Sirolimus-Embedded Silk Microneedle Wrap to Prevent Neointimal Hyperplasia in Vein Graft Model
Abstract
1. Introduction
2. Results
2.1. Drug Release of Silk MNs
2.2. Diameter and Velocity Changes of Vein Grafts
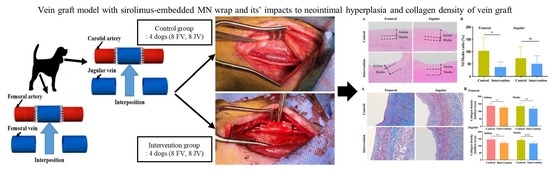
2.3. Neointimal Hyperplasia in Vein Graft
2.4. Neointimal Hyperplasia in Vein Graft
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Fabrication of Silk Wrap and Sirolimus-Embedded Silk MN Wrap
4.3. Animals and In Vivo Surgical Procedures
4.4. In Vivo Drug Release Profile of Silk MNs
4.5. Doppler Ultrasonography
4.6. Histopathological Analysis
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hillis, L.D.; Smith, P.K.; Anderson, J.L.; Bittl, J.A.; Bridges, C.R.; Byrne, J.G.; Cigarroa, J.E.; DiSesa, V.J.; Hiratzka, L.F.; Hutter, A.M.; et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011, 124, e652–e735. [Google Scholar]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e726–e779. [Google Scholar]
- Desai, M.; Seifalian, A.M.; Hamilton, G. Role of prosthetic conduits in coronary artery bypass grafting. Eur. J. Cardiothorac. Surg. 2011, 40, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Sharrock, M.; Antoniou, S.A.; Antoniou, G.A. Vein Versus Prosthetic Graft for Femoropopliteal Bypass Above the Knee: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Angiology 2019, 70, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Buxton, B.F.; Hayward, P.A.; Raman, J.; Moten, S.C.; Rosalion, A.; Gordon, I.; Seevanayagam, S.; Matalanis, G.; Benedetto, U.; Gaudino, M.; et al. Long-Term Results of the RAPCO Trials. Circulation 2020, 142, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Tondi, P.; Benedetto, U.; Milazzo, V.; Flore, R.; Glieca, F.; Romana Ponziani, F.; Luciani, N.; Girardi, L.N.; Crea, F.; et al. Radial Artery as a Coronary Artery Bypass Conduit: 20-Year Results. J. Am. Coll. Cardiol. 2016, 68, 603–610. [Google Scholar] [CrossRef]
- Gaudino, M.; Benedetto, U.; Fremes, S.; Ballman, K.; Biondi-Zoccai, G.; Sedrakyan, A.; Nasso, G.; Raman, J.; Buxton, B.; Hayward, P.A.; et al. Association of Radial Artery Graft vs Saphenous Vein Graft with Long-term Cardiovascular Outcomes Among Patients Undergoing Coronary Artery Bypass Grafting: A Systematic Review and Meta-analysis. JAMA 2020, 324, 179–187. [Google Scholar] [CrossRef]
- Harskamp, R.E.; Lopes, R.D.; Baisden, C.E.; de Winter, R.J.; Alexander, J.H. Saphenous vein graft failure after coronary artery bypass surgery: Pathophysiology, management, and future directions. Ann. Surg. 2013, 257, 824–833. [Google Scholar] [CrossRef]
- Caliskan, E.; de Souza, D.R.; Böning, A.; Liakopoulos, O.J.; Choi, Y.H.; Pepper, J.; Gibson, C.M.; Perrault, L.P.; Wolf, R.K.; Kim, K.-B.; et al. Saphenous vein grafts in contemporary coronary artery bypass graft surgery. Nat. Rev. Cardiol. 2020, 17, 155–169. [Google Scholar] [CrossRef]
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L.; et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016, 134, e123–e155. [Google Scholar] [CrossRef]
