Serum Neurofilament Light Chain and Glial Fibrillary Acidic Protein as Potential Diagnostic Biomarkers in Autism Spectrum Disorders: A Preliminary Study
Abstract
1. Introduction
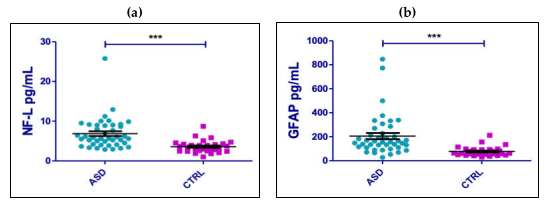
2. Results
3. Discussion
4. Materials and Methods
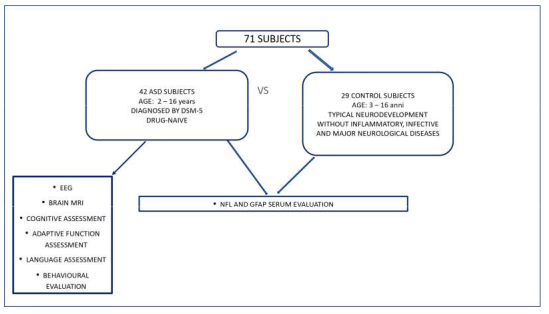
4.1. Study Samples
4.2. Serum Nfl and GFAP Assessment
4.3. Neuropsychiatric Assessment
4.4. Statistical Analysis
5. Conclusions
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013; pp. 5–25. [Google Scholar]
- Hodges, H.; Fealko, C.; Soares, N. Autism spectrum disorder: Definition, epidemiology, causes, and clinical evaluation. Transl. Pediatr. 2020, 9, S55–S65. [Google Scholar] [CrossRef]
- Afif, I.Y.; Manik, A.R.; Munthe, K.; Maula, M.I.; Ammarullah, M.I.; Jamari, J.; Winarni, T.I. Physiological Effect of Deep Pressure in Reducing Anxiety of Children with ASD during Traveling: A Public Transportation Setting. Bioengineering 2022, 9, 157. [Google Scholar] [CrossRef]
- Afif, I.Y.; Farkhan, M.; Kurdi, O.; Maula, M.I.; Ammarullah, M.I.; Setiyana, B.; Jamari, J.; Winarni, T.I. Effect of Short-Term Deep-Pressure Portable Seat on Behavioral and Biological Stress in Children with Autism Spectrum Disorders: A Pilot Study. Bioengineering 2022, 9, 48. [Google Scholar] [CrossRef]
- Russell, G.; Rodgers, L.R.; Ukoumunne, O.C.; Ford, T. Prevalence of Parent-Reported ASD and ADHD in the UK: Findings from the Millennium Cohort Study. J. Autism Dev. Disord. 2013, 44, 31–40. [Google Scholar] [CrossRef]
- Narzisi, A.; Posada, M.; Barbieri, F.; Chericoni, N.; Ciuffolini, D.; Pinzino, M.; Romano, R.; Scattoni, M.L.; Tancredi, R.; Calderoni, S.; et al. Prevalence of Autism Spectrum Disorder in a large Italian catchment area: A school-based population study within the ASDEU project. Epidemiol. Psychiatr. Sci. 2018, 29, e5. [Google Scholar] [CrossRef]
- Willsey, H.R.; Willsey, A.J.; Wang, B.; State, M.W. Genomics, convergent neuroscience and progress in understanding autism spectrum disorder. Nat. Rev. Neurosci. 2022, 23, 323–341. [Google Scholar] [CrossRef]
- Hickman, R.A.; O’Shea, S.A.; Mehler, M.F.; Chung, W.K. Neurogenetic disorders across the lifespan: From aberrant development to degeneration. Nat. Rev. Neurol. 2022, 18, 117–124. [Google Scholar] [CrossRef]
- Matsuzaki, H.; Fukunaga, K. Editorial: Environmental risk factors in autism spectrum disorder. Front. Psychiatry 2022, 13. [Google Scholar] [CrossRef]
- Pham, C.; Symeonides, C.; O’Hely, M.; Sly, P.D.; Knibbs, L.D.; Thomson, S.; Vuillermin, P.; Saffery, R.; Ponsonby, A.-L. The Barwon Infant Study Investigator Group Early life environmental factors associated with autism spectrum disorder symptoms in children at age 2 years: A birth cohort study. Autism 2022, 26, 1864–1881. [Google Scholar] [CrossRef]
- Schaefer, G.B. Clinical Genetic Aspects of ASD Spectrum Disorders. Int. J. Mol. Sci. 2016, 17, 180. [Google Scholar] [CrossRef]
- Rylaarsdam, L.E.; Guemez-Gamboa, A. Genetic Causes and Modifiers of Autism Spectrum Disorder. Front. Cell. Neurosci. 2019, 13, 385. [Google Scholar] [CrossRef]
