The EPH/Ephrin System in Pancreatic Ductal Adenocarcinoma (PDAC): From Pathogenesis to Treatment
Abstract
1. Introduction
Molecular Characteristics of the EPH/Ephrin Signaling Pathway
2. The Role of EPH/Ephrin System in the Pancreas
2.1. The EPH/Ephrin System in Pancreatic Embryology and Physiology
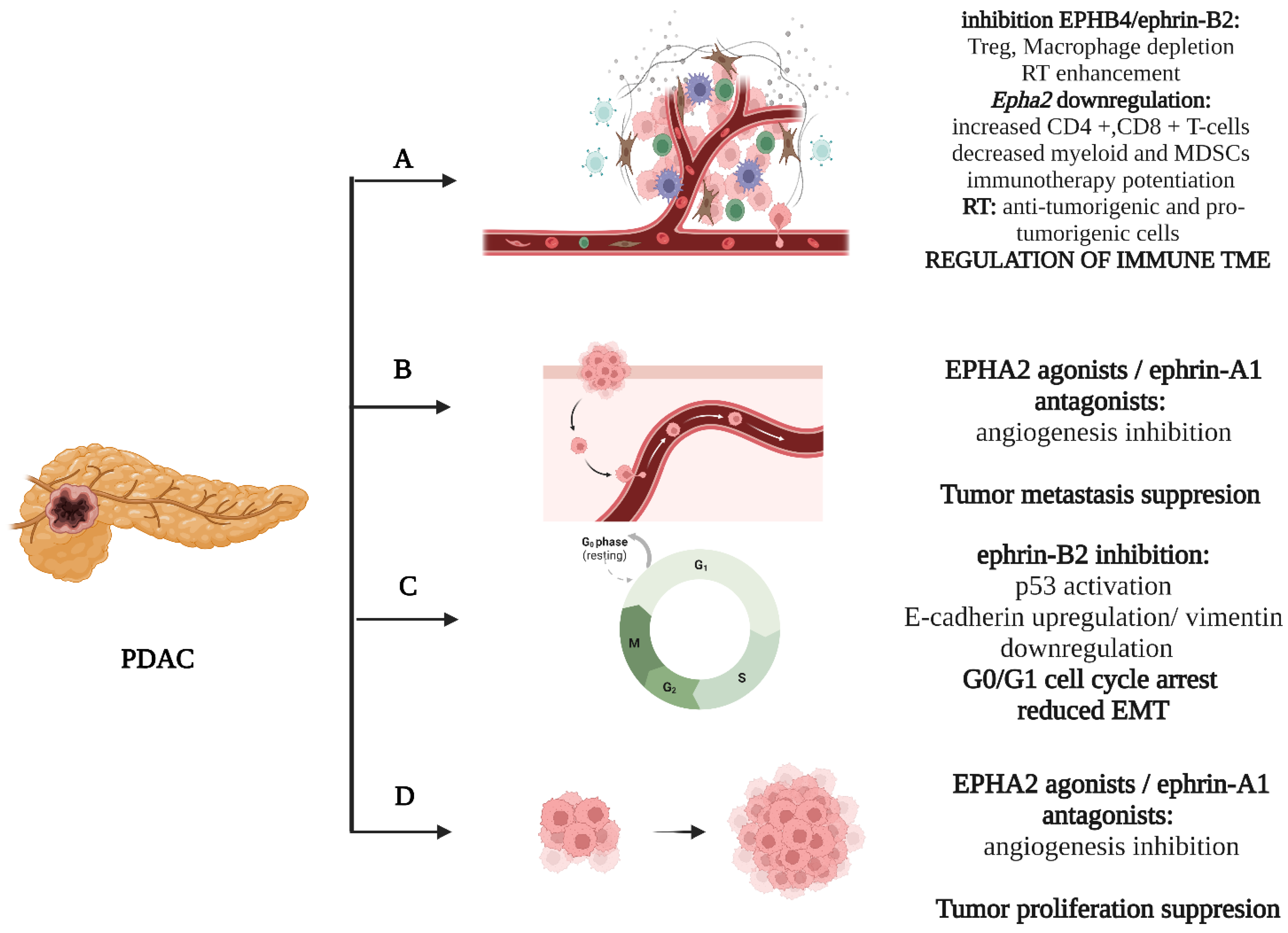
2.2. The EPH/Ephrin System in PDAC—Preclinical Data
2.3. The EPH/Ephrin System in PDAC—Clinical Data
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evan, T.; Wang, V.M.Y.; Behrens, A. The roles of intratumour heterogeneity in the biology and treatment of pancreatic ductal adenocarcinoma. Oncogene 2022, 41, 4686–4695. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, Y.; Yang, F.; Zhu, L.; Zhu, X.Q.; Wang, Z.F.; Wu, X.L.; Zhou, C.H.; Yan, J.Y.; Hu, B.Y.; et al. The molecular biology of pancreatic adenocarcinoma: Translational challenges and clinical perspectives. Signal Transduct. Target. Ther. 2021, 6, 249. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, T.; Kikuta, K.; Hamada, S.; Kume, K.; Miura, S.; Yoshida, N.; Tanaka, Y.; Matsumoto, R.; Ikeda, M.; Kataoka, F.; et al. Clinical features and prognostic impact of asymptomatic pancreatic cancer. Sci. Rep. 2022, 12, 4262. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.; Grippo, P.J. Pancreatic Cancer Subtypes: A Roadmap for Precision Medicine. Ann. Med. 2018, 50, 277. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Gong, Y.; Fan, Z.; Luo, G.; Huang, Q.; Deng, S.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. Molecular alterations and targeted therapy in pancreatic ductal adenocarcinoma. J. Hematol. Oncol. 2020, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Sarantis, P.; Koustas, E.; Papadimitropoulou, A.; Papavassiliou, A.G.; Karamouzis, M.V. Pancreatic ductal adenocarcinoma: Treatment hurdles, tumor microenvironment and immunotherapy. World J. Gastrointest. Oncol. 2020, 12, 173–181. [Google Scholar] [CrossRef]
- Thomas, A.M.; Santarsiero, L.M.; Lutz, E.R.; Armstrong, T.D.; Chen, Y.C.; Huang, L.Q.; Laheru, D.A.; Goggins, M.; Hruban, R.H.; Jaffee, E.M. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J. Exp. Med. 2004, 200, 297–306. [Google Scholar] [CrossRef]
- Beatty, G.L.; Chiorean, E.G.; Fishman, M.P.; Saboury, B.; Teitelbaum, U.R.; Sun, W.; Huhn, R.D.; Song, W.; Li, D.; Sharp, L.L.; et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011, 331, 1612–1616. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Beatty, G.L.; Dougan, S.K. Broadening the Impact of Immunotherapy to Pancreatic Cancer: Challenges and Opportunities. Gastroenterology 2019, 156, 2056–2072. [Google Scholar] [CrossRef] [PubMed]
- Bayne, L.J.; Beatty, G.L.; Jhala, N.; Clark, C.E.; Rhim, A.D.; Stanger, B.Z.; Vonderheide, R.H. Tumor-Derived Granulocyte-Macrophage Colony-Stimulating Factor Regulates Myeloid Inflammation and T Cell Immunity in Pancreatic Cancer. Cancer Cell 2012, 21, 822–835. [Google Scholar] [CrossRef]
- Rosati, A.; Basile, A.; DAuria, R.; DAvenia, M.; De Marco, M.; Falco, A.; Festa, M.; Guerriero, L.; Iorio, V.; Parente, R.; et al. BAG3 promotes pancreatic ductal adenocarcinoma growth by activating stromal macrophages. Nat. Commun. 2015, 6, 8695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Velez-Delgado, A.; Mathew, E.; Li, D.; Mendez, F.M.; Flannagan, K.; Rhim, A.D.; Simeone, D.M.; Beatty, G.L.; Di Magliano, M.P. Myeloid cells are required for PD-1/PD-L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer. Gut 2017, 66, 124–136. [Google Scholar] [CrossRef]
