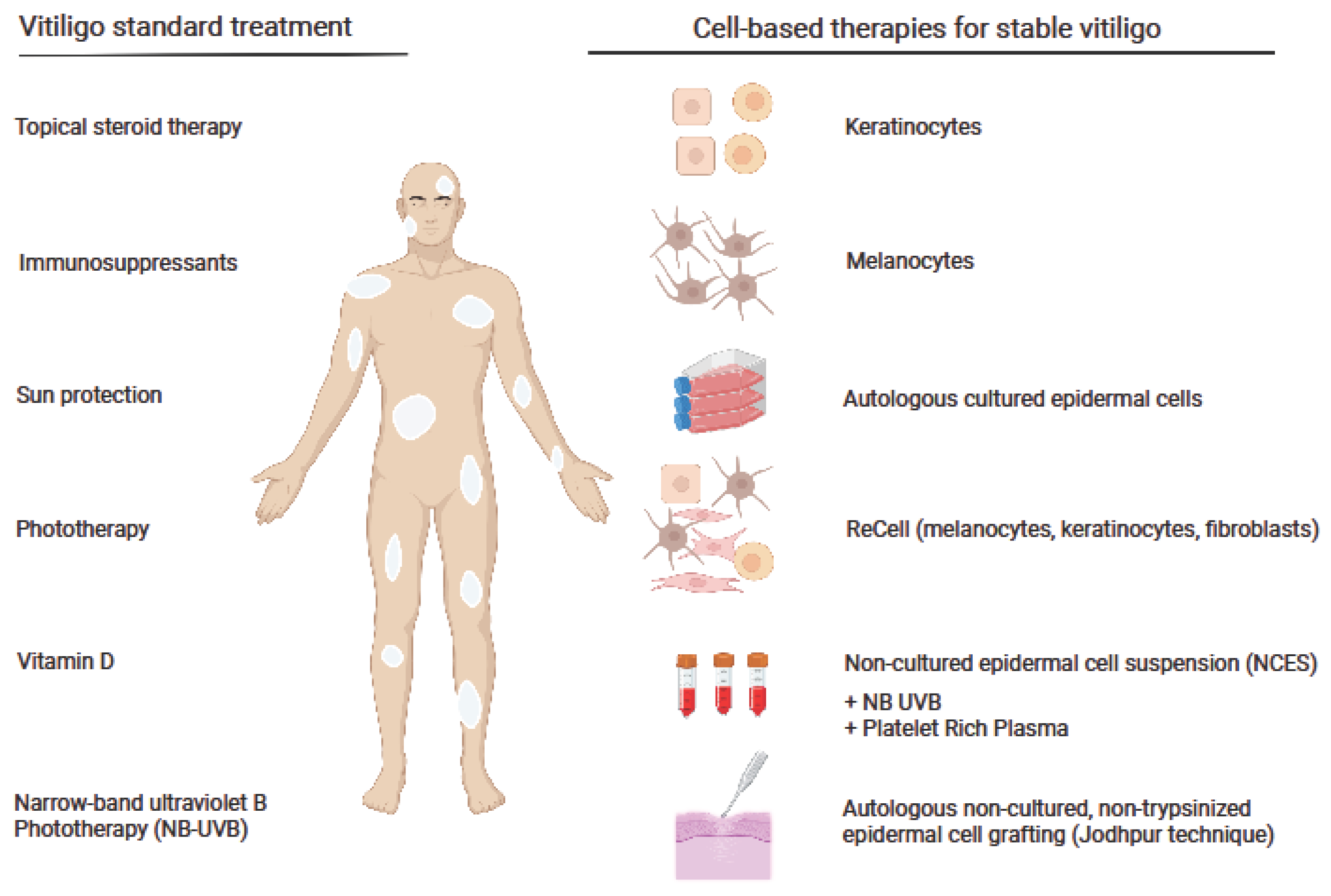
Current Status of Cell-Based Therapies for Vitiligo
Abstract
:1. Background
2. Melanocytes and Keratinocytes as the Targets for Vitiligo Therapy
3. Melanocyte–Keratinocyte Cell Transplantation (MKCT)
4. ReCell System in Vitiligo Therapy
5. Autologous Non-Cultured, Non-Trypsinized Epidermal Cell Grafting
6. Cell Transplantations in Combination Therapy with a Narrowband Ultraviolet B (NB-UVB) or Autologous Platelet-Rich Plasma
7. Are Cell-Based Therapies Appropriate for All Vitiligo Patients? Limitations and Challenges
8. Conclusions
| Therapy | Vitiligo Characteristics | Patients Number | Age Range (Years) | Clinical Results | Evaluation Time | Reference | Year |
|---|---|---|---|---|---|---|---|
| Melanocyte transplantation | |||||||
| Cultured autologous melanocytes | Stable vitiligo | 25 | 13–72 | Almost complete repigmentation in 6 out of 11 cases, in 4 cases, 40–71% of the grafted achromatic area was repigmented | 1–20 months | Chen et al. [16] | 2000 |
| Autologous transplanted epidermal cell suspensions | Stable vitiligo or vitiligo with doubts about stability | 28 | 5–65 | A total of 77% repigmentation | 12 months | Van Geel et al. [1] | 2004 |
| Autologous cultured pure melanocyte suspension | Stable vitiligo | 120 | 7–72 | Overall, 84% of patients with localized vitiligo experienced 90% to 100% coverage | 6–66 months | Chen et al. [28] | 2004 |
| Autologous melanocyte rich cell suspension (non-cultured) and cultured melanocyte technique | Stable vitiligo | 27 | 21–30 | Over 90% repigmentation in 52.17% of cases with the autologous melanocyte-rich cell suspension technique and in 50% with the melanocyte culture technique | Up to 6 months | Pandya et al. [12] | 2005 |
| Autologous cultured pure melanocytes | Stable vitiligo for at least 6 months | 102 | 8–12, 13–17, Adults: mean 29 | Repigmentation of 50% or more in children, adolescents, and adults were 83.3%, 95.0% and 84.0% cases, respectively | At least 6 months | Hong et al. [17] | 2011 |
| Autologous melanocytes culture on a denuded amniotic membrane | Stable vitiligo | 4 | 13–29 | A total of 90–95% repigmentation in all patients | Up to 6 months | Redondo et al. [18] | 2011 |
| Autologous melanocytes transplantation | Stable vitiligo and developing vitiligo | 16 | 19–40 | In 87.5% of lesions, a repigmentation of >50%; no relapse was observed | 5 years | Zhu et al. [26] | 2017 |
| Autologous epidermal cell suspension | Stable vitiligo | 300 | Range not stated, mean 27.1 | Repigmentation stability remained in most treated patches | Up to 30 months | Orouji et al. [35] | 2017 |
| Autologous non-cultured and trypsinized melanocyte grafting | Stable vitiligo | 28 | Range not stated, mean 25.9 | Over 50% in the face and neck, trunk, upper extremity, and genitals in 57.4%, 20.4%, 16.7%, and 5.5% patients, respectively | 18 months | Ghorbani et al. [14] | 2022 |
| Hair follicle cell transplantation | |||||||
| Non-cultured extracted hair follicular outer root sheath (ORS) cell suspension transplantation | Stable vitiligo for at least 3 months | 14 | 17–32 | Nine out of fourteen patients achieved >75% repigmentation | 1–15 months | Mohanty et al. [19] | 2011 |
| Autologous non-cultured outer root sheath hair follicle cell suspension (NCORSHFS) | Stable vitiligo | 30 | 8–38 | The number of melanocytes and HFSC transplanted were significantly higher among patients achieving optimum (>75%) repigmentation | 24 weeks | Vinay et al. [20] | 2015 |
| Hair follicle outer root sheath cell transplantation (EHF ORS) | Stable vitiligo | 20 | 18–43 | Mean repigmentation of 80.15% with 90–100% in 60% of patients | 6 months | Shah et al. [21] | 2016 |
| Non-cultured, extracted follicular outer root sheath suspension (NC-EHF-ORS-CS) | Stable vitiligo | 2 | 18–36 | The mean repigmentation was 52% and >75% repigmentation in 32% of patients | 6 months | Kumar et al. [22] | 2018 |
| Autologous hair follicle cell derived melanocytes transplantation | Stable vitiligo | 26 | 19–50 | Overall, 34.6% of patients achieved excellent repigmentation, 50% had good, 11.5% had fair, and 3.9% had poor repigmentation | 1 year | Shi et al. [23] | 2020 |
| Keratinocyte transplantation | |||||||
