Pharmacometabolomics for the Study of Lipid-Lowering Therapies: Opportunities and Challenges
Abstract
:1. Introduction
2. Methodological Approaches for Pharmacometabolomics and Pharmacolipidomics
2.1. Nuclear Magnetic Resonance (NMR)
2.2. Gas Chromatography-Mass Spectrometry (GC-MS)
2.3. Liquid Chromatography-Mass Spectrometry (LC-MS)
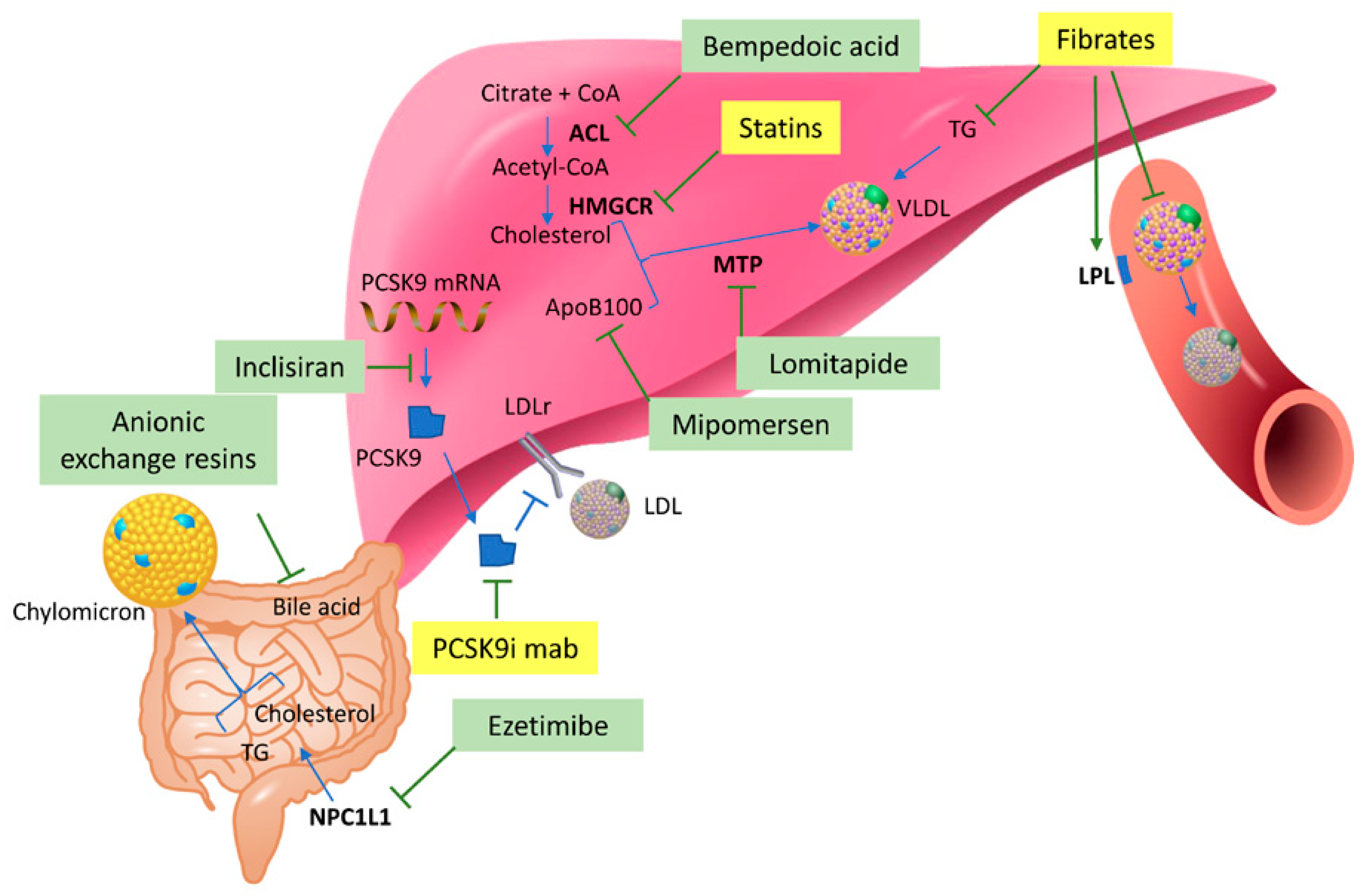
3. Lipid-Lowering Therapies and Metabolomics
3.1. Statins
3.1.1. Statin Response Variability
3.1.2. Alterations in Gut Microbiota by Statin Therapy
3.1.3. Adverse Effects of Statins
3.1.4. Beneficial Effects of Statins
3.2. PCSK9 Inhibitors
3.3. Fibrates
Combination Therapy of Statins and Fibrates
3.4. Nutraceutical and Dietary Habits
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAE | Annurca apple polyphenolic extract |
| ACS | acute coronary syndrome |
| ASCVD | atherosclerotic cardiovascular disease |
| ATP | adenosine triphosphate |
| CAP | Cholesterol and Pharmacogenetic |
| CE | capillary electrophoresis |
| CPT-1 | carnitine palmitoyltransferase I |
| CVD | cardiovascular disease |
| FH | familial hypercholesterolemia |
| FT-ICR | Fourier transform-ion cyclotron resonance |
| GC | gas chromatography |
| GlycA | glycoprotein acetylation |
| GSK-3β | glycogen synthase kinase-3β |
| HDL | high-density lipoprotein |
| HK-2 | hexokinase 2 |
| HMG-CoA | 3-hydroxy-3-methyl-glutaryl-coenzyme A |
| IDL | intermediate-density lipoprotein |
| LC | liquid chromatography |
| LDL | low-density lipoprotein |
| LDL-C | low-density lipoprotein cholesterol |
| Lp(a) | lipoprotein(a) |
| MRM | multiple reaction monitoring |
| MS | mass spectrometry |
| NIST | National Institute of Standards and Technology |
| NMR | nuclear magnetic resonance |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| PAH | pulmonary arterial hypertension |
| PCSK9 | proprotein convertase subtilisin/kexin type 9 |
| PPARα | peroxisome proliferator-activated receptor alpha |
| PUFAs | polyunsaturated fatty acids |
| Q-TOF | quadrupole-time of flight |
| ROS | reactive oxygen species |
| SNP | single nucleotide polymorphism |
| SREBP-1c | sterol regulatory element-binding protein 1c |
| TC | total cholesterol |
| TGs | triglycerides |
| TOF | time of flight |
| UHPLC | ultra-high-performance liquid chromatography |
| UPLC | ultra-performance liquid chromatography |
| VLDL | very-low-density lipoprotein |
| WHHL | Watanabe heritable hyperlipidemic |
References
- Larsen, L.E.; Stoekenbroek, R.M.; Kastelein, J.J.P.; Holleboom, A.G. Moving Targets: Recent Advances in Lipid-Lowering Therapies. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 349–359. [Google Scholar] [CrossRef]
- Banfi, C.; Baetta, R.; Gianazza, E.; Tremoli, E. Technological advances and proteomic applications in drug discovery and target deconvolution: Identification of the pleiotropic effects of statins. Drug Discov. Today 2017, 22, 848–869. [Google Scholar] [CrossRef]
- Pontremoli, M.; Brioschi, M.; Baetta, R.; Ghilardi, S.; Banfi, C. Identification of DKK-1 as a novel mediator of statin effects in human endothelial cells. Sci. Rep. 2018, 8, 16671. [Google Scholar] [CrossRef]
- Brioschi, M.; Lento, S.; Tremoli, E.; Banfi, C. Proteomic analysis of endothelial cell secretome: A means of studying the pleiotropic effects of Hmg-CoA reductase inhibitors. J. Proteom. 2013, 78, 346–361. [Google Scholar] [CrossRef]
- Chevli, P.A.; Freedman, B.I.; Hsu, F.C.; Xu, J.; Rudock, M.E.; Ma, L.; Parks, J.S.; Palmer, N.D.; Shapiro, M.D. Plasma metabolomic profiling in subclinical atherosclerosis: The Diabetes Heart Study. Cardiovasc. Diabetol. 2021, 20, 231. [Google Scholar] [CrossRef]
- Gowda, G.A.; Zhang, S.; Gu, H.; Asiago, V.; Shanaiah, N.; Raftery, D. Metabolomics-based methods for early disease diagnostics. Expert Rev. Mol. Diagn. 2008, 8, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Lindon, J.C. Systems biology: Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar] [CrossRef]
- Holmes, E.; Wilson, I.D.; Nicholson, J.K. Metabolic phenotyping in health and disease. Cell 2008, 134, 714–717. [Google Scholar] [CrossRef]
- Burt, T.; Nandal, S. Pharmacometabolomics in Early-Phase Clinical Development. Clin. Transl. Sci. 2016, 9, 128–138. [Google Scholar] [CrossRef]
- Kim, H.; Yoon, Y. Pharmacometabolomics: Current Applications and Future Perspectives. Transl. Clin. Pharmacol. 2014, 22, 8–10. [Google Scholar] [CrossRef] [Green Version]
- Rattray, N.; Daouk, R. Pharmacometabolomics and Precision Medicine Special Issue Editorial. Metabolomics 2017, 13, 59. [Google Scholar] [CrossRef]
- Huang, Q.; Aa, J.; Jia, H.; Xin, X.; Tao, C.; Liu, L.; Zou, B.; Song, Q.; Shi, J.; Cao, B.; et al. A Pharmacometabonomic Approach To Predicting Metabolic Phenotypes and Pharmacokinetic Parameters of Atorvastatin in Healthy Volunteers. J. Proteome Res. 2015, 14, 3970–3981. [Google Scholar] [CrossRef]
- Clayton, T.A.; Lindon, J.C.; Cloarec, O.; Antti, H.; Charuel, C.; Hanton, G.; Provost, J.P.; Le Net, J.L.; Baker, D.; Walley, R.J.; et al. Pharmaco-metabonomic phenotyping and personalized drug treatment. Nature 2006, 440, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Wilson, I.D. Drugs, bugs, and personalized medicine: Pharmacometabonomics enters the ring. Proc. Natl. Acad. Sci. USA 2009, 106, 14187–14188. [Google Scholar] [CrossRef]
