Host-Derived Cytotoxic Agents in Chronic Inflammation and Disease Progression
Abstract
:1. Introduction
2. The Balance between the Action of Cytotoxic Agents and Protective Principles
2.1. Major Classes of Host-Derived Cytotoxic Agents
2.2. Control of Cytotoxic Agents by Protective Principles
2.3. Disturbed Balance between De Novo Synthesis and Damage of Tissue Components during Resolution of Inflammation
2.4. Selected Environmental Cytotoxic Agents
3. Selected Cytotoxic Agents and Their Counter-Regulating Principles
3.1. Small Reactive Species and Metal Ions
3.1.1. Superoxide Anion Radicals
3.1.2. Hydrogen Peroxide
3.1.3. Transition Metal Ions and Hydroxyl Radicals
3.1.4. Peroxynitrite
3.1.5. Heme Peroxidases and Hypohalous Acids
3.2. Hemoglobin and Myoglobin Metabolites
3.3. Oxidation of Cell and Tissue Components
3.4. Serine Proteases
3.4.1. Release of Serine Proteases from Immune Cells
3.4.2. Activities of Neutrophil Serine Proteases
3.4.3. Mast Cell Serine Proteases
3.4.4. Antiproteases
3.5. Small Pro-Inflammatory Peptides
3.5.1. Angiotensin II
3.5.2. Bradykinin
3.6. Inhibition of Matrix Metalloproteases
4. Enhanced Cell and Tissue Damage during Chronic Inflammatory Diseases
4.1. Most Prominent Degradative Agents
4.2. Diminished Control over Transition Metal Ions
4.3. Haptoglobin and Hemopexin Exhaustion
4.4. Inactivation of Antiproteases
4.5. Disastrous Action of Angiotensin II
4.6. Disturbed Balance between MMPs and TIMPs
4.7. Cytotoxic Agents in Tumorigenesis
4.8. Diminished Protection during Sepsis
5. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.R.; Kaminski, J.J.; Kurt-Jones, E.A.; Fitzgerald, K.A. Pattern recognition receptors and the innate immune response to viral infection. Viruses 2011, 3, 920–940. [Google Scholar] [CrossRef]
- Suresh, R.; Moser, D.M. Pattern recognition in innate immunity, host defense, and immunopathology. Adv. Physiol. Educ. 2013, 37, 284–291. [Google Scholar] [CrossRef]
- Gewurz, H.; Mold, C.; Siegel, J.; Fiedel, B. C-Reactive protein and the acute phase response. Adv. Intern. Med. 1982, 27, 345–372. [Google Scholar] [CrossRef]
- Pepys, M.B.; Baltz, M.I. Acute phase proteins with special reference to C-reactive protein and related proteins (pentraxins) and serum amyloid A protein. Adv. Immunol. 1983, 34, 141–212. [Google Scholar] [CrossRef]
- Vandivier, R.W.; Henson, P.M.; Douglas, I.S. Burying the death: The impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest 2006, 129, 1673–1682. [Google Scholar] [CrossRef]
- Pober, J.S.; Sessa, W.C. Inflammation and the blood microvascular system. Cold Spring Harbor Perspect. Biol. 2015, 7, 016345. [Google Scholar] [CrossRef]
- Arnhold, J. Immune response and tissue damage. In Cell and Tissue Destruction. Mechanisms, Protection, Disorders; Academic Press: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK, 2020; pp. 155–204. [Google Scholar] [CrossRef]
- Arnhold, J. Acute-phase proteins and additional protective systems. In Cell and Tissue Destruction. Mechanisms, Protection, Disorders; Academic Press: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK, 2020; pp. 205–228. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Yoshimura, A.; Wakabayashi, Y.; Mori, T. Cellular and molecular basis for the regulation of inflammation by TGF-β. J. Biochem. 2010, 147, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef]
- Li, M.O.; Flavell, R.A. Contextual regulation of inflammation: A duet by transforming growth factor-beta and interleukin-10. Immunity 2008, 28, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, J. Cells and organisms as open systems. In Cell and Tissue Destruction. Mechanisms, Protection, Disorders; Academic Press: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK, 2020; pp. 3–22. [Google Scholar] [CrossRef]
- Arnhold, J. The dual role of myeloperoxidase in immune response. Int. J. Mol. Sci. 2020, 21, 8057. [Google Scholar] [CrossRef]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.K.; Flavell, R.A. Transforming growth factor-β regulation of immune responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Transforming growth factor-β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef]
- Budi, E.H.; Schaub, J.R.; Decaris, M.; Turner, S.; Derynck, R. TGF-β as a driver of fibrosis: Physiological roles and therapeutic opportunities. J. Pathol. 2021, 254, 358–373. [Google Scholar] [CrossRef]
- Cadet, J.; Teoule, R. Comparative study of oxidation of nucleic acid components by hydroxyl radicals, singlet oxygen and superoxide anion radicals. Photochem. Photobiol. 1978, 28, 661–667. [Google Scholar] [CrossRef]
- Agnez-Lima, L.F.; Melo, J.T.A.; Silva, A.E.; Oliviera, A.H.S.; Timoteo, A.R.S.; Lima-Bessa, K.M.; Martinez, G.R.; Medeiros, M.H.G.; Di Mascio, P.; Galhardo, R.S.; et al. DNA damage by singlet oxygen and cellular protective mechanisms. Mutat. Res. 2012, 751, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, P.; Devasagayam, T.P.A.; Kaiser, S.; Sies, H. Carotenoids, tocopherols and thiols as singlet oxygen quenchers. Biochem. Soc. Trans. 1990, 18, 1054–1056. [Google Scholar] [CrossRef] [PubMed]
- Conn, P.F.; Schalch, W.; Truscott, T.G. The singlet oxygen and carotenoid interaction. J. Photochem. Photobiol. B Biol. 1991, 11, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, P.; Bechara, E.J.H.; Medeiros, H.G.; Briviba, K.; Sies, H. Singlet molecular oxygen production in the reaction of peroxynitrite with hydrogen peroxide. FEBS Lett. 1994, 355, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A. The formation of free radicals and the consequences of their reactions in vivo. Photochem. Photobiol. 1978, 28, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Kermani, S.; Ben-Jebria, A.; Ultman, J.S. Kinetics of ozone reaction with uric acid, ascorbic acid, and glutathione at physiologically relevant conditions. Arch. Biochem. Biophys. 2006, 451, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Behndig, A.F.; Blomberg, A.; Helleday, R.; Duggan, S.T.; Kelly, F.J.; Mudway, I.S. Antioxidant responses to acute ozone challenge in the healthy human airway. Inhal. Toxicol. 2009, 21, 933–942. [Google Scholar] [CrossRef]
- Devlin, R.B.; Folinsbee, L.J.; Biscardi, F.; Hatch, G.; Becker, S.; Madden, M.C.; Robbins, M.; Koren, H.S. Inflammation and cell damage induced by repeated exposure of humans to ozone. Inhal. Toxicol. 1997, 9, 211–234. [Google Scholar] [CrossRef]
- Frank, R.; Liu, M.C.; Spannhake, E.W.; Mlynarek, S.; Macri, K.; Weinmann, G.G. Repetitive ozone exposure of young adults. Evidence of persistent small airway dysfunction. Am. J. Respir. Crit. Care Med. 2001, 164, 1257–1260. [Google Scholar] [CrossRef] [Green Version]
- Tyrrell, R.M. UVA (320–380 nm) radiation as an oxidative stress. In Oxidative Stress: Oxidants and Antioxidants; Sies, H., Ed.; Academic Press: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK, 1991; pp. 57–83. [Google Scholar]
- Korać, R.R.; Khambholja, K.M. Potential of herbs in skin protection from ultraviolet radiation. Pharmacogn. Rev. 2011, 5, 164–173. [Google Scholar] [CrossRef]
- Von Sonntag, C.; Schuchmann, H.-P. Pulse radiolysis. Meth. Enzymol. 1994, 233, 3–56. [Google Scholar] [CrossRef]
- Biakov, V.M.; Stepanov, S.V. Mechanism of primary radiobiologic action. Radiat. Biol. Radioecol. 1997, 37, 469–474. [Google Scholar]
- Nair, C.K.K.; Parida, D.K.; Nomura, T. Radioprotectors in radiotherapy. J. Radiat. Res. 2001, 42, 21–37. [Google Scholar] [CrossRef]
- Arnhold, J. Role of reactive species in destructions. In Cell and Tissue Destruction. Mechanisms, Protection, Disorders; Academic Press: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK, 2020; pp. 23–54. [Google Scholar]
- Segal, A.W.; Jones, O.T.G. Novel cytochrome b system in phagocytic vacuoles from human granulocytes. Nature 1976, 276, 515–517. [Google Scholar] [CrossRef]
- Babior, B.M. NADPH oxidase. Curr. Opin. Immunol. 2004, 16, 42–47. [Google Scholar] [CrossRef]
- Cheng, G.; Cao, Z.; Xu, X.; van Meir, E.G.; Lambeth, J.D. Homologs of gp91phox: Cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 2001, 269, 131–140. [Google Scholar] [CrossRef]
- Geiszt, M.; Witta, J.; Baffi, J.; Lekstrom, K.; Leto, T.L. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal host defense. FASEB J. 2003, 17, 1502–1504. [Google Scholar] [CrossRef]
- Harper, R.W.; Xu, C.; Eiserich, J.P.; Chen, Y.; Kao, C.Y.; Thai, P.; Setiadi, H.; Wu, R. Differential regulation of dual NADPH/peroxidases. Duox1 and Duox2, by Th1 and Th2 cytokines in the respiratory tract epithelium. FEBS Lett. 2005, 579, 4911–4917. [Google Scholar] [CrossRef]
- Olson, J.S.; Ballou, D.P.; Palmer, G.; Massey, V. The reaction of xanthine oxidase with molecular oxygen. J. Biol. Chem. 1974, 249, 4350–4362. [Google Scholar] [CrossRef]
- Anderson, R.F.; Hille, R.; Massey, V. The radical chemistry of milk xanthine oxidase as studies by radiation chemistry technique. J. Biol. Chem. 1986, 261, 15870–15876. [Google Scholar] [CrossRef]
- Carrell, R.W.; Winterbourn, C.C.; Rachmilewitz, E.A. Activated oxygen and hemolysis. Br. J. Hematol. 1975, 30, 259–264. [Google Scholar] [CrossRef]
- Harel, S.; Kanner, J. Hemoglobin and myoglobin as inhibitors of hydroxyl radical generation in a model system of “iron redox” cycle. Free Radic. Res. Commun. 1989, 6, 1–10. [Google Scholar] [CrossRef]
- Land, E.J.; Mukherfee, T.; Swallow, A.J.; Bruce, J.M. One-electron reduction of adriamycin: Properties of the semiquinone. Arch. Biochem. Biophys. 1983, 225, 116–121. [Google Scholar] [CrossRef]
- Camhi, S.L.; Lee, P.; Choi, A.M.K. The oxidative stress response. New Horizons 1995, 3, 170–182. [Google Scholar] [PubMed]
- Goncalves, R.L.; Quinlan, C.L.; Perevoshchikova, I.V.; Hey-Mogensen, M.; Brand, M.D. Site of superoxide and hydrogen peroxide production by muscle mitochondria assessed ex vivo under conditions mimicking rest and exercise. J. Biol. Chem. 2015, 290, 209–227. [Google Scholar] [CrossRef]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef]
