Fetuin-A: A Novel Biomarker of Bone Damage in Early Axial Spondyloarthritis. Results of an Interim Analysis of the SPACE Study
Abstract
1. Introduction
2. Results
2.1. Characteristic of the Patients in the Cohort (Descriptive Analysis)
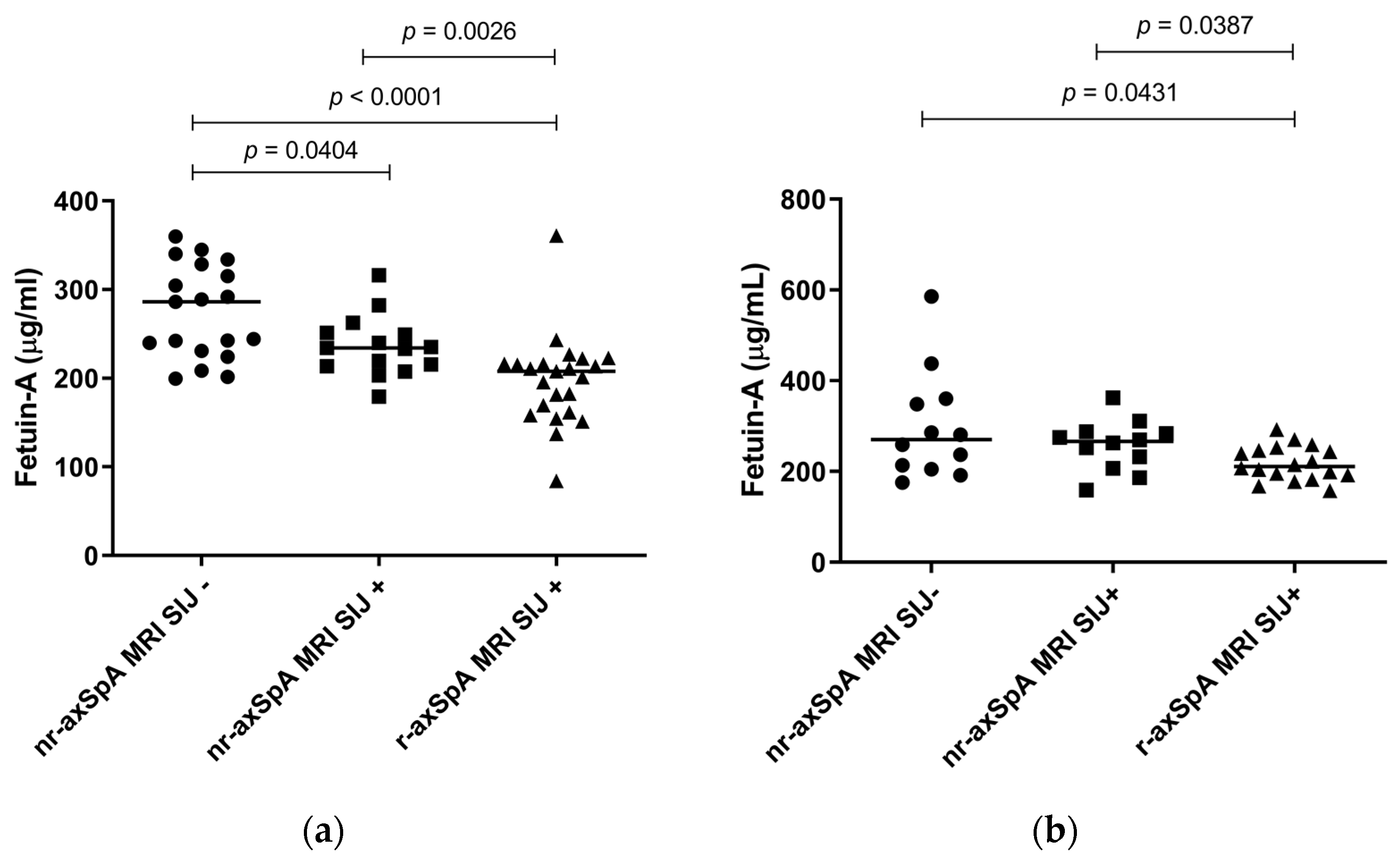
2.2. Levels of Fetuin-A in the Cohort (Descriptive Analysis)
2.3. Factors Associated with Radiographic Sacroiliitis at T0 (Univariate Analysis and Multivariate Logistic Regression)
2.4. Factors Associated with Syndesmophytes at T0 (Univariate Analysis and Multivariate Logistic Regression)
2.5. Factors Associated with Radiographic Damage in the SIJs (mNY) at T0 and at T24 (Univariate Analysis and Multivariate Linear Regression)
2.6. Baseline Predictors of Radiographic Damage in the SIJs (mNY T24) (Univariate Analysis and Multivariate Linear Regression)
3. Discussion
4. Materials and Methods
4.1. Study Population, Clinical Assessment and Radiographic Evaluation
4.2. Fetuin-A testing
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ortolan, A.; Kiltz, U.; Doria, A.; Aggarwal, A.; Ramonda, R. Do we believe in non-radiographic axial spondyloarthritis? A debate. Autoimmun. Rev. 2021, 20, 102703. [Google Scholar] [CrossRef] [PubMed]
- Sieper, J.; Poddubnyy, D. Axial spondyloarthritis. Lancet 2017, 390, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Khmelinskii, N.; Regel, A.; Baraliakos, X. The Role of Imaging in Diagnosing Axial Spondyloarthritis. Front. Med. 2018, 5, 106. [Google Scholar] [CrossRef] [PubMed]
- Lorenzin, M.; Ortolan, A.; Felicetti, M.; Vio, S.; Favero, M.; Polito, P.; Lacognata, C.; Scapin, V.; Doria, A.; Ramonda, R. Spine and Sacroiliac Joints Lesions on Magnetic Resonance Imaging in Early Axial-Spondyloarthritis During 24-Months Follow-Up (Italian Arm of SPACE Study). Front. Immunol. 2020, 11, 936. [Google Scholar] [CrossRef]
- Lorenzin, M.; Ortolan, A.; Frallonardo, P.; Vio, S.; Lacognata, C.; Oliviero, F.; Punzi, L.; Ramonda, R. Spine and sacroiliac joints on magnetic resonance imaging in patients with early axial spondyloarthritis: Prevalence of lesions and association with clinical and disease activity indices from the Italian group of the SPACE study. Reumatismo 2016, 68, 72–82. [Google Scholar] [CrossRef]
- Prajzlerová, K.; Grobelná, K.; Pavelka, K.; Šenolt, L.; Filková, M. An update on biomarkers in axial spondyloarthritis. Autoimmun. Rev. 2016, 15, 501–509. [Google Scholar] [CrossRef] [PubMed]
