A Cross-Species Analysis Reveals Dysthyroidism of the Ovaries as a Common Trait of Premature Ovarian Aging
Abstract
1. Introduction
2. Results
2.1. Ovarian Dysthyroidism in Genetically Different Mice Exposed to Different Diets
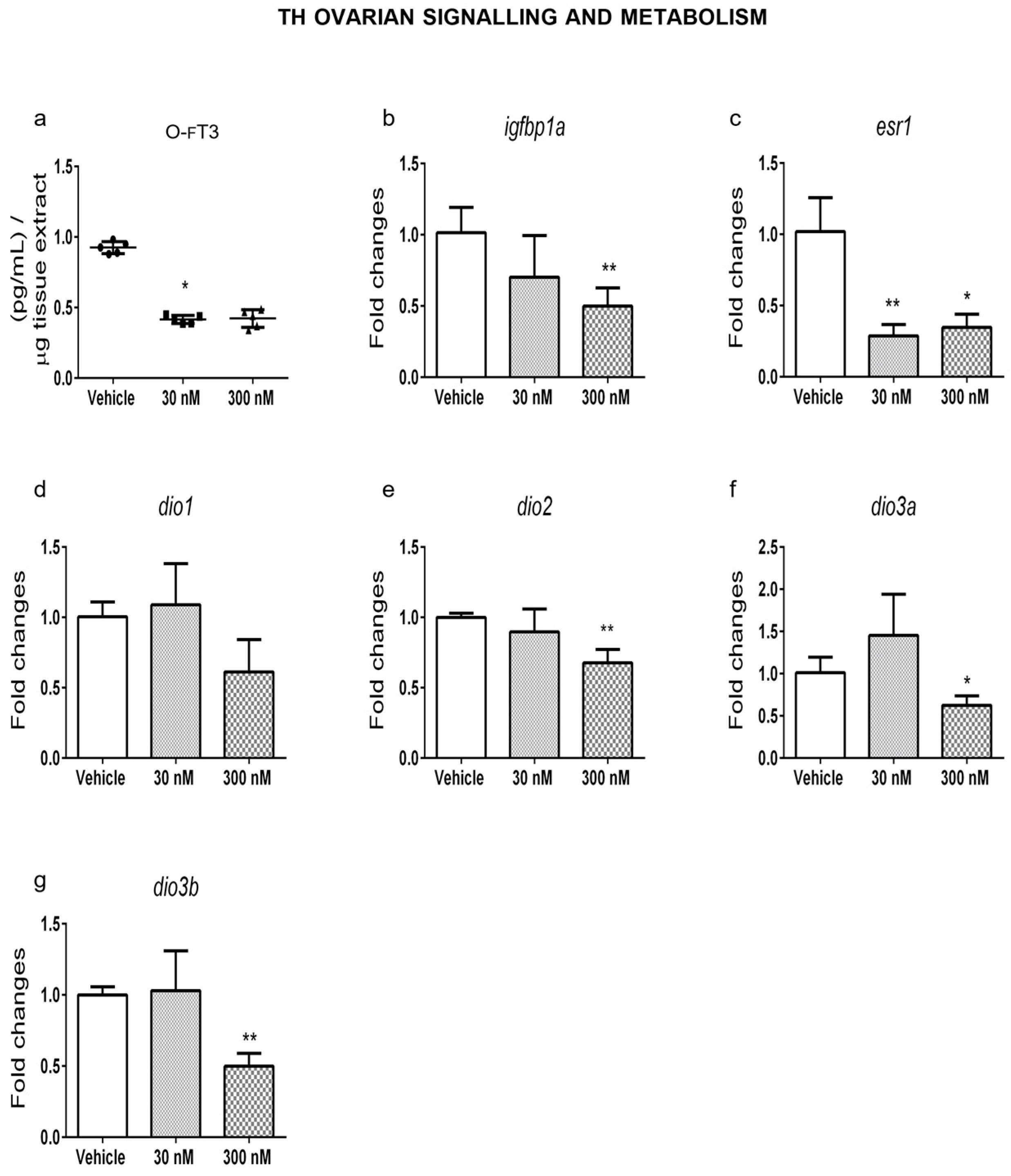
2.2. Ovarian Dysthyroidism Is a Trait of POA Induced by Developmental and Lifelong Exposure to CPF in Zebrafish
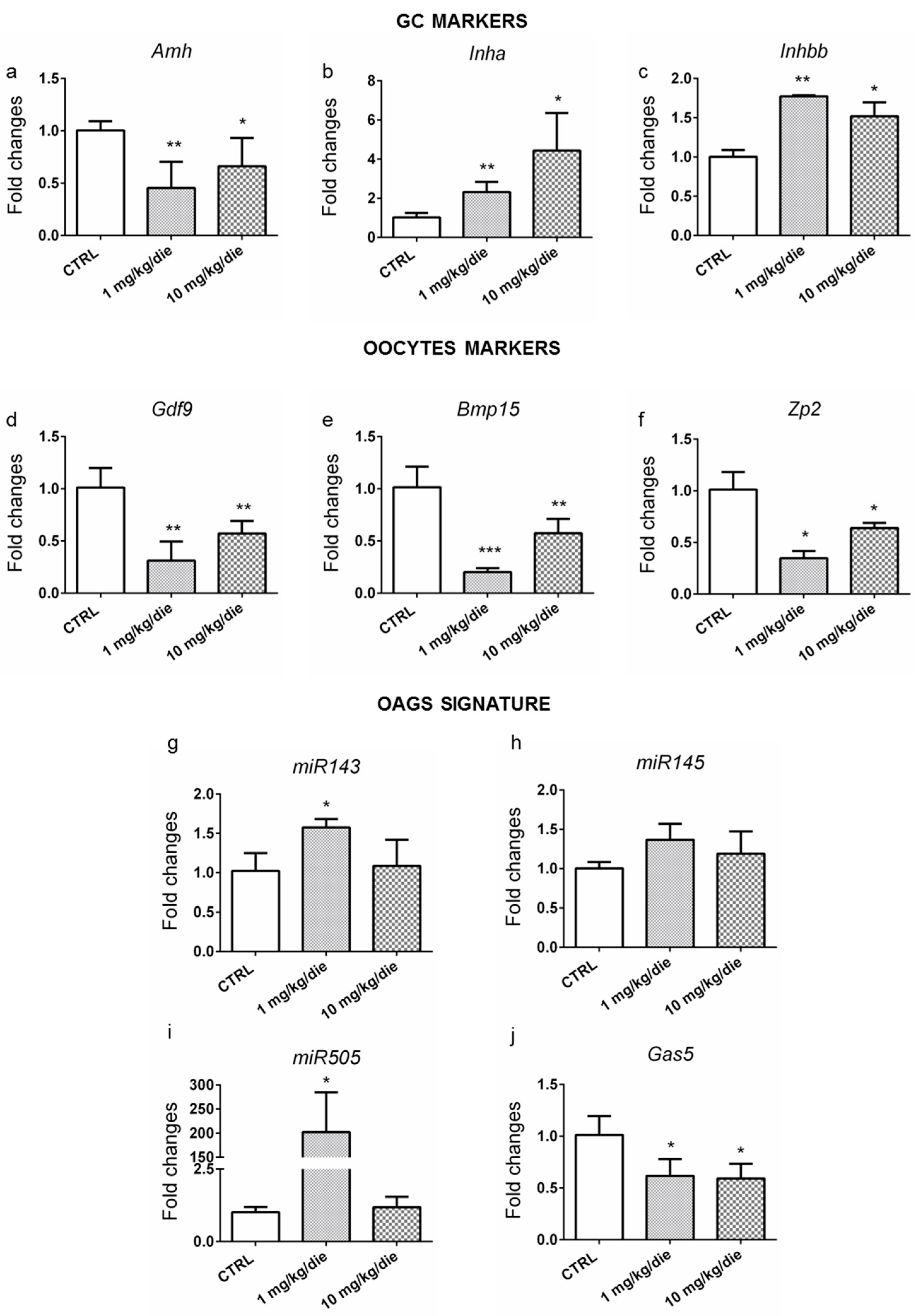
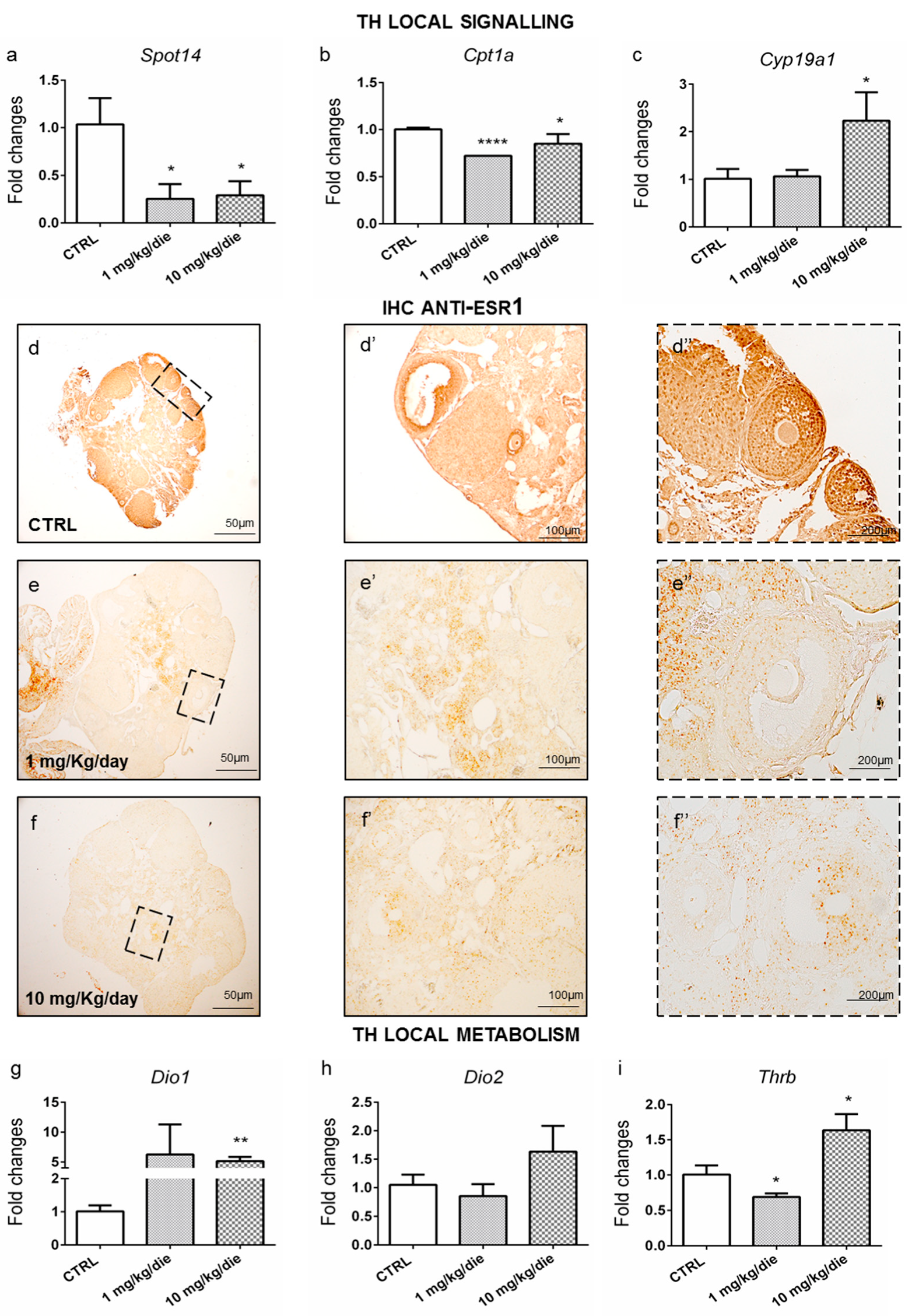
2.3. Ovarian Dysthyroidism Is a Trait of POA Induced by Developmental and Lifelong Exposure to CPF in Mice
2.4. Ovarian Dysthyroidism Is an Evolutionary Conserved Trait of POA Retrieved Also in Humans
3. Discussion
4. Materials and Methods
4.1. Mouse Treatment to Different Diets and CPF
4.2. Zebrafish Husbandry and Treatment
4.3. Human Samples
4.4. Hormones Detection
4.5. RT-qPCR Analysis
4.6. miRNA Isolation, cDNA Synthesis, and RT-qPCR for miRNA Detection
4.7. Telomere Length Detection
4.8. Western Blotting Analysis
4.9. Hematoxylin and Eosin Stining and Immuno-Histochemistry (IHC)
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rocca, W.A.; Rocca, L.G.; Smith, C.Y.; Grossardt, B.R.; Faubion, S.S.; Shuster, L.T.; Kirkland, J.L.; Lebrasseur, N.K.; Schafer, M.J.; Mielke, M.M.; et al. Loss of Ovarian Hormones and Accelerated Somatic and Mental Aging. Physiology 2018, 33, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Meng, T.; Zhai, Y.; Zhao, L.; Luo, W.; Liu, P.; Qin, Y. Ovarian Reserve Markers in Premature Ovarian Insufficiency: Within Different Clinical Stages and Different Etiologies. Front. Endocrinol. 2021, 12, 601752. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Kitahara, Y.; Osuka, S.; Tsukui, Y.; Kobayashi, M.; Iwase, A. Effect of hypothyroidism and thyroid autoimmunity on the ovarian reserve: A systematic review and meta-analysis. Reprod. Med. Biol. 2022, 21, e12427. [Google Scholar] [CrossRef] [PubMed]
- Colella, M.; Cuomo, D.; Giacco, A.; Mallardo, M.; De Felice, M.; Ambrosino, C. Thyroid Hormones and Functional Ovarian Reserve: Systemic vs. Peripheral Dysfunctions. J. Clin. Med. 2020, 9, 1679. [Google Scholar] [CrossRef]
- Brehm, E.; Flaws, J.A. Transgenerational Effects of Endocrine-Disrupting Chemicals on Male and Female Reproduction. Endocrinology 2019, 160, 1421–1435. [Google Scholar] [CrossRef]
- Cabry, R.; Merviel, P.; Madkour, A.; Lefranc, E.; Scheffler, F.; Desailloud, R.; Bach, V.; Benkhalifa, M. The impact of endocrine disruptor chemicals on oocyte/embryo and clinical outcomes in IVF. Endocr. Connect. 2020, 9, R134–R142. [Google Scholar] [CrossRef]
- Poppe, K. Management of Endocrine Disease: Thyroid and female infertility: More questions than answers?! Eur. J. Endocrinol. 2021, 184, R123–R135. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Liu, Y.; Xing, Y.; Miao, C.; Zhao, Y.; Chang, X.; Zhang, Q. The Role of Oxidative Stress and Natural Antioxidants in Ovarian Aging. Front. Pharmacol. 2021, 11, 617843. [Google Scholar] [CrossRef]
