Heat-Killed Staphylococcus aureus Induces Bone Mass Loss through Telomere Erosion
Abstract
1. Introduction
2. Results
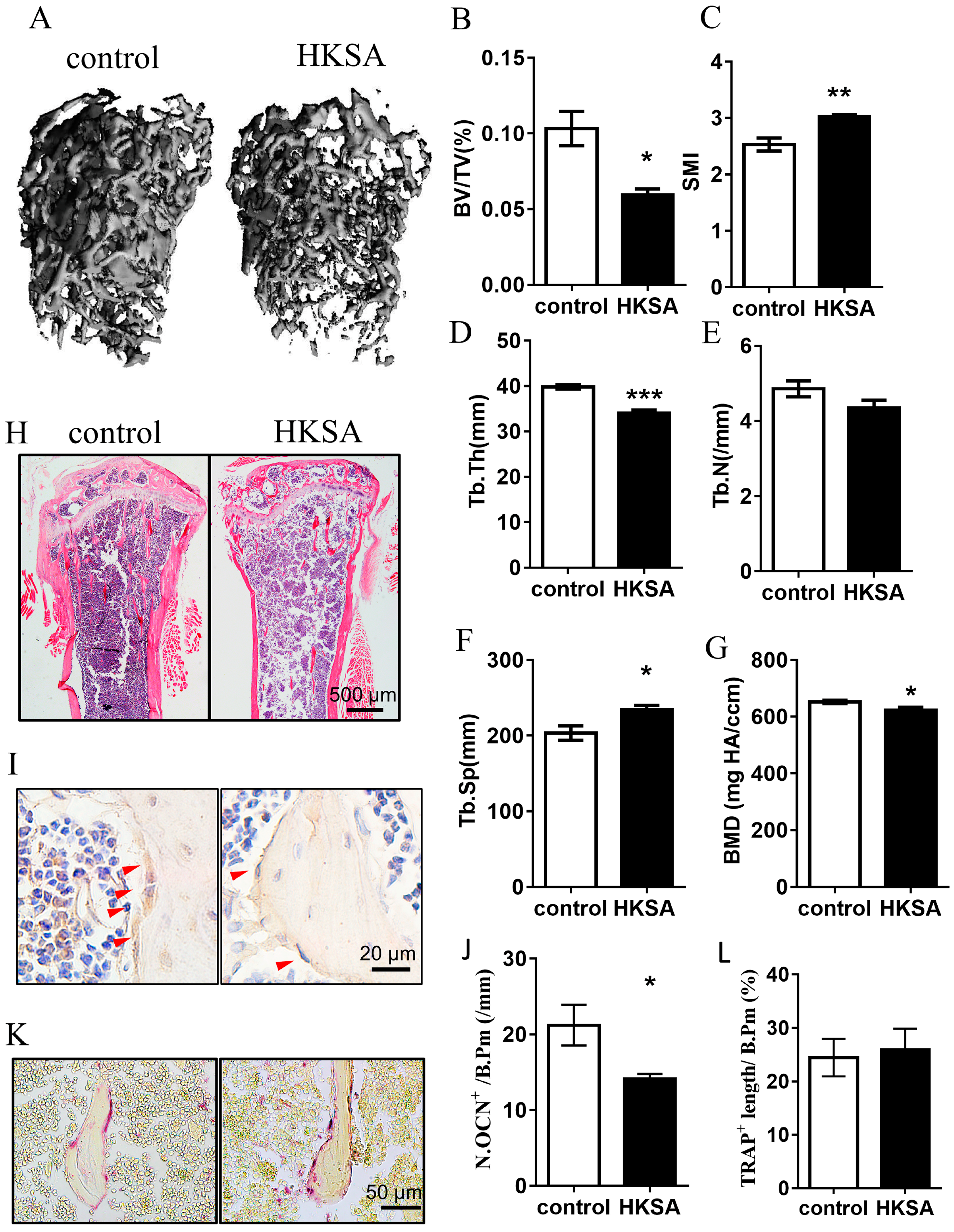
2.1. HKSA Decreased Bone Volume in Mice
2.2. HKSA Induced Cellular Senescence in Bone
2.3. HKSA Caused Telomere Erosion in Cells of Bone
2.4. CAG Protected HKSA-Treated Mice from Telomere Erosion in Cells of Bone
2.5. CAG Protected HKSA-Treated Mice from Cellular Senescence
2.6. CAG Protected HKSA-Treated Mice from Bone Loss
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Preparation of HKSA
4.2. Animals and Experiment Protocol
4.3. Micro-Computed Tomography (μCT) Analysis
4.4. Histochemistry
4.5. SA-β-Galactosidase Staining
4.6. Quantification of TIFs
4.7. Telomere Length Measurement
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hsieh, E.; Shiau, S.; Nolan, O.; Gibert, C.L.; Bedimo, R.J.; Rodriguez-Barradas, M.C.; Justice, A.C.; Womack, J.A.; Yin, M.T. Increased Fragility Fracture Rates in Older Men with Osteomyelitis. Clin. Infect. Dis. 2019, 69, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Tan, Q.; Wei, Z.; Chen, Q.; Wang, C.; Qi, L.; Wen, L.; Zhang, C.; Yao, C. Toll-like receptor 9 deficiency induces osteoclastic bone loss via gut microbiota-associated systemic chronic inflammation. Bone Res. 2022, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Farr, J.N.; Tchkonia, T.; Kirkland, J.L. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 2020, 16, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Liu, S.; Xu, S.; He, Y.; Zhou, X.; Ni, G. Shorter Telomere Length in Peripheral Blood Leukocytes Is Associated with Post-Traumatic Chronic Osteomyelitis. Surg. Infect. 2020, 21, 773–777. [Google Scholar] [CrossRef]
- Roger, L.; Tomas, F.; Gire, V. Mechanisms and Regulation of Cellular Senescence. Int. J. Mol. Sci. 2021, 22, 13173. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Lin, J.; Epel, E. Stress and telomere shortening: Insights from cellular mechanisms. Ageing Res. Rev. 2022, 73, 101507. [Google Scholar] [CrossRef]
- Gao, J.; Pickett, H.A. Targeting telomeres: Advances in telomere maintenance mechanism-specific cancer therapies. Nat. Rev. Cancer 2022, 22, 515–532. [Google Scholar] [CrossRef]
- Victorelli, S.; Passos, J.F. Telomeres and Cell Senescence—Size Matters Not. EBioMedicine 2017, 21, 14–20. [Google Scholar] [CrossRef]
- Jurk, D.; Wilson, C.; Passos, J.F.; Oakley, F.; Correia-Melo, C.; Greaves, L.; Saretzki, G.; Fox, C.; Lawless, C.; Anderson, R.; et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat. Commun. 2014, 2, 4172. [Google Scholar] [CrossRef]
- Kavanagh, N.; Ryan, E.J.; Widaa, A.; Sexton, G.; Fennell, J.; O’Rourke, S.; Cahill, K.C.; Kearney, C.J.; O’Brien, F.J.; Kerrigan, S.W. Staphylococcal Osteomyelitis: Disease Progression, Treatment Challenges, and Future Directions. Clin. Microbiol. Rev. 2018, 31, e00084-17. [Google Scholar] [CrossRef] [PubMed]
