Current State of Modeling Human Psychiatric Disorders Using Zebrafish
Abstract
:1. Introduction
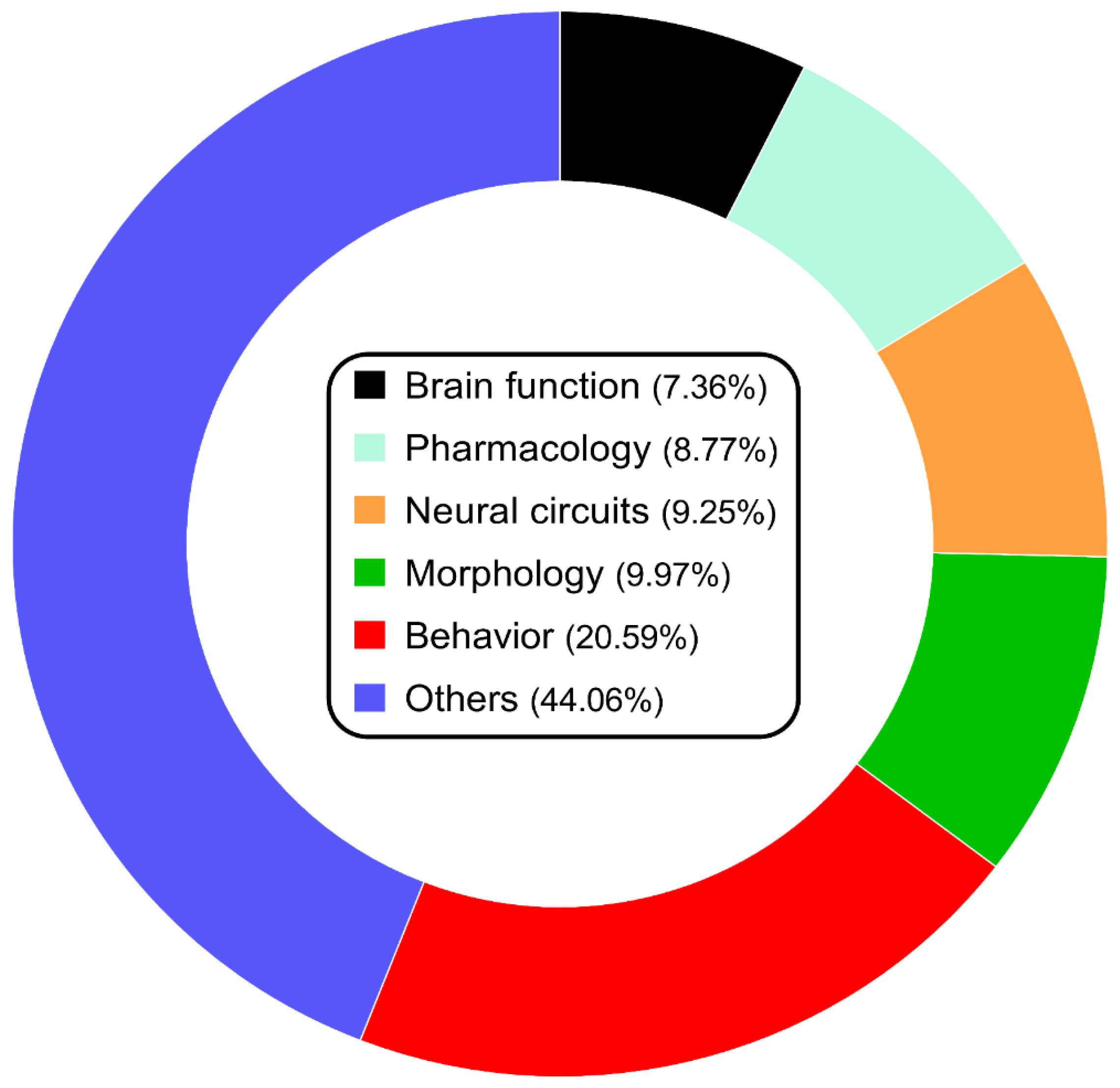
2. Current State of Studying Zebrafish Model of Psychiatric Disorders
3. Case in Point: Molecular Approaches to Modeling CNS Disorders in Zebrafish—Insights from Chronic Unpredictable Stress
4. Discussion: Where Next?
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, Z.; Li, X. Non-human Primate Models for Brain Disorders—Towards Genetic Manipulations via Innovative Technology. Neurosci. Bull. 2017, 33, 247–250. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Schmidt, M.V. Novel experimental models and paradigms for neuropsychiatric disorders: Editorial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1355–1356. [Google Scholar] [CrossRef]
- Stewart, A.M.; Kalueff, A.V. Developing better and more valid animal models of brain disorders. Behav. Brain Res. 2015, 276, 28–31. [Google Scholar] [CrossRef]
- de Hert, M.; Correll, C.U.; Bobes, J.; Cetkovich-Bakmas, M.; Cohen, D.; Asai, I.; Detraux, J.; Gautam, S.; Möller, H.J.; Ndetei, D.M.; et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011, 10, 52–77. [Google Scholar] [CrossRef]
- Demyttenaere, K.; Bruffaerts, R.; Posada-Villa, J.; Gasquet, I.; Kovess, V.; Lepine, J.P.; Angermeyer, M.C.; Bernert, S.; de Girolamo, G.; Morosini, P.; et al. Prevalence, Severity, and Unmet Need for Treatment of Mental Disorders in the World Health Organization World Mental Health Surveys. J. Am. Med. Assoc. 2004, 291, 2581–2590. [Google Scholar]
- McCammon, J.M.; Sive, H. Challenges in understanding psychiatric disorders and developing therapeutics: A role for zebrafish. Dis. Model. Mech. 2015, 8, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Ren-Patterson, R.F.; LaPorte, J.L.; Murphy, D.L. Domain interplay concept in animal models of neuropsychiatric disorders: A new strategy for high-throughput neurophenotyping research. Behav. Brain Res. 2008, 188, 243–249. [Google Scholar] [CrossRef] [PubMed]
- McGonigle, P.; Ruggeri, B. Animal models of human disease: Challenges in enabling translation. Biochem. Pharmacol. 2014, 87, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Hyman, S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010, 13, 1161–1169. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef]
- Curado, S.; Anderson, R.M.; Jungblut, B.; Mumm, J.; Schroeter, E.; Stainier, D.Y. Conditional targeted cell ablation in zebrafish: A new tool for regeneration studies. Dev. Dyn. 2007, 236, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Nagel, G.; Szellas, T.; Huhn, W.; Kateriya, S.; Adeishvili, N.; Berthold, P.; Ollig, D.; Hegemann, P.; Bamberg, E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 2003, 100, 13940–13945. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.P.; Brauner, M.; Liewald, J.F.; Kay, K.; Watzke, N.; Wood, P.G.; Bamberg, E.; Nagel, G.; Gottschalk, A.; et al. Multimodal fast optical interrogation of neural circuitry. Nature 2007, 446, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Higashijima, S.; Masino, M.A.; Mandel, G.; Fetcho, J.R. Imaging neuronal activity during zebrafish behavior with a genetically encoded calcium indicator. J. Neurophysiol. 2003, 90, 3986–3997. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S.; et al. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office on Drugs and Crime. World Drug Report, E.21.XI.8 ed.; Boom Koninklijke Uitgevers: Boston, MA, USA, 2021; Volume 1. [Google Scholar]
- Gonzalez-Nunez, V.; Rodriguez, R.E. The zebrafish: A model to study the endogenous mechanisms of pain. ILAR J. 2009, 50, 373–386. [Google Scholar] [CrossRef]
- Sundstrom, G.; Dreborg, S.; Larhammar, D. Concomitant duplications of opioid peptide and receptor genes before the origin of jawed vertebrates. PLoS ONE 2010, 5, e10512. [Google Scholar] [CrossRef]
- Gonzalez-Nunez, V.; Jimenez Gonzalez, A.; Barreto-Valer, K.; Rodriguez, R.E. In vivo regulation of the mu opioid receptor: Role of the endogenous opioid agents. Mol. Med. 2013, 19, 7–17. [Google Scholar] [CrossRef]
