Neonatal Plasma Exosomes Contribute to Endothelial Cell-Mediated Angiogenesis and Cardiac Repair after Acute Myocardial Infarction
Abstract
1. Introduction
2. Results
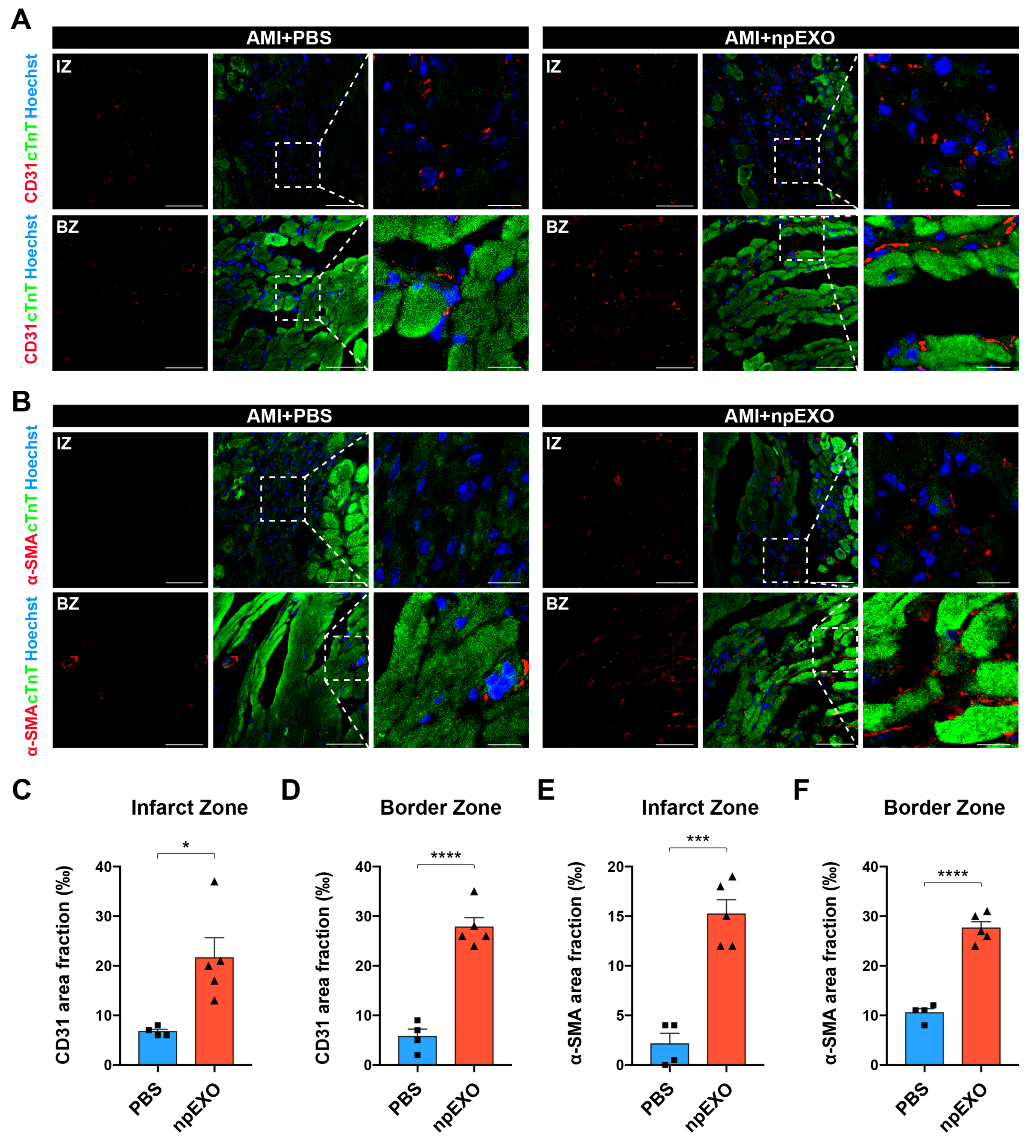
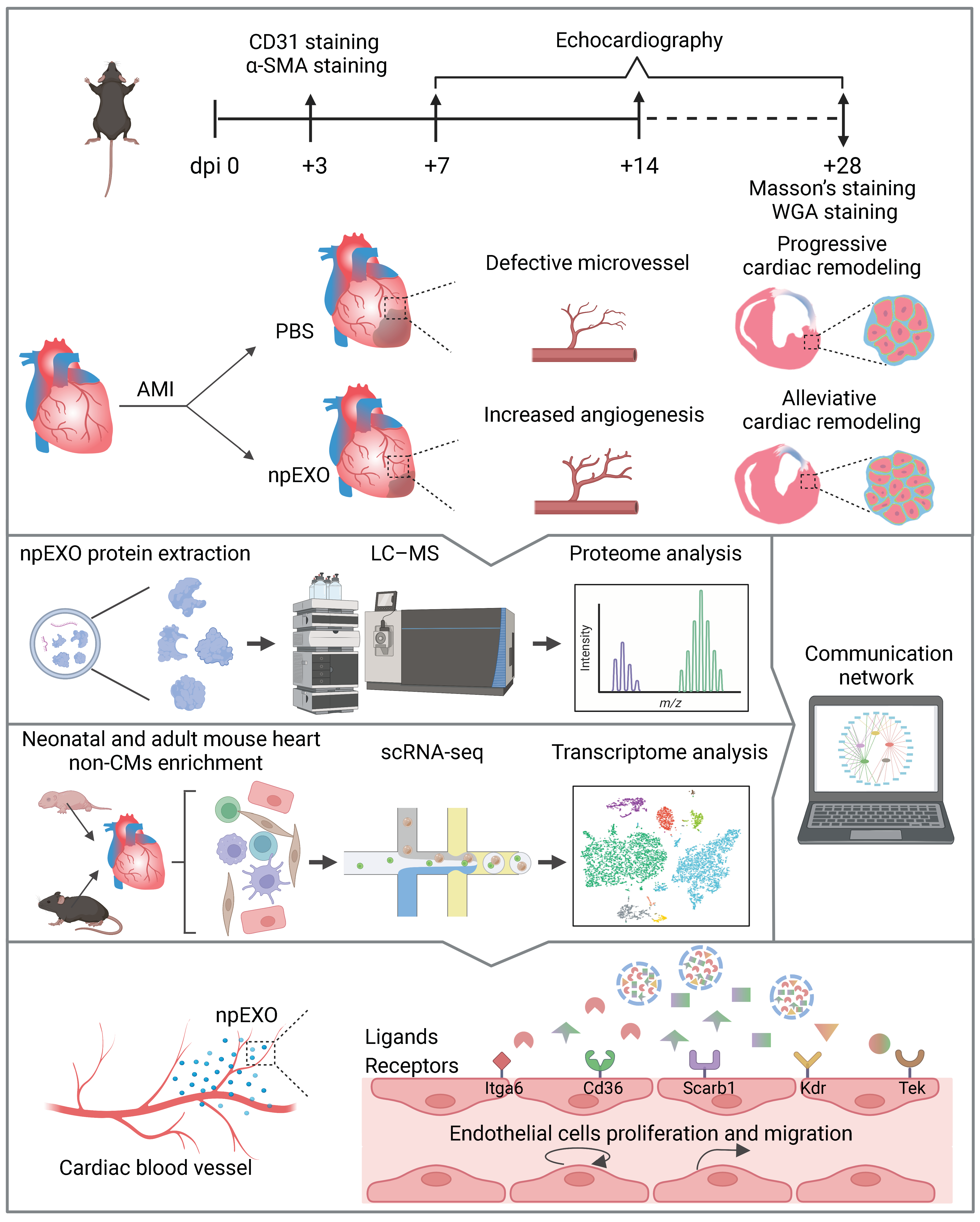
2.1. Plasma Exosomes from Neonatal Mice (npEXO) Facilitated the Structural and Functional Recovery of Adult Hearts after Acute Myocardial Infarction (AMI)
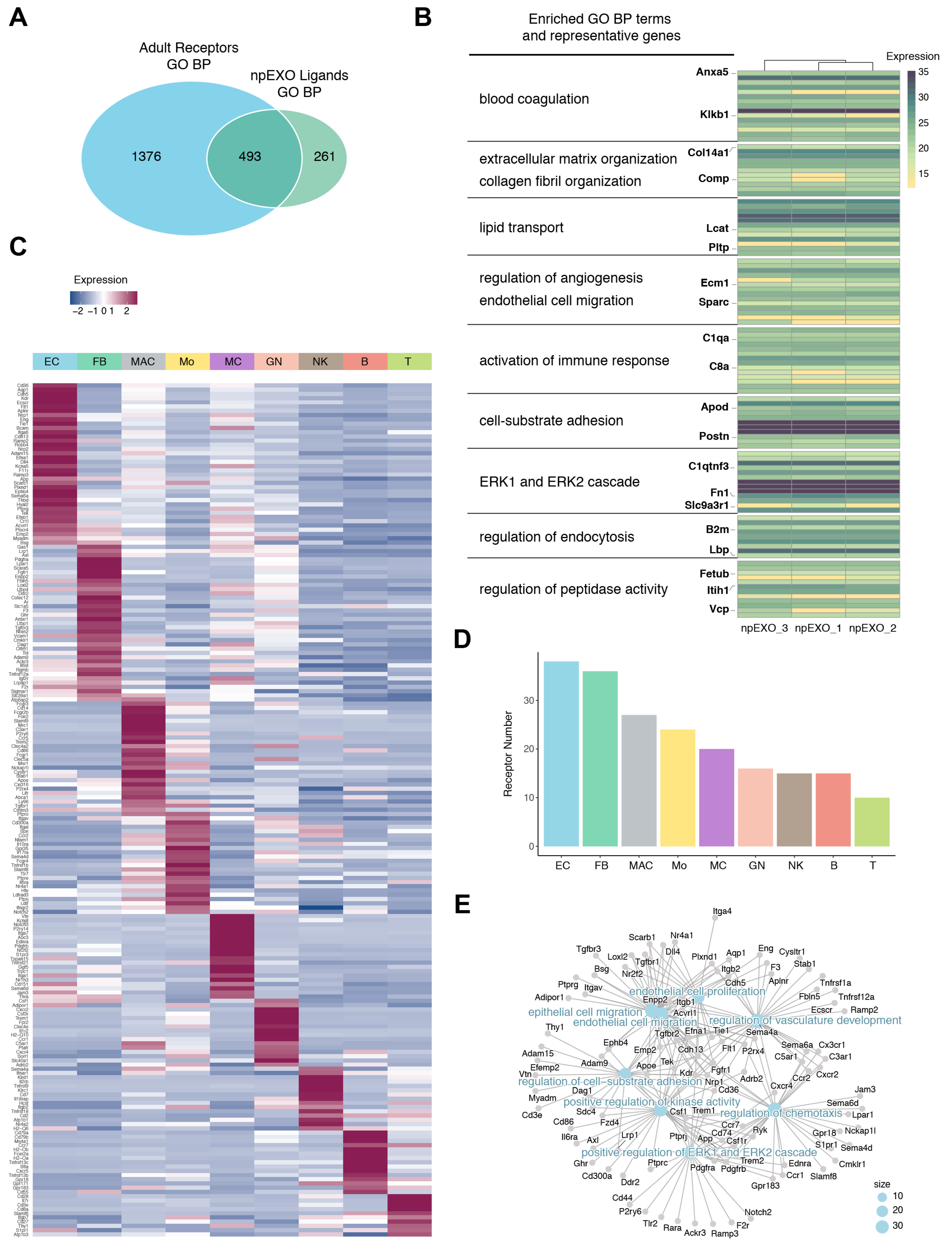
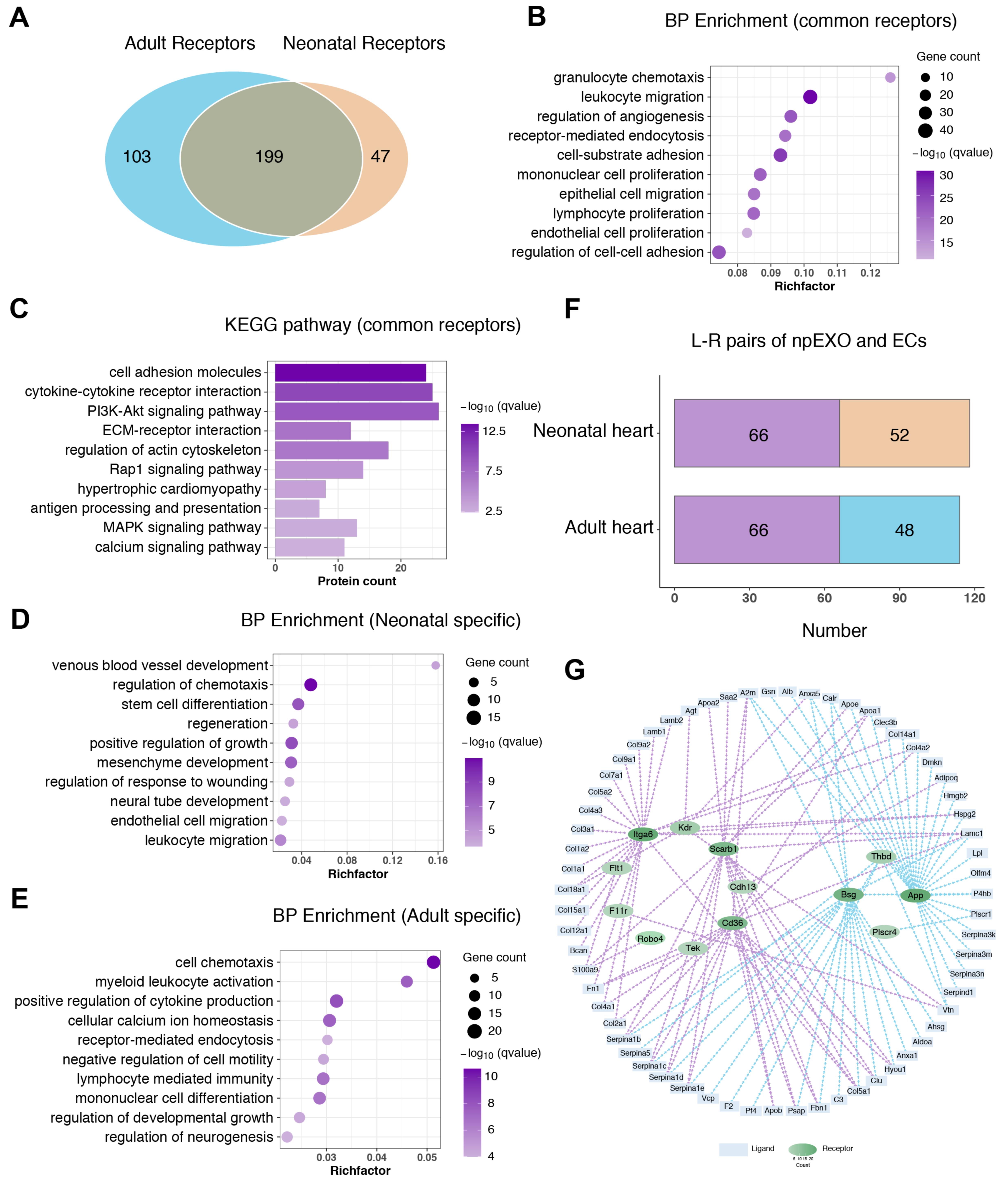
2.2. Functional Enrichment and Pathway Analyses of the npEXO Proteins
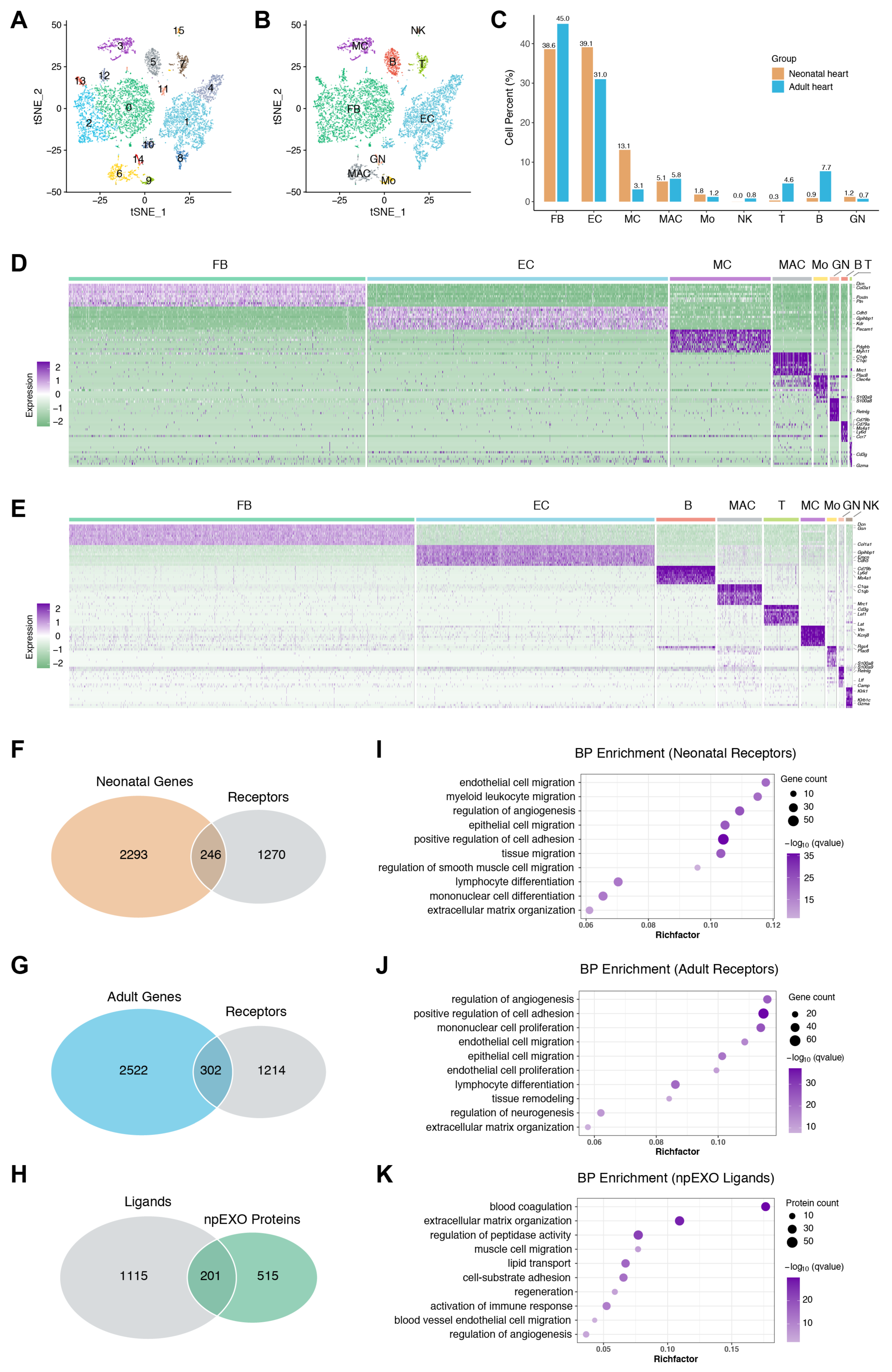
2.3. The Endothelial cells (ECs) as the Main Non-Cardiomyocyte (non-CM) Cell Type Was Responsible for Receiving npEXO Signals in the Mouse Heart
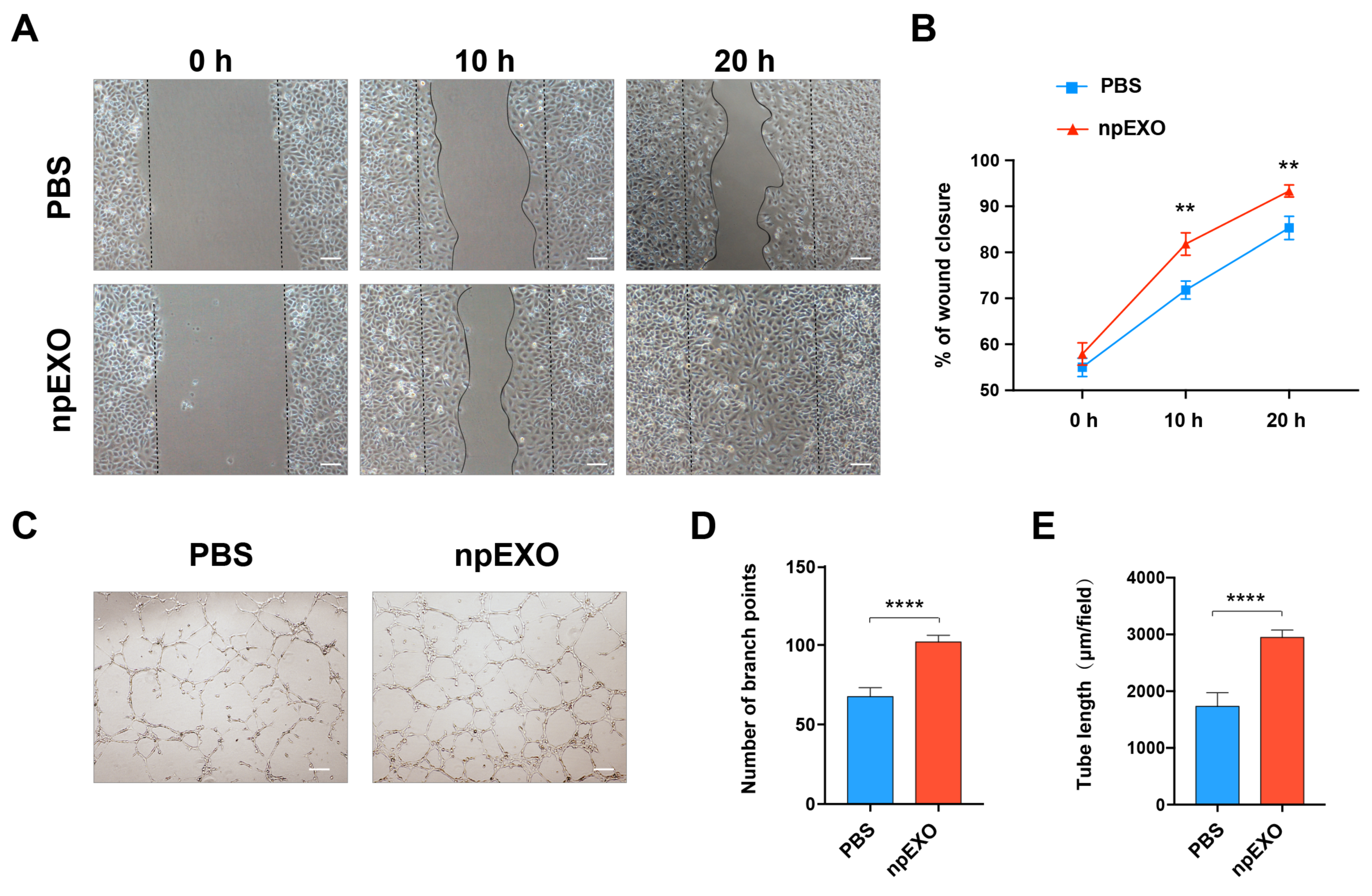
2.4. npEXO Protected the Infarcted Hearts by Inducing EC Behaviors and Angiogenesis
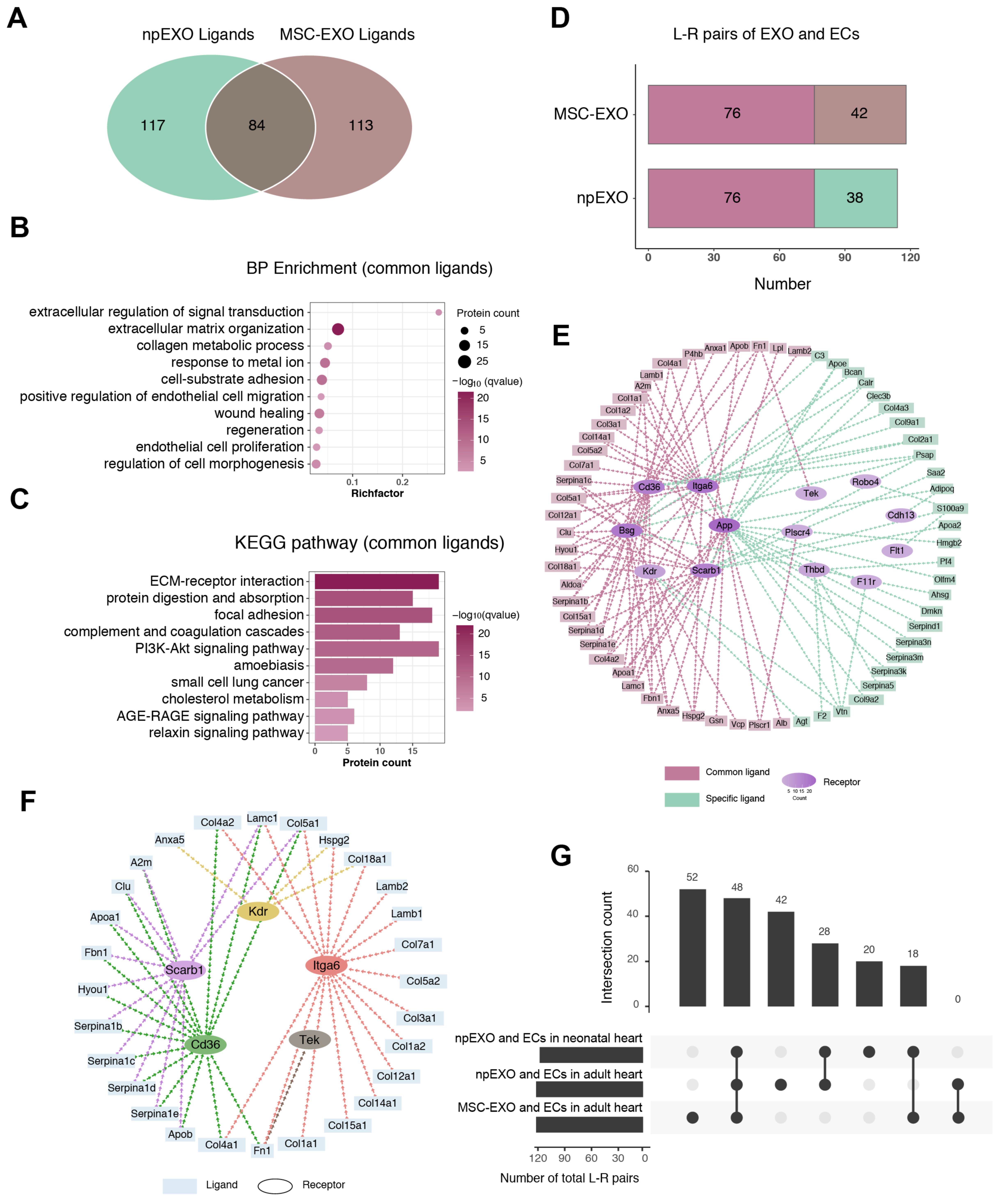
2.5. Identification of the Common npEXO Ligand–Receptor Pairs in Both Neonatal and Adult Heart
2.6. The Common Ligand–Receptor Pairs Shared by npEXO and MSC-EXO with Cardiac ECs
3. Discussion
4. Materials and Methods
4.1. npEXO Isolation
4.2. npEXO Characterization
4.3. Western Blot
4.4. Mouse Model of AMI and npEXO Treatment
4.5. Masson’s Staining
4.6. WGA Staining and Immunofluorescence Staining
4.7. Transthoracic Echocardiography Detection
4.8. hUVEC Migration and Tube Formation Assay
4.9. Mass Spectrometry Analysis of npEXO Proteins
4.10. Pre-Analysis of Proteome Data
4.11. scRNA-Seq Data Analysis
4.12. Preparation of Ligand and Receptor Datasets
4.13. Functional Enrichment Analysis
4.14. Construction of Exosome-Heart Interaction Network
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tzahor, E.; Poss, K.D. Cardiac regeneration strategies: Staying young at heart. Science 2017, 356, 1035–1039. [Google Scholar] [CrossRef]
- Yu, H.; Lu, K.; Zhu, J.; Wang, J. Stem cell therapy for ischemic heart diseases. Br. Med. Bull. 2017, 121, 135–154. [Google Scholar] [CrossRef]
- Balbi, C.; Vassalli, G. Exosomes: Beyond stem cells for cardiac protection and repair. Stem Cells 2020, 38, 1387–1399. [Google Scholar] [CrossRef]
- Sahoo, S.; Adamiak, M.; Mathiyalagan, P.; Kenneweg, F.; Kafert-Kasting, S.; Thum, T. Therapeutic and Diagnostic Translation of Extracellular Vesicles in Cardiovascular Diseases. Circulation 2021, 143, 1426–1449. [Google Scholar] [CrossRef]