- Van der Reest, J.; Cecchino, G.N.; Haigis, M.C.; Kordowitzki, P. Mitochondria: Their relevance during oocyte ageing. Ageing Res. Rev. 2021, 70, 101378. [Google Scholar] [CrossRef]
- Colella, M.; Cuomo, D.; Peluso, T.; Falanga, I.; Mallardo, M.; De Felice, M.; Ambrosino, C. Ovarian Aging: Role of Pituitary-Ovarian Axis Hormones and ncRNAs in Regulating Ovarian Mitochondrial Activity. Front. Endocrinol. 2021, 12, 1737. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Bargiel, P.; Janda-Milczarek, K. The Influence of Oxidative Stress on Thyroid Diseases. Antioxidants 2021, 10, 1442. [Google Scholar] [CrossRef]
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Currò, D. Thyroid Hormones, Oxidative Stress, and Inflammation. Mediat. Inflamm. 2016, 2016, 6757154. [Google Scholar] [CrossRef]
- Gereben, B.; Zavacki, A.M.; Ribich, S.; Kim, B.W.; Huang, S.A.; Simonides, W.S.; Zeöld, A.; Bianco, A.C. Cellular and Molecular Basis of Deiodinase-Regulated Thyroid Hormone Signaling1. Endocr. Rev. 2008, 29, 898–938. [Google Scholar] [CrossRef]
- Bianco, A.C.; Salvatore, D.; Gereben, B.; Berry, M.J.; Larsen, P.R. Biochemistry, Cellular and Molecular Biology, and Physiological Roles of the Iodothyronine Selenodeiodinases. Endocr. Rev. 2002, 23, 38–89. [Google Scholar] [CrossRef]
- Bianco, A.C.; Dumitrescu, A.; Gereben, B.; Ribeiro, M.O.; Fonseca, T.L.; Fernandes, G.W.; Bocco, B.M.L.C. Paradigms of Dynamic Control of Thyroid Hormone Signaling. Endocr. Rev. 2019, 40, 1000–1047. [Google Scholar] [CrossRef]
- Cuomo, D.; Porreca, I.; Ceccarelli, M.; Threadgill, D.W.; Barrington, W.T.; Petriella, A.; D’Angelo, F.; Cobellis, G.; De Stefano, F.; D’Agostino, M.N.; et al. Transcriptional landscape of mouse-aged ovaries reveals a unique set of non-coding RNAs associated with physiological and environmental ovarian dysfunctions. Cell Death Discov. 2018, 4, 112. [Google Scholar] [CrossRef]
- Albers, J.W.; Cole, P.; Greenberg, R.S.; Mandel, J.S.; Monson, R.R.; Snodgrass, W.R.; Spurgeon, A.; Van Gemert, M. Analysis of chlorpyrifos exposure and human health: Expert panel report. J. Toxicol. Environ. Health-Part B Crit. Rev. 1999, 2, 301–324. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Mehrpour, O.; Sadeghi, M.; Aschner, M.; Aramjoo, H.; Roshanravan, B.; Samarghandian, S. A systematic review on the metabolic effects of chlorpyrifos. Rev. Environ. Health 2022, 37, 137–151. [Google Scholar] [CrossRef]
- Rauh, V.A.; Garcia, W.E.; Whyatt, R.M.; Horton, M.K.; Barr, D.B.; Louis, E.D. Prenatal exposure to the organophosphate pesticide chlorpyrifos and childhood tremor. Neurotoxicology 2015, 51, 80–86. [Google Scholar] [CrossRef]