| Autologous cultured keratinocytes | Stable vitiligo | 27 | 9–48 | Twelve patients had 90% or more repigmentation after the first surgery, which increased by two cases when patients with multiple surgeries were included | At least 1 year | Matsuzaki and Kumagai [30] | 2013 |
| Melanocyte–keratinocyte transplantation | |||||||
| Cultured epithelial autographs with keratinocytes seeded at high density | Stable or active vitiligo | 5 | 32–71 | Out of five patients, repigmentation was only achieved in one | N/A | Phillips et al. [33] | 2001 |
| Melanocyte–keratinocyte cell transplantation (MKT) | Stable vitiligo | 184 | 9 to 70 | Overall, 53% in the generalized vitiligo and 84% in the segmental vitiligo group showed 95–100% repigmentation | Up to 1 year | Mulekar et al. [43] | 2003 |
| Autologous non-cultured melanocyte–keratinocyte cell transplantation (MKT) | Stale vitiligo for at least 1 year | 134 | At least 12 | Overall, 84% in the segmental and 73% in the focal vitiligo group showed 95–100% repigmentation | Up to 5 years | Mulekar et al. [47] | 2004 |
| Autologous non-cultured melanocyte–keratinocyte cell transplantation (MKT) | Stable genital vitiligo | 3 | 24–39 | Near-complete repigmentation observed in all patients | Up to 1 year | Mulekar et al. [49] | 2005 |
| Autologus melanocyte–keratinocyte cell transplantation (MKT) | Stable vitiligo (for at least 6 months) | 142 | 18–70 | Overall, 56% patients showed 95–100% repigmentation | Up to 6 years | Mulekar et al. [48] | 2005 |
| Noncultured melanocyte–keratinocyte cell transplantation (MKT) | Stable vitiligo (for at least 6 months) | 49 | 7–65 | For bilateral vitiligo, more than 50% of patients showed >65% repigmentation. For unilateral vitiligo, all but two patients treated for the eyelids vitiligo showed >65% repigmentation | N/A | Mulekar et al. [55] | 2009 |
| Autologous dissociated epidermal cell suspensions | Stable vitiligo | 10 | 17–52 | Overall, 76–100% repigmentation in 40% of patients | 6 months | Khodadadi et al. [34] | 2010 |
| Non-cultured melanocyte–keratinocyte transplantation (MKT) | Stable vitiligo | 25 | 8–45 | Overall, 23% of patients showed 90–100% repigmentation | 6–17 months | El-Zawahry et al. [56] | 2011 |
| Non-cultured melanocyte–keratinocyte transplantation (MKT) | Stable vitiligo | 8 | 13–43 | Of eight lesions treated with non-cultured MKT, four lesions showed 96–100%, one lesion 65–95%, and three lesions 0–25% repigmentation | 4 months | Toossi et al. [50] | 2011 |
| Melanocytes and keratinocytes transplantation using the sandpaper technique combined with dermabrasion or only dermabrasion | Stable vitiligo | 11 | 21–65 | At the end of treatment, both techniques showed similar repigmentation with repigmentation in nine cases (87% to 6%) of pigmenation in the transfer group and nine cases (94% to 5%) in the dermabrasion group | 3 months | Quezada et al. [52] | 2011 |
| Melanocyte–keratinocyte cell suspension with dermabrasion (MKT+ DA) and dermabrasion alone (DA) | Stable vitiligo | 11 | 35–48 | Slightly better pigmentation occurred with DA+MKT in 7 out of 11 patients | 12 months | Vazquez-Martinez et al. [51] | 2011 |
| Autologous melanocyte–keratinocyte transplantation (MKT) | Stable vitiligo | 23 | 18–60 | Overall, 95–100% repigmentation in 17% | 3–6 months | Huggins et al. [53] | 2012 |
| Autologous transplantation of non-cultured melanocyte–keratinocyte cell suspension (MKT) | Stable vitiligo | 20 | 10–50 | Overall, 25% of patients showed ≥90% Repigmentation; the best results were observed in face and neck | Up to 24 months | Ramos et al. [58] | 2017 |
| Autologous melanocyte–keratinocyte transplantation (MKT) | Stable vitiligo without fingertip involvement | 100 | 9–60 | MKT could maintain repigmentation for at least 72 months | 12–72 months | Silpa-Archa et al. [44] | 2017 |
| Autologous non-cultured keratinocyte–melanocyte suspension (MKT) | Stable vitiligo | 5 | Range not stated, mean 20 | Overall, 76–100% repigmentation in 60% of patients | 6 months | Benzekri and Gauthier [25] | 2017 |
| Autologous non-cultured melanocyte–keratinocyte transplantation (MKT) | Stable vitiligo | 602 | 4–67 | Overall, 84.3% of patients achieved ≥50% repigmentation at the 6th month evaluation; at 6 years, 23% showed relapse | 6 years | Altalhab et al. [46] | 2019 |
| Combination therapies | |||||||
| Non-cultured autologous melanocytes and keratinocytes combined with UVA or UVB | Stable vitiligo | 4 | 30–52 | Overall, 85–100% repigmentation achieved at 6 to 20 months | 6–20 months | van Geel et al. [68] | 2001 |