- Krug, R.G., 2nd; Clark, K.J. Elucidating cannabinoid biology in zebrafish (Danio rerio). Gene 2015, 570, 168–179. [Google Scholar] [CrossRef]
- van Staden, C.; de Brouwer, G.; Botha, T.L.; Finger-Baier, K.; Brand, S.J.; Wolmarans, D. Dopaminergic and serotonergic modulation of social reward appraisal in zebrafish (Danio rerio) under circumstances of motivational conflict: Towards a screening test for anti-compulsive drug action. Behav. Brain Res. 2020, 379, 112393. [Google Scholar] [CrossRef]
- Sim, H.R.; Choi, T.Y.; Lee, H.J.; Kang, E.Y.; Yoon, S.; Han, P.L.; Choi, S.Y.; Baik, J.H. Role of dopamine D2 receptors in plasticity of stress-induced addictive behaviours. Nat. Commun. 2013, 4, 1579. [Google Scholar] [CrossRef] [Green Version]
- Diana, M. The dopamine hypothesis of drug addiction and its potential therapeutic value. Front. Psychiatry 2011, 2, 64. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Kysil, E.V.; Meshalkina, D.A.; Frick, E.E.; Echevarria, D.J.; Rosemberg, D.B.; Maximino, C.; Lima, M.G.; Abreu, M.S.; Giacomini, A.C.; Barcellos, L.J.G.; et al. Comparative Analyses of Zebrafish Anxiety-Like Behavior Using Conflict-Based Novelty Tests. Zebrafish 2017, 14, 197–208. [Google Scholar] [CrossRef]
- Maximino, C.; Marques de Brito, T.; Dias, C.A.; Gouveia, A., Jr.; Morato, S. Scototaxis as anxiety-like behavior in fish. Nat. Protoc. 2010, 5, 209–216. [Google Scholar] [CrossRef]
- Stewart, A.M.; Gaikwad, S.; Kyzar, E.; Kalueff, A.V. Understanding spatio-temporal strategies of adult zebrafish exploration in the open field test. Brain Res. 2012, 1451, 44–52. [Google Scholar] [CrossRef]
- Stewart, A.M.; Nguyen, M.; Poudel, M.K.; Warnick, J.E.; Echevarria, D.J.; Beaton, E.A.; Song, C.; Kalueff, A.V. The failure of anxiolytic therapies in early clinical trials: What needs to be done. Expert Opin. Investig. Drugs 2015, 24, 543–556. [Google Scholar] [CrossRef]
- Porter, B.A.; Mueller, T. The zebrafish amygdaloid complex–functional ground plan, molecular delineation, and everted topology. Front. Neurosci. 2020, 14, 608. [Google Scholar] [CrossRef] [PubMed]
- Amo, R.; Aizawa, H.; Takahoko, M.; Kobayashi, M.; Takahashi, R.; Aoki, T.; Okamoto, H. Identification of the zebrafish ventral habenula as a homolog of the mammalian lateral habenula. J. Neurosci. 2010, 30, 1566–1574. [Google Scholar] [CrossRef]
- Mathuru, A.S.; Jesuthasan, S. The medial habenula as a regulator of anxiety in adult zebrafish. Front. Neural Circuits 2013, 7, 99. [Google Scholar] [CrossRef]
- Jesuthasan, S. Fear, anxiety, and control in the zebrafish. Dev. Neurobiol. 2012, 72, 395–403. [Google Scholar] [CrossRef]
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef] [PubMed]
- Demin, K.A.; Taranov, A.S.; Ilyin, N.P.; Lakstygal, A.M.; Volgin, A.D.; de Abreu, M.S.; Strekalova, T.; Kalueff, A.V. Understanding neurobehavioral effects of acute and chronic stress in zebrafish. Stress 2021, 24, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lakstygal, A.M.; de Abreu, M.S.; Lifanov, D.A.; Wappler-Guzzetta, E.A.; Serikuly, N.; Alpsyshov, E.T.; Wang, D.; Wang, M.; Tang, Z.; Yan, D.; et al. Zebrafish models of diabetes-related CNS pathogenesis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 92, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Vermeirssen, E.L.; Scott, A.P. Excretion of free and conjugated steroids in rainbow trout (Oncorhynchus mykiss): Evidence for branchial excretion of the maturation-inducing steroid, 17,20 beta-dihydroxy-4-pregnen-3-one. Gen. Comp. Endocrinol. 1996, 101, 180–194. [Google Scholar] [CrossRef]
- Borba, J.V.; Biasuz, E.; Sabadin, G.R.; Savicki, A.C.; Canzian, J.; Luchiari, A.C.; Adedara, I.A.; Rosemberg, D.B. Influence of acute and unpredictable chronic stress on spatio-temporal dynamics of exploratory activity in zebrafish with emphasis on homebase-related behaviors. Behav. Brain Res. 2022, 435, 114034. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Diseases (ICD) Eleventh Revision; U.S. Department of Health and Human Services: Washington, DC, USA, 2021.
