Growth and Molecular Responses of Tomato to Prolonged and Short-Term Heat Exposure
Abstract
1. Introduction
2. Results
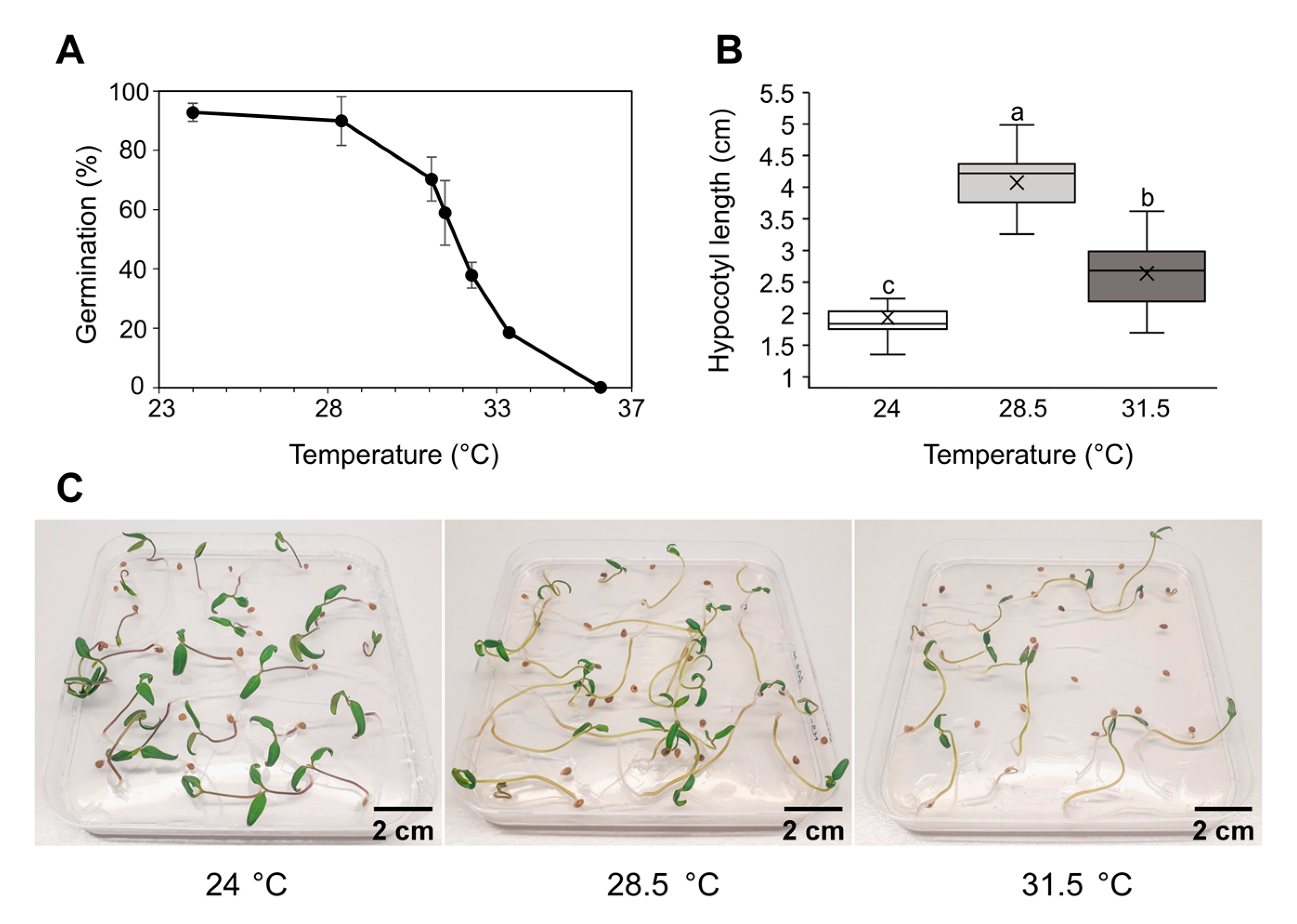
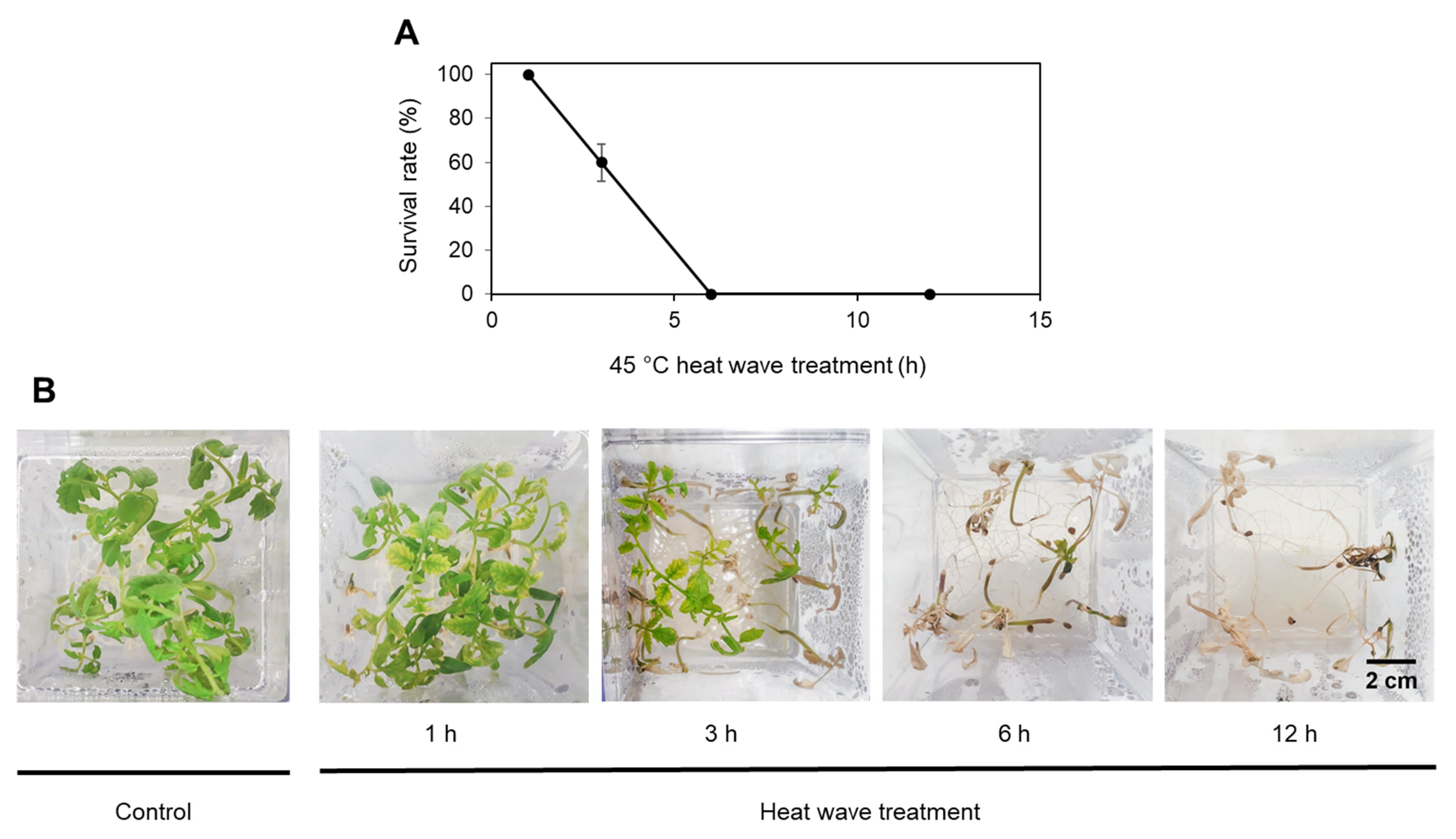
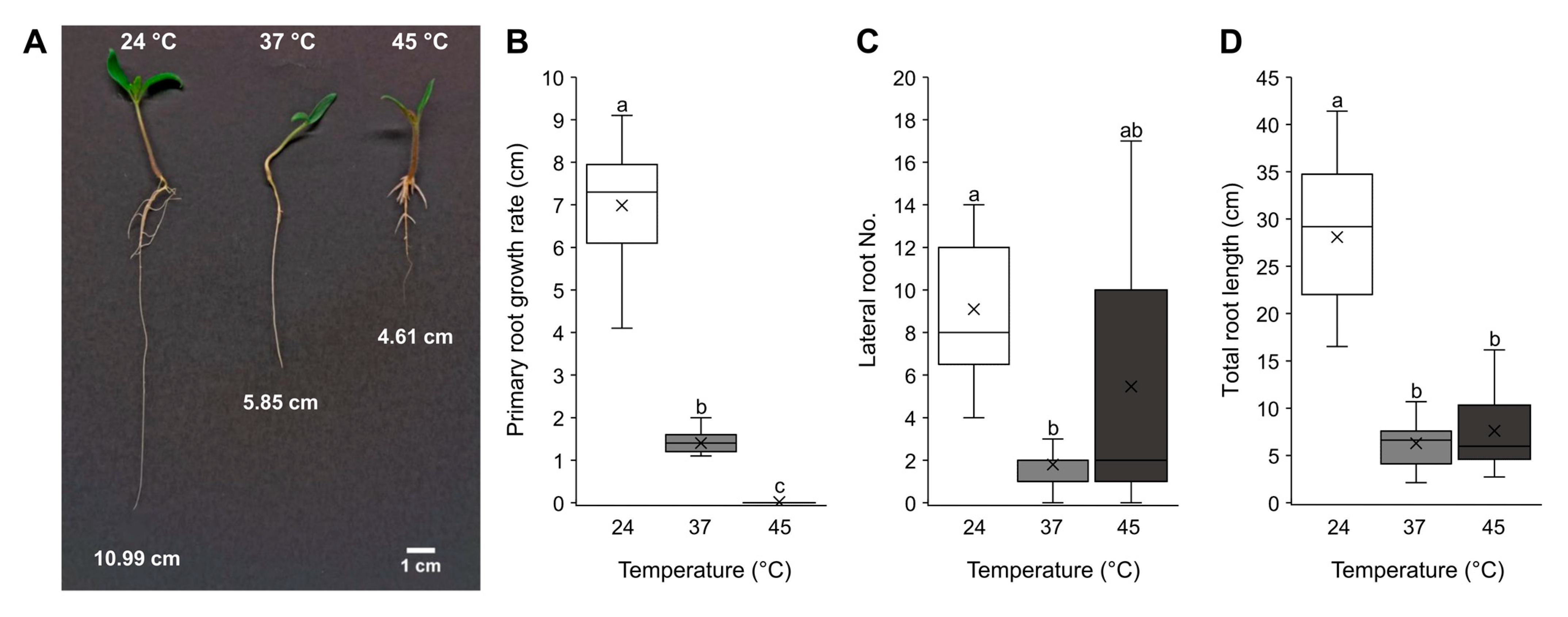
2.1. Tomato Germination and Plant Morphology following Heat Stress
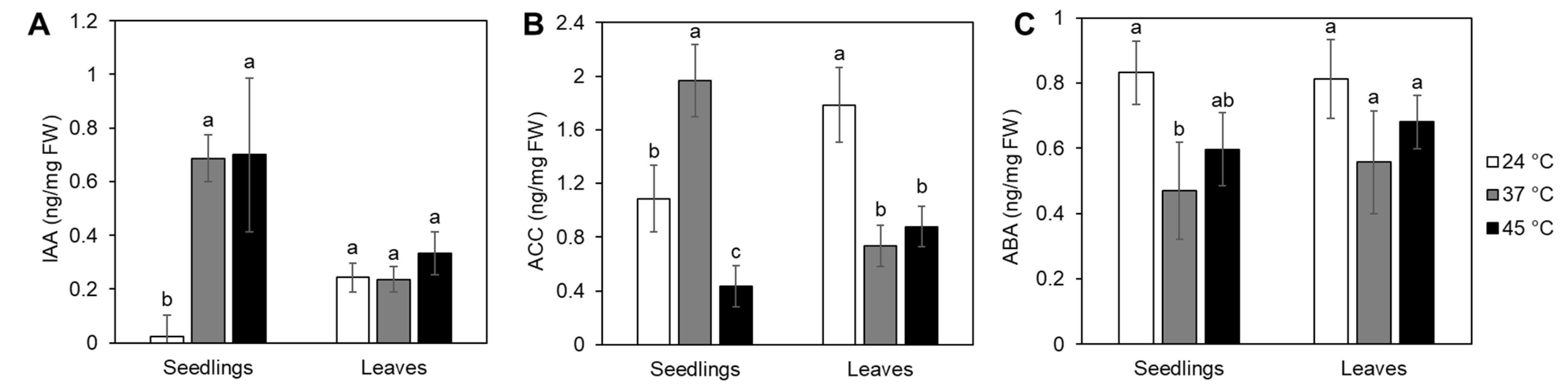
2.2. Heat Stress Effects on IAA, ACC and ABA Accumulation
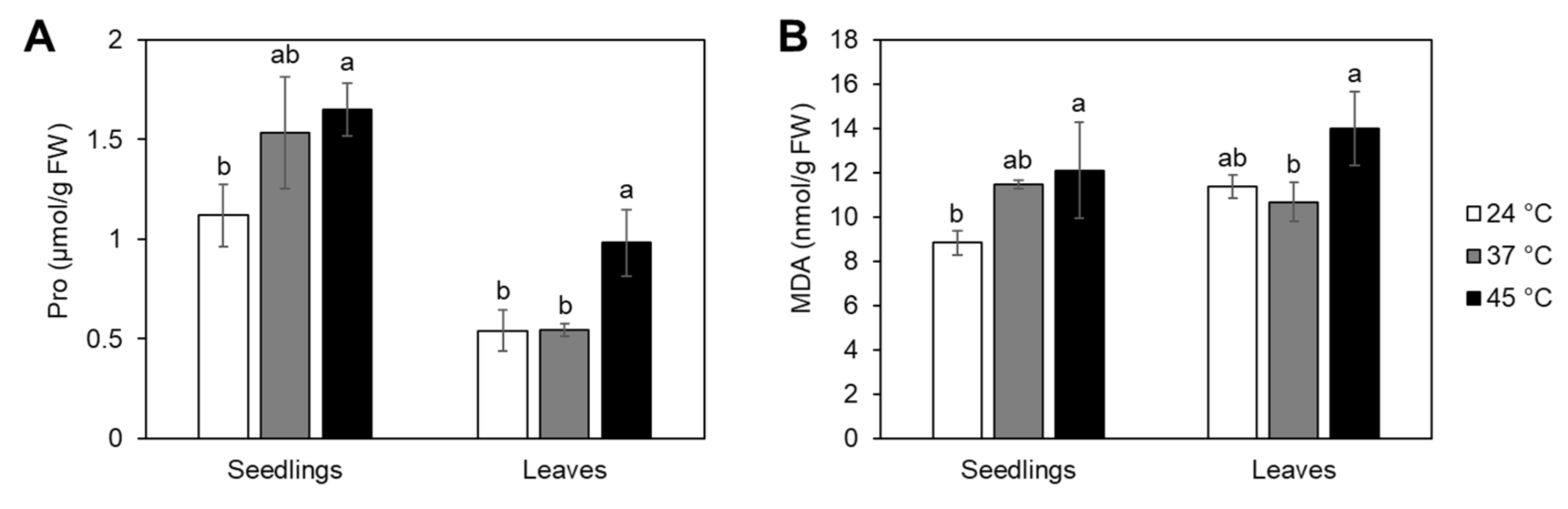
2.3. Heat Stress Effects on Proline and Malondialdehyde Accumulation
2.4. Heat Stress Responsive Transcription Factor Expression Quantification
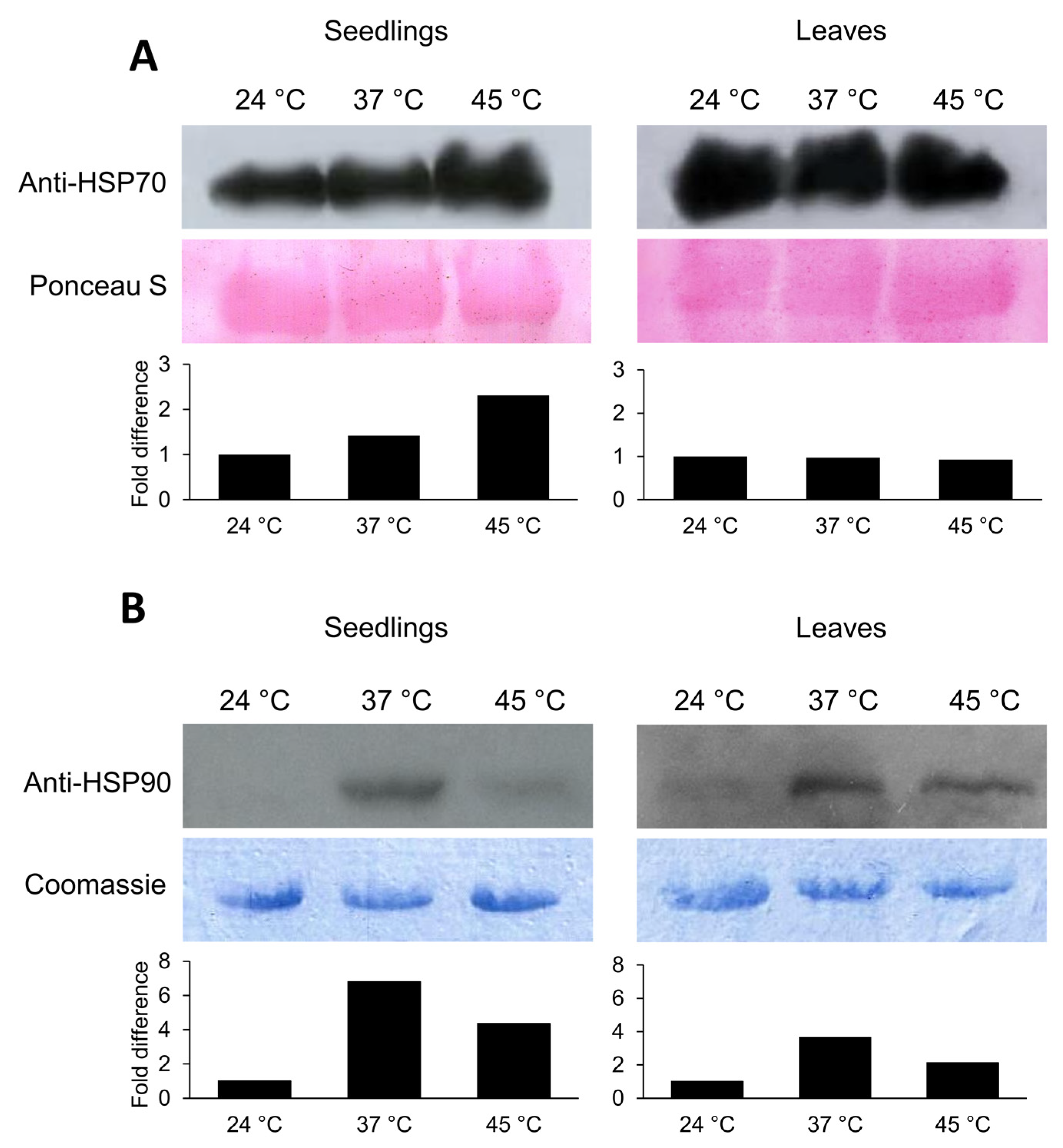
2.5. HSP70 and HSP90 Accumulation after Heat Stress Exposure
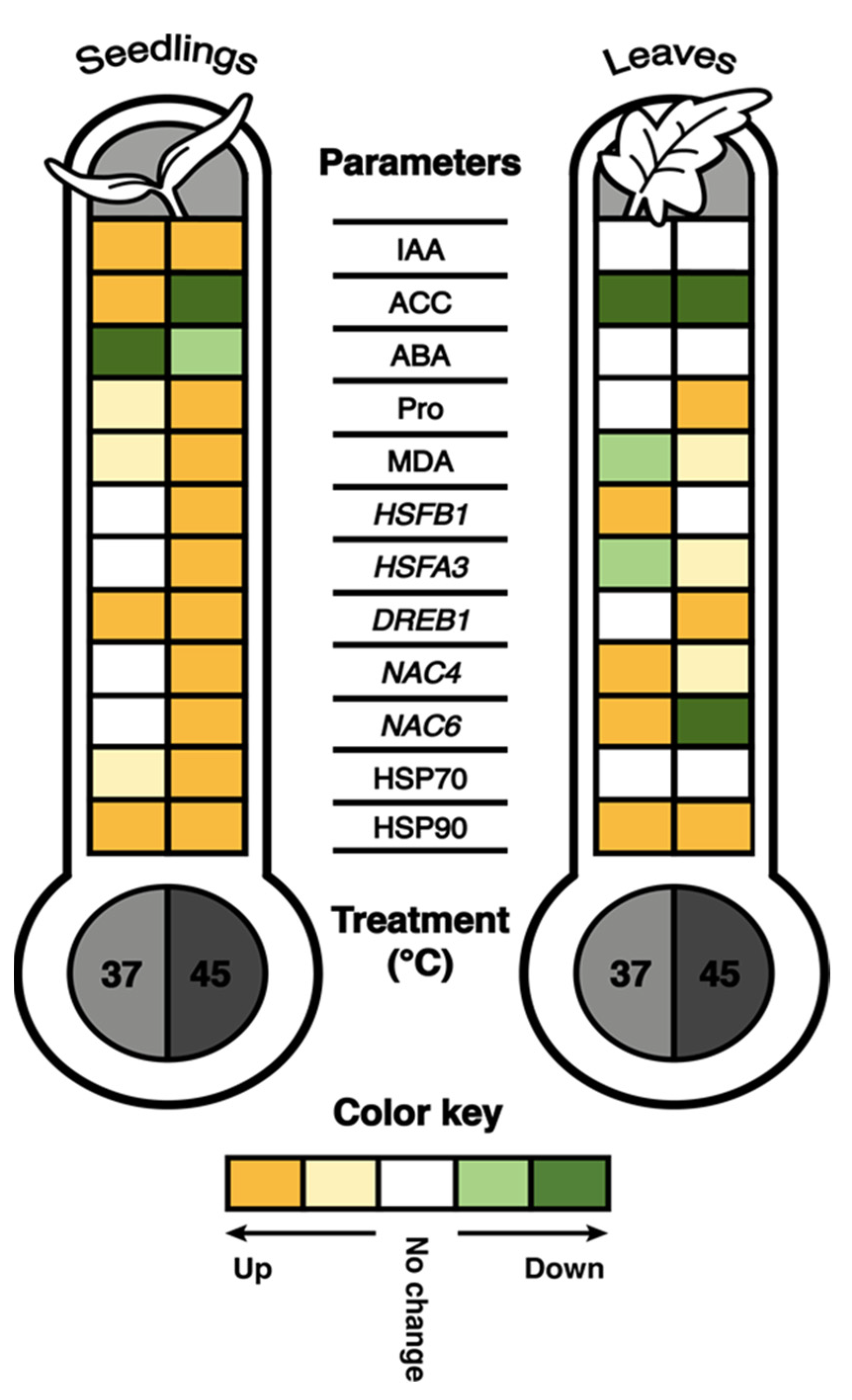
3. Discussion
4. Materials and Methods
4.1. Plant Material and Cultivation
4.2. Heat Stress Procedure
4.3. Plant Growth Measurements
4.4. Phytohormones and Stress Parameters
4.4.1. Phytohormone Content
4.4.2. Proline and Malondialdehyde Content
4.5. RNA Isolation and Gene Expression Analysis
4.6. Heat Shock Protein Detection
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centomani, I.; Sgobba, A.; D’Addabbo, P.; Dipierro, N.; Paradiso, A.; De Gara, L.; Dipierro, S.; Viggiano, L.; de Pinto, M.C. Involvement of DNA Methylation in the Control of Cell Growth during Heat Stress in Tobacco BY-2 Cells. Protoplasma 2015, 252, 1451–1459. [Google Scholar] [CrossRef]
- Van Der Ploeg, A.; Heuvelink, E. Influence of Sub-Optimal Temperature on Tomato Growth and Yield: A Review. J. Hortic. Sci. Biotechnol. 2005, 80, 652–659. [Google Scholar] [CrossRef]
- Ro, S.; Chea, L.; Ngoun, S.; Stewart, Z.P.; Roeurn, S.; Theam, P.; Lim, S.; Sor, R.; Kosal, M.; Roeun, M.; et al. Response of Tomato Genotypes under Different High Temperatures in Field and Greenhouse Conditions. Plants 2021, 10, 449. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat Tolerance in Plants: An Overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Howarth, C.J. Genetic Improvements of Tolerance to High Temperature. In Abiotic Stresses: Plant Resistance Through Breeding and Molecular Approaches; Ashraf, M., Harris, P.J.C., Eds.; Haworth Press Inc.: New York, NY, USA, 2005; pp. 277–300. ISBN 1560229640. [Google Scholar]
- Mittler, R.; Blumwald, E. The Roles of ROS and ABA in Systemic Acquired Acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef]