- de Winter, J.; de Hooge, M.; van de Sande, M.; de Jong, H.; van Hoeven, L.; de Koning, A.; Berg, I.J.; Ramonda, R.; Baeten, D.; van der Heijde, D.; et al. Magnetic Resonance Imaging of the Sacroiliac Joints Indicating Sacroiliitis According to the Assessment of SpondyloArthritis international Society Definition in Healthy Individuals, Runners, and Women With Postpartum Back Pain. Arthritis Rheumatol. 2018, 70, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Lorenzin, M.; Ortolan, A.; Vio, S.; Favero, M.; Oliviero, F.; Zaninotto, M.; Cosma, C.; Lacognata, C.; Punzi, L.; Ramonda, R. Biomarkers, imaging and disease activity indices in patients with early axial spondyloarthritis: The Italian arm of the SpondyloArthritis-Caught-Early (SPACE) Study. Reumatismo 2017, 69, 65–74. [Google Scholar] [CrossRef]
- Turina, M.C.; Yeremenko, N.; van Gaalen, F.; van Oosterhout, M.; Berg, I.J.; Ramonda, R.; Lebre, C.; Landewé, R.; Baeten, D. Serum inflammatory biomarkers fail to identify early axial spondyloarthritis: Results from the SpondyloArthritis Caught Early (SPACE) cohort. RMD Open 2017, 3, e000319. [Google Scholar] [CrossRef] [PubMed]
- Lorenzin, M.; Ometto, F.; Ortolan, A.; Felicetti, M.; Favero, M.; Doria, A.; Ramonda, R. An update on serum biomarkers to assess axial spondyloarthritis and to guide treatment decision. Ther Adv Musculoskelet Dis 2020, 12, 1759720x20934277. [Google Scholar] [CrossRef]
- Moz, S.; Lorenzin, M.; Ramonda, R.; Aneloni, V.; La Raja, M.; Plebani, M.; Basso, D. Emerging role of monocytes and their intracellular calcium pattern in spondyloarthritis. Clin Chim Acta 2020, 500, 180–188. [Google Scholar] [CrossRef]
- Lorenzin, M.; Ortolan, A.; Felicetti, M.; Favero, M.; Vio, S.; Zaninotto, M.; Polito, P.; Cosma, C.; Scapin, V.; Lacognata, C.; et al. Serological Biomarkers in Early Axial Spondyloarthritis During 24-Months Follow Up (Italian Arm of Space Study). Front. Med. 2019, 6, 177. [Google Scholar] [CrossRef] [PubMed]
- Chekol Abebe, E.; Tilahun Muche, Z.; Behaile, T.M.A.; Mengie Ayele, T.; Mekonnen Agidew, M.; Teshome Azezew, M.; Abebe Zewde, E.; Asmamaw Dejenie, T.; Asmamaw Mengstie, M. The structure, biosynthesis, and biological roles of fetuin-A: A review. Front. Cell Dev. Biol. 2022, 10, 945287. [Google Scholar] [CrossRef] [PubMed]
- Komsa-Penkova, R.S.; Golemanov, G.M.; Radionova, Z.V.; Tonchev, P.T.; Iliev, S.D.; Penkov, V.V. Fetuin-A–Alpha2-Heremans-Schmid Glycoprotein: From Structure to a Novel Marker of Chronic Diseases Part 1. Fetuin-A as a Calcium Chaperone and Inflammatory Marker. J. Biomed. Clin. Res. 2017, 10, 90–97. [Google Scholar] [CrossRef]