- Qiu, S.; Qiu, Y.; Li, Y.; Cong, X. Genetics of autism spectrum disorder: An umbrella review of systematic reviews and meta-analyses. Transl. Psychiatry 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Isaksson, J.; Ruchkin, V.; Aho, N.; Remnélius, K.L.; Marschik, P.B.; Bölte, S. Nonshared environmental factors in the aetiology of autism and other neurodevelopmental conditions: A monozygotic co-twin control study. Mol. Autism 2022, 13, 1–11. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Liang, Y.; Yao, P. Rethinking Autism: The Impact of Maternal Risk Factors on Autism Development. Am. J. Transl. Res. 2022, 14, 1136–1145. [Google Scholar]
- Dietert, R.R.; Dietert, J.M.; DeWitt, J. Environmental risk factors for autism. Emerg. Heal. Threat. J. 2011, 4, 7111. [Google Scholar] [CrossRef]
- Massarali, A.; Adhya, D.; Srivastava, D.P.; Baron-Cohen, S.; Kotter, M.R. Virus-Induced Maternal Immune Activation as an Environmental Factor in the Etiology of Autism and Schizophrenia. Front. Neurosci. 2022, 16, 834058. [Google Scholar] [CrossRef]
- Han, V.X.; Patel, S.; Jones, H.F.; Dale, R.C. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 2021, 17, 564–579. [Google Scholar] [CrossRef]
- Zawadzka, A.; Cieślik, M.; Adamczyk, A. The Role of Maternal Immune Activation in the Pathogenesis of Autism: A Review of the Evidence, Proposed Mechanisms and Implications for Treatment. Int. J. Mol. Sci. 2021, 22, 11516. [Google Scholar] [CrossRef]
- Abou-Donia, M.B.; Suliman, H.B.; Siniscalco, D.; Antonucci, N.; ElKafrawy, P. De novo Blood Biomarkers in Autism: Autoantibodies against Neuronal and Glial Proteins. Behav. Sci. 2019, 9, 47. [Google Scholar] [CrossRef]
- Ha, S.; Sohn, I.-J.; Kim, N.; Sim, H.J.; Cheon, K.-A. Characteristics of Brains in Autism Spectrum Disorder: Structure, Function and Connectivity across the Lifespan. Exp. Neurobiol. 2015, 24, 273–284. [Google Scholar] [CrossRef]
- Lee, J.K.; Andrews, D.S.; Ozturk, A.; Solomon, M.; Rogers, S.; Amaral, D.G.; Nordahl, C.W. Altered Development of Amygdala-Connected Brain Regions in Males and Females with Autism. J. Neurosci. 2022, 42, 6145–6155. [Google Scholar] [CrossRef]
- Andrews, D.S.; Aksman, L.; Kerns, C.M.; Lee, J.K.; Winder-Patel, B.M.; Harvey, D.J.; Waizbard-Bartov, E.; Heath, B.; Solomon, M.; Rogers, S.J.; et al. Association of Amygdala Development With Different Forms of Anxiety in Autism Spectrum Disorder. Biol. Psychiatry 2022, 91, 977–987. [Google Scholar] [CrossRef]
- Girault, J.B.; Piven, J. The Neurodevelopment of Autism from Infancy Through Toddlerhood. Neuroimaging Clin. North Am. 2020, 30, 97–114. [Google Scholar] [CrossRef]
- Eissa, N.; Sadeq, A.; Sasse, A.; Sadek, B. Role of Neuroinflammation in Autism Spectrum Disorder and the Emergence of Brain Histaminergic System. Lessons Also for BPSD? Front. Pharmacol. 2020, 11, 886. [Google Scholar] [CrossRef]
- Kern, J.K.; A Geier, D.; Sykes, L.K.; Geier, M.R. Evidence of neurodegeneration in autism spectrum disorder. Transl. Neurodegener. 2013, 2, 17. [Google Scholar] [CrossRef]