- Zhu, D.; Liu, S.; Huang, K.; Wang, Z.; Hu, S.; Li, J.; Li, Z.; Cheng, K. Intrapericardial Exosome Therapy Dampens Cardiac Injury via Activating Foxo3. Circ. Res. 2022, 131, e135–e150. [Google Scholar] [CrossRef]
- Zhu, D.; Li, Z.; Huang, K.; Caranasos, T.G.; Rossi, J.S.; Cheng, K. Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Hirai, K.; Ousaka, D.; Fukushima, Y.; Kondo, M.; Eitoku, T.; Shigemitsu, Y.; Hara, M.; Baba, K.; Iwasaki, T.; Kasahara, S.; et al. Cardiosphere-derived exosomal microRNAs for myocardial repair in pediatric dilated cardiomyopathy. Sci. Transl. Med. 2020, 12, eabb3336. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Sharma, S.; Korutla, L.; Datla, S.R.; Shoja-Taheri, F.; Mishra, R.; Bigham, G.E.; Sarkar, M.; Morales, D.; Bittle, G.; et al. Circulating exosomes derived from transplanted progenitor cells aid the functional recovery of ischemic myocardium. Sci. Transl. Med. 2019, 11, eaau1168. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, J.; Tan, W.L.W.; Jiang, Y.; Wang, S.; Li, Q.; Yu, X.; Tan, J.; Liu, S.; Zhang, P.; et al. Extracellular vesicles from human embryonic stem cell-derived cardiovascular progenitor cells promote cardiac infarct healing through reducing cardiomyocyte death and promoting angiogenesis. Cell Death Dis. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Gao, L.; Wang, L.; Wei, Y.; Krishnamurthy, P.; Walcott, G.P.; Menasché, P.; Zhang, J.; Lightcap, E.S.; Yu, P.; Schneider, M.A.; et al. Exosomes secreted by hiPSC-derived cardiac cells improve recovery from myocardial infarction in swine. Sci. Transl. Med. 2020, 12, eaay1318. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Nakada, Y.; Wei, Y.; Bian, W.; Chu, Y.; Borovjagin, A.V.; Xie, M.; Zhu, W.; Nguyen, T.; Zhou, Y.; et al. Cyclin D2 Overexpression Enhances the Efficacy of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Myocardial Repair in a Swine Model of Myocardial Infarction. Circulation 2021, 144, 210–228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bolli, R.; Garry, D.J.; Marbán, E.; Menasché, P.; Zimmermann, W.-H.; Kamp, T.J.; Wu, J.C.; Dzau, V.J. Basic and Translational Research in Cardiac Repair and Regeneration. J. Am. Coll. Cardiol. 2021, 78, 2092–2105. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, M.; Sahoo, S. Exosomes in Myocardial Repair: Advances and Challenges in the Development of Next-Generation Therapeutics. Mol. Ther. 2018, 26, 1635–1643. [Google Scholar] [CrossRef]