- Sharma, E.; Sharma, R.; Borah, P.; Jain, M.; Khurana, J.P. Emerging Roles of Auxin in Abiotic Stress Responses. In Elucidation of Abiotic Stress Signaling in Plants: Functional Genomics Perspectives; Springer: New York, NY, USA, 2015; Volume 1, pp. 299–328. ISBN 9781493922116. [Google Scholar]
- Wu, Y.S.; Yang, C.Y. Ethylene-Mediated Signaling Confers Thermotolerance and Regulates Transcript Levels of Heat Shock Factors in Rice Seedlings under Heat Stress. Bot. Stud. 2019, 60, 23–35. [Google Scholar] [CrossRef]
- Li, N.; Euring, D.; Cha, J.Y.; Lin, Z.; Lu, M.; Huang, L.J.; Kim, W.Y. Plant Hormone-Mediated Regulation of Heat Tolerance in Response to Global Climate Change. Front. Plant Sci. 2021, 11, 627969. [Google Scholar] [CrossRef]
- Al-Whaibi, M.H. Plant Heat-Shock Proteins: A Mini Review. J. King Saud Univ.-Sci. 2011, 23, 139–150. [Google Scholar] [CrossRef]
- Benarroch, E.E. Heat Shock Proteins: Multiple Neuroprotective Functions and Implications for Neurologic Disease. Neurology 2011, 76, 660–667. [Google Scholar] [CrossRef]
- Ul Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.X.; Zhang, H.X.; Wei, A.M.; Gong, Z.H. Heat Shock Proteins: Dynamic Biomolecules to Counter Plant Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef]
- Åkerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat Shock Factors: Integrators of Cell Stress, Development and Lifespan. Nat. Rev. Mol. Cell Biol. 2010, 11, 545–555. [Google Scholar] [CrossRef]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and Drought Stresses in Crops and Approaches for Their Mitigation. Front. Chem. 2018, 6, 26–40. [Google Scholar] [CrossRef]
- Lobell, D.B.; Field, C.B. Global Scale Climate-Crop Yield Relationships and the Impacts of Recent Warming. Environ. Res. Lett. 2007, 2, 014002–014009. [Google Scholar] [CrossRef]
- Hoshikawa, K.; Pham, D.; Ezura, H.; Schafleitner, R.; Nakashima, K. Genetic and Molecular Mechanisms Conferring Heat Stress Tolerance in Tomato Plants. Front. Plant Sci. 2021, 12, 786688. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Kjær, K.H.; Rosenqvist, E.; Yu, X.; Wu, Z.; Ottosen, C.O. Physiological Response to Heat Stress During Seedling and Anthesis Stage in Tomato Genotypes Differing in Heat Tolerance. J. Agron. Crop Sci. 2017, 203, 68–80. [Google Scholar] [CrossRef]
- Biggs, M.S.; Woodson, R.W.; Handa, A.K. Biochemical Basis of High-temperature Inhibition of Ethylene Biosynthesis in Ripening Tomato Fruits. Physiol. Plant. 1988, 72, 572–578. [Google Scholar] [CrossRef]