- Mori, K.; Emoto, M.; Inaba, M. Fetuin-A: A multifunctional protein. Recent Pat. Endocr. Metab. Immune Drug Discov. 2011, 5, 124–146. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, G.; Benedetti, F.; Wang, S.-M.; Lee, S.-J.; Jun, T.-Y.; Masand, P.S.; Patkar, A.A.; Han, C.; Serretti, A.; Pae, C.-U.; et al. Reduced plasma Fetuin-A is a promising biomarker of depression in the elderly. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-W.; Lin, C.-G.; Wu, L.-Z.; Luo, Y.-K.; Fan, L.; Dong, X.-f.; Zheng, H. Serum fetuin-A levels are associated with the presence and severity of coronary artery disease in patients with type 2 diabetes. Biomarkers 2013, 18, 160–164. [Google Scholar] [CrossRef]
- Ix, J.H.; Shlipak, M.G.; Brandenburg, V.M.; Ali, S.; Ketteler, M.; Whooley, M.A. Association Between Human Fetuin-A and the Metabolic Syndrome. Circulation 2006, 113, 1760–1767. [Google Scholar] [CrossRef]
- Di Minno, A.; Zanobini, M.; Myasoedova, V.A.; Valerio, V.; Songia, P.; Saccocci, M.; Di Minno, M.N.D.; Tremoli, E.; Poggio, P. Could circulating fetuin A be a biomarker of aortic valve stenosis? Int. J. Cardiol. 2017, 249, 426–430. [Google Scholar] [CrossRef]
- Roshanzamir, F.; Miraghajani, M.; Rouhani, M.H.; Mansourian, M.; Ghiasvand, R.; Safavi, S.M. The association between circulating fetuin-A levels and type 2 diabetes mellitus risk: Systematic review and meta-analysis of observational studies. J. Endocrinol. Investig. 2018, 41, 33–47. [Google Scholar] [CrossRef]
- Tekeoğlu, İ.; Harman, H.; Sağ, S.; Altındiş, M.; Kamanlı, A.; Nas, K. Levels of serum pentraxin 3, IL-6, fetuin A and insulin in patients with rheumatoid arthritis. Cytokine 2016, 83, 171–175. [Google Scholar] [CrossRef]
- Harman, H.; Tekeoglu, I.; Gurol, G.; Sag, M.S.; Karakece, E.; IH, C.I.; Kamanli, A.; Nas, K. Comparison of fetuin-A and transforming growth factor beta 1 levels in patients with spondyloarthropathies and rheumatoid arthritis. Int. J. Rheum. Dis. 2017, 20, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Przepiera-Bedzak, H.; Fischer, K.; Brzosko, M. Serum Interleukin-18, Fetuin-A, Soluble Intercellular Adhesion Molecule-1, and Endothelin-1 in Ankylosing Spondylitis, Psoriatic Arthritis, and SAPHO Syndrome. Int. J. Mol. Sci. 2016, 17, 1255. [Google Scholar] [CrossRef] [PubMed]