- Yano, B.L.; Young, J.T.; Mattsson, J.L. Lack of carcinogenicity of chlorpyrifos insecticide in a high-dose, 2-year dietary toxicity study in Fischer 344 rats. Toxicol. Sci. 2000, 53, 135–144. [Google Scholar] [CrossRef]
- Ubaid ur Rahman, H.; Asghar, W.; Nazir, W.; Sandhu, M.A.; Ahmed, A.; Khalid, N. A comprehensive review on chlorpyrifos toxicity with special reference to endocrine disruption: Evidence of mechanisms, exposures and mitigation strategies. Sci. Total. Environ. 2021, 755, 142649. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pang, G.; Ren, F.; Fang, B. Chlorpyrifos-induced reproductive toxicity in rats could be partly relieved under high-fat diet. Chemosphere 2019, 229, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, B.; Philip, G.H. Reproductive toxicity of chlorpyrifos tested in zebrafish (Danio rerio): Histological and hormonal end points. Toxicol. Ind. Health 2016, 32, 1808–1816. [Google Scholar] [CrossRef]
- Nittoli, V.; Colella, M.; Porciello, A.; Reale, C.; Roberto, L.; Russo, F.; Russo, N.A.; Porreca, I.; De Felice, M.; Mallardo, M.; et al. Multi Species Analyses Reveal Testicular T3 Metabolism and Signalling as a Target of Environmental Pesticides. Cells 2021, 10, 2187. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, S.; Tassinari, R.; Maranghi, F.; Eusepi, A.; Di Virgilio, A.; Chiarotti, F.; Ricceri, L.; Venerosi Pesciolini, A.; Gilardi, E.; Moracci, G.; et al. Developmental Exposure to Chlorpyrifos Induces Alterations in Thyroid and Thyroid Hormone Levels Without Other Toxicity Signs in CD-1 Mice. Toxicol. Sci. 2009, 108, 311–319. [Google Scholar] [CrossRef]
- Colella, M.; Nittoli, V.; Porciello, A.; Porreca, I.; Reale, C.; Russo, F.; Russo, N.A.; Roberto, L.; Albano, F.; De Felice, M.; et al. Peripheral T3 signaling is the target of pesticides in zebrafish larvae and adult liver. J. Endocrinol. 2020, 247, 53–68. [Google Scholar] [CrossRef]
- Sarapura, V.D.; Wood, W.M.; Gordon, D.F.; Ridgway, E.C. Effect of thyroid hormone on T3-receptor mRNA levels and growth of thyrotropic tumors. Mol. Cell. Endocrinol. 1993, 91, 75–81. [Google Scholar] [CrossRef]
- Seelig, S.; Liaw, C.; Towle, H.C.; Oppenheimer, J.H. Thyroid hormone attenuates and augments hepatic gene expression at a pretranslational level. Proc. Natl. Acad. Sci. USA 1981, 78, 4733–4737. [Google Scholar] [CrossRef]
- Ren, J.; Xu, N.; Zheng, H.; Tian, W.; Li, H.; Li, Z.; Wang, Y.; Tian, Y.; Kang, X.; Liu, X. Expression of Thyroid Hormone Responsive SPOT 14 Gene Is Regulated by Estrogen in Chicken (Gallus gallus). Sci. Rep. 2017, 7, 10243. [Google Scholar] [CrossRef]
- Colbert, C.L.; Kim, C.-W.; Moon, Y.-A.; Henry, L.; Palnitkar, M.; McKean, W.B.; Fitzgerald, K.; Deisenhofer, J.; Horton, J.D.; Kwon, H.J. Crystal structure of Spot 14, a modulator of fatty acid synthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 18820–18825. [Google Scholar] [CrossRef]