- Eve, M.; Gandawijaya, J.; Yang, L.; Oguro-Ando, A. Neuronal Cell Adhesion Molecules May Mediate Neuroinflammation in Autism Spectrum Disorder. Front. Psychiatry 2022, 13, 842755. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Xia, X.; Zhou, X.; Du, Y.; Yin, Z.; Wang, J. Integration of Urine Proteomic and Metabolomic Profiling Reveals Novel Insights Into Neuroinflammation in Autism Spectrum Disorder. Front. Psychiatry 2022, 13, 780747. [Google Scholar] [CrossRef]
- Liao, X.; Yang, J.; Wang, H.; Li, Y. Microglia mediated neuroinflammation in autism spectrum disorder. J. Psychiatr. Res. 2020, 130, 167–176. [Google Scholar] [CrossRef]
- Jin, X.-R.; Chen, X.-S.; Xiao, L. MeCP2 Deficiency in Neuroglia: New Progress in the Pathogenesis of Rett Syndrome. Front. Mol. Neurosci. 2017, 10, 316. [Google Scholar] [CrossRef]
- Delacourte, A. General and dramatic glial reaction in Alzheimer brains. Neurology 1990, 40, 33. [Google Scholar] [CrossRef]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’Anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef]
- Heimfarth, L.; Passos, F.R.S.; Monteiro, B.S.; Araújo, A.A.D.S.; Júnior, L.J.Q.; Quintans, J.D.S.S. Serum glial fibrillary acidic protein is a body fluid biomarker: A valuable prognostic for neurological disease – A systematic review. Int. Immunopharmacol. 2022, 107, 108624. [Google Scholar] [CrossRef]
- Wang, J.; Zou, Q.; Han, R.; Li, Y.; Wang, Y. Serum levels of Glial fibrillary acidic protein in Chinese children with autism spectrum disorders. Int. J. Dev. Neurosci. 2016, 57, 41–45. [Google Scholar] [CrossRef]
- Vakilzadeh, G.; Falcone, C.; Dufour, B.; Hong, T.; Noctor, S.C.; Martínez-Cerdeño, V. Decreased number and increased activation state of astrocytes in gray and white matter of the prefrontal cortex in autism. Cereb. Cortex 2022, 32, 4902–4912. [Google Scholar] [CrossRef]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef]
- Bridel, C.; van Wieringen, W.; Zetterberg, H.; Tijms, B.M.; Teunissen, C.E.; Alvarez-Cermeño, J.C.; Andreasson, U.; Axelsson, M.; Bäckström, D.C.; Bartos, A.; et al. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology. JAMA Neurol. 2019, 76, 1035–1048. [Google Scholar] [CrossRef]
- Gaetani, L.; Blennow, K.; Calabresi, P.; Di Filippo, M.; Parnetti, L.; Zetterberg, H. Neurofilament light chain as a biomarker in neurological disorders. J. Neurol. Neurosurg. Psychiatry 2019, 90, 870–881. [Google Scholar] [CrossRef]
- Mielke, M.M.; Syrjanen, J.A.; Blennow, K.; Zetterberg, H.; Vemuri, P.; Skoog, I.; Machulda, M.M.; Kremers, W.K.; Knopman, D.S.; Jack, C.; et al. Plasma and CSF neurofilament light. Neurology 2019, 93, e252–e260. [Google Scholar] [CrossRef]
- Shahim, P.; Zetterberg, H.; Blennow, K. Neurofilament Protein and Antineurofilament Antibodies Following Traumatic Brain Injury-Reply. JAMA Neurol. 2017, 74, 363–364. [Google Scholar] [CrossRef]
- Sanchez, J.D.; Martirosian, R.A.; Mun, K.T.; Chong, D.S.; Llorente, I.L.; Uphaus, T.; Gröschel, K.; Wölfer, T.A.; Tiedt, S.; Hinman, J.D.; et al. Temporal Patterning of Neurofilament Light as a Blood-Based Biomarker for Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 2022, 13, 841898. [Google Scholar] [CrossRef]