- Hiraoka, N.; Onozato, K.; Kosuge, T.; Hirohashi, S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin. Cancer Res. 2006, 12, 5423–5434. [Google Scholar] [CrossRef]
- Pylayeva-Gupta, Y.; Das, S.; Handler, J.S.; Hajdu, C.H.; Coffre, M.; Koralov, S.B.; Bar-Sagi, D. IL35-producing b cells promote the development of pancreatic neoplasia. Cancer Discov. 2016, 6, 247–255. [Google Scholar] [CrossRef]
- Gunderson, A.J.; Kaneda, M.M.; Tsujikawa, T.; Nguyen, A.V.; Affara, N.I.; Ruffell, B.; Gorjestani, S.; Liudahl, S.M.; Truit, M.; Olson, P.; et al. Bruton tyrosine kinase–Dependent immune cell cross-talk drives pancreas cancer. Cancer Discov. 2016, 6, 270–285. [Google Scholar] [CrossRef]
- Lee, K.E.; Spata, M.; Bayne, L.J.; Buza, E.L.; Durham, A.C.; Allman, D.; Vonderheide, R.H.; Simon, M.C. Hif1a deletion reveals pro-neoplastic function of B cells in pancreatic neoplasia. Cancer Discov. 2016, 6, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Feig, C.; Jones, J.O.; Kraman, M.; Wells, R.J.B.; Deonarine, A.; Chan, D.S.; Connell, C.M.; Roberts, E.W.; Zhao, Q.; Caballero, O.L.; et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 20212–20217. [Google Scholar] [CrossRef]
- Jiang, H.; Hegde, S.; Knolhoff, B.L.; Zhu, Y.; Herndon, J.M.; Meyer, M.A.; Nywening, T.M.; Hawkins, W.G.; Shapiro, I.M.; Weaver, D.T.; et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med. 2016, 22, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Cuevas, C.; Chang, A.E.; Goel, V.K.; Von Hoff, D.D.; Hingorani, S.R. Enzymatic Targeting of the Stroma Ablates Physical Barriers to Treatment of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2012, 21, 418–429. [Google Scholar] [CrossRef]
- Pasquale, E.B. Eph-Ephrin Bidirectional Signaling in Physiology and Disease. Cell 2008, 133, 38–52. [Google Scholar] [CrossRef]
- Psilopatis, I.; Pergaris, A.; Vrettou, K.; Tsourouflis, G.; Theocharis, S. The EPH/Ephrin System in Gynecological Cancers: Focusing on the Roots of Carcinogenesis for Better Patient Management. Int. J. Mol. Sci. 2022, 23, 3249. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, E.B. Eph receptors and ephrins in cancer: Bidirectional signalling and beyond. Nat. Rev. Cancer 2010, 10, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Pergaris, A.; Danas, E.; Goutas, D.; Sykaras, A.G.; Soranidis, A.; Theocharis, S. The clinical impact of the eph/ephrin system in cancer: Unwinding the thread. Int. J. Mol. Sci. 2021, 22, 8412. [Google Scholar] [CrossRef]
- Papadakos, S.P.; Tsagkaris, C.; Papadakis, M.; Papazoglou, A.S.; Moysidis, D.V.; Zografos, C.G.; Theocharis, S. Angiogenesis in gastrointestinal stromal tumors: From bench to bedside. World J. Gastrointest. Oncol. 2022, 14, 1469–1477. [Google Scholar] [CrossRef]
- Hadjimichael, A.C.; Pergaris, A.; Kaspiris, A.; Foukas, A.F.; Kokkali, S.; Tsourouflis, G.; Theocharis, S. The EPH/Ephrin System in Bone and Soft Tissue Sarcomas’ Pathogenesis and Therapy: New Advancements and a Literature Review. Int. J. Mol. Sci. 2022, 23, 5171. [Google Scholar] [CrossRef]