- Alföldi, J.; Lindblad-Toh, K. Comparative genomics as a tool to understand evolution and disease. Genome Res. 2013, 23, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a Model Vertebrate for Investigating Chemical Toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Zayed, Y.; Qi, X.; Peng, C. Identification of Novel MicroRNAs and Characterization of MicroRNA Expression Profiles in Zebrafish Ovarian Follicular Cells. Front. Endocrinol. 2019, 10, 518. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, F.; Zhao, J.; Wang, Z. Expression Regulation and Physiological Role of Transcription Factor FOXO3a During Ovarian Follicular Development. Front. Physiol. 2020, 11, 595086. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, E.; Omari, S.; Michel, M.; Ding, J.; Amano, T.; Forabosco, A.; Schlessinger, D.; Ottolenghi, C. Constitutively active Foxo3 in oocytes preserves ovarian reserve in mice. Nat. Commun. 2013, 4, 1843. [Google Scholar] [CrossRef]
- Zhang, M.; Bener, M.B.; Jiang, Z.; Wang, T.; Esencan, E.; Scott, R.; Horvath, T.; Seli, E. Mitofusin 2 plays a role in oocyte and follicle development, and is required to maintain ovarian follicular reserve during reproductive aging. Aging 2019, 11, 3919–3938. [Google Scholar] [CrossRef]
- Hanna, C.W.; Bretherick, K.L.; Gair, J.L.; Fluker, M.R.; Stephenson, M.D.; Robinson, W.P. Telomere length and reproductive aging. Hum. Reprod. 2009, 24, 1206–1211. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, H.; Wang, L.; He, J.; Guo, Y.; Liu, Y.; Liu, X.; Lin, H. Fertility Enhancement but Premature Ovarian Failure in esr1-Deficient Female Zebrafish. Front. Endocrinol. 2018, 9, 567. [Google Scholar] [CrossRef]
- Marlatt, V.L.; Gerrie, E.; Wiens, S.; Jackson, F.; Moon, T.W.; Trudeau, V.L. Estradiol and triiodothyronine differentially modulate reproductive and thyroidal genes in male goldfish. Fish Physiol. Biochem. 2012, 38, 283–296. [Google Scholar] [CrossRef]
- Porreca, I.; D’Angelo, F.; De Franceschi, L.; Mattè, A.; Ceccarelli, M.; Iolascon, A.; Zamò, A.; Russo, F.; Ravo, M.; Tarallo, R.; et al. Pesticide toxicogenomics across scales: In vitro transcriptome predicts mechanisms and outcomes of exposure in vivo. Sci. Rep. 2016, 6, 38131. [Google Scholar] [CrossRef]
- Noli, L.; Khorsandi, S.E.; Pyle, A.; Giritharan, G.; Fogarty, N.; Capalbo, A.; Devito, L.; Jovanovic, V.M.; Khurana, P.; Rosa, H.; et al. Effects of thyroid hormone on mitochondria and metabolism of human preimplantation embryos. Stem Cells 2020, 38, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Hatsuta, M.; Tamura, K.; Shimizu, Y.; Toda, K.; Kogo, H. Effect of Thyroid Hormone on CYP19 Expression in Ovarian Granulosa Cells from Gonadotropin-Treated Immature Rats. J. Pharmacol. Sci. 2004, 94, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Falzacappa, C.V.; Mangialardo, C.; Patriarca, V.; Bucci, B.; Amendola, D.; Raffa, S.; Torrisi, M.R.; Silvestrini, G.; Ballanti, P.; Moriggi, G.; et al. Thyroid hormones induce cell proliferation and survival in ovarian granulosa cells COV434. J. Cell. Physiol. 