Change in the Nature of ZSM-5 Zeolite Depending on the Type of Metal Adsorbent—The Analysis of DOS and Orbitals for Iron Species
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Computational Details
3.2. Geometrical Models
4. Conclusions
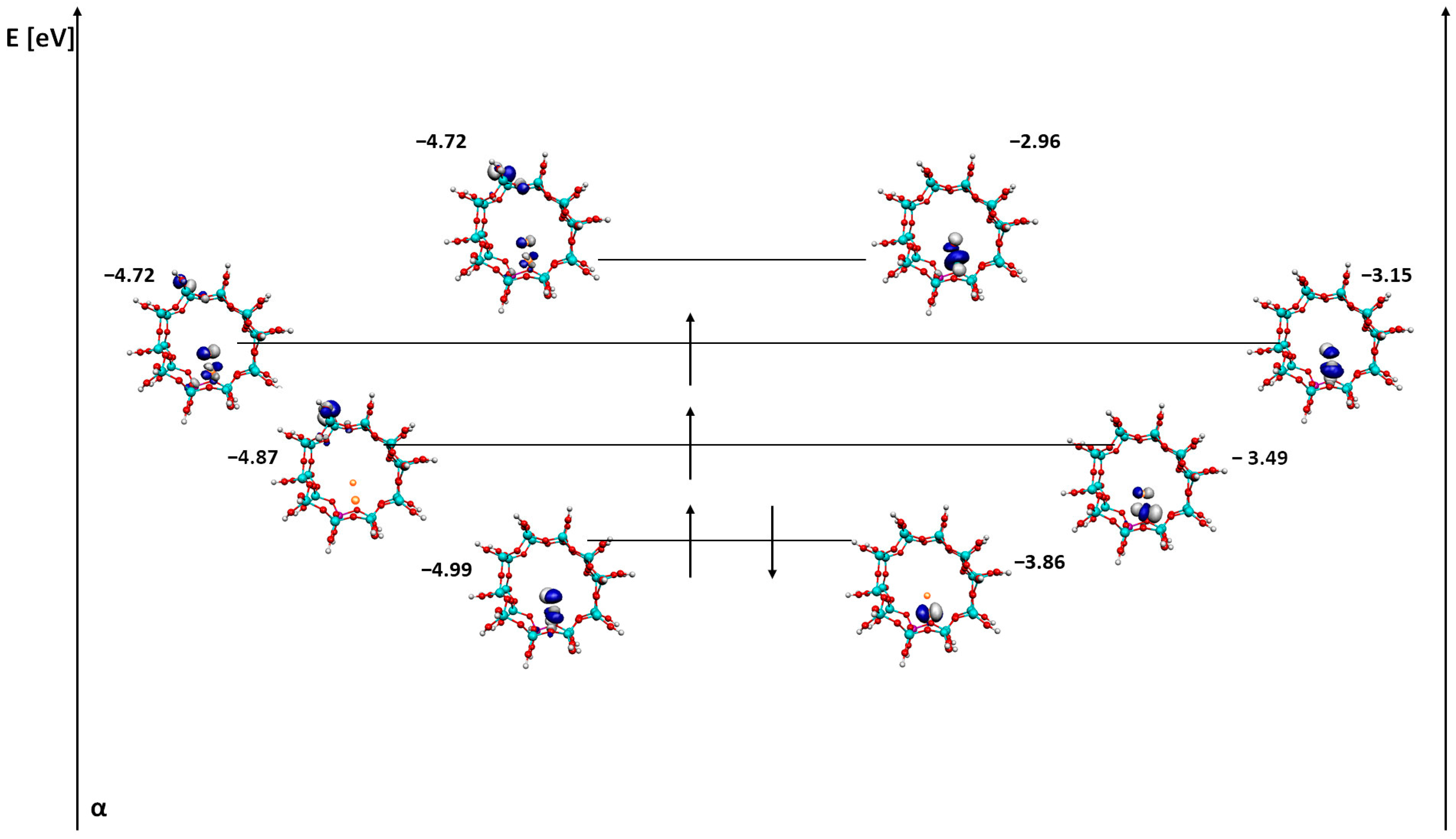
- The most stable structure is the structure with the hydroxyl group on the iron.
