Key Role of Mesenchymal Stromal Cell Interaction with Macrophages in Promoting Repair of Lung Injury
Abstract
1. Introduction
2. The Role of Lung Tissue Macrophages in the Pathogenesis of Lung Injury
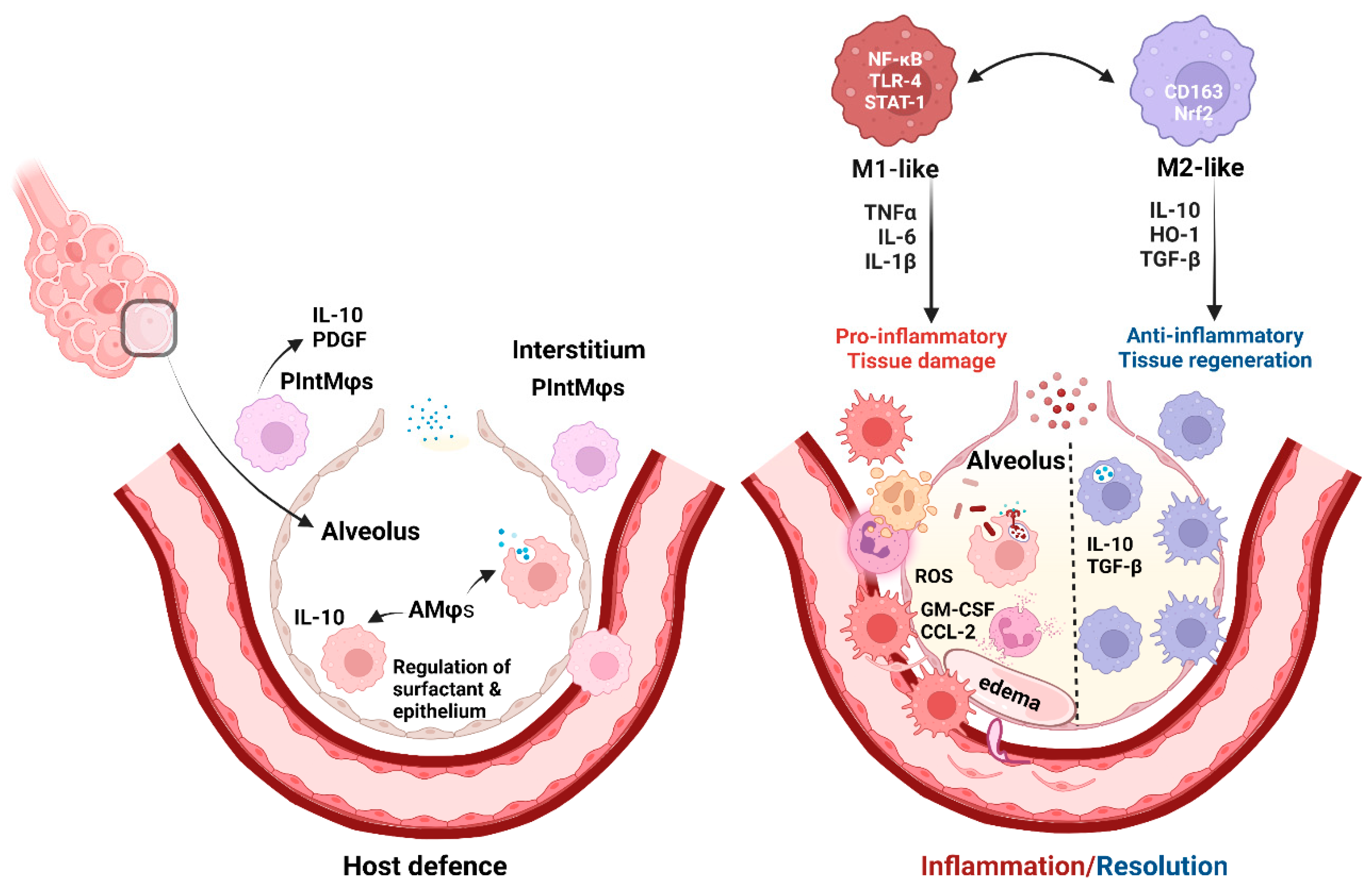
2.1. Pulmonary Mφs as a Host Defense Mechanism
2.2. Role of Mφ Plasticity in Lung Injury and Repair
3. MSCs in Lung Injury Treatment
3.1. Definition and Characteristics of MSCs
3.2. Preclinical Studies—MSC Action, Licensing, and Genetic Modifications
| Preclinical Model | Intervention (MSCs/EVs) | Outcome | Mechanism | References |
|---|---|---|---|---|
| Human-monocyte-derived Mφs in noncontact co-culture with hMSCs. | Stimulation of co-cultured cells with LPS or BALF from patients with ARDS. | MSCs suppressed pro-inflammatory cytokine production by Mφ. | Increased M2 Mφ marker expression and augmented phagocytic capacity of Mφs. | [44] Morrison et al., 2017 |
| MSCs cultured under different temperatures in vitro in co-culture with Mφ. | hBM-MSCs and Mφ. | MSCs cultured at higher temperatures induce more IL-10 and less TNFα production in Mφs (M2-like phenotype). | Nuclear translocation of HSF-1 and induction of COX2/PGE2 pathways by hyperthermia in MSCs promoted M2-like Mφ phenotype change. | [56] McClain-Caldwell et al., 2018 |
| Polymicrobial sepsis-induced lung injury in mice and in vitro. | Murine MSCs or MSC-conditioned media. | Attenuation of sepsis and TNF-induced miR- 193b-5p upregulation. | miR-193b-5p was decreased by MSCs while its target gene OCLN was increased in lungs from septic mice and in vitro. | [51] DosSantos et al., 2022 |
| Escherichia coli (E. coli)-induced ARDS in rats. | hUC-MSCs and hBM-MSCs. | Improved animal survival, systemic oxygenation, and lung compliance by both hUC- and BM-MSCs. | Decrease in pro-inflammatory cytokines in BALF, increase in IL-10, and ROS reduction in lung tissue. | [43] Curley et al., 2017 |
| LPS-induced ALI in mice. | Adoptive transfer of AMφs pretreated with hMSC-derived EVs. | Reduced inflammation and lung injury in LPS mice. | Mφ changes induced by mitochondrial transfer from EVs to AMφs during pretreatment. | [44] Morrison et al., 2017 |
| LPS-induced ALI in mice. | MSC-EVs derived from young and aging MSCs. | Young MSC-EVs alleviated LPS-ALI, while aging MSC-EVs did not. | Aging MSC-EVs failed to be internalized and did not induce Mφ phenotypic change. | [45] Huang et al., 2019 |
| LPS-induced ALI in mice. | MSC-EVs. | EVs reduce lung injury. | Restoration of mitochondrial respiration in the lung tissue. | [46] Dutra Silva 2021 |
| Ventilator-induced ALI in rats. | Rodent BM-MSCs or their secretome. | Restored systemic oxygenation, lung function, and structure by both MSCs and their secretome. | Decreased lung inflammation (TNFα, IL-6), and increase in IL-10; role of KGF in lung repair. | [47] Curley et al., 2012 |
| Ventilator-induced ALI in mice. | Murine BM-MSCs. | Lungs were protected from injury. | Improved lung function and reduced oxidative stress and collagen-1 expression. | [48] Islam et al., 2019 |
| Ventilator-induced ALI in rats. | Rodent BM-MSCs or their secretome. | MSCs were more effective in reducing lung injury than their secretome. | Improved oxygenation; reduction in lung edema, alveolar inflammation, and IL-6 levels. | [49] Hayes et al., 2015 |
| Polymicrobial sepsis-induced lung injury in mice. | Murine MSCs. | MicroRNA (miRNA) and transcriptome analysis of septic mouse lungs showed that MSCs induced a shift in transcription profiles favoring reconstitution of ‘sham-like’ expression patterns. | MSCs downregulated miR-27a-5p and upregulated its target gene VAV3 in septic lungs. | [50] Younes et al., 2020 |
| Ventilator-induced ALI in rats. | hBM-MSCs, naïve and cytokine-pre-activated (with IL-1β, TNF-α, IFN-γ). | Cytokine pre-activation enhanced the capacity of MSCs to promote injury resolution. | Mechanism dependent on KGF secreted by MSCs. | [52] Horie et al., 2020 |
| Radiation-induced pneumonia and late fibrosis in mice. | Murine BM-MSCs cultured in normoxic and hypoxic environment. | Therapeutic effect of MSCs exposed to hypoxia was more pronounced compared to MSCs exposed to normoxia. | Hypoxia-treated MSCs were more viable and resistant to hypoxia decreasing oxidative stress in lungs by HIF1-α. | [54] Li et al., 2017 |
| Chronic asthma mouse model—challenged with ovalbumin (OVA). | hUC-MSCs-derived EVs from MSCs cultured in normoxic (Nor-EVs) and hypoxic (Hypo-EVs) conditions. | Hypo-EVs were more effective than Nor-EVs in attenuation of chronic asthma. | TGFβ1 pathway was decreased and miR-146-5p increased. The effect was more pronounced if Hypo-EVs were used. | [55] Dong et al., 2021 |
| E. coli-induced pneumonia in rats. | EVs from naïve or interferon (IFN)-γ-primed hUC-MSCs. | EVs from IFN-γ-primed hUC-MSCs more effectively attenuated lung injury than EVs from naïve MSCs. | Enhancements of Mφ phagocytosis and bacterial killing. | [53] Varkouhi et al., 2019 |
| E. coli-induced ARDS. | Naïve and IL-10 over-expressing hUC-MSCs. | IL-10-UC-MSCs were more efficient in decreasing structural lung injury compared to naïve UC-MSC or vehicle therapy. | AMφs from naïve and especially from IL-10-UC-MSC-treated rats enhanced Mφ phagocytosis via increased Mφ HO-1, an effect blocked by PGE2 and LXA4 inhibition. | [58] Jerkic et al., 2019 |
| Acid-primed lung injury in mice. | Murine BM-MSCs, environment correction, or MSC-carrying human IL-10 or HGF gene. | MSCs worsened acid-primed lung injuries associated with fibrosis and high levels of ROS and IL-6. | Correction of oxidative stress with GPx-1, or treatment with MSCs carrying IL-10 or HGF after injury reversed the detrimental effects of naïve MSCs. | [48] Islam et al., 2019 |
| COPD rat cigarette smoke model. | hUC-MSCs and hUC-EVs. | Both transplantation of hUC-MSCs and application of EVs reduced lung inflammation and ameliorated the loss of alveolar septa and their thickening. | Both hUC-MSCs and EVs decreased mononuclear infiltration and reduced the levels of NF-κB subunit p65 in COPD lungs. | [63] Ridzuan et al., 2021 |
| Hyperoxia-induced bronchopulmonary dysplasia (BPD) in rats. | hUC-MSC-EVs. | EVs ameliorated the impaired alveolarization and pulmonary artery remodeling. | MSC-EV prevented hyperoxia-induced reduction in CD163-positive (M2-like) Mφ both in alveolar and interstitial compartment. | [64] Porzionato et al., 2021 |
| Mouse-bleomycin-induced pulmonary fibrosis. | hUC-MSCs. | MSCs attenuated pulmonary fibrosis and promoted lung repair by interacting with Mφs. | Mφs interferon-sensitive sub-cluster induced by MSC infusion caused T-regulatory cell recruitment by CXCL9/10. Number of CD206 Mφs involved in fibrosis was reduced. | [65] Tang et al., 2021 |
4. Crosstalk between MSCs and Mφs—Mechanisms of Action
4.1. Contact-Dependent MSC-Mφ Interaction
4.1.1. Receptor-Dependent Interaction
4.1.2. Microtubular Network
4.2. MSCs and Mφ Secretome—Paracrine-Mediated Mechanisms
4.2.1. Role of Cytokines, the COX/PGE2/EP4 Axis, Heme Oxygenase, and Chemokines in MSC-Mφ Interaction
Cytokines
COX/PGE2/EP4 Axis
Growth Factors and HO-1
Chemokines
4.2.2. Role of MSC-Derived Extracellular Vesicles (EVs), mRNA, MicroRNA, and Mitochondrial Transfer in Immunomodulation through Mφs
4.3. Role of Autophagy, Mitophagy, and Oxidative Stress in MSC-Mφ Interplay
5. Therapeutic Potential of MSC-Mφ Interaction and Lung Injury Resolution
5.1. Reparatory Potential of MSC-Mφ Interaction in Chronic Lung Diseases
5.2. Clinical Studies
5.3. MSC-Mφ Interaction in COVID-19—More Studies Are Needed
| Study Type/Patient Cohort | Intervention | Outcomes Measured and Results | Reference/Trial Number |
|---|---|---|---|
| Phase 1 moderate–severe ARDS 12 patients (pts). | Adipose MSCs—allogeneic 1x i.v. -1 million cells/kg or placebo. | No cell toxicity or SAEs. No improvement in length of hospital stay or ventilator-free days or change in biomarkers. | [199] Zheng et al., 2014 NCT01902082 |
