Silicon Application for the Modulation of Rhizosphere Soil Bacterial Community Structures and Metabolite Profiles in Peanut under Ralstonia solanacearum Inoculation
Abstract
1. Introduction
2. Results
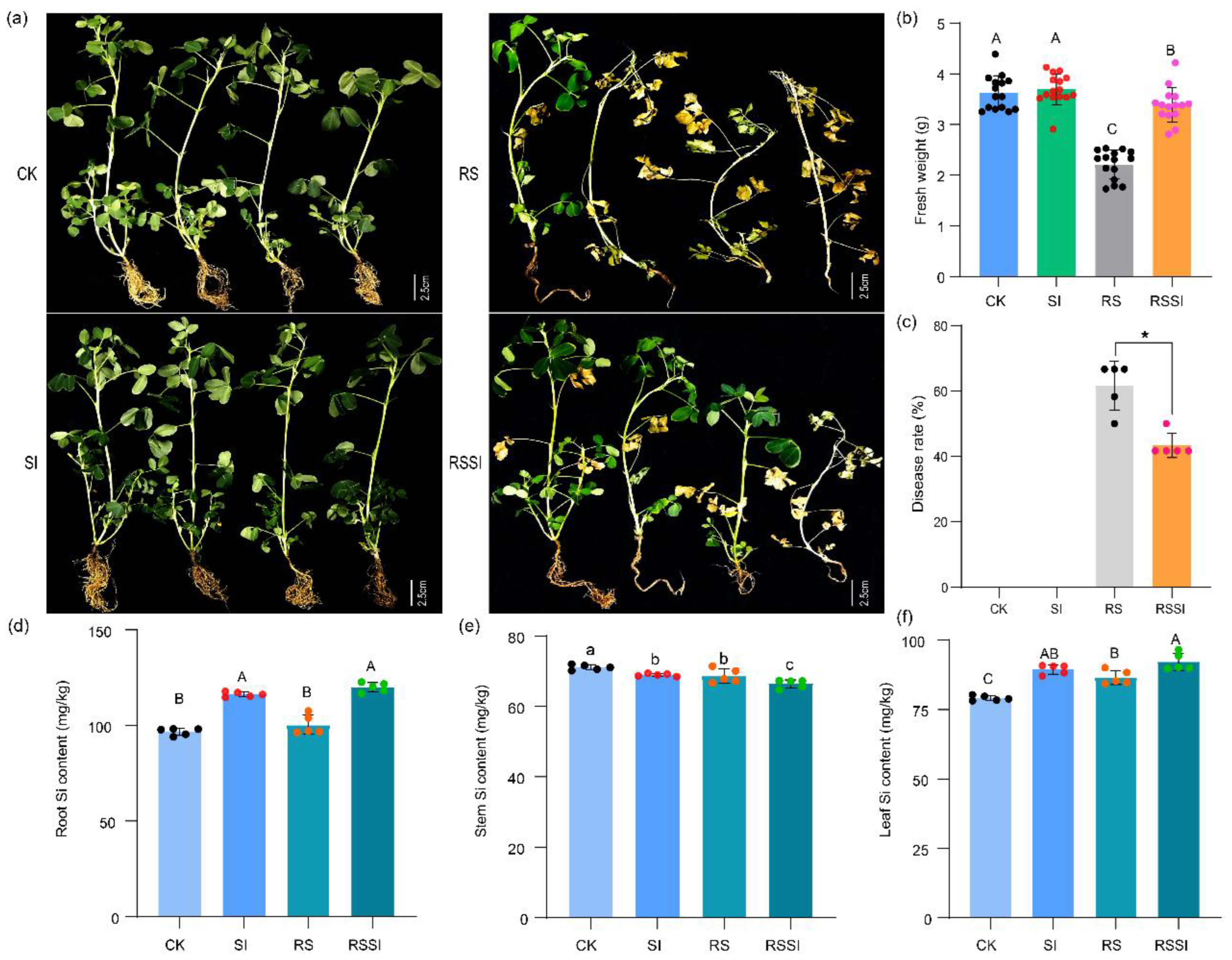
2.1. PBW Disease Severity and Peanut Growth Responses
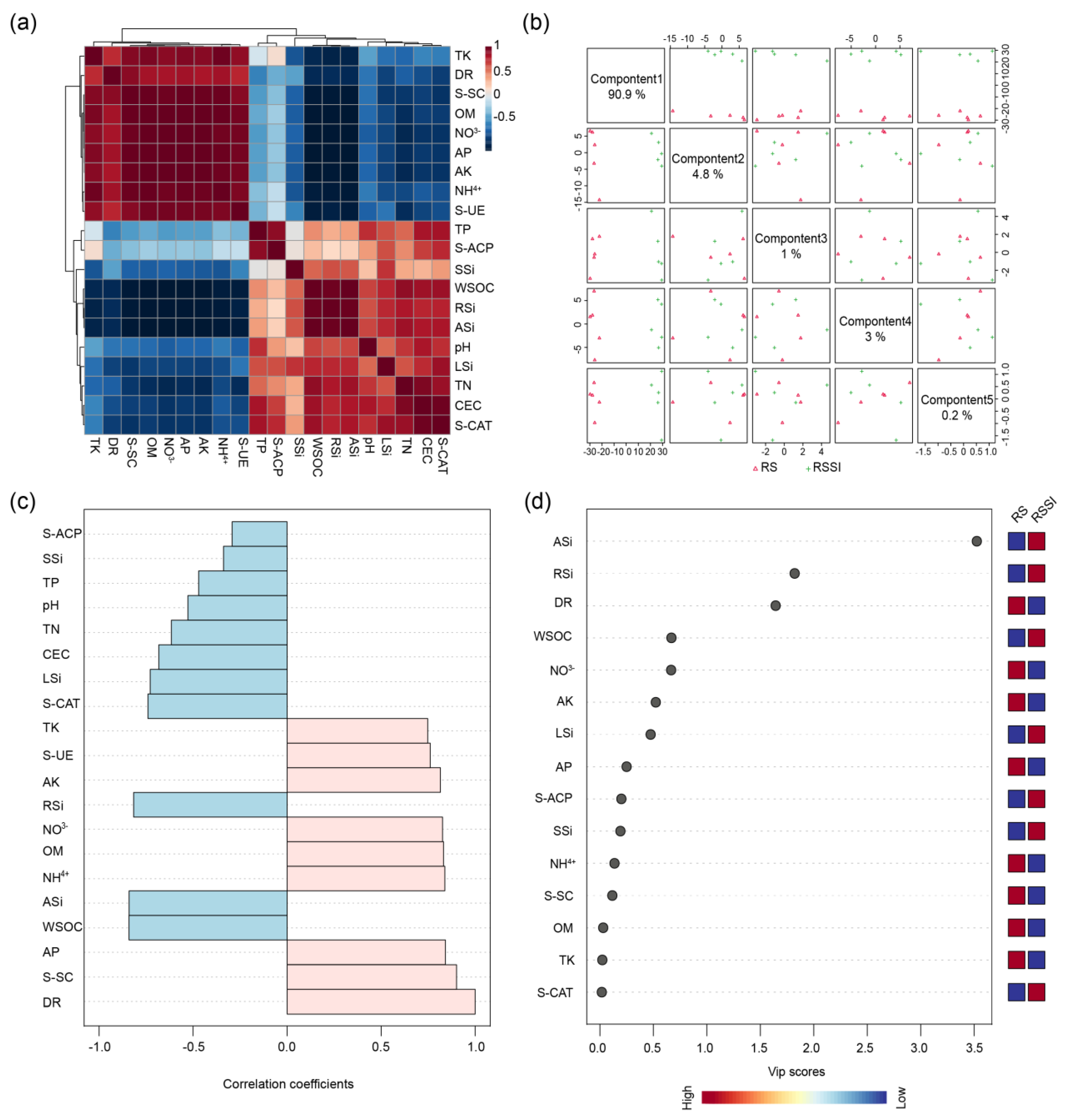
2.2. Correlation between Disease Rate and the Other Investigated Parameters
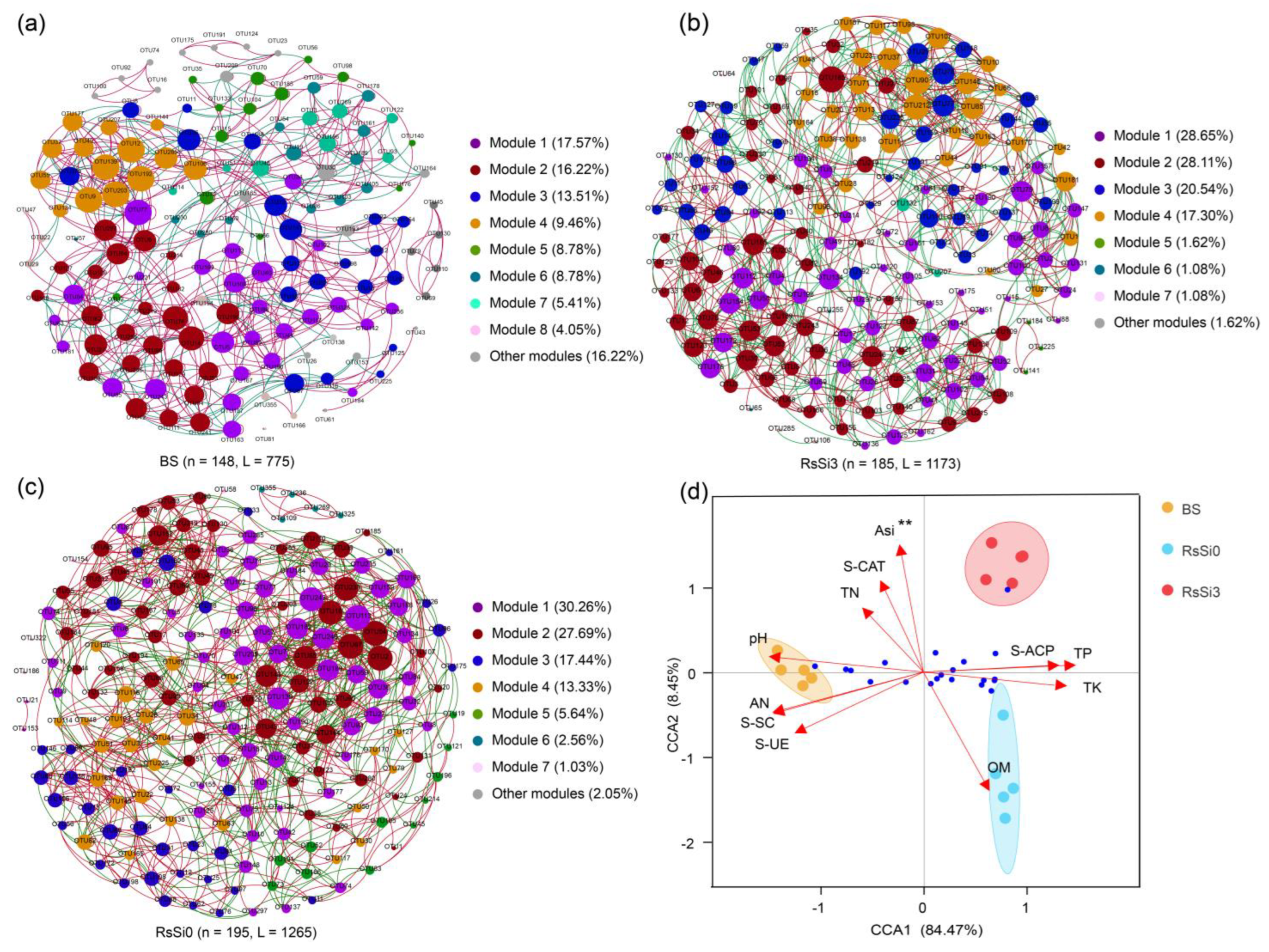
2.3. Impact of Si on the Rhizosphere Soil Bacterial Community
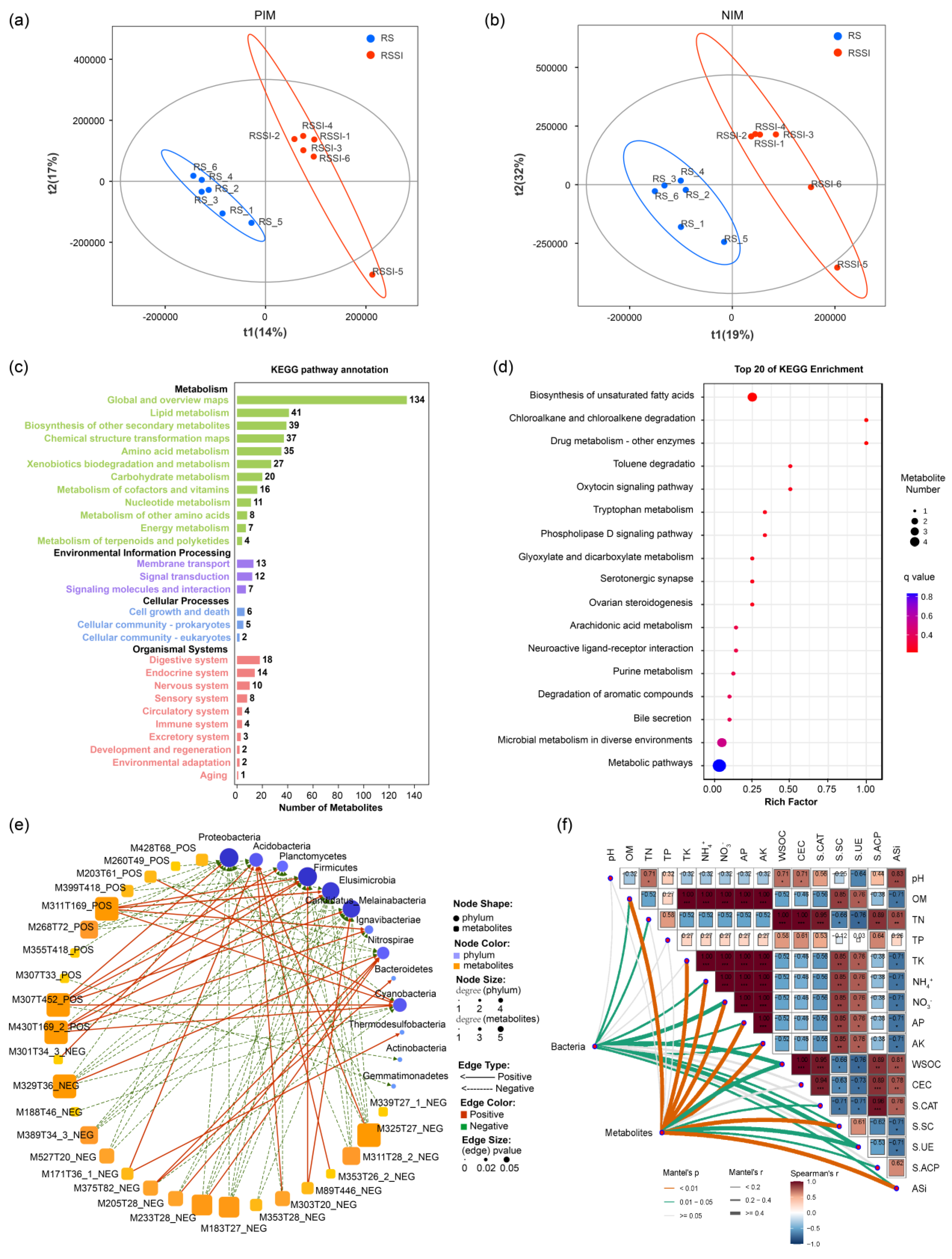
2.4. Impact of Si on Rhizosphere Soil Metabolomics
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. Experimental Treatments and Phenotype Assays
4.3. Rhizosphere Soil Sampling and Measurement
4.4. PacBio Sequencing
4.5. LC-MS/MS Based Soil Metabolomics
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, Y.; Pandey, M.K.; Lu, Q.; Liu, H.; Gangurde, S.S.; Li, S.; Liu, H.; Li, H.; Liang, X.; Varshney, R.K.; et al. Genetic diversity and distinctness based on morphological and SSR markers in peanut. Agrono. J. 2021, 113, 4648–4660. [Google Scholar] [CrossRef]
- Gangurde, S.S.; Khan, A.W.; Janila, P.; Variath, M.T.; Manohar, S.S.; Singam, P.; Chitikineni, A.; Varshney, R.K.; Pandey, M.K. Whole-genome sequencing based discovery of candidate genes and diagnostic markers for seed weight in groundnut. Plant Genome 2022, 00, e20265. [Google Scholar] [CrossRef]
- Ghosh, S.; Mahadevaiah, S.S.; Gowda, S.A.; Gangurde, S.S.; Jadhav, M.P.; Hake, A.A.; Latha, P.; Anitha, T.; Chimmad, V.P.; Mirajkar, K.K.; et al. Genetic mapping of drought tolerance traits phenotyped under varying drought stress environments in peanut (Arachis hypogaea L.). Euphytica 2022, 218, 168. [Google Scholar] [CrossRef]
- Tan, X.; Dai, X.; Chen, T.; Wu, Y.; Yang, D.; Zheng, Y.; Chen, H.; Wan, X.; Yang, Y. Complete genome sequence analysis of Ralstonia solanacearum strain PeaFJ1 provides insights into its strong virulence in peanut plants. Front. Microbiol. 2022, 13, 830900. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.A.X.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Qi, F.; Sun, Z.; Liu, H.; Zheng, Z.; Qin, L.; Shi, L.; Chen, Q.; Liu, H.; Lin, X.; Miao, L.; et al. QTL identification, fine mapping, and marker development for breeding peanut (Arachis hypogaea L.) resistant to bacterial wilt. Theor. Appl. Genet. 2022, 135, 1319–1330. [Google Scholar] [CrossRef]
- Jiang, G.; Wei, Z.; Xu, J.; Chen, H.; Zhang, Y.; She, X.; Macho, A.P.; Ding, W.; Liao, B. Bacterial Wilt in China: History, current status, and future perspectives. Front. Plant Sci. 2017, 8, 1549. [Google Scholar] [CrossRef]