- Foglio, E.; Puddighinu, G.; Fasanaro, P.; D’Arcangelo, D.; Perrone, G.A.; Mocini, D.; Campanella, C.; Coppola, L.; Logozzi, M.; Azzarito, T.; et al. Exosomal clusterin, identified in the pericardial fluid, improves myocardial performance following MI through epicardial activation, enhanced arteriogenesis and reduced apoptosis. Int. J. Cardiol. 2015, 197, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Qin, X.; Hu, Y.; Zhang, X.; Li, G.; Wu, J.; Li, J.; Sha, J.; Chen, J.; Xia, J.; et al. Longterm Exercise-Derived Exosomal miR-342-5p. Circ. Res. 2019, 124, 1386–1400. [Google Scholar] [CrossRef]
- Bei, Y.; Xu, T.; Lv, D.; Yu, P.; Xu, J.; Che, L.; Das, A.; Tigges, J.; Toxavidis, V.; Ghiran, I.; et al. Exercise-induced circulating extracellular vesicles protect against cardiac ischemia-reperfusion injury. Basic Res. Cardiol. 2017, 112, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vicencio, J.M.; Yellon, D.M.; Sivaraman, V.; Das, D.; Boi-Doku, C.; Arjun, S.; Zheng, Y.; Riquelme, J.A.; Kearney, J.; Sharma, V.; et al. Plasma Exosomes Protect the Myocardium From Ischemia-Reperfusion Injury. J. Am. Coll. Cardiol. 2015, 65, 1525–1536. [Google Scholar] [CrossRef]
- Sluijter, J.P.G.; Davidson, S.M.; Boulanger, C.M.; Buzás, E.I.; De Kleijn, D.P.V.; Engel, F.B.; Giricz, Z.; Hausenloy, D.J.; Kishore, R.; Lecour, S.; et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc. Res. 2018, 114, 19–34. [Google Scholar] [CrossRef]
- Zhu, W.; Sun, J.; Bishop, S.P.; Sadek, H.; Zhang, J. Turning back the clock: A concise viewpoint of cardiomyocyte cell cycle activation for myocardial regeneration and repair. J. Mol. Cell. Cardiol. 2022, 170, 15–21. [Google Scholar] [CrossRef]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient Regenerative Potential of the Neonatal Mouse Heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef]
- Haubner, B.J.; Schneider, J.; Schweigmann, U.; Schuetz, T.; Dichtl, W.; Velik-Salchner, C.; Stein, J.-I.; Penninger, J.M. Functional Recovery of a Human Neonatal Heart After Severe Myocardial Infarction. Circ. Res. 2016, 118, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Goldstone, A.B.; Wang, H.; Farry, J.; D’Amato, G.; Paulsen, M.J.; Eskandari, A.; Hironaka, C.E.; Phansalkar, R.; Sharma, B.; et al. A Unique Collateral Artery Development Program Promotes Neonatal Heart Regeneration. Cell 2019, 176, 1128–1142. [Google Scholar] [CrossRef]