- Lurie, S. The Effect of High Temperature Treatment on Quality of Fruits and Vegetables. Acta Hortic. 2006, 712, 165–174. [Google Scholar] [CrossRef]
- Pan, C.; Ye, L.; Zheng, Y.; Wang, Y.; Yang, D.; Liu, X.; Chen, L.; Zhang, Y.; Fei, Z.; Lu, G. Identification and Expression Profiling of MicroRNAs Involved in the Stigma Exsertion under High-Temperature Stress in Tomato. BMC Genom. 2017, 18, 843. [Google Scholar] [CrossRef]
- Giorno, F.; Wolters-Arts, M.; Mariani, C.; Rieu, I. Ensuring Reproduction at High Temperatures: The Heat Stress Response during Anther and Pollen Development. Plants 2013, 2, 489–506. [Google Scholar] [CrossRef]
- Ito, H.; Kanayama, Y.; Shibuya, T.; Mohammed, S.A.; Nishiyama, M.; Kato, K. Effect of Short-Term Temperature Stress on Fruit Set and the Expression of an Auxin Reporter Gene and Auxin Synthesis Genes in Tomato. Sci. Hortic. 2022, 300, 111039. [Google Scholar] [CrossRef]
- Li, S.; Li, F.; Wang, J.; Zhang, W.; Meng, Q.; Chen, T.H.H.; Murata, N.; Yang, X. Glycinebetaine Enhances the Tolerance of Tomato Plants to High Temperature during Germination of Seeds and Growth of Seedlings. Plant Cell Environ. 2011, 34, 1931–1943. [Google Scholar] [CrossRef] [PubMed]
- Su, P.H.; Li, H.M. Arabidopsis Stromal 70-KD Heat Shock Proteins Are Essential for Plant Development and Important for Thermotolerance of Germinating Seeds. Plant Physiol. 2008, 146, 1231–1241. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, M.K.; Singh, V.; Singh, R.; Raghuvanshi, T.; Singh, C. Debilitation in Tomato (Solanum lycopersicum L.) as Result of Heat Stress. J. Pharmacogn. Phytochem. 2017, 6, 1917–1922. [Google Scholar]
- Gray, W.M.; Östin, A.; Sandberg, G.; Romano, C.P.; Estelle, M. High Temperature Promotes Auxin-Mediated Hypocotyl Elongation in Arabidopsis. Proc. Natl. Acad. Sci. USA 1998, 95, 7197–7202. [Google Scholar] [CrossRef]
- Küpers, J.J.; Oskam, L.; Pierik, R. Photoreceptors Regulate Plant Developmental Plasticity through Auxin. Plants 2020, 9, 940. [Google Scholar] [CrossRef]
- Lynch, J.P. Root Phenotypes for Improved Nutrient Capture: An Underexploited Opportunity for Global Agriculture. N. Phytol. 2019, 223, 548–564. [Google Scholar] [CrossRef]
- Garay-Arroyo, A.; De La Paz Sánchez, M.; García-Ponce, B.; Azpeitia, E.; Álvarez-Buylla, E.R. Hormone Symphony during Root Growth and Development. Dev. Dyn. 2012, 241, 1867–1885. [Google Scholar] [CrossRef] [PubMed]