- Bergman, J.; Dring, A.; Zetterberg, H.; Blennow, K.; Norgren, N.; Gilthorpe, J.; Bergenheim, T.; Svenningsson, A. Neurofilament light in CSF and serum is a sensitive marker for axonal white matter injury in MS. Neurol.-Neuroimmunol. Neuroinflammation 2016, 3, e271. [Google Scholar] [CrossRef]
- Weber, C.F.; Lake, E.M.R.; Haider, S.P.; Mozayan, A.; Mukherjee, P.; Scheinost, D.; Bamford, N.S.; Ment, L.; Constable, T.; Payabvash, S. Age-dependent white matter microstructural disintegrity in autism spectrum disorder. Front. Neurosci. 2022, 16, 957018. [Google Scholar] [CrossRef]
- Karahanoğlu, F.I.; Baran, B.; Nguyen, Q.T.H.; Meskaldji, D.-E.; Yendiki, A.; Vangel, M.; Santangelo, S.L.; Manoach, D.S. Diffusion-weighted imaging evidence of altered white matter development from late childhood to early adulthood in Autism Spectrum Disorder. NeuroImage: Clin. 2018, 19, 840–847. [Google Scholar] [CrossRef]
- He, W.-C.; Zhang, X.-J.; Zhang, Y.-Q.; Zhang, W.-J. Elevated serum neurofilament light chain in children autism spectrum disorder: A case control study. Neurotoxicology 2020, 80, 87–92. [Google Scholar] [CrossRef]
- Lord, C.; Rutter, M.; DiLavore, P.; Risi, S.; Gotham, K.; Bishop, S. Autism Diagnostic Observation Schedule–2nd ed. (ADOS-2); 2012; Volume 284. [Google Scholar]
- Gullotta, F.; Schindler, F.; Schmutzler, R.; Weeks-Seifert, A. GFAP in Brain Tumor Diagnosis: Possibilities and Limitations. Pathol.-Res. Pr. 1985, 180, 54–60. [Google Scholar] [CrossRef]
- Foerch, C.; Curdt, I.; Yan, B.; Dvorak, F.; Hermans, M.; Berkefeld, J.; Raabe, A.; Neumann-Haefelin, T.; Steinmetz, H.; Sitzer, M. Serum glial fibrillary acidic protein as a biomarker for intracerebral haemorrhage in patients with acute stroke. J. Neurol. Neurosurg. Psychiatry 2006, 77, 181–184. [Google Scholar] [CrossRef]
- Feneberg, E.; Steinacker, P.; Lehnert, S.; Böhm, B.; Mayer, G.; Otto, M. Elevated glial fibrillary acidic protein levels in the cerebrospinal fluid of patients with narcolepsy. Sleep Med. 2013, 14, 692–694. [Google Scholar] [CrossRef]
- Papa, L.; Lewis, L.M.; Falk, J.L.; Zhang, Z.; Silvestri, S.; Giordano, P.; Brophy, G.M.; Demery, J.A.; Dixit, N.K.; Ferguson, I.; et al. Elevated Levels of Serum Glial Fibrillary Acidic Protein Breakdown Products in Mild and Moderate Traumatic Brain Injury Are Associated With Intracranial Lesions and Neurosurgical Intervention. Ann. Emerg. Med. 2011, 59, 471–483. [Google Scholar] [CrossRef]
- Alirezaei, Z.; Pourhanifeh, M.H.; Borran, S.; Nejati, M.; Mirzaei, H.; Hamblin, M.R. Neurofilament Light Chain as a Biomarker, and Correlation with Magnetic Resonance Imaging in Diagnosis of CNS-Related Disorders. Mol. Neurobiol. 2019, 57, 469–491. [Google Scholar] [CrossRef]
- Crawford, J.D.; Chandley, M.J.; Szebeni, K.; Szebeni, A.; Waters, B.; Ordway, G.A. Elevated GFAP Protein in Anterior Cingulate Cortical White Matter in Males With Autism Spectrum Disorder. Autism Res. 2015, 8, 649–657. [Google Scholar] [CrossRef]