- Cecchini, A.; Cornelison, D.D.W. Eph/Ephrin-Based Protein Complexes: The Importance of cis Interactions in Guiding Cellular Processes. Front. Mol. Biosci. 2022, 8, 809364. [Google Scholar] [CrossRef]
- Peng, Q.; Chen, L.; Wu, W.; Wang, J.; Zheng, X.; Chen, Z.; Jiang, Q.; Han, J.; Wei, L.; Wang, L.; et al. EPH receptor A2 governs a feedback loop that activates Wnt/β-catenin signaling in gastric cancer. Cell Death Dis. 2018, 9, 1146. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yuan, W.; Lai, C.; Zhong, S.; Yang, C.; Wang, R.; Mao, L.; Chen, Z.; Chen, Z. EphA2-to-YAP pathway drives gastric cancer growth and therapy resistance. Int. J. Cancer 2020, 146, 1937–1949. [Google Scholar] [CrossRef]
- Webb, D.J.; Donais, K.; Whitmore, L.A.; Thomas, S.M.; Turner, C.E.; Parsons, J.T.; Horwitz, A.F. FAK–Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 2004, 6, 154–161. [Google Scholar] [CrossRef]
- Shi, L.; Fu, W.Y.; Hung, K.W.; Porchetta, C.; Hall, C.; Fu, A.K.Y.; Ip, N.Y. Alpha2-chimaerin interacts with EphA4 and regulates EphA4-dependent growth cone collapse. Proc. Natl. Acad. Sci. USA 2007, 104, 16347–16352. [Google Scholar] [CrossRef]
- Wegmeyer, H.; Egea, J.; Rabe, N.; Gezelius, H.; Filosa, A.; Enjin, A.; Varoqueaux, F.; Deininger, K.; Schnütgen, F.; Brose, N.; et al. EphA4-Dependent Axon Guidance Is Mediated by the RacGAP α2-Chimaerin. Neuron 2007, 55, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Kania, A.; Klein, R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat. Rev. Mol. Cell Biol. 2016, 17, 240–256. [Google Scholar] [CrossRef]
- Papadakos, S.P.; Petrogiannopoulos, L.; Pergaris, A.; Theocharis, S. The EPH/Ephrin System in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 2761. [Google Scholar] [CrossRef]
- Bartolomé, A.; Suda, N.; Yu, J.; Zhu, C.; Son, J.; Ding, H.; Califano, A.; Accili, D.; Pajvani, U.B. Notch-mediated Ephrin signaling disrupts islet architecture and β cell function. JCI Insight 2022, 7, e157694. [Google Scholar] [CrossRef]
- Pan, F.C.; Wright, C. Pancreas organogenesis: From bud to plexus to gland. Dev. Dyn. 2011, 240, 530–565. [Google Scholar] [CrossRef] [PubMed]
- Dorrell, C.; Schug, J.; Lin, C.F.; Canaday, P.S.; Fox, A.J.; Smirnova, O.; Bonnah, R.; Streeter, P.R.; Stoeckert, C.J.; Kaestner, K.H.; et al. Transcriptomes of the major human pancreatic cell types. Diabetologia 2011, 54, 2832. [Google Scholar] [CrossRef] [PubMed]
- Villasenor, A.; Chong, D.C.; Henkemeyer, M.; Cleaver, O. Epithelial dynamics of pancreatic branching morphogenesis. Development 2010, 137, 4295–4305. [Google Scholar] [CrossRef]
- van Eyll, J.M.; Passante, L.; Pierreux, C.E.; Lemaigre, F.P.; Vanderhaeghen, P.; Rousseau, G.G. Eph receptors and their ephrin ligands are expressed in developing mouse pancreas. Gene Expr. Patterns 2006, 6, 353–359. [Google Scholar] [CrossRef]
- Thestrup, M.I.; Caviglia, S.; Cayuso, J.; Heyne, R.L.S.; Ahmad, R.; Hofmeister, W.; Satriano, L.; Wilkinson, D.G.; Andersen, J.B.; Ober, E.A. A morphogenetic EphB/EphrinB code controls hepatopancreatic duct formation. Nat. Commun. 2019, 10, 5220. [Google Scholar] [CrossRef]
- Maechler, P.; Wollheim, C.B. Mitochondrial function in normal and diabetic β-cells. Nature 2001, 414, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Luther, M.J.; Hauge-Evans, A.; Souza, K.L.A.; Jörns, A.; Lenzen, S.; Persaud, S.J.; Jones, P.M. MIN6 β-cell-β-cell interactions influence insulin secretory responses to nutrients and non-nutrients. Biochem. Biophys. Res. Commun. 2006, 343, 99–104. [Google Scholar] [CrossRef]