- Luo, H.; Pandey, M.K.; Khan, A.W.; Wu, B.; Guo, J.; Ren, X.; Zhou, X.; Chen, Y.; Chen, W.; Huang, L.; et al. Next-generation sequencing identified genomic region and diagnostic markers for resistance to bacterial wilt on chromosome B02 in peanut (Arachis hypogaea L.). Plant Biotechnol. J. 2019, 17, 2356–2369. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, X.; Ren, X.; Huang, L.; Luo, H.; Chen, Y.; Chen, W.; Liu, N.; Liao, B.; Lei, Y.; et al. A major and stable QTL for bacterial wilt resistance on chromosome B02 identified using a high-density SNP-based genetic linkage map in cultivated peanut Yuanza 9102 derived population. Front. Genet. 2018, 9, 652. [Google Scholar] [CrossRef]
- Chen, W.; Teng, Y.; Li, Z.; Liu, W.; Ren, W.; Luo, Y.; Christie, P. Mechanisms by which organic fertilizer and effective microbes mitigate peanut continuous cropping yield constraints in a red soil of south China. Appl. Soil Ecol. 2018, 128, 23–34. [Google Scholar] [CrossRef]
- Sang, S.; Li, S.; Fan, W.; Wang, N.; Gao, M.; Wang, Z. Zinc thiazole enhances defense enzyme activities and increases pathogen resistance to Ralstonia solanacearum in peanut (Arachis hypogaea) under salt stress. PLoS ONE 2019, 14, e0226951. [Google Scholar] [CrossRef]
- Chen, S.; Qi, G.; Ma, G.; Zhao, X. Biochar amendment controlled bacterial wilt through changing soil chemical properties and microbial community. Microbiol. Res. 2020, 231, 126373. [Google Scholar] [CrossRef]
- Chen, D.; Wang, X.; Zhang, W.; Zhou, Z.; Ding, C.; Liao, Y.; Li, X. Persistent organic fertilization reinforces soil-borne disease suppressiveness of rhizosphere bacterial community. Plant Soil 2020, 452, 313–328. [Google Scholar] [CrossRef]
- Shen, G.; Zhang, S.; Liu, X.; Jiang, Q.; Ding, W. Soil acidification amendments change the rhizosphere bacterial community of tobacco in a bacterial wilt affected field. Appl. Microbiol. Biotechnol. 2018, 102, 9781–9791. [Google Scholar] [CrossRef]
- Hao, J.; Ashley, K. Irreplaceable role of amendment-based strategies to enhance soil health and disease suppression in potato production. Microorganisms 2021, 9, 1006. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Wang, J.; Yang, L.; Zhang, S.; Xu, C.; Ding, W. Soil acidification aggravates the occurrence of bacterial wilt in south China. Front. Microbiol. 2017, 8, 703. [Google Scholar] [CrossRef]
- Lin, W.; Jiang, N.; Peng, L.; Fan, X.; Gao, Y.; Wang, G.; Cai, K. Silicon impacts on soil microflora under Ralstonia Solanacearum inoculation. J. Integr. Agric. 2020, 19, 251–264. [Google Scholar] [CrossRef]
