Abstract
Wheat (Triticum aestivum L.) growing areas in many regions of the world are subject to heat waves which are predicted to increase in frequency because of climate change. The engineering of crop plants can be a useful strategy to mitigate heat stress-caused yield losses. Previously, we have shown that heat shock factor subclass C (TaHsfC2a-B)-overexpression significantly increased the survival of heat-stressed wheat seedlings. Although previous studies have shown that the overexpression of Hsf genes enhanced the survival of plants under heat stress, the molecular mechanisms are largely unknown. To understand the underlying molecular mechanisms involved in this response, a comparative analysis of the root transcriptomes of untransformed control and TaHsfC2a-overexpressing wheat lines by RNA-sequencing have been performed. The results of RNA-sequencing indicated that the roots of TaHsfC2a-overexpressing wheat seedlings showed lower transcripts of hydrogen peroxide-producing peroxidases, which corresponds to the reduced accumulation of hydrogen peroxide along the roots. In addition, suites of genes from iron transport and nicotianamine-related gene ontology categories showed lower transcript abundance in the roots of TaHsfC2a-overexpressing wheat roots than in the untransformed control line following heat stress, which are in accordance with the reduction in iron accumulation in the roots of transgenic plants under heat stress. Overall, these results suggested the existence of ferroptosis-like cell death under heat stress in wheat roots, and that TaHsfC2a is a key player in this mechanism. To date, this is the first evidence to show that a Hsf gene plays a key role in ferroptosis under heat stress in plants. In future, the role of Hsf genes could be further studied on ferroptosis in plants to identify root-based marker genes to screen for heat-tolerant genotypes.
Keywords:
climate change; heat stress; heat shock factor; ferroptosis; peroxidases; roots; thermotolerance; wheat 1. Introduction
Heat is a major abiotic stress factor that affects crop production in many regions of the world. Depending on the intensity and duration of heat stress and the physiological and developmental status of the plant, most plant species are negatively affected by heat stress. However, heat stress is especially problematic for cool season crops, such as wheat (Triticum aestivum L.). Because wheat is a crop that meets the calorie demand of a significant portion of the world’s population, a reduced wheat production caused by heat stress can potentially be a significant threat to global food security. Indeed, according to one estimate, the average cereal yield across the globe declined by 7.6% due to heat stress during extreme weather events that occurred between 1964 and 2007 [1].
In addition to the elevated temperatures currently experienced in many wheat-growing regions, the increased atmospheric concentration of CO2 and other greenhouse gases is predicted to cause even more intense heat waves and dramatically alter rainfall patterns [2]. Indeed, the frequency, duration, and severity of episodes with exceptionally high temperatures have already been seen to rise in recent years [3,4]. These unusual climatic changes, which are projected to become more severe in future, have the potential to further threaten global food security by severely limiting crop productivity [1]. Global wheat yield is projected to decline by 4.1 to 6.4% with every 1 °C rise in global temperatures [5]. Deryng et al. [6] predicted that strong heat stress during anthesis could be responsible for up to a 52% reduction from the expected yield of spring wheat by 2080.
Plants respond to heat stress by activating many genes involved in hormone biosynthesis, protein degradation, transcriptional activation and repression, and an unfolded-protein response [7,8,9]. In addition, heat stress induces the production of reactive oxygen species (ROS), which can damage membranes and other cellular systems through lipid peroxidation [10]. Distefano et al. [11] demonstrated that heat stress induces ferroptosis-like cell death in Arabidopsis (Arabidopsis thaliana (L.) Heynh.) roots. Ferroptosis is a non-apoptotic form of cell death and is dependent on intracellular iron and ROS [12,13,14,15]. Excess free iron (Fe) ions can generate ROS within the cell [16], resulting in lipid peroxidation and ferroptotic cell death, and the inhibition of this response was shown to have a beneficial effect on heat stress tolerance.
Heat stress also induces the production of heat shock proteins (HSPs) that act as molecular chaperones under stress conditions to maintain cellular homeostasis [17,18]. Heat shock factors (HsfA, HsfB and HsfC) play a key role in the regulation of heat-inducible genes, including HSPs [19]. HsfA and HsfB have a central role in the regulation of heat stress-related genes in plants [20]. However, the monocot-specific HsfC2 subclass HSPs have been poorly studied [21].
To date, most studies have considered the detrimental effects of heat stress on above-ground plant parts [22,23], although the roots can also be affected by high temperatures under natural conditions, especially when plant canopies are not dense enough and sunlight can directly contact the soil surface [24]. Given that heat and drought stress often occur simultaneously, maintaining a functional root system, that can adsorb water and nutrients from the soil, could be essential to alleviate the damaging effects of heat and drought stress, especially during the early developmental stages of plant growth. Indeed, reducing root heat stress was found to increase shoot biomass by 33 to 160% and grain yield by 18 to 147% in wheat under field conditions [25]. However, currently very little is known about the molecular responses of plant roots to heat stress. Recently, it has been reported that exogenous trehalose modulates gene expression and alleviates the oxidative damage of high temperature stress in wheat roots [26]. In our previous study, it has been shown that the survival rates of TaHsfC2a-B-overexpressing wheat seedlings were significantly improved (90%) relative to those of untransformed plants (15%) after exposure to heat stress (2 h at 43 °C) [27]. In addition, a significant correlation was observed between recovery rates and the average root dry weights of the recovered transgenic lines which were 8.7 and 7.8-fold higher than those of the recovered parental line, suggesting that physiological changes in the roots of transgenic plants may have contributed to the heat stress recovery phenomenon.
The aim of this study was firstly to investigate transcriptional responses of wheat roots to heat stress, and secondly to understand the potential roles of TaHsfC2a over-expressing roots in contributing to plant recovery after heat stress that can kill most untransformed control plants [27]. Therefore, this study undertook RNA-seq analyses to identify heat stress-inducible root genes that are potentially associated with heat stress tolerance in wheat. A comparative analysis of untransformed control plants (hereafter called wild-type) and TaHsfC2a root transcriptomes indicated that TaHsfC2a negatively regulates ROS levels under heat stress in roots through class III peroxidases. In addition, the reduced transcripts of Fe transport genes and nicotianamine synthases caused a lower accumulation of Fe in TaHsfC2a roots under heat stress. These results suggested the existence of ferroptosis-like cell death in wheat. To the best of our knowledge, no functional genes have been identified in the ferroptosis-like cell death mechanism seen under heat stress in plants. Therefore, this study is the first report that functionally characterizes a gene in the ferroptosis-like cell death pathway under heat stress in plants. Overall, these results provide new insights into heat stress tolerance in plants.
2. Results
2.1. Root Transcriptome of Wild-Type Wheat Seedlings under Heat Stress
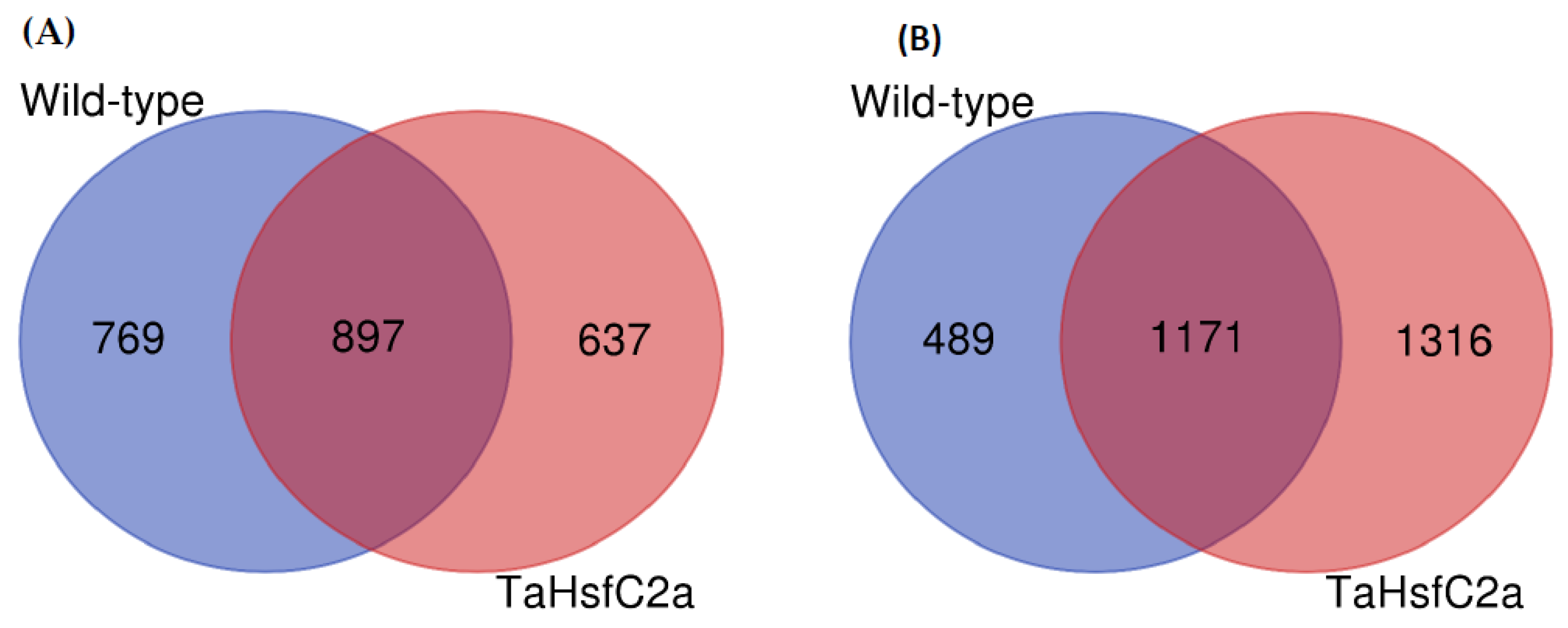
To identify root transcriptional responses to heat stress, the RNA-seq of wild-type roots with and without heat stress was performed. On average, 90% of the reads were aligned to the reference RefSeq v1.0 (Supplementary Table S1). RNA-seq analysis was carried out with log2 (Fold Change, FC ≥ 2; p < 0.05), unless otherwise stated to identify the genes that are at least twofold induced or repressed with a p value of < 0.05. We found that 1666 and 1660 genes were up- and down-regulated, respectively, in wild-type roots after heat treatment (Figure 1; Supplementary Table S2).