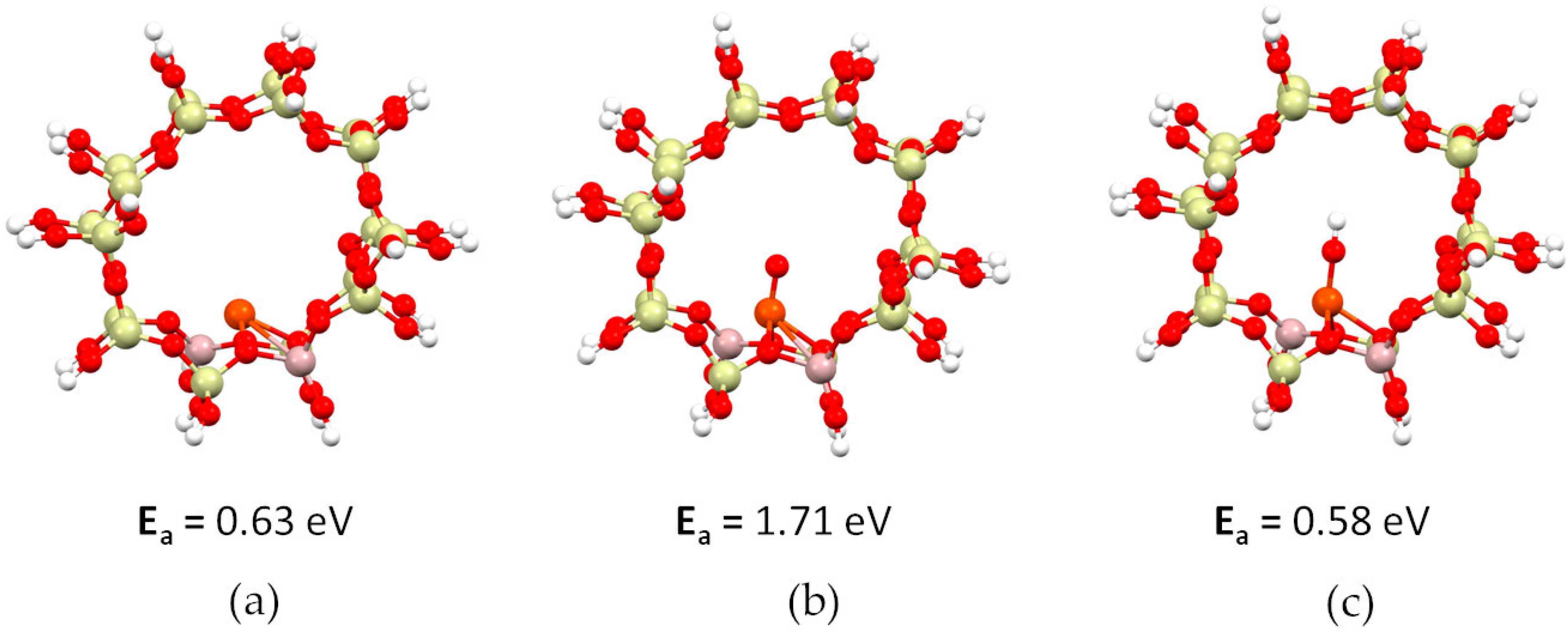
- The formation of iron monomers is highly endothermic and they increase in the following order: Fe-OH < Fe << FeO.
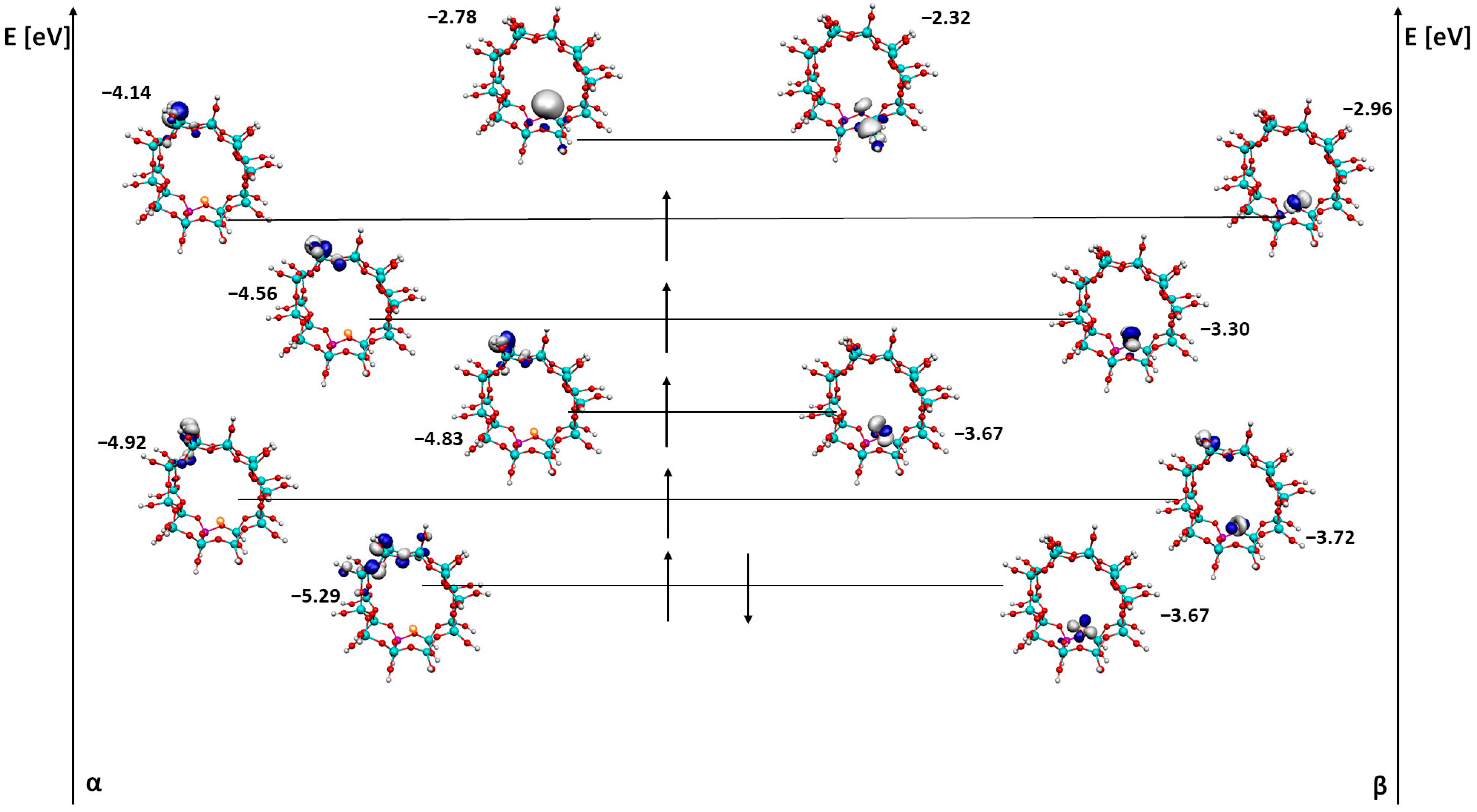
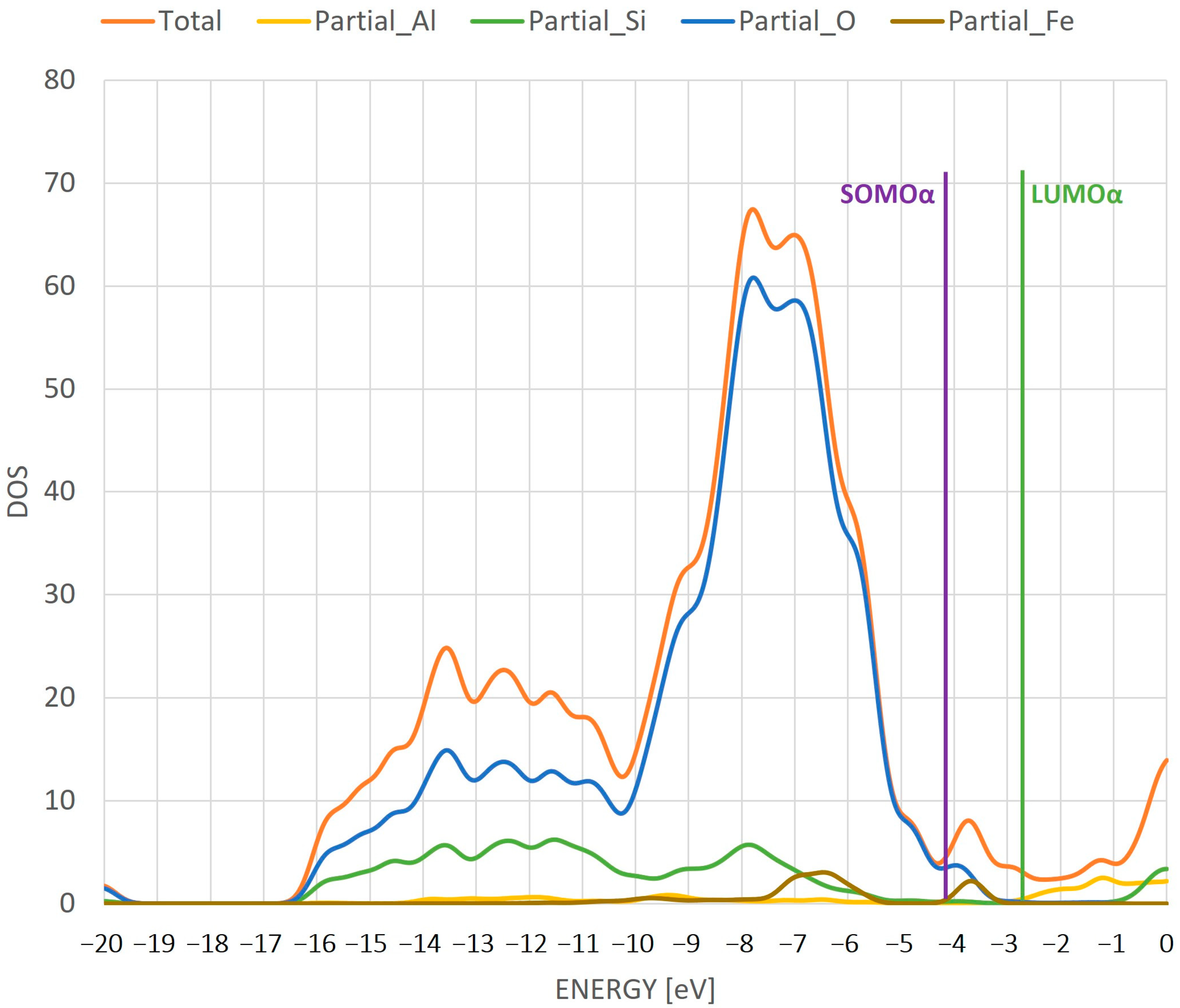
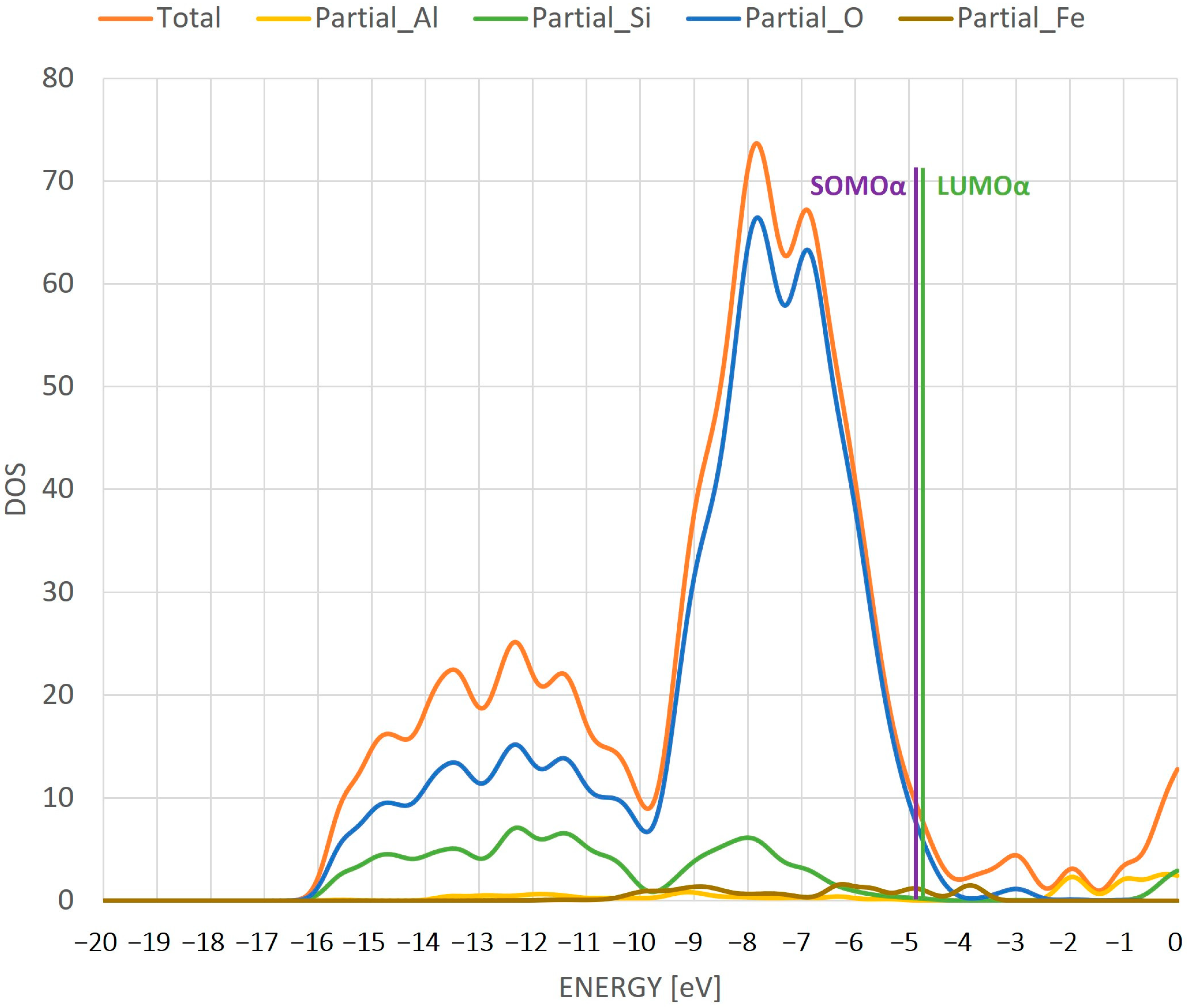
- The difference in the HOMO/SOMO and LUMO orbital energy gives us information about a conductive nature of the iron species inside the zeolite. This character changes from an insulator (Fe/ZSM-5) to a conductor (FeO/ZSM-5) to an insulator (FeOH/ZSM-5), which will have an important effect on the reactivity of the whole system during a catalytic reaction.
- From the molecular orbital visualization, it can be seen that the catalyst with iron absorbed inside the ZSM-5 pore is likely to be the most active—the LUMO orbital is located directly on the iron.
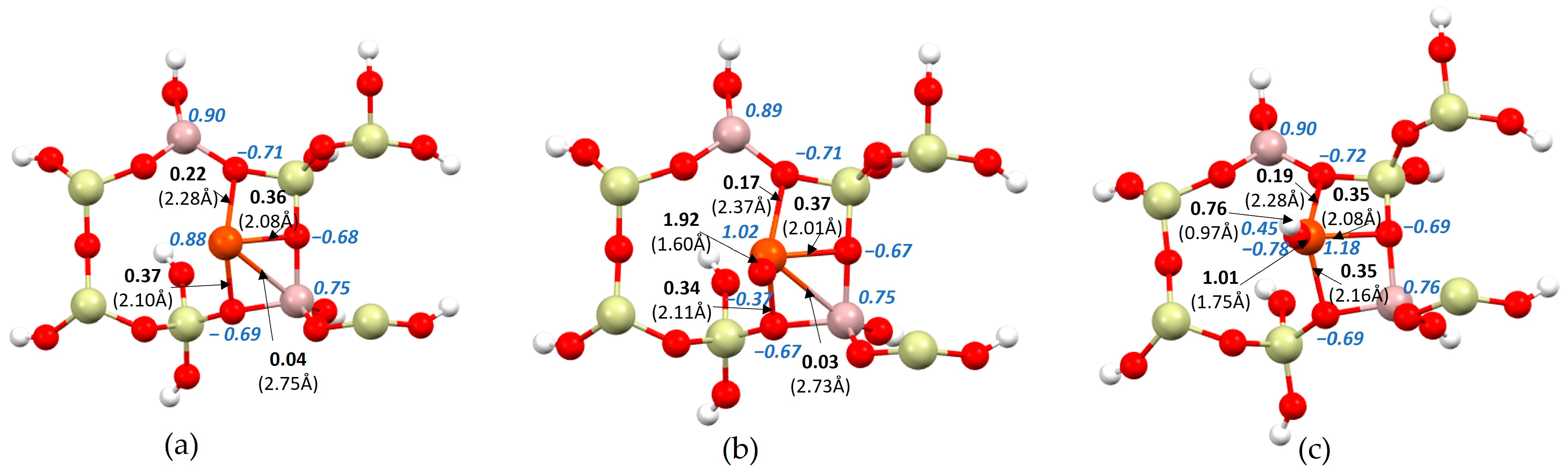
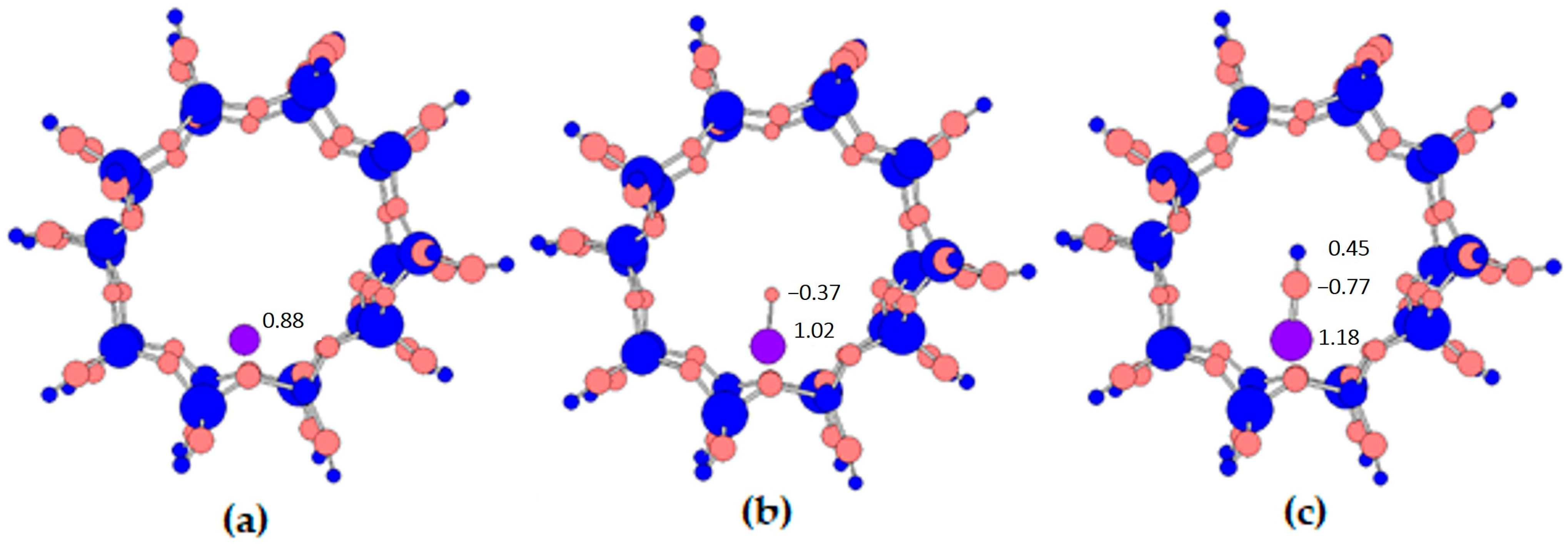
- The ionicity of iron increases after bonding with oxygen or an OH group. It starts at 0.88 for the iron atom, then amounts to 1.02 for iron in the oxide and to 1.18 for iron with the OH group.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, P.; Fan, J.; Cheng, X.; Qian, Z.; Wang, Z.; Wang, L.; Jen, T.C. Decoupled NOx adsorption and reduction by CO over catalyst Fe/ZSM-5: A DFT study. Chem. Phys. Lett. 2021, 766, 138344. [Google Scholar] [CrossRef]