- Sun, S.; Yang, Z.; Song, Z.; Wang, N.; Guo, N.; Niu, J.; Liu, A.; Bai, B.; Ahammed, G.; Chen, S. Silicon enhances plant resistance to Fusarium wilt by promoting antioxidant potential and photosynthetic capacity in cucumber (Cucumis sativus L.). Front. Plant Sci. 2022, 13, 1011859. [Google Scholar] [CrossRef]
- Keeping, M.G. Uptake of silicon by sugarcane from applied sources may not reflect plant-available soil silicon and total silicon content of sources. Front. Plant Sci. 2017, 8, 760. [Google Scholar] [CrossRef]
- Wang, M.; Gao, L.M.; Dong, S.Y.; Sun, Y.M.; Shen, Q.R.; Guo, S.W. Role of silicon on plant-pathogen interactions. Front. Plant Sci. 2017, 8, 701. [Google Scholar] [CrossRef]
- Jiang, N.; Fan, X.; Lin, W.; Wang, G.; Cai, K. Transcriptome analysis reveals new insights into the bacterial wilt resistance mechanism mediated by silicon in tomato. Int. J. Mol. Sci. 2019, 20, 761. [Google Scholar] [CrossRef]
- Dong, Z.; Li, Y.; Xiao, X.; Chen, Y.; Shen, X. Silicon effect on growth, nutrient uptake, and yield of peanut (Arachis hypogaea L.) under aluminum stress. J. Plant Nutr. 2018, 41, 2001–2008. [Google Scholar] [CrossRef]
- de Souza Junior, J.P.; Frazão, J.J.; de Morais, T.C.B.; Espoti, C.D.; dos Santos Sarah, M.M.; de Mello Prado, R. Foliar spraying of silicon associated with salicylic acid increases silicon absorption and peanut growth. Silicon 2021, 13, 1269–1275. [Google Scholar] [CrossRef]
- Fan, X.; Lin, W.; Liu, R.; Jiang, N.; Cai, K. Physiological response and phenolic metabolism in tomato (Solanum lycopersicum) mediated by silicon under Ralstonia solanacearum infection. J. Integr. Agric. 2018, 17, 2160–2171. [Google Scholar] [CrossRef]
- Liang, Y.; Nikolic, M.; Bélanger, R.; Gong, H.; Song, A. Silicon in Agriculture: From Theory to Practice; Springer Publishing: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Ma, C.; Ci, K.; Zhu, J.; Sun, Z.; Liu, Z.; Li, X.; Zhu, Y.; Tang, C.; Wang, P.; Liu, Z. Impacts of exogenous mineral silicon on cadmium migration and transformation in the soil-rice system and on soil health. Sci. Total Environ. 2021, 759, 143501. [Google Scholar] [CrossRef]
- Le, C.N.; Hoang, T.K.; Thai, T.H.; Tran, T.L.; Phan, T.P.N.; Raaijmakers, J.M. Isolation, characterization and comparative analysis of plant-associated bacteria for suppression of soil-borne diseases of field-grown groundnut in Vietnam. Biol. Control 2018, 121, 256–262. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, W.; Xie, Q.; Liu, N.; Liu, L.; Wang, D.; Zhang, X.; Yang, C.; Chen, X.; Tang, D.; et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175. [Google Scholar] [CrossRef]