- Winnike, J.H.; Li, Z.; Wright, F.A.; Macdonald, J.M.; O’Connell, T.M.; Watkins, P.B. Use of pharmaco-metabonomics for early prediction of acetaminophen-induced hepatotoxicity in humans. Clin. Pharmacol. Ther. 2010, 88, 45–51. [Google Scholar] [CrossRef]
- Alarcon-Barrera, J.C.; Kostidis, S.; Ondo-Mendez, A.; Giera, M. Recent advances in metabolomics analysis for early drug development. Drug Discov. Today 2022, 27, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Harrieder, E.M.; Kretschmer, F.; Bocker, S.; Witting, M. Current state-of-the-art of separation methods used in LC-MS based metabolomics and lipidomics. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2022, 1188, 123069. [Google Scholar] [CrossRef]
- Macedo, A.N.; Faccio, A.T.; Fukuji, T.S.; Canuto, G.A.B.; Tavares, M.F.M. Analytical Platforms for Mass Spectrometry-Based Metabolomics of Polar and Ionizable Metabolites. Adv. Exp. Med. Biol. 2021, 1336, 215–242. [Google Scholar] [CrossRef]
- Banoei, M.M.; Donnelly, S.J.; Mickiewicz, B.; Weljie, A.; Vogel, H.J.; Winston, B.W. Metabolomics in critical care medicine: A new approach to biomarker discovery. Clin. Investig. Med. 2014, 37, E363–E376. [Google Scholar] [CrossRef]
- Paglia, G.; Del Greco, F.M.; Sigurdsson, B.B.; Rainer, J.; Volani, C.; Hicks, A.A.; Pramstaller, P.P.; Smarason, S.V. Influence of collection tubes during quantitative targeted metabolomics studies in human blood samples. Clin. Chim. Acta 2018, 486, 320–328. [Google Scholar] [CrossRef]
- Volani, C.; Caprioli, G.; Calderisi, G.; Sigurdsson, B.B.; Rainer, J.; Gentilini, I.; Hicks, A.A.; Pramstaller, P.P.; Weiss, G.; Smarason, S.V.; et al. Pre-analytic evaluation of volumetric absorptive microsampling and integration in a mass spectrometry-based metabolomics workflow. Anal. Bioanal. Chem. 2017, 409, 6263–6276. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cebrián, N.; Ferreiro, P.V.; Hueso, F.J.C.; Andrés, J.L.P.; Puchades-Carrasco, L.; Pineda-Lucena, A. Pharmacometabolomics by NMR in Oncology: A Systematic Review. Pharmaceuticals 2021, 14, 1015. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Villaret-Cazadamont, J.; Claus, S.P.; Canlet, C.; Guillou, H.; Cabaton, N.J.; Ellero-Simatos, S. Important Considerations for Sample Collection in Metabolomics Studies with a Special Focus on Applications to Liver Functions. Metabolites 2020, 10, 104. [Google Scholar] [CrossRef]
- Zierer, J.; Jackson, M.A.; Kastenmuller, G.; Mangino, M.; Long, T.; Telenti, A.; Mohney, R.P.; Small, K.S.; Bell, J.T.; Steves, C.J.; et al. The fecal metabolome as a functional readout of the gut microbiome. Nat. Genet. 2018, 50, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics by Gas Chromatography-Mass Spectrometry: Combined Targeted and Untargeted Profiling. Curr. Protoc. Mol. Biol. 2016, 114, 30.4.1–30.4.32. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies—Challenges and Emerging Directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef]
- Marshall, D.D.; Powers, R. Beyond the paradigm: Combining mass spectrometry and nuclear magnetic resonance for metabolomics. Prog. Nucl. Magn. Reson. Spectrosc. 2017, 100, 1–16. [Google Scholar] [CrossRef]
- Emwas, A.H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR Spectroscopy for Metabolomics Research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef]
- Au, A. Metabolomics and Lipidomics of Ischemic Stroke. Adv. Clin. Chem. 2018, 85, 31–69. [Google Scholar] [CrossRef]
- Han, X.; Yang, K.; Gross, R.W. Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass Spectrom. Rev. 2012, 31, 134–178. [Google Scholar] [CrossRef] [Green Version]
- Belhaj, M.R.; Lawler, N.G.; Hoffman, N.J. Metabolomics and Lipidomics: Expanding the Molecular Landscape of Exercise Biology. Metabolites 2021, 11, 151. [Google Scholar] [CrossRef]
- Kostara, C.E.; Karakitsou, K.S.; Florentin, M.; Bairaktari, E.T.; Tsimihodimos, V. Progressive, Qualitative, and Quantitative Alterations in HDL Lipidome from Healthy Subjects to Patients with Prediabetes and Type 2 Diabetes. Metabolites 2022, 12, 683. [Google Scholar] [CrossRef]
- Kostara, C.E.; Papathanasiou, A.; Psychogios, N.; Cung, M.T.; Elisaf, M.S.; Goudevenos, J.; Bairaktari, E.T. NMR-based lipidomic analysis of blood lipoproteins differentiates the progression of coronary heart disease. J. Proteome Res. 2014, 13, 2585–2598. [Google Scholar] [CrossRef]
- Haslauer, K.E.; Hemmler, D.; Schmitt-Kopplin, P.; Heinzmann, S.S. Guidelines for the Use of Deuterium Oxide (D2O) in (1)H NMR Metabolomics. Anal. Chem. 2019, 91, 11063–11069. [Google Scholar] [CrossRef] [PubMed]
- Bodi, V.; Marrachelli, V.G.; Husser, O.; Chorro, F.J.; Vina, J.R.; Monleon, D. Metabolomics in the diagnosis of acute myocardial ischemia. J. Cardiovasc. Transl. Res. 2013, 6, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Amor, A.J.; Vinagre, I.; Valverde, M.; Urquizu, X.; Meler, E.; Lopez, E.; Alonso, N.; Pane, A.; Gimenez, M.; Codina, L.; et al. Nuclear magnetic resonance-based metabolomic analysis in the assessment of preclinical atherosclerosis in type 1 diabetes and preeclampsia. Diabetes Res. Clin. Pract. 2021, 171, 108548. [Google Scholar] [CrossRef] [PubMed]