- Liang, W.Z.; Bell, A.T.; Head-Gordon, M.; Chakraborty, A. Density Functional Theory Investigations of the Direct Oxidation of Methane on an Fe-Exchanged Zeolite. J. Phys. Chem. B 2004, 108, 4362–4368. [Google Scholar] [CrossRef]
- Chatterjee, A.; Iwasaki, T.; Ebina, T.; Miyamoto, A. Density functional study for estimating Brsnsted acid site strength in isomorphously substituted ZSM-5. Microporous Mesoporous Mater. 1998, 21, 421–428. [Google Scholar] [CrossRef]
- Vetrivel, R.; Pal, S.; Krishnan, S. Properties of iron-containing ZSM-5 zeolite: A theoretical study based on quantum chemical calculations. J. Mol. Catal. 1991, 66, 385–397. [Google Scholar] [CrossRef]
- Fellah, M.F.; Onal, I. DFT Study of Direct Methanol Oxidation to Formaldehyde by N2O on the [Fe]2+−ZSM-5 Zeolite Cluster. J. Phys. Chem. C 2012, 116, 13616–13622. [Google Scholar] [CrossRef]
- Fellah, M.F.; van Santen, R.A.; Onal, I. Oxidation of Benzene to Phenol by N2O on an Fe2+-ZSM-5 Cluster: A Density Functional Theory Study. J. Phys. Chem. C 2009, 113, 15307–15313. [Google Scholar] [CrossRef]
- Sun, K.; Xia, H.; Feng, Z.; Santen, R.; Hensen, E.; Li, C. Active sites in Fe/ZSM-5 for nitrous oxide decomposition and benzene hydroxylation with nitrous oxide. J. Catal. 2008, 254, 383–396. [Google Scholar] [CrossRef]
- Fellah, M.F. Direct oxidation of methanol to formaldehyde by N2O on [Fe]1+ and [FeO]1+ sites in Fe–ZSM-5 zeolite: A density functional theory study. J. Catal. 2011, 282, 191–200. [Google Scholar] [CrossRef]