| Phase 1 (STAR) 9 (pts.); moderate-to-severe ARDS. | BM-MSCs—allogeneic 1x i.v.: 1, 5, or 10 million cells/kg (3 pt./each dose). | Safety trial: single dose of allogeneic BM-MSCs was safe and well tolerated. | [180] Wilson et al., 2015 NCT01775774 |
| Phase 2a (STAR) Moderate–severe ARDS 60 ventilated pts. | BM-MSCs allogeneic—1x i.v.: 2:1 either 10 million/kg of MSCs or placebo. | 28-day mortality did not differ after adjustments for APACHE III score. | [80] Matthay et al., 2019 NCT02097641 |
| Nested cohort within a phase 2a (STAR); moderate–severe ARDS 27 pts. | BM-MSCs—allogeneic 1x i.v.: 10 million/kg, n = 17 pts, and placebo, n = 10 pts. | MSC treatment significantly reduced airspace total protein, Ang-2, IL-6, and soluble TNF receptor-1 concentrations. | [181] Wick et al., 2021 NCT02097641 |
| Phase 1/2a COVID-19 ARDS 24 pts (1:1). | UC-MSCs—2x i.v. (100 million cells/infusion) + heparin; placebo vehicle + heparin. | No AEs and SAEs with cell treatment; improvement of patient survival and time to recovery. | [195] Lanzoni et al., 2021 NCT04355728 |
| Phase 1 COVID-19 with mild–severe ARDS (REALIST) 9 pts. | UC-MSCs-CD362 (Syndecan-2) enriched (ORBCEL-3)—1x i.v.: 100, 200, or 400 million cells/infusion (3 pts/each dose). | Well tolerated and no dose-limiting toxicity. Safe to proceed to Phase 2 trial. | [192] Gorman et al., 2021 NCT03042143 |
| Phase 1/2a COVID-19; critically ill 40 pts (1:1). | UC-MSCs + standard care (Oseltamivir and Azithromycin)—1x i.v. 1 million cells/kg or placebo + standard care. | Improved survival rate, no changes in ICU stay or ventilator use, and no AEs. IL-6 reduced | [194] Dilogo et al., 2021 NCT04457609 |
| Phase 2 COVID-19 with severe ARDS 100 pts, (2:1). | UC-MSCs—3x i.v. (40 million cells/infusion) or placebo. | Improvement in whole-lung lesion volume and no difference in SAEs. | [191] Shi et al., 2021 NCT04288102 |
| Phase2b COVID-19 with mild–severe ARDS 45 pts. | UC-MSCs—3x i.v. 1 million/kg = 21 pts, or placebo = 24 pts. | No SAEs associated with repeated cell infusions. PaO2/FiO2 changes did not differ between the groups. | [193] Monsel et al., 2022 NCT04333368 |
6. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations in Alphabetical Order
References
- Kumar, V. Pulmonary Innate Immune Response Determines the Outcome of Inflammation During Pneumonia and Sepsis-Associated Acute Lung Injury. Front. Immunol. 2020, 11, 1722. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Christman, J.W. Editorial: Alveolar Macrophages in Lung Inflammation and Resolution. Front. Immunol. 2019, 10, 2275. [Google Scholar] [CrossRef] [PubMed]
- Perdiguero, E.G.; Klapproth, K.; Schulz, C.; Busch, K.; de Bruijn, M.; Rodewald, H.-R.; Geissmann, F. The Origin of Tissue-Resident Macrophages: When an Erythro-myeloid Progenitor Is an Erythro-myeloid Progenitor. Immunity 2015, 43, 1023–1024. [Google Scholar] [PubMed]
- Wu, Y.; Hirschi, K.K. Tissue-Resident Macrophage Development and Function. Front. Cell Dev. Biol. 2021, 8, 617879. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gu, Y.; Chakarov, S.; Bleriot, C.; Kwok, I.; Chen, X.; Shin, A.; Huang, W.; Dress, R.J.; Dutertre, C.-A.; et al. Fate Mapping via Ms4a3-Expression History Traces Monocyte-Derived Cells. Cell 2019, 178, 1509–1525.e19. [Google Scholar] [CrossRef]
- Byrne, A.J.; Powell, J.E.; O’Sullivan, B.J.; Ogger, P.P.; Hoffland, A.; Cook, J.; Bonner, K.L.; Hewitt, R.J.; Wolf, S.; Ghai, P.; et al. Dynamics of human monocytes and airway macrophages during healthy aging and after transplant. J. Exp. Med. 2020, 217, e20191236. [Google Scholar] [CrossRef]
- Hortobágyi, L.; Kierstein, S.; Krytska, K.; Zhu, X.; Das, A.M.; Poulain, F.; Haczku, A. Surfactant Protein D Inhibits Tnf-Alpha Production by Macrophages and Dendritic Cells in Mice. J. Allergy Clin. Immunol. 2008, 122, 521–528. [Google Scholar] [CrossRef]
- Nakamura, A.; Ebina-Shibuya, R.; Itoh-Nakadai, A.; Muto, A.; Shima, H.; Saigusa, D.; Aoki, J.; Ebina, M.; Nukiwa, T.; Igarashi, K. Transcription repressor Bach2 is required for pulmonary surfactant homeostasis and alveolar macrophage function. J. Exp. Med. 2013, 210, 2191–2204. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Wang, X.; Zhang, S.; Gao, P.; Shi, Z. The Role of Pulmonary Surfactants in the Treatment of Acute Respiratory Distress Syndrome in COVID-19. Front. Pharmacol. 2021, 12, 698905. [Google Scholar] [CrossRef]
- Rubins, J.B. Alveolar Macrophages: Wielding the Double-Edged Sword of Inflammation. Am. J. Respir. Crit. Care Med. 2003, 167, 103–104. [Google Scholar] [CrossRef]
- Berenson, C.S.; Kruzel, R.L.; Eberhardt, E.; Sethi, S. Phagocytic Dysfunction of Human Alveolar Macrophages and Severity of Chronic Obstructive Pulmonary Disease. J. Infect. Dis. 2013, 208, 2036–2045. [Google Scholar] [CrossRef]
- Bain, C.C.; MacDonald, A.S. The impact of the lung environment on macrophage development, activation and function: Diversity in the face of adversity. Mucosal Immunol. 2022, 15, 223–234. [Google Scholar] [CrossRef]
- Bedoret, D.; Wallemacq, H.; Marichal, T.; Desmet, C.; Quesada Calvo, F.; Henry, E.; Closset, R.; Dewals, B.G.; Thielen, C.; Gustin, P.; et al. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J. Clin. Investig. 2009, 119, 3723–3738. [Google Scholar] [CrossRef]
- Hoppstädter, J.; Diesel, B.; Zarbock, R.; Breinig, T.; Monz, D.; Koch, M.; Meyerhans, A.; Gortner, L.; Lehr, C.-M.; Huwer, H.; et al. Differential cell reaction upon Toll-like receptor 4 and 9 activation in human alveolar and lung interstitial macrophages. Respir. Res. 2010, 11, 124. [Google Scholar] [CrossRef]
- Kawano, H.; Kayama, H.; Nakama, T.; Hashimoto, T.; Umemoto, E.; Takeda, K. IL-10-producing lung interstitial macrophages prevent neutrophilic asthma. Int. Immunol. 2016, 28, 489–501. [Google Scholar] [CrossRef]
- Sabatel, C.; Radermecker, C.; Fievez, L.; Paulissen, G.; Chakarov, S.; Fernandes, C.; Olivier, S.; Toussaint, M.; Pirottin, D.; Xiao, X.; et al. Exposure to Bacterial CpG DNA Protects from Airway Allergic Inflammation by Expanding Regulatory Lung Interstitial Macrophages. Immunity 2017, 46, 457–473. [Google Scholar] [CrossRef]
- Brody, A.R.; Bonner, J.C.; Overby, L.H.; Badgett, A.; Kalter, V.; Kumar, R.; Bennett, R.A. Interstitial pulmonary macrophages produce platelet-derived growth factor that stimulates rat lung fibroblast proliferation in vitro. J. Leukoc. Biol. 1992, 51, 640–648. [Google Scholar] [CrossRef]
- Jaguin, M.; Houlbert, N.; Fardel, O.; Lecureur, V. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell. Immunol. 2013, 281, 51–61. [Google Scholar] [CrossRef]
- Dong, Y.; Poon, G.F.T.; Arif, A.; Lee-Sayer, S.S.M.; Dosanjh, M.; Johnson, P. The survival of fetal and bone marrow monocyte-derived alveolar macrophages is promoted by CD44 and its interaction with hyaluronan. Mucosal Immunol. 2018, 11, 601–614. [Google Scholar] [CrossRef]
- Sierra-Filardi, E.; Nieto, C.; Domínguez-Soto, Á.; Barroso, R.; Sánchez-Mateos, P.; Puig-Kroger, A.; López-Bravo, M.; Joven, J.; Ardavín, C.; Rodríguez-Fernández, J.L.; et al. Ccl2 Shapes Macrophage Polarization by Gm-Csf and M-Csf: Identification of Ccl2/Ccr2-Dependent Gene Expression Profile. J Immunol 2014, 192, 3858–3867. [Google Scholar] [CrossRef]
- Stein, M.; Keshav, S.; Harris, N.; Gordon, S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. J. Exp. Med. 1992, 176, 287–292. [Google Scholar] [CrossRef]
- Scull, C.M.; Hays, W.D.; Fischer, T.H. Macrophage pro-inflammatory cytokine secretion is enhanced following interaction with autologous platelets. J. Inflamm. 2010, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Song, Y.; Li, F.; Fan, Y.; Li, Y.; Zhang, C.; Hou, H.; Shi, M.; Zhao, Z.; Chen, Z. Act001 Suppressing M1 Polarization against Inflammation Via Nf-Κb and Stat1 Signaling Pathways Alleviates Acute Lung Injury in Mice. Int. Immunopharmacol. 2022, 110, 108944. [Google Scholar] [CrossRef] [PubMed]