2009, 221, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Abu-Halima, M.; Becker, L.S.; Ayesh, B.M.; Baus, S.L.; Hamza, A.; Fischer, U.; Hammadeh, M.; Keller, A.; Meese, E. Characterization of micro-RNA in women with different ovarian reserve. Sci. Rep. 2021, 11, 13351. [Google Scholar] [CrossRef] [PubMed]
- Van den Beld, A.W.; Kaufman, J.-M.; Zillikens, M.C.; Lamberts, S.W.J.; Egan, J.M.; Van Der Lely, A.J. The physiology of endocrine systems with ageing. Lancet Diabetes Endocrinol. 2018, 6, 647–658. [Google Scholar] [CrossRef]
- Louzada, R.A.; Carvalho, D.P. Similarities and Differences in the Peripheral Actions of Thyroid Hormones and Their Metabolites. Front. Endocrinol. 2018, 9, 394. [Google Scholar] [CrossRef]
- Hernandez, A. Thyroid hormone deiodination and action in the gonads. Curr. Opin. Endocr. Metab. Res. 2018, 2, 18–23. [Google Scholar] [CrossRef]
- Ren, B.; Zhu, Y. A New Perspective on Thyroid Hormones: Crosstalk with Reproductive Hormones in Females. Int. J. Mol. Sci. 2022, 23, 2708. [Google Scholar] [CrossRef]
- Kose, E.; Guzel, O.; Demir, K.; Arslan, N. Changes of thyroid hormonal status in patients receiving ketogenic diet due to intractable epilepsy. J. Pediatr. Endocrinol. Metab. 2017, 30, 411–416. [Google Scholar] [CrossRef]
- Dittrich, R.; Beckmann, M.W.; Oppelt, P.G.; Hoffmann, I.; Lotz, L.; Kuwert, T.; Mueller, A. Thyroid hormone receptors and reproduction. J. Reprod. Immunol. 2011, 90, 58–66. [Google Scholar] [CrossRef]
- Aghajanova, L.; Lindeberg, M.; Carlsson, I.B.; Stavreus-Evers, A.; Zhang, P.; Scott, J.E.; Hovatta, O.; Skjöldebrand-Sparre, L. Receptors for thyroid-stimulating hormone and thyroid hormones in human ovarian tissue. Reprod. Biomed. Online 2009, 18, 337–347. [Google Scholar] [CrossRef]
- Göthe, S.; Wang, Z.; Ng, L.; Kindblom, J.M.; Barros, A.C.; Ohlsson, C.; Vennström, B.; Forrest, D. Mice devoid of all known thyroid are viable hormone receptors but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. Genes Dev. 1999, 13, 1329–1341. [Google Scholar] [CrossRef]
- Pawlik-Pachucka, E.; Budzinska, M.; Wicik, Z.; Domaszewska-Szostek, A.; Owczarz, M.; Roszkowska-Gancarz, M.; Gewartowska, M.; Puzianowska-Kuznicka, M. Age-associated increase of thyroid hormone receptor β gene promoter methylation coexists with decreased gene expression. Endocr. Res. 2018, 43, 246–257. [Google Scholar] [CrossRef]
- Berry, M.J.; Grieco, D.; Taylor, B.A.; Maia, A.L.; Kieffer, J.D.; Beamer, W.; Glover, E.; Poland, A.; Larsen, P.R. Physiological and genetic analyses of inbred mouse strains with a type I iodothyronine 5′ deiodinase deficiency. J. Clin. Investig. 1993, 92, 1517–1528. [Google Scholar] [CrossRef]