- Stafler, P.; Zaks-Hoffer, G.; Scheuerman, O.; Ben-Zvi, H.; Mussaffi, H.; Mei-Zahav, M.; Steuer, G.; Levine, H.; Bar-On, O.; Mantin, H.; et al. Diagnostic value of sputum cultures in children under 2 years of age with chronic suppurative lung diseases. Pediatr. Pulmonol. 2020, 55, 3421–3428. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.C.; Vallejo, J.G.; Kok, E.Y.; Sommer, L.M.; Hultén, K.G.; Kaplan, S.L. Clinical and Microbiologic Variables Predictive of Orthopedic Complications Following Staphylococcus aureus Acute Hematogenous Osteoarticular Infections in Children. Clin. Infect. Dis. 2019, 69, 1955–1961. [Google Scholar] [CrossRef]
- Sousa, M.G.C.; Xavier, P.D.; Cantuária, A.P.C.; Porcino, R.A.; Almeida, J.A.; Franco, O.L.; Rezende, T.M.B. Host defense peptide IDR-1002 associated with ciprofloxacin as a new antimicrobial and immunomodulatory strategy for dental pulp revascularization therapy. Microb. Pathog. 2021, 152, 104634. [Google Scholar] [CrossRef]
- Sun, H.N.; Liu, Y.; Wang, J.N.; Wang, C.; Liu, R.; Kong, L.Z.; Zhen, X.; Chandimali, N.; Cui, Y.D.; Kim, S.U.; et al. Protective Role of Peroxiredoxin I in Heat-Killed Staphylococcus Aureus-infected Mice. In Vivo 2019, 33, 749–755. [Google Scholar] [CrossRef]
- Chen, Y.T.; Du, Y.; Zhao, B.; Gan, L.X.; Yu, K.K.; Sun, L.; Wang, J.; Qian, F. Costunolide alleviates HKSA-induced acute lung injury via inhibition of macrophage activation. Acta Pharmacol. Sin. 2019, 40, 1040–1048. [Google Scholar] [CrossRef]
- Irazoqui, J.E.; Troemel, E.R.; Feinbaum, R.L.; Luhachack, L.G.; Cezairliyan, B.O.; Ausubel, F.M. Distinct pathogenesis and host responses during infection of C. elegans by P. aeruginosa and S. aureus. PLoS Pathog. 2010, 6, e1000982. [Google Scholar] [CrossRef] [PubMed]
- Chau, T.A.; McCully, M.L.; Brintnell, W.; An, G.; Kasper, K.J.; Vinés, E.D.; Kubes, P.; Haeryfar, S.M.; McCormick, J.K.; Cairns, E.; et al. Toll-like receptor 2 ligands on the staphylococcal cell wall downregulate superantigen-induced T cell activation and prevent toxic shock syndrome. Nat. Med. 2009, 15, 641–648. [Google Scholar] [CrossRef]
- Fiedler, T.; Salamon, A.; Adam, S.; Herzmann, N.; Taubenheim, J.; Peters, K. Impact of bacteria and bacterial components on osteogenic and adipogenic differentiation of adipose-derived mesenchymal stem cells. Exp. Cell Res. 2013, 319, 2883–2892. [Google Scholar] [CrossRef]
- Idrees, M.; Kumar, V.; Khan, A.M.; Joo, M.D.; Lee, K.W.; Sohn, S.H.; Kong, I.K. Cycloastragenol activation of telomerase improves β-Klotho protein level and attenuates age-related malfunctioning in ovarian tissues. Mech. Ageing Dev. 2023, 209, 111756. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, Z.; Li, Y.; Qin, H.; Zuo, C.; Zhou, C.; Xu, D. Cycloastragenol protects against glucocorticoid-induced osteogenic differentiation inhibition by activating telomerase. Phytother. Res. 2021, 35, 2034–2044. [Google Scholar] [CrossRef] [PubMed]