| Narrow-band ultraviolet B therapy for cultured autologous melanocyte transplantation patients | Stable vitiligo | 437 | 5–55 | A total of 20 sessions of NB-UVB treatment before transplantation and 30 sessions after transplantation gave the best repigmentation | 6 months | Zhang et al. [66] | 2014 |
| Cultured autologous melanocyte transplantation (CMT combined with narrowband ultraviolet B (NB-UVB) | Stable vitiligo | 8 | 7–28 | All patients treated with low-density CMT combined with NB-UVB obtained more than 90% repigmentation | 1 year | Yao et al. [67] | 2017 |
| Platelet-rich plasma (PRP) used to suspend non-cultured epidermal cell suspension (NCES) before transplantation | Stable vitiligo | 21 | Range not stated, mean 23.1 | Suspending NCES in PRP can result in significantly greater mean repigmentation | 6 months | Parambath et al. [70] | 2019 |
| ReCell | |||||||
| ReCell vs. conventional melanocyte–keratinocyte transplantation (MKT) | Stable vitiligo | 5 | 18–40 | Overall, 40% of lesions treated with ReCell showed 100% repigmentation, while 20% of lesions failed to repigment. Overall, 60% of lesions treated with conventional MKT showed 100% repigmentation and 20% failed to repigment | 4 months | Mulekar et al. [62] | 2008 |
| ReCell | Stable vitiligo | 15 | 18–45 | Repigmentation greater than 75% was recorded in 12 (80%) patients | Minimum 6 months | Cervelli et al. [61] | 2009 |
| ReCell | Stable vitiligo | 1 | N/A | The patient had >90% repigmentation | N/A | Cervelli et al. [62] | 2010 |
| Non-cultured cellular grafting | |||||||
| Autologous, non-cultured cellular grafting (MKTP) | Stable vitiligo for at least 6 months | 25 | 4–16 | Overall, 95–100% repigmentation in 62% of patients | 9–54 months | Mulekar et al. [60] | 2010 |
| Autologous non-cultured epidermal suspension (NCES) | Stable vitiligo | 13 | 8–17 | Overall, 79% of lesions had >90% repigmentation | 1 year | Sahni et al. [61] | 2011 |
| Non-cultured cellular grafting | Stable vitiligo | 13 | 15–52 | Overall, 91% of the patients achieved >50% repigmentation | 3–12 months | Gan et al. [59] | 2012 |
| Non-cultured epidermal cell suspension (NCES) | Stable vitiligo | 36 | 16–47 | More than 75% repigmentation in 63.75% of lesions | 6–18 months | Holla et al. [57] | 2013 |
| Non-cultured epidermal cell suspension (NCES) | Stable vitiligo | 37 | Range not stated, mean: 28.3 (group 1) 24.1(group 2) 22.4 (group 3) | Cell count significantly lower in the ORSHFS compared with NCES with no significant difference in the repigmentation outcome | 18 months | El-Zawahry et al. [39] | 2017 |
| Autologous non-cultured epidermal cell suspension (NCES) | Stable vitiligo | 41 | 8–50 | Overall, 80.5% of patients showed 51–75% repigmentation, and 17.1% showed complete or almost complete repigmentation | 6–9 months | Liu et al. [72] | 2019 |
| Non-cultured autologous epidermal cell grafting resuspended in hyaluronic acid (A ready-to-use kit, Viticell®) | Stable vitiligo | 36 | 17–67 | For difficult-to-treat lesions, no repigmentation ≥50% was observed; for other locations, the success rate was significantly higher | 12 months | Bertolotti et al. [76] | 2020 |
| Comparative studies | |||||||
| Three different transplantation methods: autologous cultured melanocytes, ultrathin epidermal sheets, and basal layer cell suspension | Stable or unstable vitiligo | 132 | 8–61 | Stable vitiligo patients responded in most cases with 100% repigmentation in all studied treatments | 1–7 years | Olsson et al. [27] | 2002 |
| Autologous non-cultured epidermal cell suspension (NCES) compared to suction blister epidermal grafting (SBEG) | Stable vitiligo | 41 | 12–40 | Overall, 90–100% repigmentation in 71% of lesions in the NCES group and 27% of lesions in the SBEG group | 4 months | Budania et al. [37] | 2012 |
| Autologous non-cultured epidermal cell suspension (NCES) compared to autologous non-cultured extracted hair follicle outer root sheath cell suspension (NCORSHFS) | Stable vitiligo | 30 | 13–35 | Overall, 90–100% repigmentation in 83% of lesions in the NCES group and 65% of lesions in the NCORSHFS group | 4 months | Singh et al. [38] | 2013 |