- Mansell, T.; Saffery, R.; Burugupalli, S.; Ponsonby, A.L.; Tang, M.L.K.; O’Hely, M.; Bekkering, S.; Smith, A.A.T.; Rowland, R.; Ranganathan, S.; et al. Early life infection and proinflammatory, atherogenic metabolomic and lipidomic profiles in infancy: A population-based cohort study. eLife 2022, 11, e75170. [Google Scholar] [CrossRef] [PubMed]
- Kostara, C.E.; Ferrannini, E.; Bairaktari, E.T.; Papathanasiou, A.; Elisaf, M.; Tsimihodimos, V. Early Signs of Atherogenic Features in the HDL Lipidomes of Normolipidemic Patients Newly Diagnosed with Type 2 Diabetes. Int. J. Mol. Sci. 2020, 21, 8835. [Google Scholar] [CrossRef]
- Huang, Z.; Klaric, L.; Krasauskaite, J.; McLachlan, S.; Strachan, M.W.J.; Wilson, J.F.; Price, J.F. Serum metabolomic profiles associated with subclinical and clinical cardiovascular phenotypes in people with type 2 diabetes. Cardiovasc. Diabetol. 2022, 21, 62. [Google Scholar] [CrossRef]
- Hanafi, R.S.; Lammerhofer, M. Quality-by-design approach for development of aqueous headspace microextraction GC-MS method for targeted metabolomics of small aldehydes in plasma of cardiovascular patients. Anal. Chim. Acta 2022, 1221, 340176. [Google Scholar] [CrossRef]
- Sobczak, A.I.S.; Pitt, S.J.; Smith, T.K.; Ajjan, R.A.; Stewart, A.J. Lipidomic profiling of plasma free fatty acids in type-1 diabetes highlights specific changes in lipid metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158823. [Google Scholar] [CrossRef]
- Paglia, G.; Smith, A.J.; Astarita, G. Ion mobility mass spectrometry in the omics era: Challenges and opportunities for metabolomics and lipidomics. Mass Spectrom. Rev. 2021, 41, 722–765. [Google Scholar] [CrossRef]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’Auria, J.; Ewald, J.; Ewald, J.C.; Fraser, P.D.; Giavalisco, P.; Hall, R.D.; et al. Mass spectrometry-based metabolomics: A guide for annotation, quantification and best reporting practices. Nat. Methods 2021, 18, 747–756. [Google Scholar] [CrossRef]
- Gowda, S.G.B.; Gowda, D.; Hou, F.; Chiba, H.; Parcha, V.; Arora, P.; Halade, G.V.; Hui, S.P. Temporal lipid profiling in the progression from acute to chronic heart failure in mice and ischemic human hearts. Atherosclerosis 2022, 363, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Gowda, S.G.B.; Gowda, D.; Kain, V.; Chiba, H.; Hui, S.P.; Chalfant, C.E.; Parcha, V.; Arora, P.; Halade, G.V. Sphingosine-1-phosphate interactions in the spleen and heart reflect extent of cardiac repair in mice and failing human hearts. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H599–H611. [Google Scholar] [CrossRef]
- Paavola, T.; Bergmann, U.; Kuusisto, S.; Kakko, S.; Savolainen, M.J.; Salonurmi, T. Distinct Fatty Acid Compositions of HDL Phospholipids Are Characteristic of Metabolic Syndrome and Premature Coronary Heart Disease—Family Study. Int. J. Mol. Sci. 2021, 22, 4908. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Banach, M.; Sahebkar, A.; Corsini, A.; Sirtori, C.R. ETC-1002 (Bempedoic acid) for the management of hyperlipidemia: From preclinical studies to phase 3 trials. Expert Opin. Pharmacother. 2019, 20, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Bilen, O.; Ballantyne, C.M. Bempedoic Acid (ETC-1002): An Investigational Inhibitor of ATP Citrate Lyase. Curr. Atheroscler. Rep. 2016, 18, 61. [Google Scholar] [CrossRef]
- Xu, Q.Y.; Liu, Y.H.; Zhang, Q.; Ma, B.; Yang, Z.D.; Liu, L.; Yao, D.; Cui, G.B.; Sun, J.J.; Wu, Z.M. Metabolomic analysis of simvastatin and fenofibrate intervention in high-lipid diet-induced hyperlipidemia rats. Acta Pharmacol. Sin. 2014, 35, 1265–1273. [Google Scholar] [CrossRef] [Green Version]
- Muller, A.L.; Freed, D.H. Basic and Clinical Observations of Mevalonate Depletion on the Mevalonate Signaling Pathway. Curr. Mol. Pharmacol. 2017, 10, 6–12. [Google Scholar] [CrossRef]
- Liao, J.K.; Laufs, U. Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 89–118. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hu, H.; Yang, J.; Yao, Q.; Xu, H.; Yu, Y.; Liu, T.; Lin, S. The efficacy and safety of statin in combination with ezetimibe compared with double-dose statin in patients with high cardiovascular risk: A meta-analysis. Bosn. J. Basic Med. Sci. 2020, 20, 169–182. [Google Scholar] [CrossRef]
- Jones, M.R.; Nwose, O.M. Role of colesevelam in combination lipid-lowering therapy. Am. J. Cardiovasc. Drugs 2013, 13, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef] [PubMed]
- Bellosta, S.; Corsini, A. Statin drug interactions and related adverse reactions: An update. Expert Opin. Drug Saf. 2018, 17, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.; Ravi, R.; Vangipurapu, J.; Laakso, M. Metabolite Signature of Simvastatin Treatment Involves Multiple Metabolic Pathways. Metabolites 2022, 12, 753. [Google Scholar] [CrossRef]
- Krauss, R.M.; Zhu, H.; Kaddurah-Daouk, R. Pharmacometabolomics of statin response. Clin. Pharmacol. Ther. 2013, 94, 562–565. [Google Scholar] [CrossRef]
- Morris, M.E.; Felmlee, M.A. Overview of the proton-coupled MCT (SLC16A) family of transporters: Characterization, function and role in the transport of the drug of abuse gamma-hydroxybutyric acid. AAPS J. 2008, 10, 311–321. [Google Scholar] [CrossRef]
- Felmlee, M.A.; Jones, R.S.; Rodriguez-Cruz, V.; Follman, K.E.; Morris, M.E. Monocarboxylate Transporters (SLC16): Function, Regulation, and Role in Health and Disease. Pharmacol. Rev. 2020, 72, 466–485. [Google Scholar] [CrossRef]