- Araujo, R.L.; Andrade, B.M.; Padrón, A.S.; Gaidhu, M.P.; Perry, R.L.S.; Carvalho, D.P.; Ceddia, R.C. High-Fat Diet Increases Thyrotropin and Oxygen Consumption without Altering Circulating 3,5,3′-Triiodothyronine (T3) and Thyroxine in Rats: The Role of Iodothyronine Deiodinases, Reverse T3 Production, and Whole-Body Fat Oxidation. Endocrinology 2010, 151, 3460–3469. [Google Scholar] [CrossRef]
- Gauthier, B.R.; Sola-García, A.; Cáliz-Molina, M.; Lorenzo, P.I.; Cobo-Vuilleumier, N.; Capilla-González, V.; Martin-Montalvo, A. Thyroid hormones in diabetes, cancer, and aging. Aging Cell 2020, 19, e13260. [Google Scholar] [CrossRef]
- Britt, K.L.; Findlay, J.K. Estrogen actions in the ovary revisited. J. Endocrinol. 2002, 175, 269–276. [Google Scholar] [CrossRef]
- Peiris, D.C.; Dhanushka, T. Low doses of chlorpyrifos interfere with spermatogenesis of rats through reduction of sex hormones. Environ. Sci. Pollut. Res. 2017, 24, 20859–20867. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Kim, B.-Y.; Kang, H.-G.; Ku, H.-O.; Cho, J.-H. Effect of chlorpyrifos-methyl on steroid and thyroid hormones in rat F0- and F1-generations. Toxicology 2006, 220, 189–202. [Google Scholar] [CrossRef]
- Hapon, M.B.; Gamarra-Luques, C.; Jahn, G.A. Short term hypothyroidism affects ovarian function in the cycling rat. Reprod. Biol. Endocrinol. 2010, 8, 14. [Google Scholar] [CrossRef]
- Parakh, T.N.; Hernandez, J.A.; Grammer, J.C.; Weck, J.; Hunzicker-Dunn, M.; Zeleznik, A.J.; Nilson, J.H. Follicle-stimulating hormone/cAMP regulation of aromatase gene expression requires β-catenin. Proc. Natl. Acad. Sci. USA 2006, 103, 12435–12440. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yao, Y.; Ma, X.; Fu, B.; Li, N.; Zhang, C. Mechanisms of OCT4 on 3,5,3′-Tri-iodothyronine and FSH-induced Granulosa Cell Development in Female Mice. Endocrinology 2021, 162, bqab183. [Google Scholar] [CrossRef] [PubMed]
- Kanasaki, H.; Purwana, I.N.; Mijiddorj, T.; Sukhbaatar, U.; Oride, A.; Miyazaki, K. Effects of estradiol and progesterone on gonadotropin LHβ- and FSHβ-subunit promoter activities in gonadotroph LβT2 cells. Neuroendocrinol. Lett. 2012, 33, 608–613. [Google Scholar] [PubMed]
- Stocco, C. Aromatase expression in the ovary: Hormonal and molecular regulation. Steroids 2008, 73, 473–487. [Google Scholar] [CrossRef]
- Rae, M.T.; Gubbay, O.; Kostogiannou, A.; Price, D.; Critchley, H.O.D.; Hillier, S.G. Thyroid Hormone Signaling in Human Ovarian Surface Epithelial Cells. J. Clin. Endocrinol. Metab. 2007, 92, 322–327. [Google Scholar] [CrossRef]
- Dalgård, C.; Benetos, A.; Verhulst, S.; Labat, C.; Kark, J.D.; Christensen, K.; Kimura, M.; Kyvik, K.O.; Aviv, A. Leukocyte telomere length dynamics in women and men: Menopause vs age effects. Leuk. Res. 2015, 44, 1688–1695. [Google Scholar] [CrossRef]