- Kellner, M.; Noonepalle, S.; Lu, Q.; Srivastava, A.; Zemskov, E.; Black, S.M. ROS Signaling in the Pathogenesis of Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS). Adv. Exp. Med. Biol. 2017, 967, 105–137. [Google Scholar] [PubMed]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of macrophage polarization in autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef]
- Hussell, T.; Bell, T.J. Alveolar macrophages: Plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014, 14, 81–93. [Google Scholar] [CrossRef]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 123–147. [Google Scholar] [CrossRef]
- Jerkic, M.; Litvack, M.L.; Gagnon, S.; Otulakowski, G.; Zhang, H.; Rotstein, O.; Kavanagh, B.P.; Post, M.; Laffey, J.G. Laffey. Embryonic-Derived Myb(-) Macrophages Enhance Bacterial Clearance and Improve Survival in Rat Sepsis. Int. J. Mol. Sci. 2021, 22, 3190. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage Plasticity, Polarization, and Function in Health and Disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Shen, K.; Jia, Y.; Wang, X.; Zhang, J.; Liu, K.; Wang, J.; Cai, W.; Li, J.; Li, S.; Zhao, M.; et al. Exosomes from Adipose-Derived Stem Cells Alleviate the Inflammation and Oxidative Stress Via Regulating Nrf2/Ho-1 Axis in Macrophages. Free Radic. Biol. Med. 2021, 165, 54–66. [Google Scholar] [CrossRef]
- Jerkic, M.; Gagnon, S.; Rabani, R.; Ward-Able, T.; Masterson, C.; Otulakowski, G.; Curley, G.F.; Marshall, J.; Kavanagh, B.P.; Laffey, J.G. Human Umbilical Cord Mesenchymal Stromal Cells Attenuate Systemic Sepsis in Part by Enhancing Peritoneal Macrophage Bacterial Killing via Heme Oxygenase-1 Induction in Rats. Anesthesiology 2020, 132, 140–154. [Google Scholar] [CrossRef]
- Fleming, B.D.; Mosser, D.M. Regulatory macrophages: Setting the Threshold for Therapy. Eur. J. Immunol. 2011, 41, 2498–2502. [Google Scholar] [CrossRef]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar]
- Shi, C.; Jia, T.; Mendez-Ferrer, S.; Hohl, T.M.; Serbina, N.V.; Lipuma, L.; Leiner, I.; Li, M.O.; Frenette, P.S.; Pamer, E.G. Bone Marrow Mesenchymal Stem and Progenitor Cells Induce Monocyte Emigration in Response to Circulating Toll-like Receptor Ligands. Immunity 2011, 34, 590–601. [Google Scholar] [CrossRef]
- Zeytin, I.C.; Alkan, B.; Ozdemir, C.; Cetinkaya, D.U.; Okur, F.V. Alterations in Hematopoietic and Mesenchymal Stromal Cell Components of the Osteopetrotic Bone Marrow Niche. STEM CELLS Transl. Med. 2022, 11, 310–321. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The Development of Fibroblast Colonies in Monolayer Cultures of Guinea-Pig Bone Marrow and Spleen Cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef]
- Lan, T.; Luo, M.; Wei, X. Mesenchymal Stem/Stromal Cells in Cancer Therapy. J. Hematol. Oncol. 2021, 14, 195. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Huppert, L.A.; Matthay, M.A.; Ware, L.B. Pathogenesis of Acute Respiratory Distress Syndrome. Semin. Respir. Crit. Care Med. 2019, 40, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Curley, G.; Jerkic, M.; Dixon, S.; Hogan, G.; Masterson, C.; O’Toole, D.; Devaney, J.; Laffey, J. Cryopreserved, Xeno-Free Human Umbilical Cord Mesenchymal Stromal Cells Reduce Lung Injury Severity and Bacterial Burden in Rodent Escherichia coli–Induced Acute Respiratory Distress Syndrome. Crit. Care Med. 2017, 45, e202–e212. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.J.; Jackson, M.V.; Cunningham, E.K.; Kissenpfennig, A.; McAuley, D.F.; O’Kane, C.M.; Krasnodembskaya, A.D. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am. J. Respir. Crit. Care Med. 2017, 196, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Qin, C.; Wang, J.; Hu, Y.; Zheng, G.; Qiu, G.; Ge, M.; Tao, H.; Shu, Q.; Xu, J. Differential effects of extracellular vesicles from aging and young mesenchymal stem cells in acute lung injury. Aging 2019, 11, 7996–8014. [Google Scholar] [CrossRef] [PubMed]
- Dutra Silva, J.; Su, Y.; Calfee, C.S.; Delucchi, K.L.; Weiss, D.; McAuley, D.F.; O’Kane, C.; Krasnodembskaya, A.D. Mesenchymal stromal cell extracellular vesicles rescue mitochondrial dysfunction and improve barrier integrity in clinically relevant models of ARDS. Eur. Respir. J. 2020, 58, 2002978. [Google Scholar] [CrossRef] [PubMed]
- Curley, G.F.; Hayes, M.; Ansari, B.; Shaw, G.; Ryan, A.; Barry, F.; O’Brien, T.; O’Toole, D.; Laffey, J.G. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax 2011, 67, 496–501. [Google Scholar] [CrossRef]
- Islam, D.; Huang, Y.; Fanelli, V.; Delsedime, L.; Wu, S.; Khang, J.; Han, B.; Grassi, A.; Li, M.; Xu, Y.; et al. Identification and Modulation of Microenvironment Is Crucial for Effective Mesenchymal Stromal Cell Therapy in Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2019, 199, 1214–1224. [Google Scholar] [CrossRef]
- Hayes, M.; Curley, G.F.; Masterson, C.; Devaney, J.; O’Toole, D.; Laffey, J.G. Mesenchymal stromal cells are more effective than the MSC secretome in diminishing injury and enhancing recovery following ventilator-induced lung injury. Intensiv. Care Med. Exp. 2015, 3, 29. [Google Scholar] [CrossRef]
- Younes, N.; Zhou, L.; Amatullah, H.; Mei, S.H.J.; Herrero, R.; Lorente, J.A.; Stewart, D.J.; Marsden, P.; Liles, W.C.; Hu, P.; et al. Mesenchymal stromal/stem cells modulate response to experimental sepsis-induced lung injury via regulation of miR-27a-5p in recipient mice. Thorax 2020, 75, 556–567. [Google Scholar] [CrossRef]
- dos Santos, C.C.; Amatullah, H.; Vaswani, C.M.; Maron-Gutierrez, T.; Kim, M.; Mei, S.H.; Szaszi, K.; Monteiro, A.P.T.; Varkouhi, A.K.; Herreroz, R.; et al. Mesenchymal Stromal (stem) Cell (MSC) therapy modulates miR-193b-5p expression to attenuate sepsis-induced acute lung injury. Eur. Respir. J. 2021, 59, 2004216. [Google Scholar] [CrossRef]
- Horie, S.; Gaynard, S.; Murphy, M.; Barry, F.; Scully, M.; O’Toole, D.; Laffey, J.G. Cytokine pre-activation of cryopreserved xenogeneic-free human mesenchymal stromal cells enhances resolution and repair following ventilator-induced lung injury potentially via a KGF-dependent mechanism. Intensiv. Care Med. Exp. 2020, 8, 8–15. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Jerkic, M.; Ormesher, L.; Gagnon, S.; Goyal, S.; Rabani, R.; Masterson, C.; Spring, C.; Chen, P.Z.; Gu, F.X.; et al. Extracellular Vesicles from Interferon-γ–primed Human Umbilical Cord Mesenchymal Stromal Cells Reduce Escherichia coli–induced Acute Lung Injury in Rats. Anesthesiology 2019, 130, 778–790. [Google Scholar] [CrossRef]
- Li, B.; Li, C.; Zhu, M.; Zhang, Y.; Du, J.; Xu, Y.; Liu, B.; Gao, F.; Liu, H.; Cai, J.; et al. Hypoxia-Induced Mesenchymal Stromal Cells Exhibit an Enhanced Therapeutic Effect on Radiation-Induced Lung Injury in Mice due to an Increased Proliferation Potential and Enhanced Antioxidant Ability. Cell. Physiol. Biochem. 2017, 44, 1295–1310. [Google Scholar] [CrossRef]
- Dong, L.; Wang, Y.; Zheng, T.; Pu, Y.; Ma, Y.; Qi, X.; Zhang, W.; Xue, F.; Shan, Z.; Liu, J.; et al. Hypoxic Hucmsc-Derived Extracellular Vesicles Attenuate Allergic Airway Inflammation and Airway Remodeling in Chronic Asthma Mice. Stem Cell Res. Ther. 2021, 12, 4. [Google Scholar] [CrossRef]
- Mcclain-Caldwell, I.; Vitale-Cross, L.; Mayer, B.; Krepuska, M.; Boyajian, M.; Myneni, V.; Martin, D.; Marko, K.; Nemeth, K.; Mezey, E. Immunogenic potential of human bone marrow mesenchymal stromal cells is enhanced by hyperthermia. Cytotherapy 2018, 20, 1437–1444. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Monteiro, A.P.T.; Tsoporis, J.N.; Mei, S.H.J.; Stewart, D.J.; Dos Santos, C.C. Genetically Modified Mesenchymal Stromal/Stem Cells: Application in Critical Illness. Stem Cell Rev. Rep. 2020, 16, 812–827. [Google Scholar] [CrossRef]