- Koppenol, W.H. Thermodynamics of reactions involving oxyradicals and hydrogen peroxide. Bioelectrochem. Bioenerg. 1987, 18, 3–11. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L. Reactivity of HO2/O2− radicals in aqueous solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- Huie, R.E.; Padmaja, S. Reactions of NO and O2−. Free Radic. Res. Commun. 1993, 18, 195–199. [Google Scholar] [CrossRef]
- Kissner, R.; Nauser, T.; Bugnon, P.; Lye, P.G.; Koppenol, W.H. Formation and properties of peroxynitrite as studied by laser flash photolysis, high-pressure stopped-flow technique, and pulse radiolysis. Chem. Res. Toxicol. 1997, 10, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Gardner, P.R. Superoxide-driven aconitase Fe-S cycling. Biosci. Rep. 1997, 17, 33–42. [Google Scholar] [CrossRef]
- Gardner, P.R. Aconitase: Sensitive target and measure of superoxide. Meth. Enzymol. 2002, 349, 9–23. [Google Scholar] [CrossRef]
- McCord, J.; Fridovich, I. Superoxide dismutase: An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1960, 224, 6049–6055. [Google Scholar]
- Chang, L.Y.; Slot, J.W.; Geuze, H.J.; Crapo, J.D. Molecular immunocytochemistry of the CuZn superoxide dismutase in rat hepatocytes. J. Cell Biol. 1988, 107, 2169–2179. [Google Scholar] [CrossRef] [PubMed]
- Weisiger, R.A.; Fridovich, I. Mitochondrial superoxide dismutase. Site of synthesis and intramolecular localization. J. Biol. Chem. 1973, 248, 4793–4796. [Google Scholar] [CrossRef] [PubMed]
- Antonyuk, S.V.; Strange, R.W.; Marklund, S.L.; Hasnain, S.S. The structure of human extracellular copper-zinc superoxide dismutase at 1.7 Å resolution: Insights into heparin and collagen binding. J. Mol. Biol. 2009, 388, 310–326. [Google Scholar] [CrossRef]
- Starkov, A.A. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. N. Y. Acad. Sci. 2008, 1147, 37–52. [Google Scholar] [CrossRef]
- Pasdois, P.; Parker, J.E.; Griffiths, E.J.; Halestrap, A.P. The role of oxidized cytochrome c in regulating mitochondrial reactive species production and its perturbation in ischemia. Biochem. J. 2011, 436, 493–505. [Google Scholar] [CrossRef]
- Schrader, M.; Fahimi, H.D. Peroxisomes and oxidative stress. Biochim. Biophys. Acta 2006, 1763, 1755–1766. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Butler, J. Mechanisms of reactions involving singlet oxygen and the superoxide anion. FEBS Lett. 1977, 83, 1–6. [Google Scholar] [CrossRef]
- Reich, H.J.; Hondal, R.J. Why nature chose selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Iron as a biological pro-oxidant. ISI Atlas Sci. Biochem. 1988, 1, 48–52. [Google Scholar]
- Koppenol, W.H. The centennial of the Fenton reaction. Free Radic. Biol. Chem. 1993, 15, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, J.; Flemmig, J. Human myeloperoxidase in innate and acquired immunity. Arch. Biochem. Biophys. 2010, 500, 92–106. [Google Scholar] [CrossRef]
- Flemmig, J.; Gau, J.; Schlorke, D.; Arnhold, J. Lactoperoxidase as potential drug target. Expert Opin. Ther. Targets 2016, 20, 447–461. [Google Scholar] [CrossRef]
- Arnhold, J. Heme peroxidases at unperturbed and inflamed mucous surfaces. Antioxidants 2021, 10, 1805. [Google Scholar] [CrossRef]
- Arnhold, J.; Malle, E. Halogenation activity of mammalian heme peroxidases. Antioxidants 2022, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Maiorino, M.; Roveri, A. Phospholipid hydroperoxide glutathione peroxidase (PHGPx): More than an antioxidant enzyme? Biomed. Environm. Sci. 1997, 10, 327–332. [Google Scholar]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Meister, A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988, 263, 17205–17208. [Google Scholar] [CrossRef]
- Low, F.M.; Hampton, M.P.; Winterbourn, C.C. Prx2 and peroxide metabolism in the erythrocyte. Antioxid. Redox Signal. 2008, 10, 1621–1630. [Google Scholar] [CrossRef]
- Goyal, M.M.; Basak, A. Human catalase: Looking for complete identity. Prot. Cell 2010, 1, 888–897. [Google Scholar] [CrossRef]
- Gunther, M.R.; Hanna, P.M.; Mason, R.P.; Cohen, M.S. Hydroxyl radical formation from cuprous ion and hydrogen peroxide: A spin-trapping study. Arch. Biochem. Biophys. 1995, 316, 515–522. [Google Scholar] [CrossRef]
- Ryan, T.P.; Aust, S.D. The role of iron in oxygen-mediated toxicities. Crit. Rev. Toxicol. 1992, 22, 119–141. [Google Scholar] [CrossRef]
- Reinke, L.A.; Rau, J.M.; McCay, P.B. Characteristics of an oxidant formed during iron(II) autoxidation. Free Radic. Biol. Med. 1994, 16, 485–492. [Google Scholar] [CrossRef]
- Qian, S.Y.; Buettner, G.R. Iron and dioxygen chemistry is an important route to initiation of biological free radical oxidations: An electron paramagnetic resonance spin trapping study. Free Radic. Biol. Med. 1999, 26, 1447–1456. [Google Scholar] [CrossRef]
- Urbanski, N.K.; Beresewicz, A. Generation of •OH initiated by interaction of Fe2+ and Cu+ with dioxygen; comparison with the Fenton chemistry. Acta Biochim. Pol. 2000, 47, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Flemmig, J.; Arnhold, J. Ferrous ion-induced strand breaks in the DNA plasmid pBR322 are not mediated by hydrogen peroxide. Eur. Biophys. J. 2007, 36, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Bors, W.; Erben-Russ, M.; Saran, M. Fatty acid peroxyl radicals: Their generation and reactivities. Bioelectrochem. Bioenerg. 1987, 18, 37–49. [Google Scholar] [CrossRef]
- Crichton, R.R.; Ward, R.J. Iron homeostasis. Metal Ions Biol. Syst. 1998, 35, 633–665. [Google Scholar]
- Ponka, P. Cellular iron metabolism. Kidney Int. 1999, 55, S2–S11. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, A.-S.; Enns, C.A. Iron regulation by hepcidin. J. Clin. Investig. 2013, 123, 2337–2343. [Google Scholar] [CrossRef]
- Gkouvatsos, K.; Papanikolaou, G.; Pantopoulos, K. Regulation of iron transport and the role of transferrin. Biochim. Biophys. Acta 2011, 1820, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Gamella, E.; Buratti, P.; Cairo, G.; Recalcati, S. The transferrin receptor: The cellular iron gate. Metallomics 2017, 9, 1367–1375. [Google Scholar] [CrossRef]
- Massover, W.H. Ultrastructure of ferritin and apoferritin: A review. Micron 1993, 24, 389–437. [Google Scholar] [CrossRef]
- Prohaska, J.R. Role of copper transporters in copper homeostasis. Am. J. Clin. Nutr. 2008, 88, 826S–829S. [Google Scholar] [CrossRef] [PubMed]
- Stoj, C.; Kosman, D.J. Cuprous oxidase activity of yeast Fet3p and human ceruloplasmin: Implication for function. FEBS Lett. 2003, 554, 422–426. [Google Scholar] [CrossRef]
- Sokolov, A.V.; Pulina, M.O.; Ageeva, K.V.; Runova, O.I.; Zakharova, E.T.; Vasilyev, V.B. Identification of leukocyte cationic proteins that interact with ceruloplasmin. Biochemistry 2007, 72, 872–877. [Google Scholar] [CrossRef]
- Radi, R.; Beckman, J.S.; Bush, K.M.; Freeman, B.A. Peroxynitrite-induced membrane lipid peroxidation: The cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 1991, 288, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Darley-Usmar, V.M.; Hogg, N.; O’Leary, V.J.; Wilson, M.T.; Moncada, S. The simultaneous generation of superoxide and nitric oxide can initiate lipid peroxidation in human low density lipoprotein. Free Radic. Res. Commun. 1992, 17, 9–20. [Google Scholar] [CrossRef]
- Goldstein, S.; Czapski, G. Mechanism of the nitrosation of thiols and amines by oxygenated ∙NO solutions: The nature of the nitrosating intermediates. J. Am. Chem. Soc. 1996, 118, 3419–3425. [Google Scholar] [CrossRef]
- Schopfer, F.J.; Baker, P.R.S.; Freeman, B.A. NO-dependent protein nitration: A cell signaling event or an oxidative inflammatory response? Trends Biochem. Sci. 2003, 28, 646–654. [Google Scholar] [CrossRef]
- Augusto, O.; Bonini, M.G.; Amanso, A.M.; Linares, E.; Santos, C.C.; de Menezes, S.L. Nitrogen dioxide and carbonate radical anion: Two emerging radicals in biology. Free Radic. Biol. Med. 2002, 32, 841–859. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Sueta, G.; Radi, R. Chemical biology of ONOO-: Kinetics, diffusion, and radicals. ACS Chem. Biol. 2009, 4, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Furtmüller, P.G.; Jantschko, W.; Zederbauer, M.; Schwanninger, M.; Jakoptisch, C.; Herold, S.; Koppenol, W.H.; Obinger, C. Peroxynitrite efficiently mediates the interconverion of redox intermediates of myeloperoxidase. Biochem. Biophys. Res. Commun. 2005, 337, 944–954. [Google Scholar] [CrossRef]
- Galijasevic, S.; Maitra, D.; Lu, T.; Sliskovic, I.; Abdulhamid, I.; Abu-Soud, H.M. Myeloperoxidase interaction with peroxynitrite: Chloride deficiency and heme depletion. Free Radic. Biol. Med. 2009, 47, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Koyani, C.N.; Flemmig, J.; Malle, E.; Arnhold, J. Myeloperoxidase scavenges peroxynitrite: A novel anti-inflammatory action of the heme enzyme. Arch. Biochem. Biophys. 2015, 571, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Groves, J.T. Mechanisms of peroxynitrite interactions with heme proteins. Inorg. Chem. 2010, 49, 6317–6329. [Google Scholar] [CrossRef]
- Keng, T.; Privalle, C.T.; Gilkeson, G.S.; Weinberg, J.B. Peroxynitrite formation and decreased catalase activity in autoimmune MRL-lpr/lpr mice. Mol. Med. 2000, 6, 779–792. [Google Scholar] [CrossRef]
- Gebicka, L.; Gebicki, J.L. Reaction of heme peroxidases with peroxynitrite. IUBMB Life 2000, 49, 11–15. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef]
- Rothenberg, M.E.; Hogan, S.P. The eosinophil. Annu. Rev. Immunol. 2006, 24, 147–174. [Google Scholar] [CrossRef]
- Pattison, D.I.; Davies, M.J. Absolute rate constants for the reaction of hypochlorous acid with protein side chains and peptide bonds. Chem. Res. Toxicol. 2001, 14, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.L.; Pattison, D.I.; Davies, M.J. Hypochlorite-induced oxidation of amino acids, peptides, and proteins. Amino Acids 2003, 25, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.I.; Davies, M.J. Kinetic analysis of the reaction of hypobromous acid with protein components: Implication for cellular damage and the use of 3-bromotyrosine as a marker of oxidative stress. Biochemistry 2004, 43, 4799–4809. [Google Scholar] [CrossRef] [PubMed]