- Sari, I.; Kebapcilar, L.; Taylan, A.; Bilgir, O.; Kozaci, D.L.; Yildiz, Y.; Yuksel, A.; Gunay, N.; Akkoc, N. Fetuin-A and interleukin-18 levels in ankylosing spondylitis. Int. J. Rheum. Dis. 2010, 13, 75–81. [Google Scholar] [CrossRef]
- Tuylu, T.; Sari, I.; Solmaz, D.; Kozaci, D.L.; Akar, S.; Gunay, N.; Onen, F.; Akkoc, N. Fetuin-A is related to syndesmophytes in patients with ankylosing spondylitis: A case control study. Clinics 2014, 69, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Punzi, L.; Ramonda, R.; Todesco, S. Is the alpha-2-HS-glycoprotein a negative acute phase reactant. Clin. Rheumatol. 1987, 6, 299. [Google Scholar] [CrossRef]
- Przepiera-Będzak, H.; Fischer, K.; Brzosko, M. Axial spondyloarthritis and inflammatory bowel disease: Association between disease activity and endothelial dysfunction markers. Rheumatol. Int. 2022, 42, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Lories, R.J.; Haroon, N. Bone formation in axial spondyloarthritis. Best Pract. Res. Clin. Rheumatol. 2014, 28, 765–777. [Google Scholar] [CrossRef]
- Ez-Zaitouni, Z.; Bakker, P.A.; van Lunteren, M.; de Hooge, M.; van den Berg, R.; Reijnierse, M.; Fagerli, K.M.; Landewé, R.B.; Ramonda, R.; Jacobsson, L.T.; et al. The yield of a positive MRI of the spine as imaging criterion in the ASAS classification criteria for axial spondyloarthritis: Results from the SPACE and DESIR cohorts. Ann. Rheum. Dis. 2017, 76, 1731–1736. [Google Scholar] [CrossRef]
- Calin, A.; Elswood, J. The relationship between pelvic, spinal and hip involvement in ankylosing spondylitis--one disease process or several? Br. J. Rheumatol. 1988, 27, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Jahnen-Dechent, W.; Heiss, A.; Schäfer, C.; Ketteler, M. Fetuin-A regulation of calcified matrix metabolism. Circ. Res. 2011, 108, 1494–1509. [Google Scholar] [CrossRef]
- Favero, M.; Belluzzi, E.; Frallonardo, P.; Peruzzo, L.; Tauro, L.; Oliviero, F.; Ramonda, R.; Punzi, L. Synovial fluid fetuin-A levels in patients affected by osteoarthritis with or without evidence of calcium crystals. Rheumatology 2019, 58, 729–730. [Google Scholar] [CrossRef]
- Rasul, S.; Ilhan, A.; Reiter, M.H.; Todoric, J.; Farhan, S.; Esterbauer, H.; Kautzky-Willer, A. Levels of fetuin-A relate to the levels of bone turnover biomarkers in male and female patients with type 2 diabetes. Clin. Endocrinol. 2012, 76, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Özkan, E.; Özkan, H.; Bilgiç, S.; Odabaşi, E.; Can, N.; Akgül, E.; Yanmiş, I.; Yurttaş, Y.; Kürklü, M.; Başbozkurt, M.; et al. Serum fetuin-A levels in postmenopausal women with osteoporosis. Turk. J. Med. Sci. 2014, 44, 985–988. [Google Scholar] [CrossRef]