- Demin, K.A.; Lakstygal, A.M.; Krotova, N.A.; Masharsky, A.; Tagawa, N.; Chernysh, M.V.; Ilyin, N.P.; Taranov, A.S.; Galstyan, D.S.; Derzhavina, K.A. Understanding complex dynamics of behavioral, neurochemical and transcriptomic changes induced by prolonged chronic unpredictable stress in zebrafish. Sci. Rep. 2020, 10, 19981. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, J.H.; Duffy, J.F. Circadian Rhythm Sleep Disorders. J. Clin. Outcomes Manag. 2013, 20, 513–528. [Google Scholar]
- Yokogawa, T.; Marin, W.; Faraco, J.; Pezeron, G.; Appelbaum, L.; Zhang, J.; Rosa, F.; Mourrain, P.; Mignot, E. Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants. PLoS Biol. 2007, 5, e277. [Google Scholar] [CrossRef]
- Zhdanova, I.V. Sleep in zebrafish. Zebrafish 2006, 3, 215–226. [Google Scholar] [CrossRef]
- Prober, D.A.; Rihel, J.; Onah, A.A.; Sung, R.J.; Schier, A.F. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J. Neurosci. 2006, 26, 13400–13410. [Google Scholar] [CrossRef]
- Appelbaum, L.; Wang, G.X.; Maro, G.S.; Mori, R.; Tovin, A.; Marin, W.; Yokogawa, T.; Kawakami, K.; Smith, S.J.; Gothilf, Y.; et al. Sleep-wake regulation and hypocretin-melatonin interaction in zebrafish. Proc. Natl. Acad. Sci. USA 2009, 106, 21942–21947. [Google Scholar] [CrossRef] [Green Version]
- Leung, L.C.; Wang, G.X.; Madelaine, R.; Skariah, G.; Kawakami, K.; Deisseroth, K.; Urban, A.E.; Mourrain, P. Neural signatures of sleep in zebrafish. Nature 2019, 571, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.R.; Skariah, G.; Maro, G.S.; Mignot, E.; Mourrain, P. Characterization of two melanin-concentrating hormone genes in zebrafish reveals evolutionary and physiological links with the mammalian MCH system. J. Comp. Neurol. 2009, 517, 695–710. [Google Scholar] [CrossRef]
- Sullivan, P.F.; Kendler, K.S.; Neale, M.C. Schizophrenia as a complex trait: Evidence from a meta-analysis of twin studies. Arch. Gen. Psychiatry 2003, 60, 1187–1192. [Google Scholar] [CrossRef]
- Bailey, A.J.; Braeutigam, S.; Jousmäki, V.; Swithenby, S.J. Abnormal activation of face processing systems at early and intermediate latency in individuals with autism spectrum disorder: A magnetoencephalographic study. Eur. J. Neurosci. 2005, 21, 2575–2585. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R.E.; Daniels, A.M.; Law, J.K.; Law, P.A.; Kaufmann, W.E. Trends in autism spectrum disorder diagnoses: 1994–2007. J. Autism Dev. Disord. 2009, 39, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Banaschewski, T.; Becker, K.; Scherag, S.; Franke, B.; Coghill, D. Molecular genetics of attention-deficit/hyperactivity disorder: An overview. Eur. Child Adolesc. Psychiatry 2010, 19, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, J.M.; Nurnberger, J.I. Polygenic risk scores in psychiatry: Will they be useful for clinicians? F1000 Res. 2019, 8, F1000. [Google Scholar] [CrossRef]
- Palk, A.C.; Dalvie, S.; De Vries, J.; Martin, A.R.; Stein, D.J. Potential use of clinical polygenic risk scores in psychiatry–ethical implications and communicating high polygenic risk. Philos. Ethics Humanit. Med. 2019, 14, 4. [Google Scholar] [CrossRef]
- Levey, D.F.; Stein, M.B.; Wendt, F.R.; Pathak, G.A.; Zhou, H.; Aslan, M.; Quaden, R.; Harrington, K.M.; Nuñez, Y.Z.; Overstreet, C. Bi-ancestral depression GWAS in the Million Veteran Program and meta-analysis in> 1.2 million individuals highlight new therapeutic directions. Nat. Neurosci. 2021, 24, 954–963. [Google Scholar] [CrossRef]
- Adams, M.J.; Howard, D.M.; Luciano, M.; Clarke, T.-K.; Davies, G.; Hill, W.D.; Smith, D.; Deary, I.J.; Porteous, D.J.; McIntosh, A.M. Genetic stratification of depression by neuroticism: Revisiting a diagnostic tradition. Psychol. Med. 2020, 50, 2526–2535. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, T.; Lam, K.; Chen, Y.; Xia, Y.; Liu, C. A decade in psychiatric GWAS research. Mol. Psychiatry 2019, 24, 378–389. [Google Scholar] [CrossRef]
- Mehta, D.; Menke, A.; Binder, E.B. Gene expression studies in major depression. Curr. Psychiatry Rep. 2010, 12, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Shakya, M. Transcriptomics and sequencing analysis of gene expression profiling for major depressive disorder. Indian J. Psychiatry 2021, 63, 549. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, G.J.; Vallender, E.J.; Garrett, M.R.; Challagundla, L.; Overholser, J.C.; Jurjus, G.; Dieter, L.; Syed, M.; Romero, D.G.; Benghuzzi, H. Altered neuro-inflammatory gene expression in hippocampus in major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 82, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.; Penninx, B.; Madar, V.; Xia, K.; Milaneschi, Y.; Hottenga, J.; Hammerschlag, A.; Beekman, A.; Van Der Wee, N.; Smit, J. Gene expression in major depressive disorder. Mol. Psychiatry 2016, 21, 339–347. [Google Scholar] [CrossRef]