- Ashby, M.T.; Carlson, A.C.; Scott, M.J. Redox buffering of hypochlorous acid by thiocyanate in physiologic fluids. J. Am. Chem. Soc. 2004, 126, 15976–15977. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.; Beal, J.L.; Ashby, M.T. Thiocyanate is an efficient endogenous scavenger of the phagocytic killing agent hypobromous acid. Chem. Res. Toxicol. 2006, 19, 587–593. [Google Scholar] [CrossRef]
- Davies, M.J.; Hawkins, C.L. The role of myeloperoxidase in biomolecule modification, chronic inflammation, and disease. Antioxid. Redox Signal. 2020, 32, 957–981. [Google Scholar] [CrossRef]
- Sokolov, A.V.; Ageeva, K.V.; Pulina, M.O.; Cherkalina, O.S.; Samygina, V.R.; Vlasova, I.I.; Panasenko, O.M.; Zakharova, E.T.; Vasilyev, V.B. Ceruloplasmin and myeloperoxidase in complex affect the enzymatic properties of each other. Free Radic. Res. 2008, 42, 989–998. [Google Scholar] [CrossRef]
- Chapman, A.L.P.; Mocatta, T.J.; Shiva, S.; Seidel, A.; Chen, B.; Khalilova, I.; Paumann-Page, M.E.; Jameson, G.N.L.; Winterbourn, C.C.; Kettle, A.J. Ceruloplasmin is an endogenous inhibitor of myeloperoxidase. J. Biol. Chem. 2013, 288, 6464–6477. [Google Scholar] [CrossRef]
- Samygina, V.R.; Sokolov, A.V.; Bourenkov, G.; Petoukhov, M.V.; Pulina, M.O.; Zakharova, E.T.; Vasilyev, V.B.; Bartunik, H.; Svergun, D.I. Ceruloplasmin: Macromolecular assemblies with iron-containing acute phase proteins. PLoS ONE 2013, 8, e67145. [Google Scholar] [CrossRef]
- Sokolov, A.V.; Kostevich, V.A.; Zakharova, E.T.; Samygina, V.R.; Panasenko, O.M.; Vasilyev, V.B. Interaction of ceruloplasmin with eosinophil peroxidase as compared to its interplay with myeloperoxidase: Reciprocal effect on enzymatic properties. Free Radic. Res. 2015, 49, 800–811. [Google Scholar] [CrossRef]
- Reiter, C.D.; Wang, X.; Tanus-Santos, J.E.; Hogg, N.; Cannon III, R.O.; Schechter, A.N.; Gladwin, M.T. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat. Med. 2002, 8, 1383–1389. [Google Scholar] [CrossRef]
- Rother, R.P.; Bell, L.; Hillman, P.; Gladwin, M.T. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin. J. Am. Med. Assoc. 2005, 293, 1653–1662. [Google Scholar] [CrossRef]
- Kristiansen, M.; Graversen, J.H.; Jacobsen, C.; Sonne, O.; Hoffmann, H.J.; Law, S.K.; Moestrup, S.K. Identification of the haemoglobin scavenger receptor. Nature 2001, 409, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Chiabrando, D.; Vinchi, F.; Fiorito, V.; Tolosano, E. Haptoglobin and hemopexin in heme detoxification and iron recycling. In Acute Phase Proteins- Regulation and Functions of Acute Phase Proteins; Veas, F., Ed.; Intech: Rijeka, Croatia, 2011; pp. 261–288. [Google Scholar] [CrossRef]
- Bunn, H.F.; Jandl, J.H. Exchange of heme among hemoglobins and hemoglobin and albumin. J. Biol. Chem. 1968, 243, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Balla, G.; Jacob, H.S.; Eaton, J.W.; Belcher, J.D.; Vercelotti, G.M. Hemin: A possible physiological mediator of low density lipoprotein oxidation and endothelial injury. Arterioscler. Thromb. 1991, 11, 1700–1711. [Google Scholar] [CrossRef]
- Jeney, V.; Balla, J.; Yachie, A.; Varga, Z.; Vercelotti, G.M.; Eaton, J.W.; Balla, G. Pro-oxidant and cytotoxic effects of circulating heme. Blood 2002, 100, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bandyopadhyay, U. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 2005, 157, 175–188. [Google Scholar] [CrossRef]
- Flemmig, J.; Schlorke, D.; Kühne, F.-W.; Arnhold, J. Inhibition of the heme-induced hemolysis of red blood cells by the chlorite-based drug WF10. Free Radic. Res. 2016, 50, 1386–1395. [Google Scholar] [CrossRef]
- Lin, T.; Sammy, F.; Yang, H.; Thundivalappil, S.; Hellman, J.; Tracey, K.C.; Warren, H.S. Identification of hemopexin as an anti-inflammatory factor that inhibits synergy of hemoglobin with HMGB1 in sterile and infectious inflammation. J. Immunol. 2012, 189, 2017–2022. [Google Scholar] [CrossRef]
- Schaer, D.J.; Buehler, P.W.; Alayash, A.I.; Belcher, J.D.; Vercelotti, G.M. Hemolysis and free hemoglobin revisited: Exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 2013, 121, 1276–1284. [Google Scholar] [CrossRef]
- Figueiredo, R.T.; Fernandez, P.L.; Mourao-Sa, D.S.; Porto, B.N.; Dutra, F.F.; Alves, L.S.; Oliviera, M.F.; Oliviera, P.L.; Graca-Souza, A.V.; Bozza, M.T. Characterization of heme as activator of toll-like receptor 4. J. Biol. Chem. 2007, 282, 20221–20229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belcher, J.D.; Chen, C.; Nguyen, J.; Milbauer, L.; Abdulla, F.; Alayash, A.I.; Smith, A.; Nath, K.A.; Hebbel, R.P.; Vercelotti, G.M. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood 2014, 123, 377–390. [Google Scholar] [CrossRef]
- Poon, L.C.; Methot, S.P.; Morabi-Pazocki, W.; Pio, F.; Bennet, A.J.; Sen, D. Guanine-rich RNAs and DNAs that bind heme robustly catalyze oxygen transfer reactions. J. Am. Chem. Soc. 2011, 133, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.T.; Lombardi, E.P.; Verga, D.; Nicolas, A.; Teulade-Fichou, M.-P.; Londoño-Vallejo, A.; Maizels, N. G-Quadruplexes sequester free heme in living cells. Cell Chem. Biol. 2019, 26, 1681–1689. [Google Scholar] [CrossRef]
- Hvidberg, V.; Maniecki, M.B.; Jacobson, C.; Hojrup, P.; Moller, H.J.; Moestrup, S.K. Identification of the receptor scavenging hemopexin-heme complexes. Blood 2005, 106, 2572–2579. [Google Scholar] [CrossRef]
- Lin, T.; Maita, D.; Thundivalappil, S.R.; Riley, F.E.; Hambsch, J.; van Marter, L.J.; Christou, H.A.; Berra, L.; Fagan, S.; Christiani, D.C.; et al. Hemopexin in severe inflammation and infection: Mouse models and human diseases. Crit. Care 2015, 19, 166. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.M.; Lo Giudice, M.C.; D’Urso, A.; Lauceri, R.; Purello, R.; Milardi, D. Cationic porphyrins are reversible proteasome inhibitors. J. Am. Chem. Soc. 2012, 134, 10451–10457. [Google Scholar] [CrossRef]
- Vallelian, F.; Deuel, J.W.; Opitz, L.; Schaer, C.A.; Puglia, M.; Lönn, M.; Engelsberger, W.; Schauer, S.; Karnaukhova, E.; Spahn, D.R.; et al. Proteasome inhibition and oxidative reactions disrupt cellular homeostasis during heme stress. Cell Death Differ. 2015, 22, 597–611. [Google Scholar] [CrossRef]
- Arnhold, J. Oxidation and reduction of biological material. In Cell and Tissue Destruction. Mechanisms, Protection, Disorders; Academic Press: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK, 2020; pp. 55–97. [Google Scholar]
- Hayward, L.D.; Angyal, S.J. A symmetry rule for the circular dichroism of reducing sugar, and the proportion of carbonyl forms in aqueous solutions thereof. Carbohydr. Res. 1977, 53, 13–20. [Google Scholar] [CrossRef]
- Pamplona, R. Advanced lipoxidation end-products. Chem. Biol. Interact. 2011, 192, 14–20. [Google Scholar] [CrossRef]
- Vistoli, G.; de Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs amd ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47 (Suppl. 1), 3–27. [Google Scholar] [CrossRef] [Green Version]
- Burton, G.W.; Joyce, A.; Ingold, K.U. Is vitamin E the only lipid-soluble, chain-breaking antioxidant in human blood plasma and erythrocyte membrane? Arch. Biochem. Biophys. 1983, 221, 281–290. [Google Scholar] [CrossRef]
- Buettner, G.R. The pecking order of free radicals and antioxidants: Lipid peroxidation, α-tocopherol, and ascorbate. Arch. Biochem. Biophys. 1993, 300, 535–543. [Google Scholar] [CrossRef]
- Niki, E. Antioxidants in relation to lipid peroxidation. Chem. Phys. Lipids 1987, 44, 227–253. [Google Scholar] [CrossRef]
- Van Kujik, F.J.G.M.; Sevanian, A.; Handelman, G.J.; Dratz, E.A. A new role for phospholipase A2: Protection of membranes from lipid peroxidation damage. Trends Biochem. Sci. 1987, 12, 31–34. [Google Scholar] [CrossRef]
- Hochstein, P.; Hatch, L.; Sevanian, A. Uric acid: Functions and determinations. Methods Enzymol. 1984, 105, 162–166. [Google Scholar]
- Nyyssönen, K.; Porkkala-Sarataho, E.; Kaikkonen, J.; Salonen, J.T. Ascorbate and urate are the strongest determinants of plasma antioxidative capacity and serum lipid resistance to oxidation in Finnish men. Atherosclerosis 1997, 130, 223–233. [Google Scholar] [CrossRef]
- Spencer, J.P.E.; Abd El Mohsen, M.M.; Minihane, A.-M.; Mathers, J.C. Biomarkers of the intake of dietary polyphenols: Strengths, limitations and application in nutrition research. Br. J. Nutr. 2008, 99, 12–22. [Google Scholar] [CrossRef]
- Steenken, S.; Neta, P. One-electron redox potentials of phenols. Hydroxy- and aminophenols and related compounds of biological interest. J. Phys. Chem. 1982, 86, 3661–3667. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Gau, J.; Furtmüller, P.G.; Obinger, C.; Prévost, M.; van Antwerpen, P.; Arnhold, J.; Flemmig, J. Flavonoids as promoters of the (pseudo-)halogenating activity of lactoperoxidase and myeloperoxidase. Free Radic. Biol. Med. 2016, 97, 307–319. [Google Scholar] [CrossRef]
- Wardlaw, A.C. The complement-dependent bacteriolytic activity of normal human serum. I. The effect of pH and ionic strength and the role of lysozyme. J. Exp. Med. 1962, 115, 1231–1249. [Google Scholar] [CrossRef]
- Korkmaz, B.; Horwitz, M.S.; Jenne, D.E.; Gauthier, F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol. Rev. 2010, 62, 726–759. [Google Scholar] [CrossRef] [PubMed]
- Weinrauch, Y.; Drujan, D.; Shapiro, S.D.; Weiss, J.; Zychlinsky, A. Neutrophil elastase targets virulence factors of enterobacteria. Nature 2002, 417, 91–94. [Google Scholar] [CrossRef]