- Lebeis, S.L.; Paredes, S.H.; Lundberg, D.S.; Breakfield, N.; Gehring, J.; McDonald, M.; Malfatti, S.; Glavina del Rio, T.; Jones, C.D.; Tringe, S.G.; et al. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science 2015, 349, 860–864. [Google Scholar] [CrossRef]
- Cha, J.Y.; Han, S.; Hong, H.J.; Cho, H.; Kim, D.; Kwon, Y.; Kwon, S.K.; Crüsemann, M.; Bok Lee, Y.; Kim, J.F.; et al. Microbial and biochemical basis of a Fusarium wilt-suppressive soil. ISME J. 2016, 10, 119–129. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Yao, S.; Yang, X.; Jiang, X. Correlations between soil metabolomics and bacterial community structures in the pepper rhizosphere under plastic greenhouse cultivation. Sci. Total Environ. 2020, 728, 138439. [Google Scholar] [CrossRef]
- Kwak, M.J.; Kong, H.G.; Choi, K.; Kwon, S.K.; Song, J.Y.; Lee, J.; Lee, P.A.; Choi, S.Y.; Seo, M.; Lee, H.J.; et al. Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat. Biotechnol. 2018, 36, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, J.M.; Badri, D.V.; Vivanco, J.M. Rhizosphere microbiome assemblage is affected by plant development. ISME J. 2014, 8, 790–803. [Google Scholar] [CrossRef]
- Pétriacq, P.; Williams, A.; Cotton, A.; McFarlane, A.E.; Rolfe, S.A.; Ton, J. Metabolite profiling of non-sterile rhizosphere soil. Plant J. 2017, 92, 147–162. [Google Scholar] [CrossRef]
- Mohanram, S.; Kumar, P. Rhizosphere microbiome: Revisiting the synergy of plant-microbe interactions. Ann. Microbiol. 2019, 69, 307–320. [Google Scholar] [CrossRef]
- Sugiyama, A. The soybean rhizosphere: Metabolites, microbes, and beyond—A review. J. Adv. Res. 2019, 19, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Wu, J.; Chen, J.; Shen, W. Physiological mechanisms of improved smut resistance in sugarcane through application of silicon. Front. Plant Sci. 2020, 11, 568130. [Google Scholar] [CrossRef] [PubMed]
- Van Bockhaven, J.; De Vleesschauwer, D.; Höfte, M. Silicon-induced brown spot resistance in rice (Oryza sativa L.). Commun. Agric. Appl. Biol. Sci. 2011, 76, 137–140. [Google Scholar] [PubMed]
- Debona, D.; Rodrigues, F.; Rios, J.; Nascimento, K.; de Castro Silva, L. The effect of silicon on antioxidant metabolism of wheat leaves infected by Pyricularia oryzae. Plant Pathol. 2014, 63, 581–589. [Google Scholar] [CrossRef]
- Win, K.T.; Maeda, S.; Kobayashi, M.; Jiang, C.J. Silicon enhances resistance to red crown rot caused by Calonectria ilicicola in soybean. Agronomy 2021, 11, 899. [Google Scholar] [CrossRef]