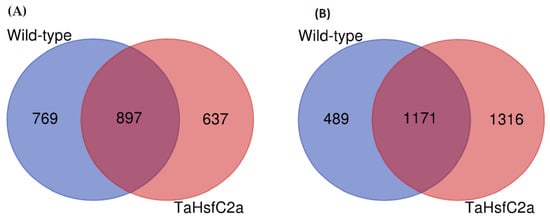
Figure 1.
Differentially regulated genes in the wild-type and the transgenic roots under heat stress. (A) upregulated genes (B) downregulated genes. Wild-type (control v heat) and TaHsfC2a (control v heat). Fold Change log2 >2; False Discovery Rate corrected p < 0.05.
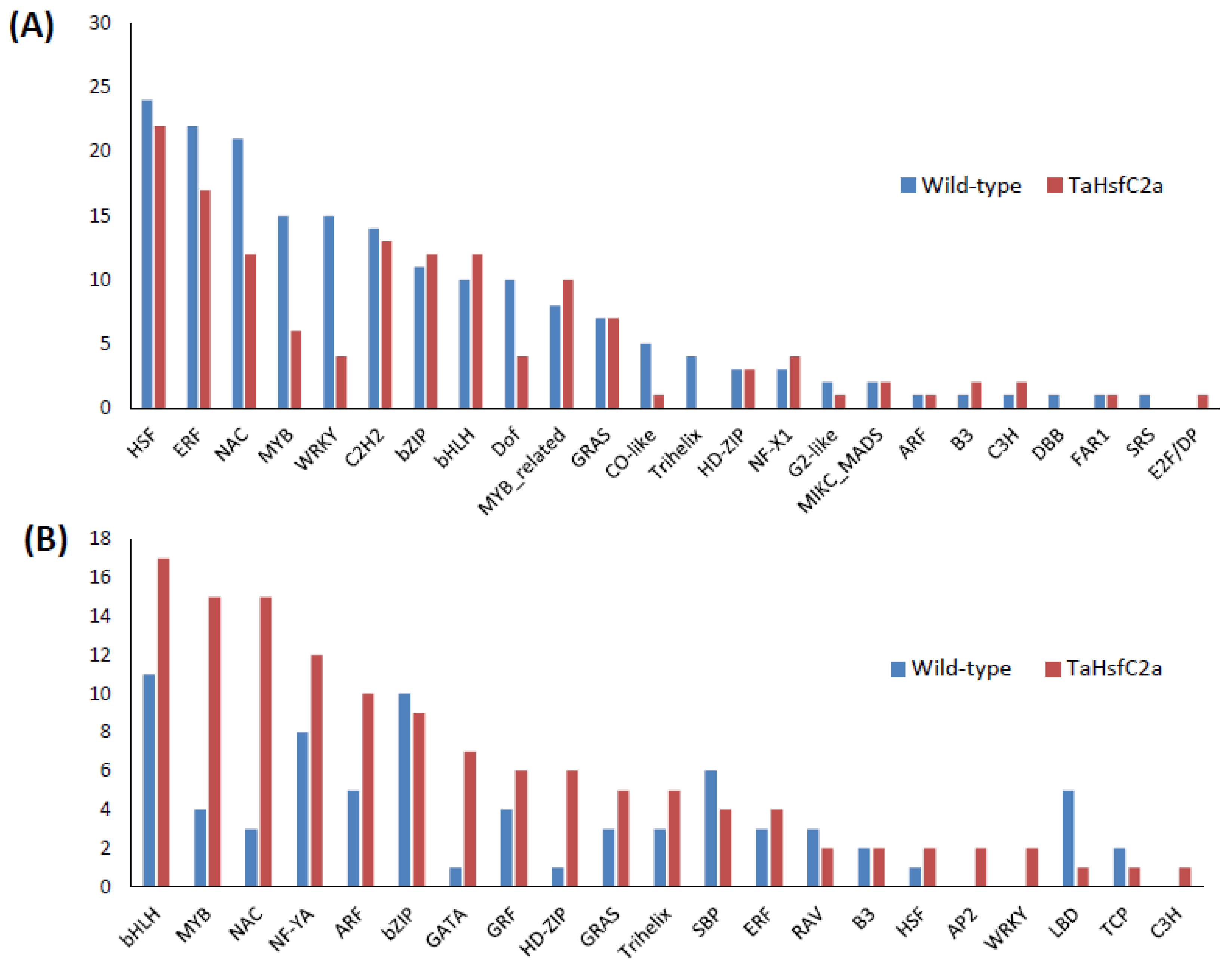
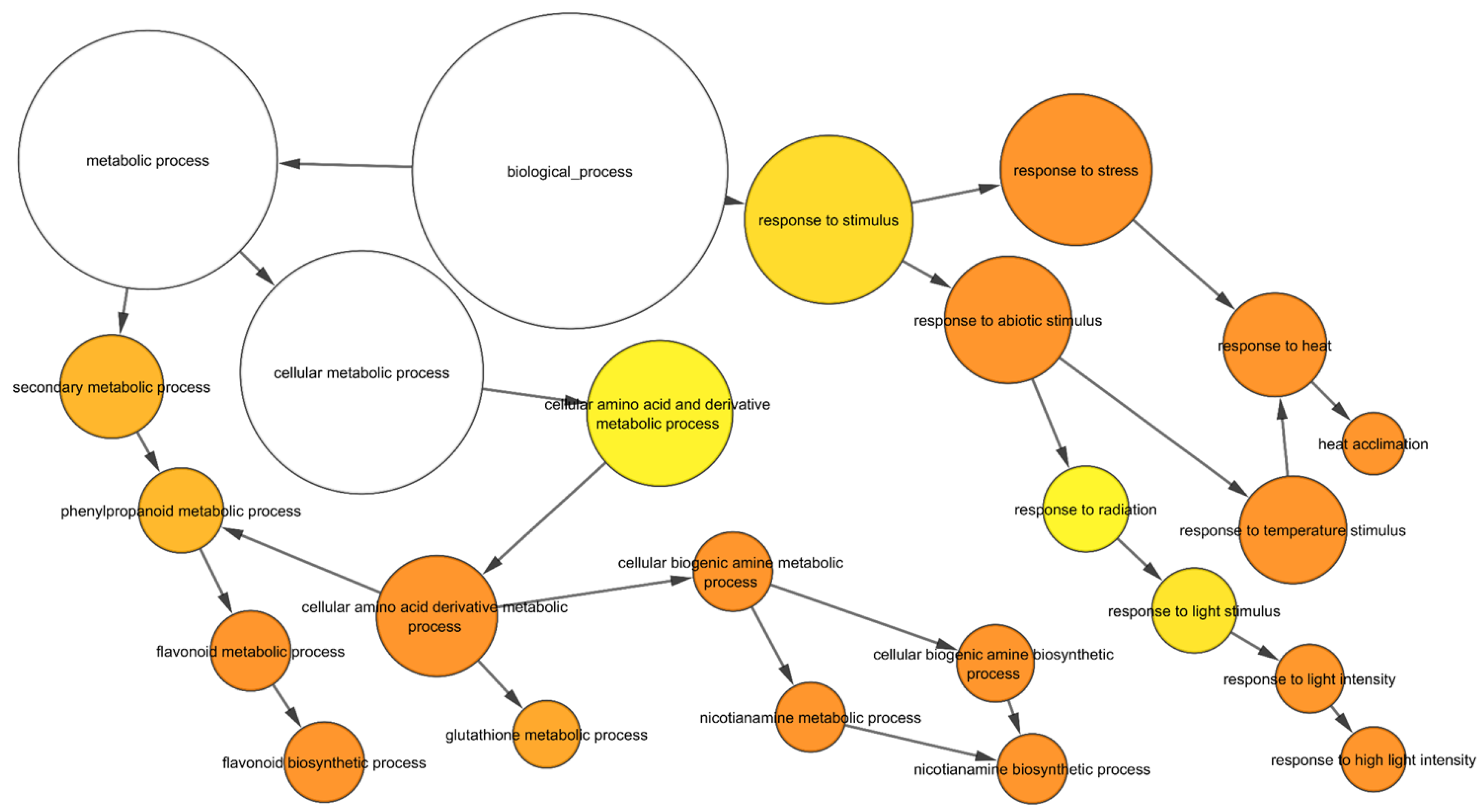
To gain an insight into the nature of molecular responses to heat, gene ontology (GO) enrichment analysis of the differentially expressed genes (DEGs) in the root tissue was performed, and found that the genes belonging to 46 GO categories were up-regulated under heat stress in wild-type roots (Supplementary Table S3). Some of these GO categories include “response to heat, nicotianamine synthase activity, the nicotianamine biosynthetic process, the tricarboxylic acid biosynthetic process, protein folding, response to hydrogen peroxide, ROS, iron ion transport, glutathione transferase activity, DNA binding transcription factor activity, heat shock protein binding, transcriptional repressor activity, and RNA polymerase II proximal promoter sequence-specific DNA binding” (Supplementary Table S3). In the down-regulated gene sets, 86 GO categories were over-represented. Some of these categories include “ROS, oxidative stress and hydrogen peroxide-related, cellular oxidant detoxification, peroxidase activity, antioxidant activity, cell proliferation and cell wall-related, cellulose and signalling-related” (Supplementary Table S3). In addition, in wild-type roots, 182 and 75 transcription factors (TF) encoding genes were up- and down-regulated, respectively after heat stress (Figure 2; Supplementary Table S4). The most abundant TF families up-regulated in wild-type roots were HSFs (24), ERFs (22), NACs (21), MYBs (15), and WRKYs (15) (Figure 2A) while those down-regulated in wild-type roots were bHLHs (11), bZIPs (10), and NF-YAs (8) (Figure 2B).
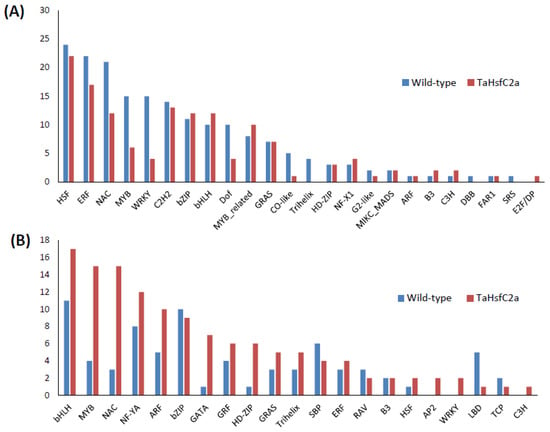
Figure 2.