- Nikiphorou, E.; Baraliakos, X. Treat to Target in Axial Spondyloarthritis. Rheum. Dis. Clin. N. Am. 2019, 45, 519–535. [Google Scholar] [CrossRef] [PubMed]
- Moz, S.; Aita, A.; Basso, D.; Ramonda, R.; Plebani, M.; Punzi, L. Spondyloarthritis: Matrix Metalloproteinasesas Biomarkers of Pathogenesis and Response to Tumor Necrosis Factor (TNF) Inhibitors. Int. J. Mol. Sci. 2017, 18, 830. [Google Scholar] [CrossRef] [PubMed]
- Lorenzin, M.; Ortolan, A.; Frallonardo, P.; Oliviero, F.; Punzi, L.; Ramonda, R. Predictors of response and drug survival in ankylosing spondylitis patients treated with infliximab. BMC Musculoskelet. Disord. 2015, 16, 166. [Google Scholar] [CrossRef]
- Ramiro, S.; Nikiphorou, E.; Sepriano, A.; Ortolan, A.; Webers, C.; Baraliakos, X.; Landewé, R.B.M.; Van den Bosch, F.E.; Boteva, B.; Bremander, A.; et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann. Rheum. Dis. 2023, 82, 19–34. [Google Scholar] [CrossRef]
- Heuft-Dorenbosch, L.; Spoorenberg, A.; van Tubergen, A.; Landewé, R.; van ver Tempel, H.; Mielants, H.; Dougados, M.; van der Heijde, D. Assessment of enthesitis in ankylosing spondylitis. Ann. Rheum. Dis. 2003, 62, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, M.; Lato, V.; Carotti, M.; Salaffi, F. Clinimetric properties of the ASAS health index in a cohort of Italian patients with axial spondyloarthritis. Health Qual. Life Outcomes 2016, 14, 78. [Google Scholar] [CrossRef]
- Garrett, S.; Jenkinson, T.; Kennedy, L.G.; Whitelock, H.; Gaisford, P.; Calin, A. A new approach to defining disease status in ankylosing spondylitis: The Bath Ankylosing Spondylitis Disease Activity Index. J. Rheumatol. 1994, 21, 2286–2291. [Google Scholar] [PubMed]
- Calin, A.; Garrett, S.; Whitelock, H.; Kennedy, L.G.; O’Hea, J.; Mallorie, P.; Jenkinson, T. A new approach to defining functional ability in ankylosing spondylitis: The development of the Bath Ankylosing Spondylitis Functional Index. J. Rheumatol. 1994, 21, 2281–2285. [Google Scholar] [PubMed]
- van der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984, 27, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Creemers, M.C.; Franssen, M.J.; van’t Hof, M.A.; Gribnau, F.W.; van de Putte, L.B.; van Riel, P.L. Assessment of outcome in ankylosing spondylitis: An extended radiographic scoring system. Ann. Rheum. Dis. 2005, 64, 127–129. [Google Scholar] [CrossRef]