- Pirngruber, G.D.; Roy, P.K.; Prins, R. The role of autoreduction and of oxygen mobility in N2O decomposition over Fe-ZSM-5. J. Catal. 2007, 246, 147–157. [Google Scholar] [CrossRef]
- Hansen, N.; Heyden, A.; Bell, A.T.; Keil, F.J. Microkinetic modeling of nitrous oxide decomposition on dinuclear oxygen bridged iron sites in Fe-ZSM-5. J. Catal. 2007, 248, 213–225. [Google Scholar] [CrossRef]
- Zecchina, A.; Rivallan, M.; Berlier, G.; Lambertia, C.; Ricchiardia, G. Structure and nuclearity of active sites in Fe-zeolites: Comparison with iron sites in enzymes and homogeneous catalysts. Phys. Chem. Chem. Phys. 2007, 9, 3483–3499. [Google Scholar] [CrossRef]
- Schwidder, M.; Kumar, M.S.; Brucknerb, A.; Grunert, W. Active sites for NO reduction over Fe-ZSM-5 catalysts. Chem. Commun. 2005, 805–807. [Google Scholar] [CrossRef]
- Louwersea, M.J.; Baerends, E.J. Oxidative properties of FeO2+: Electronic structure and solvation effects. Phys. Chem. Chem. Phys. 2007, 9, 156–166. [Google Scholar] [CrossRef]
- Rizwana, B.F.; Prasana, J.C.; Muthu, S.; Abraham, C.S. Spectroscopic (FT-IR, FT-Raman, NMR) investigation on 2-[(2-amino-6-oxo-6,9-dihydro-3H-purin-9-yl)methoxy]ethyl(2S)-2-amino-3-methylbutanoate by Density Functional Theory. Mater. Today Proc. 2019, 18, 1770–1782. [Google Scholar] [CrossRef]