Number of transcription factors identified in the wild-type and the transgenic roots in response to heat stress (A) upregulated genes (B) downregulated genes. Wild-type (control v heat) and TaHsfC2a (control v heat).
2.2. Heat Stress Regulation of Genes in the Roots of TaHsfC2a-Overexpressing Wheat Seedlings
As indicated above, previously, it has been reported that the TaHsfC2a-overexpressing plants exposed to heat stress (2 h of heat treatment at 43 °C) showed significantly better recovery rates (> 90%) in the subsequent 3 weeks following the heat treatment than wild-type plants (15%) [27]. The evidence presented by Hu et al. [27] also indicated that the roots played a role in the recovery phenomenon. To understand the potential mechanisms involved in the recovery of TaHsfC2a-overexpressing lines after heat stress, we performed an RNA-seq analysis of the roots. The analysis showed that 1535 and 2487 genes were up- and down-regulated in transgenic roots in response to heat stress (Figure 1; Supplementary Table S2).
In the up-regulated gene sets, several GO categories were observed, including secondary metabolic processes and responses to stress, and amine metabolic processes were overrepresented in the wild-type plants (Figure 3), which were also overrepresented in the transgenic line. To further understand the differences in GO enrichment between wild-type and transgenic plants, we have selected highly significant GO categories (FDR ≤ 0.001) from the BLAST2GO enrichment analysis. We found 38 categories over-represented relative to the wild-type in the transgenic up-regulated gene dataset, including the regulation of hydrogen peroxide metabolic process, the regulation of reactive oxygen species metabolic process, glutathione metabolic process, flavonoid biosynthetic process, calcium ion binding, transition metal ion transport, UDP-glucosyltransferase activity, and DNA binding and regulation of the RNA biosynthetic process (Supplementary Table S3). A total of 42 GO categories down-regulated in the transgenic roots relative to the heat-treated wild-type, including the nucleosome and chromatin assembly, protein-DNA packaging related, oxidation-reduction process, cell wall polysaccharide, metal ion binding, and membrane transporter activity (Supplementary Table S3). Among the DEGs between the control and heat-treated seedlings, 137 TFs were up-regulated, and 128 TFs were down-regulated in the transgenic roots, respectively (Figure 2; Supplementary Table S4). The most abundant TF families up-regulated in the roots of TaHsfC2a-overexpressing plants were HSFs (22), ERFs (17), C2H2 zinc fingers (13), NACs (12), bZIPs (12), and bHLHs (12) (Figure 2A), while the most abundant TFs down-regulated were bHLHs (17), MYBs (15), NACs (15), and NF-YA (12) (Figure 2B). These results indicated that several TFs were differentially regulated in the transgenic roots.
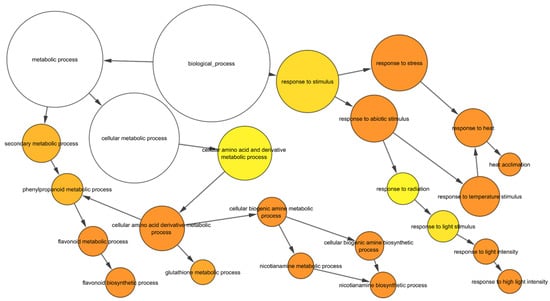
Figure 3.
GO enrichment of upregulated genes in response to heat in wild-type. The yellow to orange colour of the circles correspond to the level of significance of the overrepresented GO categories. The size of the circle is proportional to the number of genes in the category. Benjamini & Hochberg false discovery rate corrected p value < 0.05.
2.3. TaHsfC2a Negatively Regulates Root ROS Levels after Heat Treatment
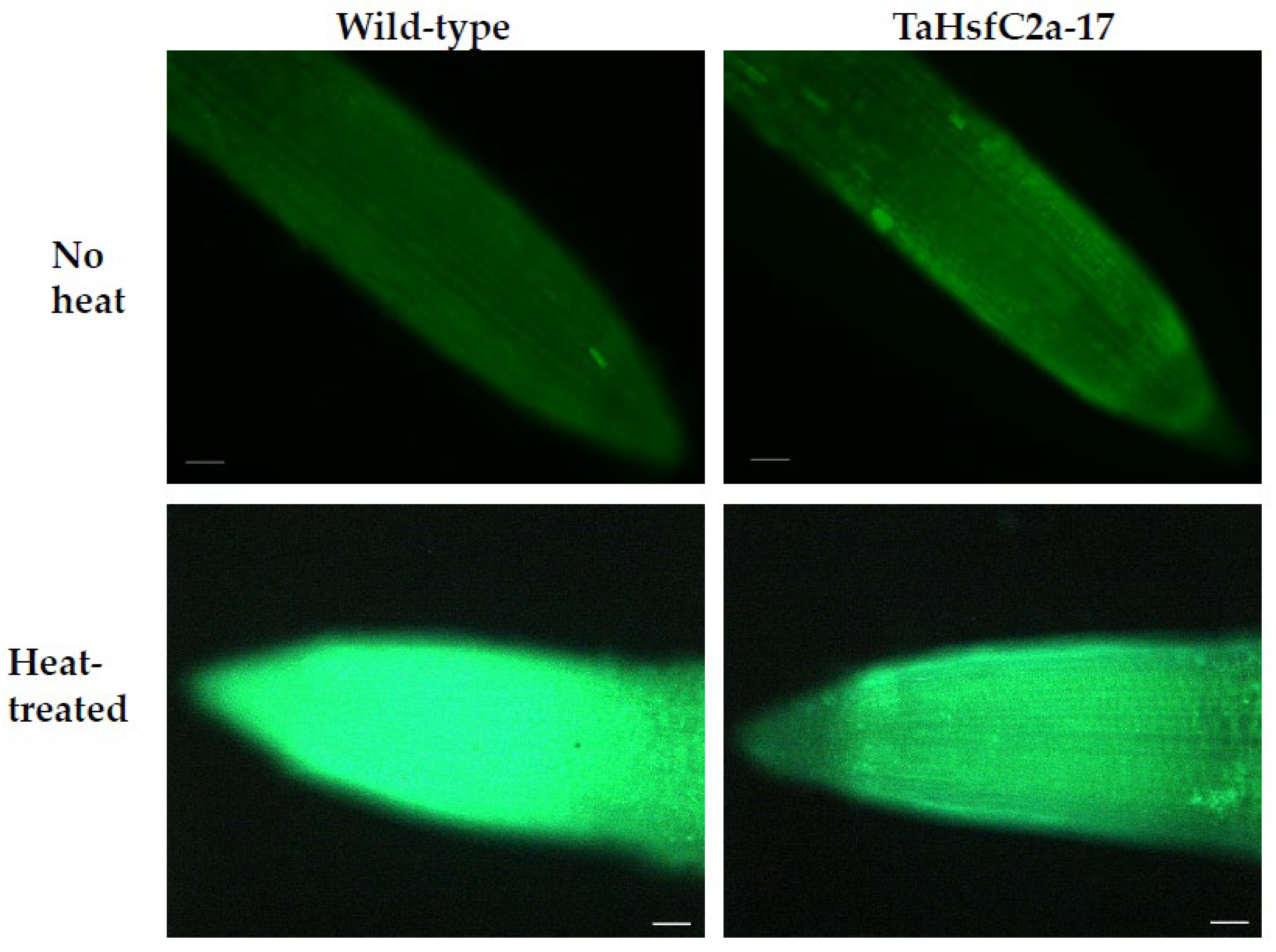
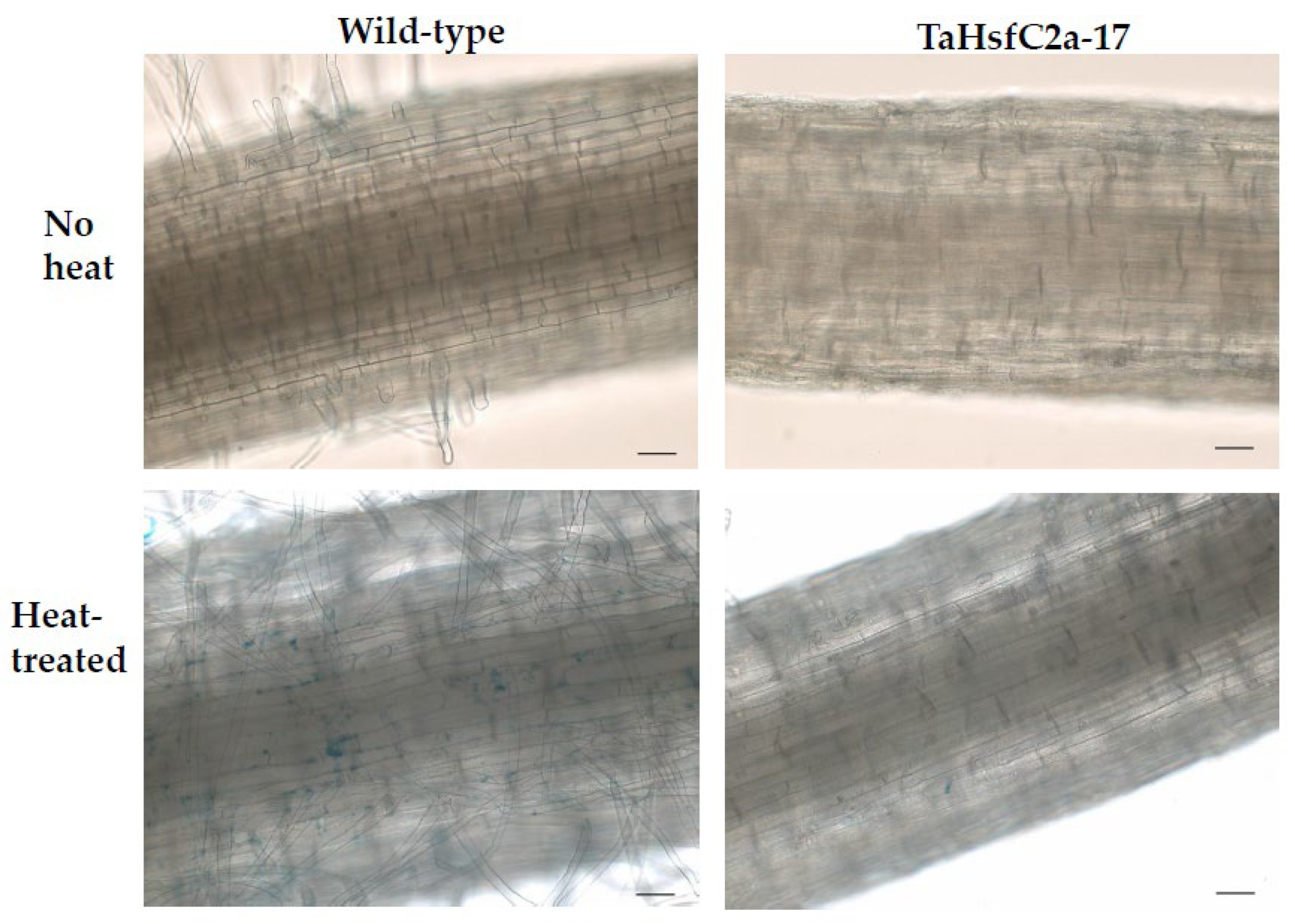
As indicated above, the analyses found several GO categories related to ROS, such as those involved in the regulation of hydrogen peroxide metabolism, regulation of ROS metabolic processes, and glutathione metabolism, in both the wild-type and TaHsfC2a-overexpressing plants (Supplementary Table S3). It is well known that heat stress generates ROS [28,29], and class III peroxidases have important functions in maintaining the ROS balance in the plant cell by consuming and/or producing ROS [30]. Therefore, a comparative analysis was performed, and it revealed the differences in peroxidase expressions between wild-type and the transgenic line after heat treatment. At least 70 class III peroxidases were significantly down-regulated in the roots of TaHsfC2a-overexpressing plants, while only four peroxidases significantly up-regulated when compared with the wild-type after heat treatment (Table 1). To understand whether the down-regulation of peroxidases in the roots of TaHsfC2a-overexpressing plants caused a difference in the accumulation of ROS, hydrogen peroxide levels were measured in the roots of the wild-type and transgenic line before and after heat treatment and found that transgenic roots accumulated lower hydrogen peroxide than wild-type roots (Figure 4). These results indicated that the overexpression of TaHsfC2a may be negatively regulating hydrogen peroxide level in transgenic roots by down-regulating the hydrogen peroxide-producing class III peroxidases.

