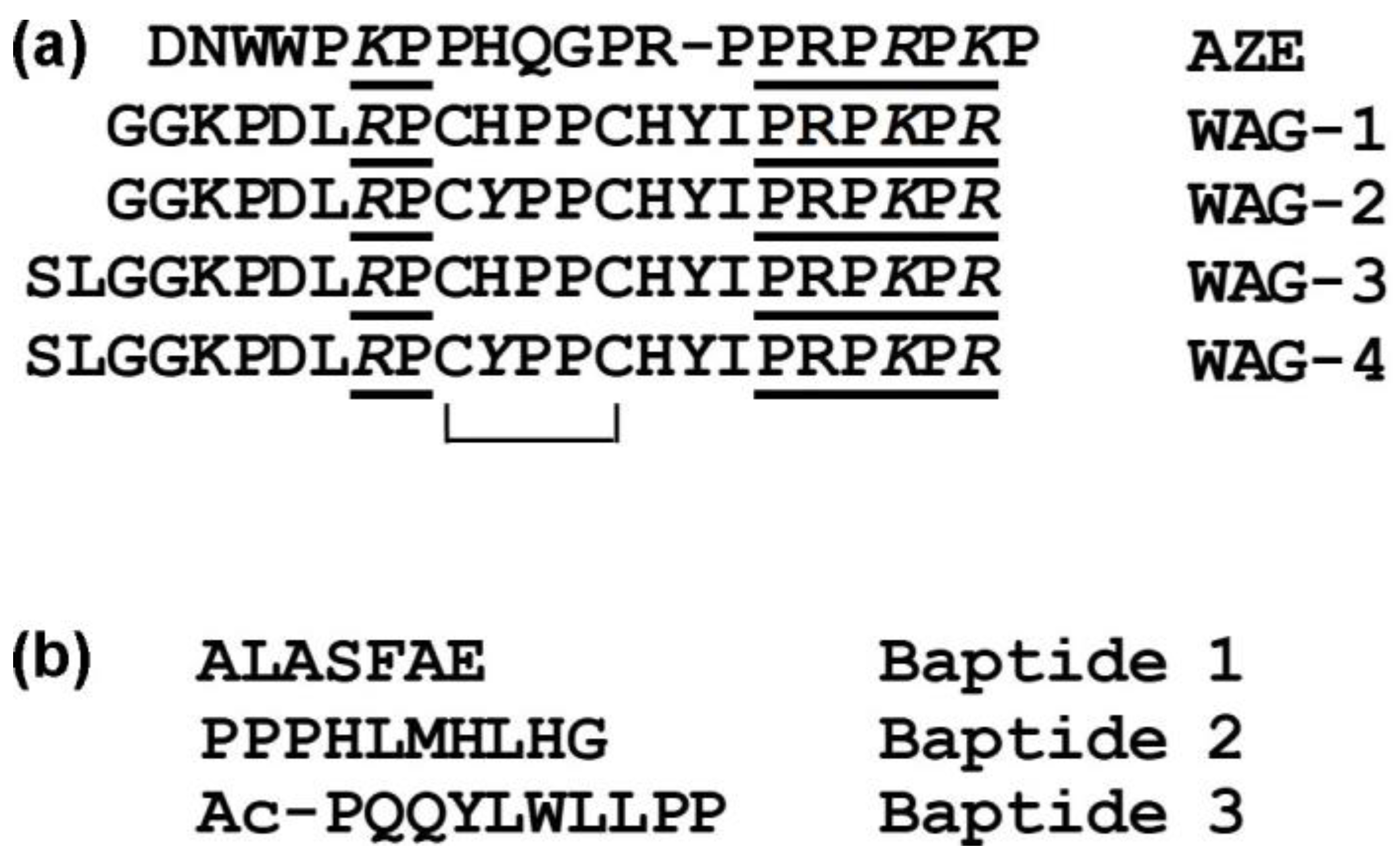
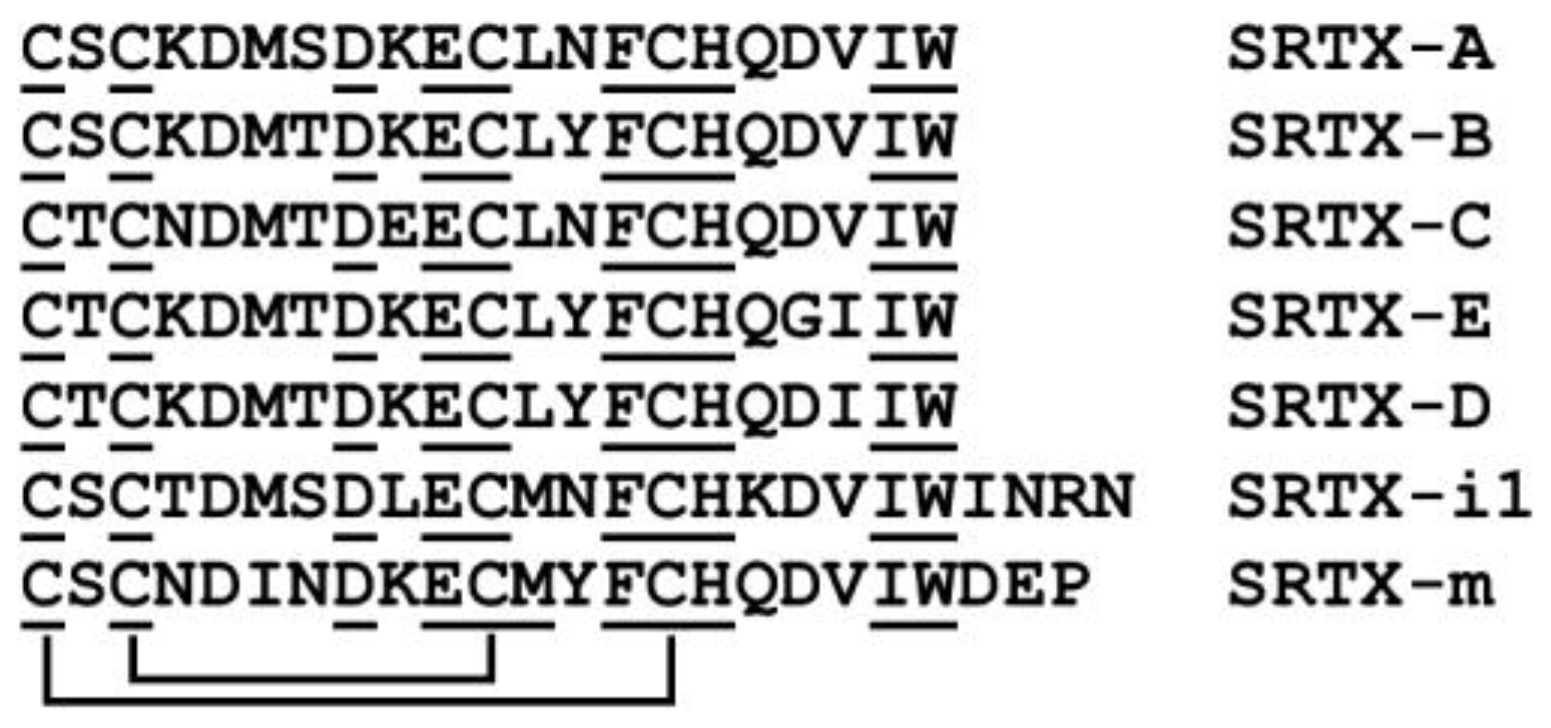
| Name | Species (Common Name) | Biological Activity(es) | Protein Family | Structural Type | Size, mol. Weight | Subunit Composition | Other Structural Features | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ablomin | A. blomhoffi (Japanese Mamushi snake) | K+-blocker | CRiSP | 221 aa, 24.9 kDa | monomer | [106] | ||
| α-Agkistrodotoxin | A. halys pallas (Pallas’s pit viper) | Postsynaptic neurotoxin | putative TFT | Putative long-chain α-neurotoxin | 8.0 kDa | monomer | [76] | |
| β-Agkistrodotoxin | A. halys pallas (Pallas’s pit viper) | β-neurotoxin | sPLA2 | Group IIA sPLA2 | monomer | [50] | ||
| Ammodytoxin A | V. ammodytes ammodytes (nose-horned viper) | β-neurotoxin, phospholipolysis, anticoagulant, | sPLA2 | Group IIA sPLA2 | 122 aa, 13.8 kDa | monomer | [40,47,51] | |
| Ammodytoxin C | V. ammodytes ammodytes (nose-horned viper) | Anticoagulant, phospholipolysis | sPLA2 | Group IIA sPLA2 | 122 aa, 13.8 kDa | monomer | [40] | |
| BaltCRP | B. alternatus (Urutu pit viper) | K+-blocker, Immune stimulation | CRiSP | 24.4 kDa | monomer | [104] | ||
| Baptides 1, 2 and 3 | B. arietans (puff adder) | Postsynaptic neurotoxin | peptides | 7 or 10 aa | monomer | Acetylated N-terminus, without Cys residues | [100] | |
| Bitanarin | B. arietans (puff adder) | Phospholipolysis, Postsynaptic neurotoxin | sPLA2 | Group II sPLA2 | 27.4 kDa | monomer | 14 disulfides within a single chain | [59] |
| BomoTx | B. moojeni (Brazilian lancehead pit viper) | Myotoxin, induce pain, unable to phospholipolysis | sPLA2 | sPLA2 of IIA group | 122 aa, 13.84 kDa | Lys substitutes Asp49 in active center | [74] | |
| α-Bungarotoxin | B. multicinctus (many-banded krait), Elapidae | α-neurotoxin | TFT | long-chain α-neurotoxin | 76 aa, 8.2 kDa | monomer | Additional fifth disulfide in loop II | |