- Konstantinova, I.; Nikolova, G.; Ohara-Imaizumi, M.; Meda, P.; Kučera, T.; Zarbalis, K.; Wurst, W.; Nagamatsu, S.; Lammert, E. EphA-Ephrin-A-Mediated β Cell Communication Regulates Insulin Secretion from Pancreatic Islets. Cell 2007, 129, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Hutchens, T.; Piston, D.W. EphA4 receptor forward signaling inhibits glucagon secretion from α-cells. Diabetes 2015, 64, 3839–3851. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Xiao, Y.; Wang, W.; Tang, Y.Y.; Xiao, Z.; Su, M. Targeting EphA2 in cancer. J. Hematol. Oncol. 2020, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Pasqualini, R.; Lindberg, R.A.; Kain, R.; Freeman, A.L.; Pasquale, E.B. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene 2000, 19, 6043–6052. [Google Scholar] [CrossRef] [PubMed]
- van Geer, M.A.; Bakker, C.T.; Koizumi, N.; Mizuguchi, H.; Wesseling, J.G.; Elferink, R.P.J.O.; Bosma, P.J. Ephrin A2 receptor targeting does not increase adenoviral pancreatic cancer transduction in vivo. World J. Gastroenterol. 2009, 15, 2754–2762. [Google Scholar] [CrossRef]
- Vitelli, A.; Ansuini, H.; Meola, A.; Gunes, Z.; Paradisi, V.; Pezzanera, M.; Acali, S.; Santini, C.; Luzzago, A.; Mori, F.; et al. Anti-EphA2 antibodies with distinct in vitro properties have equal in vivo efficacy in pancreatic cancer. J. Oncol. 2009, 2009, 951917. [Google Scholar] [CrossRef]
- Markosyan, N.; Li, J.; Sun, Y.H.; Richman, L.P.; Lin, J.H.; Yan, F.; Quinones, L.; Sela, Y.; Yamazoe, T.; Gordon, N.; et al. Tumor cell-intrinsic EPHA2 suppresses antitumor immunity by regulating PTGS2 (COX-2). J. Clin. Investig. 2019, 129, 3594–3609. [Google Scholar] [CrossRef]
- Müller-Decker, K.; Fürstenberger, G.; Annan, N.; Kucher, D.; Pohl-Arnold, A.; Steinbauer, B.; Esposito, I.; Chiblak, S.; Friess, H.; Schirmacher, P.; et al. Preinvasive Duct-Derived Neoplasms in Pancreas of Keratin 5–Promoter Cyclooxygenase-2 Transgenic Mice. Gastroenterology 2006, 130, 2165–2178. [Google Scholar] [CrossRef]
- Hill, R.; Li, Y.; Tran, L.M.; Dry, S.; Calvopina, J.H.; Garcia, A.; Kim, C.; Wang, Y.; Donahue, T.R.; Herschman, H.R.; et al. Cell intrinsic role of COX-2 in pancreatic cancer development. Mol. Cancer Ther. 2012, 11, 2127–2137. [Google Scholar] [CrossRef]
- Philip, B.; Roland, C.L.; Daniluk, J.; Liu, Y.; Chatterjee, D.; Gomez, S.B.; Ji, B.; Huang, H.; Wang, H.; Fleming, J.B.; et al. A High-Fat Diet Activates Oncogenic Kras and COX2 to Induce Development of Pancreatic Ductal Adenocarcinoma in Mice. Gastroenterology 2013, 145, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Conejo-Garcia, J.R. Breaking barriers for T cells by targeting the EPHA2/ TGF-β/COX-2 axis in pancreatic cancer. J. Clin. Investig. 2019, 129, 3521–3523. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Hu, T.; Han, G.; Wu, Y.; Hua, X.; Su, J.; Jin, W.; Mou, Y.; Mou, X.; Li, Q.; et al. Accurate Cancer Diagnosis and Stage Monitoring Enabled by Comprehensive Profiling of Different Types of Exosomal Biomarkers: Surface Proteins and miRNAs. Small 2020, 16, e2004492. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Ino, Y.; Naito, C.; Nara, S.; Shimasaki, M.; Ishimoto, U.; Iwasaki, T.; Doi, N.; Esaki, M.; Kishi, Y.; et al. Neoadjuvant therapy alters the collagen architecture of pancreatic cancer tissue via Ephrin-A5. Br. J. Cancer 2022, 126, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Renuse, S.; Madamsetty, V.S.; Mun, D.G.; Madugundu, A.K.; Singh, S.; Udainiya, S.; Mangalaparthi, K.K.; Kim, M.S.; Liu, R.; Kumar, S.R.; et al. Tyrosine phosphoproteomics of patient-derived xenografts reveals ephrin type-b receptor 4 tyrosine kinase as a therapeutic target in pancreatic cancer. Cancers 2021, 13, 3404. [Google Scholar] [CrossRef]
- Djokovic, D.; Trindade, A.; Gigante, J.; Badenes, M.; Silva, L.; Liu, R.; Li, X.; Gong, M.; Krasnoperov, V.; Gill, P.S.; et al. Combination of Dll4/Notch and Ephrin-B2/EphB4 targeted therapy is highly effective in disrupting tumor angiogenesis. BMC Cancer 2010, 10, 641. [Google Scholar] [CrossRef]
- Zhu, F.; Dai, S.N.; Xu, D.L.; Hou, C.Q.; Liu, T.T.; Chen, Q.Y.; Wu, J.L.; Miao, Y. EFNB2 facilitates cell proliferation, migration, and invasion in pancreatic ductal adenocarcinoma via the p53/p21 pathway and EMT. Biomed. Pharmacother. 2020, 125, 109972. [Google Scholar] [CrossRef]
- Demaria, S.; Coleman, C.N.; Formenti, S.C. Radiotherapy: Changing the Game in Immunotherapy. Trends Cancer 2016, 2, 286–294. [Google Scholar] [CrossRef]
- Lennon, S.; Oweida, A.; Milner, D.; Phan, A.V.; Bhatia, S.; Van Court, B.; Darragh, L.; Mueller, A.C.; Raben, D.; Martínez-Torrecuadrada, J.L.; et al. Pancreatic tumor microenvironment modulation by EphB4-ephrinB2 inhibition and radiation combination. Clin. Cancer Res. 2019, 25, 3352–3365. [Google Scholar] [CrossRef]
- Giaginis, C.; Tsourouflis, G.; Zizi-Serbetzoglou, A.; Kouraklis, G.; Chatzopoulou, E.; Dimakopoulou, K.; Theocharis, S.E. Clinical Significance of Ephrin (Eph)-A1, -A2, -A4, -A5 and -A7 Receptors in Pancreatic Ductal Adenocarcinoma. Pathol. Oncol. Res. 2010, 16, 267–276. [Google Scholar] [CrossRef]
- Van Den Broeck, A.; Vankelecom, H.; Van Eijsden, R.; Govaere, O.; Topal, B. Molecular markers associated with outcome and metastasis in human pancreatic cancer. J. Exp. Clin. Cancer Res. 2012, 31, 68. [Google Scholar] [CrossRef] [PubMed]
- Koshikawa, N.; Minegishi, T.; Kiyokawa, H.; Seiki, M. Specific detection of soluble EphA2 fragments in blood as a new biomarker for pancreatic cancer. Cell Death Dis. 2017, 8, e3134. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhang, J.; Li, Z.; Wei, L.; Ren, L. Serum Exo-EphA2 as a Potential Diagnostic Biomarker for Pancreatic Cancer. Pancreas 2020, 49, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.K.; Parakh, S.; Lee, F.T.; Tebbutt, N.C.; Ameratunga, M.; Lee, S.T.; O’Keefe, G.J.; Gong, S.J.; Vanrenen, C.; Caine, J.; et al. A phase 1 safety and bioimaging trial of antibody DS-8895a against EphA2 in patients with advanced or metastatic EphA2 positive cancers. Investig. New Drugs 2022, 40, 747–755. [Google Scholar] [CrossRef]