- Menassa, D.A.; Sloan, C.; Chance, S.A. Primary olfactory cortex in autism and epilepsy: Increased glial cells in autism. Brain Pathol. 2016, 27, 437–448. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, J.; Tang, L.; Mikhailova, T.; Liang, Q.; Li, M.; Zhou, J.; Kopp, R.F.; Weickert, C.; Chen, C.; et al. Neuroimmune transcriptome changes in patient brains of psychiatric and neurological disorders. Mol. Psychiatry 2022, 28, 710–721. [Google Scholar] [CrossRef]
- Cetin, I.; Tezdig, I.; Tarakcioglu, M.C.; Kadak, M.T.; Demirel, O.F.; Ozer, O.F. Serum levels of glial fibrillary acidic protein and Nogo-A in children with autism spectrum disorders. Biomarkers 2016, 21, 614–618. [Google Scholar] [CrossRef]
- Hughes, H.; Moreno, R.J.; Ashwood, P. Innate immune dysfunction and neuroinflammation in autism spectrum disorder (ASD). Brain, Behav. Immun. 2023, 108, 245–254. [Google Scholar] [CrossRef]
- Hu, C.; Li, H.; Li, J.; Luo, X.; Hao, Y. Microglia: Synaptic modulator in autism spectrum disorder. Front. Psychiatry 2022, 13, 958661. [Google Scholar] [CrossRef]
- Erbescu, A.; Papuc, S.M.; Budisteanu, M.; Arghir, A.; Neagu, M. Re-emerging concepts of immune dysregulation in autism spectrum disorders. Front. Psychiatry 2022, 13, 2332. [Google Scholar] [CrossRef]
- Lewis, M.; Norbury, C.; Luyster, R.; Schmitt, L.; McDuffie, A.; Haebig, E.; Murray, D.S.; Timler, G.; Frazier, T.; Holmes, D.L.; et al. Leiter International Performance Scale-Revised (Leiter-R); Volkmar, F.R., Ed.; Springer: Berlin, Germany, 2013. [Google Scholar]
- Weschler, D.; Sattler, J.M. Wppsi-III Administration and Scoring Manual, 1st ed.; The Psychological Corporation: San Antonio, TX, USA, 2002. [Google Scholar]
- Wechsler, D. Wechsler Intelligence Scale for Children, 4th ed.; The Psychological Corporation: San Antonio, TX, USA,, 2012. [Google Scholar]
- Cianchetti, C.; Fancello, G.S. Test TVL. Test Di Valutazione Del Linguaggio; Edizioni Centro Studi Erickson: Trento, Italy, 2003. [Google Scholar]
- Cornoldi, C.; Cerretti, B.; Alvaro, P.B.; Giofrè, D. Prove MT-3 Clinica. Il Test Sviluppato Da Cesare Cornoldi per La Valutazione Delle Abilità Di Lettura, Comprensione, Scrittura e Matematica; Giunti Psychometrics: Firenze, Italy, 2022. [Google Scholar]
- Cornoldi, C.; Mammarella, I.C.; Caviola, S. AC-MT-3 6-14 Anni Prove per La Clinica. Test Di Valutazione Delle Abilità Di Calcolo e Del Ragionamento Matematico; Edizioni Centro Studi Erickson S.p.A.: Trento, Italy, 2020. [Google Scholar]
- Sartori, G.; Job, R. DDE-2: Batteria per la valutazione della dislessia e della disortografia evolutiva-2 [Assessment battery for Developmental Reading and Spelling Disorders]; Giunti O.S.: Florence, Italy, 2007. [Google Scholar]
- Conners, C.K. Conners’ Rating Scales-Revised; Multi-Heatlh System: Toronto, Canada, 1997. [Google Scholar]
| Number | 42 |
|---|---|
Age (yrs) (mean value; median) | 6.98; 6 |
| Gender Male Female | 33 9 |
| Level of Severity ASD (%) 1 2 3 | 42.86% 38.1% 19.05% |
| Frequency of EEG abnormalities (%) | 9.52% |
| Frequency of MRI alterations (%) | 4.76% |
| Learning disorder (%) | 2.38% |
| ADHD (%) | 16.67% |