- Hoffmann, R. Solids and Surfaces: A Chemist’s View of Bonding in Extended Structures; VCH Publishers: New York, NY, USA, 1988. [Google Scholar]
- Fukui, K. Role of Frontier Orbitals in Chemical Reactions. Science 1982, 218, 747–754. [Google Scholar] [CrossRef]
- Fleming, I. Frontier Orbitals and Organic Chemical Reactions; John Wiley and Sons: New York, NY, USA, 1976. [Google Scholar] [CrossRef]
- Denardin, F.G.; Muniz, A.R.; Perez-Lopez, O.W. Experimental and DFT analysis of the acid and reduction properties of Fe-Cu/ZSM-5. Microporous Mesoporous Mater. 2021, 314, 110860. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Rossmeisl, J.; Christensen, C.H. Towards the computational design of solid catalysts. Nat. Chem. 2009, 1, 37–46. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Hammer, B.; Hansen, L.B.; Nørskov, J.K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 1999, 59, 7413. [Google Scholar] [CrossRef]
- Hammer, B.; Nørskov, J.K. Theoretical surface science and catalysis—Calculations and concepts. Adv. Catal. 2000, 45, 71–129. [Google Scholar] [CrossRef]
- Schlexer, P. Computational Modeling in Heterogeneous Catalysis. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Sabatier, P. La Catalyse en Chimie Organique; Librairie Polytechnique: Paris, France, 1920. [Google Scholar] [CrossRef]
- Li, G.; Pidko, E.A. The Nature and Catalytic Function of Cation Sites in Zeolites: A Computational Perspective. ChemCatChem. 2019, 11, 134–156. [Google Scholar] [CrossRef]