| Bothropstoxin-I | B. jararacussu (Jararacussu) | Myotoxin, Ca2+-channel blocker? | sPLA2 | sPLA2 of IIA group | 121 aa, 13.72 kDa | monomer | Lys substitutes Asp49 in active center | [49] |
| β-Bungarotoxins | Bungarus spp., (kraits) Elapidae | β-neurotoxin | sPLA2 | Bungarotoxin | 120aa + 60 aa, ~20 kDa | basic IA group sPLA2 subunit disulfide bound to KSPI-like subunit | ||
| Calciseptine | Dendroaspis polylepis (black mamba) Elapidae | L-type Ca2+ channel blocker | TFT | Short-chain TFT | 60 aa, 7.0 kDa | |||
| Catrin-1 and catrin-2 | C. atrox (Western diamondback rattlesnake) | K+-blocker | CRiSP | 121 aa, 24.7 kDa | monomer | Differ by ionic strength at elution from a cation-exchange column | [107] | |
| α-Colubritoxin | C. radiatus (radiated ratsnake), Colubridae | α-neurotoxin | TFT | 77 aa, 8.5 kDa | N-terminally blocked by a pyroglutamic acid residue | [86] | ||
| Crotamine | C. durissus terrificus (South American rattlesnake) | Analgetics; K+-blocker | β-defensin | small basic myotoxins | 42 aa, 4.9 kDa | monomer | [112,113,114,115,116,117,118,119,120,121] | |
| Crotoxin | C. durissus terrificus (South American rattlesnake) | β-neurotoxin, phospholipolysis, anti-nociception | sPLA2 | sPLA2 of IIA group | CB of 122 residues; CA of 88 residues | basic CB subunit of (CBa, CBb, Cbc, CBd) non-covalently bound to acidic CA subunit (CA1-CA4) | CA subunit consists of 3 covalently linked chains (α, 39 residues, β, 35 residues, and γ, 14 residues) | [35,40,42,54,55,56,61,63,64,65,66,67,68,69,70] |
| Dendrotoxins | Dendroaspis spp.(mambas), Elapidae | block voltage-dependent K+-channels | KSPI | 57–60 aa | ||||
| DNTx I; DNTx-III | D. russelli (Russell’s viper) | α-neurotoxins | TFT | Short-chain TFT | 6675 Da; 6849 Da | monomer | Cytotoxin-related; α-neurotoxin-related | [77,78] |
| Emunarecins EM1 and EM2 | E. multisquamatus (saw-scaled viper) | Anticoagulants, Presynaptic neurotoxicity | C-type lectin-like proteins | 70–160 kDa | Multimers of heterodimers | Composed of α and β subunits of 13 kDa and 18 kDa | [101] | |