- Oweida, A.; Bhatia, S.; Hirsch, K.; Calame, D.; Griego, A.; Keysar, S.; Pitts, T.; Sharma, J.; Eckhardt, G.; Jimeno, A.; et al. Ephrin-B2 overexpression predicts for poor prognosis and response to therapy in solid tumors. Mol. Carcinog. 2017, 56, 1189–1196. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, Y.; Li, Z.; Yu, S.; Zhao, G.; Li, M.; Wang, Z.; Wang, Q.; Yang, Y. Overexpression of the B-type Eph and ephrin genes correlates with progression and pain in human pancreatic cancer. Oncol. Lett. 2012, 3, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Chee, C.E.; Krishnamurthi, S.; Nock, C.J.; Meropol, N.J.; Gibbons, J.; Fu, P.; Bokar, J.; Teston, L.; O’Brien, T.; Gudena, V.; et al. Phase II Study of Dasatinib (BMS-354825) in Patients With Metastatic Adenocarcinoma of the Pancreas. Oncologist 2013, 18, 1091–1092. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.; Nakamura, T.; Tsuchikawa, T.; Kushibiki, T.; Hontani, K.; Inoko, K.; Takahashi, M.; Sato, S.; Abe, H.; Takeuchi, S.; et al. Inhibition of Eph receptor A4 by 2,5-dimethylpyrrolyl benzoic acid suppresses human pancreatic cancer growing orthotopically in nude mice. Oncotarget 2015, 6, 41063–41076. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.C.; Piper, M.; Goodspeed, A.; Bhuvane, S.; Williams, J.S.; Bhatia, S.; Phan, A.V.; Van Court, B.; Zolman, K.L.; Peña, B.; et al. Induction of ADAM10 by Radiation Therapy Drives Fibrosis, Resistance, and Epithelial-to-Mesenchyal Transition in Pancreatic Cancer. Cancer Res. 2021, 81, 3255–3269. [Google Scholar] [CrossRef]
- Shitara, K.; Satoh, T.; Iwasa, S.; Yamaguchi, K.; Muro, K.; Komatsu, Y.; Nishina, T.; Esaki, T.; Hasegawa, J.; Kakurai, Y.; et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the afucosylated, humanized anti-EPHA2 antibody DS-8895a: A first-in-human phase i dose escalation and dose expansion study in patients with advanced solid tumors. J. Immunother. Cancer 2019, 7, 219. [Google Scholar] [CrossRef]
- Huang, Z.R.; Tipparaju, S.K.; Kirpotin, D.B.; Pien, C.; Kornaga, T.; Noble, C.O.; Koshkaryev, A.; Tran, J.; Kamoun, W.S.; Drummond, D.C. Formulation optimization of an ephrin A2 targeted immunoliposome encapsulating reversibly modified taxane prodrugs. J. Control. Release 2019, 310, 47–57. [Google Scholar] [CrossRef]
- Wagner, M.J.; Mitra, R.; Mcarthur, M.J.; Baze, W.; Barnhart, K.; Wu, S.Y.; Rodriguez-Aguayo, C.; Zhang, X.; Coleman, R.L.; Lopez-Berestein, G.; et al. Preclinical Mammalian Safety Studies of EPHARNA (DOPC Nanoliposomal EphA2-Targeted siRNA). Mol. Cancer Ther. 2017, 16, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Chatzizacharias, N.A.; Giaginis, C.T.; Agapitos, E.; Theocharis, S.E. The role of ephrins’ receptors and ephrins’ ligands in normal placental development and disease. Expert Opin. Ther. Targets 2014, 18, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Vasileiou, I.; Adamakis, I.; Patsouris, E.; Theocharis, S. Ephrins and pain. Expert Opin. Ther. Targets 2013, 17, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, D.; Thomas, J.A.; Ali, A.; Kumar, R. Mechanistic and therapeutic implications of EphA-4 receptor tyrosine kinase in the pathogenesis of Alzheimer’s disease. Eur. J. Neurosci. 2022, 56, 5532–5546. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, S.; Tang, A.; Al-Rabadi, L.; Henkemeyer, M.; Mimche, P.N.; Huang, Y. Key role for EphB2 receptor in kidney fibrosis. Clin. Sci. 2021, 135, 2127–2142. [Google Scholar] [CrossRef] [PubMed]