| Hyperactivity (%) | 40.48% |
| Language impairment (%) | 69.05% |
| Intellectual disability (%) | 50% |
| ADOS-2 score (%) 8–11 11–16 >16 | 23.81% 45.24% 30.95% |
| Age | Gender | ASD Level | EEG Abnormalities | MRI Abnormalities | Learning Disorder | ADHD | Hyperactivity | Language Impairment | Intellectual Disability | Nfl | GFAP | Total Scores ADOS-2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1 | ||||||||||||
| Gender | −0.26 | 1 | |||||||||||
| ASD level | −0.09 | −0.15 | 1 | ||||||||||
| EEG Abnormalities | −0.19 | −0.03 | −0.1 | 1 | |||||||||
| MRI Abnormalities | 0.01 | −0.16 | −0.24 | −0.07 | 1 | ||||||||
| Learning Disorder | 0.18 | 0.08 | −0.17 | −0.05 | −0.03 | 1 | |||||||
| ADHD | −0.05 | 0.08 | 0.11 | −0.15 | −0.1 | −0.07 | 1 | ||||||
| Hyperactivity | −0.08 | −0.04 | 0.28 | −0.1 | 0.04 | −0.13 | 0.37 | 1 | |||||
| Language impairment | −0.4 | 0.03 | 0.5 | 0.04 | −0.09 | −0.23 | 0.02 | 0.24 | 1 | ||||
| Intellectual Disability | −0.13 | −0.06 | 0.44 | 0 | −0.22 | −0.16 | −0.06 | 0.24 | 0.36 | 1 | |||
| sNfl | −0.39 | 0.05 | 0.17 | 0.04 | −0.13 | −0.06 | 0.14 | 0.17 | 0.21 | 0.21 | 1 | ||
| sGFAP | −0.72 | 0.22 | 0.14 | 0.06 | 0.03 | −0.23 | −0.04 | 0.31 | 0.22 | 0.26 | 0.56 | 1 | |
| Total Scores ADOS-2 | −0.22 | −0.15 | 0.63 | −0.04 | −0.15 | −0.1 | 0.12 | 0.08 | 0.34 | 0.26 | 0.11 | 0.21 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simone, M.; De Giacomo, A.; Palumbi, R.; Palazzo, C.; Lucisano, G.; Pompamea, F.; Micella, S.; Pascali, M.; Gabellone, A.; Marzulli, L.; et al. Serum Neurofilament Light Chain and Glial Fibrillary Acidic Protein as Potential Diagnostic Biomarkers in Autism Spectrum Disorders: A Preliminary Study. Int. J. Mol. Sci. 2023, 24, 3057. https://doi.org/10.3390/ijms24033057
Simone M, De Giacomo A, Palumbi R, Palazzo C, Lucisano G, Pompamea F, Micella S, Pascali M, Gabellone A, Marzulli L, et al. Serum Neurofilament Light Chain and Glial Fibrillary Acidic Protein as Potential Diagnostic Biomarkers in Autism Spectrum Disorders: A Preliminary Study. International Journal of Molecular Sciences. 2023; 24(3):3057. https://doi.org/10.3390/ijms24033057
Chicago/Turabian StyleSimone, Marta, Andrea De Giacomo, Roberto Palumbi, Claudia Palazzo, Giuseppe Lucisano, Francesco Pompamea, Stefania Micella, Mara Pascali, Alessandra Gabellone, Lucia Marzulli, and et al. 2023. "Serum Neurofilament Light Chain and Glial Fibrillary Acidic Protein as Potential Diagnostic Biomarkers in Autism Spectrum Disorders: A Preliminary Study" International Journal of Molecular Sciences 24, no. 3: 3057. https://doi.org/10.3390/ijms24033057
APA StyleSimone, M., De Giacomo, A., Palumbi, R., Palazzo, C., Lucisano, G., Pompamea, F., Micella, S., Pascali, M., Gabellone, A., Marzulli, L., Giordano, P., Gargano, C. D., Margari, L., Frigeri, A., & Ruggieri, M. (2023). Serum Neurofilament Light Chain and Glial Fibrillary Acidic Protein as Potential Diagnostic Biomarkers in Autism Spectrum Disorders: A Preliminary Study. International Journal of Molecular Sciences, 24(3), 3057. https://doi.org/10.3390/ijms24033057