| Helicopsin | Helicops angulatus (Brown-banded watersnake), Colubridae | CRiSP | ~20 kDa | monomer | [105] | |||
| HDP-2 | V. nikolskii (Nikolsky’s viper) | Phospholipolysis, β-neurotoxin (?) | sPLA2 | sPLA2 of IIA group | 122 aa + 122 aa 13.7 + 13.8 kDa | acidic subunit non-covalently bound to basic subunit | Non-enzymatic acid subunit is inhibitor of basic subunit | [71] |
| Lys49 sPLA2s | Agkistrodon, Bothrops, Trimeresurus spp. | Myotoxin, induce pain, β-neurotoxin, unable to phospholipolysis | sPLA2 | sPLA2 of IIA group | About 13–14 kDa or 27–28 kDa | Monomers or dimers | Lys substitutes Asp49 in active center | [48,73] |
| Piscivorin | A. piscivorus piscivorus (Northern cottonmouth) | K+-blocker | CRiSP | 221 aa, 24.8 kDa | monomer | [107] | ||
| Rusvikunin | D. russelii russelii (Russell’s viper) | Anticoagulant, possible neurotoxicity | KSPI | 6.9 kDa + 7.1 kDa | heterodimer | Composed of two KSPIs, rusvikunin and rusvikunin-II | [110] | |
| Sarafotoxins, S6a-d | Atractaspis spp., Atractaspididae, Colubroidea | Activators of endothelin type A and B receptors | endothelin | peptides | 15–30 aa; 21 aa, ~2500 Da | monomer | Have two disulfides. 4 major isopeptides S6a-d are composed of 21 aa | [123,124,125,126,127] |
| TFT-AF; TFT-VN, | A. feae; V. nikolskii | α-neurotoxins | TFT | non-conventional α-neurotoxin | 68–70 aa | monomer | Additional fifth disulfide in loop I | [83] |
| Triflin | Habu snake (Trimeresurus flavoviridis, P. flavoviridis) | K+-blocker | CRiSP | 221 aa, 24.8 kDa | monomer | [106] | ||
| Trimucrotoxin | P. mucrosquamatus (brown-spotted pit viper) | Presynaptic neurotoxin | sPLA2 | Group IIA sPLA2 | 122 aa | monomer | Asn6-containing sPLA2 | [53] |
| U1-viperitoxin-Dr1a | D. russelii (Russell’s viper) | Phospholipolysis, presynaptic neurotoxin | sPLA2s | Group IIA sPLA2 | 13.6 kDa | monomer | [24] | |