| Comparison between autologous melanocyte rich cell suspension (NCMT technique) and cultured melanocyte technique (CMT) | Stable vitiligo | 25 | N/A | More than 70% repigmentation in 62.17% cases treated with NCMT and in 52% with the CMT | 6 months | Verma et al. [13] | 2014 |
| Blister roof grafting (BG), cultured melanocytes transplantation (CMT) and non-cultured epidermal cell suspension transplantation (NCES) | Stable vitiligo | 83 | Range not stated, mean 25 | More than 50% repigmentation in 92%, 82%, and 81% of the 83 patients treated with the BG, CMT, and NCES methods, respectively | 12 months | Bao et al. [36] | 2015 |
| Autologous cultured melanocytes transplantation (CMT) and non-cultured epidermal cell suspension transplantation (NCES) | Stable vitiligo | 30 | Range not stated, mean 26.1 | Overall, 66.7% of cases showed more than 70% repigmentation with CMT; NCES resulted in less than 40% repigmentation in most of the cases | 3–6 months | Verma et al. [40] | 2015 |
| Excimer laser alone compared to non-cultured melanocyte–keratinocyte transplantation (MKCT) alone and combination therapy | Stable vitiligo | 10 | 21–48 | Excimer laser combined with non-cultured MKCT improves the repigmentation rate, with an average of 41.9% reduction of depigmented area surface | 2 weeks | Ebadi et al. [54] | 2015 |
| Epidermal melanocyte transfer (EMT) compared to hair follicular melanocyte transfer (HFMT) | Stable vitiligo | 11 | 12–42 | More than 90% repigmentation observed in 83.33% patches of the EMT group and 43.33% in the HFMT group | 6 months | Donaparthi et al. [77] | 2016 |
| Autologous non-cultured epidermal cell suspension (ECS) and follicular cell suspension (FCS) | Stable vitiligo | 5 | 21–33 | Superior repigmentation obtained in combined ECS and FCS treatment | 4 months | Razmi et al. [41] | 2017 |
| Hair follicle transplantation (follicular unit transplantation, FUT) and autologous non-cultured, non-trypsinized epidermal cells grafting (Jodhpur Technique-JT) | Stable vitiligo | 30 | 21–25 | More than 75% repigmentation was observed in 70% lesions in the FUT group and 72% of lesions in the JT group | 20 weeks | Lamoria et al. [24] | 2020 |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Geel, N.; Ongenae, K.; De Mil, M.; Vander Haeghen, Y.; Vervaet, C.; Naeyaert, J.M. Double-Blind Placebo-Controlled Study of Autologous Transplanted Epidermal Cell Suspensions for Repigmenting Vitiligo. Arch. Dermatol. 2004, 140, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Alkhateeb, A.; Fain, P.R.; Thody, A.; Bennett, D.C.; Spritz, R.A. Epidemiology of Vitiligo and Associated Autoimmune Diseases in Caucasian Probands and Their Families. Pigment. Cell Res. 2003, 16, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Alikhan, A.; Felsten, L.M.; Daly, M.; Petronic-Rosic, V. Vitiligo: A Comprehensive Overview. J. Am. Acad. Dermatol. 2011, 65, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Felsten, L.M.; Alikhan, A.; Petronic-Rosic, V. Vitiligo: A Comprehensive Overview. J. Am. Acad. Dermatol. 2011, 65, 493–514. [Google Scholar] [CrossRef]
- Sarkar, S.; Sarkar, T.; Sarkar, A.; Das, S. Vitiligo and Psychiatric Morbidity: A Profile from a Vitiligo Clinic of a Rural-Based Tertiary Care Center of Eastern India. Indian J. Dermatol. 2018, 63, 281. [Google Scholar] [CrossRef]
- Bonotis, K.; Pantelis, K.; Karaoulanis, S.; Katsimaglis, C.; Papaliaga, M.; Zafiriou, E.; Tsogas, P. Investigation of Factors Associated with Health-Related Quality of Life and Psychological Distress in Vitiligo: Quality of Life and Psychological Distress in Vitiligo. JDDG J. Dtsch. Dermatol. Ges. 2016, 14, 45–48. [Google Scholar] [CrossRef]
- Khaitan, B.; Kathuria, S.; Ramam, M. A Descriptive Study to Characterize Segmental Vitiligo. Indian J. Dermatol. Venereol. Leprol. 2012, 78, 715. [Google Scholar] [CrossRef]
- Toriyama, K.; Kato, H.; Sato, H.; Tanaka, T.; Inoie, M.; Morita, A. Cultured Epidermal Autografts for Treatment of Stable Vitiligo: Quantitative Analysis of Color Matching with Surrounding Normally Pigmented Skin. J. Dermatol. 2021, 48, 1405–1408. [Google Scholar] [CrossRef]
- Picardo, M.; Bastonini, E. A New View of Vitiligo: Looking at Normal-Appearing Skin. J. Investig. Dermatol. 2015, 135, 1713–1714. [Google Scholar] [CrossRef]
- Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 20 November 2022).
- Available online: https://clinicaltrials.gov/ (accessed on 20 November 2022).