- Torrey, J.G. Root Hormones and Plant Growth. Annu. Rev. Plant Physiol. 1976, 27, 435–459. [Google Scholar] [CrossRef]
- Du, Y.; Scheres, B. Lateral Root Formation and the Multiple Roles of Auxin. J. Exp. Bot. 2018, 69, 155–167. [Google Scholar] [CrossRef]
- Negi, S.; Sukumar, P.; Liu, X.; Cohen, J.D.; Muday, G.K. Genetic Dissection of the Role of Ethylene in Regulating Auxin-Dependent Lateral and Adventitious Root Formation in Tomato. Plant J. 2010, 61, 3–15. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Fatma, M.; Asgher, M.; Iqbal, N.; Rasheed, F.; Sehar, Z.; Sofo, A.; Khan, N.A. Ethylene Signaling under Stressful Environments: Analyzing Collaborative Knowledge. Plants 2022, 11, 2211. [Google Scholar] [CrossRef]
- Larkindale, J.; Knight, M.R. Protection against Heat Stress-Induced Oxidative Damage in Arabidopsis Involves Calcium, Abscisic Acid, Ethylene, and Salicylic Acid. Plant Physiol. 2002, 128, 682–695. [Google Scholar] [CrossRef]
- Peiser, G.; Yang, S.F. Evidence for 1-(Malonylamino)Cyclopropane-1-Carboxylic Acid Being the Major Conjugate of Aminocyclopropane-1-Carboxylic Acid in Tomato Fruit. Plant Physiol. 1998, 116, 1527–1532. [Google Scholar] [CrossRef][Green Version]
- Staswick, P.E.; Tiryaki, I. The Oxylipin Signal Jasmonic Acid Is Activated by an Enzyme That Conjugates It to Isoleucine in Arabidopsis. Plant Cell 2004, 16, 2117–2127. [Google Scholar] [CrossRef]
- Harris, J.M. Abscisic Acid: Hidden Architect of Root System Structure. Plants 2015, 4, 548–572. [Google Scholar] [CrossRef]
- Lehr, P.P.; Hernández-Montes, E.; Ludwig-Müller, J.; Keller, M.; Zörb, C. Abscisic Acid and Proline Are Not Equivalent Markers for Heat, Drought and Combined Stress in Grapevines. Aust. J. Grape Wine Res. 2022, 28, 119–130. [Google Scholar] [CrossRef]
- Daie, J.; Campbell, W.F. Response of Tomato Plants to Stressful Temperatures. Plant Physiol. 1981, 67, 26–29. [Google Scholar] [CrossRef]
- Claussen, W. Proline as a Measure of Stress in Tomato Plants. Plant Sci. 2005, 168, 241–248. [Google Scholar] [CrossRef]
- Francesca, S.; Vitale, L.; Arena, C.; Raimondi, G.; Olivieri, F.; Cirillo, V.; Paradiso, A.; de Pinto, M.C.; Maggio, A.; Barone, A.; et al. The Efficient Physiological Strategy of a Novel Tomato Genotype to Adapt to Chronic Combined Water and Heat Stress. Plant Biol. 2022, 24, 62–74. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K.; Kern, R.; Mistou, M.Y.; Dandekar, A.M.; Uratsu, S.L.; Richarme, G. The Chemical Chaperone Proline Relieves the Thermosensitivity of a DnaK Deletion Mutant at 42 °C. J. Bacteriol. 2004, 186, 8149–8152. [Google Scholar] [CrossRef]