- Liu, X.; De la Cruz, E.; Gu, X.; Balint, L.; Oxendine-Burns, M.; Terrones, T.; Ma, W.; Kuo, H.-H.; Lantz, C.; Bansal, T.; et al. Lymphoangiocrine signals promote cardiac growth and repair. Nature 2020, 588, 705–711. [Google Scholar] [CrossRef]
- Lavine, K.J.; Epelman, S.; Uchida, K.; Weber, K.J.; Nichols, C.G.; Schilling, J.D.; Ornitz, D.M.; Randolph, G.J.; Mann, D.L. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl. Acad. Sci. USA 2014, 111, 16029–16034. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, J.; Song, S.; Li, H.; Yang, H.; Zhou, B.; Li, Y.; Yue, Z.; Lian, H.; Liu, L.; et al. gp130 Controls Cardiomyocyte Proliferation and Heart Regeneration. Circulation 2020, 142, 967–982. [Google Scholar] [CrossRef] [PubMed]
- Bassat, E.; Mutlak, Y.E.; Genzelinakh, A.; Shadrin, I.Y.; Umansky, K.B.; Yifa, O.; Kain, D.; Rajchman, D.; Leach, J.; Bassat, D.R.; et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 2017, 547, 179–184. [Google Scholar] [CrossRef]
- Baehr, A.; Umansky, K.B.; Bassat, E.; Jurisch, V.; Klett, K.; Bozoglu, T.; Hornaschewitz, N.; Solyanik, O.; Kain, D.; Ferraro, B.; et al. Agrin Promotes Coordinated Therapeutic Processes Leading to Improved Cardiac Repair in Pigs. Circulation 2020, 142, 868–881. [Google Scholar] [CrossRef]
- Cardoso, A.C.; Pereira, A.H.M.; Sadek, H.A. Mechanisms of Neonatal Heart Regeneration. Curr. Cardiol. Rep. 2020, 22, 1–11. [Google Scholar] [CrossRef]
- Agarwal, U.; George, A.; Bhutani, S.; Ghosh-Choudhary, S.; Maxwell, J.T.; Brown, M.E.; Mehta, Y.; Platt, M.O.; Liang, Y.; Sahoo, S.; et al. Experimental, Systems, and Computational Approaches to Understanding the MicroRNA-Mediated Reparative Potential of Cardiac Progenitor Cell-Derived Exosomes From Pediatric Patients. Circ. Res. 2017, 120, 701–712. [Google Scholar] [CrossRef]
- Yoshida, M.; Satoh, A.; Lin, J.B.; Mills, K.F.; Sasaki, Y.; Rensing, N.; Wong, M.; Apte, R.S.; Imai, S.-I. Extracellular Vesicle-Contained eNAMPT Delays Aging and Extends Lifespan in Mice. Cell Metab. 2019, 30, 329–342. [Google Scholar] [CrossRef]