- Rudwaleit, M.; Jurik, A.G.; Hermann, K.G.A.; Landewé, R.; van der Heijde, D.; Baraliakos, X.; Marzo-Ortega, H.; Østergaard, M.; Braun, J.; Sieper, J. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: A consensual approach by the ASAS/OMERACT MRI group. Ann. Rheum. Dis. 2009, 68, 1520. [Google Scholar] [CrossRef]



| All Patients | No Radiographic Sacroiliitis | Radiographic Sacroiliitis | p Value | No Syndesmophytes | Syndesmophytes | p Value | |
|---|---|---|---|---|---|---|---|
| Number of individuals | 57 | 36 | 21 | 51 | 6 | ||
| Male sex | 24 (42.1) | 12 (33.3) | 12 (57.1) | 0.08 * | 19 (37.3) | 5 (83.3) | 0.03 ** |
| BMI | 23.5 (21.1–26.1) | 23.5 (21–26.8) | 23.5 (21.1–24.8) | 0.92 | 23.3 (20.9–25.4) | 26.3 (23.8–32.3) | 0.05 ** |
| Smoking | 19 (33.3) | 9 (25) | 10 (47.6) | 0.08 * | 16 (31.4) | 3 (50) | 0.36 |
| Age of onset CBP | 28 (22–36) | 29.5 (21.8–37.3) | 26 (23–32) | 0.49 | 28 (21.5–34) | 41 (37–42.8) | 0.01 ** |
| Duration CBP | 12 (8–18) | 12 (9.5–18) | 12 (8–20) | 0.75 | 12 (8–18) | 15 (12.5–19) | 0.25 |
| HLA-B27 positivity | 22 (38.6) | 12 (33.3) | 10 (47.6) | 0.29 | 21 (41.2) | 1 (16.7) | 0.24 |
| IBP | 57 (100) | 36 (100) | 21 (100) | - | 51 (100) | 6 (100) | - |
| Heel enthesitis | 46 (80.7) | 32 (88.9) | 14 (66.7) | 0.04 ** | 40 (78.4) | 6 (100) | 0.21 |
| Dactilitis | 13 (22.8) | 7 (19.4) | 6 (28.6) | 0.43 | 13 (25.5) | 0 (0) | 0.16 |
| IBD | 8 (14) | 5 (13.9) | 3 (14.3) | 0.97 | 7 (13.7) | 1 (16.7) | 0.84 |
| Psoriasis | 21 (36.8) | 15 (41.7) | 6 (28.6) | 0.32 | 17 (33.3) | 4 (66.7) | 0.11 |
| Peripheral arthritis | 24 (42.1) | 13 (36.1) | 11 (52.4) | 0.23 | 20 (39.2) | 4 (66.7) | 0.2 |
| Family history | 28 (49.1) | 19 (52.8) | 9 (42.9) | 0.47 | 26 (51) | 2 (33.3) | 0.41 |
| Response to NSAIDs | 55 (96.5) | 35 (97.2) | 20 (95.2) | 0.69 | 49 (96.1) | 6 (100) | 0.62 |
| Uveitis | 4 (7) | 4 (11.1) | 0 (0) | 0.11 | 3 (5.9) | 1 (16.7) | 0.33 |
| Fetuin-A, µg/mL | 222.8 (203.3–251) | 239.9 (217.9–286.9) | 207.9 (181.7–215.9) | <0.001 ** | 224.1 (204.4–256.7) | 209.8 (204.6–239.7) | 0.68 |
| Elevated CRP or ESR | 30 (52.6) | 16 (44.4) | 14 (66.7) | 0.11 | 27 (52.9) | 3 (50) | 0.89 |
| CRP, mg/L | 2 (1–5) | 2.5 (1–5) | 2 (1–5) | 0.97 | 2 (1–5) | 3.5 (1.3–5.8) | 0.97 |
| ESR mm/h | 17.6 ± 15.6 | 12 (7.8–20) | 14 (8–25) | 0.87 | 13 (7.5–20) | 16 (9.8–28.3) | 0.5 |
| 0.9 | |||||||
| BASDAI | 4.4 ± 2.5 | 4.9 (3.2–7.2) | 3.1 (1.5–5.2) | 0.03 ** | 4.4 (2.4–6.9) | 2.3 (1.1–5.3) | 0.2 |