- Olsbye, U.; Svelle, S.; Bjorgen, M.; Beato, P.; Janssens, T.W.V.; Joensen, F.; Bordiga, S.; Lillerud, K.P. Conversion of Methanol to Hydrocarbons: How Zeolite Cavity and Pore Size Controls Product Selectivity. Angew. Chem. Int. Ed. 2012, 51, 5810–5831. [Google Scholar] [CrossRef]
- Tomkins, P.; Ranocchiari, M.; van Bokhoven, J.A. Direct Conversion of Methane to Methanol under Mild Conditions over Cu-Zeolites and beyond. Acc. Chem. Res. 2017, 50, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Riisager, A.; Saravanamurugan, S.; Pandey, A.; Sangwan, R.S.; Yang, S.; Luque, R. Carbon-Increasing Catalytic Strategies for Upgrading Biomass into Energy-Intensive Fuels and Chemicals. ACS Catal. 2018, 8, 148–187. [Google Scholar] [CrossRef]
- Szecsenyi, A.; Li, G.; Gascon, J.; Pidko, E.A. Mechanistic Complexity of Methane Oxidation with H2O2 by SingleSite Fe/ZSM-5 Catalyst. ACS Catal. 2018, 8, 7961–7972. [Google Scholar] [CrossRef]
- Meher, S.K.; Rao, G.R. Tuning, via counter anions, the morphology and catalytic activity of CeO2 prepared under mild conditions. J. Colloid Interface Sci. 2012, 373, 46–56. [Google Scholar] [CrossRef]
- Lv, P.; Lu, Z.; Li, S.; Ma, D.; Zhang, W.; Zhang, Y.; Yang, Z. Tuning metal cluster catalytic activity with morphology and composition: A DFT study of O2 dissociation at the global minimum of PtmPdn (m + n = 5) clusters. RSC Adv. 2016, 6, 104388–104397. [Google Scholar] [CrossRef]