- Belaaouaj, A. Neutrophil elastase-mediated killing of bacteria: Lessons from targeted mutagenesis. Microb. Infect. 2002, 4, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Chakraborty, K.; Tang, X.A.; Zhou, G.; Schoenfelt, K.Q.; Becker, K.M.; Hoffmann, A.; Chang, Y.-F.; Blank, A.; Reardon, C.A.; et al. Neutrophil elastase selectively kills cancer cells and attenuates tumorigenesis. Cell 2021, 184, 3163–3177. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Akula, S.; Thorpe, M.; Hellman, L. Potent and broad but not unselectively cleavage of cytokines and chemokines by human neutrophil elastase and proteinase 3. Int. J. Mol. Sci. 2020, 21, 651. [Google Scholar] [CrossRef]
- Fu, Z.; Thorpe, M.; Alemayehu, R.; Roy, A.; Kervinen, J.; de Garavilla, L.; Abrink, M.; Hellman, L. Highly selective cleavage of cytokines and chemokines by the human mast cell chymase and neutrophil cathepsin G. J. Immunol. 2017, 198, 1474–1483. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Zychlinsky, A. NETs: A new strategy for using old weapons. Trends Immunol. 2009, 30, 513–521. [Google Scholar] [CrossRef]
- Caughey, G.H. Mast cell tryptases and chymases in inflammation and host defense. Immunol. Rev. 2007, 217, 141–151. [Google Scholar] [CrossRef]
- Korkmaz, B.; Moreau, T.; Gauthier, F. Neutrophil elastase, proteinase 3 and cathepsin G: Physicochemical properties, activity and physiopathological functions. Biochimie 2008, 90, 227–242. [Google Scholar] [CrossRef]
- Ramaha, A.; Patston, P.A. Release and degradation of angiotensin I and II from angiotensinogen by neutrophil serine proteases. Arch. Biochem. Biophys. 2002, 397, 77–83. [Google Scholar] [CrossRef]
- Vidotti, D.B.; Casarini, D.E.; Christovam, P.C.; Leite, C.A.; Schor, N.; Boim, M.A. High glucose concentration stimulates intracellular renin activity and angiotensin II generation in rat mesangial cells. Am. J. Physiol. Renal Physiol. 2004, 286, F1039–F1045. [Google Scholar] [CrossRef]
- Penafuerte, C.A.; Gagnon, B.; Sirois, J.; Murphy, J.; MacDonald, N.; Tremblay, M.L. Identification of neutrophil-derived proteases and angiotensin II as biomarkers of cancer cachexia. Br. J. Cancer 2016, 114, 680–687. [Google Scholar] [CrossRef]
- Okada, Y.; Nakanishi, I. Activation of matrix metalloproteinase 3 (stromelysin) and matrix metalloproteinase 2 (‘gelatinase’) by human neutrophil elastase and cathepsin G. FEBS Lett. 1989, 249, 353–356. [Google Scholar] [CrossRef]
- Shamanian, P.; Schwartz, J.D.; Pocock, B.J.Z.; Monea, S.; Whiting, D.; Marcus, S.G.; Mignatti, P. Activation of progelatinase (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: A role for inflammatory cells in tumor invasion and angiogenesis. J. Cell. Physiol. 2001, 189, 197–206. [Google Scholar] [CrossRef]
- Gaggar, A.; Li, Y.; Weathington, N.; Winkler, M.; Kong, M.; Jackson, P.; Blalock, J.E.; Clancy, J.P. Matrix metalloprotease-9 dysregulation in lower airway secretions of cystic fibrosis patients. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, L96–L104. [Google Scholar] [CrossRef]
- Garratt, L.W.; Sutanto, E.N.; Ling, K.-M.; Looi, K.; Iosifidis, T.; Martinovich, K.M.; Shaw, N.C.; Kicic-Starcevich, E.; Knight, D.A.; Ranganathan, S.; et al. on behalf of the Australian respiratory early surveillance team for cystic fibrosis. Matrix metalloproteinase activation by free neutrophil elastase contributes to bronchiectasis progression in early cystic fibrosis. Eur. Respir. J. 2015, 46, 384–394. [Google Scholar] [CrossRef]
- Jackson, P.I.; Xu, X.; Wilson, L.; Weathington, N.M.; Clancy, J.P.; Blalock, J.E.; Gaggar, A. Human neutrophil elastase-mediated cleavage sites of MMP-9 and TIMP-1: Implications to cystic fibrosis proteolytic dysfunction. Mol. Med. 2010, 16, 159–166. [Google Scholar] [CrossRef]
- Cairns, J.A.; Walls, A.F. Mast cell tryptase is a mitogen for epithelial cells. Stimulation of IL-8 production and intercellular adhesion molecule-1 expression. J. Immunol. 1996, 156, 275–283. [Google Scholar] [CrossRef]
- Dougherty, R.H.; Sidhu, S.S.; Raman, K.; Solon, M.; Solberg, O.D.; Caughey, G.H.; Woodruff, P.G.; Fahy, J.V. Accumulation of intraepithelial mast cells with a unique protease phenotype in T(H)2-high asthma. J. Allergy Clin. Immunol. 2010, 125, 1046–1053. [Google Scholar] [CrossRef] [Green Version]
- Ramu, S.; Akbarshshi, H.; Mogren, S.; Berlin, F.; Cerps, S.; Menzel, M.; Hvidtfeldt, M.; Porsbjerg, C.; Uller, L.; Andersson, C.K. Direct effects of mast cell proteases, tryptase and chymase, on bronchial epithelial integrity proteins and anti-viral responses. BMC Immunol. 2021, 22, 35. [Google Scholar] [CrossRef]
- Karimi, N.; Morovati, S.; Chan, L.; Napoleoni, C.; Mehrani, Y.; Bridle, B.W.; Karimi, K. Mast cell tryptase and implications for SARS-CoV-2 pathogenesis. Bio. Med. 2021, 1, 136–149. [Google Scholar] [CrossRef]
- He, A.; Shi, G.-P. Mast cell chymase and tryptase as targets for cardiovascular and metabolic diseases. Curr. Pharm. Des. 2013, 19, 1114–1125. [Google Scholar] [CrossRef]
- Gruber, B.L.; Marchese, M.J.; Suzuki, K.; Schwartz, L.B.; Okada, Y.; Nagae, H.; Ramamurthy, N.S. Synovial procollagenase activation by human mast cell tryptase dependence upon matrix metalloproteinase 3 activation. J. Clin. Investig. 1989, 84, 1657–1662. [Google Scholar] [CrossRef]
- Lees, M.; Taylor, D.J.; Woolley, D.E. Mast cell proteinases activate precursor forms of collagenase and stromelysin, but not of gelatinases A and B. Eur. J. Biochem. 1994, 223, 171–177. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kumagai, N.; Fukuda, K.; Fujitsu, Y.; Nishida, T. Activation of corneal fibroblast-derived matrix metalloproteinase-2 by tryptase. Curr. Eye Res. 2006, 31, 313–317. [Google Scholar] [CrossRef]
- Pyo, R.; Lee, J.K.; Shipley, J.M.; Curci, J.A.; Mao, D.; Ziporin, S.J.; Ennis, T.L.; Shapiro, S.D.; Senior, R.M.; Thompson, R.W. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J. Clin. Investig. 2000, 105, 1641–1649. [Google Scholar] [CrossRef]
- Galis, Z.S.M.; Johnson, C.; Godin, D.; Magid, R.; Shipley, J.M.; Senior, R.M.; Ivan, E. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ. Res. 2002, 91, 852–859. [Google Scholar] [CrossRef]
- Longo, G.M.; Xiong, W.; Greiner, T.C.; Zhao, Y.; Fiotti, N.; Baxter, B.T. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J. Clin. Investig. 2002, 110, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, M.; Nakamura, K.; Sasaki, T.; Cheng, X.W.; Itohara, S.; Iguchi, A. Effect of MMP-2 deficiency on atherosclerotic lesion formation in apoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1120–1125. [Google Scholar] [CrossRef] [Green Version]
- Wågsäter, D.; Zhu, C.; Björkegren, J.; Skogsberg, J.; Eriksson, P. MMP-2 and MMP-9 are prominent matrix metalloproteinases during atherosclerosis development in the Ldlr(−/−)Apob(100/100) mouse. Int. J. Mol. Med. 2011, 28, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Berlin, F.; Mogren, S.; Tutzauer, J.; Andersson, C.K. Mast cell proteases tryptase and chymase induce migratory and morphological alterations in bronchial epithelial cells. Int. J. Mol. Sci. 2021, 22, 5250. [Google Scholar] [CrossRef]
- De Souza Junior, D.A.; Santana, A.C.; da Silva, E.Z.M.; Oliver, C.; Jamur, M.C. The role of mast cell specific chymases and tryptases in tumor angiogenesis. BioMed Res. Int. 2015, 2015, 142359. [Google Scholar] [CrossRef] [PubMed]
- Arooj, M.; Kim, S.; Sakkiah, S.; Cao, G.P.; Lee, Y.; Lee, K.W. Molecular modeling study for inhibition mechanism of human chymase and its application in inhibitor design. PLoS ONE 2013, 8, e62740. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.B.; Bradford, T.R. Regulation of tryptase from human lung mast cells by heparin. Stabilization of the active tetramer. J. Biol. Chem. 1986, 261, 7372–7379. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.B.; Lewis, R.A.; Austen, K.F. Tryptase from human pulmonary mast cells. Purification and characterization. J. Biol. Chem. 1981, 256, 11939–11943. [Google Scholar] [CrossRef]
- Alter, S.C.; Yates, P.; Margolius, H.S.; Schwartz, L.B. Tryptase and kinin generation: Tryptase from human mast cells does not activate human urinary prokallikrein. Int. Arch. Allergy Appl. Immunol. 1987, 83, 321–324. [Google Scholar] [CrossRef]
- Cregar, L.; Elrod, K.C.; Putnam, D.; Moore, W.R. Neutrophil myeloperoxidase is a potent and selective inhibitor of mast cell tryptase. Arch. Biochem. Biophys. 1999, 366, 125–130. [Google Scholar] [CrossRef]
- Elrod, K.C.; Moore, W.R.; Abraham, W.M.; Tanaka, R.D. Lactoferrin, a potent tryptase inhibitor, abolishes late-phase airway responses in allergic sheep. Am. J. Respir. Crit. Care Med. 1997, 156, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Alter, S.C.; Kramps, J.A.; Janoff, A.; Schwartz, L.B. Interactions of human mast cell tryptase with biological protease inhibitors. Arch. Biochem. Biophys. 1990, 276, 26–31. [Google Scholar] [CrossRef]
- Samoszuk, M.; Corwin, M.; Hazen, S.L. Effects of human mast cell tryptase and eosinophil granule proteins on the kinetics of blood clotting. Am. J. Hematol. 2003, 73, 18–25. [Google Scholar] [CrossRef]
- Schechter, N.M.; Eng, G.Y.; McCaslin, D.R. Human skin tryptase: Kinetic characterization of its spontaneous inactivation. Biochemistry 1993, 32, 2617–2625. [Google Scholar] [CrossRef] [PubMed]
- Frommherz, K.J.; Faller, B.; Bieth, J.G. Heparin strongly decreases the rate of inhibition of neutrophil elastase by α1-proteinase inhibitor. J. Biol. Chem. 1991, 266, 15356–15362. [Google Scholar] [CrossRef] [PubMed]
- Ermolieff, J.; Boudier, C.; Laine, A.; Meyer, B.; Bieth, J.G. Heparin protects cathepsin G against inhibition by protein proteinase inhibitors. J. Biol. Chem. 1994, 269, 29502–29508. [Google Scholar] [CrossRef]
- Fleddermann, J.; Pichert, A.; Arnhold, J. Interaction of serine proteases from polymorphonuclear leucocytes with the cell surface and heparin. Inflammation 2012, 35, 81–88. [Google Scholar] [CrossRef]
- Taggart, C.; Cervantes-Laurean, D.; Kim, G.; McElvaney, N.G.; Wehr, N.; Moss, J.; Levine, R.L. Oxidation of either methionine 351 or methionine 358 in alpha 1-antitrypsin causes loss of anti-neutrophil elastase activity. J. Biol. Chem. 2000, 275, 27258–27265. [Google Scholar] [CrossRef]