- Pandya, V.; Parmar, K.; Shah, B.; Bilimoria, F. A Study of Autologous Melanocyte Transfer in Treatment of Stable Vitiligo. Indian J. Dermatol. Venereol. Leprol. 2005, 71, 393. [Google Scholar] [CrossRef]
- Verma, R.; Grewal, R.S.; Chatterjee, M.; Pragasam, V.; Vasudevan, B.; Mitra, D. A Comparative Study of Efficacy of Cultured versus Non Cultured Melanocyte Transfer in the Management of Stable Vitiligo. Med. J. Armed Forces India 2014, 70, 26–31. [Google Scholar] [CrossRef]
- Ghorbani, I.; Khazaei, M.; Kavoussi, H.; Ebrahimi, A.; Rezaei, M.; Kavoussi, R.; Mansouri, K. Treatment of Recalcitrant Vitiligo by Autologous Non-Cultured and Trypsinized Melanocyte Grafting in the West of Iran. An. Bras. Dermatol. 2022, 97, 315–320. [Google Scholar] [CrossRef]
- Thingnes, J.; Lavelle, T.J.; Hovig, E.; Omholt, S.W. Understanding the Melanocyte Distribution in Human Epidermis: An Agent-Based Computational Model Approach. PLoS ONE 2012, 7, e40377. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Chang, J.S.; Yang, P.-Y.; Hung, C.-M.; Huang, M.-H.; Hu, D.-N. Transplant of Cultured Autologous Pure Melanocytes after Laser-Abrasion for the Treatment of Segmental Vitiligo. J. Dermatol. 2000, 27, 434–439. [Google Scholar] [CrossRef]
- Hong, W.; Hu, D.; Qian, G.; McCormick, S.; Xu, A. Treatment of Vitiligo in Children and Adolescents by Autologous Cultured Pure Melanocytes Transplantation with Comparison of Efficacy to Results in Adults: Melanocyte Transplantation in Children with Vitiligo. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 538–543. [Google Scholar] [CrossRef]
- Redondo, P.; Giménez de Azcarate, A.; Marqués, L.; García-Guzman, M.; Andreu, E.; Prósper, F. Amniotic Membrane as a Scaffold for Melanocyte Transplantation in Patients with Stable Vitiligo. Dermatol. Res. Pract. 2011, 2011, 1–6. [Google Scholar] [CrossRef]
- Mohanty, S.; Kumar, A.; Dhawan, J.; Sreenivas, V.; Gupta, S. Noncultured Extracted Hair Follicle Outer Root Sheath Cell Suspension for Transplantation in Vitiligo: ORS Cell Transplantation in Vitiligo. Br. J. Dermatol. 2011, 164, 1241–1246. [Google Scholar] [CrossRef]
- Vinay, K.; Dogra, S.; Parsad, D.; Kanwar, A.J.; Kumar, R.; Minz, R.W.; Saikia, U.N. Clinical and Treatment Characteristics Determining Therapeutic Outcome in Patients Undergoing Autologous Non-Cultured Outer Root Sheath Hair Follicle Cell Suspension for Treatment of Stable Vitiligo. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 31–37. [Google Scholar] [CrossRef]
- Shah, A.; Marfatia, R.; Saikia, S. A Study of Noncultured Extracted Hair Follicle Outer Root Sheath Cell Suspension for Transplantation in Vitiligo. Int. J. Trichol. 2016, 8, 67. [Google Scholar] [CrossRef]
- Kumar, P.; Bhari, N.; Tembhre, M.K.; Mohanty, S.; Arava, S.; Sharma, V.K.; Gupta, S. Study of Efficacy and Safety of Noncultured, Extracted Follicular Outer Root Sheath Cell Suspension Transplantation in the Management of Stable Vitiligo. Int. J. Dermatol. 2018, 57, 245–249. [Google Scholar] [CrossRef]
- Shi, H.-X.; Zhang, R.-Z.; Xu, B.; Xu, C.-X.; Li, D.; Wang, L.; Xiao, L. Experimental Study and Clinical Observations of Autologous Hair Follicle Cell Transplants to Treat Stable Vitiligo. Indian J. Dermatol. Venereol. Leprol. 2020, 86, 124. [Google Scholar] [CrossRef] [PubMed]
- Lamoria, A.; Agrawal, A.; Rao, P.; Kachhawa, D. A Comparative Study between Follicular Unit Transplantation and Autologous Non-Cultured Non-Trypsinized Epidermal Cells Grafting (Jodhpur Technique) in Stable Vitiligo. J. Cutan. Aesthet. Surg. 2020, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Benzekri, L.; Gauthier, Y. The First Transepidermal Transplantation of Non-Cultured Epidermal Suspension Using a Dermarolling System in Vitiligo: A Sequential Histological and Clinical Study. Pigment Cell Melanoma Res. 2017, 30, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-C.; Ma, H.-Y.; Zhan, Z.; Liu, C.-G.; Luo, W.; Zhao, G. Detection of Auto Antibodies and Transplantation of Cultured Autologous Melanocytes for the Treatment of Vitiligo. Exp. Ther. Med. 2017, 13, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.J.; Juhlin, L. Long-Term Follow-up of Leucoderma Patients Treated with Transplants of Autologous Cultured Melanocytes, Ultrathin Epidermal Sheets and Basal Cell Layer Suspension. Br. J. Dermatol. 2002, 147, 893–904. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Yang, P.-Y.; Hu, D.-N.; Kuo, F.-S.; Hung, C.-S.; Hung, C.-M. Treatment of Vitiligo by Transplantation of Cultured Pure Melanocyte Suspension: Analysis of 120 Cases. J. Am. Acad. Dermatol. 2004, 51, 68–74. [Google Scholar] [CrossRef]