- Tang, H.; Lv, W.; Sun, W.; Bi, Q.; Hao, Y. miR-505 inhibits cell growth and EMT by targeting MAP3K3 through the AKT-NFκB pathway in NSCLC cells. Int. J. Mol. Med. 2019, 43, 1203–1216. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef]
- García-García, V.A.; Alameda, J.P.; Page, A.; Casanova, M.L. Role of nf-κb in ageing and age-related diseases: Lessons from genetically modified mouse models. Cells 2021, 10, 1906. [Google Scholar] [CrossRef]
- Achache, H.; Falk, R.; Lerner, N.; Beatus, T.; Tzur, Y.B. Oocyte aging is controlled by mitogen-activated protein kinase signaling. Aging Cell 2021, 20, e13386. [Google Scholar] [CrossRef]
- Li, J.; Ge, W. Zebrafish as a model for studying ovarian development: Recent advances from targeted gene knockout studies. Mol. Cell. Endocrinol. 2020, 507, 110778. [Google Scholar] [CrossRef]
- Porazzi, P.; Calebiro, D.; Benato, F.; Tiso, N.; Persani, L. Thyroid gland development and function in the zebrafish model. Mol. Cell. Endocrinol. 2009, 312, 14–23. [Google Scholar] [CrossRef]
- Rosales, M.; Nuñez, M.; Abdala, A.; Mesch, V.; Mendeluk, G. Thyroid hormones in ovarian follicular fluid: Association with oocyte retrieval in women undergoing assisted fertilization procedures. J. Bras. Reprod. Assist. 2020, 24, 245. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Y.-J.; Wang, M.; Tang, M.-Q.; Liu, Y.-F. Correlation Analysis Between Ovarian Reserve and Thyroid Hormone Levels in Infertile Women of Reproductive Age. Front. Endocrinol. 2021, 12, 745199. [Google Scholar] [CrossRef]
- Barrington, W.T.; Wulfridge, P.; Wells, A.E.; Rojas, C.M.; Howe, S.Y.F.; Perry, A.; Hua, K.; Pellizzon, M.A.; Hansen, K.D.; Voy, B.H.; et al. Improving Metabolic Health Through Precision Dietetics in Mice. Genetics 2018, 208, 399–417. [Google Scholar] [CrossRef]
- Marotta, P.; Amendola, E.; Scarfò, M.; De Luca, P.; Zoppoli, P.; Amoresano, A.; De Felice, M.; Di Lauro, R. The paired box transcription factor Pax8 is essential for function and survival of adult thyroid cells. Mol. Cell. Endocrinol. 2014, 396, 26–36. [Google Scholar] [CrossRef]
- McCurley, A.T.; Callard, G.V. Characterization of housekeeping genes in zebrafish: Male-female differences and effects of tissue type, developmental stage and chemical treatment. BMC Mol. Biol. 2008, 9, 102. [Google Scholar] [CrossRef]
- Gong, Z.-K.; Wang, S.-J.; Huang, Y.-Q.; Zhao, R.-Q.; Zhu, Q.-F.; Lin, W.-Z. Identification and validation of suitable reference genes for RT-qPCR analysis in mouse testis development. Mol. Genet. Genom. 2014, 289, 1157–1169. [Google Scholar] [CrossRef]
- Yang, L.-H.; Wang, S.-L.; Tang, L.-L.; Liu, B.; Ye, W.-L.; Wang, L.-L.; Wang, Z.-Y.; Zhou, M.-T.; Chen, B.-C. Universal stem-loop primer method for screening and quantification of microRNA. PLoS ONE 2014, 9, e115293. [Google Scholar] [CrossRef]