- Evans, M.D.; Pryor, W.A. Cigarette smoking. emphysema and damage to alpha 1-proteinase inhibitor. Am. J. Physiol. Lung Cell. Mol. Physiol. 1994, 266, L593–L611. [Google Scholar] [CrossRef]
- Dittrich, A.S.; Kühbandner, I.; Gehrig, S.; Rickert-Zacharias, V.; Twigg, M.; Wege, S.; Taggart, C.C.; Herth, F.; Schultz, C.; Mall, M.A. Elastase activity on sputum neutrophils correlates with severity of lung disease in cystic fibrosis. Eur. Respir. J. 2018, 51, 1701910. [Google Scholar] [CrossRef]
- Duranton, J.; Adam, C.; Blieth, J.G. Kinetic mechanism of the inhibition of cathepsin G by α1-antichymotrypsin and α1-proteinase inhibitor. Biochemistry 1997, 37, 11239–11245. [Google Scholar] [CrossRef]
- Travis, J.; Bowen, J.; Baugh, R. Human α1-antichymotrypsin: Interaction with chymotrypsin-like proteinases. Biochemistry 1978, 26, 5651–5656. [Google Scholar] [CrossRef] [PubMed]
- Kalsheker, N.A. α1-Antichymotrypsin. Int. J. Biochem. Cell Biol. 1996, 28, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Ohlsson, K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc. Natl. Acad. Sci. USA 1986, 83, 6692–6696. [Google Scholar] [CrossRef]
- Franken, C.; Meijer, C.J.L.M.; Dijkman, J.H. Tissue distribution of antileukoprotease and lysozyme in humans. J. Histochem. Cytochem 1989, 37, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Igarashi, Y.; Hohman, R.J.; Kaulbach, H.; White, M.V.; Kaliner, M.A. Distribution of secretory leukoprotease inhibitor in the human nasal airway. Am. Rev. Respir Dis 1993, 147, 710–716. [Google Scholar] [CrossRef] [PubMed]
- McGarry, N.; Greene, C.M.; McElvaney, N.G.; Weldon, S.; Taggart, C.C. The ability of secretory leukocyte protease inhibitor to inhibit apoptosis in monocytes is independent of its antiprotease activity. J. Immunol. Res. 2015, 2015, 507315. [Google Scholar] [CrossRef]
- Williams, S.E.; Brown, T.I.; Roghanian, A.; Sallenave, J.M. SLPI and elafin: One glove, many fingers. Clin. Sci. 2006, 110, 21–35. [Google Scholar] [CrossRef]
- Verrier, T.; Solhonne, B.; Sallenave, J.M.; Garcia-Verdugo, I. The WAP protein Trappin-2/Elafin: A handyman in the regulation of inflammatory and immune responses. Int. J. Biochem. Cell Biol. 2012, 44, 1377–1380. [Google Scholar] [CrossRef]
- Labidi-Galy, S.I.; Clauss, A.; Ng, V.; Duraisamy, S.; Elias, K.M.; Piao, H.Y.; Bilal, E.; Davidowitz, R.A.; Lu, Y.; Badalian-Very, G.; et al. Elafin drives poor outcome in high-grade serous ovarian cancers and basal-like breast tumors. Oncogene 2015, 34, 373–383. [Google Scholar] [CrossRef]
- Hunt, K.K.; Wingate, H.; Yokota, T.; Liu, Y.; Mills, G.B.; Zhang, F.; Fang, B.; Su, C.H.; Zhang, M.; Yi, M.; et al. Elafin, an inhibitor of elastase, is a prognostic indicator in breast cancer. Breast Cancer Res. 2013, 15, R3. [Google Scholar] [CrossRef]
- Wang, C.; Liao, Y.; He, W.; Zhang, H.; Zuo, D.; Liu, W.; Yang, Z.; Qiu, J.; Yuan, Y.; Li, K.; et al. Elafin promotes tumour metastasis and attenuates the anti-metastatic effects of erlotinib via binding to EGFR in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 113. [Google Scholar] [CrossRef]
- Cooley, J.; Takayama, T.K.; Shapiro, S.D.; Schechter, N.M.; Remold-O’Donnell, E. The serpin MNEI inhibits elastase-like and chymotrypsin-like serine proteases through efficient reactions at two active sites. Biochemistry 2001, 40, 15762–15770. [Google Scholar] [CrossRef]
- Marrero, A.; Duquerroy, S.; Trapani, S.; Goulas, T.; Guevara, T.; Andersen, G.R.; Navaza, J.; Sottrup-Jensen, L.; Gomis-Rüth, F.X. The crystal structure of human alpha2-macroglobulin reveals a unique molecular cage. Angew. Chem. Int. Ed. Engl. 2012, 51, 3340–3344. [Google Scholar] [CrossRef]
- Vandooren, J.; Itoh, Y. Alpha-2-macroglobulin in inflammation, immunity and infections. Front. Immunol. 2021, 12, 803244. [Google Scholar] [CrossRef] [PubMed]
- Salvesen, G.; Virca, G.D.; Travis, J. Interaction of alpha 2-macroglobulin with neutrophil and plasma proteinases. Ann. N. Y. Acad. Sci. 1983, 421, 316–326. [Google Scholar] [CrossRef]
- Wewers, M.D.; Herzyk, D.J.; Gadek, J.E. Alveolar fluid neutrophil elastase activity in the adult respiratory distress syndrome is complexed to alpha-2-macroglobulin. J. Clin. Investig. 1988, 82, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.V.; Wehner, N.G.; Marshall, B.C.; Gray, W.R.; Gray, B.H.; Hoidal, J.R. Characterization of proteinase-3 (PR-3), a neutrophil serine proteinase. Structural and functional properties. J. Biol. Chem. 1991, 266, 9540–9548. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, T.; Zia, M.K.; Ali, S.S.; Ahsan, H.; Khan, F.H. Insight into the interactions of proteinase inhibitor- alpha-2-macroglobulin with hypochlorite. Int. J. Biol. Macromol. 2018, 117, 401–406. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Desorchers, P.E.; Pizzo, S.V.; Gonias, S.L.; Sahakian, J.A.; Levine, R.L.; Weiss, S.J. Oxidative dissociation of human alpha 2-macroglobulin tetramers into dysfunctional dimers. J. Biol. Chem. 1994, 269, 4683–4691. [Google Scholar] [CrossRef]
- Ahmad, S.; Simmons, T.; Varagic, J.; Moniwa, N.; Chappell, M.C.; Ferrario, C.M. Chymase-dependent generation of angiotensin II from angiotensin-(1-12) in human atrial tissue. PLoS ONE 2011, 6, e28501. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Li, Y.; Du, J.; Mitch, W.E.; Rosenthal, N.; Delafontaine, P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J. Clin. Investig. 2005, 115, 451–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, P.M.; Russell, S.T.; Tisdale, M.J. Angiotensin II directly induces muscle protein catabolism through the ubiquitin-proteasome proteolytic pathway and may play a role in cancer cachexia. Br. J. Cancer 2005, 93, 425–434. [Google Scholar] [CrossRef]
- Trobec, K.; von Haehling, S.; Anker, S.D.; Lainsack, M. Growth hormone, insulin-like growth factor 1, and insulin signaling—A pharmacological target in body wasting and cachexia. J. Cachexia Sarcopenia Muscle 2011, 2, 191–200. [Google Scholar] [CrossRef]
- Semprun-Prieto, L.C.; Sukhanov, S.; Yoshida, T.; Rezk, B.M.; Gonzalez-Villalobos, R.A.; Vaughn, C.; Tabony, A.M.; Delafontaine, P. Angiotensin II induced catabolic effect and muscle atrophy are redox dependent. Biochem. Biophys. Res. Commun. 2011, 409, 217–221. [Google Scholar] [CrossRef]
- Benigni, A.; Cassis, P.; Remuzzi, G. Angiotensin II revisited: New roles in inflammation, immunology, and aging. EMBO Mol. Med. 2010, 2, 247–257. [Google Scholar] [CrossRef]
- Crackower, M.A.; Sarao, R.; Oudit, G.Y.; Yagil, C.; Kozieradzki, I.; Scanga, S.E.; Oliveira-dos-Santos, A.J.; da Costa, J.; Zhang, L.; Pey, Y.; et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 2002, 417, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.M.; Chappell, M.C. Novel angiotensin peptides. Cell. Mol. Life Sci. 2004, 61, 2720–2727. [Google Scholar] [CrossRef]
- Muller, F.; Renné, T. Novel roles for factor XII-driven plasma contact activation system. Curr. Opin. Hematol. 2008, 15, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Oehmcke-Hecht, S.; Köhler, J. Interaction of the human contact system with pathogens—An update. Front. Immunol. 2018, 9, 312. [Google Scholar] [CrossRef]
- Marceau, F.; Hess, H.J.; Rachvarov, D.R. The B1 receptors for kinins. Pharmacol. Rev. 1998, 50, 357–386. [Google Scholar]
- Cyr, M.; Lepage, Y.; Blais Jr., C.; Gervais, N.; Cugno, M.; Rouleau, J.-L.; Adam, A. Bradykinin and des-Arg9-bradykinin metabolic pathways and kinetics of activation of human plasma. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H275–H283. [Google Scholar] [CrossRef]
- Hamza, M.; Wang, X.M.; Adam, A.; Brahim, J.S.; Rowan, J.S.; Carmona, G.N.; Dionne, R.A. Kinin B1 receptors contributes to acute pain following minor surgery in humans. Mol. Pain 2010, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Murphy, G.; Nagase, H. Progress in matrix metalloproteinase research. Mol. Aspects Med. 2008, 29, 290–308. [Google Scholar] [CrossRef]
- Löffek, S.; Schilling, O.; Franzke, C.W. Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef]
- Ra, H.-J.; Parks, W.C. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007, 26, 587–596. [Google Scholar] [CrossRef]
- Fu, X.; Kao, J.L.; Bergt, C.; Kassim, S.Y.; Huq, N.P.; d’Avignon, A.; Parks, W.C.; Mecham, R.P.; Heinecke, J.W. Oxidative cross-linking of tryptophan to glycine restrains matrix metalloproteinase activity: Specific structural motifs control protein oxidation. J. Biol. Chem. 2004, 279, 6209–6912. [Google Scholar] [CrossRef]
- Thompson, R.W.; Holmes, D.R.; Mertens, R.A.; Liao, S.; Botney, M.D.; Mecham, R.P.; Welgus, H.G.; Parks, W.C. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J. Clin. Investig. 1995, 96, 318–326. [Google Scholar] [CrossRef]
- Davis, V.; Persidskaia, R.; Baca-Regen, L.; Itoh, Y.; Nagase, H.; Persidsky, Y.; Ghorpade, A.; Baxter, B.T. Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1625–1633. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Y.H.; LeMaire, S.A. Thoracic aortic dissection: Are matrix metalloproteinases involved? Vascular 2009, 17, 147–157. [Google Scholar] [CrossRef]
- Strickland, D.K.; Ashcom, J.D.; Williams, S.; Burgess, W.H.; Migliorini, M.; Argraves, W.S. Sequence identity between the alpha 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J. Biol. Chem. 1990, 265, 17401–17404. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Atkinson, S.; Murphy, G. Identification of the extracellular matrix (ECM) binding motifs of tissue inhibitor of metalloproteinases (TIMP)-3 and effective transfer to TIMP-1. J. Biol. Chem. 2007, 282, 6887–6898. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. The expanding role of mitochondria in apoptosis. Genes Develop. 2001, 15, 2922–2933. [Google Scholar]
- Kagan, V.E.; Tyurin, V.A.; Jiang, J.; Tyurina, Y.Y.; Ritov, V.B.; Amoscato, A.A.; Osipov, A.N.; Belikova, N.A.; Kapralov, A.A.; Kini, V.; et al. Cytochrome c acts as a cardiolipin oxygenase required for the release of proapoptotic factors. Nat. Chem. Biol. 2005, 1, 223–232. [Google Scholar] [CrossRef]