- Jerkic, M.; Masterson, C.; Ormesher, L.; Gagnon, S.; Goyal, S.; Rabani, R.; Otulakowski, G.; Zhang, H.; Kavanagh, B.P.; Laffey, J.G. Overexpression of IL-10 Enhances the Efficacy of Human Umbilical-Cord-Derived Mesenchymal Stromal Cells in E. coli Pneumosepsis. J. Clin. Med. 2019, 8, 847. [Google Scholar] [CrossRef]
- Dunbar, H.; Weiss, D.J.; Enes, S.R.; Laffey, J.G.; English, K. The Inflammatory Lung Microenvironment; a Key Mediator in MSC Licensing. Cells 2021, 10, 2982. [Google Scholar] [CrossRef]
- Rendin, L.E.; Löfdahl, A.; Kadefors, M.; Söderlund, Z.; Tykesson, E.; Enes, S.R.; Wigén, J.; Westergren-Thorsson, G. Harnessing the ECM Microenvironment to Ameliorate Mesenchymal Stromal Cell-Based Therapy in Chronic Lung Diseases. Front. Pharmacol. 2021, 12, 645558. [Google Scholar] [CrossRef]
- Antunes, M.A.; e Silva, J.R.L.; Rocco, P.R. Mesenchymal stromal cell therapy in COPD: From bench to bedside. Int. J. Chronic Obstr. Pulm. Dis. 2017, ume 12, 3017–3027. [Google Scholar] [CrossRef]
- Cruz, F.F.; Rocco, P.R.M. The potential of mesenchymal stem cell therapy for chronic lung disease. Expert Rev. Respir. Med. 2019, 14, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Ridzuan, N.; Zakaria, N.; Widera, D.; Sheard, J.; Morimoto, M.; Kiyokawa, H.; Isa, S.A.M.; Singh, G.K.C.; Then, K.-Y.; Ooi, G.-C.; et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles ameliorate airway inflammation in a rat model of chronic obstructive pulmonary disease (COPD). Stem Cell Res. Ther. 2021, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Zaramella, P.; Dedja, A.; Guidolin, D.; Bonadies, L.; Macchi, V.; Pozzobon, M.; Jurga, M.; Perilongo, G.; De Caro, R.; et al. Intratracheal administration of mesenchymal stem cell-derived extracellular vesicles reduces lung injuries in a chronic rat model of bronchopulmonary dysplasia. Am. J. Physiol. Cell. Mol. Physiol. 2021, 320, L688–L704. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Gao, J.; Wu, J.; Zeng, G.; Liao, Y.; Song, Z.; Liang, X.; Hu, J.; Hu, Y.; Liu, M.; et al. Human Umbilical Cord Mesenchymal Stromal Cells Attenuate Pulmonary Fibrosis Via Regulatory T Cell through Interaction with Macrophage. Stem Cell Res. Ther. 2021, 12, 397. [Google Scholar] [CrossRef]
- Galipeau, J. Macrophages at the Nexus of Mesenchymal Stromal Cell Potency: The Emerging Role of Chemokine Cooperativity. Stem Cells 2021, 39, 1145–1154. [Google Scholar] [CrossRef]
- Falcones, B.; Söderlund, Z.; Ibáñez-Fonseca, A.; Almendros, I.; Otero, J.; Farré, R.; Rolandsson Enes, S.; Elowsson Rendin, L.; Westergren-Thorsson, G. Hlmsc Secretome Affects Macrophage Activity Differentially Depending on Lung-Mimetic Environments. Cells 2022, 11, 12. [Google Scholar] [CrossRef]
- Chanteux, H.; Guisset, A.C.; Pilette, C.; Sibille, Y. LPS induces IL-10 production by human alveolar macrophages via MAPKinases- and Sp1-dependent mechanisms. Respir. Res. 2007, 8, 71. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Sun, L.; Gao, W.; Xiong, Y.; Ma, A.; Liu, X.; Shen, L.; Li, Q.; Yang, H. Manipulation of macrophage polarization by peptide-coated gold nanoparticles and its protective effects on acute lung injury. J. Nanobiotechnol. 2020, 18, 38. [Google Scholar] [CrossRef]
- Jackson, M.V.; Morrison, T.J.; Doherty, D.F.; McAuley, D.F.; Matthay, M.A.; Kissenpfennig, A.; O’Kane, C.M.; Krasnodembskaya, A.D. Krasnodembskaya. Mitochondrial Transfer Via Tunneling Nanotubes Is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the in Vitro and in Vivo Models of Ards. Stem Cells 2016, 34, 2210–2223. [Google Scholar] [CrossRef]
- Evans, J.F.; Ricigliano, A.E.; Morante, A.V.; Martinez, E.; Vargas, D.; Thyagaraj, J. Mesenchymal Stem Cell Regulation of Macrophage Phagocytosis; Quantitation and Imaging. J. Vis. Exp. 2021, 173, e62729. [Google Scholar]
- Shirin, M.; Agharezaeei, M.; Alizadeh, S.; Bashash, D.; Sheikhsaran, F.; Chahardouli, B.; A Mousavi, S.; Ahmadvand, M.; Ghaffari, S.H. A Comparative Study of the Bone Marrow- and Umbilical Cord-Derived Mesenchymal Stem Cells (MSCs) Efficiency on Generating MSC-Educated Macrophages (MEMs). Asian Pac. J. Cancer Prev. 2022, 23, 3083–3092. [Google Scholar] [CrossRef]
- Dong, B.; Wang, C.; Zhang, J.; Zhang, J.; Gu, Y.; Guo, X.; Zuo, X.; Pan, H.; Hsu, A.C.-Y.; Wang, G.; et al. Exosomes from human umbilical cord mesenchymal stem cells attenuate the inflammation of severe steroid-resistant asthma by reshaping macrophage polarization. Stem Cell Res. Ther. 2021, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Saldaña, L.; Bensiamar, F.; Vallés, G.; Mancebo, F.J.; García-Rey, E.; Vilaboa, N. Immunoregulatory Potential of Mesenchymal Stem Cells Following Activation by Macrophage-Derived Soluble Factors. Stem Cell Res. Ther. 2019, 10, 58. [Google Scholar] [CrossRef]
- Mathias, L.J.; Khong, S.M.L.; Spyroglou, L.; Payne, N.L.; Siatskas, C.; Thorburn, A.N.; Boyd, R.L.; Heng, T.S.P. Alveolar Macrophages Are Critical for the Inhibition of Allergic Asthma by Mesenchymal Stromal Cells. J. Immunol. 2013, 191, 5914–5924. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhou, X.; Li, J.; Meng, Q.; Cao, H.; Kang, L.; Ni, Y.; Fan, H.; Liu, Z. Mesenchymal stem cells reprogram host macrophages to attenuate obliterative bronchiolitis in murine orthotopic tracheal transplantation. Int. Immunopharmacol. 2013, 15, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Webb, T.L.; Ning, F.; Shiraishi, Y.; Regan, D.P.; Chow, L.; Smith, M.J.; Ashino, S.; Guth, A.M.; Hopkins, S.; et al. Mesenchymal Stem Cells Recruit CCR2+ Monocytes To Suppress Allergic Airway Inflammation. J. Immunol. 2018, 200, 1261–1269. [Google Scholar] [CrossRef]
- Chang, C.-L.; Leu, S.; Sung, H.-C.; Zhen, Y.-Y.; Cho, C.-L.; Chen, A.; Tsai, T.-H.; Chung, S.-Y.; Chai, H.-T.; Sun, C.-K.; et al. Impact of apoptotic adipose-derived mesenchymal stem cells on attenuating organ damage and reducing mortality in Rat sepsis syndrome induced by cecal puncture and ligation. J. Transl. Med. 2012, 10, 244. [Google Scholar] [CrossRef]
- Sung, P.-H.; Chang, C.-L.; Tsai, T.-H.; Chang, L.-T.; Leu, S.; Chen, Y.-L.; Yang, C.-C.; Chua, S.; Yeh, K.-H.; Chai, H.-T.; et al. Apoptotic adipose-derived mesenchymal stem cell therapy protects against lung and kidney injury in sepsis syndrome caused by cecal ligation puncture in rats. Stem Cell Res. Ther. 2013, 4, 155. [Google Scholar] [CrossRef]
- Matthay, M.A.; Calfee, C.S.; Zhuo, H.; Thompson, B.T.; Wilson, J.G.; E Levitt, J.; Rogers, A.J.; E Gotts, J.; Wiener-Kronish, J.P.; Bajwa, E.K.; et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): A randomised phase 2a safety trial. Lancet Respir. Med. 2018, 7, 154–162. [Google Scholar] [CrossRef]
- Weiss, D.J.; English, K.; Krasnodembskaya, A.; Isaza-Correa, J.M.; Hawthorne, I.J.; Mahon, B.P. The Necrobiology of Mesenchymal Stromal Cells Affects Therapeutic Efficacy. Front. Immunol. 2019, 10, 1228. [Google Scholar] [CrossRef]
- Cheung, T.S.; Dazzi, F. Mesenchymal-myeloid interaction in the regulation of immunity. Semin. Immunol. 2018, 35, 59–68. [Google Scholar] [CrossRef]
- Giri, J.; Galipeau, J. Mesenchymal stromal cell therapeutic potency is dependent upon viability, route of delivery, and immune match. Blood Adv. 2020, 4, 1987–1997. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Xu, L.; Dong, L.; Zheng, J.; Lin, Y.; Huang, J.; Zhang, Y.; Tao, Y.; Zang, X.; et al. Cell–cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cell. Mol. Immunol. 2019, 16, 908–920. [Google Scholar] [CrossRef]
- Jackson, M.; Krasnodembskaya, A. Analysis of Mitochondrial Transfer in Direct Co-cultures of Human Monocyte-derived Macrophages (MDM) and Mesenchymal Stem Cells (MSC). Bio-Protocol 2017, 7, e2255. [Google Scholar] [CrossRef]
- Liu, K.; Ji, K.; Guo, L.; Wu, W.; Lu, H.; Shan, P.; Yan, C. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia–reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc. Res. 2014, 92, 10–18. [Google Scholar] [CrossRef]