- Shetty, R.; Jensen, B.; Shetty, N.; Hansen, M.; Hansen, C.; Starkey, K.; Jørgensen, H.J.L. Silicon induced resistance against powdery mildew of roses caused by Podosphaera pannosa. Plant Pathol. 2011, 61, 120–131. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.; Jiang, N.; Yan, J.; Lin, W.; Cai, K. Silicon controls bacterial wilt disease in tomato plants and inhibits the virulence-related gene expression of Ralstonia solanacearum. Int. J. Mol. Sci. 2022, 23, 6965. [Google Scholar] [CrossRef]
- Fu, Q.; Lai, J.L.; Ji, X.H.; Luo, Z.; Wu, G.; Luo, X. Alterations of the rhizosphere soil microbial community composition and metabolite profiles of Zea mays by polyethylene-particles of different molecular weights. J. Hazard. Mater. 2022, 423, 127062. [Google Scholar] [CrossRef]
- Fernandes, S.; Pereira Da Silva, G.; Prado, R.D.M.; Rossatto, D.R. Association of root and leaf silicon application decreases the C/Si ratio, increasing carbon gain and dry mass production in peanut plants. Commun. Soil Sci. Plan. 2021, 52, 2349–2357. [Google Scholar] [CrossRef]
- Yang, S.; Chen, X.; Jiang, Z.; Ding, J.; Sun, X.; Xu, J. Effects of biochar application on soil organic carbon composition and enzyme activity in paddy soil under water-saving irrigation. Int. J. Environ. Res. Public Health 2020, 17, 333. [Google Scholar] [CrossRef] [PubMed]
- Bailey, V.L.; Fansler, S.J.; Smith, J.L.; Bolton, H. Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol. Biochem. 2011, 43, 296–301. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Mazzola, M. Soil immune responses. Science 2016, 352, 1392–1393. [Google Scholar] [CrossRef]
- Deng, Q.; Yu, T.; Zeng, Z.; Ashraf, U.; Shi, Q.; Huang, S.; Lian, T.; Chen, J.; Muzaffar, W.; Shen, W. Silicon application modulates the growth, rhizosphere soil characteristics, and bacterial community structure in sugarcane. Front. Plant Sci. 2021, 12, 710139. [Google Scholar] [CrossRef]
- Yuan, Z.; Pang, Z.; Fallah, N.; Zhou, Y.; Dong, F.; Lin, W.; Hu, C. Silicon fertilizer mediated structural variation and niche differentiation in the rhizosphere and endosphere bacterial microbiome and metabolites of sugarcane. Front. Microbiol. 2022, 13, 1009505. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Q.; Liu, Z.; Pan, X.; Zhang, Y. Silicon application and related changes in soil bacterial community dynamics reduced ginseng black spot incidence in Panax ginseng in a short-term study. BMC Microbiol. 2019, 19, 263. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Zeng, W.; Xiao, Y.; Li, P.; Gu, S.; Wu, S.; Zhai, Z.; Feng, K.; Deng, Y.; Hu, Q. Fungi-bacteria associations in wilt diseased rhizosphere and endosphere by interdomain ecological network analysis. Front. Microbiol. 2021, 12, 722626. [Google Scholar] [CrossRef] [PubMed]