- Kakhlon, O.; Cabantchik, Z.I. The labile iron pool: Characterization, measurement, and participation in cellular processes. Free Radic. Biol. Med. 2002, 33, 1037–1046. [Google Scholar] [CrossRef]
- Kruszewski, M. Labile iron pool: The main determinant of cellular response to oxidative stress. Mutat. Res. 2003, 53, 81–92. [Google Scholar] [CrossRef]
- Frankel, E.N. Secondary products of lipid oxidation. Chem. Phys. Lipids 1987, 44, 73–85. [Google Scholar] [CrossRef]
- Stables, M.J.; Gillroy, D.W. Old and new generation of lipid mediators in acute inflammation and resolution. Prog. Lipid Res. 2011, 50, 35–51. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Cantley, A.M.; Yang, W.S.; Morrison, B., 3rd; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by lipid peroxidation. Trends Cell. Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- De Bie, P.; Muller, P.; Wijmenga, C.; Klomp, L.W.J. Molecular pathogenesis of Wilson and Menkes disease: Correlation of mutations with molecular defects and disease phenotypes. J. Med. Genet. 2007, 44, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Squitti, R.; Pasqualetti, P.; Dal Forno, G.; Moffa, F.; Cassetta, E.; Lupoi, D.; Vernieri, F.; Rossi, L.; Baldassini, M.; Rossini, P.M. Excess of serum copper not related to ceruloplasmin in Alzheimer disease. Neurology 2005, 64, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Squitti, R.; Bressi, F.; Pasqualetti, P.; Bonomini, C.; Ghidoni, R.; Binetti, G.; Cassetta, E.; Moffa, F.; Ventriglia, M.; Vernieri, F.; et al. Longitudinal prognostic value of serum ‘‘free” copper in patients with Alzheimer disease. Neurology 2009, 72, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. Application of pulse radiolysis methods to study the reactions and structure of biomolecules. Rep. Prog. Phys. 1978, 41, 259–302. [Google Scholar] [CrossRef]
- Sarkar, S.; Prakash, D.; Marwaha, R.K.; Garewal, G.; Kumar, L.; Singhi, S.; Walia, B.N. Acute intravascular haemolysis in glucose-6-phosphate dehydrogenase deficiency. Ann. Trop. Paediatr. 1993, 13, 391–394. [Google Scholar] [CrossRef]
- Parker, C. Paroxysmal nocturnal hemoglobinuria. Curr. Opin. Hematol. 2012, 19, 141–148. [Google Scholar] [CrossRef]
- Kato, G.J.; Steinberg, M.H.; Gladwin, M.T. Intravascular hemolysis and the pathophysiology of sickle cell disease. J. Clin. Investig. 2017, 127, 750–760. [Google Scholar] [CrossRef]
- Sauret, J.M.; Marinides, G.; Wang, G.K. Rhabdomyolysis. Am. Fam. Physican 2002, 65, 907–912. [Google Scholar]
- Hunter, J.D.; Gregg, K.; Damani, Z. Rhabdomyolysis. Cont. Educ. Anaesth. Crit. Care Pain 2006, 6, 141–143. [Google Scholar] [CrossRef]
- Körmöczi, G.F.; Säemann, M.D.; Buchta, C.; Peck-Radosavljevic, M.; Mayr, W.R.; Schwartz, D.W.; Dunkler, D.; Spitzauer, S.; Panzer, S. Influence of clinical factors on the haemolysis marker haptoglobin. Eur. J. Clin. Invest. 2006, 36, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Schaer, D.J.; Vinchi, F.; Ingoglia, G.; Tolosano, E.; Buehler, P.W. Haptoglobin, hemopexin, and related defense pathways—Basic science, clinical perspectives, and drug development. Front. Physiol. 2014, 5, 415. [Google Scholar] [CrossRef]
- Muller-Eberhard, U.; Javid, J.; Liem, H.H.; Hanstein, A.; Hanna, M. Plasma concentrations of hemopexin, haptoglobin and heme in patients with various hemolytic diseases. Blood 1968, 32, 811–815. [Google Scholar] [CrossRef]
- Tombe, M. Images in clinical medicine. Hemoglobinuria with malaria. N. Engl. J. Med. 2008, 358, 1837. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Sever, M.S.; Erek, E.; Lameire, N. Acute renal failure related to the crush syndrome: Towards an era of seismo-nephrology? Nephrol. Dial. Transplant. 2000, 15, 1517–1521. [Google Scholar] [CrossRef]
- Genthon, A.; Wilcox, S.R. Crush syndrome: A case report and review of the literature. J. Emerg. Med. 2014, 46, 313–319. [Google Scholar] [CrossRef]
- Deuel, J.W.; Schaer, C.A.; Boretti, F.S.; Opitz, L.; Garcia-Rubio, I.; Baek, J.H.; Spahn, D.R.; Buehler, P.W.; Schaer, D.J. Hemoglobinuria-related acute kidney injury is driven by intrarenal oxidative reactions triggering a heme toxicity response. Cell Death Dis. 2016, 7, e2064. [Google Scholar] [CrossRef]
- Tracz, M.J.; Alam, J.; Nath, K.A. Physiology and pathophysiology of heme: Implications for kidney disease. J. Am. Soc. Nephrol. 2007, 18, 414–420. [Google Scholar] [CrossRef]
- Tabibzadeh, N.; Estournet, C.; Placier, S.; Perez, J.; Bilbault, H.; Girshovich, A.; Vandermeersch, A.; Jouanneau, C.; Letavenier, E.; Hammoudi, N.; et al. Plasma-heme induced renal toxicity is related to capillary rarefaction. Sci. Rep. 2017, 7, 40156. [Google Scholar] [CrossRef]
- Schröder, M.; Kaufman, R.J. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005, 74, 730–789. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Hogg, J.C. A brief review of chronic obstructive pulmonary disease. Can. Respir. J. 2012, 19, 381–384. [Google Scholar] [CrossRef] [PubMed]
- McDonough, J.E.; Yuan, R.; Suzuki, M.; Seyednejad, N.; Elliott, W.M.; Sanchez, P.G.; Wright, A.C.; Gefter, W.B.; Litzky, L.; Coxson, H.O.; et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N. Engl. J. Med. 2011, 365, 1567–1575. [Google Scholar] [CrossRef]
- Taggart, C.C.; Greene, C.M.; Carroll, T.P.; O’Neill, S.J.; McElvaney, N.G. Elastolytic proteases. Inflammation resolution and dysregulation in chronic infective lung disease. Am. J. Respir. Crit. Care Med. 2005, 171, 1070–1076. [Google Scholar] [CrossRef]
- Brode, S.K.; Ling, S.C.; Chapman, K.R. Alpha-1 antitrypsin deficiency: A commonly overlooked cause of lung disease. Can. Med. Ass. J. 2012, 184, 1365–1371. [Google Scholar] [CrossRef]
- Gompertz, S.; O’Brien, C.; Bayley, D.L.; Hill, S.L.; Stockley, R.A. Changes in bronchial inflammation during acute exacerbations of chronic bronchitis. Eur. Respir. J. 2001, 17, 1112–1119. [Google Scholar] [CrossRef]
- Paone, G.; Conti, V.; Vestry, A.; Leone, A.; Puglisi, G.; Benassi, F.; Brunetti, G.; Schmid, G.; Cammarella, I.; Terzano, C. Analysis of sputum markers in the evaluation of lung inflammation and functional impairment in symptomatic smokers and COPD patients. Dis. Markers 2011, 31, 91–100. [Google Scholar] [CrossRef]
- Gramegna, A.; Amati, F.; Terranova, L.; Sotgui, G.; Tarsia, P.; Miglietta, D.; Calderazzo, M.A.; Aliberti, S.; Blasi, F. Neutrophil elastase in bronchiectasis. Respir. Res. 2017, 18, 211. [Google Scholar] [CrossRef]
- Weldon, S.; McNally, P.; McElvaney, N.G.; Elborn, J.S.; McAuley, D.F.; Wartelle, J.; Belaaouaj, A.; Levine, R.L.; Taggart, C.C. Decreased levels of secretory leucoprotease inhibitor in the Pseudomonas-infected cystic fibrosis lung are due to neutrophil elastase degradation. J. Immunol. 2009, 183, 8148–8156. [Google Scholar] [CrossRef]
- Guerra, M.; Frey, D.; Hagner, M.; Dittrich, S.; Paulsen, M.; Mall, M.A.; Schultz, C. Cathepsin G activity as a new marker for detecting airway inflammation by microscopy and flow cytometry. ACS Cent. Sci. 2019, 5, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Janus, E.D.; Philipps, N.D.; Carrell, R.W. Smoking, lung function and alpha 1-antitrypsin deficiency. Lancet 1985, 8421, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Kuba, K.; Ohto-Nakanishi, T.; Penninger, J.M. Angiotensin-converting enzyme 3 (ACE2) in disease pathogenesis. Circ. J. 2010, 74, 405–410. [Google Scholar] [CrossRef]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef]
- Lumbers, E.R.; Delforce, S.J.; Pringle, K.G.; Smith, G.R. The lung, the heart, the novel coronavirus, and the renin-angiotensin system; the need for clinical trials. Front. Med. 2020, 7, 248. [Google Scholar] [CrossRef]
- Lamas-Barreiro, J.M.; Alonso-Suárez, M.; Fernández-Martín, J.J.; Saavedra-Alonso, J.A. Angiotensin II suppression in SARS-CoV-2 infection; a therapeutic approach. Nefrologia 2020, 40, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Briquez, P.S.; Rouhani, S.J.; Yu, J.; Pyzer, A.R.; Trujillo, J.; Dugan, H.L.; Stamper, C.T.; Changrob, S.; Sperling, A.I.; Wilson, P.C.; et al. Severe COVID-19 induces autoantibodies against angiotensin II that correlate with blood pressure dysregulation and disease severity. Sci. Adv. 2022, 8, eabn3777. [Google Scholar] [CrossRef]
- Garvin, M.R.; Alvarez, C.; Miller, J.I.; Prates, E.T.; Walker, A.M.; Amos, B.K.; Mast, A.E.; Justice, A.; Aronow, B.; Jacobson, D. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. eLife 2020, 9, e59177. [Google Scholar] [CrossRef]
- Gough, P.J.; Gomez, I.G.; Wille, P.T.; Raines, E.W. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J. Clin. Investig. 2006, 116, 59–69. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Liapis, C.D. Matrix metalloproteinases: Contribution to pathogenesis, diagnosis, surveillance and treatment of abdominal aortic aneurysms. Curr. Med. Res. Opin. 2004, 20, 419–432. [Google Scholar] [CrossRef]
- Cohen, J.R.; Parikh, S.; Grella, L.; Sarfati, I.; Corbie, G.; Danna, D.; Wise, L. Role of the neutrophil in abdominal aortic aneurysm development. Cardiovasc. Surg. 1993, 1, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Lysgaard Poulsen, J.; Stubbe, J.; Lindholt, J.S. Animal Models Used to Explore Abdominal Aortic Aneurysms: A Systematic Review. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 487–499. [Google Scholar] [CrossRef]
- Maguire, E.M.; Pearce, S.W.A.; Xiao, R.; Oo, A.Y.; Xiao, Q. Matrix metalloproteinase in abdominal aortic aneurysm and aortic dissection. Pharmaceuticals 2019, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ares, W.J.; Taussky, P.; Ducruet, A.; Grandhi, R. Role of matrix metalloproteinases in the pathogenesis of intracranial aneurysms. Neurosurg. Focus 2019, 47, E4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freestone, T.; Turner, R.J.; Coady, A.; Higman, D.J.; Greenhalgh, R.M.; Powell, J.T. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1145–1151. [Google Scholar] [CrossRef]