- Armour, K.J.; Armour, K.E.; van’t Hof, R.J.; Reid, D.M.; Wei, X.Q.; Liew, F.Y.; Ralston, S.H. Activation of the inducible nitric oxide synthase pathway contributes to inflammation-induced osteoporosis by suppressing bone formation and causing osteoblast apoptosis. Arthritis Rheum. 2001, 44, 2790–2796. [Google Scholar] [CrossRef]
- Ginaldi, L.; Di Benedetto, M.C.; De Martinis, M. Osteoporosis, inflammation and ageing. Immun. Ageing 2005, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.P.; Lerner, U.H. The role of cytokines in inflammatory bone loss. Immunol. Investig. 2013, 42, 555–622. [Google Scholar] [CrossRef] [PubMed]
- Meirow, Y.; Jovanovic, M.; Zur, Y.; Habib, J.; Colombo, D.F.; Twaik, N.; Ashkenazi-Preiser, H.; Ben-Meir, K.; Mikula, I., Jr.; Reuven, O.; et al. Specific inflammatory osteoclast precursors induced during chronic inflammation give rise to highly active osteoclasts associated with inflammatory bone loss. Bone Res. 2022, 10, 36. [Google Scholar] [CrossRef]
- Bonnell, E.; Pasquier, E.; Wellinger, R.J. Telomere Replication: Solving Multiple End Replication Problems. Front. Cell Dev. Biol. 2021, 9, 668171. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Fidan, K.; Um, J.Y.; Ahn, K.S. Telomerase: Key regulator of inflammation and cancer. Pharmacol. Res. 2020, 155, 104726. [Google Scholar] [CrossRef] [PubMed]
- Kamal, S.; Junaid, M.; Ejaz, A.; Bibi, I.; Akash, M.S.H.; Rehman, K. The secrets of telomerase: Retrospective analysis and future prospects. Life Sci. 2020, 257, 118115. [Google Scholar] [CrossRef]
- Li, T.; Zhu, Y.; Lin, C.; Chen, J.; Yin, Y.; Tang, X.; Chen, Y.; Guo, A.; Hu, C. N6-Methyladenosine Modification Profile in Bovine Mammary Epithelial Cells Treated with Heat-Inactivated Staphylococcus aureus. Oxid. Med. Cell. Longev. 2022, 2022, 1704172. [Google Scholar] [CrossRef]
- Borysowski, J.; Wierzbicki, P.; Kłosowska, D.; Korczak-Kowalska, G.; Weber-Dabrowska, B.; Górski, A. The effects of T4 and A3/R phage preparations on whole-blood monocyte and neutrophil respiratory burst. Viral Immunol. 2010, 23, 541–544. [Google Scholar] [CrossRef]
- Penev, A.; Markiewicz-Potoczny, M.; Sfeir, A.; Lazzerini Denchi, E. Stem cells at odds with telomere maintenance and protection. Trends Cell Biol. 2022, 32, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Ghilain, C.; Gilson, E.; Giraud-Panis, M.J. Multifunctionality of the Telomere-Capping Shelterin Complex Explained by Variations in Its Protein Composition. Cells 2021, 10, 1753. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Yao, H.; Lai, J.; Zeng, Y.; Guo, X.; Lin, S.; Hu, W.; Chen, J.; Chen, X. Cycloastragenol Confers Cerebral Protection after Subarachnoid Hemorrhage by Suppressing Oxidative Insults and Neuroinflammation via the SIRT1 Signaling Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 3099409. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, K.; Chen, H.; Zhao, X.; Wang, J.; Li, L.; Cong, Y.; Ju, Z.; Xu, D.; Williams, B.R.; et al. Telomerase Deficiency Causes Alveolar Stem Cell Senescence-associated Low-grade Inflammation in Lungs. J. Biol. Chem. 2015, 290, 30813–30829. [Google Scholar] [CrossRef]