- Wang, Z.-G.; He, Z.-Y.; Liang, S.; Yang, Q.; Cheng, P.; Chen, A.-M. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Ma, H.; Liu, Z.; Yang, Y.; Feng, D.; Dong, Y.; A Garbutt, T.; Hu, Z.; Wang, L.; Luan, C.; Cooper, C.D.; et al. Functional coordination of non-myocytes plays a key role in adult zebrafish heart regeneration. EMBO Rep. 2021, 22, e52901. [Google Scholar] [CrossRef]
- Aurora, A.B.; Porrello, E.; Tan, W.; Mahmoud, A.I.; Hill, J.A.; Bassel-Duby, R.; Sadek, H.A.; Olson, E.N. Macrophages are required for neonatal heart regeneration. J. Clin. Investig. 2014, 124, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Li, Y.; Shen, Y.; Liu, Y.; Cai, J.; Liu, N.; Ma, G.; Tang, Y. Transplantation of Cardiac Mesenchymal Stem Cell-Derived Exosomes for Angiogenesis. J. Cardiovasc. Transl. Res. 2018, 11, 429–437. [Google Scholar] [CrossRef]
- Ma, T.; Chen, Y.; Chen, Y.; Meng, Q.; Sun, J.; Shao, L.; Yu, Y.; Huang, H.; Hu, Y.; Yang, Z.; et al. MicroRNA-132, Delivered by Mesenchymal Stem Cell-Derived Exosomes, Promote Angiogenesis in Myocardial Infarction. Stem Cells Int. 2018, 2018, 3290372. [Google Scholar] [CrossRef] [PubMed]
- Humeres, C.; Frangogiannis, N.G. Fibroblasts in the Infarcted, Remodeling, and Failing Heart. JACC: Basic Transl. Sci. 2019, 4, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Zhao, Z.; Liu, L.; Xia, G.; Ye, T.; Chen, Y.; Xu, C.; Jin, X.; Shen, C. Nephronectin promotes cardiac repair post myocardial infarction via activating EGFR/JAK2/STAT3 pathway. Int. J. Med Sci. 2022, 19, 878–892. [Google Scholar] [CrossRef] [PubMed]
- Benwell, C.J.; Taylor, J.A.G.E.; Robinson, S.D. Endothelial neuropilin-2 influences angiogenesis by regulating actin pattern development and α5-integrin-p-FAK complex recruitment to assembling adhesion sites. FASEB J. 2021, 35, e21679. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, J.; Hao, H.; Lin, H.; Wang, L.; Zhang, Y.; Chen, L.; Cao, S.; Huang, X.; Liao, W.; et al. Ablation of periostin inhibits post-infarction myocardial regeneration in neonatal mice mediated by the phosphatidylinositol 3 kinase/glycogen synthase kinase 3β/cyclin D1 signalling pathway. Cardiovasc. Res. 2017, 113, 620–632. [Google Scholar] [CrossRef]
- Litviňuková, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the adult human heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef]