- Goutas, D.; Pergaris, A.; Goutas, N.; Theocharis, S. Utilizing Exosomal-EPHs/Ephrins as Biomarkers and as a Potential Platform for Targeted Delivery of Therapeutic Exosomes. Int. J. Mol. Sci. 2022, 23, 3551. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Venkitachalam, S.; Babu, D.; Ravillah, D.; Katabathula, R.M.; Joseph, P.; Singh, S.; Udhayakumar, B.; Miao, Y.; Martinez-Uribe, O.; Hogue, J.A.; et al. The Ephrin B2 Receptor Tyrosine Kinase Is a Regulator of Proto-oncogene MYC and Molecular Programs Central to Barrett’s Neoplasia. Gastroenterology 2022, 163, 1228–1241. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekera, P.; Perfetto, M.; Lu, C.; Zhuo, M.; Bahudhanapati, H.; Li, J.; Chen, W.-C.; Kulkarni, P.; Christian, L.; Liu, J.; et al. Metalloprotease ADAM9 cleaves ephrin-B ligands and differentially regulates Wnt and mTOR signaling downstream of Akt kinase in colorectal cancer cells. J. Biol. Chem. 2022, 298, 102225. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Nguyen, D.; Darragh, L.B.; Van Court, B.; Sharma, J.; Knitz, M.W.; Piper, M.; Bukkapatnam, S.; Gadwa, J.; Bickett, T.E.; et al. EphB4 and ephrinB2 act in opposition in the head and neck tumor microenvironment. Nat. Commun. 2022, 13, 3535. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, L.; Li, X.; Xu, S. EphA10 drives tumor progression and immune evasion by regulating the MAPK/ERK cascade in lung adenocarcinoma. Int. Immunopharmacol. 2022, 110, 109031. [Google Scholar] [CrossRef] [PubMed]
- Papadakos, S.P.; Dedes, N.; Pergaris, A.; Gazouli, M.; Theocharis, S. Exosomes in the Treatment of Pancreatic Cancer: A Moonshot to PDAC Treatment? Int. J. Mol. Sci. 2022, 23, 3620. [Google Scholar] [CrossRef]
- Gulhati, P.; Schalck, A.; Jiang, S.; Shang, X.; Wu, C.J.; Hou, P.; Ruiz, S.H.; Soto, L.S.; Parra, E.; Ying, H.; et al. Targeting T cell checkpoints 41BB and LAG3 and myeloid cell CXCR1/CXCR2 results in antitumor immunity and durable response in pancreatic cancer. Nat. Cancer 2022, 4, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Eun, S.-Y.; Lee, S.-W.; Xu, Y.; Croft, M. 4-1BB ligand signaling to T cells limits T cell activation. J. Immunol. 2015, 194, 134–141. [Google Scholar] [CrossRef]
- Maruhashi, T.; Sugiura, D.; Okazaki, I.-M.; Shimizu, K.; Maeda, T.K.; Ikubo, J.; Yoshikawa, H.; Maenaka, K.; Ishimaru, N.; Kosako, H.; et al. Binding of LAG-3 to stable peptide-MHC class II limits T cell function and suppresses autoimmunity and anti-cancer immunity. Immunity 2022, 55, 912–924. [Google Scholar] [CrossRef]
- von Ahrens, D.; Bhagat, T.D.; Nagrath, D.; Maitra, A.; Verma, A. The role of stromal cancer-associated fibroblasts in pancreatic cancer. J. Hematol. Oncol. 2017, 10, 76. [Google Scholar] [CrossRef]
- Mirlekar, B.; Michaud, D.; Searcy, R.; Greene, K.; Pylayeva-Gupta, Y. IL35 Hinders Endogenous Antitumor T-cell Immunity and Responsiveness to Immunotherapy in Pancreatic Cancer. Cancer Immunol. Res. 2018, 6, 1014–1024. [Google Scholar] [CrossRef]
- Mirlekar, B.; Wang, Y.; Li, S.; Zhou, M.; Entwistle, S.; De Buysscher, T.; Morrison, A.; Herrera, G.; Harris, C.; Vincent, B.G.; et al. Balance between immunoregulatory B cells and plasma cells drives pancreatic tumor immunity. Cell Rep. Med. 2022, 0, 100744. [Google Scholar] [CrossRef]
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020, 577, 549–555. [Google Scholar] [CrossRef]