- Li, G.; Vassilev, P.; Sanchez-Sanchez, M.; Lercher, J.; Hensen, E.J.M.; Pidko, E.A. Stability and Reactivity of Copper Oxo-Clusters in ZSM-5 Zeolite for Selective Methane Oxidation to Methanol. J. Catal. 2016, 338, 305–312. [Google Scholar] [CrossRef]
- Hermann, K.; Pettersson, L.G.M.; Casida, M.E.; Daul, C.; Goursot, A.; Koester, A.; Proynov, E.; St-Amant, A.; Salahub, D.R.; Carravetta, V.; et al. StoBe-deMon; deMon Software: Stockholm, Sweden; Berlin, Germany, 2005. [Google Scholar]
- Broclawik, E.; Salahub, F.R. Density functional theory and quantum chemistry: Metals and metal oxides. J. Mol. Catal. 1993, 82, 117. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO–MO Molecular Wave Functions. II. Overlap Populations, Bond Orders, and Covalent Bond Energies. J. Chem. Phys. 1955, 23, 1833. [Google Scholar] [CrossRef]
- Mayer, I. Charge, bond order and valence in the AB initio SCF theory. Chem. Phys. Lett. 1983, 97, 270. [Google Scholar] [CrossRef]
- Mayer, I. Bond orders and valences: Role of d-orbitals for hypervalent Sulphur. J. Mol. Struct. THEOCHEM 1987, 149, 81–89. [Google Scholar] [CrossRef]
- Portmann, S.; Lüthi, H.P. MOLEKEL: An Interactive Molecular Graphic Tool. Chimia 2000, 54, 766–769. [Google Scholar] [CrossRef]
- Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 17 January 2023).