- Lee, A.-Y. Role of Keratinocytes in the Development of Vitiligo. Ann. Dermatol. 2012, 24, 115. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Kumagai, N. Treatment of Vitiligo with Autologous Cultured Keratinocytes in 27 Cases. Eur. J. Plast. Surg. 2013, 36, 651–656. [Google Scholar] [CrossRef]
- Gauthier, Y.; Surleve-Bazeille, J.-E. Autologous Grafting with Non-cultured Melanocytes: A Simplified Method for Treatment of Depigmented Lesions. J. Am. Acad. Dermatol. 1992, 26, 191–194. [Google Scholar] [CrossRef]
- Billingham, R.E.; Medawar, P.B. Pigment Spread and Cell Heredity in Guinea-Pigs’ Skin. Heredity 1948, 2, 29–47. [Google Scholar] [CrossRef]
- Phillips, J.; Gawkrodger, D.J.; Caddy, C.M.; Hedley, S.; Dawson, R.A.; Smith-Thomas, L.; Freedlander, E.; Mac Neil, S. Keratinocytes Suppress TRP-1 Expression and Reduce Cell Number of Co-Cultured Melanocytes—Implications For Grafting of Patients with Vitiligo: Keratinocytes Down-Regulate Melanocyte Number and Pigmentation. Pigment. Cell Res. 2001, 14, 116–125. [Google Scholar] [CrossRef]
- Khodadadi, L.; Shafieyan, S.; Sotoudeh, M.; Dizaj, A.V.; Shahverdi, A.; Aghdami, N.; Baharvand, H. Intraepidermal Injection of Dissociated Epidermal Cell Suspension Improves Vitiligo. Arch. Dermatol. Res. 2010, 302, 593–599. [Google Scholar] [CrossRef]
- Orouji, Z.; Bajouri, A.; Ghasemi, M.; Mohammadi, P.; Fallah, N.; Shahbazi, A.; Rezvani, M.; Vaezirad, F.; Khalajasadi, Z.; Alizadeh, A.; et al. A Single-Arm Open-Label Clinical Trial of Autologous Epidermal Cell Transplantation for Stable Vitiligo: A 30-Month Follow-Up. J. Dermatol. Sci. 2018, 89, 52–59. [Google Scholar] [CrossRef]
- Bao, H.; Hong, W.; Fu, L.; Wei, X.; Qian, G.; Xu, A. Blister Roof Grafting, Cultured Melanocytes Transplantation and Non-Cultured Epidermal Cell Suspension Transplantation in Treating Stable Vitiligo: A Mutual Self-Control Study. J. Dermatol. Treat. 2015, 26, 571–574. [Google Scholar] [CrossRef]
- Budania, A.; Parsad, D.; Kanwar, A.J.; Dogra, S. Comparison between Autologous Noncultured Epidermal Cell Suspension and Suction Blister Epidermal Grafting in Stable Vitiligo: A Randomized Study. Br. J. Dermatol. 2012, 167, 1295–1301. [Google Scholar] [CrossRef]
- Singh, C.; Parsad, D.; Kanwar, A.J.; Dogra, S.; Kumar, R. Comparison between Autologous Noncultured Extracted Hair Follicle Outer Root Sheath Cell Suspension and Autologous Noncultured Epidermal Cell Suspension in the Treatment of Stable Vitiligo: A Randomized Study. Br. J. Dermatol. 2013, 169, 287–293. [Google Scholar] [CrossRef]
- El-Zawahry, B.M.; Esmat, S.; Bassiouny, D.; Zaki, N.S.; Sobhi, R.; Saleh, M.A.; Abdel-Halim, D.; Hegazy, R.; Gawdat, H.; Samir, N.; et al. Effect of Procedural-Related Variables on Melanocyte–Keratinocyte Suspension Transplantation in Nonsegmental Stable Vitiligo: A Clinical and Immunocytochemical Study. Dermatol. Surg. 2017, 43, 226–235. [Google Scholar] [CrossRef]
- Verma, G.; Varkhande, S.R.; Kar, H.K.; Rani, R. Evaluation of Repigmentation with Cultured Melanocyte Transplantation (CMT) Compared with Non-Cultured Epidermal Cell Transplantation in Vitiligo at 12th Week Reveals Better Repigmentation with CMT. J. Investig. Dermatol. 2015, 135, 2533–2535. [Google Scholar] [CrossRef]
- Razmi, T.M.; Parsad, D.; Kumaran, S.M. Combined Epidermal and Follicular Cell Suspension as a Novel Surgical Approach for Acral Vitiligo. J. Am. Acad. Dermatol. 2017, 76, 564–567. [Google Scholar] [CrossRef]
- Olsson, M.J.; Juhlin, L. Juhlin Leucoderma Treated by Transplantation of a Basal Cell Layer Enriched Suspension: BASAL CELL TRANSPLANT FOR LEUCODERMA. Br. J. Dermatol. 1998, 138, 644–648. [Google Scholar] [CrossRef]
- Mulekar, S.V. Melanocyte-Keratinocyte Cell Transplantation for Stable Vitiligo. Int. J. Dermatol. 2003, 42, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Silpa-Archa, N.; Griffith, J.L.; Huggins, R.H.; Henderson, M.D.; Kerr, H.A.; Jacobsen, G.; Mulekar, S.V.; Lim, H.W.; Hamzavi, I.H. Long-Term Follow-up of Patients Undergoing Autologous Noncultured Melanocyte-Keratinocyte Transplantation for Vitiligo and Other Leukodermas. J. Am. Acad. Dermatol. 2017, 77, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Nahhas, A.F.; Mohammad, T.F.; Hamzavi, I.H. Vitiligo surgery: Shuffling melanocytes. J. Investig. Dermatol. Symp. Proc. 2017, 18, S34–S37. [Google Scholar] [CrossRef] [PubMed]
- Altalhab, S.; AlJasser, M.I.; Mulekar, S.V.; Al Issa, A.; Mulekar, S.; Diaz, J.; Diallo, A.; Ezzedine, K. Six-year Follow-up of Vitiligo Patients Successfully Treated with Autologous Non-cultured Melanocyte–Keratinocyte Transplantation. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Mulekar, S.V. Long-Term Follow-up Study of Segmental and Focal Vitiligo Treated by Autologous, Noncultured Melanocyte-Keratinocyte Cell Transplantation. Arch. Dermatol. 2004, 140, 1211–1215. [Google Scholar] [CrossRef]