| BASFI | 0.8 (0.2–2.3) | 0.8 (0.2–2.3) | 1.2 (0.3–2.3) | 0.19 | 0.8 (0.2–2.6) | 1.2 (0.5–1.7) | 0.82 |
| BASMI | 0 (0–1) | 0 (0–1) | 0 (0–1.3) | 0.8 | 0 (0–1) | 0 (0–2.3) | 0.98 |
| ASDAS | 2.5 ± 0.8 | 2.8 (1.8–3.1) | 2.4 (1.8–2.9) | 0.6 | 2.6 (1.9–3.1) | 2 (1.3–2.7) | 0.18 |
| HAQ | 0.1 (0–0.5) | 0.2 (0–0.6) | 0.1 (0–0.4) | 0.38 | 0.1 (0–0.5) | 0.3 (0–0.4) | 0.97 |
| MASES | 3 (1–5) | 4 (1.8–5.3) | 3 (1–3) | 0.04 ** | 3 (1–5) | 3 (1–5) | 0.53 |
| VAS pain | 4 (1–6) | 4 (2–7) | 2 (1–5) | 0.13 | 4 (1.5–6) | 2.5 (1–6.3) | 0.53 |
| VAS disease activity | 3 (1–7) | 4 (1.8–7) | 2 (1–5) | 0.17 | 4 (1–6.5) | 2 (0.3–6) | 0.36 |
| Night pain | 3 (0–6) | 3 (0.8–6.3) | 1 (0–6) | 0.45 | 3 (0–6.5) | 2 (0.3–3.8) | 0.62 |
| Radiographic sacroliliitis | 21 (36.8) | 0 (0) | 21 (100) | - | 19 (37.3) | 2 (33.3) | 0.85 |
| Syndesmophytes | 6 (10.5) | 4 (11.1) | 2 (9.5) | 0.85 | 0 (0) | 6 (100) | - |
| mNY | 0 (0–1) | 0 (0–0) | 1 (1–1) | - | 0 (0–1) | 0 (0–0.8) | 0.61 |
| mSASSS | 2 (0–5) | 2 (0–5) | 3 (2–5) | 0.36 | 2 (0–4) | 9.5 (5.8–11.8) | <0.001 ** |
| SPARCC SIJ | 2 (0–5) | 0 (0–2) | 5 (0–9) | 0.09 † | 0 (0–5) | 2.5 (0.5–3.8) | 0.89 |
| SPARCC spine | 3 (0–16) | 1 (0–12) | 8 (0–21) | 0.09 † | 0 (0–1) | 0 (0–0.8) | 0.63 |
| Factors Associated with Radiographic Sacroiliitis at T0 | ||
| OR (95%C.I.) | p value | |
| Male sex | 1.3 (0.3; 5.6) | 0.76 |
| Smoking | 1.2 (0.3; 4.6) | 0.824 |
| Heel enthesitis at T0 | 0.4 (0.1; 2) | 0.278 |
| BASDAI at T0. per unit increase | 0.9 (0.7; 1.2) | 0.361 |
| MASES at T0 | 0.8 (0.6; 1.2) | 0.284 |
| Fetuin-A at T0 (µg/mL), per 10-unit increase | 0.9 (0.8; 0.999) | 0.048 |
| Constant | 78.8 (0; 0) | 0.032 |
| X2 (df = 6, N = 57) 15.61, p = 0.016; Nagelkerke R2 32.7% The model classified correctly 63.2% cases. | ||
| Factors associated with syndesmophytes at T0 | ||
| OR (95%C.I.) | p value | |
| Male sex | 90.48 (1.45; 5644.87) | 0.033 |
| BMI | 1.28 (0.94; 1.75) | 0.118 |
| Age at CBP onset, per year increase | 1.28 (1.04; 1.58) | 0.019 |
| Constant | 0 (0; 0) | 0.004 |
| X2 (df = 3, N = 57) 22.02, p < 0.001; Nagelkerke R2 64.5% The model classified correctly 89.5% cases. | ||
| Factors Associated with mNY Score at T0 (Variables at T0) | ||||
| B (95%C.I.) | β | t | p Value | |
| Constant | 2.2 (1.4; 3.1) | 0 | 5.5 | <0.001 |
| Male sex | 0.1 (−0.3; 0.5) | 0.1 | 0.7 | 0.464 |
| BASDAI at T0, per unit increase | −0.03 (−0.1; 0.02) | −0.1 | −0.9 | 0.347 |
| Fetuin-A at T0 (µg/mL), per 10-unit increase | −0.07 (−0.098; −0.04) | −0.5 | −4.6 | <0.001 |