- Czekaj, I.; Brandenberger, S.; Kröcher, O. Theoretical studies of HNCO adsorption at stabilized iron complexes in the ZSM-5 framework. Microporous Mesoporous Mater. 2013, 169, 97–102. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurzydym, I.; Garbujo, A.; Biasi, P.; Czekaj, I. Change in the Nature of ZSM-5 Zeolite Depending on the Type of Metal Adsorbent—The Analysis of DOS and Orbitals for Iron Species. Int. J. Mol. Sci. 2023, 24, 3374. https://doi.org/10.3390/ijms24043374
Kurzydym I, Garbujo A, Biasi P, Czekaj I. Change in the Nature of ZSM-5 Zeolite Depending on the Type of Metal Adsorbent—The Analysis of DOS and Orbitals for Iron Species. International Journal of Molecular Sciences. 2023; 24(4):3374. https://doi.org/10.3390/ijms24043374
Chicago/Turabian StyleKurzydym, Izabela, Alberto Garbujo, Pierdomenico Biasi, and Izabela Czekaj. 2023. "Change in the Nature of ZSM-5 Zeolite Depending on the Type of Metal Adsorbent—The Analysis of DOS and Orbitals for Iron Species" International Journal of Molecular Sciences 24, no. 4: 3374. https://doi.org/10.3390/ijms24043374
APA StyleKurzydym, I., Garbujo, A., Biasi, P., & Czekaj, I. (2023). Change in the Nature of ZSM-5 Zeolite Depending on the Type of Metal Adsorbent—The Analysis of DOS and Orbitals for Iron Species. International Journal of Molecular Sciences, 24(4), 3374. https://doi.org/10.3390/ijms24043374