- Yu, X.; Takayama, T.; Goel, S.A.; Shi, X.; Zhou, Y.; Kent, K.C.; Murphy, W.L.; Guo, L.-W. A rapamycin-releasing perivascular polymeric sheath produces highly effective inhibition of intimal hyperplasia. J. Control. Release 2014, 191, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Terry, C.M.; Li, L.; Li, H.; Zhuplatov, I.; Blumenthal, D.K.; Kim, S.-E.; Owen, S.C.; Kholmovski, E.G.; Fowers, K.D.; Rathi, R.; et al. In vivo evaluation of the delivery and efficacy of a sirolimus-laden polymer gel for inhibition of hyperplasia in a porcine model of arteriovenous hemodialysis graft stenosis. J. Control. Release 2012, 160, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Skalský, I.; Filová, E.; Szárszoi, O.; Pařízek, M.; Lytvynets, A.; Malušková, J.; Lodererová, A.; Brynda, E.; Lisá, V.; Burdíková, Z.; et al. A periadventitial sirolimus-releasing mesh decreased intimal hyperplasia in a rabbit model. Physiol. Res. 2011, 60, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Kanjickal, D.; Lopina, S.; Evancho-Chapman, M.; Schmidt, S.; Donovan, D. Sustained local drug delivery from a novel polymeric ring to inhibit intimal hyperplasia. J. Biomed. Mater. Res. A 2010, 93, 656–665. [Google Scholar] [CrossRef]
- Rajathurai, T.; Rizvi, S.; Lin, H.; Angelini, G.; Newby, A.; Murphy, G. Periadventitial rapamycin-eluting microbeads promote vein graft disease in longterm pig vein-into-artery interposition grafts clinical perspective. Circ. Cardiovasc. Interv. 2010, 3, 157–165. [Google Scholar] [CrossRef]
- Lee, K.J.; Park, S.H.; Lee, J.Y.; Joo, H.C.; Jang, E.H.; Youn, Y.-N.; Ryu, W. Perivascular biodegradable microneedle cuff for reduction of neointima formation after vascular injury. J. Control. Release 2014, 192, 174–181. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jang, E.H.; Lee, K.; Lee, J.Y.; Park, S.H.; Seo, I.H.; Lee, S.H.; Ryu, W.; Youn, Y.-N. A biodegradable microneedle cuff for comparison of drug effects through perivascular delivery to balloon-injured arteries. Polymers 2017, 9, 56. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Ding, F.; Zhang, P.; Liu, J.; Gu, X. Biocompatibility evaluation of silk fibroin with peripheral nerve tissues and cells in vitro. Biomaterials 2007, 28, 1643–1652. [Google Scholar] [CrossRef]
- Raja, W.K.; MacCorkle, S.; Diwan, I.M.; Abdurrob, A.; Lu, J.; Omenetto, F.G.; Kaplan, D.L. Transdermal delivery devices: Fabrication, mechanics and drug release from silk. Small 2013, 9, 3704–3713. [Google Scholar] [CrossRef] [PubMed]
- Tsioris, K.; Raja, W.K.; Pritchard, E.M.; Panilaitis, B.; Kaplan, D.L.; Omenetto, F.G. Fabrication of silk microneedles for controlled-release drug delivery. Adv. Funct. Mater. 2012, 22, 330–335. [Google Scholar] [CrossRef]
- Lee, J.; Jang, E.H.; Kim, J.H.; Park, S.; Kang, Y.; Park, S.; Lee, K.; Kim, J.-H.; Youn, Y.-N.; Ryu, W. Highly flexible and porous silk fibroin microneedle wraps for perivascular drug delivery. J. Control. Release 2021, 340, 125–135. [Google Scholar] [CrossRef] [PubMed]
- De Vries, M.R.; Simons, K.H.; Jukema, J.W.; Braun, J.; Quax, P.H. Vein graft failure: From pathophysiology to clinical outcomes. Nat. Rev. Cardiol. 2016, 13, 451–470. [Google Scholar] [CrossRef] [PubMed]
- Kalra, M.; Miller, V.M. Early remodeling of saphenous vein grafts: Proliferation, migration and apoptosis of adventitial and medial cells occur simultaneously with changes in graft diameter and blood flow. J. Vasc. Res. 2000, 37, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.K.; Gangahar, D.M.; Agrawal, D.K. Cellular, molecular and immunological mechanisms in the pathophysiology of vein graft intimal hyperplasia. Immunol. Cell. Biol. 2006, 84, 115–124. [Google Scholar] [CrossRef]