- Wu, X.; Whitfield, L.R.; Stewart, B.H. Atorvastatin transport in the Caco-2 cell model: Contributions of P-glycoprotein and the proton-monocarboxylic acid co-transporter. Pharm. Res. 2000, 17, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.A.; Lin, F.; Hulley, S.B.; Blanche, P.J.; Waters, D.; Shiboski, S.; Rotter, J.I.; Nickerson, D.A.; Yang, H.; Saad, M.; et al. Phenotypic predictors of response to simvastatin therapy among African-Americans and Caucasians: The Cholesterol and Pharmacogenetics (CAP) Study. Am. J. Cardiol. 2006, 97, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Kaddurah-Daouk, R.; Baillie, R.A.; Zhu, H.; Zeng, Z.B.; Wiest, M.M.; Nguyen, U.T.; Watkins, S.M.; Krauss, R.M. Lipidomic analysis of variation in response to simvastatin in the Cholesterol and Pharmacogenetics Study. Metabolomics 2010, 6, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Kaddurah-Daouk, R.; Baillie, R.A.; Zhu, H.; Zeng, Z.B.; Wiest, M.M.; Nguyen, U.T.; Wojnoonski, K.; Watkins, S.M.; Trupp, M.; Krauss, R.M. Enteric microbiome metabolites correlate with response to simvastatin treatment. PLoS ONE 2011, 6, e25482. [Google Scholar] [CrossRef]
- Peyser, B.; Perry, E.P.; Singh, K.; Gill, R.D.; Mehan, M.R.; Haga, S.B.; Musty, M.D.; Milazzo, N.A.; Savard, D.; Li, Y.J.; et al. Effects of Delivering SLCO1B1 Pharmacogenetic Information in Randomized Trial and Observational Settings. Circ. Genom. Precis. Med. 2018, 11, e002228. [Google Scholar] [CrossRef]
- Stewart, A. SLCO1B1 Polymorphisms and Statin-Induced Myopathy. PLoS Curr. 2013, 5, ecurrents.eogt.d21e7f0c58463571bb0d9d3a19b82203. [Google Scholar] [CrossRef]
- Trupp, M.; Zhu, H.; Wikoff, W.R.; Baillie, R.A.; Zeng, Z.B.; Karp, P.D.; Fiehn, O.; Krauss, R.M.; Kaddurah-Daouk, R. Metabolomics reveals amino acids contribute to variation in response to simvastatin treatment. PLoS ONE 2012, 7, e38386. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Kiaie, N.; Hajighasemi, S.; Banach, M.; Penson, P.E.; Jamialahmadi, T.; Sahebkar, A. Statin-Induced Nitric Oxide Signaling: Mechanisms and Therapeutic Implications. J. Clin. Med. 2019, 8, 2051. [Google Scholar] [CrossRef]
- Golomb, B.A.; Dimsdale, J.E.; White, H.L.; Ritchie, J.B.; Criqui, M.H. Reduction in blood pressure with statins: Results from the UCSD Statin Study, a randomized trial. Arch. Intern. Med. 2008, 168, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Vasankari, T.; Ahotupa, M.; Viikari, J.; Nuotio, I.; Strandberg, T.; Vanhanen, H.; Gylling, H.; Miettinen, T.; Tikkanen, M.J. Effect of 12-month statin therapy on antioxidant potential of LDL and serum antioxidant vitamin concentrations. Ann. Med. 2004, 36, 618–622. [Google Scholar] [CrossRef]
- Pal, S.; Thomson, A.M.; Bottema, C.D.; Roach, P.D. Alpha-tocopherol modulates the low density lipoprotein receptor of human HepG2 cells. Nutr. J. 2003, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, M.; Herrmann, J.; Tolle, M.; van der Giet, M. Xanthine Oxidase and its Role as Target in Cardiovascular Disease: Cardiovascular Protection by Enzyme Inhibition? Curr. Pharm. Des. 2017, 23, 3391–3404. [Google Scholar] [CrossRef] [PubMed]
- Bredemeier, M.; Lopes, L.M.; Eisenreich, M.A.; Hickmann, S.; Bongiorno, G.K.; d’Avila, R.; Morsch, A.L.B.; da Silva Stein, F.; Campos, G.G.D. Xanthine oxidase inhibitors for prevention of cardiovascular events: A systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2018, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Zhang, J.L. Mass-spectrometry-based lipidomics. J. Sep. Sci. 2018, 41, 351–372. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, L.; Li, H.; Han, L.; Jing, W.; Wu, X.; Ullah, S.; Liu, R.; Wu, Y.; Xu, J. The Novel Interplay between Commensal Gut Bacteria and Metabolites in Diet-Induced Hyperlipidemic Rats Treated with Simvastatin. J. Proteome Res. 2022, 21, 808–821. [Google Scholar] [CrossRef]
- Hu, X.; Li, H.; Zhao, X.; Zhou, R.; Liu, H.; Sun, Y.; Fan, Y.; Shi, Y.; Qiao, S.; Liu, S.; et al. Multi-omics study reveals that statin therapy is associated with restoration of gut microbiota homeostasis and improvement in outcomes in patients with acute coronary syndrome. Theranostics 2021, 11, 5778–5793. [Google Scholar] [CrossRef]
- Hussain, K.; Xavier, A. Rosuvastatin-related rhabdomyolysis causing severe proximal paraparesis and acute kidney injury. BMJ Case Rep. 2019, 12, e229244. [Google Scholar] [CrossRef]
- Graham, D.J.; Staffa, J.A.; Shatin, D.; Andrade, S.E.; Schech, S.D.; La Grenade, L.; Gurwitz, J.H.; Chan, K.A.; Goodman, M.J.; Platt, R. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA 2004, 292, 2585–2590. [Google Scholar] [CrossRef]
- Obayashi, H.; Kobayashi, N.; Nezu, Y.; Yamoto, T.; Shirai, M.; Asai, F. Plasma 2-hydroxyglutarate and hexanoylcarnitine levels are potential biomarkers for skeletal muscle toxicity in male Fischer 344 rats. J. Toxicol. Sci. 2017, 42, 385–396. [Google Scholar] [CrossRef]
- Laaksonen, R. STOMPing forward: Statins, muscle complaints and CK. Atherosclerosis 2013, 230, 256–257. [Google Scholar] [CrossRef]