- Ahmad, T.; Mukherjee, S.; Pattnaik, B.; Kumar, M.; Singh, S.; Rehman, R.; Tiwari, B.K.; Jha, K.A.; Barhanpurkar, A.P.; Wani, M.R.; et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014, 33, 994–1010. [Google Scholar]
- Tseng, N.; Lambie, S.C.; Huynh, C.Q.; Sanford, B.; Patel, M.; Herson, P.S.; Ormond, D.R. Mitochondrial transfer from mesenchymal stem cells improves neuronal metabolism after oxidant injury in vitro: The role of Miro1. J. Cereb. Blood Flow Metab. 2020, 41, 761–770. [Google Scholar] [CrossRef]
- Yao, Y.; Fan, X.L.; Jiang, D.; Zhang, Y.; Li, X.; Xu, Z.B.; Fang, S.B.; Chiu, S.; Tse, H.F.; Lian, Q.; et al. Connexin 43-Mediated Mitochondrial Transfer of Ipsc-Mscs Alleviates Asthma Inflammation. Stem Cell Rep. 2018, 11, 1120–1135. [Google Scholar] [CrossRef]
- Stevens, H.Y.; Bowles, A.C.; Yeago, C.; Roy, K. Molecular Crosstalk Between Macrophages and Mesenchymal Stromal Cells. Front. Cell Dev. Biol. 2020, 8, 600160. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef]
- Chen, X.; Tang, J.; Shuai, W.; Meng, J.; Feng, J.; Han, Z. Macrophage polarization and its role in the pathogenesis of acute lung injury/acute respiratory distress syndrome. Inflamm. Res. 2020, 69, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Li, S.; Chen, H. Macrophages in Lung Injury, Repair, and Fibrosis. Cells 2021, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Arabpour, M.; Saghazadeh, A.; Rezaei, N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int. Immunopharmacol. 2021, 97, 107823. [Google Scholar] [CrossRef]
- Vrančić, M.; Banjanac, M.; Nujić, K.; Bosnar, M.; Murati, T.; Munić, V.; Stupin Polančec, D.; Belamarić, D.; Parnham, M.J.; Eraković Haber, V. Azithromycin Distinctively Modulates Classical Activation of Human Monocytes in Vitro. Br. J. Pharmacol. 2012, 165, 1348–1360. [Google Scholar] [CrossRef]
- Rabani, R.; Volchuk, A.; Jerkic, M.; Ormesher, L.; Garces-Ramirez, L.; Canton, J.; Masterson, C.; Gagnon, S.; Tatham, K.C.; Marshall, J.; et al. Mesenchymal stem cells enhance NOX2-dependent reactive oxygen species production and bacterial killing in macrophages during sepsis. Eur. Respir. J. 2018, 51, 1702021. [Google Scholar] [CrossRef]
- Tsai, C.F.; Chen, G.W.; Chen, Y.C.; Shen, C.K.; Lu, D.Y.; Yang, L.Y.; Chen, J.H.; Yeh, W.L. Regulatory Effects of Quercetin on M1/M2 Macrophage Polarization and Oxidative/Antioxidative Balance. Nutrients 2021, 14, 67. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lin, Y.C.; Tsai, M.L.; Tsai, Y.G.; Kuo, C.H.; Hung, C.H. Il-33 Regulates M1/M2 Chemokine Expression Via Mitochondrial Redox-Related Mitophagy in Human Monocytes. Chem. Biol. Interact. 2022, 359, 109915. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, Z.; Zhong, Q.; Zheng, Z.; Chen, Y.; Shangguan, W.; Zhang, Y.; Yang, J.; Zhu, D.; Xie, W. M2 macrophages secrete CXCL13 to promote renal cell carcinoma migration, invasion, and EMT. Cancer Cell Int. 2021, 21, 677. [Google Scholar] [CrossRef]
- Iwakura, T.; Zhao, Z.; A Marschner, J.; Devarapu, S.K.; Yasuda, H.; Anders, H.J. Dipeptidyl peptidase-4 inhibitor teneligliptin accelerates recovery from cisplatin-induced acute kidney injury by attenuating inflammation and promoting tubular regeneration. Nephrol. Dial. Transplant. 2019, 34, 1669–1680. [Google Scholar] [CrossRef]
- Kohler, J.B.; Cervilha, D.A.D.B.; Moreira, A.R.; Santana, F.R.; Farias, T.M.; Vale, M.I.C.A.; Martins, M.D.A.; Prado, C.M.; Tibério, I.C.; Ito, J.T.; et al. Microenvironmental stimuli induce different macrophage polarizations in experimental models of emphysema. Biol. Open 2019, 8, bio040808. [Google Scholar] [CrossRef]
- Dong, F.; Ruan, S.; Wang, J.; Xia, Y.; Le, K.; Xiao, X.; Hu, T.; Wang, Q. M2 macrophage-induced lncRNA PCAT6 facilitates tumorigenesis and angiogenesis of triple-negative breast cancer through modulation of VEGFR2. Cell Death Dis. 2020, 11, 728. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, T.; Wu, T.; Xu, W.; Dou, G.; Wang, Y.; Guo, C. M2 Macrophages Promote Vasculogenesis During Retinal Neovascularization by Regulating Bone Marrow-Derived Cells Via Sdf-1/Vegf. Cell Tissue. Res. 2020, 380, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tredget, E.E.; Wu, P.Y.G.; Wu, Y. Paracrine Factors of Mesenchymal Stem Cells Recruit Macrophages and Endothelial Lineage Cells and Enhance Wound Healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef]
- Zhang, S.; Fang, J.; Liu, Z.; Hou, P.; Cao, L.; Zhang, Y.; Liu, R.; Li, Y.; Shang, Q.; Chen, Y.; et al. Inflammatory cytokines-stimulated human muscle stem cells ameliorate ulcerative colitis via the IDO-TSG6 axis. Stem Cell Res. Ther. 2021, 12, 50. [Google Scholar] [CrossRef]
- Wang, G.; Cao, K.; Liu, K.; Xue, Y.; Roberts, A.I.; Li, F.; Han, Y.; Rabson, A.B.; Wang, Y.; Shi, Y. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2017, 25, 1209–1223. [Google Scholar] [CrossRef]
- Abreu, S.C.; Antunes, M.A.; Xisto, D.G.; Cruz, F.F.; Branco, V.C.; Bandeira, E.; Kitoko, J.Z.; de Araújo, A.F.; Dellatorre-Texeira, L.; Olsen, P.C.; et al. Bone Marrow, Adipose, and Lung Tissue-Derived Murine Mesenchymal Stromal Cells Release Different Mediators and Differentially Affect Airway and Lung Parenchyma in Experimental Asthma. STEM CELLS Transl. Med. 2017, 6, 1557–1567. [Google Scholar] [CrossRef]
- Tang, X.-D.; Shi, L.; Monsel, A.; Li, X.-Y.; Zhu, H.-L.; Zhu, Y.-G.; Qu, J.-M. Mesenchymal Stem Cell Microvesicles Attenuate Acute Lung Injury in Mice Partly Mediated by Ang-1 Mrna. Stem Cells 2017, 35, 1849–1859. [Google Scholar] [CrossRef]
- Kwon, H.M.; Hur, S.-M.; Park, K.-Y.; Kim, C.-K.; Kim, Y.-M.; Kim, H.-S.; Shin, H.-C.; Won, M.-H.; Ha, K.-S.; Kwon, Y.-G.; et al. Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vasc. Pharmacol. 2014, 63, 19–28. [Google Scholar] [CrossRef]
- Harrell, C.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.T.; Hosseini-Beheshti, E.; Afrose, D.; Ding, X.; Xia, B.; Grau, G.; Little, C.; McClements, L.; Li, J. Extracellular Vesicles from Mesenchymal Stromal Cells for the Treatment of Inflammation-Related Conditions. Int. J. Mol. Sci. 2021, 22, 3023. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Van Balkom, B.W.M.; Bruno, S.; Choo, A.; Dominici, M.; Gimona, M.; Hill, A.F.; De Kleijn, D.; Koh, M.; Lai, R.C.; et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles 2019, 8, 1609206. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal Stem Cell-Mediated Immunosuppression Occurs via Concerted Action of Chemokines and Nitric Oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.; Oh, M.-K.; Kang, J.; Park, H.; Chae, D.-H.; Kim, J.; Lee, J.-H.; Yoo, H.; Choi, U.; Kim, D.-K.; et al. Extracellular Vesicles from Thapsigargin-Treated Mesenchymal Stem Cells Ameliorated Experimental Colitis via Enhanced Immunomodulatory Properties. Biomedicines 2021, 9, 209. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-W.; Wu, X. Mesenchymal Stem Cells Ameliorate Lps-Induced Acute Lung Injury through Kgf Promoting Alveolar Fluid Clearance of Alveolar Type Ii Cells. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2368–2378. [Google Scholar]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal Stromal Cells: Sensors and Switchers of Inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef]
- Choi, H.; Lee, R.H.; Bazhanov, N.; Oh, J.Y.; Prockop, D.J. Anti-Inflammatory Protein Tsg-6 Secreted by Activated Mscs Attenuates Zymosan-Induced Mouse Peritonitis by Decreasing Tlr2/Nf-Κb Signaling in Resident Macrophages. Blood 2011, 118, 330–338. [Google Scholar] [CrossRef]
- Krampera, M.; Le Blanc, K. Mesenchymal stromal cells: Putative microenvironmental modulators become cell therapy. Cell Stem Cell 2021, 28, 1708–1725. [Google Scholar] [CrossRef]