- Ujita, Y.; Sakata, M.; Yoshihara, A.; Hikichi, Y.; Kai, K. Signal production and response specificity in the phc quorum sensing systems of Ralstonia solanacearum species complex. ACS Chem. Biol. 2019, 14, 2243–2251. [Google Scholar] [PubMed]
- Yin, Y.; Li, R.; Liang, W.T.; Zhang, W.B.; Hu, Z.; Ma, J.C.; Wang, H.H. Of its five acyl carrier proteins, only AcpP1 functions in Ralstonia solanacearum fatty acid synthesis. Front. Microbiol. 2022, 13, 1014971. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Yuan, S.; Xun, G.; Shi, W.; Pan, B.; Guan, H.; Shen, B.; Shen, Q. Root exudates from two tobacco cultivars affect colonization of Ralstonia solanacearum and the disease index. Eur. J. Plant Pathol. 2015, 141, 667–677. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, C.; Chen, H.; Yuan, M.; Nipper, R.; Prakash, C.S.; Zhuang, W.; He, G. QTL mapping for bacterial wilt resistance in peanut (Arachis hypogaea L.). Mol. Breed. 2016, 36, 13. [Google Scholar] [CrossRef]
- Pan, X.; Chen, J.; Yang, A.; Yuan, Q.; Zhao, W.; Xu, T.; Chen, B.; Ren, M.; Geng, R.; Zong, Z.; et al. Comparative transcriptome profiling reveals defense-related genes against Ralstonia solanacearum infection in tobacco. Front. Plant Sci. 2021, 12, 767882. [Google Scholar] [CrossRef]
- Yu, T.; Cheng, L.; Liu, Q.; Wang, S.; Zhou, Y.; Zhong, H.; Tang, M.; Nian, H.; Lian, T. Effects of waterlogging on soybean rhizosphere bacterial community using V4, LoopSeq, and PacBio 16S rRNA Sequence. Microbiol. Spectr. 2022, 10, 767882. [Google Scholar] [CrossRef]
- Abdul Rahman, N.S.; Abdul Hamid, N.W.; Nadarajah, K. Effects of abiotic stress on soil microbiome. Int. J. Mol. Sci. 2021, 22, 9036. [Google Scholar] [CrossRef]
- Mosher, J.J.; Bernberg, E.L.; Shevchenko, O.; Kan, J.; Kaplan, L.A. Efficacy of a 3rd generation high-throughput sequencing platform for analyses of 16S rRNA genes from environmental samples. J. Microbiol. Methods 2013, 95, 175–181. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Yun, K.; Mun, S.; Chung, W.H.; Choi, S.Y.; Nam, Y.; Lim, M.Y.; Hong, C.P.; Park, C.; Ahn, Y.J.; et al. The effect of taxonomic classification by full-length 16S rRNA sequencing with a synthetic long-read technology. Sci. Rep. 2021, 11, 1727. [Google Scholar] [CrossRef] [PubMed]