- Tomlinson, L.; Tirmenstein, M.A.; Janovitz, E.B.; Aranibar, N.; Ott, K.H.; Kozlosky, J.C.; Patrone, L.M.; Achanzar, W.E.; Augustine, K.A.; Brannen, K.C.; et al. Cannabinoid receptor antagonist-induced striated muscle toxicity and ethylmalonic-adipic aciduria in beagle dogs. Toxicol. Sci. 2012, 129, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Aguer, C.; McCoin, C.S.; Knotts, T.A.; Thrush, A.B.; Ono-Moore, K.; McPherson, R.; Dent, R.; Hwang, D.H.; Adams, S.H.; Harper, M.E. Acylcarnitines: Potential implications for skeletal muscle insulin resistance. FASEB J. 2015, 29, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.S.; Lee, Y.J.; Yi, H.J.; Chung, B.C.; Jung, B.H. Discovery of safety biomarkers for atorvastatin in rat urine using mass spectrometry based metabolomics combined with global and targeted approach. Anal. Chim. Acta 2010, 661, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi-Sidhu, M.; Baillie, R.A.; Zhu, H.; Chen, Y.I.; Goodarzi, M.O.; Rotter, J.I.; Krauss, R.M.; Fiehn, O.; Kaddurah-Daouk, R. Pharmacometabolomic signature links simvastatin therapy and insulin resistance. Metabolomics 2017, 13, 11. [Google Scholar] [CrossRef]
- Lee, H.; Choi, J.M.; Cho, J.Y.; Kim, T.E.; Lee, H.J.; Jung, B.H. Regulation of endogenic metabolites by rosuvastatin in hyperlipidemia patients: An integration of metabolomics and lipidomics. Chem. Phys. Lipids 2018, 214, 69–83. [Google Scholar] [CrossRef]
- Sliz, E.; Shin, J.; Ahmad, S.; Williams, D.M.; Frenzel, S.; Gauss, F.; Harris, S.E.; Henning, A.K.; Hernandez, M.V.; Hu, Y.H.; et al. Circulating Metabolome and White Matter Hyperintensities in Women and Men. Circulation 2022, 145, 1040–1052. [Google Scholar] [CrossRef]
- Pallares-Mendez, R.; Aguilar-Salinas, C.A.; Cruz-Bautista, I.; Del Bosque-Plata, L. Metabolomics in diabetes, a review. Ann. Med. 2016, 48, 89–102. [Google Scholar] [CrossRef]
- Ooga, T.; Sato, H.; Nagashima, A.; Sasaki, K.; Tomita, M.; Soga, T.; Ohashi, Y. Metabolomic anatomy of an animal model revealing homeostatic imbalances in dyslipidaemia. Mol. Biosyst. 2011, 7, 1217–1223. [Google Scholar] [CrossRef]
- Christensen, J.J.; Ulven, S.M.; Retterstol, K.; Narverud, I.; Bogsrud, M.P.; Henriksen, T.; Bollerslev, J.; Halvorsen, B.; Aukrust, P.; Holven, K.B. Comprehensive lipid and metabolite profiling of children with and without familial hypercholesterolemia: A cross-sectional study. Atherosclerosis 2017, 266, 48–57. [Google Scholar] [CrossRef]
- Davignon, J. Beneficial cardiovascular pleiotropic effects of statins. Circulation 2004, 109, III39–III43. [Google Scholar] [CrossRef] [Green Version]
- Graziano, T.S.; Cuzzullin, M.C.; Franco, G.C.; Schwartz-Filho, H.O.; de Andrade, E.D.; Groppo, F.C.; Cogo-Muller, K. Statins and Antimicrobial Effects: Simvastatin as a Potential Drug against Staphylococcus aureus Biofilm. PLoS ONE 2015, 10, e0128098. [Google Scholar] [CrossRef]
- Hennessy, E.; Adams, C.; Reen, F.J.; O’Gara, F. Is There Potential for Repurposing Statins as Novel Antimicrobials? Antimicrob. Agents Chemother. 2016, 60, 5111–5121. [Google Scholar] [CrossRef]
- Rana, R.; Sharma, R.; Kumar, A. Repurposing of Existing Statin Drugs for Treatment of Microbial Infections: How Much Promising? Infect. Disord. Drug Targets 2019, 19, 224–237. [Google Scholar] [CrossRef]
- Kocak, E.; Nemutlu, E.; Kir, S.; Sagiroglu, M.; Ozkul, C. Integrative proteomics and metabolomics approach to elucidate the antimicrobial effect of simvastatin on Escherichia coli. Biomed. Chromatogr. 2021, 35, e5180. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Parvathaneni, V.; Kulkarni, N.S.; Muth, A.; Gupta, V. Drug repurposing: A promising tool to accelerate the drug discovery process. Drug Discov. Today 2019, 24, 2076–2085. [Google Scholar] [CrossRef]
- Lin, J.B.; Halawa, O.A.; Husain, D.; Miller, J.W.; Vavvas, D.G. Dyslipidemia in age-related macular degeneration. Eye 2022, 36, 312–318. [Google Scholar] [CrossRef]
- Wurtz, P.; Wang, Q.; Soininen, P.; Kangas, A.J.; Fatemifar, G.; Tynkkynen, T.; Tiainen, M.; Perola, M.; Tillin, T.; Hughes, A.D.; et al. Metabolomic Profiling of Statin Use and Genetic Inhibition of HMG-CoA Reductase. J. Am. Coll. Cardiol. 2016, 67, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wu, J.; Lin, T.; Lian, G.; Wang, H.; Gao, G.; Xie, L. Influence of atorvastatin on metabolic pattern of rats with pulmonary hypertension. Aging 2021, 13, 11954–11968. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Du, S.; Shen, S.; Luo, P.; Ding, S.; Wang, G.; Wang, L. Comparative efficacy and safety of lipid-lowering agents in patients with hypercholesterolemia: A frequentist network meta-analysis. Medicine 2019, 98, e14400. [Google Scholar] [CrossRef] [PubMed]
- Artenstein, A.W.; Opal, S.M. Proprotein convertases in health and disease. N. Engl. J. Med. 2011, 365, 2507–2518. [Google Scholar] [CrossRef] [PubMed]
- Pecin, I.; Hartgers, M.L.; Hovingh, G.K.; Dent, R.; Reiner, Z. Prevention of cardiovascular disease in patients with familial hypercholesterolaemia: The role of PCSK9 inhibitors. Eur. J. Prev. Cardiol. 2017, 24, 1383–1401. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Ference, B.A.; Robinson, J.G.; Brook, R.D.; Catapano, A.L.; Chapman, M.J.; Neff, D.R.; Voros, S.; Giugliano, R.P.; Smith, G.D.; Fazio, S.; et al. Variation in PCSK9 and HMGCR and Risk of Cardiovascular Disease and Diabetes. N. Engl. J. Med. 2016, 375, 2144–2153. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef]
- Gallego-Colon, E.; Daum, A.; Yosefy, C. Statins and PCSK9 inhibitors: A new lipid-lowering therapy. Eur. J. Pharmacol. 2020, 878, 173114. [Google Scholar] [CrossRef]