- Lv, W.T.; Lin, B.; Zhang, M.; Hua, X.J. Proline Accumulation Is Inhibitory to Arabidopsis Seedlings during Heat Stressspi. Plant Physiol. 2011, 156, 1921–1933. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef]
- Kou, X.; Wang, S.; Wu, M.; Guo, R.; Xue, Z.; Meng, N.; Tao, X.; Chen, M.; Zhang, Y. Molecular Characterization and Expression Analysis of NAC Family Transcription Factors in Tomato. Plant Mol. Biol. Report. 2014, 32, 501–516. [Google Scholar] [CrossRef]
- Puranik, S.; Sahu, P.P.; Srivastava, P.S.; Prasad, M. NAC Proteins: Regulation and Role in Stress Tolerance. Trends Plant Sci. 2012, 17, 369–381. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, G.; Zhou, S.; Tu, Y.; Wang, Y.; Dong, T.; Hu, Z. A New Tomato NAC (NAM ATAF1/2/CUC2) Transcription Factor, SlNAC4, Functions as a Positive Regulator of Fruit Ripening and Carotenoid Accumulation. Plant Cell Physiol. 2014, 55, 119–135. [Google Scholar] [CrossRef]
- Zhu, M.; Hu, Z.; Zhou, S.; Wang, L.; Dong, T.; Pan, Y.; Chen, G. Molecular Characterization of Six Tissue-Specific or Stress-Inducible Genes of NAC Transcription Factor Family in Tomato (Solanum lycopersicum). J. Plant Growth Regul. 2014, 33, 730–744. [Google Scholar] [CrossRef]
- Scharf, K.D.; Berberich, T.; Ebersberger, I.; Nover, L. The Plant Heat Stress Transcription Factor (Hsf) Family: Structure, Function and Evolution. Biochim. Biophys. Acta-Gene Regul. Mech. 2012, 1819, 104–119. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, W.; Zhang, H.; Liu, N.; Tian, S. Heat Shock Factors in Tomatoes: Genome-Wide Identification, Phylogenetic Analysis and Expression Profiling under Development and Heat Stress. PeerJ 2016, 2016, e1961. [Google Scholar] [CrossRef] [PubMed]
- Fragkostefanakis, S.; Mesihovic, A.; Simm, S.; Paupière, M.J.; Hu, Y.; Paul, P.; Mishra, S.K.; Tschiersch, B.; Theres, K.; Bovy, A.; et al. HsfA2 Controls the Activity of Developmentally and Stress-Regulated Heat Stress Protection Mechanisms in Tomato Male Reproductive Tissues. Plant Physiol. 2016, 170, 2461–2477. [Google Scholar] [CrossRef]
- Fragkostefanakis, S.; Simm, S.; El-Shershaby, A.; Hu, Y.; Bublak, D.; Mesihovic, A.; Darm, K.; Mishra, S.K.; Tschiersch, B.; Theres, K.; et al. The Repressor and Co-Activator HsfB1 Regulates the Major Heat Stress Transcription Factors in Tomato. Plant Cell Environ. 2019, 42, 874–890. [Google Scholar] [CrossRef]