- Qiang, B.; Lim, S.Y.; Lekas, M.; Kuliszewski, M.A.; Wolff, R.; Osherov, A.B.; Rudenko, D.; Leong-Poi, H.; Noyan, H.; Husain, M.; et al. Perlecan Heparan Sulfate Proteoglycan Is a Critical Determinant of Angiogenesis in Response to Mouse Hind-Limb Ischemia. Can. J. Cardiol. 2014, 30, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M.; Andreadou, I.; Barile, L.; Birnbaum, Y.; Cabrera-Fuentes, H.A.; Cohen, M.V.; Downey, J.M.; Girao, H.; Pagliaro, P.; Penna, C.; et al. Circulating blood cells and extracellular vesicles in acute cardioprotection. Cardiovasc. Res. 2019, 115, 1156–1166. [Google Scholar] [CrossRef]
- Toh, W.S.; Lai, R.C.; Zhang, B.; Lim, S.K. MSC exosome works through a protein-based mechanism of action. Biochem. Soc. Trans. 2018, 46, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Hayıroğlu, M.İ.; Keskin, M.; Uzun, A.O.; Yıldırım, D.I.; Kaya, A.; Çinier, G.; Bozbeyoğlu, E.; Yıldırımtürk, Ö.; Kozan, Ö.; Pehlivanoğlu, S. Predictors of In-Hospital Mortality in Patients With ST-Segment Elevation Myocardial Infarction Complicated With Cardiogenic Shock. Heart Lung Circ. 2019, 28, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Çinar, T.; Hayiroğlu, M.İ.; Şeker, M.; Doğan, S.; Çiçek, V.; Öz, A.; Uzun, M.; Orhan, A.L. The predictive value of age, creatinine, ejection fraction score for in-hospital mortality in patients with cardiogenic shock. Coron. Artery Dis. 2019, 30, 569–574. [Google Scholar] [CrossRef]
- Feng, J.; Li, Y.; Nie, Y. Non-Cardiomyocytes in Heart Regeneration. Curr. Drug Targets 2018, 19, 1077–1086. [Google Scholar] [CrossRef]
- Edelberg, J.M.; Lee, S.H.; Kaur, M.; Tang, L.; Feirt, N.M.; McCabe, S.; Bramwell, O.; Wong, S.C.; Hong, M.K. Platelet-Derived Growth Factor-AB Limits the Extent of Myocardial Infarction in a Rat Model. Circulation 2002, 105, 608–613. [Google Scholar] [CrossRef]
- Wu, X.; Reboll, M.R.; Korf-Klingebiel, M.; Wollert, K.C. Angiogenesis after acute myocardial infarction. Cardiovasc. Res. 2020, 117, 1257–1273. [Google Scholar] [CrossRef]
- Reboll, M.R.; Klede, S.; Taft, M.H.; Cai, C.-L.; Field, L.J.; Lavine, K.J.; Koenig, A.L.; Fleischauer, J.; Meyer, J.; Schambach, A.; et al. Meteorin-like promotes heart repair through endothelial KIT receptor tyrosine kinase. Science 2022, 376, 1343–1347. [Google Scholar] [CrossRef]