- Sliz, E.; Kettunen, J.; Holmes, M.V.; Williams, C.O.; Boachie, C.; Wang, Q.; Mannikko, M.; Sebert, S.; Walters, R.; Lin, K.; et al. Metabolomic consequences of genetic inhibition of PCSK9 compared with statin treatment. Circulation 2018, 138, 2499–2512. [Google Scholar] [CrossRef]
- Shepherd, J.; Blauw, G.J.; Murphy, M.B.; Bollen, E.L.; Buckley, B.M.; Cobbe, S.M.; Ford, I.; Gaw, A.; Hyland, M.; Jukema, J.W.; et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): A randomised controlled trial. Lancet 2002, 360, 1623–1630. [Google Scholar] [CrossRef]
- Zhang, X.; Stiekema, L.C.A.; Stroes, E.S.G.; Groen, A.K. Metabolic effects of PCSK9 inhibition with Evolocumab in subjects with elevated Lp(a). Lipids Health Dis. 2020, 19, 91. [Google Scholar] [CrossRef]
- Di Minno, A.; Orsini, R.C.; Chiesa, M.; Cavalca, V.; Calcaterra, I.; Tripaldella, M.; Anesi, A.; Fiorelli, S.; Eligini, S.; Colombo, G.I.; et al. Treatment with PCSK9 Inhibitors in Patients with Familial Hypercholesterolemia Lowers Plasma Levels of Platelet-Activating Factor and Its Precursors: A Combined Metabolomic and Lipidomic Approach. Biomedicines 2021, 9, 1073. [Google Scholar] [CrossRef] [PubMed]
- Di Minno, A.; Gentile, M.; Iannuzzo, G.; Calcaterra, I.; Tripaldella, M.; Porro, B.; Cavalca, V.; Di Taranto, M.D.; Tremoli, E.; Fortunato, G.; et al. Endothelial function improvement in patients with familial hypercholesterolemia receiving PCSK-9 inhibitors on top of maximally tolerated lipid lowering therapy. Thromb. Res. 2020, 194, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Masutomi, N.; Tsutsui, N.; Sakairi, T.; Mitchell, M.; Milburn, M.V.; Ryals, J.A.; Beebe, K.D.; Guo, L. Untargeted metabolomic profiling as an evaluative tool of fenofibrate-induced toxicology in Fischer 344 male rats. Toxicol. Pathol. 2009, 37, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.D.; Slanar, O.; Krausz, K.W.; Li, F.; Hofer, C.C.; Perlik, F.; Gonzalez, F.J.; Idle, J.R. Human urinary metabolomic profile of PPARalpha induced fatty acid beta-oxidation. J. Proteome Res. 2009, 8, 4293–4300. [Google Scholar] [CrossRef]
- Lu, Y.; Boekschoten, M.V.; Wopereis, S.; Muller, M.; Kersten, S. Comparative transcriptomic and metabolomic analysis of fenofibrate and fish oil treatments in mice. Physiol. Genom. 2011, 43, 1307–1318. [Google Scholar] [CrossRef]
- Strauss, V.; Mellert, W.; Wiemer, J.; Leibold, E.; Kamp, H.; Walk, T.; Looser, R.; Prokoudine, A.; Fabian, E.; Krennrich, G.; et al. Increased toxicity when fibrates and statins are administered in combination--a metabolomics approach with rats. Toxicol. Lett. 2012, 211, 187–200. [Google Scholar] [CrossRef]
- Han, J.S.; Kim, K.; Jung, Y.; Lee, J.H.; Namgung, J.; Lee, H.Y.; Suh, J.; Hwang, G.S.; Lee, S.H. Metabolic Alterations Associated with Atorvastatin/Fenofibric Acid Combination in Patients with Atherogenic Dyslipidaemia: A Randomized Trial for Comparison with Escalated-Dose Atorvastatin. Sci. Rep. 2018, 8, 14642. [Google Scholar] [CrossRef]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Mink, P.J.; Scrafford, C.G.; Barraj, L.M.; Harnack, L.; Hong, C.P.; Nettleton, J.A.; Jacobs, D.R., Jr. Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007, 85, 895–909. [Google Scholar] [CrossRef]
- Grosso, G. Effects of Polyphenol-Rich Foods on Human Health. Nutrients 2018, 10, 1089. [Google Scholar] [CrossRef] [Green Version]
- Sommella, E.; Badolati, N.; Riccio, G.; Salviati, E.; Bottone, S.; Dentice, M.; Campiglia, P.; Tenore, G.C.; Stornaiuolo, M.; Novellino, E. A Boost in Mitochondrial Activity Underpins the Cholesterol-Lowering Effect of Annurca Apple Polyphenols on Hepatic Cells. Nutrients 2019, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Caruso, D.; Buonomo, G.; D’Avino, M.; Campiglia, P.; Marinelli, L.; Novellino, E. A Healthy Balance of Plasma Cholesterol by a Novel Annurca Apple-Based Nutraceutical Formulation: Results of a Randomized Trial. J. Med. Food 2017, 20, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, S.; Feng, M.; Shete, V.; Chu, Y.; Kamil, A.; Yang, C.; Liu, H.; Xia, H.; Wang, X.; et al. Serum Metabolomics Reveals Underlying Mechanisms of Cholesterol-Lowering Effects of Oat Consumption: A Randomized Controlled Trial in a Mildly Hypercholesterolemic Population. Mol. Nutr. Food Res. 2021, 65, e2001059. [Google Scholar] [CrossRef]
- Johansson-Persson, A.; Barri, T.; Ulmius, M.; Onning, G.; Dragsted, L.O. LC-QTOF/MS metabolomic profiles in human plasma after a 5-week high dietary fiber intake. Anal. Bioanal. Chem. 2013, 405, 4799–4809. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Jing, L.; Ma, X.; Zhang, Z.; Guo, Q.; Li, Y. GC-TOF-MS-based serum metabolomic investigations of naked oat bran supplementation in high-fat-diet-induced dyslipidemic rats. J. Nutr. Biochem. 2015, 26, 1509–1519. [Google Scholar] [CrossRef]
- Keski-Rahkonen, P.; Kolehmainen, M.; Lappi, J.; Micard, V.; Jokkala, J.; Rosa-Sibakov, N.; Pihlajamaki, J.; Kirjavainen, P.V.; Mykkanen, H.; Poutanen, K.; et al. Decreased plasma serotonin and other metabolite changes in healthy adults after consumption of wholegrain rye: An untargeted metabolomics study. Am. J. Clin. Nutr. 2019, 109, 1630–1639. [Google Scholar] [CrossRef]