- Chang, X.; Liu, Y.; Hahn, C.; Gur, R.; Sleiman, P.; Hakonarson, H. RNA-seq analysis of amygdala tissue reveals characteristic expression profiles in schizophrenia. Transl. Psychiatry 2017, 7, e1203. [Google Scholar] [CrossRef]
- Xu, J.; Sun, J.; Chen, J.; Wang, L.; Li, A.; Helm, M.; Dubovsky, S.L.; Bacanu, S.-A.; Zhao, Z.; Chen, X. RNA-Seq analysis implicates dysregulation of the immune system in schizophrenia. BMC Genom. 2012, 13, S2. [Google Scholar] [CrossRef]
- Myers, A.; McGonigle, P. Overview of transgenic mouse models for Alzheimer’s disease. Curr. Protoc. Neurosci. 2019, 89, e81. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Aβ and synaptic dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, V.; Butler, A.A.; Lubin, F.D. Telencephalon transcriptome analysis of chronically stressed adult zebrafish. Sci. Rep. 2019, 9, 1379. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Fei, F.; Sun, S.; Zhang, D.; Dong, Q.; Wang, X.; Wang, L. Increased anxiety was found in serpini1 knockout zebrafish larval. Biochem. Biophys. Res. Commun. 2021, 534, 1013–1019. [Google Scholar] [CrossRef]
- Genario, R.; de Abreu, M.S.; Giacomini, A.C.; Demin, K.A.; Kalueff, A.V. Sex differences in behavior and neuropharmacology of zebrafish. Eur. J. Neurosci. 2019, 52, 2586–2603. [Google Scholar] [CrossRef]
- Jaffee, S.R.; Price, T.S. Gene–environment correlations: A review of the evidence and implications for prevention of mental illness. Mol. Psychiatry 2007, 12, 432–442. [Google Scholar] [CrossRef]
- Baye, T.M.; Abebe, T.; Wilke, R.A. Genotype–environment interactions and their translational implications. Pers. Med. 2011, 8, 59–70. [Google Scholar] [CrossRef]
- Duncan, L.E.; Pollastri, A.R.; Smoller, J.W. Mind the gap: Why many geneticists and psychological scientists have discrepant views about gene–environment interaction (G × E) research. Am. Psychol. 2014, 69, 249. [Google Scholar] [CrossRef]
- Hein, R.; Beckmann, L.; Chang-Claude, J. Sample size requirements for indirect association studies of gene–environment interactions (G × E). Genet. Epidemiol. Off. Publ. Int. Genet. Epidemiol. Soc. 2008, 32, 235–245. [Google Scholar] [CrossRef]
- Le Strat, Y.; Ramoz, N.; Gorwood, P. The role of genes involved in neuroplasticity and neurogenesis in the observation of a gene-environment interaction (GxE) in schizophrenia. Curr. Mol. Med. 2009, 9, 506–518. [Google Scholar] [CrossRef]
- Kalueff, A.; Wheaton, M.; Murphy, D. What’s wrong with my mouse model?: Advances and strategies in animal modeling of anxiety and depression. Behav. Brain Res. 2007, 179, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Caspi, A.; Sugden, K.; Moffitt, T.E.; Taylor, A.; Craig, I.W.; Harrington, H.; McClay, J.; Mill, J.; Martin, J.; Braithwaite, A. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science 2003, 301, 386–389. [Google Scholar] [CrossRef]
- Ancelin, M.-L.; Ryan, J. 5-HTTLPR× stress hypothesis: Is the debate over? Mol. Psychiatry 2018, 23, 2116–2117. [Google Scholar] [CrossRef] [PubMed]
- Jaaro-Peled, H.; Ayhan, Y.; Pletnikov, M.V.; Sawa, A. Review of pathological hallmarks of schizophrenia: Comparison of genetic models with patients and nongenetic models. Schizophr. Bull. 2010, 36, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Homberg, J.R.; Kyzar, E.J.; Nguyen, M.; Norton, W.H.; Pittman, J.; Poudel, M.K.; Gaikwad, S.; Nakamura, S.; Koshiba, M.; Yamanouchi, H. Understanding autism and other neurodevelopmental disorders through experimental translational neurobehavioral models. Neurosci. Biobehav. Rev. 2016, 65, 292–312. [Google Scholar] [CrossRef]
- Murray, C.J.; Lopez, A.D. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997, 349, 1498–1504. [Google Scholar] [CrossRef]
- Cryan, J.F.; Mombereau, C. In search of a depressed mouse: Utility of models for studying depression-related behavior in genetically modified mice. Mol. Psychiatry 2004, 9, 326–357. [Google Scholar] [CrossRef]
- APA. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Publication: Washington, DC, USA, 2013. [Google Scholar]