- Mishra, S.K.; Tripp, J.; Winkelhaus, S.; Tschiersch, B.; Theres, K.; Nover, L.; Scharf, K.D. In the Complex Family of Heat Stress Transcription Factors, HsfA1 Has a Unique Role as Master Regulator of Thermotolerance in Tomato. Genes Dev. 2002, 16, 1555–1567. [Google Scholar] [CrossRef]
- Röth, S.; Mirus, O.; Bublak, D.; Scharf, K.D.; Schleiff, E. DNA-Binding and Repressor Function Are Prerequisites for the Turnover of the Tomato Heat Stress Transcription Factor HsfB1. Plant J. 2017, 89, 31–44. [Google Scholar] [CrossRef]
- Friedrich, T.; Oberkofler, V.; Trindade, I.; Altmann, S.; Brzezinka, K.; Lämke, J.; Gorka, M.; Kappel, C.; Sokolowska, E.; Skirycz, A.; et al. Heteromeric HSFA2/HSFA3 Complexes Drive Transcriptional Memory after Heat Stress in Arabidopsis. Nat. Commun. 2021, 12, 3426. [Google Scholar] [CrossRef]
- Guo, M.; Liu, J.H.; Ma, X.; Luo, D.X.; Gong, Z.H.; Lu, M.H. The Plant Heat Stress Transcription Factors (HSFS): Structure, Regulation, and Function in Response to Abiotic Stresses. Front. Plant Sci. 2016, 7, 114. [Google Scholar] [CrossRef]
- Sakuma, Y.; Maruyama, K.; Qin, F.; Osakabe, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Dual Function of an Arabidopsis Transcription Factor DREB2A in Water-Stress-Responsive and Heat-Stress-Responsive Gene Expression. Proc. Natl. Acad. Sci. USA 2006, 103, 18822–18827. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Wang, A.; Xu, X.; Li, J. Ectopic Overexpression of SlHsfA3, a Heat Stress Transcription Factor from Tomato, Confers Increased Thermotolerance and Salt Hypersensitivity in Germination in Transgenic Arabidopsis. PLoS ONE 2013, 8, e54880. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.; Kieffer, M.; Yu, H.; Kepinski, S.; Estelle, M. HSP90 Regulates Temperature-Dependent Seedling Growth in Arabidopsis by Stabilizing the Auxin Co-Receptor F-Box Protein TIR1. Nat. Commun. 2016, 7, 10269. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant Tolerance to High Temperature in a Changing Environment: Scientific Fundamentals and Production of Heat Stress-Tolerant Crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Li, X.; Ahammed, G.J.; Zhang, Y.Q.; Zhang, G.Q.; Sun, Z.H.; Zhou, J.; Zhou, Y.H.; Xia, X.J.; Yu, J.Q.; Shi, K. Carbon Dioxide Enrichment Alleviates Heat Stress by Improving Cellular Redox Homeostasis through an ABA-Independent Process in Tomato Plants. Plant Biol. 2015, 17, 81–89. [Google Scholar] [CrossRef]