- Miceli, V.; Bulati, M.; Iannolo, G.; Zito, G.; Gallo, A.; Conaldi, P.G. Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 763. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Park, W.S.; Kim, Y.E.; Sung, D.K.; Sung, S.I.; Ahn, J.Y.; Chang, Y.S. Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell-derived extracellular vesicles against neonatal hyperoxic lung injury. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Virumbrales, M.; Menta, R.; Pérez, L.M.; Lucchesi, O.; Mancheño-Corvo, P.; Avivar-Valderas, Á.; Palacios, I.; Herrero-Mendez, A.; Dalemans, W.; de la Rosa, O.; et al. Human Adipose Mesenchymal Stem Cells Modulate Myeloid Cells toward an Anti-Inflammatory and Reparative Phenotype: Role of Il-6 and Pge2. Stem Cell Res. Ther. 2020, 11, 462. [Google Scholar] [CrossRef] [PubMed]
- Németh, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Mezey. Bone Marrow Stromal Cells Attenuate Sepsis Via Prostaglandin E(2)-Dependent Reprogramming of Host Macrophages to Increase Their Interleukin-10 Production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Huang, H.; Guo, Z.; Chang, Y.; Li, Z. Role of prostaglandin E2 in tissue repair and regeneration. Theranostics 2021, 11, 8836–8854. [Google Scholar] [CrossRef]
- Silva, J.D.; Lopes-Pacheco, M.; De Castro, L.L.; Kitoko, J.Z.; Trivelin, S.A.; Amorim, N.R.; Capelozzi, V.L.; Morales, M.M.; Gutfilen, B.; De Souza, S.A.L.; et al. Eicosapentaenoic acid potentiates the therapeutic effects of adipose tissue-derived mesenchymal stromal cells on lung and distal organ injury in experimental sepsis. Stem Cell Res. Ther. 2019, 10, 264. [Google Scholar] [CrossRef]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019, 2019, 7132708. [Google Scholar] [CrossRef]
- Rezakhani, L.; Kelishadrokhi, A.F.; Soleimanizadeh, A.; Rahmati, S. Mesenchymal stem cell (MSC)-derived exosomes as a cell-free therapy for patients Infected with COVID-19: Real opportunities and range of promises. Chem. Phys. Lipids 2020, 234, 105009. [Google Scholar] [CrossRef]
- Silva, J.D.; De Castro, L.L.; Braga, C.L.; Oliveira, G.P.; Trivelin, S.A.; Barbosa-Junior, C.M.; Morales, M.M.; Dos Santos, C.C.; Weiss, D.J.; Lopes-Pacheco, M.; et al. Mesenchymal Stromal Cells Are More Effective Than Their Extracellular Vesicles at Reducing Lung Injury Regardless of Acute Respiratory Distress Syndrome Etiology. Stem Cells Int. 2019, 2019, 8262849. [Google Scholar] [CrossRef]
- Mahrouf-Yorgov, M.; Augeul, L.; Da Silva, C.C.; Jourdan, M.; Rigolet, M.; Manin, S.; Ferrera, R.; Ovize, M.; Henry, A.; Guguin, A.; et al. Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ. 2017, 24, 1224–1238. [Google Scholar] [CrossRef]
- Willis, G.R.; Fernandez-Gonzalez, A.; Anastas, J.; Vitali, S.H.; Liu, X.; Ericsson, M.; Kwong, A.; Mitsialis, S.A.; Kourembanas, S. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am. J. Respir. Crit. Care Med. 2018, 197, 104–116. [Google Scholar] [CrossRef]
- Mansouri, N.; Willis, G.R.; Fernandez-Gonzalez, A.; Reis, M.; Nassiri, S.; Mitsialis, S.A.; Kourembanas, S. Mesenchymal Stromal Cell Exosomes Prevent and Revert Experimental Pulmonary Fibrosis through Modulation of Monocyte Phenotypes. JCI Insight 2019, 4, e128060. [Google Scholar] [CrossRef]
- Nakazaki, M.; Morita, T.; Lankford, K.L.; Askenase, P.W.; Kocsis, J.D. Small Extracellular Vesicles Released by Infused Mesenchymal Stromal Cells Target M2 Macrophages and Promote Tgf-Β Upregulation, Microvascular Stabilization and Functional Recovery in a Rodent Model of Severe Spinal Cord Injury. J. Extracell. Vesicles 2021, 10, e12137. [Google Scholar] [CrossRef]
- Philipp, D.; Suhr, L.; Wahlers, T.; Choi, Y.-H.; Paunel-Görgülü, A. Preconditioning of bone marrow-derived mesenchymal stem cells highly strengthens their potential to promote IL-6-dependent M2b polarization. Stem Cell Res. Ther. 2018, 9, 286. [Google Scholar] [CrossRef]
- Dong, N.; Zhou, P.-P.; Li, D.; Zhu, H.-S.; Liu, L.-H.; Ma, H.-X.; Shi, Q.; Ju, X.-L. Intratracheal administration of umbilical cord-derived mesenchymal stem cells attenuates hyperoxia-induced multi-organ injury via heme oxygenase-1 and JAK/STAT pathways. World J. Stem Cells 2022, 14, 556–576. [Google Scholar] [CrossRef]
- Liu, W.; Yu, M.; Xie, D.; Wang, L.; Ye, C.; Zhu, Q.; Liu, F.; Yang, L. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res. Ther. 2020, 11, 259. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Zhou, Y.; Obulkasim, H.; Zhang, Z.; Dai, B.; Zhu, W.; Shi, X. BM-MSCs protect against liver ischemia/reperfusion injury via HO-1 mediated autophagy. Mol. Med. Rep. 2018, 18, 2253–2262. [Google Scholar] [CrossRef]
- Eggenhofer, E.; Hoogduijn, M.J. Mesenchymal stem cell-educated macrophages. Transplant. Res. 2012, 1, 12. [Google Scholar] [CrossRef]
- Lee, J.W.; Gupta, N.; Serikov, V.; A Matthay, M. Potential application of mesenchymal stem cells in acute lung injury. Expert Opin. Biol. Ther. 2009, 9, 1259–1270. [Google Scholar] [CrossRef]
- Prockop, D.J. Concise Review: Two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells 2013, 31, 2042–2046. [Google Scholar] [CrossRef]
- Park, J.Y.; Pillinger, M.H.; Abramson, S. Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clin. Immunol. 2006, 119, 229–240. [Google Scholar] [CrossRef]
- Ylöstalo, J.H.; Bartosh, T.J.; Coble, K.; Prockop, D.J. Human Mesenchymal Stem/Stromal Cells Cultured as Spheroids Are Self-Activated to Produce Prostaglandin E2 That Directs Stimulated Macrophages into an Anti-Inflammatory Phenotype. Stem Cells 2012, 30, 2283–2296. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, M.; Liu, Y. Are Mesenchymal Stem Cells Major Sources of Safe Signals in Immune System? Cell Immunol. 2012, 272, 112–116. [Google Scholar] [CrossRef]
- Walker, N.M.; Badri, L.N.; Wadhwa, A.; Wettlaufer, S.; Peters-Golden, M.; Lama, V.N. Prostaglandin E2 As an Inhibitory Modulator of Fibrogenesis in Human Lung Allografts. Am. J. Respir. Crit. Care Med. 2012, 185, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, R.; Rajan, D.; Qayed, M.; Arafat, D.; Garcia, M.; Liu, Y.; Kugathasan, S.; Anderson, L.J.; Gibson, G.; Galipeau, J. Potency Analysis of Mesenchymal Stromal Cells Using a Combinatorial Assay Matrix Approach. Cell Rep. 2018, 22, 2504–2517. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Frezzetti, D.; Roma, C.; Chicchinelli, N.; Barbieri, A.; Arra, C.; Scognamiglio, G.; Botti, G.; De Luca, A.; Normanno, N. RANTES and IL-6 cooperate in inducing a more aggressive phenotype in breast cancer cells. Oncotarget 2018, 9, 17543–17553. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Gimona, M.; Brizzi, M.F.; Choo, A.B.H.; Dominici, M.; Davidson, S.M.; Grillari, J.; Hermann, D.M.; Hill, A.F.; de Kleijn, D.; Lai, R.C.; et al. Critical considerations for the development of potency tests for therapeutic applications of mesenchymal stromal cell-derived small extracellular vesicles. Cytotherapy 2021, 23, 373–380. [Google Scholar] [CrossRef]
- Andjus, P.; Kosanović, M.; Milićević, K.; Gautam, M.; Vainio, S.J.; Jagečić, D.; Kozlova, E.N.; Pivoriūnas, A.; Chachques, J.C.; Sakaj, M.; et al. Extracellular Vesicles as Innovative Tool for Diagnosis, Regeneration and Protection against Neurological Damage. Int. J. Mol. Sci. 2020, 21, 6859. [Google Scholar] [CrossRef]
- Tieu, A.; Hu, K.; Gnyra, C.; Montroy, J.; Fergusson, D.A.; Allan, D.S.; Stewart, D.J.; Thébaud, B.; Lalu, M.M. Mesenchymal stromal cell extracellular vesicles as therapy for acute and chronic respiratory diseases: A meta-analysis. J. Extracell. Vesicles 2021, 10, e12141. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Mscs Exosomes. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=MSC+exosomes&cntry=&state=&city=&dist= (accessed on 5 January 2023).
- U.S. National Library of Medicine. Mscs Extracellular vesicles. Available online: https://clinicaltrials.gov/ct2/results?cond=MSC+extracellular+vesicles&term=&cntry=&state=&city=&dist= (accessed on 5 January 2023).