- Schmidt, M.V.; Wang, X.-D.; Meijer, O.C. Early life stress paradigms in rodents: Potential animal models of depression? Psychopharmacology 2011, 214, 131–140. [Google Scholar] [CrossRef]
- Huynh, N.N.; McIntyre, R.S. What are the implications of the STAR* D trial for primary care? A review and synthesis. Prim. Care Companion J. Clin. Psychiatry 2008, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.R.; Charney, D.S. Research on major depression: Strategies and priorities. J. Am. Med. Assoc. 2003, 289, 3167–3168. [Google Scholar] [CrossRef]
- Wong, M.-L.; Licinio, J. From monoamines to genomic targets: A paradigm shift for drug discovery in depression. Nat. Rev. Drug Discov. 2004, 3, 136–151. [Google Scholar] [CrossRef]
- Bechtholt, A.J.; Lucki, I. Effects of serotonin-related gene deletion on measures of anxiety, depression, and neurotransmission. Serotonin Recept. 2006, 577–606. [Google Scholar] [CrossRef]
- Mohammad, F.; Ho, J.; Woo, J.H.; Lim, C.L.; Poon, D.J.J.; Lamba, B.; Claridge-Chang, A. Concordance and incongruence in preclinical anxiety models: Systematic review and meta-analyses. Neurosci. Biobehav. Rev. 2016, 68, 504–529. [Google Scholar] [CrossRef]
- Kane, M.J.; Angoa-Peréz, M.; Briggs, D.I.; Sykes, C.E.; Francescutti, D.M.; Rosenberg, D.R.; Kuhn, D.M. Mice genetically depleted of brain serotonin display social impairments, communication deficits and repetitive behaviors: Possible relevance to autism. PLoS ONE 2012, 7, e48975. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Stewart, A.M.; Kalueff, A.V. Aquatic blues: Modeling depression and antidepressant action in zebrafish. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 55, 26–39. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, M.S.; Friend, A.J.; Demin, K.A.; Amstislavskaya, T.G.; Bao, W.; Kalueff, A.V. Zebrafish models: Do we have valid paradigms for depression? J. Pharmacol. Toxicol. Methods 2018, 94, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Demin, K.A.; Kolesnikova, T.O.; Khatsko, S.L.; Meshalkina, D.A.; Efimova, E.V.; Morzherin, Y.Y.; Kalueff, A.V. Acute effects of amitriptyline on adult zebrafish: Potential relevance to antidepressant drug screening and modeling human toxidromes. Neurotoxicol. Teratol. 2017, 62, 27–33. [Google Scholar] [CrossRef]
- Meshalkina, D.A.; Kysil, E.V.; Antonova, K.A.; Demin, K.A.; Kolesnikova, T.O.; Khatsko, S.L.; Gainetdinov, R.R.; Alekseeva, P.A.; Kalueff, A.V. The Effects of Chronic Amitriptyline on Zebrafish Behavior and Monoamine Neurochemistry. Neurochem. Res. 2018, 435, 1191–1199. [Google Scholar] [CrossRef]
- Demin, K.A.; Lakstygal, A.M.; Chernysh, M.V.; Krotova, N.A.; Taranov, A.S.; Ilyin, N.P.; Seredinskaya, M.V.; Tagawa, N.; Savva, A.K.; Mor, M.S. The zebrafish tail immobilization (ZTI) test as a new tool to assess stress-related behavior and a potential screen for drugs affecting despair-like states. J. Neurosci. Methods 2020, 337, 108637. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Demin, K.A.; Kolesnikova, T.O.; Kharsko, S.L.; Zhu, X.; Yuan, X.; Song, C.; Meshalkina, D.A.; Leonard, B.E.; Tian, L. Animal inflammation-based models of depression and their application to drug discovery. Expert Opin. Drug Discov. 2017, 12, 995–1009. [Google Scholar] [CrossRef]
- Demin, K.A.; Kalueff, A.V. Understanding translational and evolutionary conservative molecular biomarkers of affective disorders in the zebrafish, rat and human. In Proceedings of the 28th Multidisciplinary International Neuroscience and Biological Psychiatry Conference “Stress and Behavior”, Saint Petersburg, Russia, 16–18 May 2021. [Google Scholar]
- Belzung, C.; Lemoine, M. Criteria of validity for animal models of psychiatric disorders: Focus on anxiety disorders and depression. Biol. Mood Anxiety Disord. 2011, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, B.-P.; Zhang, Y.-P.; Peng, Z.; Wang, J.; Collier, A.D.; Echevarria, D.J.; Savelieva, K.V.; Lawrence, R.F.; Rex, C.S. Modeling consequences of prolonged strong unpredictable stress in zebrafish: Complex effects on behavior and physiology. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 81, 384–394. [Google Scholar] [CrossRef]
- Piato, Â.L.; Capiotti, K.M.; Tamborski, A.R.; Oses, J.P.; Barcellos, L.J.; Bogo, M.R.; Lara, D.R.; Vianna, M.R.; Bonan, C.D. Unpredictable chronic stress model in zebrafish (Danio rerio): Behavioral and physiological responses. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 561–567. [Google Scholar] [CrossRef]