- Mazorra, L.M.; Holton, N.; Bishop, G.J.; Núñez, M. Heat Shock Response in Tomato Brassinosteroid Mutants Indicates That Thermotolerance Is Independent of Brassinosteroid Homeostasis. Plant Physiol. Biochem. 2011, 49, 1420–1428. [Google Scholar] [CrossRef]
- Xu, W.; Cai, S.Y.; Zhang, Y.; Wang, Y.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Reiter, R.J.; et al. Melatonin Enhances Thermotolerance by Promoting Cellular Protein Protection in Tomato Plants. J. Pineal Res. 2016, 61, 457–469. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Rattunde, R.; Rößler, S.; Liedel, K.; Benade, F.; Rost, A.; Becker, J. Two Auxinic Herbicides Affect Brassica Napus Plant Hormone Levels and Induce Molecular Changes in Transcription. Biomolecules 2021, 11, 1153. [Google Scholar] [CrossRef]
- Rawlinson, C.; Kamphuis, L.G.; Gummer, J.P.A.; Singh, K.B.; Trengove, R.D. A Rapid Method for Profiling of Volatile and Semi-Volatile Phytohormones Using Methyl Chloroformate Derivatisation and GC–MS. Metabolomics 2015, 11, 1922–1933. [Google Scholar] [CrossRef]
- Villas-Bôas, S.G.; Delicado, D.G.; Åkesson, M.; Nielsen, J. Simultaneous Analysis of Amino and Nonamino Organic Acids as Methyl Chloroformate Derivatives Using Gas Chromatography-Mass Spectrometry. Anal. Biochem. 2003, 322, 134–138. [Google Scholar] [CrossRef]
- Migowska, N.; Stepnowski, P.; Paszkiewicz, M.; Gołębiowski, M.; Kumirska, J. Trimethylsilyldiazomethane (TMSD) as a New Derivatization Reagent for Trace Analysis of Selected Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) by Gas Chromatography Methods. Anal. Bioanal. Chem. 2010, 397, 3029–3034. [Google Scholar] [CrossRef]
- Cohen, J.D.; Baldi, B.G.; Slovin, J.P. 13C6-[Benzene Ringj-Indole-3-Acetic Acid. Plant Physiol. 1986, 80, 14–19. [Google Scholar] [CrossRef]
- Carillo, P.; Gibon, Y. PROTOCOL: Extraction and Determination of Proline. Prometh. Wiki 2011, 2011. Available online: https://www.researchgate.net/publication/211353600_PROTOCOL_Extraction_and_determination_of_proline (accessed on 19 December 2022).