- Zhao, J.; Li, X.; Hu, J.; Chen, F.; Qiao, S.; Sun, X.; Gao, L.; Xie, J.; Xu, B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc. Res. 2019, 115, 1205–1216. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Feng, X.-M.; Abbott, J.; Fang, X.-H.; Hao, Q.; Monsel, A.; Qu, J.-M.; Matthay, M.A.; Lee, J.W. Human Mesenchymal Stem Cell Microvesicles for Treatment of Escherichia coli Endotoxin-Induced Acute Lung Injury in Mice. Stem Cells 2014, 32, 116–125. [Google Scholar] [CrossRef]
- Hyvärinen, K.; Holopainen, M.; Skirdenko, V.; Ruhanen, H.; Lehenkari, P.; Korhonen, M.; Käkelä, R.; Laitinen, S.; Kerkelä, E. Mesenchymal Stromal Cells and Their Extracellular Vesicles Enhance the Anti-Inflammatory Phenotype of Regulatory Macrophages by Downregulating the Production of Interleukin (IL)-23 and IL-22. Front. Immunol. 2018, 9, 771. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, L.; Li, L.; Liu, F.; Liu, J.; Chen, Y.; Cheng, J.; Lu, Y. Mitochondrial Transfer from Mesenchymal Stem Cells to Macrophages Restricts Inflammation and Alleviates Kidney Injury in Diabetic Nephropathy Mice Via Pgc-1α Activation. Stem Cells 2021, 39, 913–928. [Google Scholar] [CrossRef]
- Su, Y.; Silva, J.D.; Doherty, D.; Simpson, D.A.; Weiss, D.J.; Rolandsson-Enes, S.; McAuley, D.F.; O’Kane, C.M.; Brazil, D.P.; Krasnodembskaya, A.D. Mesenchymal Stromal Cells-Derived Extracellular Vesicles Reprogramme Macrophages in Ards Models through the Mir-181a-5p-Pten-Pstat5-Socs1 Axis. Thorax 2022, 1–14. [Google Scholar] [CrossRef]
- Shi, M.; Zhu, Y.; Yan, J.; Rouby, J.; Summah, H.; Monsel, A.; Qu, J. Role of miR-466 in mesenchymal stromal cell derived extracellular vesicles treating inoculation pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. Clin. Transl. Med. 2021, 11, e287. [Google Scholar] [CrossRef]
- Ceccariglia, S.; Cargnoni, A.; Silini, A.R.; Parolini, O. Autophagy: A potential key contributor to the therapeutic action of mesenchymal stem cells. Autophagy 2019, 16, 28–37. [Google Scholar] [CrossRef]
- Li, S.; Wu, H.; Han, D.; Ma, S.; Fan, W.; Wang, Y.; Zhang, R.; Fan, M.; Huang, Y.; Fu, X.; et al. A Novel Mechanism of Mesenchymal Stromal Cell-Mediated Protection against Sepsis: Restricting Inflammasome Activation in Macrophages by Increasing Mitophagy and Decreasing Mitochondrial ROS. Oxidative Med. Cell. Longev. 2018, 2018, 3537609. [Google Scholar] [CrossRef]
- Moya, A.; Larochette, N.; Paquet, J.; Deschepper, M.; Bensidhoum, M.; Izzo, V.; Kroemer, G.; Petite, H.; Logeart-Avramoglou, D. Quiescence Preconditioned Human Multipotent Stromal Cells Adopt a Metabolic Profile Favorable for Enhanced Survival under Ischemia. Stem Cells 2016, 35, 181–196. [Google Scholar] [CrossRef]
- Ghanta, S.; Tsoyi, K.; Liu, X.; Nakahira, K.; Ith, B.; Coronata, A.A.; Fredenburgh, L.E.; Englert, J.A.; Piantadosi, C.A.; Choi, A.M.K.; et al. Mesenchymal Stromal Cells Deficient in Autophagy Proteins Are Susceptible to Oxidative Injury and Mitochondrial Dysfunction. Am. J. Respir. Cell Mol. Biol. 2017, 56, 300–309. [Google Scholar] [CrossRef]
- Wang, N.-F.; Bai, C.-X. Bone marrow-derived mesenchymal stem cells modulate autophagy in RAW264.7 macrophages via the phosphoinositide 3-kinase/protein kinase B/heme oxygenase-1 signaling pathway under oxygen-glucose deprivation/restoration conditions. Chin. Med. J. 2021, 134, 699–707. [Google Scholar] [CrossRef]
- Phinney, D.G.; Di Giuseppe, M.; Njah, J.; Sala, E.; Shiva, S.; St Croix, C.M.; Stolz, D.B.; Watkins, S.C.; Di, Y.P.; Leikauf, G.D.; et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun. 2015, 6, 8472. [Google Scholar] [CrossRef] [PubMed]
- Siegel, E.; Croze, R.; Fang, X.; Matthay, M.; Gotts, J. Inhibition of the Lipoxin A4 and Resolvin D1 Receptor Impairs Host Response to Acute Lung Injury Caused by Pneumococcal Pneumonia in Mice. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2021, 320, L1085–L1092. [Google Scholar] [CrossRef] [PubMed]
- Pasula, R.; Azad, A.K.; Gardner, J.C.; Schlesinger, L.S.; McCormack, F.X. Keratinocyte Growth Factor Administration Attenuates Murine Pulmonary Mycobacterium tuberculosis Infection through Granulocyte-Macrophage Colony-stimulating Factor (GM-CSF)-dependent Macrophage Activation and Phagolysosome Fusion. J. Biol. Chem. 2015, 290, 7151–7159. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Guo, X.; Li, R.; Zhou, J.; Yu, F.; Yan, X. p-Coumaric acid regulates macrophage polarization in myocardial ischemia/reperfusion by promoting the expression of indoleamine 2, 3-dioxygenase. Bioengineered 2021, 12, 10971–10981. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-P.; Wu, K.-H.; Chao, W.-R.; Lee, Y.-J.; Yang, S.-F.; Chao, Y.-H. Immunomodulation of Mesenchymal Stem Cells in Acute Lung Injury: From Preclinical Animal Models to Treatment of Severe COVID-19. Int. J. Mol. Sci. 2022, 23, 8196. [Google Scholar] [CrossRef] [PubMed]
- Casanova, J.L.; Abel, L. Revisiting Crohn’s Disease as a Primary Immunodeficiency of Macrophages. J. Exp. Med. 2009, 206, 1839–1843. [Google Scholar] [CrossRef]
- Litvack, M.L.; Wigle, T.J.; Lee, J.; Wang, J.; Ackerley, C.; Grunebaum, E.; Post, M. Alveolar-Like Stem Cell-Derived Myb(-) Macrophages Promote Recovery and Survival in Airway Disease. Am. J. Respir. Crit. Care Med. 2016, 193, 1219–1229. [Google Scholar] [CrossRef]
- Abreu, S.C.; Antunes, M.A.; de Castro, J.C.; de Oliveira, M.V.; Bandeira, E.; Ornellas, D.S.; Diaz, B.L.; Morales, M.M.; Xisto, D.G.; Rocco, P.R. Bone marrow-derived mononuclear cells vs. mesenchymal stromal cells in experimental allergic asthma. Respir. Physiol. Neurobiol. 2013, 187, 190–198. [Google Scholar] [CrossRef]
- Dai, R.; Yu, Y.; Yan, G.; Hou, X.; Ni, Y.; Shi, G. Intratracheal administration of adipose derived mesenchymal stem cells alleviates chronic asthma in a mouse model. BMC Pulm. Med. 2018, 18, 131. [Google Scholar] [CrossRef]
- Nemeth, K.; Keane-Myers, A.; Brown, J.M.; Metcalfe, D.D.; Gorham, J.D.; Bundoc, V.G.; Hodges, M.G.; Jelinek, I.; Madala, S.; Karpati, S.; et al. Bone Marrow Stromal Cells Use Tgf-Beta to Suppress Allergic Responses in a Mouse Model of Ragweed-Induced Asthma. Proc. Natl. Acad. Sci. USA 2010, 107, 5652–5657. [Google Scholar] [CrossRef]
- Ren, J.; Liu, Y.; Yao, Y.; Feng, L.; Zhao, X.; Li, Z.; Yang, L. Intranasal delivery of MSC-derived exosomes attenuates allergic asthma via expanding IL-10 producing lung interstitial macrophages in mice. Int. Immunopharmacol. 2020, 91, 107288. [Google Scholar] [CrossRef]
- Averyanov, A.; Koroleva, I.; Konoplyannikov, M.; Revkova, V.; Lesnyak, V.; Kalsin, V.; Danilevskaya, O.; Nikitin, A.; Sotnikova, A.; Kotova, S.; et al. First-in-human high-cumulative-dose stem cell therapy in idiopathic pulmonary fibrosis with rapid lung function decline. Stem Cells Transl. Med. 2019, 9, 6–16. [Google Scholar] [CrossRef]
- de Oliveira, H.G.; Cruz, F.F.; Antunes, M.A.; Macedo Neto, A.V.; Oliveira, G.A.; Svartman, F.M.; Borgonovo, T.; Rebelatto, C.L.K.; Weiss, D.J.; Brofman, P.R.S.; et al. Combined Bone Marrow-Derived Mesenchymal Stromal Cell Therapy and One-Way Endobronchial Valve Placement in Patients with Pulmonary Emphysema: A Phase I Clinical Trial. Stem Cells Transl. Med. 2017, 6, 962–969. [Google Scholar] [CrossRef]
- E Fishman, J.; Kim, G.-H.J.; Kyeong, N.-Y.; Goldin, J.G.; Glassberg, M.K. Intravenous Stem Cell Dose and Changes in Quantitative Lung Fibrosis and Dlco in the Aether Trial: A Pilot Study. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7568–7572. [Google Scholar]
- Behnke, J.; Kremer, S.; Shahzad, T.; Chao, C.-M.; Böttcher-Friebertshäuser, E.; Morty, R.E.; Bellusci, S.; Ehrhardt, H. MSC Based Therapies—New Perspectives for the Injured Lung. J. Clin. Med. 2020, 9, 682. [Google Scholar] [CrossRef]
- Ntolios, P.; Manoloudi, E.; Tzouvelekis, A.; Bouros, E.; Steiropoulos, P.; Anevlavis, S.; Bouros, D.; Froudarakis, M.E. Longitudinal outcomes of patients enrolled in a phase Ib clinical trial of the adipose-derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. Clin. Respir. J. 2018, 12, 2084–2089. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Msc & Lung. Available online: https://clinicaltrials.gov/ct2/results?cond=MSC+%26+lung&term=&cntry=&state=&city=&dist= (accessed on 5 January 2023).