- Lian, T.; Ma, Q.; Shi, Q.; Cai, Z.; Zhang, Y.; Cheng, Y.; Nian, H. High aluminum stress drives different rhizosphere soil enzyme activities and bacterial community structure between aluminum-tolerant and aluminum-sensitive soybean genotypes. Plant Soil 2019, 440, 409–425. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, H.; White, J.; Chen, X.; Li, H.; Qu, X.; Ji, R. Metabolomics reveal that engineered nanomaterial exposure in soil alters both soil rhizosphere metabolite profiles and maize metabolic pathways. Environ. Sci. Nano 2019, 6, 1716–1727. [Google Scholar] [CrossRef]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using metaboanalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef] [PubMed]

| Treatment | S-CAT | S-SC | S-UE | S-ACP |
|---|---|---|---|---|
| mL/g/20 min | mg/g/24 h | mg/g/24 h | µg/g/2 h | |
| CK | 2.33 ± 0.07 ab | 4.23 ± 0.03 C | 0.64 ± 0.01 A | 134.88 ± 1.67 A |
| SI | 2.26 ± 0.08 ab | 4.70 ± 0.01 B | 0.58 ± 0.01 B | 138.30 ± 2.90 A |
| RS | 2.19 ± 0.05 b | 4.98 ± 0.06 A | 0.61 ± 0.01 A | 109.58 ± 3.33 B |
| RSSI | 2.40 ± 0.02 a | 3.61 ± 0.07 C | 0.53 ± 0.01 C | 111.95 ± 1.33 B |
| Groups | R_Value | p_Value | Sig |
|---|---|---|---|
| BS_vs_RSSI | 1 | 0.014 | * |
| BS_vs_RSSI | 1 | 0.013 | * |
| RS_vs_RSSI | 0.864 | 0.007 | ** |
| BS_vs_RS_vs_RSSI | 0.8293 | 0.001 | ** |
| ID | Name | Class | Pathway | Fold |
|---|---|---|---|---|
| PIM | ||||
| M260T49_POS | Venlafaxine | Organooxygen compounds | Global and overview maps | 1.42 |
| M430T169_2_POS | Sorbitane monostearate | Fatty acid | Lipid metabolism | 2.12 |
| M307T33_POS | 11,14,17-Eicosatrienoic acid | Fatty acid | 0.68 | |
| M311T169_POS | Oleic acid ethyl ester | Fatty acid | 2.24 | |
| M268T72_POS | Auramine o | Organic chloride salt | Biosynthesis of other secondary metabolites | 0.68 |
| M533T33_POS | Manumycin a | Organooxygen compound | Chemical structure transformation maps | 0.68 |
| M223T597_POS | Tetradecanedioic acid | Organooxygen compound | 0.83 | |
| M399T418_POS | Ethiprole | Monocyclic heteroarene | 0.64 | |
| M428T68_POS | Peimisine | Alkaloid | 0.68 | |
| M307T452_POS | Perchloroterephthalic acid | Organooxygen compound | Carbohydrate metabolism | 1.9 |
| M355T418_POS | Carboplatin | Cyclobutanedicarboxylate | Metabolism of other amino acids | 0.64 |
| M203T61_POS | Cyclohexanone | Ketone | Metabolism of terpenoids and polyketides | 1.68 |
| NIM | ||||
| M353T26_2_NEG | Prostaglandin f2.alpha. | Fatty acid | Lipid metabolism | 0.23 |
| M618T34_NEG | N-palmitoyl-d-erythro-dihydroceramide-1-phosphate | Fatty acid | 0.55 | |
| M375T82_NEG | (R)-butaprost | Fatty acid | 0.63 | |
| M375T37_NEG | Hexaenoic acid | Fatty acid | 0.62 | |
| M527T20_NEG | Pachymic acid | Fatty acid | 0.18 | |
| M301T34_3_NEG | Eicosapentaenoic acid | Fatty acid | 0.56 | |
| M389T34_3_NEG | Ilicicolin a | Phenolate | Chemical structure transformation maps | 0.78 |