Table 1.
Differential expression of class III peroxidases in the roots of TaHsfC2a overexpressing plants compared with the wild-type after heat treatment.
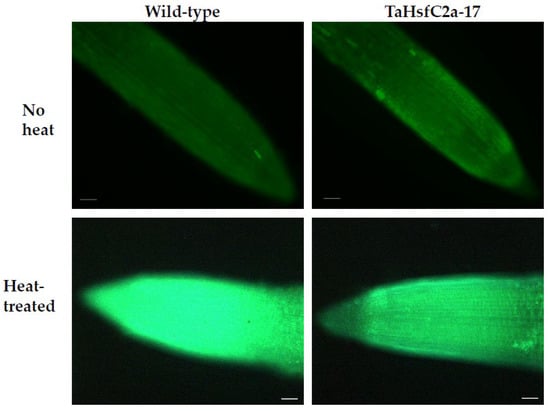
Figure 4.
Hydrogen peroxide along the roots of wild-type and transgenic line with and without heat treatment. Roots of eight-day-old wheat plants were stained with 3′-(phydroxyphenyl) fluorescein (HPF) for hydrogen peroxide. Scale bar, 100 µm.
2.4. Tahsfc2a-Overexpression Affected Iron Transport Genes and Iron Accumulation in Roots after Heat Treatment
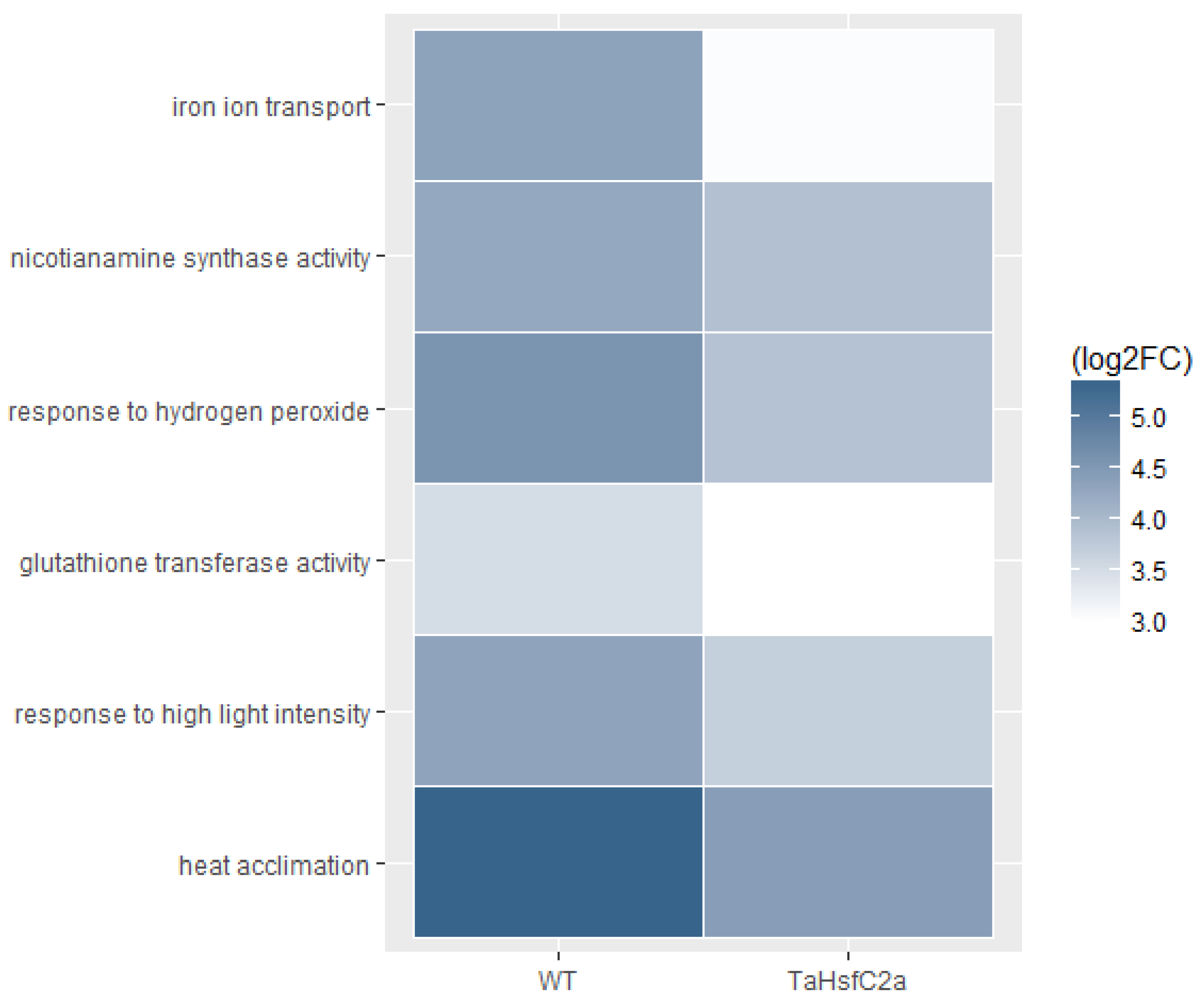
In addition, Fe ion transport and nicotianamine-related GO categories were highly up-regulated in both wild-type and TaHsfC2a-overexpressing line transcriptomes after heat stress (Supplementary Table S3). Nicotianamine is a precursor of phytosiderophores, which are excreted by the roots of Poaceae species, and mediate the chelation and acquisition of Fe ions [31]. Recently, Distefano et al. [11] identified Fe and ROS-dependent cell death could be induced by heat stress in Arabidopsis roots. To explore a possible involvement of Fe-mediated cell death phenomenon in heat stress tolerance, we examined the expression profiling of genes related to Fe transport and nicotianamine synthase activity and found heat to down-regulate the expression profiles of these genes in TaHsfC2a over-expressing plants when compared with wild-type roots (Figure 5). Further, the TaHsfC2a over-expressing plant roots were found to accumulate low Fe ions when compared with the wild-type roots after heat treatment (Figure 6). Therefore, these results suggested that TaHsfC2a-overexpression was reducing Fe accumulation in the roots, and thus, in turn, reducing ROS- and iron-mediated cell death response, enabling transgenic roots to recover from the detrimental effects of heat stress.
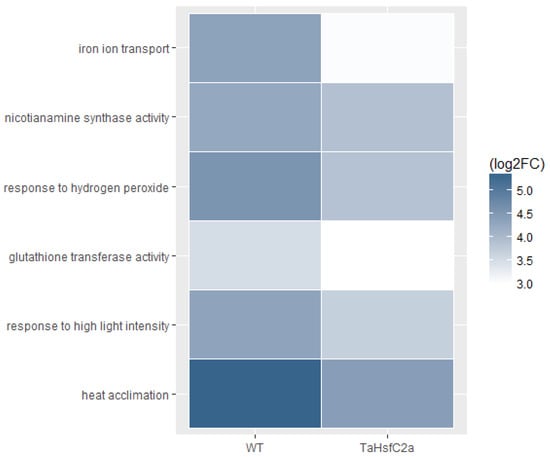
Figure 5.
Transcripts from iron transport, nicotianamine synthase, reactive oxygen species and heat stress GO categories were low abundant in the transgenic roots. WT, wild-type (control v heat); TaHsfC2a (control v heat); log2 FC, Fold Change log2 ≥ 2.