| Uveitis at T0 | −0.6 (−1.3; 0.1) | −0.2 | −1.8 | 0.085 |
| R2 = 0.37, F (4) = 7.45; p < 0.001, N = 57 | ||||
| Factors Associated with mNY Score at T24 (Variables at T24) | ||||
| B (95%C.I.) | Β | t | p Value | |
| Constant | 1.6 (0.8; 2.4) | 4.1 | <0.001 | |
| BASDAI at T24, per unit increase | −0.1 (−0.2; 0.1) | −0.2 | −1.2 | 0.2 |
| Fetuin-A at T24 (µg/mL), per 10-unit increase | −0.030 (−0.1; −0.060) | −0.3 | −2.1 | <0.001 |
| R2 = 0.16, F (2) = 3.51; p = 0.40; N = 41 | ||||
| Factors associated with mNY score at T24 (variables at T0, predictors) | ||||
| B (95%C.I.) | Β | t | p Value | |
| Constant | 0.2 (−0.4; 0.8) | 0 | 0.8 | 0.444 |
| BASDAI at T0 | −0.03 (−0.1; 0.02) | −0.1 | −1.4 | 0.183 |
| mNY at T0, per unit increase | 0.9 (0.7; 1.1) | 0.9 | 11.6 | <0.001 |
| Fetuin-A at T0 (µg/mL), per 10-unit increase | −0.001 (−0.02; 0.02) | 0 | 0.1 | 0.937 |
| R2 = 0.85; F (5) = 40.69; p < 0.00; N = 41 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Favero, M.; Ometto, F.; Belluzzi, E.; Cozzi, G.; Scagnellato, L.; Oliviero, F.; Ruggieri, P.; Doria, A.; Lorenzin, M.; Ramonda, R. Fetuin-A: A Novel Biomarker of Bone Damage in Early Axial Spondyloarthritis. Results of an Interim Analysis of the SPACE Study. Int. J. Mol. Sci. 2023, 24, 3203. https://doi.org/10.3390/ijms24043203
Favero M, Ometto F, Belluzzi E, Cozzi G, Scagnellato L, Oliviero F, Ruggieri P, Doria A, Lorenzin M, Ramonda R. Fetuin-A: A Novel Biomarker of Bone Damage in Early Axial Spondyloarthritis. Results of an Interim Analysis of the SPACE Study. International Journal of Molecular Sciences. 2023; 24(4):3203. https://doi.org/10.3390/ijms24043203
Chicago/Turabian StyleFavero, Marta, Francesca Ometto, Elisa Belluzzi, Giacomo Cozzi, Laura Scagnellato, Francesca Oliviero, Pietro Ruggieri, Andrea Doria, Mariagrazia Lorenzin, and Roberta Ramonda. 2023. "Fetuin-A: A Novel Biomarker of Bone Damage in Early Axial Spondyloarthritis. Results of an Interim Analysis of the SPACE Study" International Journal of Molecular Sciences 24, no. 4: 3203. https://doi.org/10.3390/ijms24043203
APA StyleFavero, M., Ometto, F., Belluzzi, E., Cozzi, G., Scagnellato, L., Oliviero, F., Ruggieri, P., Doria, A., Lorenzin, M., & Ramonda, R. (2023). Fetuin-A: A Novel Biomarker of Bone Damage in Early Axial Spondyloarthritis. Results of an Interim Analysis of the SPACE Study. International Journal of Molecular Sciences, 24(4), 3203. https://doi.org/10.3390/ijms24043203