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Sunkar, R.; Bartels, D.; Kirch, H.H. Overexpression of a Stress-Inducible Aldehyde Dehydrogenase Gene from Arabidopsis Thaliana in Transgenic Plants Improves Stress Tolerance. Plant J. 2003, 35, 452–464. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT–PCR. Nucleic Acids Res. 2001, 29, E45. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, 00341–003412. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Yu, J.Q.; Chen, Z. Role and Regulation of Autophagy in Heat Stress Responses of Tomato Plants. Front. Plant Sci. 2014, 5, 174–186. [Google Scholar] [CrossRef]
- Hichri, I.; Muhovski, Y.; Clippe, A.; Žižková, E.; Dobrev, P.I.; Motyka, V.; Lutts, S. SlDREB2, a Tomato Dehydration-Responsive Element-Binding 2 Transcription Factor, Mediates Salt Stress Tolerance in Tomato and Arabidopsis. Plant Cell Environ. 2016, 39, 62–79. [Google Scholar] [CrossRef]
- Frank, G.; Pressman, E.; Ophir, R.; Althan, L.; Shaked, R.; Freedman, M.; Shen, S.; Firon, N. Transcriptional Profiling of Maturing Tomato (Solanum lycopersicum L.) Microspores Reveals the Involvement of Heat Shock Proteins, ROS Scavengers, Hormones, and Sugars in the Heat Stress Response. J. Exp. Bot. 2009, 60, 3891–3908. [Google Scholar] [CrossRef]
- Staples, R.C.; Stahmann, M.A. Changes in Proteins and Several Enzymes in Susceptible Bean Leaves after Infection by the Bean Rust Fungus. Phytopathology 1964, 54, 760–764. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Taylor, S.C.; Posch, A. The Design of a Quantitative Western Blot Experiment. Biomed Res. Int. 2014, 2014, 361590–361598. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokić, M.; Leljak Levanić, D.; Ludwig-Müller, J.; Bauer, N. Growth and Molecular Responses of Tomato to Prolonged and Short-Term Heat Exposure. Int. J. Mol. Sci. 2023, 24, 4456. https://doi.org/10.3390/ijms24054456
Tokić M, Leljak Levanić D, Ludwig-Müller J, Bauer N. Growth and Molecular Responses of Tomato to Prolonged and Short-Term Heat Exposure. International Journal of Molecular Sciences. 2023; 24(5):4456. https://doi.org/10.3390/ijms24054456
Chicago/Turabian StyleTokić, Mirta, Dunja Leljak Levanić, Jutta Ludwig-Müller, and Nataša Bauer. 2023. "Growth and Molecular Responses of Tomato to Prolonged and Short-Term Heat Exposure" International Journal of Molecular Sciences 24, no. 5: 4456. https://doi.org/10.3390/ijms24054456
APA StyleTokić, M., Leljak Levanić, D., Ludwig-Müller, J., & Bauer, N. (2023). Growth and Molecular Responses of Tomato to Prolonged and Short-Term Heat Exposure. International Journal of Molecular Sciences, 24(5), 4456. https://doi.org/10.3390/ijms24054456