| M339T27_1_NEG | Gly-His-Lys | Amino acids | Amino acid metabolism | 0.72 |
| M89T446_NEG | Oxalate | Amino acids, peptides, and analogues | 1.4 | |
| M325T27_NEG | Hydroquinidine | Organonitrogen compound | Xenobiotics biodegradation and metabolism | 0.78 |
| M205T28_NEG | Hydroxybenzoic acid | Benzoic acids and derivatives | 0.73 | |
| M171T36_1_NEG | p-Toluenesulfonic acid | Benzoic acids and derivatives | 0.61 | |
| M329T36_NEG | Aurantio-obtusin | Anthraquinones | 0.54 | |
| M311T28_2_NEG | Thymol-beta-d-glucoside | Carbohydrate derivative | Carbohydrate metabolism | 0.83 |
| M183T27_NEG | Thiouric acid | Purines and purine derivatives | Nucleotide metabolism | 0.77 |
| M353T28_NEG | Rauwolscine | Alkaloid | Energy metabolism | 0.84 |
| M188T46_NEG | Kynurenic acid | Quinoline carboxylic acids | 0.69 | |
| M303T20_NEG | 2,6-Dihydroxy-7-methoxy-1,1,4a-trimethyl-3,4,10,10a-tetrahydro-2h-phenanthren-9-one | Terpenoid | Metabolism of terpenoids and polyketides | 0.11 |
| M233T28_NEG | Valerenic acid | Terpenoid | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Q.; Liu, H.; Lu, Q.; Gangurde, S.S.; Du, P.; Li, H.; Li, S.; Liu, H.; Wang, R.; Huang, L.; et al. Silicon Application for the Modulation of Rhizosphere Soil Bacterial Community Structures and Metabolite Profiles in Peanut under Ralstonia solanacearum Inoculation. Int. J. Mol. Sci. 2023, 24, 3268. https://doi.org/10.3390/ijms24043268
Deng Q, Liu H, Lu Q, Gangurde SS, Du P, Li H, Li S, Liu H, Wang R, Huang L, et al. Silicon Application for the Modulation of Rhizosphere Soil Bacterial Community Structures and Metabolite Profiles in Peanut under Ralstonia solanacearum Inoculation. International Journal of Molecular Sciences. 2023; 24(4):3268. https://doi.org/10.3390/ijms24043268
Chicago/Turabian StyleDeng, Quanqing, Hao Liu, Qing Lu, Sunil S. Gangurde, Puxuan Du, Haifen Li, Shaoxiong Li, Haiyan Liu, Runfeng Wang, Lu Huang, and et al. 2023. "Silicon Application for the Modulation of Rhizosphere Soil Bacterial Community Structures and Metabolite Profiles in Peanut under Ralstonia solanacearum Inoculation" International Journal of Molecular Sciences 24, no. 4: 3268. https://doi.org/10.3390/ijms24043268
APA StyleDeng, Q., Liu, H., Lu, Q., Gangurde, S. S., Du, P., Li, H., Li, S., Liu, H., Wang, R., Huang, L., Chen, R., Fan, C., Liang, X., Chen, X., & Hong, Y. (2023). Silicon Application for the Modulation of Rhizosphere Soil Bacterial Community Structures and Metabolite Profiles in Peanut under Ralstonia solanacearum Inoculation. International Journal of Molecular Sciences, 24(4), 3268. https://doi.org/10.3390/ijms24043268