| VaaChi | V. ammodytes ammodytes (nose-horned viper) | Putative K+-blocker | KSPI | 7.5–7.6 kDa | monomer | Mix of 6 closely related isoforms | [111] | |
| Vipoxin | V. ammodytes ammodytes (nose-horned viper) | β-neurotoxin, presynaptic neurotoxin? | sPLA2 | Group IIA sPLA2 | [51] | |||
| VRV-PL-IIIc, VRV-PL-VII, VRV-PL-IX | D. russelii (Russell’s viper) | Phospholipolysis, Pre- and postsynaptic neurotoxins | sPLA2 | Group IIA sPLA2 | 13.0 kDa; 13.1 kDa; 12.5 kDa | monomer | [62] | |
| Vurtoxin; Vur-PL | V. ursinii (meadow viper) | Phospholipolysis, anti-coagulant, Postsynaptic neurotoxin | sPLA2s | Group IIA sPLA2 | 122 aa, 13.9 kDa; | monomer | [61] | |
| Vur-S49 | V. ursinii (meadow viper) | Postsynaptic neurotoxin, unable to phospholipolysis | sPLA2 | Group IIA sPLA2 | 122 aa, 13.9 kDa | monomer | Ser substitutes Asp49 in active center | [61] |
| Waglerins | T. wagleri | α-neurotoxin, GABAA receptor modulation | Waglerins | Peptide from pre-pro region of the C-type natriuretic peptide | 22–24 aa, 2.7–2.8 kDa | monomer | Basic proline-rich peptides with 1 disulfide | [90,91,92,93,94,95,96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osipov, A.; Utkin, Y. What Are the Neurotoxins in Hemotoxic Snake Venoms? Int. J. Mol. Sci. 2023, 24, 2919. https://doi.org/10.3390/ijms24032919
Osipov A, Utkin Y. What Are the Neurotoxins in Hemotoxic Snake Venoms? International Journal of Molecular Sciences. 2023; 24(3):2919. https://doi.org/10.3390/ijms24032919
Chicago/Turabian StyleOsipov, Alexey, and Yuri Utkin. 2023. "What Are the Neurotoxins in Hemotoxic Snake Venoms?" International Journal of Molecular Sciences 24, no. 3: 2919. https://doi.org/10.3390/ijms24032919
APA StyleOsipov, A., & Utkin, Y. (2023). What Are the Neurotoxins in Hemotoxic Snake Venoms? International Journal of Molecular Sciences, 24(3), 2919. https://doi.org/10.3390/ijms24032919