- Räsänen, M.; Sultan, I.; Paech, J.; Hemanthakumar, K.A.; Yu, W.; He, L.; Tang, J.; Sun, Y.; Hlushchuk, R.; Huan, X.; et al. VEGF-B Promotes Endocardium-Derived Coronary Vessel Development and Cardiac Regeneration. Circulation 2021, 143, 65–77. [Google Scholar] [CrossRef]
- Rai, A.; Fang, H.; Claridge, B.; Simpson, R.J.; Greening, D.W. Proteomic dissection of large extracellular vesicle surfaceome unravels interactive surface platform. J. Extracell. Vesicles 2021, 10, e12164. [Google Scholar] [CrossRef]
- Jiang, Z.; Lu, Z.; Kou, S.; Feng, T.; Wei, Y.; Gao, Z.; Deng, D.; Meng, J.; Lin, C.-P.; Zhou, B.; et al. Overexpression of Kdr in adult endocardium induces endocardial neovascularization and improves heart function after myocardial infarction. Cell Res. 2020, 31, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, R.; Hemanthakumar, K.A.; Vaparanta, K.; Robciuc, M.; Izumiya, Y.; Kidoya, H.; Takakura, N.; Peng, X.; Sawyer, D.B.; Elenius, K.; et al. Endothelial Cells Regulate Physiological Cardiomyocyte Growth via VEGFR2-Mediated Paracrine Signaling. Circulation 2019, 139, 2570–2584. [Google Scholar] [CrossRef]
- Tan, J.T.; Prosser, H.C.; Dunn, L.L.; Vanags, L.Z.; Ridiandries, A.; Tsatralis, T.; Leece, L.; Clayton, Z.E.; Yuen, S.C.G.; Robertson, S.; et al. High-Density Lipoproteins Rescue Diabetes-Impaired Angiogenesis via Scavenger Receptor Class B Type I. Diabetes 2016, 65, 3091–3103. [Google Scholar] [CrossRef]
- Shu, H.; Peng, Y.; Hang, W.; Nie, J.; Zhou, N.; Wang, D.W. The role of CD36 in cardiovascular disease. Cardiovasc. Res. 2020, 118, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Bassat, E.; Perez, D.E.; Tzahor, E. Myocardial Infarction Techniques in Adult Mice. Card. Regen. Methods Protoc. 2020, 2158, 3–21. [Google Scholar] [CrossRef]
- Takagawa, J.; Zhang, Y.; Wong, M.L.; Sievers, R.E.; Kapasi, N.K.; Wang, Y.; Yeghiazarians, Y.; Lee, R.J.; Grossman, W.; Springer, M.L. Myocardial infarct size measurement in the mouse chronic infarction model: Comparison of area- and length-based approaches. J. Appl. Physiol. 2007, 102, 2104–2111. [Google Scholar] [CrossRef]
- Li, Q.; Xu, Y.; Lv, K.; Wang, Y.; Zhong, Z.; Xiao, C.; Zhu, K.; Ni, C.; Wang, K.; Kong, M.; et al. Small extracellular vesicles containing miR-486-5p promote angiogenesis after myocardial infarction in mice and nonhuman primates. Sci. Transl. Med. 2021, 13, eabb0202. [Google Scholar] [CrossRef]