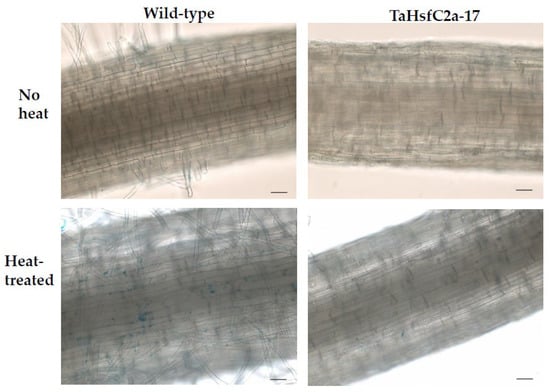
Figure 6.
Iron accumulation along the roots of wild-type and transgenic line with and without heat treatment. Roots of eight-day-old wheat plants were stained with Prussian blue for ferric ions. Scale bar, 100 µm.
2.5. Wheat Root and Leaf Transcriptomes Show Low Correlation under Heat Stress
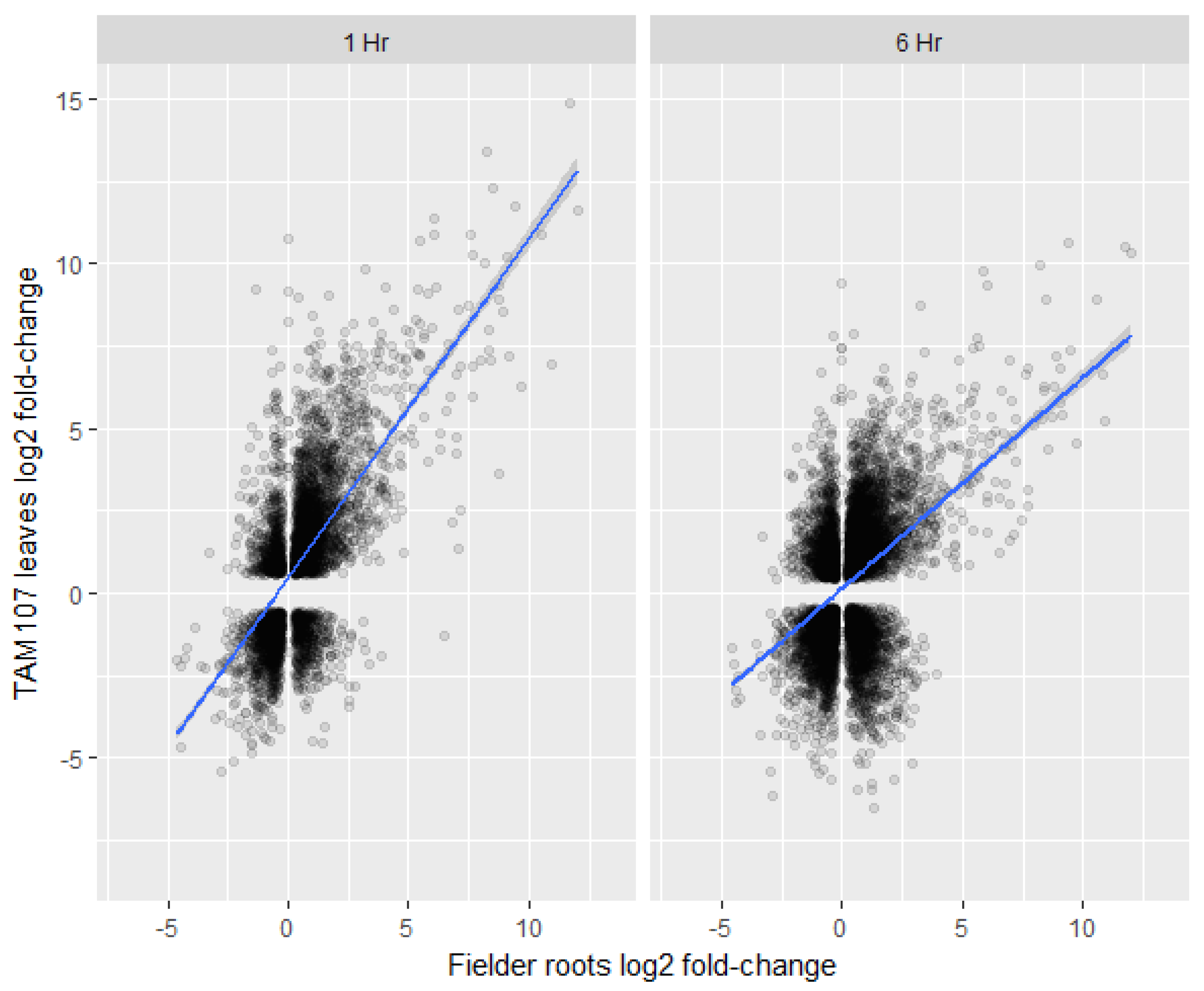
To explore if wheat roots and leaves/shoots show similar transcriptional responses to heat, we have compared our root transcriptional data to recently published leaf transcriptome data [32]. The cultivars used in these two studies were different; however, the age of the seedlings and the temperature and duration of heat treatment were similar between the two experiments. Overall, the root transcriptome after heat treatment showed relatively low correlations with the transcriptome of 1 h (r = 0.42) and 6 h (r = 0.27) heat-treated leaves when both up- and down-regulated genes were included in the analysis (Figure 7). Furthermore, our analysis also showed that 3331 genes were at least twofold or more differentially regulated (log2 FC ≥ 2) in the roots under heat stress. Of these 3331 genes, only 474 (14%) and 546 (16%) genes were significantly differentially regulated in 1- and 6-h in heat-treated leaves, respectively (Supplementary Table S5). Surprisingly, 29 and 104 genes showed opposite transcript levels in the 1- and 6-h heat-treated leaves, respectively (Supplementary Table S5). In addition, several NAS genes were up-regulated in the roots; hence, we looked at the transcript levels of the nicotianamine synthases (NAS) in the leaves. However, none of the NAS-related genes were significantly up-regulated in the leaves (Table 2). These results indicated that the overall transcriptomic responses of heat-treated leaves and heat-treated roots may not be similar, and root-specific iron-related mechanism may exist under heat stress.
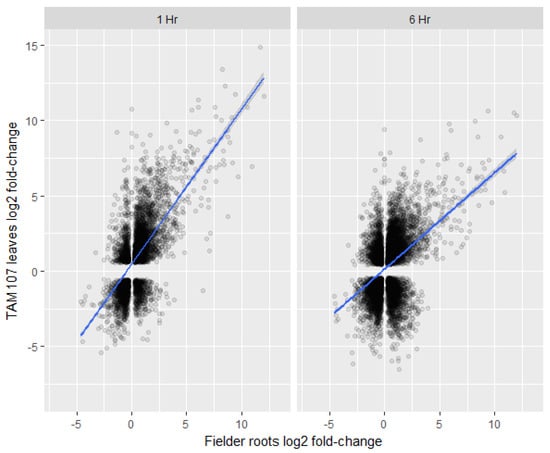
Figure 7.
Comparison of differentially expressed gene log fold-changes between Fielder roots (wild-type) (x-axis) 2 h post heat treatment and TAM107 leaves (y-axis) 1 h post heat treatment (left panel) or 6 h post heat treatment (right panel). Significant Kendall’s rank correlations of 0.42 and 0.27 were calculated for 1 hour and 6 h datasets respectively.

Table 2.
Fold changes (heat/control ratio) observed at the transcript levels of nicotianamine synthase genes in the roots and the leaves of the wheat cultivars, wild-type (this study) and TAM107 [32], respectively.
3. Discussion
Heat stress is becoming an immense problem in crop production around the world. Although heat can have a drastic effect on roots [33], most studies have focused on above-ground tissues [23]. Here, we have analyzed the effects of heat shock on seedling roots in both the wild-type and a transgenic line over-expressing TaHsfC2a. Our analyses showed that heat stress does indeed have a major effect on the root transcriptome. Further, our heat-stressed root transcriptome data were compared with those of the above-ground plant parts reported by Liu et al. [32]. These results showed that although general categories of differentially expressed root genes were like those seen in wheat leaves [32,34], very little correlation, if any, could be found between the actual genes differentially expressed in different tissues (see also below). Organ-specific (roots as compared to leaves) expressions patterns of the TaHsf gene family between roots and leaves of wheat in response to heat stress were also reported in wheat [18]. In another study, heat-responsive TF expression patterns were found different in different wheat tissues, such as flag leaves and filling-grains [22]. Similar results have been reported in root hairs and striped roots in soybeans [35]. Furthermore, He et al. [36] showed that transcriptomic responses to heat stress differ between the different tissues of maize (Zea mays L.). The alterations of photosynthesis-related proteins in above-ground parts of wheat during heat-stress could be another difference between wheat roots and leaves under heat stress [37]. Another potential difference between wheat roots and leaf transcriptomes under stress is, found by Su et al. [38], the transcriptional regulation of zeatin, brassinosteroid, and flavonoid biosynthesis pathways during heat stress response in leaves [38] but not in the root transcriptome (this study).
The GO enrichment analysis of the DEGs showed that responses to environmental stimuli (heat, light, temperature), ROS, transcriptional regulation, protein-folding, and chromatin remodeling were affected significantly by heat treatment in both the wild-type and transgenic roots. Similar categories were reported from the transcriptome analysis of wheat roots under heat stress [26]. A transcriptome and protein analysis of soybean (Glycine max (L.) Merr.) roots under heat also found similar GO categories [35]. The categories related to stress, chromatin, and ROS were also reported in heat-treated wheat seedling leaves [32]. Recently, TaHsfA6b-4D was shown to contribute to unfolded protein responses under heat stress to maintain protein homoeostasis in the cells [39]. In tomatoes, HSFA1a plays a key role in chromatin spatial reorganization and enhances the expression of heat-stress responsive genes under heat stress [40]. Together with these studies and our RNA-seq results, it can be suggested that TaHSFC2a could be involved in protein homeostasis and chromatin reorganization. In addition, our root transcriptome study identified Fe- and nicotinamide-related GO categories, and similar categories were identified by Luo et al. [26] in wheat roots under heat.