- Jiang, Z.; Tao, M.; Omalley, K.A.; Wang, D.; Ozaki, C.K.; Berceli, S.A. Established neointimal hyperplasia in vein grafts expands via TGF-β-mediated progressive fibrosis. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1200–H1207. [Google Scholar] [CrossRef]
- Wang, J.; Zohar, R.; McCulloch, C.A. Multiple roles of α-smooth muscle actin in mechanotransduction. Exp. Cell Res. 2006, 312, 205–214. [Google Scholar] [CrossRef]
- Guo, D.-C.; Papke, C.L.; Tran-Fadulu, V.; Regalado, E.S.; Avidan, N.; Johnson, R.J.; Kim, D.H.; Pannu, H.; Willing, M.C.; Sparks, E.; et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and Moyamoya disease, along with thoracic aortic disease. Am. J. Hum. Genet. 2009, 84, 617–627. [Google Scholar] [CrossRef]
- Milewicz, D.M.; Kwartler, C.S.; Papke, C.L.; Regalado, E.S.; Cao, J.; Reid, A.J. Genetic variants promoting smooth muscle cell proliferation can result in diffuse and diverse vascular diseases: Evidence for a hyperplastic vasculomyopathy. Genet. Med. 2010, 12, 196–203. [Google Scholar] [CrossRef]
- Chu, T.; Gao, C.; Zhao, Z.; Ling, F.; Sun, A.; Zheng, Y.; Cao, J.; Ge, J. Rapamycin Combined with alpha-Cyanoacrylate Contributes to Inhibiting Intimal Hyperplasia in Rat Models. Arq. Bras. Cardiol. 2019, 112, 3–10. [Google Scholar]
- Owens, C.D.; Ho, K.J.; Conte, M.S. Lower extremity vein graft failure: A translational approach. Vasc. Med. 2008, 13, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, D.E.; Smith, R.F.; Elliott, J.P. Venous autografts in femoropolpitieal arterioplasty. Obervations in the treatment of occlusive disease. Arch. Surg. 1964, 89, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.D. Adaptive changes in autogenous vein grafts for arterial reconstruction: Clinical implications. J. Vasc. Surg. 2010, 51, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Izzat, M.B.; Mehta, D.; Bryan, A.J.; Reeves, B.; Newby, A.C.; Angelini, G.D. Influence of external stent size on early medial and neointimal thickening in a pig model of saphenous vein bypass grafting. Circulation 1996, 94, 1741–1745. [Google Scholar] [CrossRef]
- Angelini, G.D.; Lloyd, C.; Bush, R.; Johnson, J.; Newby, A.C. An external, oversized, porous polyester stent reduces vein graft neointima formation, cholesterol concentration, and vascular cell adhesion molecule 1 expression in cholesterol-fed pigs. J. Thorac. Cardiovasc. Surg. 2002, 124, 950–956. [Google Scholar] [CrossRef]
- Lardenoye, J.H.; de Vries, M.R.; Grimbergen, J.M.; Havekes, L.M.; Knaapen, M.W.M.; Kockx, M.M.; van Hinsbergh, V.W.M.; van Bockel, J.H.; Quax, P.H.A. Inhibition of accelerated atherosclerosis in vein grafts by placement of external stent in apoE*3-Leiden transgenic mice. Arter. Thromb. Vasc. Biol. 2002, 22, 1433–1438. [Google Scholar] [CrossRef]
- Moodley, L.; Franz, T.; Human, P.; Wolf, M.F.; Bezuidenhout, D.; Scherman, J.; Zilla, P. Protective constriction of coronary vein grafts with knitted nitinol. Eur. J. Cardiothorac. Surg. 2013, 44, 64–71. [Google Scholar] [CrossRef]
- Longchamp, A.; Alonso, F.; Dubuis, C.; Allagnat, F.; Berard, X.; Meda, P.; Saucy, F.; Corpataux, J.-M.; Déglise, S.; Haefliger, J.-A. The use of external mesh reinforcement to reduce intimal hyperplasia and preserve the structure of human saphenous veins. Biomaterials 2014, 35, 2588–2599. [Google Scholar] [CrossRef]
- Taggart, D.P.; Gal, Y.B.; Lees, B.; Patel, N.; Webb, C.; Rehman, S.M.; Desouza, A.; Yadav, R.; De Robertis, F.; Delby, M.; et al. A randomized trial of external stenting for saphenous vein grafts in coronary artery bypass grafting. Ann. Thorac. Surg. 2015, 99, 2039–2045. [Google Scholar] [CrossRef]