- Ding, Z.; Hani, A.; Li, W.; Gao, L.; Ke, W.; Guo, X. Influence of a cholesterol-lowering strain Lactobacillus plantarum LP3 isolated from traditional fermented yak milk on gut bacterial microbiota and metabolome of rats fed with a high-fat diet. Food Funct. 2020, 11, 8342–8353. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.A. Plant polyphenols: Modifiers of immune function and risk of cardiovascular disease. Nutrition 2005, 21, 422–423. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, S.; Zhao, A.; Wang, K.; Fan, Z.; Yang, H.; Liao, W.; Bao, S.; Zhao, L.; Zhang, Y.; et al. Transcriptomic and metabonomic profiling reveal synergistic effects of quercetin and resveratrol supplementation in high fat diet fed mice. J. Proteome Res. 2012, 11, 4961–4971. [Google Scholar] [CrossRef]
- Abdelsayed, M.; Kort, E.J.; Jovinge, S.; Mercola, M. Repurposing drugs to treat cardiovascular disease in the era of precision medicine. Nat. Rev. Cardiol. 2022, 19, 751–764. [Google Scholar] [CrossRef]
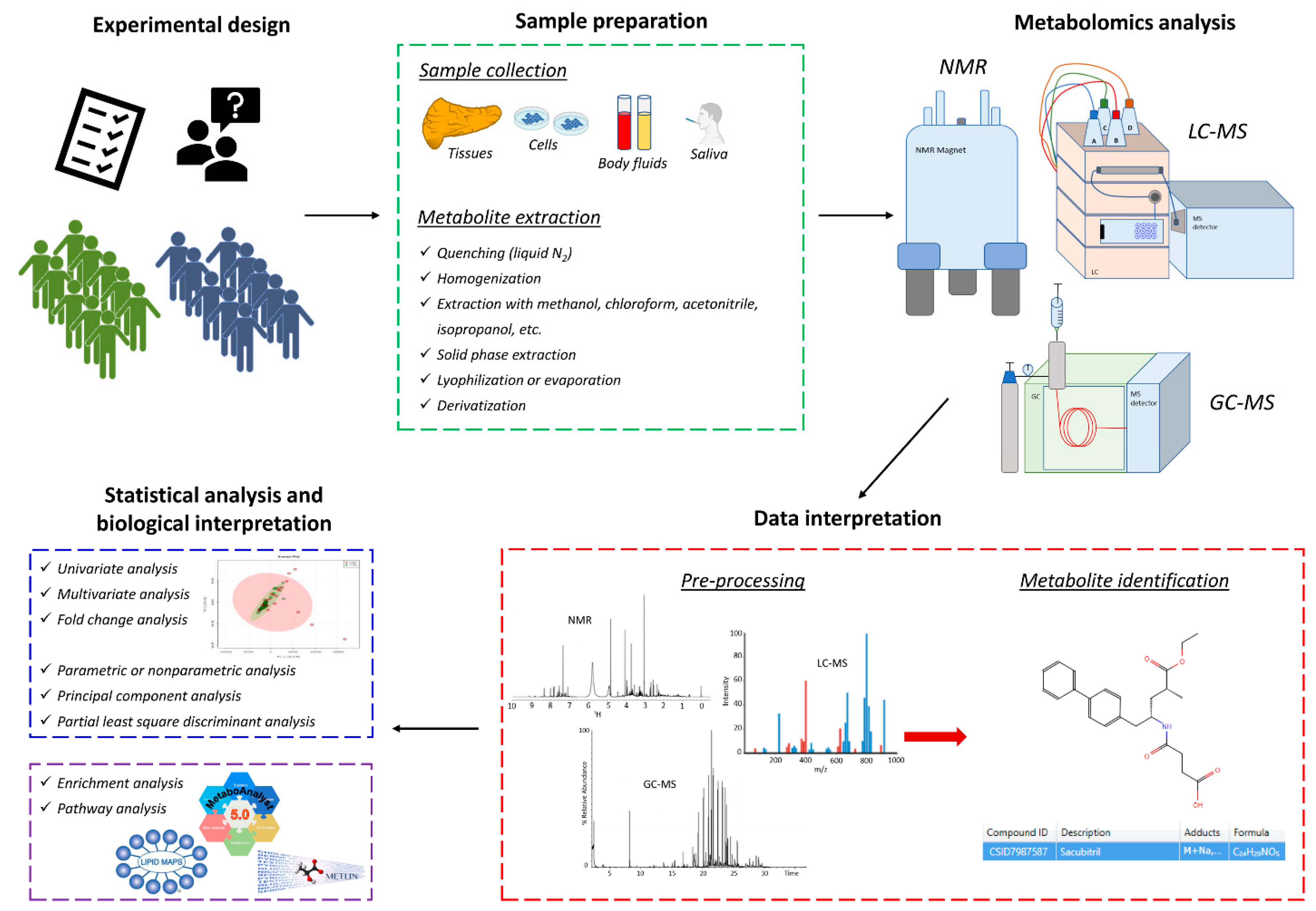
| Treatment | Sample Type | Inclusion Criteria | Exclusion Criteria | Matrix | Experimental Design | Analytical Technique | Type of Analysis | Main Biomarkers or Pathways Involved | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Statins | |||||||||
| Atorvastatin | Human healthy subjects | Selection based on medical history and routine clinical laboratory tests (i.e., hematology, urinalysis, biochemistry, serology, and physical examination). |
| Plasma | Randomized open-label clinical trial | GC-MS | Untargeted | Tryptophan, alanine, arachidonic acid, 2-hydroxybutyric acid, cholesterol, and isoleucine | [12] |
| Simvastatin | Human healthy subjects |
|
| Plasma | Non-randomized open-label clinical trial | GC-MS | Targeted | Arachidonic acid and linoleic acid within primarily phosphatidylcholine and cholesteryl esters, plasmalogens | [63] |
| Simvastatin | Human healthy subjects |
|
| Plasma | Non-randomized open-label clinical trial | GC-MS | Targeted | Lithocholic acid, taurolithocholic acid, glycolithocholic acid, and coprostanol | [64] |
| Simvastatin | Human healthy subjects |
|
| Plasma | Non-randomized open-label clinical trial | GC-MS | Untargeted |
| [67] |
| Simvastatin | Hyperlipidemic rats |
| Serum | Animal study | LC-MS | Untargeted | Phenylalanine, tyrosine, linoleic acid, 9-hydroxyoctadecadienoic acid (9-HODE), m-coumaric acid, and 3-(2-hydroxyphenyl) propionic acid | [75] | |
| Long-term and standard statins (not mentioned the type of statin) | Human healthy subjects and acute coronary syndrome patients |
|
| Serum | Interventional study (case vs. control) | LC-MS | Untargeted | Fatty acyls, steroids, and steroid derivatives, benzene and substituted derivatives, prenol lipids, and acyl carnitines | [76] |
| Atorvastatin | Hyperlipidemic rats |
| Urine | Animal study | LC-MS, GC-MS and CE-MS | Untargeted and targeted | Estrone, cortisone, proline, cystine, 3-ureidopropionic acid, and histidine | [83] | |
| Cerivastatin | Rat | Fischer male rats at 8 weeks of age who were fed a diet supplemented with cerivastatin or commercial diet only as a control. | Plasma and skeletal muscle tissue | Animal study | LC-MS and GC-MS | Untargeted | 2-Hydroxyglutarate and hexanoylcarnitine | [79] | |
| Simvastatin | Human healthy subjects |
| Plasma | Non-randomized open-label clinical trial | GC-MS | Untargeted | Ethanolamine, hydroxylamine, hydroxycarbamate, and isoleucine | [84] | |
| Rosuvastatin | Human healthy subjects and hyperlipidemic patients |
|
| Plasma and urine | Interventional study (case vs. control) | LC-MS | Untargeted and targeted | L-carnitine, diacylglycerol, acylcarnitines, fatty acids, lysophosphatidylcholines, phosphatidylcholines, arachidonic acid, linoleic acid, myristate and palmitate | [85] |
| Simvastatin | Hyperlipidemic rabbits | Japanese White male rabbits and Watanabe heritable hyperlipidemic rabbits aged 11 months. | Plasma and tissues (liver, aorta, cardiac muscle, and brain) | Animal study | CE-MS and LC-MS | Untargeted | Glutathione and phosphatidylcholine metabolism, purine compounds, and uric acid | [88] | |
| Atorvastatin, rosuvastatin and simvastatin | Children with and without familial hypercholesterolemia |
| Plasma | Cross-sectional study | NMR | Untargeted | Cholesteryl esters, free cholesterol and phospholipids in small HDL, polyunsaturated fatty acids, linoleic acid, acetoacetate and acetate | [89] | |
| Simvastatin | Escherichia coli | Escherichia coli ATCC 25922 cultured on tryptic soy agar. | Cell lysate | In vitro study | GC-MS | Untargeted | Biosynthesis of amino acids, tricarboxylic acid cycle, glyoxylate shunt, glycolysis, pyruvate metabolism, purine and pyrimidine metabolisms | [94] | |
| Statin | Human subjects who started statins and persistent nonusers during follow-up | Individuals with metabolomic profile measured at both baseline and a follow-up visit and free of statin medication at baseline. |
| Serum and plasma | Longitudinal study | NMR | Untargeted | Remnant cholesterol, omega-6 fatty acids, glycoprotein acetyl and acetate | [98] |
| Atorvastatin | Rats with pulmonary arterial hypertension |
| Serum | Animal study | NMR | Untargeted | Carnitine, glucose, glycerol, acetone, leucine, isoleucine, pyruvate, acetate and choline | [99] | |
| Pravastatin and genetic inhibition of PCSK9 | Human healthy subjects |