- Callicott, R.J.; Womack, J.E. Real-time PCR assay for measurement of mouse telomeres. Comp. Med. 2006, 56, 17–22. [Google Scholar]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.A.; Whitmore, D. Circadian Rhythmicity and Light Sensitivity of the Zebrafish Brain. PLoS ONE 2014, 9, e86176. [Google Scholar] [CrossRef] [PubMed]



| Expected Marker Regulation | ||
|---|---|---|
| Function | In Hypothyroidism [4] | In Ovarian Aging [16] |
| TH ovarian metabolism | cT3 ↓ | cT3 ↓ |
| Dio1/Dio2 ↑ | Dio1/Dio2 (?) | |
| Dio3 ↓ | Dio3 (?) | |
| TH ovarian signalling | Thra, Thrb ↓ | Thra, Thrb (?) |
| Spot14 ↓ | Spot14 (?) | |
| Granulosa cell markers | Amh (?) | Amh ↓ |
| Inha (?) | Inha ↓ | |
| Inhbb (?) | Inhbb (-) | |
| Oocytes cell markers | Gdf9 ↓ | Gdf9 ↓ |
| Bmp15 ↓ | Bmp15 ↓ | |
| Oct4 (?) | Oct4 ↓ | |
| Sycp1 (?) | Sycp1 ↓ | |
| OAGS markers | MiR143 (?) | MiR143 ↑ |
| MiR145 (?) | MiR145 ↑ | |
| MiR505 (?) | MiR505 ↑ | |
| Gas5 (?) | Gas5 ↓ | |
| Ovarian Healthspan and Lifespan | |||||
|---|---|---|---|---|---|
| Mouse Exposed to CPF | Number of Dams (n°) | Percentage (%) | Pups (n°) | Placental Buttons (n°) | Telomere Length (ATLR) |
| CTRL | 5/5 | 100% | 39 | 42 | 4225.8 ± 1005.9 |
| 1 mg/Kg/day | 5/10 | 30% | 0 | 7 | 2638.2 ± 155.3 * |
| 10 mg/Kg/day | 5/10 | 40% | 15 | 34 | 2410.9 ± 613.2 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colella, M.; Cuomo, D.; Nittoli, V.; Amoresano, A.; Porciello, A.; Reale, C.; Roberto, L.; Russo, F.; Russo, N.A.; De Felice, M.; et al. A Cross-Species Analysis Reveals Dysthyroidism of the Ovaries as a Common Trait of Premature Ovarian Aging. Int. J. Mol. Sci. 2023, 24, 3054. https://doi.org/10.3390/ijms24033054
Colella M, Cuomo D, Nittoli V, Amoresano A, Porciello A, Reale C, Roberto L, Russo F, Russo NA, De Felice M, et al. A Cross-Species Analysis Reveals Dysthyroidism of the Ovaries as a Common Trait of Premature Ovarian Aging. International Journal of Molecular Sciences. 2023; 24(3):3054. https://doi.org/10.3390/ijms24033054
Chicago/Turabian StyleColella, Marco, Danila Cuomo, Valeria Nittoli, Angela Amoresano, Alfonsina Porciello, Carla Reale, Luca Roberto, Filomena Russo, Nicola Antonino Russo, Mario De Felice, and et al. 2023. "A Cross-Species Analysis Reveals Dysthyroidism of the Ovaries as a Common Trait of Premature Ovarian Aging" International Journal of Molecular Sciences 24, no. 3: 3054. https://doi.org/10.3390/ijms24033054
APA StyleColella, M., Cuomo, D., Nittoli, V., Amoresano, A., Porciello, A., Reale, C., Roberto, L., Russo, F., Russo, N. A., De Felice, M., Mallardo, M., & Ambrosino, C. (2023). A Cross-Species Analysis Reveals Dysthyroidism of the Ovaries as a Common Trait of Premature Ovarian Aging. International Journal of Molecular Sciences, 24(3), 3054. https://doi.org/10.3390/ijms24033054