- Mulekar, S.V. Long-Term Follow-up Study of 142 Patients with Vitiligo Vulgaris Treated by Autologous, Non-Cultured Melanocyte-Keratinocyte Cell Transplantation: Vitiligo Vulgaris Patients Treated by Epidermal Cell Transplantation. Int. J. Dermatol. 2005, 44, 841–845. [Google Scholar] [CrossRef]
- Mulekar, S.V.; Issa, A.; Eisa, A.; Asaad, M. Genital Vitiligo Treated by Autologous, Noncultured Melanocyte-Keratinocyte Cell Transplantation. Dermatol. Surg. 2005, 31, 1737–1740. [Google Scholar] [CrossRef]
- Toossi, P.; Shahidi-Dadras, M.; Mahmoudi Rad, M.; Fesharaki, R.J. Non-Cultured Melanocyte-Keratinocyte Transplantation for the Treatment of Vitiligo: A Clinical Trial in an Iranian Population: Cell Grafting for Vitiligo. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1182–1186. [Google Scholar] [CrossRef]
- Vazquez-Martínez, O.T.; Martínez-Rodríguez, H.G.; Velásquez-Arenas, L.; Baños-González, D.; Ortíz-López, R.; Padilla-Rivas, G.; Welsh, O.; Ocampo-Candiani, J. Treatment of Vitiligo with a Melanocyte-Keratinocyte Cell Suspension versus Dermabrasion Only: A Pilot Study with a 12-Month Follow Up. J. Drugs Dermatol. 2011, 10, 1032–1036. [Google Scholar]
- Quezada, N.; Filho, C. App.M.; De La Sotta, P.; Uribe, P. Melanocytes and Keratinocytes Transfer Using Sandpaper Technique Combined with Dermabrasion for Stable Vitiligo. Dermatol. Surg. 2011, 37, 192–198. [Google Scholar] [CrossRef]
- Huggins, R.H.; Henderson, M.D.; Mulekar, S.V.; Ozog, D.M.; Kerr, H.A.; Jabobsen, G.; Lim, H.W.; Hamzavi, I.H. Melanocyte-Keratinocyte Transplantation Procedure in the Treatment of Vitiligo: The Experience of an Academic Medical Center in the United States. J. Am. Acad. Dermatol. 2012, 66, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, A.; Rad, M.M.; Nazari, S.; Fesharaki, R.J.; Ghalamkarpour, F.; Younespour, S. The Additive Effect of Excimer Laser on Non-Cultured Melanocyte-Keratinocyte Transplantation for the Treatment of Vitiligo: A Clinical Trial in an Iranian Population. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Mulekar, S.V.; Al Issa, A.; Al Eisa, A. Treatment of Vitiligo on Difficult-to-Treat Sites Using Autologous Noncultured Cellular Grafting. Dermatol. Surg. 2009, 35, 66–71. [Google Scholar] [CrossRef] [PubMed]
- El-Zawahry, B.; Zaki, N.; Bassiouny, D.; Sobhi, R.; Zaghloul, A.; Khorshied, M.; Gouda, H. Autologous Melanocyte-Keratinocyte Suspension in the Treatment of Vitiligo: MC-KC Suspension in Treatment of Vitiligo. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Holla, A.P.; Sahni, K.; Kumar, R.; Parsad, D.; Kanwar, A.; Mehta, S.D. Acral Vitiligo and Lesions over Joints Treated with Non-Cultured Epidermal Cell Suspension Transplantation. Clin. Exp. Dermatol. 2013, 38, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.G.; Ramos, D.G.; Ramos, C.G. Evaluation of Treatment Response to Autologous Transplantation of Noncultured Melanocyte/Keratinocyte Cell Suspension in Patients with Stable Vitiligo. An. Bras. Dermatol. 2017, 92, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Gan, E.Y.; van Geel, N.; Goh, B.K. Repigmentation of Leucotrichia in Vitiligo with Noncultured Cellular Grafting: Leucotrichia Repigmentation with Noncultured Cellular Grafting. Br. J. Dermatol. 2012, 166, 196–199. [Google Scholar] [CrossRef]
- Mulekar, S.V.; Al Eisa, A.; Delvi, M.B.; Al Issa, A.; Al Saeed, A.H. Childhood Vitiligo: A Long-Term Study of Localized Vitiligo Treated by Non-cultured Cellular Grafting. Pediatr. Dermatol. 2010, 27, 132–136. [Google Scholar] [CrossRef]
- Sahni, K.; Parsad, D.; Kanwar, A.J. Noncultured Epidermal Suspension Transplantation for the Treatment of Stable Vitiligo in Children and Adolescents: Noncultured Melanocyte Transplantation in Children and Adolescents. Clin. Exp. Dermatol. 2011, 36, 607–612. [Google Scholar] [CrossRef]
- Cervelli, V.; De Angelis, B.; Balzani, A.; Colicchia, G.; Spallone, D.; Grimaldi, M. Treatment of Stable Vitiligo by ReCell System. Acta Dermatovenerol. Croat. 2009, 17, 273–278. [Google Scholar]
- Mulekar, S.V.; Ghwish, B.; Al Issa, A.; Al Eisa, A. Treatment of Vitiligo Lesions by ReCell ® vs. Conventional Melanocyte–Keratinocyte Transplantation: A Pilot Study. Br. J. Dermatol. 2007, 19, 507–527. [Google Scholar] [CrossRef]
- Cervelli, V.; Spallone, D.; Lucarini, L.; Palla, L.; Brinci, L.; De Angelis, B. Treatment of Stable Vitiligo Hands by ReCell System: A Preliminary Report. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 691–694. [Google Scholar]