- Sharonov, G.V.; Serebrovskaya, E.O.; Yuzhakova, D.V.; Britanova, O.V.; Chudakov, D.M. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat. Rev. Immunol. 2020, 20, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Papadakos, S.P.; Dedes, N.; Kouroumalis, E.; Theocharis, S. The Role of the NLRP3 Inflammasome in HCC Carcinogenesis and Treatment: Harnessing Innate Immunity. Cancers 2022, 14, 3150. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, K.; Koletsa, T.; Mitroulis, I.; Germanidis, G. Tumor-Associated Macrophages in Hepatocellular Carcinoma Pathogenesis, Prognosis and Therapy. Cancers 2022, 14, 226. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, K.; Mitroulis, I.; Germanidis, G. Tumor-associated neutrophils in hepatocellular carcinoma pathogenesis, prognosis, and therapy. Cancers 2021, 13, 2899. [Google Scholar] [CrossRef]
- Lenzo, F.L.; Kato, S.; Pabla, S.; DePietro, P.; Nesline, M.K.; Conroy, J.M.; Burgher, B.; Glenn, S.T.; Kuvshinoff, B.; Kurzrock, R.; et al. Immune profiling and immunotherapeutic targets in pancreatic cancer. Ann. Transl. Med. 2021, 9, 119. [Google Scholar] [CrossRef]

| EPH/Ephrin | Study Material | Result | References |
|---|---|---|---|
| EPHA1/A2/A4/A5/A7 | Neoplastic tissue | EPHA1 staining intensity was significantly associated with
| [60] |
| EPHA2 | Neoplastic tissue | EPHA2 was associated with poor outcome and aggressive disease | [54,61] |
| EPHA2 | Soluble EPHA2 fragments | May be applicable as a diagnostic biomarker | [62] |
| EPHA2 | Neoplastic tissue | The expression of EPHA2 was inversely correlated with the degree of T cell infiltration in PDAC | [48] |
| EPHA2 | PC patients | Dasatinib (inhibition of EPHA2) did not show clinical activity in metastatic PDAC | [67] |
| EPHA4 | Neoplastic tissue | EPHA4 positivity was associated with lower overall survival | [68] |
| EPHB2/ephrin-B2 | Neoplastic tissue | Overexpression of EPHB2 and ephrin-B2 was associated with:
| [66] |
| ephrin-B2 | Neoplastic tissue | High ephrin-B2 expression correlated with:
| [65] |
| ephrin-B2 | Neoplastic tissue | Lower expression of ephrin-B2 and ADAM10 after neo-adjuvant therapy was associated with better:
| [69] |
| EPHB4 | PDAC patients | Significant expression of EPHB4 in >70% of patients with PDAC | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadakos, S.P.; Dedes, N.; Gkolemi, N.; Machairas, N.; Theocharis, S. The EPH/Ephrin System in Pancreatic Ductal Adenocarcinoma (PDAC): From Pathogenesis to Treatment. Int. J. Mol. Sci. 2023, 24, 3015. https://doi.org/10.3390/ijms24033015
Papadakos SP, Dedes N, Gkolemi N, Machairas N, Theocharis S. The EPH/Ephrin System in Pancreatic Ductal Adenocarcinoma (PDAC): From Pathogenesis to Treatment. International Journal of Molecular Sciences. 2023; 24(3):3015. https://doi.org/10.3390/ijms24033015
Chicago/Turabian StylePapadakos, Stavros P., Nikolaos Dedes, Nikolina Gkolemi, Nikolaos Machairas, and Stamatios Theocharis. 2023. "The EPH/Ephrin System in Pancreatic Ductal Adenocarcinoma (PDAC): From Pathogenesis to Treatment" International Journal of Molecular Sciences 24, no. 3: 3015. https://doi.org/10.3390/ijms24033015
APA StylePapadakos, S. P., Dedes, N., Gkolemi, N., Machairas, N., & Theocharis, S. (2023). The EPH/Ephrin System in Pancreatic Ductal Adenocarcinoma (PDAC): From Pathogenesis to Treatment. International Journal of Molecular Sciences, 24(3), 3015. https://doi.org/10.3390/ijms24033015