Transcription factors can play a significant role in regulating cellular responses to abiotic stress. The TF families most affected by the heat treatment were HSFs, ERFs, NACs, bHLHs, and MYBs, WRKYs in both wild-type and transgenic roots, and these TFs were also reported in wheat leaves after heat stress [32]. TFs, such as HSFs, ERFs, and WRKYs, were also found in heat-treated soybean roots and maize seedlings [9,35]. When we compared our transcriptome data with the heat-treated leaf transcriptome of wheat [32], we found that G2-like, NF-X1, GRF, RAV, and LBD TF families were differentially regulated only in the roots. G2-like TFs were also differentially expressed in Arabidopsis roots and Brassica rapa whole-plant tissues under abiotic stress conditions [41,42]. NF-X1 was differentially expressed in Arabidopsis whole-plant tissues under heat stress [43], which is consistent with our transcriptome result on NF-X1. The grf7 Arabidopsis mutants were more tolerant to salinity and drought stress, and GRF was found to regulate the expression of DREB2A [44]. DREB2A promotes plant survival under severe environmental stress, including high temperatures [44]. At least six GRFs were down-regulated in the transgenic line; however, only four were down-regulated in wild-type roots. RAV1 and LBD genes were shown to respond to environmental stimuli in Arabidopsis [45,46]. These TFs were expressed in roots (this study) but not in leaves [32], suggesting that these TFs may be root specific, and may play a role in the heat stress tolerance of wheat.
Several heat shock proteins, including HSP70 and HSP90, were highly up-regulated after heat treatment in both the wild-type and transgenic roots, indicating a strong transcriptional response of root tissues (Supplementary Table S2). In wheat, the RNA-seq of seedling leaves after heat treatment increased the expression of several Hsf (class A, B and C), and HSP genes [47]. The Hsf TFs play a crucial role under heat stress by regulating HSPs and other stress-related genes. Previous studies have reported that the overexpression of HsfA induced the expression of a large array of stress-related genes [48,49,50]. The overexpression of TaHsfA2-1 and TaHsfA2e-5D in Arabidopsis was found to up-regulate HSP genes and confers stress tolerance [51,52]. HsfC2 members are monocot specific [19], and their effects on the whole transcriptome have not been studied before. Our results showed that TaHsfC2a overexpression affects a large array of genes under heat stress in roots when compared with wild-type plants. Although several studies observed that the overexpression of Hsfs increased the survival of plants and conferred stress tolerance [27,53], the underlying molecular mechanisms are unknown. For example, the overexpression of TaHsfA2-1, TaHsfA2e-5D, and TaHsfA2-7-AS in Arabidopsis enhanced heat tolerance [51,52,54]. However, the molecular mechanisms are yet to be studied.
Here, through transcriptional profiling, we aimed to decipher the mechanism behind the survival of TaHsfC2a-overexpressed transgenic plants after heat stress as reported by Hu et al. [27]. We found that a group of peroxidases were down-regulated in transgenic lines when compared with wild-type plants after heat treatment. The down-regulation of hydrogen peroxide-producing peroxidases would decrease the hydrogen peroxide accumulation in the transgenic roots. This may be one of the reasons for the increased survival of TaHsfC2a-overexpressing plants as increased ROS levels could trigger cell death in roots. Previous studies showed that the peroxidases gene expression possibly affected the level of hydrogen peroxide in Arabidopsis roots [55,56]. This view is consistent with the observation of the reduced accumulation of hydrogen peroxide in the transgenic roots in this study, as class III peroxidases are involved in generating hydrogen peroxide [57,58]. Furthermore, a recent study in hair grass (Agrostis scabra Willd.) showed that the heat-tolerant grass accumulated lower ROS levels in the roots than in the heat-susceptible creeping bent grass (A. stolonifera L.) under heat stress [59].
In addition to peroxidases, nicotianamine-related transcripts were less abundantly expressed in the roots of TaHsfC2a-overexpressing plants in response to heat when compared with wild-type heat responses. Nicotianamine is a precursor of phytosiderophores, which are important for the iron uptake by the Poaceae family plants, including wheat [31]. Using a chelation-based strategy to form soluble Fe (III) complexes, which are then taken up by the roots [60]. Recently, Distefano et al. [11] identified ferroptosis-like cell death in Arabidopsis roots in response to heat stress that is dependent on ROS and iron accumulation. Therefore, our results suggest that reduced ROS and iron levels may be critical factors contributing to the survival of TaHsfC2a-overexpressing plants after heat treatment through the reduction of ferroptotic cell death. Ferroptosis-like cell death has been identified in diverse species with distinct features of the disruption of iron and ROS-homeostasis [61]. Ferroptosis is an ancient evolutionary mechanism, which is conserved across animal and plant species [11]. In animal cell culture studies, the knockdown of HSF1 was found to enhance ferroptosis through the accumulation of intracellular iron and the associated lipid peroxidation in cancer cells and xenograft models [62], which indicates that TaHsfC2a plays a key role in ferroptosis in plants as both genes belong to the Hsf gene family.
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
The spring wheat (Triticum aestivum L.) cultivar (cv.) Fielder (wild-type/untransformed control) and TaHsfC2a-B overexpressing transgenic line (C2a-17) in cv. Fielder background were described in Hu et al. [27]. Seeds were washed, kept on wet paper towel at 4 °C for 5 days, and then transferred to 12 °C to induce germination. Two days after germination, the seedlings retained within the moistened paper towel were transferred to room temperature for 1 day, and then transferred to plastic containers containing sterilized reverse osmosis water and allowed to grow for one further day at room temperature. Then, the 4-day-old seedlings were placed into a Hoagland and Arnon nutrient solution No.2 [63] containing plastic container and transferred to a controlled-environment facility (CEF; CSIRO, St. Lucia, Queensland, Australia), where the environmental factors, such as photoperiod, temperature, light, and relative humidity can be controlled. The seedlings were kept under a 16/8-h (day/night) thermoperiod of 22/16 °C with a matching photoperiod (500 μmol m−2s−1) and 60 to 80% relative humidity for a further 3 days in the CEF. Seven-day-old seedlings in the container with Hoagland and Arnon nutrient solution No.2 were kept in a water bath at 43 °C. Once the nutrient solution reached 43 °C, the seedlings were heat-treated for 2 h at 43 °C, while untreated seedlings were kept in the CEF as control samples. The heat-treated and untreated seedlings were then kept in the CEF for one further day before being sequenced.
4.2. RNA Preparation and Sequencing
Eight-day-old seedling roots from the wild-type and C2a-17 lines were collected from heat-treated and untreated plants. Root tissues were immediately frozen in liquid nitrogen and then stored at −80 °C for further use. Roots from at least six seedlings, from each container with nutrient solution, were pooled to make up one replicate. Total RNA was extracted from the pooled root tissues, and per replicate using RNeasy Plant Mini Kit (Qiagen, Melbourne, Victoria, Australia) according to the manufacturer’s instructions. Four biological replicates (biologically distinct samples from same treatment) per treatment were used for each of the treated and control samples of wild-type and overexpressing plants in this study. Nucleic acid quantity was measured with a NanoDrop ND-1000 UV-Vis Spectrophotometer (Nano Drop Technologies, Wilmington, DE, USA), and quality was determined using Agilent 2100 Bioanalyzer (Agilent Technologies, CA, USA).
A TruSeq stranded messenger RNA (mRNA) kit was used to generate 100 base pair (bp) paired-end libraries, according to the manufacturer’s protocol (Illumina Inc., San Diego, USA). Libraries were barcoded prior to sequencing. Sequencing was performed using an Illumina HiSeq 2500 platform with four lanes, and all samples were run on each lane which made up four technical replicates (same sample run on four lanes) per sample. Library preparation and sequencing were performed at the Australian Genome Research Facility (AGRF; Melbourne, Victoria, Australia). Sequence files were deposited to the National Centre for Biotechnology Information (NCBI) Sequence Read Archive under bioproject PRJNA498129.
4.3. RNAseq Analysis
We used the wheat reference genomic sequence of cv Chinese Spring, RefSeq v1.0 [64], and the Tuxedo package, previously described [65], to detect genes that were differentially expressed between the control and heat-treated seedlings. Firstly, we excluded base-call errors arising during the sequencing process using the software package SolexaQA. RNA-seq reads were trimmed so that all remaining bases had a PHRED score > 30 and their final read length was at least 70 bp with both right and left reads fulfilling these requirements. Filtered and trimmed paired reads were aligned to the Chinese Spring cv. RefSeq v1.0 annotation (files accessed from URGI on 13 March 2017) using Tophat2 v2.1.1 with Bowtie2 v2.2.9 as the aligner. Binary alignment maps (BAM files) were produced using the program SAMtools v1.3.1. Transcript fragments were assembled and normalized to yield fragments per kb per million reads using the package Cufflinks v2.2.2, prior to replicate concatenation by Cuffmerge, and a differential gene expression calling by Cuffdiff. A false discovery rate (FDR) and multiple comparison correction (Bonferroni correction) were performed within the Cuffdiff process, identifying which genes were significantly differentially expressed between the control and heat-treated seedlings for each genotype, the wild-type Fielder, and the TaHsfC2a-overexpressing line. Differentially expressed genes (DEG) were then filtered from each dataset using FDR adjusted p value < 0.05 and > log2 twofold change cut-off values and divided into up- and down-regulated sets based on the positive or negative fold change values, respectively. Venn diagrams were produced using these filtered datasets with the aid of a webtool produced by the Bioinformatics and Evolutionary Genomics group based at the University of Ghent (http://bioinformatics.psb.ugent.be/webtools/Venn/) (accessed on 1 December 2019).
4.4. Gene Ontology Enrichment Analysis
The set of high confidence coding sequences were identified from our RNA-seq. Then, these sequences were annotated using the BLAST2GO [66] server based at CSIRO Agriculture and Food. This produced set of ca.138,000 annotated genes and was used as a background reference to define the wheat GO categories for the enrichment analysis. The up- and down-regulated genes (≥2 log2-fold and ≤−2 log2-fold) were used as separate test inputs. Standard parameters were applied for the BLASTx, mapping, and annotation steps and for functional enrichment testing using the Fisher’s exact test module (FDR adjusted p value < 0.05). Highly significant GO terms (FDR; p value < 0.001) are presented in Supplementary Table S3. The heatmaps incorporated in this study were generated using the ggplot2 package (tidyverse 1.2.1) in R. We have used the BiNGO plugin for Cytoscape to identify significantly overrepresented GO categories from the wild-type, and used custom wheat GO term annotation as a reference (http://www.psb.ugent.be/cbd/papers/BiNGO/ [67]). The differentially expressed gene sequences were used in PlantTFDB4.0 to identify putative TFs [68].