- Ferrari, E.; Von, S.L.; Berdajs, D. Improving coronary artery bypass graft durability: Use of the external saphenous vein graft support. In Multimedia Manual of Cardio-Thoracic Surgery; Oxford University Press: Oxford, UK, 2015; pp. 1–7. [Google Scholar]
- Moser, J.; van Ark, J.; van Dijk, M.C.; Greiner, D.L.; Shultz, L.D.; van Goor, H.; Hillebrands, J.L. Distinct Differences on Neointima Formation in Immunodeficient and Humanized Mice after Carotid or Femoral Arterial Injury. Sci. Rep. 2016, 6, 35387. [Google Scholar] [CrossRef]
- Owens, C.D.; Gasper, W.J.; Rahman, A.S.; Conte, M.S. Vein graft failure. J. Vasc. Surg. 2015, 61, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; DiPietro, L.A. Apoptosis and angiogenesis: An evolving mechanism for fibrosis. FASEB J. 2013, 27, 3893–3901. [Google Scholar] [CrossRef] [PubMed]
- Stead, S.; Werstiuk, E.S.; Lee, R.M. Nifedipine induces apoptosis in cultured vascular smooth muscle cells from spontaneously hypertensive rats. Life Sci. 2000, 67, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Diez, J.; Panizo, A.; Hernandez, M.; Pardo, J. Is the regulation of apoptosis altered in smooth muscle cells of adult spontaneously hypertensive rats? Hypertension 1997, 29, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Kiffin, R.; Bandyopadhyay, U.; Cuervo, A.M. Oxidative stress and autophagy. Antioxid. Redox Signal. 2006, 8, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Martinet, W.; Schrijvers, D.M.; Timmermans, J.P.; Bult, H. Interactions between cell death induced by statins and 7-ketocholesterol in rabbit aorta smooth muscle cells. Br. J. Pharmacol. 2008, 154, 1236–1246. [Google Scholar] [CrossRef]
- Schrijvers, D.M.; De Meyer, G.R.; Martinet, W. Autophagy in atherosclerosis: A potential drug target for plaque stabilization. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2787–2791. [Google Scholar] [CrossRef]
- Jang, E.H.; Kim, J.H.; Ryu, J.Y.; Lee, J.; Kim, H.H.; Youn, Y.N. Time-dependent pathobiological and physiological changes of implanted vein grafts in a canine model. J. Cardiovasc. Transl. Res. 2022, 15, 1108–1118. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Jang, E.H.; Ryu, J.-Y.; Lee, J.; Kim, J.H.; Ryu, W.; Youn, Y.-N. Sirolimus-Embedded Silk Microneedle Wrap to Prevent Neointimal Hyperplasia in Vein Graft Model. Int. J. Mol. Sci. 2023, 24, 3306. https://doi.org/10.3390/ijms24043306
Kim J-H, Jang EH, Ryu J-Y, Lee J, Kim JH, Ryu W, Youn Y-N. Sirolimus-Embedded Silk Microneedle Wrap to Prevent Neointimal Hyperplasia in Vein Graft Model. International Journal of Molecular Sciences. 2023; 24(4):3306. https://doi.org/10.3390/ijms24043306
Chicago/Turabian StyleKim, Jung-Hwan, Eui Hwa Jang, Ji-Yeon Ryu, Jiyong Lee, Jae Ho Kim, Wonhyoung Ryu, and Young-Nam Youn. 2023. "Sirolimus-Embedded Silk Microneedle Wrap to Prevent Neointimal Hyperplasia in Vein Graft Model" International Journal of Molecular Sciences 24, no. 4: 3306. https://doi.org/10.3390/ijms24043306
APA StyleKim, J.-H., Jang, E. H., Ryu, J.-Y., Lee, J., Kim, J. H., Ryu, W., & Youn, Y.-N. (2023). Sirolimus-Embedded Silk Microneedle Wrap to Prevent Neointimal Hyperplasia in Vein Graft Model. International Journal of Molecular Sciences, 24(4), 3306. https://doi.org/10.3390/ijms24043306