- Butler, A.; Hoffman, P.; Smibert, P.; Papalexi, E.; Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 2018, 36, 411–420. [Google Scholar] [CrossRef]
- Ren, Z.; Yu, P.; Li, D.; Li, Z.; Liao, Y.; Wang, Y.; Zhou, B.; Wang, L. Single-Cell Reconstruction of Progression Trajectory Reveals Intervention Principles in Pathological Cardiac Hypertrophy. Circulation 2020, 141, 1704–1719. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cui, M.; Shah, A.M.; Tan, W.; Liu, N.; Bassel-Duby, R.; Olson, E.N. Cell-Type-Specific Gene Regulatory Networks Underlying Murine Neonatal Heart Regeneration at Single-Cell Resolution. Cell Rep. 2020, 33, 108472. [Google Scholar] [CrossRef] [PubMed]
- Farbehi, N.; Patrick, R.; Dorison, A.; Xaymardan, M.; Janbandhu, V.; Wystub-Lis, K.; Ho, J.W.; E Nordon, R.; Harvey, R.P. Single-cell expression profiling reveals dynamic flux of cardiac stromal, vascular and immune cells in health and injury. Elife 2019, 8, e43882. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Lian, Y.; Wu, Y.; Ye, Z.; Feng, J.; Zhao, Y.; Guo, X.; Kang, J. Neonatal Plasma Exosomes Contribute to Endothelial Cell-Mediated Angiogenesis and Cardiac Repair after Acute Myocardial Infarction. Int. J. Mol. Sci. 2023, 24, 3196. https://doi.org/10.3390/ijms24043196
Li X, Lian Y, Wu Y, Ye Z, Feng J, Zhao Y, Guo X, Kang J. Neonatal Plasma Exosomes Contribute to Endothelial Cell-Mediated Angiogenesis and Cardiac Repair after Acute Myocardial Infarction. International Journal of Molecular Sciences. 2023; 24(4):3196. https://doi.org/10.3390/ijms24043196
Chicago/Turabian StyleLi, Xiuya, Yilin Lian, Yukang Wu, Zihui Ye, Jiabao Feng, Yuan Zhao, Xudong Guo, and Jiuhong Kang. 2023. "Neonatal Plasma Exosomes Contribute to Endothelial Cell-Mediated Angiogenesis and Cardiac Repair after Acute Myocardial Infarction" International Journal of Molecular Sciences 24, no. 4: 3196. https://doi.org/10.3390/ijms24043196
APA StyleLi, X., Lian, Y., Wu, Y., Ye, Z., Feng, J., Zhao, Y., Guo, X., & Kang, J. (2023). Neonatal Plasma Exosomes Contribute to Endothelial Cell-Mediated Angiogenesis and Cardiac Repair after Acute Myocardial Infarction. International Journal of Molecular Sciences, 24(4), 3196. https://doi.org/10.3390/ijms24043196