4.5. Hydrogen Peroxide Measurement Using Hydroxyphenyl Fluorescein (HPF)
At least 15 heat-treated and untreated roots from 8-day-old seedlings (similar treatments were applied as described for RNA sample collection) were incubated for 2 min in 0.1 M phosphate buffer, pH 7.4, containing 5 µM 3′-(p-hydroxyphenyl) fluorescein (Sigma, St. Louis, CA, USA) [69]. Roots were then mounted on a glass slide in a drop of buffer, covered, and observed under a fluorescence microscope (Leica MZ16FA) with excitation/emission of 480/510 nm. Fluorescence images of roots were immediately captured from roots, with the microscope setting unaltered for all the treatments. ImageJ was used to measure the staining intensity of the fluorescence images.
4.6. Histochemical Detection of Fe3+
The histochemical study for the detection of Fe was carried out following the previous methods with slight modifications [70,71]. One-week-old seedlings in a container containing Hoagland and Arnon nutrient solution No.2 were exposed to heat stress in a water bath at 43 °C for 2 h, as described for the RNA sample collection, and then incubated in 7% potassium ferrocyanide and 3% HCl (1:1 v/v) for 15 h at room temperature. Roots were washed three times with running distilled water and samples collected from the root hair zone. The sections were mounted onto slides, stained with Prussian blue, covered with a cover slip, and observed under the microscope. Blue pigmentation in tissues caused by the presence of ferric ferrocyanides, formed by an interaction between Fe ions and ferrocyanides, was observed.
5. Conclusions
In conclusion, our current study showed that TaHsfC2a is modulating ROS and iron levels in response to heat stress in wheat roots. Consequently, this study found the existence of ferroptosis-like cell death in wheat, and TaHsfC2a could be a key player in this mechanism. Further studies in this area are highly warranted to test the utility of TaHsfC2a overexpression in developing thermotolerant varieties in wheat.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24043099/s1.
Author Contributions
Conceptualization and design of experiments, S.K.; performed experiments, S.K., A.K., U.K.; Analysis and interpretation of data, S.K., J.P., J.S., S.A., K.K., D.F.; writing—original draft preparation, S.K.; writing—review and editing, S.K., J.P., J.S., S.A., K.K., D.F., U.K., A.K.; supervision, D.F., K.K., J.S., S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Commonwealth Scientific and Industrial Research Organisation postdoctoral fellowship scheme with additional funding from the Australian Research Council Industrial Transformation Research Hub for wheat in a hot and dry climate (IH130200027).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All sequence files were deposited to the National Centre for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under bioproject PRJNA498129.
Acknowledgments
We thank Gangping Xue for kindly providing the seeds of TaHsfC2a-B-overexpression lines. Authors thank the Australian Genome Research Facility (AGRF) for performing RNA-sequencing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Easterling, D.R.; Meehl, G.A.; Parmesan, C. Climate extremes: Observations, modeling, and impacts. Science 2000, 289, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Tripathi, D.K.; Chauhan, D.K.; Kumar, N.; Singh, G.S. Paradigms of climate change impacts on some major food sources of the world: A review on current knowledge and future prospects. Agric. Ecosyst. Environ. 2016, 216, 356–373. [Google Scholar] [CrossRef]
- Liu, B.; Asseng, S.; Müller, C.; Ewert, F.; Elliott, J.; Lobell, D.; Martres, P.; Ruane, A.C.; Wallach, D.; Jones, J.W.; et al. Similar estimates of temperature impacts on global wheat yield by three independent methods. Nat. Clim. Chang. 2016, 6, 1130–1136. [Google Scholar] [CrossRef]
- Deryng, D.; Conway, D.; Ramankutty, N.; Price, J.; Warren, R. Global crop yield response to extreme heat stress under multiple climate change futures. Environ. Res. Lett. 2014, 9, 034011. [Google Scholar] [CrossRef]
- Li, Y.F.; Wang, Y.; Tang, Y.; Kakani, V.G.; Mahalingam, R. Transcriptome analysis of heat stress response in switchgrass (Panicum virgatum L.). BMC Plant Biol. 2013, 13, 153. [Google Scholar] [CrossRef]
- Zhang, S.S.; Yang, H.; Ding, L.; Song, Z.T.; Ma, H.; Chang, F.; Liu, J.X. Tissue-specific transcriptomics reveals an important role of the unfolded protein response in maintaining fertility upon heat stress in Arabidopsis. Plant Cell 2017, 29, 1007–1023. [Google Scholar] [CrossRef]
- Qian, Y.; Ren, Q.; Zhang, J.; Chen, L. Transcriptomic analysis of the maize (Zea mays L.) inbred line B73 response to heat stress at the seedling stage. Gene 2019, 692, 68–78. [Google Scholar] [CrossRef]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef]
- Distéfano, A.M.; Martin, M.V.; Córdoba, J.P.; Bellido, A.M.; D’Ippólito, S.; Colman, S.L.; Soto, D.; Roldán, J.A.; Bartoli, C.G.; Zabaleta, E.J.; et al. Heat stress induces ferroptosis-like cell death in plants. J. Cell Biol. 2017, 16, 463–476. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Kazan, K.; Kalaipandian, S. Ferroptosis: Yet another way to die. Trends Plant Sci. 2019, 24, 479–481. [Google Scholar] [CrossRef]
- Distefano, A.M.; López, G.A.; Bauer, V.; Zabaleta, E.; Pagnussat, G.C. Ferroptosis in plants: Regulation of lipid peroxidation and redox status. Biochem. J. 2022, 479, 857–866. [Google Scholar] [CrossRef]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global Plant Stress Signaling: Reactive Oxygen Species at the Cross-Road. Front. Plant Sci. 2016, 7, 187. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef]
- Duan, S.; Liu, B.; Zhang, Y.; Li, G.; Guo, X. Genome-wide identification and abiotic stress-responsive pattern of heat shock transcription factor family in Triticum aestivum L. BMC Genom. 2019, 20, 257. [Google Scholar] [CrossRef]
- Scharf, K.D.; Berberich, T.; Ebersberger, I.; Nover, L. The plant heat stress transcription factor (Hsf) family: Structure, function and evolution. Biochim. Biophys. Acta 2012, 1819, 104–119. [Google Scholar] [CrossRef]
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant heat stress response. Trends Plant Sci. 2017, 22, 53–65. [Google Scholar] [CrossRef]
- Guo, M.; Liu, J.H.; Ma, X.; Luo, D.X.; Gong, Z.H.; Lu, M.H. The plant heat stress transcription factors (HSFs): Structure, regulation, and function in response to abiotic stresses. Front. Plant Sci. 2016, 7, 114. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S.; Shi, X.; Liu, D.; Zhao, P.; Lu, Y.; Cheng, Y.; Liu, Z.; Nie, X.; Song, W.; et al. Hybrid sequencing reveals insight into heat sensing and signaling of bread wheat. Plant J. 2019, 98, 1015–1032. [Google Scholar] [CrossRef] [PubMed]
- Janni, M.; Gullì, M.; Maestri, E.; Marmiroli, M.; Valliyodan, B.; Nguyen, H.T.; Marmiroli, N. Molecular and genetic bases of heat stress responses in crop plants and breeding for increased resilience and productivity. J. Exp. Bot. 2020, 71, 3780–3802. [Google Scholar] [CrossRef] [PubMed]
- Heckathorn, S.A.; Giri, A.; Mishra, S.; Bista, D. Heat stress and roots. In Climate Change and Plant Abiotic Stress Tolerance; Tuteja, N., Gill, S., Eds.; Wiley Blackwell: Weinheim, Germany, 2014; pp. 109–136. [Google Scholar]
- Wang, H.; Lemke, R.; Goddard, T.; Sprout, C. Tillage and root heat stress in wheat in Central Alberta. Can. J. Soil Sci. 2007, 87, 3–10. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, Y.; Li, W.; Wei, M.; Dai, T.; Li, Z.; Wang, B. Physiological and Transcriptomic Analyses Reveal Exogenous Trehalose Is Involved in the Responses of Wheat Roots to High Temperature Stress. Plants 2021, 10, 2644. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.J.; Chen, D.; Mclntyre, L.C.; Dreccer, F.M.; Zhang, Z.B.; Drenth, J.; Kalaipandian, S.; Chang, H.; Xue, G.P. Heat shock factor C2a serves as a proactive mechanism for heat protection in developing grains in wheat via an ABA-mediated regulatory pathway. Plant Cell Environ. 2018, 41, 79–98. [Google Scholar] [CrossRef]
- Volkov, R.A.; Panchuk, I.I.; Mullineaux, P.M.; Schöffl, F. Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Mol. Biol. 2006, 61, 733–746. [Google Scholar] [CrossRef]
- Fedyaeva, A.V.; Stepanov, A.V.; Lyubushkina, I.V.; Pobezhimova, T.P.; Rikhvanov, E.G. Heat shock induces production of reactive oxygen species and increases inner mitochondrial membrane potential in winter wheat cells. Biochemistry 2014, 79, 1202–1210. [Google Scholar] [CrossRef]
- Shigeto, J.; Tsutsumi, Y. Diverse functions and reactions of class III peroxidases. N. Phytol. 2016, 209, 1395–1402. [Google Scholar] [CrossRef]
- Curie, C.; Cassin, G.; Couch, D.; Divol, F.; Higuchi, K.; Le Jean, M.; Mission, J.; Schikora, A.; Czernic, P.; Mari, S. Metal movement within the plant: Contribution of nicotianamine and yellow stripe 1-like transporters. Ann. Bot. 2009, 103, 1–11. [Google Scholar] [CrossRef]