|
| Serum and plasma | Randomized clinical trial (randomized placebo-controlled study vs. large population studies) | NMR | Untargeted | Lipoprotein subclasses, their lipid concentrations and composition, fatty acids, and amino acids | [108] |
| PCSK9 inhibitors | |||||||||
| Evolocumab | Patients with elevated Lp(a) | A selection of patients from the ANITSCHKOW trial:
|
| Plasma | Randomized placebo-controlled clinical trial | NMR | Untargeted | VLDL, IDL and LDL particles and their lipid contents, Lp(a), fatty acids (e.g., docosahexaenoic acid) | [110] |
| Evolocumab | Patients with familial hypercholesterolemia |
|
| Plasma | Interventional study | LC-MS | Untargeted | Creatine, indole, indoleacrylic acid, choline, phosphatidylcholine, and platelet-activating factor 16 | [111] |
| Evolocumab | Patients with familial hypercholesterolemia |
|
| Serum and urine | Interventional study | LC-MS | Targeted | Small dense LDL, Lp(a), 11-dehydro-thromboxane, 8-isoprostaglandin-2alpha | [112] |
| Fibrates | |||||||||
| Fenofibrate | Human healthy subjects | No medication 28 days prior enrollment and during the study. | Urine (24 h) | Interventional study (fenofibrate 200 mg; 0, 7 and 14 days) | LC-MS | Untargeted | Pantothenic acid and acetylcarnitine | [114] | |
| Fenofibrate | Mice |
| Urine | Animal study (0.1% fenofibrate in diet, for 7 days) | LC-MS | Targeted | Pantothenic acid and acetylcarnitine | [114] | |
| Fenofibrate and fish oil | Mice | C57Bl/6 mice 12 weeks old. | Plasma | Animal study (0.03% fenofibrate or fish oil in diet, for 2 weeks) | LC-MS and GC-MS | Untargeted | Krebs cycle intermediates (fumaric acid, isocitric acid, malic acid, succinic acid and α-ketoglutaric acid); amino acids | [115] | |
| Fenofibrate | Rats | Fisher 344 male rats 9 weeks old | Urine | Animal study (300 mg/kg/day fenofibrate or vehicle for 2 and 14 days) | LC-MS and GC-MS | Untargeted | Acetylcarnitine, 3-hydroxybutanoic acid, TCA cycle intermediates (i.e., malate, fumarate, alpha-ketoglutarate), glutathione metabolism (i.e., gamma glutamyltyrosine), tryptophan metabolism (kynurenine) | [113] | |
| Fenofibrate, clofibrate, atorvastatin and pravastatin | Rats | Wistar (Crl:WI(Han)) rats in standard diet. | Plasma | Animal study: two fibrates (100 mg/kg bw/d fenofibrate, 50 mg/kg bw/d clofibrate) and two statins (70 mg/kg bw/d atorvastatin, 200 mg/kg bw/d pravastatin) in monotherapy as well as each combination of a fibrate and a statin | LC-MS and GC-MS | Untargeted | 5-Oxoproline, glutamine, glycine and tryptophan | [116] | |
| Fenofibrate and atorvastatin | Hyperlipidemic patients |
|
| Serum | Randomized trial (atorvastatin escalation 20 mg vs. combined therapy, 10 mg fenofibrate and 135 mg fenofibrate, for 12 weeks) | LC-MS | Untargeted | Acylglycerols, ceramides, sphingomyelins and carnitine | [117] |
| Fenofibrate and simvastatin | Hyperlipidemic rats |
| Plasma | Animal study: simvastatin (10 mg/kg daily) and fenofibrate (150 mg/kg daily) for 2 weeks | GC-MS | Untargeted | Creatinine and tyrosine | [50] | |
| Nutraceutical treatments | |||||||||
| Annurca Apple | HuH7, hepatoma cell line | Cell lysate | In vitro study | GC-MS | Untargeted | Glutamine, acyl-carnitines, glutathione | [121] | ||
| Oat | Patients with mild cholesterol elevation |
|
| Serum | Randomized placebo-controlled clinical trial (40 g oats or rice twice daily (total of 80 g day−1, 3 g beta-glucan in the oats group) | LC-MS | Untargeted | Glycerophospholipid, alanine, aspartate and glutamate, sphingolipid, and retinol metabolism | [123] |
| Lactobacillus plantarum LP3 | Rats |
| Cecum samples | Animal study to compare (1) normal diet (2) high-fat diet or (3) high-fat diet + L. plantarum LP3 | LC-MS | Untargeted | Linoleic acid, linolenic acid and arachidonic acid | [127] | |
| Quercetin and resveratrol | Mice treated with high-fat diet | C57/6J mice 7 weeks old. | Liver tissue | Animal study to compare normal diet (Normal) group fed with normal diet; high-fat diet, for 26 weeks (HFD) group; quercetin (Quercetin) group fed with HFD and supplemented with 0.4% quercetin (4 g/kg diet); resveratrol(Resveratrol) group fed with HFD and supplemented with0.4% resveratrol (4 g/kg diet); combined quercetin and resveratrol (Combined) group fed with HFD and supplemented with 0.2% quercetin and 0.2% resveratrol (2 g quercetin + 2 g resveratrol per kg diet) | GC-MS | Untargeted | 4-aminobutiric acid, ornithine and histidine | [129] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gianazza, E.; Brioschi, M.; Iezzi, A.; Paglia, G.; Banfi, C. Pharmacometabolomics for the Study of Lipid-Lowering Therapies: Opportunities and Challenges. Int. J. Mol. Sci. 2023, 24, 3291. https://doi.org/10.3390/ijms24043291
Gianazza E, Brioschi M, Iezzi A, Paglia G, Banfi C. Pharmacometabolomics for the Study of Lipid-Lowering Therapies: Opportunities and Challenges. International Journal of Molecular Sciences. 2023; 24(4):3291. https://doi.org/10.3390/ijms24043291
Chicago/Turabian StyleGianazza, Erica, Maura Brioschi, Ada Iezzi, Giuseppe Paglia, and Cristina Banfi. 2023. "Pharmacometabolomics for the Study of Lipid-Lowering Therapies: Opportunities and Challenges" International Journal of Molecular Sciences 24, no. 4: 3291. https://doi.org/10.3390/ijms24043291