- Tamarina, N.A.; McMillan, W.D.; Shively, V.P.; Pearce, W.H. Expression of matrix metalloproteinases and their inhibitors in aneurysms and normal aorta. Surgery 1997, 122, 264–271. [Google Scholar] [CrossRef]
- Lauer-Fields, J.L.; Juska, D.; Fields, G.B. Matrix metalloproteinases and collagen catabolism. Biopolymers 2002, 66, 19–32. [Google Scholar] [CrossRef]
- Iredale, J.P.; Thompson, A.; Henderson, N.C. Extracellular matrix degradation in liver fibrosis: Biochemistry and regulation. Biochim. Biophys. Acta 2013, 1832, 876–883. [Google Scholar] [CrossRef]
- Lech, M.; Anders, H.-J. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim. Biophys. Acta 2013, 1832, 989–997. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Horowitz, J.C.; Thannickal, V.J. Mechanisms for the resolution of organ fibrosis. Physiology 2019, 34, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Otte, J.M.; Rosenberg, I.M.; Podolsky, D.K. Intestinal myofibroblasts in innate immune responses of the intestine. Gastroenterology 2003, 124, 1866–1878. [Google Scholar] [CrossRef] [PubMed]
- Meneghin, M.D.; Hogaboam, C. Infectious disease, the innate immune response, and fibrosis. J. Clin. Investig. 2007, 117, 530–538. [Google Scholar] [CrossRef]
- Varga, J.; Abraham, D. Systemic sclerosis: A prototypic multisystem fibrotic disorder. J. Clin. Investig. 2007, 117, 557–567. [Google Scholar] [CrossRef]
- Lee, C.G.; Homer, R.J.; Zhu, Z.; Lanone, S.; Wang, X.; Koteliansky, V.; Shipley, J.M.; Gotwals, P.; Noble, P.; Chen, Q.; et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor-β1. J. Exp. Med. 2001, 194, 809–821. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fujimoto, M.; Takehara, K.; Sato, S. Pathogenesis of systemic sclerosis: Altered B cell function is the key linking systemic autoimmunity and tissue fibrosis. J. Dermatol. Sci. 2005, 389, 1–17. [Google Scholar] [CrossRef]
- Mezzano, S.A.; Ruiz-Ortega, M.; Egido, J. Angiotensin II and renal fibrosis. Hypertension 2001, 38, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Barker, T.A.; Berk, B.C. Angiotensin II and the endothelium: Diverse signals and effects. Hypertension 2005, 45, 163–169. [Google Scholar] [CrossRef]
- Mu, X.; Shi, W.; Xu, Y.; Xu, C.; Zhao, T.; Geng, B.; Yang, J.; Pan, J.; Hu, S.; Zhang, C.; et al. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle 2018, 17, 428–438. [Google Scholar] [CrossRef]
- Végran, F.; Boidot, R.; Michiels, C.; Sonveaux, P.; Feron, O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NFκB/IL-8 pathway that drives angiogenesis. Cancer Res. 2011, 71, 2550–2560. [Google Scholar] [CrossRef] [PubMed]
- Chamnee, T.; Ontong, P.; Itano, N. Hyaluronan: A modulator of the tumor microenvironment. Cancer Lett. 2016, 375, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A. Malignant cells, directors of the malignant process: Role of transforming growth factor-β. Cancer Metastasis Rev. 2001, 20, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Blobe, G.C. Role of transforming growth factor-β in hematologic malignancies. Blood 2006, 107, 4589–4596. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ma, J.; Lu, W. The significance of mitochondrial dysfunction in cancer. Int. J. Mol. Sci. 2020, 21, 5598. [Google Scholar] [CrossRef]
- Brown, J.L.; Rosa-Caldwell, M.E.; Lee, D.E.; Blackwell, T.A.; Brown, L.A.; Perry, R.A.; Haynie, W.S.; Hardee, J.P.; Carson, J.A.; Wiggs, M.P.; et al. Mitochondrial degeneration precedes the development of muscle atrophy in progression cancer cachexia in tumor-bearing mice. J. Cachexia Sarcopenia Muscle 2017, 8, 926–938. [Google Scholar] [CrossRef]
- Blackwell, T.A.; Cervenka, I.; Khatri, B.; Brown, J.L.; Rosa-Caldwell, M.E.; Lee, D.E.; Perry, R.A., Jr.; Brown, L.A.; Haynie, W.S.; Wiggs, M.P.; et al. Transcriptomic analysis of the development of skeletal muscle atrophy in cancer-cachexia in tumor-bearing mice. Physiol. Genomics 2018, 50, 1071–1082. [Google Scholar] [CrossRef]
- Prokopchuk, O.; Grünwald, B.; Nitsche, U.; Jäger, C.; Prokopchuk, O.L.; Schubert, E.C.; Friess, H.; Martignoni, M.E.; Krüger, A. Elevated systemic levels of the matrix metalloproteinase inhibitor TIMP-1 correlate with clinical markers of cachexia in patients with chronic pancreatitis and pancreatic cancer. BMC Cancer 2018, 18, 128. [Google Scholar] [CrossRef]
- Cao, Z.; Zhao, K.; Jose, I.; Hoogenraad, N.J.; Osellame, L.D. Biomarkers for cancer cachexia: A mini review. Int. J. Mol. Sci. 2021, 22, 4501. [Google Scholar] [CrossRef]
- Grünwald, B.; Harant, V.; Schaten, S.; Frühschütz, M.; Spallek, R.; Höchst, B.; Stutzer, K.; Berchtold, S.; Erkan, M.; Prokopchuk, O.; et al. Pancreatic pre-malignant lesions secrete TIMP-1, which activates hepatic stellate cells via CD63 signaling to create a pre-metastatic niche in the liver. Gastroenterology 2016, 151, 1011–1024. [Google Scholar] [CrossRef]
- Tarnawski, R.; Skladowski, K.; Maciejewski, B. Prognostic value of hemoglobin concentration in radiotherapy for cancer of supraglottic larynx. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 1007–1011. [Google Scholar] [CrossRef]
- Grogan, M.; Thomas, G.M.; Malemed, J.; Wong, F.L.; Pearcey, R.G.; Joseph, P.K.; Portelance, L.; Crook, J.; Jones, K.D. The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer 1999, 86, 1528–1536. [Google Scholar] [CrossRef]
- Yin, T.; He, S.; Liu, X.; Jiang, W.; Ye, T.; Lin, Z.; Sang, Y.; Su, C.; Wan, Y.; Shen, G.; et al. Extravascular red blood cells and hemoglobin promote tumor growth and therapeutic resistance as endogenous danger signals. J. Immunol. 2015, 194, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Canesin, G.; Di Ruscio, A.; Li, M.; Ummarino, S.; Hedblom, A.; Choudhury, R.; Krzyzanowska, A.; Csizmadia, E.; Palominos, M.; Stiehm, A.; et al. Scavenging of labile heme by hemopexin is a key checkpoint in cancer growth and metastases. Cell Rep. 2020, 32, 108181. [Google Scholar] [CrossRef]
- Chang, H.Y.; Lee, S.H.; Liao, I.C.; Huang, S.H.; Cheng, H.C.; Liao, P.C. Secretomic analysis identifies alpha-1 antitrypsin (A1AT) as a required protein in cancer cell migration, invasion, and pericellular fibronectin assembly for facilitating lung colonization of lung adenocarcinoma cells. Mol. Cell. Proteomics 2012, 11, 1320–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudani, J.S.; Warren, A.D.; Bhatia, S.N. Harnessing protease activity to improve cancer care. Annu. Rev. Cancer Biol. 2018, 2, 353–376. [Google Scholar] [CrossRef]
- Lerman, I.; Hammes, S.R. Neutrophil elastase in the tumor microenvironment. Steroids 2018, 133, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Houghton, A.M.; Rzymkiewicz, D.M.; Ji, H.; Gregory, A.D.; Egea, E.E.; Metz, H.E.; Stolz, D.B.; Land, S.R.; Marconcini, L.A.; Kliment, C.R.; et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat. Med. 2010, 16, 219–223. [Google Scholar] [CrossRef]
- Peng, B.; Hu, J.; Fu, X. ELANE: An emerging lane to selective anticancer therapy. Signal Transduction Targeted Therapy 2021, 6, 358. [Google Scholar] [CrossRef]
- Xie, G.; Cheng, T.; Lin, J.; Zhang, L.; Zhneg, J.; Liu, Y.; Xie, G.; Wang, B.; Yuan, Y. Local angiotensin II contributes to tumor resistance to checkpoint immunotherapy. J. Immunother. Cancer 2018, 6, 88. [Google Scholar] [CrossRef]
- Ino, K.; Shibata, K.; Kajiyama, H.; Yamamoto, E.; Nagasaka, T.; Nawa, A.; Nomura, S.; Kikkawa, F. Angiotensin II type 1 receptor expression in ovarian cancer and its correlation with tumour angiogenesis and patient survival. Br. J. Cancer 2006, 94, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Pinter, M.; Jain, R.K. Targeting the renin-angiotensin system to improve cancer treatment: Implications for immunotherapy. Sci. Transl. Med. 2017, 9, eaan5616. [Google Scholar] [CrossRef] [PubMed]
- Pittet, D.; Wenzel, R.P. Nosocomial bloodstream infections. Secular trends in rates, mortality, and contribution to total hospital deaths. Arch. Intern. Med. 1995, 155, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Llewelyn, M.J.; Cohen, J. Tracking the microbes in sepsis: Advancements in treatment bring challenges for microbial epidemiology. Clin. Infect. Dis. 2007, 44, 1343–1348. [Google Scholar] [CrossRef]
- Kollef, K.E.; Schramm, G.E.; Wills, A.R.; Reichley, R.M.; Mirek, S.T.; Kollef, M.H. Predictors of 30-day mortality and hospital costs in patients with ventilator-associated pneumonia attributed to potentially antibiotic-resistant gram-negative bacteria. Chest 2008, 134, 281–287. [Google Scholar] [CrossRef]
- Babu, M.; Menon, V.P.; Devi, P.U. Prevalence of antimicrobial resistant pathogens in severe sepsis and septic shock patients. J. Young Pharm. 2018, 10, 358–361. [Google Scholar] [CrossRef]
- Luyt, C.E.; Combes, A.; Deback, C.; Aubriot-Lorton, N.H.; Nieszkowska, A.; Trouillet, J.L.; Capron, F.; Agut, H.; Gibert, C.; Chastre, J. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am. J. Respir. Crit. Care Med. 2007, 175, 935–942. [Google Scholar] [CrossRef]
- Limaye, A.P.; Kirby, K.A.; Rubenfeld, G.D.; Leisenring, W.M.; Bulger, E.M.; Neff, M.J.; Gibran, N.S.; Huang, M.-L.; Santo, T.K.; Corey, L.; et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. J. Am. Med. Assoc. 2008, 300, 413–422. [Google Scholar] [CrossRef]
- Lin, G.-L.; McGinley, J.P.; Drysdale, S.B.; Pollard, A.J. Epidemiology and immune pathogenesis of viral sepsis. Front. Immunol. 2018, 9, 2147. [Google Scholar] [CrossRef]
- Crouser, E.; Exline, M.; Wewers, M.D. Sepsis: Links between pathogen sensing and organ damage. Curr. Pharm. Des. 2008, 14, 1840–1852. [Google Scholar] [CrossRef]
- Rittirsch, D.; Flierl, M.A.; Ward, P.A. Harmful molecular mechanisms in sepsis. Nat. Rev. Immunol. 2008, 8, 776–787. [Google Scholar] [CrossRef]