- Demin, K.A.; Kolesnikova, T.O.; Galstyan, D.S.; Krotova, N.A.; Ilyin, N.P.; Derzhavina, K.A.; Levchenko, N.A.; Strekalova, T.; de Abreu, M.S.; Petersen, E.V. Modulation of behavioral and neurochemical responses of adult zebrafish by fluoxetine, eicosapentaenoic acid and lipopolysaccharide in the prolonged chronic unpredictable stress model. Sci. Rep. 2021, 11, 14289. [Google Scholar] [CrossRef]
- Surget, A.; Wang, Y.; Leman, S.; Ibarguen-Vargas, Y.; Edgar, N.; Griebel, G.; Belzung, C.; Sibille, E. Corticolimbic transcriptome changes are state-dependent and region-specific in a rodent model of depression and of antidepressant reversal. Neuropsychopharmacology 2009, 34, 1363–1380. [Google Scholar] [CrossRef]
- Lisowski, P.; Juszczak, G.R.; Goscik, J.; Wieczorek, M.; Zwierzchowski, L.; Swiergiel, A.H. Effect of chronic mild stress on hippocampal transcriptome in mice selected for high and low stress-induced analgesia and displaying different emotional behaviors. Eur. Neuropsychopharmacol. 2011, 21, 45–62. [Google Scholar] [CrossRef]
- Shen, M.; Song, Z.; Wang, J.-H. microRNA and mRNA profiles in the amygdala are associated with stress-induced depression and resilience in juvenile mice. Psychopharmacology 2019, 236, 2119–2142. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, N.; Smagin, D.; Kovalenko, I.; Galyamina, A.; Vishnivetskaya, G.; Babenko, V.; Orlov, Y.L. Serotonergic genes in the development of anxiety/depression-like state and pathology of aggressive behavior in male mice: RNA-seq data. Mol. Biol. 2017, 51, 251–262. [Google Scholar] [CrossRef]
- Andrus, B.; Blizinsky, K.; Vedell, P.; Dennis, K.; Shukla, P.; Schaffer, D.; Radulovic, J.; Churchill, G.A.; Redei, E. Gene expression patterns in the hippocampus and amygdala of endogenous depression and chronic stress models. Mol. Psychiatry 2012, 17, 49–61. [Google Scholar] [CrossRef]
- Carboni, L.; Marchetti, L.; Lauria, M.; Gass, P.; Vollmayr, B.; Redfern, A.; Jones, L.; Razzoli, M.; Malki, K.; Begni, V. Cross-species evidence from human and rat brain transcriptome for growth factor signaling pathway dysregulation in major depression. Neuropsychopharmacology 2018, 43, 2134–2145. [Google Scholar] [CrossRef]
- Freedman, L.P.; Cockburn, I.M.; Simcoe, T.S. The economics of reproducibility in preclinical research. PLoS Biol. 2015, 13, e1002165. [Google Scholar] [CrossRef] [PubMed]
- Jaric, I.; Voelkl, B.; Clerc, M.; Schmid, M.W.; Novak, J.; Rosso, M.; Rufener, R.; von Kortzfleisch, V.T.; Richter, S.H.; Buettner, M. The rearing environment persistently modulates mouse phenotypes from the molecular to the behavioural level. PLoS Biol. 2022, 20, e3001837. [Google Scholar] [CrossRef]
- Hackam, D.G.; Redelmeier, D.A. Translation of research evidence from animals to humans. J. Am. Med. Assoc. 2006, 296, 1727–1732. [Google Scholar] [CrossRef]
- Kola, I.; Landis, J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 2004, 3, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Pound, P.; Bracken, M.B. Is animal research sufficiently evidence based to be a cornerstone of biomedical research? BMJ 2014, 348, g3387. [Google Scholar] [CrossRef]
- Lowenstein, P.R.; Castro, M.G. Uncertainty in the translation of preclinical experiments to clinical trials. Why do most phase III clinical trials fail? Curr. Gene Ther. 2009, 9, 368–374. [Google Scholar] [CrossRef]
- Demin, K.A.; Krotova, N.A.; Ilyin, N.P.; Galstyan, D.S.; Kolesnikova, T.O.; Strekalova, T.; de Abreu, M.S.; Petersen, E.V.; Zabegalov, K.N.; Kalueff, A.V. Evolutionarily conserved gene expression patterns for affective disorders revealed using cross-species brain transcriptomic analyses in humans, rats and zebrafish. Sci. Rep. 2022, 12, 20836. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.F.; Neale, M.C.; Kendler, K.S. Genetic epidemiology of major depression: Review and meta-analysis. Am. J. Psychiatry 2000, 157, 1552–1562. [Google Scholar] [CrossRef]
- Bosker, F.; Hartman, C.; Nolte, I.; Prins, B.; Terpstra, P.; Posthuma, D.; Van Veen, T.; Willemsen, G.; DeRijk, R.; De Geus, E. Poor replication of candidate genes for major depressive disorder using genome-wide association data. Mol. Psychiatry 2011, 16, 516–532. [Google Scholar] [CrossRef]
- Griffiths, B.; Schoonheim, P.J.; Ziv, L.; Voelker, L.; Baier, H.; Gahtan, E. A zebrafish model of glucocorticoid resistance shows serotonergic modulation of the stress response. Front. Behav. Neurosci. 2012, 6, 68. [Google Scholar] [CrossRef]