- Ameri, Z.; Ghiasi, S.; Farsinejad, A.; Hassanshahi, G.; Ehsan, M.; Fatemi, A. Telomerase inhibitor MST-312 induces apoptosis of multiple myeloma cells and down-regulation of anti-apoptotic, proliferative and inflammatory genes. Life Sci. 2019, 228, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Hemann, M.T.; Strong, M.A.; Hao, L.Y.; Greider, C.W. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell 2001, 107, 67–77. [Google Scholar] [CrossRef]
- Rossiello, F.; Jurk, D.; Passos, J.F.; d’Adda di Fagagna, F. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 2022, 24, 135–147. [Google Scholar] [CrossRef]
- Frodermann, V.; van Duijn, J.; van Puijvelde, G.H.; van Santbrink, P.J.; Lagraauw, H.M.; de Vries, M.R.; Quax, P.H.; Bot, I.; Foks, A.C.; de Jager, S.C.; et al. Heat-killed Staphylococcus aureus reduces atherosclerosis by inducing anti-inflammatory macrophages. J. Intern. Med. 2016, 279, 592–605. [Google Scholar] [CrossRef]
- Liu, J.; Gao, D.; Dan, J.; Liu, D.; Peng, L.; Zhou, R.; Luo, Y. The protective effect of cycloastragenol on aging mouse circadian rhythmic disorder induced by d-galactose. J. Cell Biochem. 2019, 120, 16408–16415. [Google Scholar] [CrossRef]
- Hewitt, G.; Jurk, D.; Marques, F.D.; Correia-Melo, C.; Hardy, T.; Gackowska, A.; Anderson, R.; Taschuk, M.; Mann, J.; Passos, J.F. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat. Commun. 2012, 3, 708. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Gensch, C.; Pöss, J.; Haendeler, J.; Böhm, M.; Laufs, U. Pioglitazone activates aortic telomerase and prevents stress-induced endothelial apoptosis. Atherosclerosis 2011, 216, 23–34. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, S.; Yang, M.; Su, J.; Cui, N.; Wu, S.; Zhang, G.; Wang, L.; Hou, Y.; Chai, Y.; Yu, B. Heat-Killed Staphylococcus aureus Induces Bone Mass Loss through Telomere Erosion. Int. J. Mol. Sci. 2023, 24, 3179. https://doi.org/10.3390/ijms24043179
Deng S, Yang M, Su J, Cui N, Wu S, Zhang G, Wang L, Hou Y, Chai Y, Yu B. Heat-Killed Staphylococcus aureus Induces Bone Mass Loss through Telomere Erosion. International Journal of Molecular Sciences. 2023; 24(4):3179. https://doi.org/10.3390/ijms24043179
Chicago/Turabian StyleDeng, Songyun, Mankai Yang, Jianwen Su, Naiqian Cui, Siyuan Wu, Guangyan Zhang, Lei Wang, Yilong Hou, Yu Chai, and Bin Yu. 2023. "Heat-Killed Staphylococcus aureus Induces Bone Mass Loss through Telomere Erosion" International Journal of Molecular Sciences 24, no. 4: 3179. https://doi.org/10.3390/ijms24043179
APA StyleDeng, S., Yang, M., Su, J., Cui, N., Wu, S., Zhang, G., Wang, L., Hou, Y., Chai, Y., & Yu, B. (2023). Heat-Killed Staphylococcus aureus Induces Bone Mass Loss through Telomere Erosion. International Journal of Molecular Sciences, 24(4), 3179. https://doi.org/10.3390/ijms24043179