- Wilson, J.G.; Liu, K.D.; Zhuo, H.; Caballero, L.; McMillan, M.; Fang, X.; Cosgrove, K.; Vojnik, R.; Calfee, C.S.; Lee, J.-W.; et al. Mesenchymal stem (stromal) cells for treatment of ARDS: A phase 1 clinical trial. Lancet Respir. Med. 2014, 3, 24–32. [Google Scholar] [CrossRef]
- Wick, K.D.; Leligdowicz, A.; Zhuo, H.; Ware, L.B.; Matthay, M.A. Mesenchymal Stromal Cells Reduce Evidence of Lung Injury in Patients with Ards. JCI Insight 2021, 6, e148983. [Google Scholar] [CrossRef]
- Khatri, M.; Richardson, L.A.; Meulia, T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res. Ther. 2018, 9, 17. [Google Scholar] [CrossRef]
- Masterson, C.H.; Ceccato, A.; Artigas, A.; dos Santos, C.; Rocco, P.R.; Enes, S.R.; Weiss, D.J.; McAuley, D.; Matthay, M.A.; English, K.; et al. Mesenchymal stem/stromal cell-based therapies for severe viral pneumonia: Therapeutic potential and challenges. Intensiv. Care Med. Exp. 2021, 9, 61. [Google Scholar] [CrossRef]
- Yang, J.; Petitjean, S.J.; Koehler, M.; Zhang, Q.; Dumitru, A.C.; Chen, W.; Derclaye, S.; Vincent, S.P.; Soumillion, P.; Alsteens, D. Molecular Interaction and Inhibition of Sars-Cov-2 Binding to the Ace2 Receptor. Nat. Commun. 2020, 11, 4541. [Google Scholar] [CrossRef] [PubMed]
- Avanzini, M.A.; Mura, M.; Percivalle, E.; Bastaroli, F.; Croce, S.; Valsecchi, C.; Lenta, E.; Nykjaer, G.; Cassaniti, I.; Bagnarino, J.; et al. Human Mesenchymal Stromal Cells Do Not Express Ace2 and Tmprss2 and Are Not Permissive to Sars-Cov-2 Infection. Stem Cells Transl. Med. 2021, 10, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, R.; Spohn, G.; Bechtel, M.; Bojkova, D.; Baer, P.C.; Kuçi, S.; Seifried, E.; Ciesek, S.; Cinatl, J. Human Mesenchymal Stromal Cells Are Resistant to Sars-Cov-2 Infection under Steady-State, Inflammatory Conditions and in the Presence of Sars-Cov-2-Infected Cells. Stem Cell Rep. 2021, 16, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, J.K.; Saini, D.; Chaudhary, P.K.; Maurya, A.; Verma, G.K.; Gupta, A.K.; Roshan, R.; Vats, T.K.; Garg, N.; Yadav, D.; et al. Exploring the Immunomodulatory Aspect of Mesenchymal Stem Cells for Treatment of Severe Coronavirus Disease 19. Cells 2022, 11, 2175. [Google Scholar] [CrossRef]
- Klimczak, A. Perspectives on mesenchymal stem/progenitor cells and their derivates as potential therapies for lung damage caused by COVID-19. World J. Stem Cells 2020, 12, 1013–1022. [Google Scholar] [CrossRef]
- Chen, L.; Qu, J.; Kalyani, F.S.; Zhang, Q.; Fan, L.; Fang, Y.; Li, Y.; Xiang, C. Mesenchymal stem cell-based treatments for COVID-19: Status and future perspectives for clinical applications. Cell. Mol. Life Sci. 2022, 79, 142. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Msc and COVID-19. Clinical Trials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=MSC+and+COVID-19&term=&cntry=&state=&city=&dist= (accessed on 6 January 2023).
- Shi, L.; Huang, H.; Lu, X.; Yan, X.; Jiang, X.; Xu, R.; Wang, S.; Zhang, C.; Yuan, X.; Xu, Z.; et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: A randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct. Target. Ther. 2021, 6, 58. [Google Scholar] [CrossRef]
- Gorman, E.; Shankar-Hari, M.; Hopkins, P.; Tunnicliffe, W.S.; Perkins, G.D.; Silversides, J.; McGuigan, P.; Krasnodembskaya, A.; Jackson, C.; Boyle, R.; et al. Repair of acute respiratory distress syndrome by stromal cell administration (REALIST) trial: A phase 1 trial. Eclinicalmedicine 2021, 41, 101167. [Google Scholar] [CrossRef]
- Monsel, A.; Hauw-Berlemont, C.; Mebarki, M.; Heming, N.; Mayaux, J.; Tchoumba, O.N.; Diehl, J.-L.; Demoule, A.; Annane, D.; Marois, C.; et al. Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: A multicenter randomized double-blind trial. Crit. Care 2022, 26, 48. [Google Scholar] [CrossRef]
- Dilogo, I.H.; Aditianingsih, D.; Sugiarto, A.; Burhan, E.; Damayanti, T.; Sitompul, P.A.; Mariana, N.; Antarianto, R.D.; Liem, I.K.; Kispa, T.; et al. Umbilical Cord Mesenchymal Stromal Cells as Critical COVID-19 Adjuvant Therapy: A Randomized Controlled Trial. Stem Cells Transl. Med. 2021, 10, 1279–1287. [Google Scholar] [CrossRef]
- Lanzoni, G.; Linetsky, E.; Correa, D.; Cayetano, S.M.; Alvarez, R.A.; Kouroupis, D.; Gil, A.A.; Poggioli, R.; Ruiz, P.; Marttos, A.C.; et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 2021, 10, 660–673. [Google Scholar] [CrossRef]
- Dauletova, M.; Hafsan, H.; Mahhengam, N.; Zekiy, A.O.; Ahmadi, M.; Siahmansouri, H. Mesenchymal stem cell alongside exosomes as a novel cell-based therapy for COVID-19: A review study. Clin. Immunol. 2021, 226, 108712. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, W.; Chen, L.; Xu, Z.; Zhang, Q.; Zhu, M.; Ye, P.; Li, H.; Yu, L.; Zhou, X.; et al. Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: An exploratory clinical trial. Clin. Transl. Med. 2021, 11, e297. [Google Scholar] [CrossRef]
- Shu, L.; Niu, C.; Li, R.; Huang, T.; Wang, Y.; Huang, M.; Ji, N.; Zheng, Y.; Chen, X.; Shi, L.; et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 361. [Google Scholar] [CrossRef]
- Zheng, G.; Huang, L.; Tong, H.; Shu, Q.; Hu, Y.; Ge, M.; Deng, K.; Zhang, L.; Zou, B.; Cheng, B.; et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: A randomized, placebo-controlled pilot study. Respir. Res. 2014, 15, 39. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, K.; Xie, L. Advances in mesenchymal stromal cell therapy for acute lung injury/acute respiratory distress syndrome. Front. Cell Dev. Biol. 2022, 10, 951764. [Google Scholar] [CrossRef]
- Murugan, D.; Rangasamy, L. Pooled evidence from preclinical and clinical studies for stem cell-based therapy in ARDS and COVID-19. Mol. Cell. Biochem. 2022, 1–32. [Google Scholar] [CrossRef]

| M1-like Mφs | M2-like Mφs | MSCs | MSC-Mφ Interaction | |
|---|---|---|---|---|
| MSCs * | Mφ | |||
| IL-1α, Il-1β [92,93] IL-6, 12, 23 [69,92,94] TNF-α [92,93,94] CCL-2, 8, 10, 15, 19, 20 [66,90,92] CXCL-9, 10, 11, 16, 17 [95,96] ROS (by Nox-2), NO (by iNOS), [92,94,97,98] | IL-4,10, 13 [77,92,93] CCL-1, 17, 18, 22, 24 [92,95,99] CXCL-13 [100] CXCL-12 [101] TGF-β [94,95,102] SDF-1 and VEGF [103,104] Arg-1 [23,92] HO-1 [98] | CCL-1-2, 4-5 [66] CXCL-8, 10, 12 [66,105] KGF [47,105] INDO [106], TSG-6 [107] Ang-1, VEGF, HGF, IGF-1 [105,108,109,110] EVs, Mt, MiRs [53,111,112,113] | CCL-2, 5, 7 [66,114] CXCL-8-12 [66] KGF [52,105,115,116] NO, TGFβ [34,117] INDO, TSG-6 [107,118,119] Ang-1, VEGF, HGF, IGF-1 [108,109,110,120,121] COX-2/PGE2, LXA4 [34,97,122,123,124] Rv-D1, Rv-E1, E2, Protectins [125] EVs, Mt, MiRs [51,53,91,113,126,127,128,129,130,131,132] | ↑ M2 Mφ and their ILs (by STAT 3,6, PPARγδ [30,56,95] ↓ M1 Mφ and their ILs [109,133] HO-1, antioxidants [34,134] IL-10 [32,123] TGF-β [32,95,132] Arg-1 [92,135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerkic, M.; Szaszi, K.; Laffey, J.G.; Rotstein, O.; Zhang, H. Key Role of Mesenchymal Stromal Cell Interaction with Macrophages in Promoting Repair of Lung Injury. Int. J. Mol. Sci. 2023, 24, 3376. https://doi.org/10.3390/ijms24043376
Jerkic M, Szaszi K, Laffey JG, Rotstein O, Zhang H. Key Role of Mesenchymal Stromal Cell Interaction with Macrophages in Promoting Repair of Lung Injury. International Journal of Molecular Sciences. 2023; 24(4):3376. https://doi.org/10.3390/ijms24043376
Chicago/Turabian StyleJerkic, Mirjana, Katalin Szaszi, John G. Laffey, Ori Rotstein, and Haibo Zhang. 2023. "Key Role of Mesenchymal Stromal Cell Interaction with Macrophages in Promoting Repair of Lung Injury" International Journal of Molecular Sciences 24, no. 4: 3376. https://doi.org/10.3390/ijms24043376
APA StyleJerkic, M., Szaszi, K., Laffey, J. G., Rotstein, O., & Zhang, H. (2023). Key Role of Mesenchymal Stromal Cell Interaction with Macrophages in Promoting Repair of Lung Injury. International Journal of Molecular Sciences, 24(4), 3376. https://doi.org/10.3390/ijms24043376