- Jiminez, M.F.; Watson, R.W.; Parodo, J.; Evans, D.; Foster, D.; Steinberg, M.; Rotstein, O.D.; Marshall, J.C. Dysregulated expression of neutrophil apoptosis in the systemic inflammatory response syndrome. Arch. Surg. 1997, 132, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Demaret, J.; Venet, F.; Friggeri, A.; Cauzalis, M.-A.; Plassais, J.; Jallades, L.; Malcus, C.; Poitevin-Later, F.; Textoris, J.; Lepape, A.; et al. Marked alterations of neutrophil functions during sepsis-induced immunosuppression. J. Leukoc. Biol. 2015, 98, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Brain, S.D.; Pearson, J.D.; Edgeworth, J.D.; Lewis, S.M.; Treacher, D.F. Neutrophils in development of multiple organ failure in sepsis. Lancet 2006, 368, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Kovach, M.A.; Standiford, T.J. The function of neutrophils in sepsis. Curr. Opin. Infect. Dis. 2012, 25, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, H.; Ono, S.; Majima, T.; Kawarabayashi, N.; Takayama, E.; Kinoshita, M.; Seki, S.; Hiraide, H.; Moldawer, L.L.; Mochizuki, H. Neutrophil elastase, MIP-2, and TLR-4 expression during human and experimental sepsis. Shock 2005, 23, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Schrijver, I.T.; Kempermann, H.; Roest, M.; Kesecioglu, J.; de Lange, D.W. Myeloperoxidase can differentiate between sepsis and non-infectious SIRS and predicts mortality in intensive care patients with SIRS. Intens. Care Med. Exp. 2017, 5, 43. [Google Scholar] [CrossRef]
- Kothari, N.; Keshari, R.S.; Bogra, J.; Kohli, M.; Abbas, H.; Malik, A.; Dikshit, M.; Barthwal, M.K. Increased myeloperoxidase enzyme activity in plasma is an indicator of inflammation and onset of sepsis. J. Crit. Care 2011, 26, 435.e1–453.e7. [Google Scholar] [CrossRef]
- Cha, Y.S.; Yoon, J.M.; Jung, W.J.; Kim, Y.W.; Kim, T.H.; Kim, O.H.; Cha, K.C.; Kim, H.; Hwang, S.O.; Lee, K.H. Evaluation of usefulness of myeloperoxidase index (MPXI) for differential diagnosis of systemic inflammatory response syndrome (SIRS) in the emergency department. Emerg. Med. J. 2015, 32, 304–307. [Google Scholar] [CrossRef]
- Maruchi, Y.; Tsuda, M.; Mori, H.; Takenaka, N.; Gocho, T.; Huq, M.A.; Takeyama, N. Plasma myeloperoxidase-conjugated DNA level predicts outcomes and organ dysfunction in patients with septic shock. Crit. Care 2018, 22, 176. [Google Scholar] [CrossRef]
- Czaikoski, P.G.; Mota, J.M.S.C.; Nascimento, D.C.; Sȏnego, F.; Castanheira, F.V.S.; Melo, P.H.; Scortegagna, G.T.; Silva, R.L.; Borrosa-Sousa, R.; Souto, F.O.; et al. Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS ONE 2016, 11, e0148142. [Google Scholar] [CrossRef]
- Denning, N.-L.; Aziz, M.; Gurien, S.D.; Wang, P. DAMPs and NETs in sepsis. Front. Immunol. 2019, 10, 2536. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.; Gozzelino, R.; Jeney, V.; Tokaji, L.; Bozza, F.A.; Japiassú, A.M.; Bonaparte, D.; Cavalcante, M.M.; Chora, A.; Ferreira, A.; et al. A central role for free heme in the pathogenesis of severe sepsis. Sci. Transl. Med. 2010, 2, 51ra71-1. [Google Scholar] [CrossRef]
- Othmann, A.; Filep, J.G. Enemies at the gate: How cell-free hemoglobin and bacterial infection can cooperate to drive acute lung injury during sepsis. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H131–H134. [Google Scholar] [CrossRef] [PubMed]
- Verheij, M.W.; Bulder, I.; Wuillemin, W.A.; Voermans, C.; Zeerleder, S.S. Scavengers of hemoproteins as potential biomarkers for severe sepsis and septic shock. Transl. Med. Commun. 2021, 6, 8. [Google Scholar] [CrossRef]
- Lan, P.; Pan, K.-H.; Wang, S.-J.; Shi, Q.-C.; Yu, Y.-X.; Fu, Y.; Chen, Y.; Jiang, Y.; Hua, X.-T.; Zhou, J.-C.; et al. High serum iron level is associated with increased mortality in patients with sepsis. Sci. Rep. 2018, 8, 11072. [Google Scholar] [CrossRef] [PubMed]
- Brandtner, A.; Tymoszuk, P.; Nairz, M.; Lehner, G.F.; Fritsche, G.; Vales, A.; Falkner, A.; Schennach, H.; Theurl, I.; Joannidis, M.; et al. Linkage of alterations in systemic iron homeostasis to patients’ outcome in sepsis: A prospective study. J. Intens. Care 2020, 8, 76. [Google Scholar] [CrossRef]
- Galley, H.F. Oxidative stress and mitochondrial dysfunction in sepsis. Br. J. Anaesth. 2011, 107, 57–64. [Google Scholar] [CrossRef]
- Singer, M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 2014, 51, 66–72. [Google Scholar] [CrossRef]
- Rahmel, T.; Marko, B.; Nowak, H.; Bergmann, L.; Thon, P.; Rump, K.; Kreimendahl, S.; Rassow, J.; Peters, J.; Singer, M.; et al. Mitochondrial dysfunction in sepsis is associated with diminished intramitochondrial TFAM despite its increased cellular expression. Sci. Rep. 2020, 10, 21209. [Google Scholar] [CrossRef]
- Arnhold, J. Organ damage and failure. In Cell and Tissue Destruction. Mechanisms, Protection, Disorders; Academic Press: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK, 2020; pp. 289–307. [Google Scholar] [CrossRef]
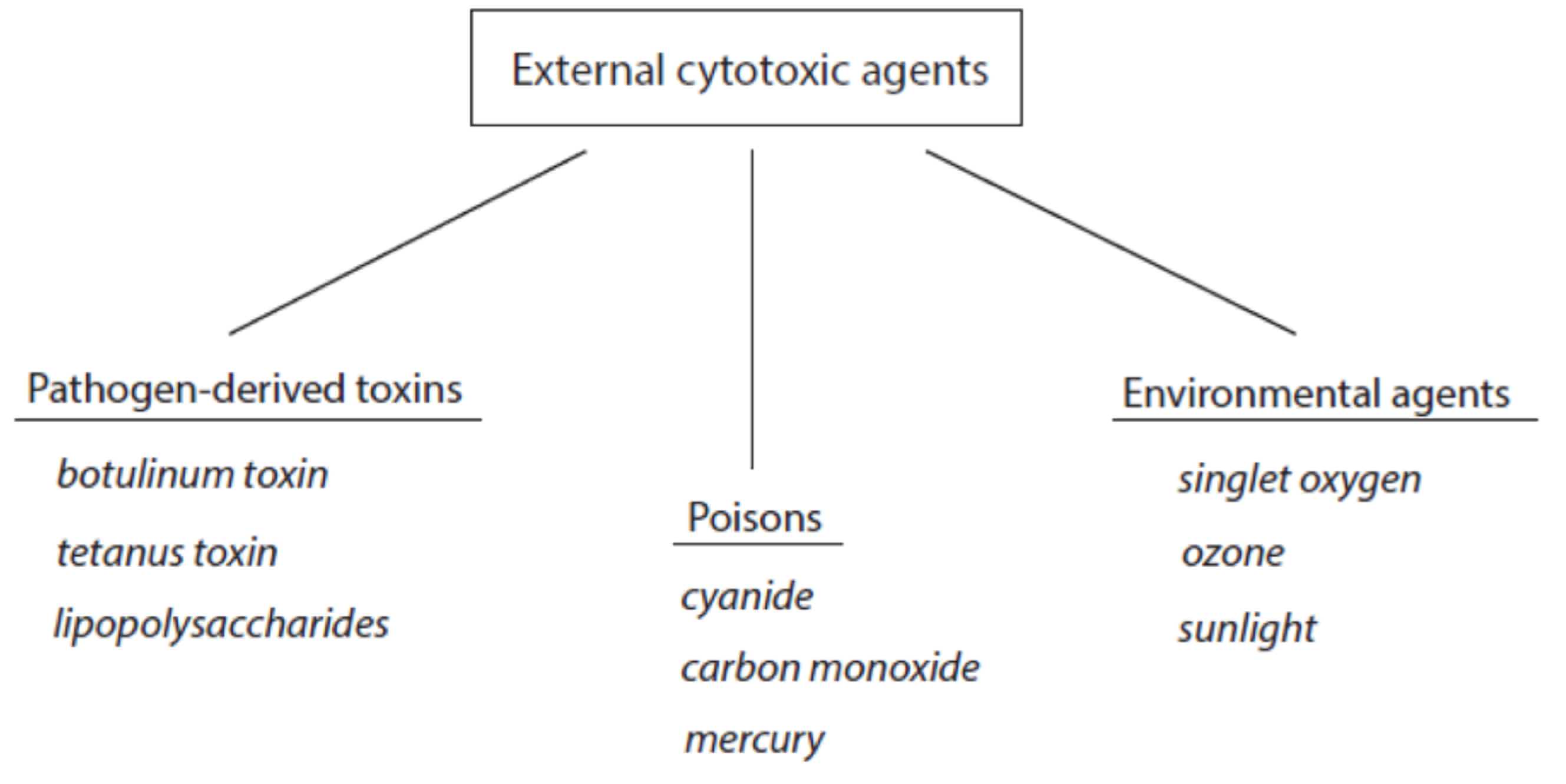
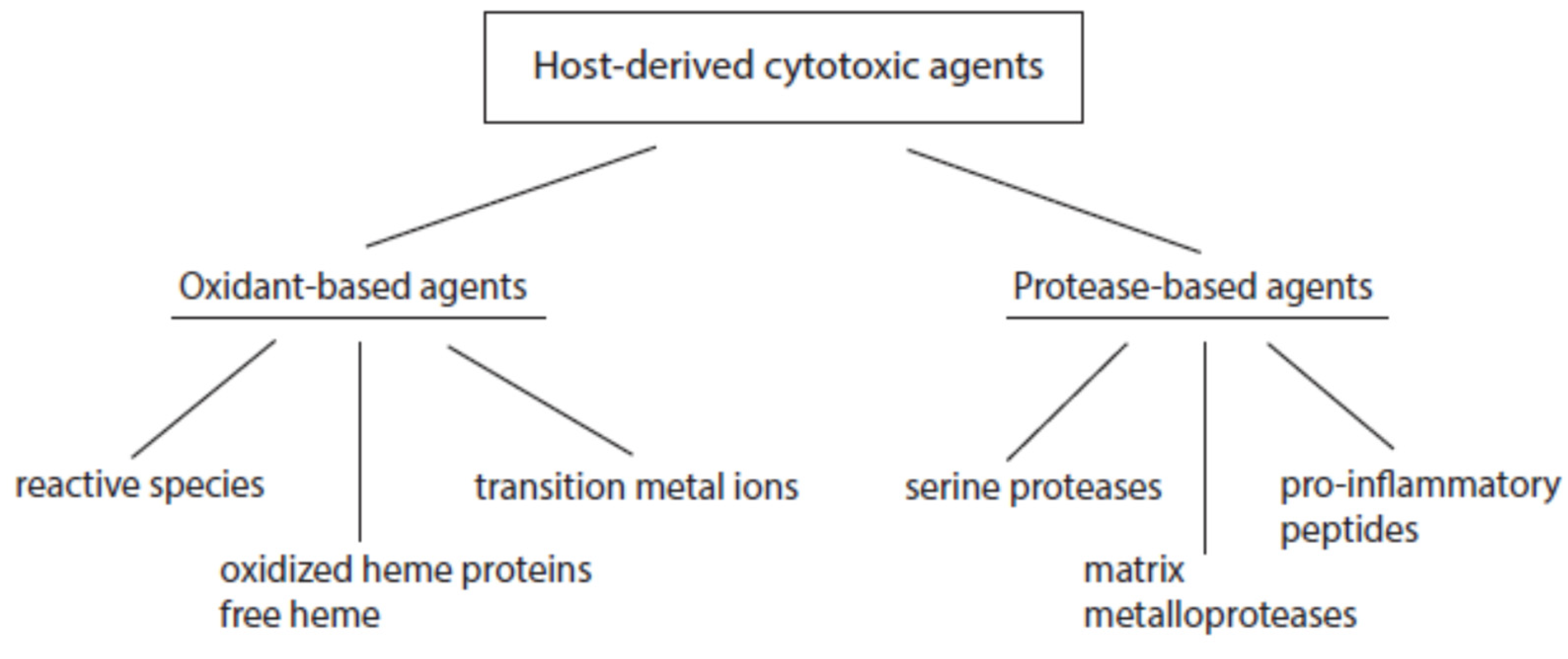
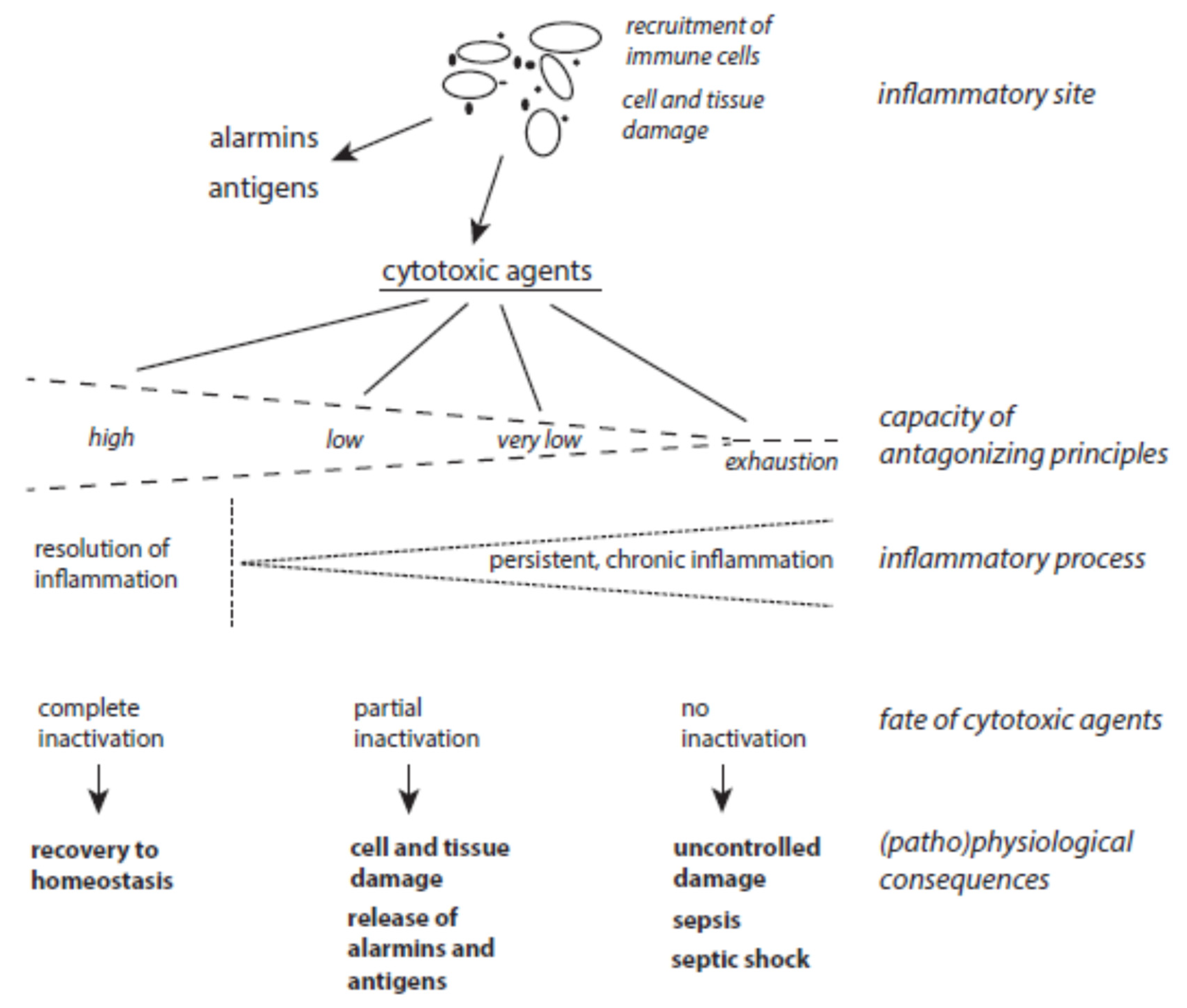
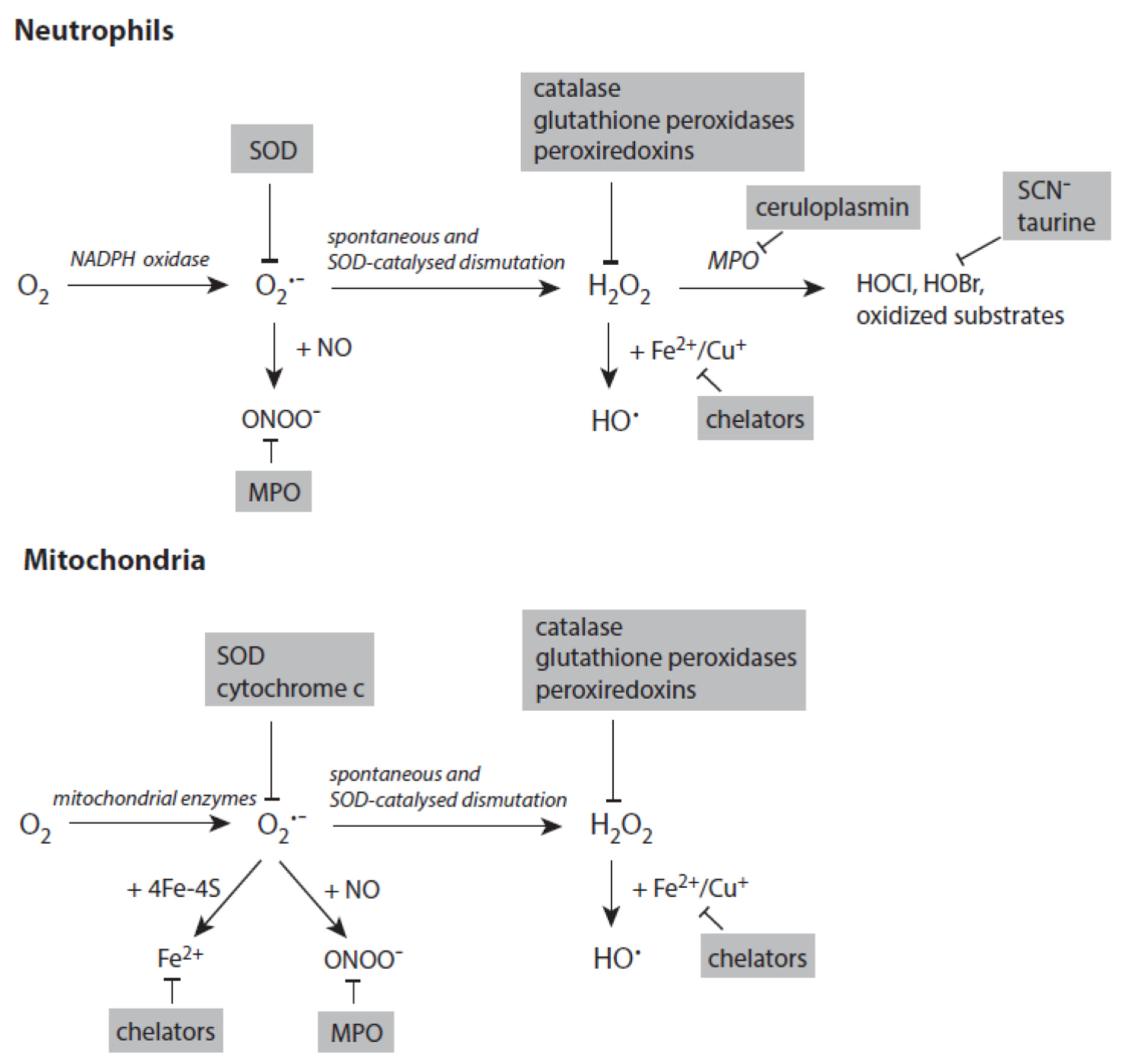
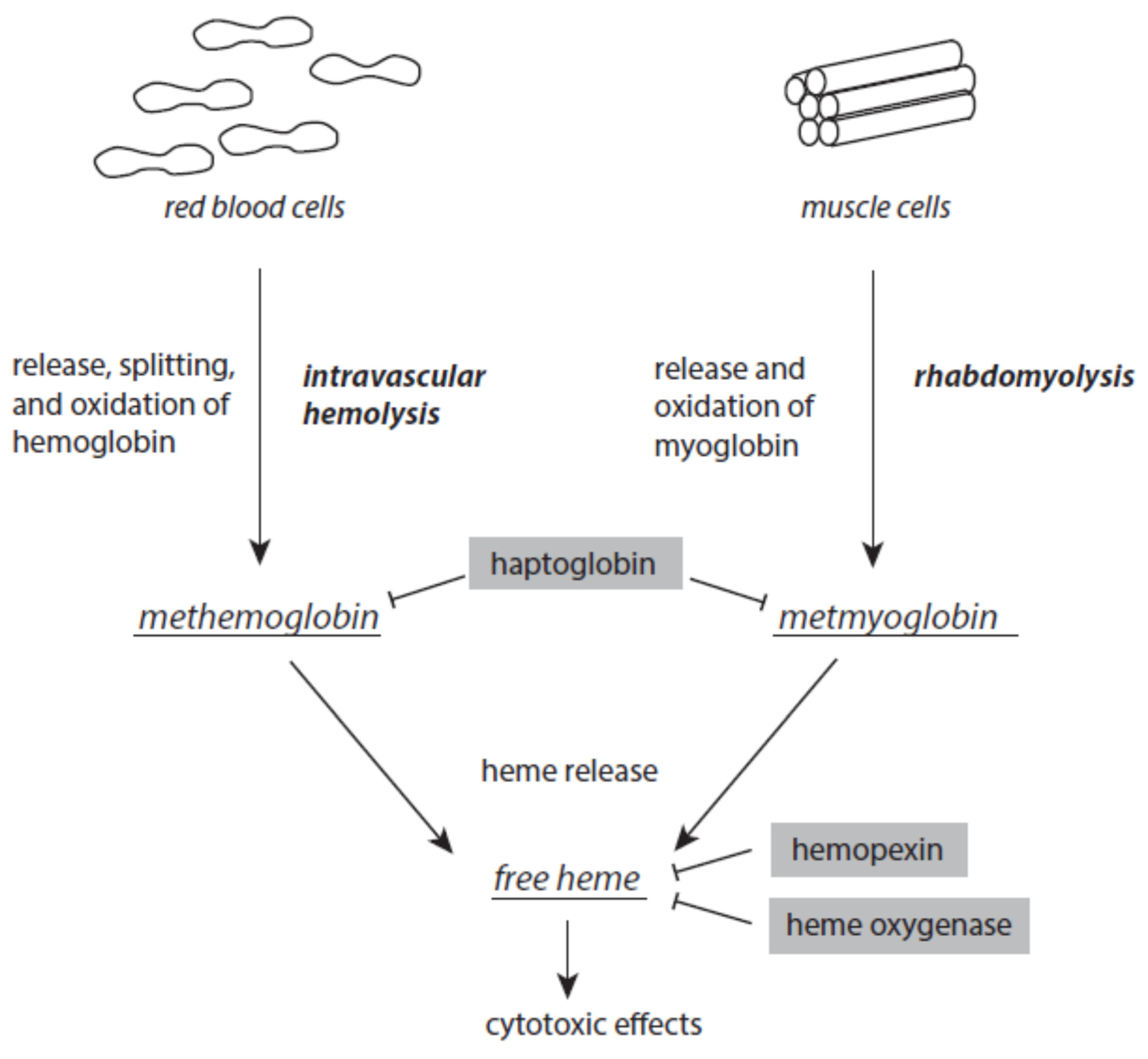
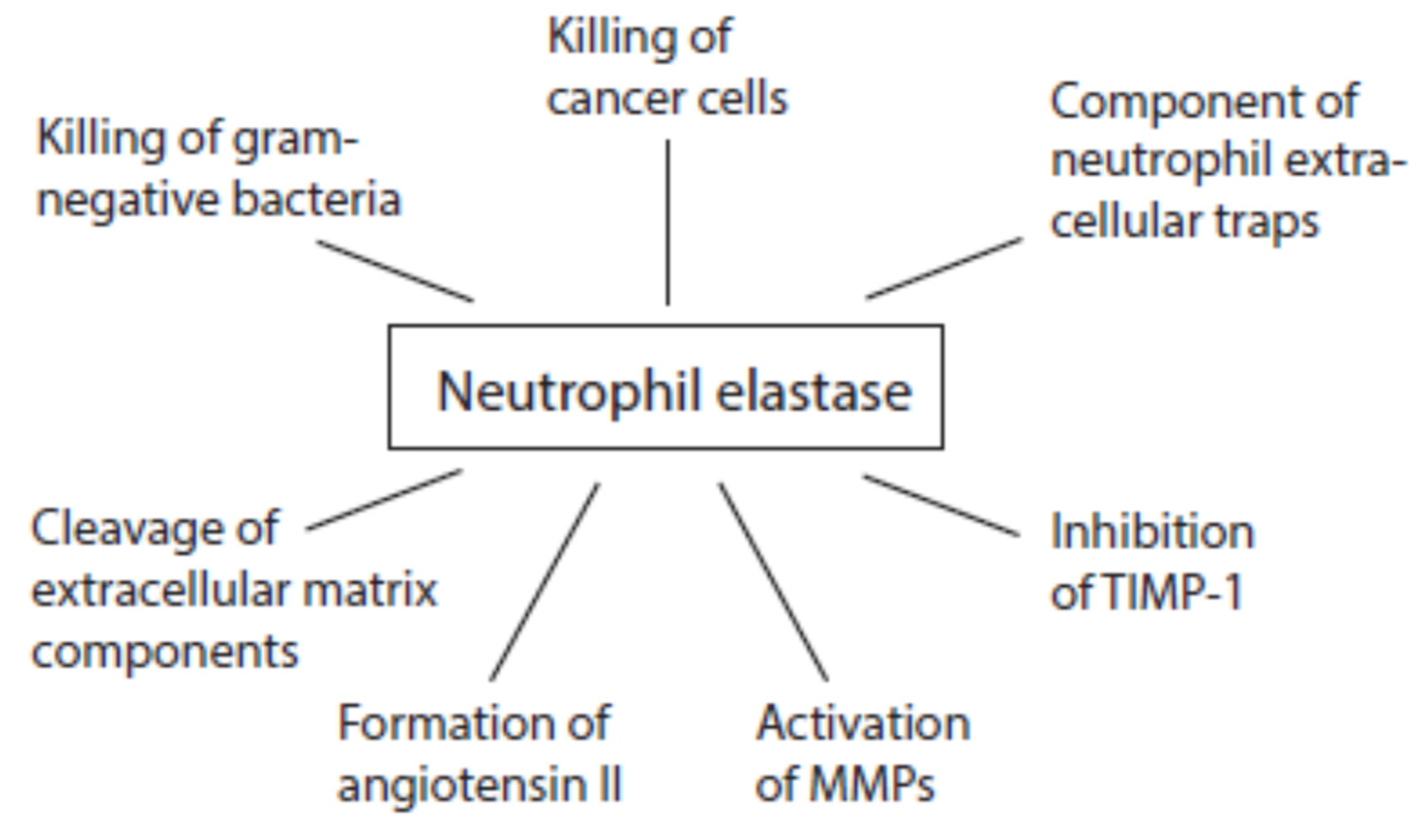

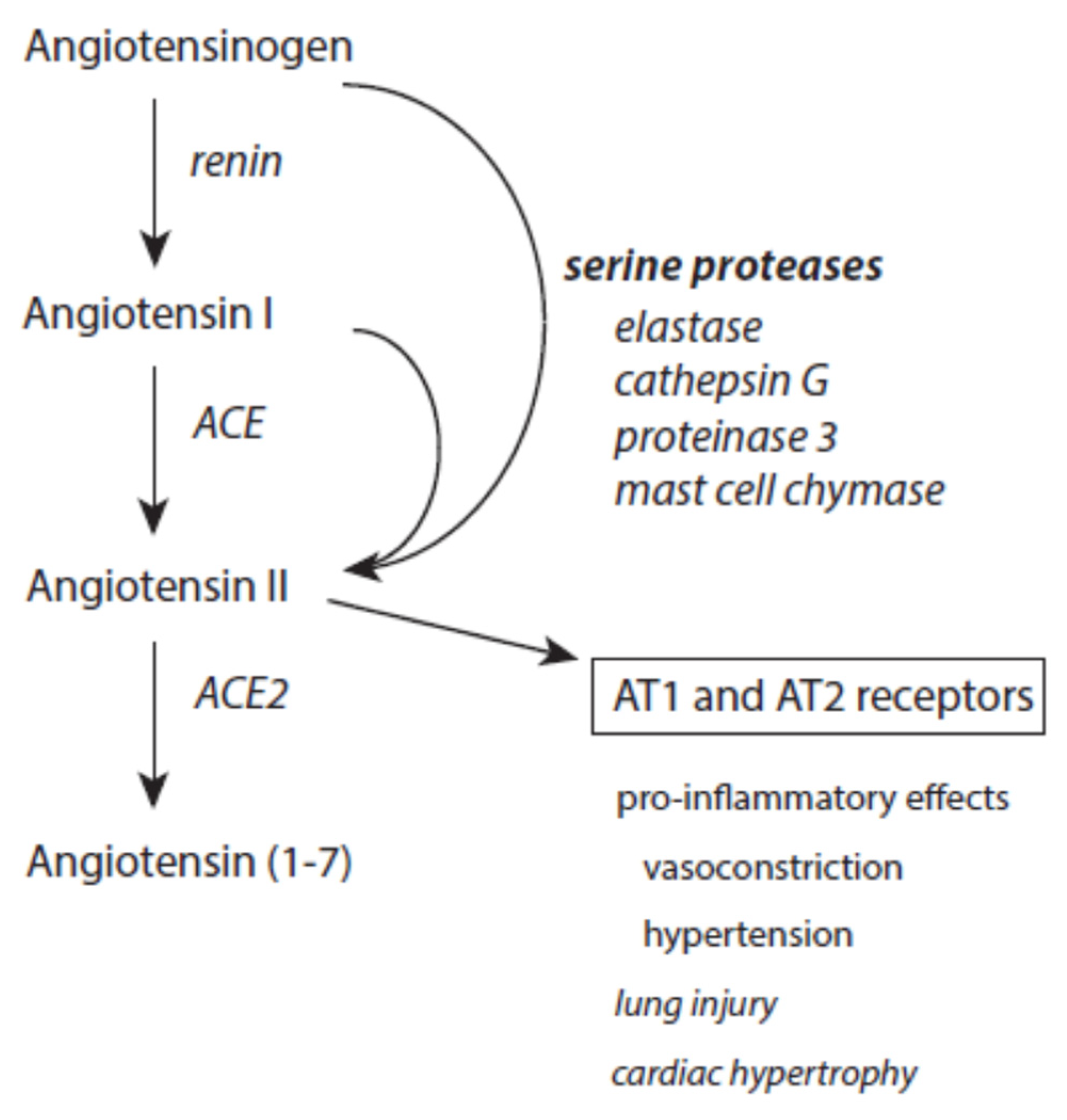

| Cytotoxic Agent | Mode of Cytotoxic Action | Antagonizing Principles | Remarks |
|---|---|---|---|
| Superoxide anion radical | Release of Fe2+ from [4Fe-4S]2+ clusters, formation of peroxynitrite | Superoxide dismutases, cytochrome c | |
| Hydrogen peroxide | Formation of hydroxyl radicals in reaction with Fe2+ or Cu+ | Catalase, peroxiredoxins, glutathione peroxidases | |
| Hydroxyl radicals | Diffusion-controlled oxidation of many substrates | No antagonizing principles; only limited protection by carbohydrates | Prevention of their formation is the main strategy Very dangerous |
| Peroxynitrite | Formation of substrate radicals, nitration of tyrosine residues, initiation of lipid peroxidation | Myeloperoxidase, heme proteins | |
| Hypochlorous acid, hypobromous acid | Preferred oxidation of cysteine, methionine residues Interaction with aromatic amino acid residues and amino groups | SCN−, taurine, glutathione (GSH), ascorbate | |
| Myeloperoxidase (MPO) | Formation of HOCl, HOBr, substrate radicals | Ceruloplasmin | |
| Free transition metal ions | Dangerous radical species in reaction with H2O2 and organic hydroperoxides | Proper control over all aspects of iron and copper ion metabolism | Enhanced yield of free transition metal ions is dangerous |
| Free methemoglobin | Formation of free heme | Haptoglobin | |
| Free metmyoglobin | Formation of free heme | Haptoglobin | |
| Free heme | Oxidation at hydrophobic loci, hemolysis of red blood cells, cytotoxic to kidney and liver, interaction with G4 structures in nucleic acids, can act as DAMP | Hemopexin Heme oxygenase | Very dangerous |
| Oxidative products in lipid phases such as lipid peroxyl radicals and lipid hydroperoxides | Induction of further oxidative modifications of yet-unperturbed molecules | Lipid antioxidants such as α-tocopherol, carotinoids, ubiquinol, dehydrolipoic acid Glutathione peroxidase 4 (GPX4), and GSHProper control over transition free metal ions | |
| Oxidative products in water-exposed molecules | Induction of further oxidative modifications of yet-unperturbed molecules | Urate, ascorbate, polyphenols Proper control over transition free metal ions | |
| Neutrophil elastase | Cleavage of many extracellular matrix components, formation of angiotensin II | α1-antitrypsin (A1AT), secretory leukocyte protease inhibitor (SLPI), elafin, serpin B1, α2-macroglobulin | Failure of anti-proteases to inhibit elastase at severe oxidative stress Very dangerous |
| Cathepsin G | Cleavage of extracellular matrix components, receptor shedding, formation of angiotensin II | A1AT, α1-antichymotrypsin, SLPI | |
| Proteinase 3 | Cleavage of extracellular matrix components, in particular elastin | A1AT, elafin | |
| Mast cell tryptases | Cleavage of extracellular matrix components | Heparin-binding proteins such as lactoferrin, MPO, antithrombin III | Protected by heparin against the action of anti-proteases |
| Mast cell chymase | Cleavage of extracellular matrix components, chemokines, and cytokines, formation of angiotensin II | α1-antichymotrypsin | |
| Angiotensin II | Receptor-mediated pro-inflammatory effects | Angiotensin converting enzyme 2 (ACE2) | Very dangerous |
| Bradykinin | Receptor-mediated pro-inflammatory effects | Aminopeptidase P, angiotensin converting enzyme (ACE) | |
| Matrix metalloproteases (MMPs) | Cleavage of extracellular matrix components | Tissue inhibitors of metalloproteases (TIMPs) | Problems at shifted balance between MMPs and TIMPs |
| Cytotoxic Agent | Mode of Cytotoxic Action | Antagonizing Principles | Remarks |
|---|---|---|---|
| Singlet oxygen (1O2) | DNA damage, especially guanine [21,22] | Carotenoids [23,24,25] | Skin and eye exposure |
| Ozone | Formation of ozonides and cytotoxic aldehydes [26] | Ascorbate, GSH, urate [27,28] | Exposure to respiratory system [29,30] |
| Sunlight | Induction of photooxidative processes, formation of 1O2 [31] | Melanin, polyphenols, [32] | Skin exposure |
| Ionizing irradiation | Water radiolysis, formation of solvated electrons, O2•−, H2O2, •OH, and substrate radicals [33,34,35] | See remarks in Table 1 | Always present at very low level |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnhold, J. Host-Derived Cytotoxic Agents in Chronic Inflammation and Disease Progression. Int. J. Mol. Sci. 2023, 24, 3016. https://doi.org/10.3390/ijms24033016
Arnhold J. Host-Derived Cytotoxic Agents in Chronic Inflammation and Disease Progression. International Journal of Molecular Sciences. 2023; 24(3):3016. https://doi.org/10.3390/ijms24033016
Chicago/Turabian StyleArnhold, Jürgen. 2023. "Host-Derived Cytotoxic Agents in Chronic Inflammation and Disease Progression" International Journal of Molecular Sciences 24, no. 3: 3016. https://doi.org/10.3390/ijms24033016
APA StyleArnhold, J. (2023). Host-Derived Cytotoxic Agents in Chronic Inflammation and Disease Progression. International Journal of Molecular Sciences, 24(3), 3016. https://doi.org/10.3390/ijms24033016