- Ziv, L.; Muto, A.; Schoonheim, P.J.; Meijsing, S.H.; Strasser, D.; Ingraham, H.A.; Schaaf, M.J.; Yamamoto, K.R.; Baier, H. An affective disorder in zebrafish with mutation of the glucocorticoid receptor. Mol. Psychiatry 2013, 18, 681. [Google Scholar] [CrossRef]
- Fjose, A.; Zhao, X.-F. Inhibition of the microRNA pathway in zebrafish by siRNA. RNA Ther. 2010, 629, 237–253. [Google Scholar]
- Wang, L.; Zhou, J.-Y.; Yao, J.-H.; Lu, D.-R.; Qiao, X.-J.; Jia, W. U6 promoter-driven siRNA injection has nonspecific effects in zebrafish. Biochem. Biophys. Res. Commun. 2010, 391, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, X.; Gao, L.; Meng, L.-Y.; Xie, J.-M.; Xiong, J.-W.; Luo, Y. Nanoparticle-mediated delivery of siRNA into zebrafish heart: A cell-level investigation on the biodistribution and gene silencing effects. Nanoscale 2019, 11, 18052–18064. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, F.; Hou, J.; Zhu, X.; Luo, Y.; Xiong, J.-W. Nanoparticle-mediated sirna gene-silencing in adult zebrafish heart. J. Vis. Exp. 2018, 137, e58054. [Google Scholar]
- Shin, L.M.; Liberzon, I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010, 35, 169–191. [Google Scholar] [CrossRef]
- Felix-Ortiz, A.C.; Tye, K.M. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J. Neurosci. 2014, 34, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Duval, E.R.; Javanbakht, A.; Liberzon, I. Neural circuits in anxiety and stress disorders: A focused review. Ther. Clin. Risk Manag. 2015, 11, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Dougnon, G.; Matsui, H. Modelling Autism Spectrum Disorder (ASD) and Attention-Deficit/Hyperactivity Disorder (ADHD) Using Mice and Zebrafish. Int. J. Mol. Sci. 2022, 23, 7550. [Google Scholar] [CrossRef] [PubMed]
- Zabegalov, K.N.; Khatsko, S.L.; Lakstyga, A.M.; Demin, K.A.; Cleal, M.; Fontana, B.D.; McBride, S.D.; Harvey, B.H.; De Abreu, M.S.; Parker, M.O. Abnormal repetitive behaviors in zebrafish and their relevance to human brain disorders. Behav. Brain Res. 2019, 367, 101–110. [Google Scholar] [CrossRef]
- Patowary, A.; Won, S.Y.; Oh, S.J.; Nesbitt, R.R.; Archer, M.; Nickerson, D.; Raskind, W.H.; Bernier, R.; Lee, J.E.; Brkanac, Z. Family-based exome sequencing and case-control analysis implicate CEP41 as an ASD gene. Transl. Psychiatry 2019, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Ruzzo, E.K.; Pérez-Cano, L.; Jung, J.-Y.; Wang, L.-k.; Kashef-Haghighi, D.; Hartl, C.; Singh, C.; Xu, J.; Hoekstra, J.N.; Leventhal, O. Inherited and de novo genetic risk for autism impacts shared networks. Cell 2019, 178, 850–866.e26. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.-H.; Cho, H.-J.; Han, E.; Hong, T.I.; Ariyasiri, K.; Choi, J.-H.; Hwang, K.-S.; Jeong, Y.-M.; Yang, S.-Y.; Yu, K. Zebrafish knockout of Down syndrome gene, DYRK1A, shows social impairments relevant to autism. Mol. Autism 2017, 8, 50. [Google Scholar] [CrossRef]
- Dalla Vecchia, E.; Di Donato, V.; Young, A.M.; Del Bene, F.; Norton, W.H. Reelin signaling controls the preference for social novelty in zebrafish. Front. Behav. Neurosci. 2019, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-X.; Li, C.-Y.; Hu, C.-C.; Wang, Y.; Lin, J.; Jiang, Y.-H.; Li, Q.; Xu, X. CRISPR/Cas9-induced shank3b mutant zebrafish display autism-like behaviors. Mol. Autism 2018, 9, 23. [Google Scholar] [CrossRef]
- Kozol, R.A.; Cukier, H.N.; Zou, B.; Mayo, V.; De Rubeis, S.; Cai, G.; Griswold, A.J.; Whitehead, P.L.; Haines, J.L.; Gilbert, J.R. Two knockdown models of the autism genes SYNGAP1 and SHANK3 in zebrafish produce similar behavioral phenotypes associated with embryonic disruptions of brain morphogenesis. Hum. Mol. Genet. 2015, 24, 4006–4023. [Google Scholar] [CrossRef] [PubMed]
- Fontana, B.D.; Franscescon, F.; Rosemberg, D.B.; Norton, W.H.; Kalueff, A.V.; Parker, M.O. Zebrafish models for attention deficit hyperactivity disorder (ADHD). Neurosci. Biobehav. Rev. 2019, 100, 9–18. [Google Scholar] [CrossRef]
- Yang, L.; Chang, S.; Lu, Q.; Zhang, Y.; Wu, Z.; Sun, X.; Cao, Q.; Qian, Y.; Jia, T.; Xu, B. A new locus regulating MICALL2 expression was identified for association with executive inhibition in children with attention deficit hyperactivity disorder. Mol. Psychiatry 2018, 23, 1014–1020. [Google Scholar] [CrossRef]