- Liu, Z.; Xin, M.; Qin, J.; Peng, H.; Ni, Z.; Yao, Y.; Sun, Q. Temporal transcriptome profiling reveals expression partitioning of homeologous genes contributing to heat and drought acclimation in wheat (Triticum aestivum L.). BMC Plant Biol. 2015, 15, 152. [Google Scholar] [CrossRef]
- Jagadish, S.K.; Way, D.A.; Sharkey, T.D. Plant heat stress: Concepts directing future research. Plant Cell Environ. 2021, 21, 1992–2005. [Google Scholar] [CrossRef]
- Qin, D.; Wu, H.; Peng, H.; Yao, Y.; Ni, Z.; Li, Z.; Zhou, C.; Sun, Q. Heat stress-responsive transcriptome analysis in heat susceptible and tolerant wheat (Triticum aestivum L.) by using wheat genome array. BMC Genom. 2008, 9, 432. [Google Scholar] [CrossRef]
- Valdés-López, O.; Batek, J.; Gomez-Hernandez, N.; Nguyen, C.T.; Isidra-Arellano, M.C.; Zhang, N.; Joshi, T.; Xu, D.; Hixson, K.K.; Weitz, K.K.; et al. Soybean roots grown under heat stress show global changes in their transcriptional and proteomic profiles. Front. Plant Sci. 2016, 7, 517. [Google Scholar] [CrossRef]
- He, J.; Jiang, Z.; Gao, L.; You, C.; Ma, X.; Wang, X.; Xu, X.; Mo, B.; Chen, X.; Liu, L. Genome-wide transcript and small RNA profiling reveals transcriptomic responses to heat stress. Plant Physiol. 2019, 181, 609–629. [Google Scholar] [CrossRef]
- Kumar, R.R.; Singh, K.; Ahuja, S.; Tasleem, M.; Singh, I.; Kumar, S.; Grover, M.; Mishra, D.; Rai, G.K.; Goswami, S.; et al. Quantitative proteomic analysis reveals novel stress-associated active proteins (SAAPs) and pathways involved in modulating tolerance of wheat under terminal heat. Funct. Integr. Genom. 2019, 19, 329–348. [Google Scholar] [CrossRef]
- Su, P.; Jiang, C.; Qin, H.; Hu, R.; Feng, J.; Chang, J.; Yang, G.; He, G. Identification of potential genes responsible for thermotolerance in wheat under high temperature stress. Genes 2019, 10, E174. [Google Scholar] [CrossRef]
- Meena, S.; Samtani, H.; Khurana, P. Elucidating the functional role of heat stress transcription factor A6b (TaHsfA6b) in linking heat stress response and the unfolded protein response in wheat. Plant Mol. Biol. 2022, 108, 621–634. [Google Scholar] [CrossRef]
- Huang, Y.; An, J.; Sircar, S.; Bergis, C.; Lopes, C.D.; He, X.; Da Costa, B.; Tan, F.-Q.; Bazin, J.; Antunez-Sanchez, J.; et al. HSFA1a modulates plant heat stress responses and alters the 3D chromatin organization of enhancer-promoter interactions. Nat. Commun. 2023, 14, 469. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Deyholos, M.K. Comprehensive transcriptional profiling of NaCl-stressed Arabidopsis roots reveals novel classes of responsive genes. BMC Plant Biol. 2006, 6, 25. [Google Scholar] [CrossRef]
- Lee, S.C.; Lim, M.H.; Kim, J.A.; Lee, S.-I.; Kim, J.S.; Jin, M.; Kwon, S.-I.; Mu, J.H.; Kim, Y.K.; Kim, H.U.; et al. Transcriptome analysis in Brassica rapa under the abiotic stresses using Brassica 24K oligo microarray. Mol. Cells 2008, 26, 595–605. [Google Scholar]
- Larkindale, J.; Vierling, E. Core genome responses involved in acclimation to high temperature. Plant Physiol. 2008, 146, 748–761. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Mizoi, J.; Kidokoro, S.; Maruyama, K.; Nakajima, J.; Nakashima, K.; Mitsuda, N.; Takiguchi, Y.; Ohme-Takagi, M.; Kondou, Y.; et al. Arabidopsis growth-regulating factor7 functions as a transcriptional repressor of abscisic acid- and osmotic stress-responsive genes, including DREB2A. Plant Cell 2012, 24, 3393–3405. [Google Scholar] [CrossRef] [PubMed]
- Kagaya, Y.; Hattori, T. Arabidopsis transcription factors, RAV1 and RAV2, are regulated by touch-related stimuli in a dose-dependent and biphasic manner. Genes Genet. Syst. 2009, 84, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Zhang, S.Z.; Su, L.; Liu, X.; Hao, Y.J. A genome-wide analysis of the LBD (Lateral Organ Boundaries Domain) gene family in Malus domestica with a functional characterization of MdLBD11. PLoS ONE 2013, 8, e57044. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, K.-M.; Sang, W.-G.; Kang, C.-S.; Choi, C. Comparison of Gene Expression Changes in Three Wheat Varieties with Different Susceptibilities to Heat Stress Using RNA-Seq Analysis. Int. J. Mol. Sci. 2022, 23, 10734. [Google Scholar] [CrossRef]
- Nishizawa, A.; Yabuta, Y.; Yoshida, E.; Maruta, T.; Yoshimura, K.; Shi-geoka, S. Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress. Plant J. 2006, 48, 535–547. [Google Scholar] [CrossRef]
- Bechtold, U.; Albihlal, W.S.; Lawson, T.; Fryer, M.J.; Sparrow, P.A.; Richard, F.; Persad, R.; Bowden, L.; Hickman, R.; Martin, C.; et al. Arabidopsis Heat Shock Transcription Factor A1b overexpression enhances water productivity, resistance to drought, and infection. J. Exp. Bot. 2013, 64, 3467–3481. [Google Scholar] [CrossRef]
- Albihlal, W.S.; Chernukhin, I.; Blein, T.; Persad, R.; Obomighie, I.; Crespi, M.; Bechtold, U.; Mullineaux, P. Arabidopsis heat shock transcription factorA1b regulates multiple developmental genes under growth and stress conditions. J. Exp. Bot. 2018, 69, 2847–2862. [Google Scholar] [CrossRef]
- Liu, Z.; Li, G.; Zhang, H.; Zhang, Y.; Zhang, Y.; Duan, S.; Sheteiwy, M.S.A.; Zhang, H.; Shao, H.; Guo, X. TaHsfA2-1, a new gene for thermotolerance in wheat seedlings: Characterization and functional roles. J. Plant Physiol. 2020, 246–247, 153135. [Google Scholar] [CrossRef]
- Bi, H.; Miao, J.; He, J.; Chen, Q.; Qian, J.; Li, H.; Xu, Y.; Ma, D.; Zhao, Y.; Tian, X.; et al. Characterization of the Wheat Heat Shock Factor TaHsfA2e-5D Conferring Heat and Drought Tolerance in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 2784. [Google Scholar] [CrossRef]
- Prandl, R.; Hinderhofer, K.; Eggers-Schumacher, G.; Schoffl, F. HSF3, a new heat shock factor from Arabidopsis thaliana, derepresses the heat shock response and confers thermotolerance when overexpressed in transgenic plants. Mol. Gen. Genet. 1998, 258, 269–278. [Google Scholar] [CrossRef]
- Ma, Z.; Li, M.; Zhang, H.; Zhao, B.; Liu, Z.; Duan, S.; Meng, X.; Li, G.; Guo, X. Alternative Splicing of TaHsfA2-7 Is Involved in the Improvement of Thermotolerance in Wheat. Int. J. Mol. Sci. 2023, 24, 1014. [Google Scholar] [CrossRef]
- Tsukagoshi, H.; Busch, W.; Benfey, P.N. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 2010, 143, 606–616. [Google Scholar] [CrossRef]
- Sundaravelpandian, K.; Chandrika, N.N.P.; Schmidt, W. PFT1, a transcriptional Mediator complex subunit, controls root hair differentiation through reactive oxygen species (ROS) distribution in Arabidopsis. N. Phytol. 2013, 197, 151–161. [Google Scholar] [CrossRef]
- Rouet, M.A.; Mathieu, Y.; Barbier-Brygoo, H.; Lauriere, C. Characterization of active oxygen-producing proteins in response to hypo-osmolarity in tobacco and Arabidopsis cell suspensions: Identification of a cell wall peroxidase. J. Exp. Bot. 2006, 57, 1323–1332. [Google Scholar] [CrossRef]
- Daudi, A.; Cheng, Z.; O’Brien, J.A.; Mammarella, N.; Khan, S.; Ausubel, F.M.; Bolwell, G.P. The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 2012, 24, 275–287. [Google Scholar] [CrossRef]
- Xu, Y.; Burgess, P.; Huang, B. Root antioxidant mechanisms in relation to root thermotolerance in perennial grass species contrasting in heat tolerance. PLoS ONE 2015, 10, e0138268. [Google Scholar] [CrossRef]
- Romheld, V.; Marschner, H. Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol. 1986, 80, 175–180. [Google Scholar] [CrossRef]
- Conrad, M.; Kagan, V.E.; Bayir, H.; Pagnussat, G.C.; Head, B.; Traber, M.G.; Stockwell, B.R. Regulation of lipid peroxidation and ferroptosis in diverse species. Genes Dev. 2018, 32, 602–619. [Google Scholar] [CrossRef]
- Sun, X.; Ou, Z.; Xie, M.; Kang, R.; Fan, Y.; Niu, X.; Wang, H.; Cao, L.; Tang, D. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene 2015, 34, 5617–5625. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. 1950, 347, 1–32. [Google Scholar]
- The International Wheat Genome Sequencing Consortium. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Daehwan, K.; Kelley, D.R.; Pimentel, H.; Salzburg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Maere, S.; Heymans, K.; Kuiper, M. BiNGO: A Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 2005, 21, 3448–3449. [Google Scholar] [CrossRef]
- Jin, J.P.; Tian, F.; Yang, D.C.; Meng, Y.Q.; Kong, L.; Luo, J.C.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017, 45, D1040–D1045. [Google Scholar] [CrossRef]
- Dunand, C.; Crevecoeur, M.; Penel, C. Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: Possible interaction with peroxidases. N. Phytol. 2007, 174, 332–341. [Google Scholar] [CrossRef]
- Liu, G.; Greenshields, D.L.; Sammynaiken, R.; Hirji, R.N.; Selvaraj, G.; Wei, Y. Targeted alterations in iron homeostasis underlie plant defense responses. J. Cell Sci. 2007, 120, 596–605. [Google Scholar] [CrossRef]
- Dangol, S.; Chen, Y.; Hwang, B.K.; Jwa, N.S. Iron- and reactive oxygen species-dependent ferroptotic cell death in rice–Magnaporthe oryzae interactions. Plant Cell 2019, 31, 189–209. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).