- Tyagi, S.; Malhotra, S.K.; Kaur, T. Comparative Evaluation of Efficacy of Non-Cultured Epidermal Cell Suspension and Epidermal Curettage in Stable Vitiligo. J. Cutan. Aesthet. Surg. 2021, 14, 32–40. [Google Scholar] [CrossRef]
- Samson Yashar, S.; Gielczyk, R.; Scherschun, L.; Lim, H.W. Narrow-Band Ultraviolet B Treatment for Vitiligo, Pruritus, and Inflammatory Dermatoses: Narrow-Band UVB Treatment. Photodermatol. Photoimmunol. Photomed. 2003, 19, 164–168. [Google Scholar] [CrossRef]
- Zhang, D.; Hong, W.; Fu, L.; Wei, X.; Xu, A. A Randomized Controlled Study of the Effects of Different Modalities of Narrow-Band Ultraviolet B Therapy on the Outcome of Cultured Autologous Melanocytes Transplantation in Treating Vitiligo. Dermatol. Surg. 2014, 40, 420–426. [Google Scholar] [CrossRef]
- Yao, L.; Liu, Y.; Song, Y.; Zhong, S.; Li, S. Successful Treatment of Stable Vitiligo by Low-Density Cultured Autologous Melanocyte Transplantation Combined With Narrowband Ultraviolet B Therapy. Dermatol. Surg. 2017, 43, 1281–1287. [Google Scholar] [CrossRef]
- van Geel, N.; Ongenae, K.; De Mil, M.; Naeyaert, J.M. Modified Technique of Autologous Noncultured Epidermal Cell Transplantation for Repigmenting Vitiligo: A Pilot Study. Dermatol. Surg. 2001, 27, 873–876. [Google Scholar] [CrossRef]
- Ibrahim, Z.A.; El-Ashmawy, A.A.; El-Tatawy, R.A.; Sallam, F.A. The Effect of Platelet-Rich Plasma on the Outcome of Short-Term Narrowband-Ultraviolet B Phototherapy in the Treatment of Vitiligo: A Pilot Study. J. Cosmet. Dermatol. 2016, 15, 108–116. [Google Scholar] [CrossRef]
- Parambath, N.; Sharma, V.K.; Parihar, A.S.; Sahni, K.; Gupta, S. Use of Platelet-Rich Plasma to Suspend Non-cultured Epidermal Cell Suspension Improves Repigmentation after Autologous Transplantation in Stable Vitiligo: A Double-Blind Randomized Controlled Trial. Int. J. Dermatol. 2019, 58, 472–476. [Google Scholar] [CrossRef]
- Liu, B.; Chen, H.-H.; Liu, Z.-H.; Liang, J.-F.; Xue, R.-J.; Chen, P.-J.; Li, C.-X.; Liang, X.-D.; Deng, J.; Ye, R.-X.; et al. The Clinical Efficacy of Treatment Using the Autologous Non-Cultured Epidermal Cell Suspension Technique for Stable Vitiligo in 41 Patients. J. Dermatol. Treat. 2021, 32, 90–94. [Google Scholar] [CrossRef]
- Available online: https://www.aafp.org/pubs/afp/issues/2017/1215/p797.html (accessed on 20 November 2022).
- Abdolahzadeh, H.; Mohammadi, P.; Ghasemi, M.; Mousavi, S.A.; Bajouri, A.; Ataei-Fashtami, L.; Totonchi, M.; Rezvani, M.; Aghdami, N.; Shafieyan, S. Comparison of Skin Transcriptome between Responder and Non-Responder Vitiligo Lesions to Cell Transplantation: A Clinical Trial Study. Cell J. 2022, 24, 316. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.J.; Bae, J.M.; Lee, R.W.; Kim, S.H.; Parsad, D.; Pourang, A.; Hamzavi, I.; Shourick, J.; Ezzedine, K. Surgical Interventions for Patients With Vitiligo: A Systematic Review and Meta-analysis. JAMA Dermatol. 2021, 157, 307–331. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, A.; Leone, G.; Taïeb, A.; Soriano, E.; Pascal, M.; Maillard, H.; Geel, N. Assessment of Non-Cultured Autologous Epidermal Cell Grafting Resuspended in Hyaluronic Acid for Repigmenting Vitiligo and Piebaldism Lesions: A Randomized Clinical Trial. Acta Derm. Venereol. 2021, 2021, 101. [Google Scholar] [CrossRef] [PubMed]
- Donaparthi, N.; Chopra, A. Comparative Study of Efficacy of Epidermal Melanocyte Transfer Versus Hair Follicular Melanocyte Transfer in Stable Vitiligo. Indian J. Dermatol. 2016, 61, 640–644. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domaszewska-Szostek, A.; Polak, A.; Słupecka-Ziemilska, M.; Krzyżanowska, M.; Puzianowska-Kuźnicka, M. Current Status of Cell-Based Therapies for Vitiligo. Int. J. Mol. Sci. 2023, 24, 3357. https://doi.org/10.3390/ijms24043357
Domaszewska-Szostek A, Polak A, Słupecka-Ziemilska M, Krzyżanowska M, Puzianowska-Kuźnicka M. Current Status of Cell-Based Therapies for Vitiligo. International Journal of Molecular Sciences. 2023; 24(4):3357. https://doi.org/10.3390/ijms24043357
Chicago/Turabian StyleDomaszewska-Szostek, Anna, Agnieszka Polak, Monika Słupecka-Ziemilska, Marta Krzyżanowska, and Monika Puzianowska-Kuźnicka. 2023. "Current Status of Cell-Based Therapies for Vitiligo" International Journal of Molecular Sciences 24, no. 4: 3357. https://doi.org/10.3390/ijms24043357
APA StyleDomaszewska-Szostek, A., Polak, A., Słupecka-Ziemilska, M., Krzyżanowska, M., & Puzianowska-Kuźnicka, M. (2023). Current Status of Cell-Based Therapies for Vitiligo. International Journal of Molecular Sciences, 24(4), 3357. https://doi.org/10.3390/ijms24043357