- Lange, M.; Froc, C.; Grunwald, H.; Norton, W.H.J.; Bally-Cuif, L. Pharmacological analysis of zebrafish lphn3. 1 morphant larvae suggests that saturated dopaminergic signaling could underlie the ADHD-like locomotor hyperactivity. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 84, 181–189. [Google Scholar] [CrossRef]
- Hoffman, E.J.; Turner, K.J.; Fernandez, J.M.; Cifuentes, D.; Ghosh, M.; Ijaz, S.; Jain, R.A.; Kubo, F.; Bill, B.R.; Baier, H. Estrogens suppress a behavioral phenotype in zebrafish mutants of the autism risk gene, CNTNAP2. Neuron 2016, 89, 725–733. [Google Scholar] [CrossRef]
- Huang, J.; Zhong, Z.; Wang, M.; Chen, X.; Tan, Y.; Zhang, S.; He, W.; He, X.; Huang, G.; Lu, H. Circadian modulation of dopamine levels and dopaminergic neuron development contributes to attention deficiency and hyperactive behavior. J. Neurosci. 2015, 35, 2572–2587. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Collins, C.; Kyzar, E.J.; Pham, M.; Roth, A.; Gaikwad, S.; Cachat, J.; Stewart, A.M.; Landsman, S.; Grieco, F. Automated high-throughput neurophenotyping of zebrafish social behavior. J. Neurosci. Methods 2012, 210, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.M.; Grieco, F.; Tegelenbosch, R.A.; Kyzar, E.J.; Nguyen, M.; Kaluyeva, A.; Song, C.; Noldus, L.P.; Kalueff, A.V. A novel 3D method of locomotor analysis in adult zebrafish: Implications for automated detection of CNS drug-evoked phenotypes. J. Neurosci. Methods 2015, 255, 66–74. [Google Scholar] [CrossRef]
- Romero-Ferrero, F.; Bergomi, M.G.; Hinz, R.C.; Heras, F.J.; De Polavieja, G.G. Idtracker. ai: Tracking all individuals in small or large collectives of unmarked animals. Nat. Methods 2019, 16, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Bozhko, D.V.; Myrov, V.O.; Kolchanova, S.M.; Polovian, A.I.; Galumov, G.K.; Demin, K.A.; Zabegalov, K.N.; Strekalova, T.; de Abreu, M.S.; Petersen, E.V. Artificial intelligence-driven phenotyping of zebrafish psychoactive drug responses. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 112, 110405. [Google Scholar] [CrossRef]
- Fontana, B.D.; Gibbon, A.J.; Cleal, M.; Norton, W.H.; Parker, M.O. Chronic unpredictable early-life stress (CUELS) protocol: Early-life stress changes anxiety levels of adult zebrafish. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 108, 110087. [Google Scholar] [CrossRef]
- Eachus, H.; Choi, M.-K.; Ryu, S. The effects of early life stress on the brain and behaviour: Insights from zebrafish models. Front. Cell Dev. Biol. 2021, 9, 1209. [Google Scholar] [CrossRef] [PubMed]

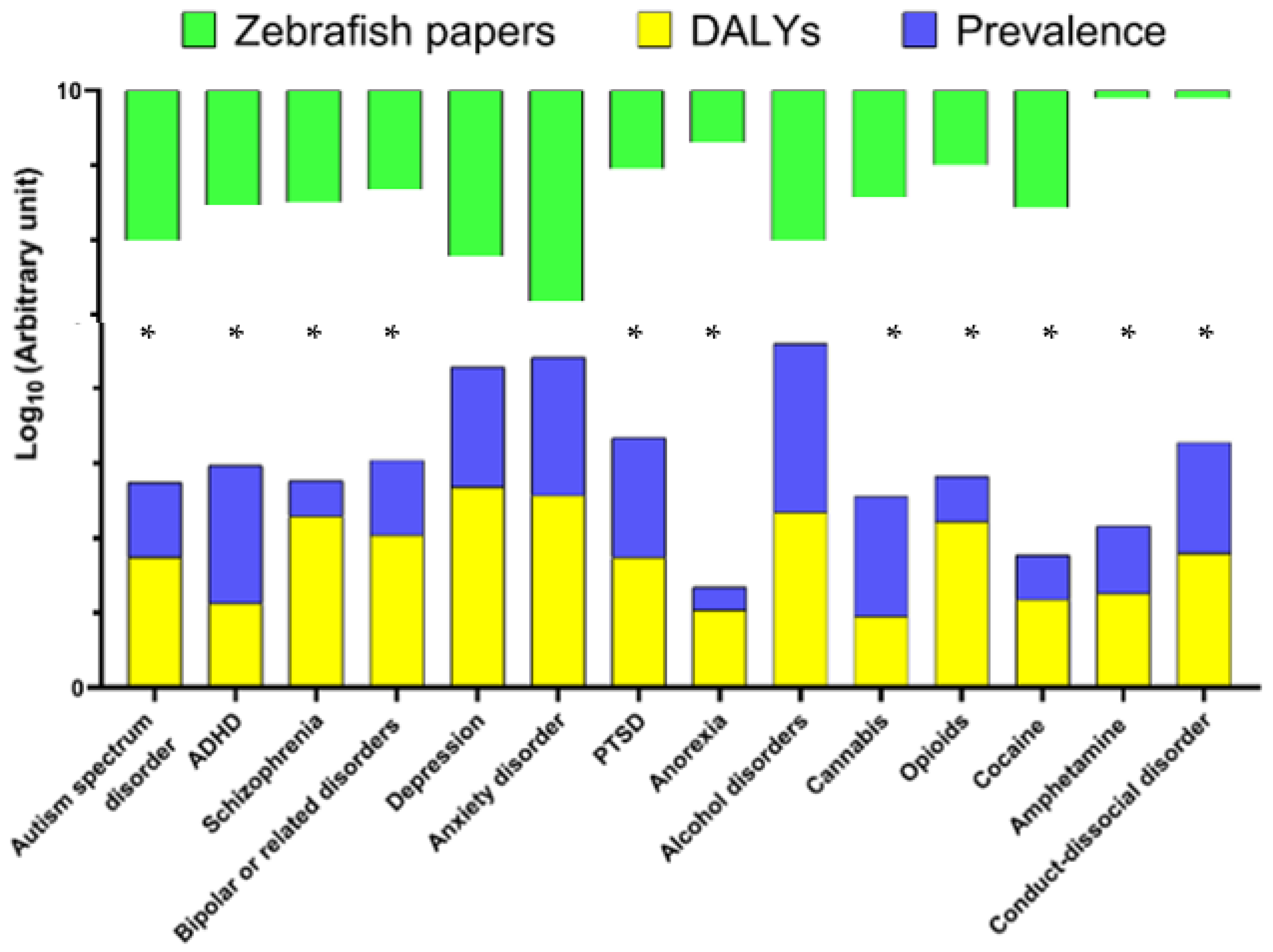
| Human Psychiatric Disorders | DALYs (100.000 Individuals) | Global Prevalence, % | Zebrafish Papers in Pubmed (n) |
|---|---|---|---|
| Autism spectrum disorder | 55.66 | 1 | 103 |
| ADHD | 13.32 | 7 | 34 |
| Schizophrenia | 195.27 | 0.3 | 31 |
| Bipolar or related disorders | 109.89 | 1 | 21 |
| Depression | 480.81 | 4 | 167 |
| Anxiety disorder * | 370.61 | 7 | 665 |
| PTSD | 55 | 4 | 11 |
| Anorexia | 10.96 | 0.2 | 5 |
| Alcohol | 219.96 | 18 | 103 |
| Cannabis | 8.92 | 4 | 27 |
| Opioids | 167.07 | 0.4 | 10 |
| Cocaine | 14.9 | 0.4 | 37 |
| Amphetamine | 18.08 | 0.8 | 1 |
| Conduct-dissocial disorder | 62.97 | 3 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, F.V.; Kolesnikova, T.O.; Galstyan, D.S.; Ilyin, N.P.; de Abreu, M.S.; Petersen, E.V.; Demin, K.A.; Yenkoyan, K.B.; Kalueff, A.V. Current State of Modeling Human Psychiatric Disorders Using Zebrafish. Int. J. Mol. Sci. 2023, 24, 3187. https://doi.org/10.3390/ijms24043187
Costa FV, Kolesnikova TO, Galstyan DS, Ilyin NP, de Abreu MS, Petersen EV, Demin KA, Yenkoyan KB, Kalueff AV. Current State of Modeling Human Psychiatric Disorders Using Zebrafish. International Journal of Molecular Sciences. 2023; 24(4):3187. https://doi.org/10.3390/ijms24043187
Chicago/Turabian StyleCosta, Fabiano V., Tatiana O. Kolesnikova, David S. Galstyan, Nikita P. Ilyin, Murilo S. de Abreu, Elena V. Petersen, Konstantin A. Demin, Konstantin B. Yenkoyan, and Allan V. Kalueff. 2023. "Current State of Modeling Human Psychiatric Disorders Using Zebrafish" International Journal of Molecular Sciences 24, no. 4: 3187. https://doi.org/10.3390/ijms24043187
APA StyleCosta, F. V., Kolesnikova, T. O., Galstyan, D. S., Ilyin, N. P., de Abreu, M. S., Petersen, E. V., Demin, K. A., Yenkoyan, K. B., & Kalueff, A. V. (2023). Current State of Modeling Human Psychiatric Disorders Using Zebrafish. International Journal of Molecular Sciences, 24(4), 3187. https://doi.org/10.3390/ijms24043187








