Abstract
Vascular remodeling is a common pathological hallmark of many cardiovascular diseases. Vascular smooth muscle cells (VSMCs) are the predominant cell type lining the tunica media and play a crucial role in maintaining aortic morphology, integrity, contraction and elasticity. Their abnormal proliferation, migration, apoptosis and other activities are tightly associated with a spectrum of structural and functional alterations in blood vessels. Emerging evidence suggests that mitochondria, the energy center of VSMCs, participate in vascular remodeling through multiple mechanisms. For example, peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α)-mediated mitochondrial biogenesis prevents VSMCs from proliferation and senescence. The imbalance between mitochondrial fusion and fission controls the abnormal proliferation, migration and phenotypic transformation of VSMCs. Guanosine triphosphate-hydrolyzing enzymes, including mitofusin 1 (MFN1), mitofusin 2 (MFN2), optic atrophy protein 1 (OPA1) and dynamin-related protein 1 (DRP1), are crucial for mitochondrial fusion and fission. In addition, abnormal mitophagy accelerates the senescence and apoptosis of VSMCs. PINK/Parkin and NIX/BINP3 pathways alleviate vascular remodeling by awakening mitophagy in VSMCs. Mitochondrial DNA (mtDNA) damage destroys the respiratory chain of VSMCs, resulting in excessive ROS production and decreased ATP levels, which are related to the proliferation, migration and apoptosis of VSMCs. Thus, maintaining mitochondrial homeostasis in VSMCs is a possible way to relieve pathologic vascular remodeling. This review aims to provide an overview of the role of mitochondria homeostasis in VSMCs during vascular remodeling and potential mitochondria-targeted therapies.
1. Introduction
Vascular remodeling refers to the structural and functional changes in the vasculature in response to injury, ageing and diseases [1]. This process is initially adaptive but is likely to develop to be pathogenic and unrecoverable under sustained disturbance. The vascular wall is typically formed by endothelial cells, smooth muscle cells and fibroblasts. During pathogenic remodeling, vascular cell proliferation, death, migration and phenotypic transformation are involved in the response to oxidative stress, inflammation, vascular calcification and other stimuli [2]. Pathogenic vascular remodeling contributes to numerous cardiovascular diseases. Here, we will focus on vascular remodeling in the aorta and its associated diseases, especially hypertension, atherosclerosis and arterial aneurysm [3,4].
Hypertension is an inward eutrophic remodeling disease involving the artery and is pathophysiologically characterized by increased vascular resistance, which is determined by a reduced vascular diameter due to increased vascular contraction and arterial remodeling [5]. Vascular smooth muscle cells (VSMCs) are one of the mediators of complex systems, including the renin–angiotensin–aldosterone system, the sympathetic nervous system, immune activation and oxidative stress, which regulate hypertension. The increase in intracellular calcium ion concentration leads to VSMC contraction, which promotes vascular contraction, triggering acute and rapid adaptation of blood vessel diameter [5]. This adaptive diameter change becomes maladaptive under chronic pathological conditions, contributing to vascular remodeling and stiffness [6]. Physiologically, VSMCs are of contractility phenotype and exhibit low levels of proliferation. In contrast, VSMCs can dedifferentiate or undergo a phenotypic transformation in the pathological status [5]. In hypertension, VSMC phenotypic switch contributes to vascular dysfunction and arterial remodeling. Adaptive vascular remodeling is responsible for vascular repair in the early stage of hypertension when the proliferation of VSMC is strictly regulated. However, with the aggravation of hypertension, VSMC proliferation is out of control and the dedifferentiated VSMC accumulates on the vascular wall, leading to media thickening, intima hyperplasia and vascular sclerosis [7], which indicates that pathological vascular remodeling develops. During the process, diverse signal mediators, such as AngII, endothelin 1 (ET-1) [8] and aldosterone [9], initiate vascular remodeling of VSMCs.
Atherosclerosis is a chronic disease characterized by lipid-laden plaques and damaged arterial walls [10]. Unlike hypertension, atherosclerosis is associated with a positive vascular remodeling in the early stage and the lumen is dilated to stabilize the blood flow. As the disease progresses, negative remodeling leads to lumen stenosis and plaque blockage with compensatory imbalance [11]. Furthermore, pathological vascular remodeling can cause postoperative lumen stenosis, graft lumen lesions and post-lesion restenosis [12]. VSMCs, located in the medial layer of the vascular wall, participate in atherosclerosis and invade the fibrous cap and plaque center [10]. With the stimulation of inflammation or lipids, the damaged vascular wall exposes the media layer together with VSMC migration to the intima layer. These VSMCs accumulate and abnormally proliferate in the intima [13]. Otherwise, the pathological environment stimulates VSMCs’ phenotypic switch from contraction to synthetic, during which some cells are transformed into dedifferentiated states [13], associating with lumen thickening and plaque stabilization.
Arterial aneurysm and arterial dissection are life-threatening diseases characterized by structural vascular wall changes [14]. The integrity of the aortic wall can be damaged by factors such as the degradation of the extracellular matrix and inflammation. Together with the impact of blood flow, the vascular wall is prone to tear, leading to blood vessel rupture or the formation of a false lumen between the intima and the media layer [15]. There are some typical chronic aortic dissection pathological features: (1) increased aortic diameter, the aorta dilated quickly at first, then slowly; (2) thrombus slowly forms in the false lumen; (3) the dissection flap thickened initially, then deteriorated, straightened and lost its activity; and (4) there was significant vascular remodeling at the beginning, and as the disease aggravates, the remodeling slowed down and translated into fibrosis [16]. During the process, elastin fragments and fibrosis rating in medium layer are crucial markers of dissection progression. Damage to VSMCs leads to increased collagen deposition and fibrosis, whereas loss of VSMCs’ nuclei leads to necrosis of the medium layer [16].
As a critical component of the intima-medial of the vessel wall, VSMCs are mainly responsible for the contraction and relaxation of the vessel to control blood pressure and blood flow [17]. In vascular remodeling, VSMCs are characterized by hypertrophy, proliferation, phenotype transformation, migration, senescence and excess extracellular matrix [18,19]. In atherosclerosis, VSMCs migrate from the media into the intima and proliferate with the induction of platelet-derived growth factors [11]. Intimal hyperplasia is a severe consequence of VSMC proliferation, leading to luminal stenosis and endangering patients undergoing revascularization [20]. The abnormal status of VSMCs is tightly related to vascular growth factors, vasoactive substances, hemodynamics, oxidative stress and inflammatory factors [21,22].
VSMCs undergo contraction to regulate blood vessel width and blood pressure. Most of the energy for muscle contraction is provided by mitochondria [23]. Mitochondria not only produce ATP but also provide various metabolic substrates through the respiratory chain. Thus, the quality of mitochondria is crucial to keep VSMCs healthy. The morphology and function of mitochondria in VSMCs were changed responding to vascular injury [24]. Mitochondrial homeostasis is maintained by mitochondrial biogenesis, fission and fusion, and mitophagy. The disruption of these processes and the mitochondrial genome may affect mitochondrial quality and functions and are considered to promote VSMC-mediated vascular remodeling [25,26]. For instance, in both human and rodent atherosclerosis, mitochondrial respiration in VSMCs is impaired, resulting in increased glycolytic activity and decreased OXPHOS. Restoring mitochondrial function in VSMCs can limit or even reverse cardiovascular diseases [27,28].
In this article, we will focus on how mitochondrial homeostasis in VSMC drives the development of vascular remodeling and the corresponding treatments for restoring mitochondrial function.
2. Mitochondrial Biogenesis and Vascular Remodeling
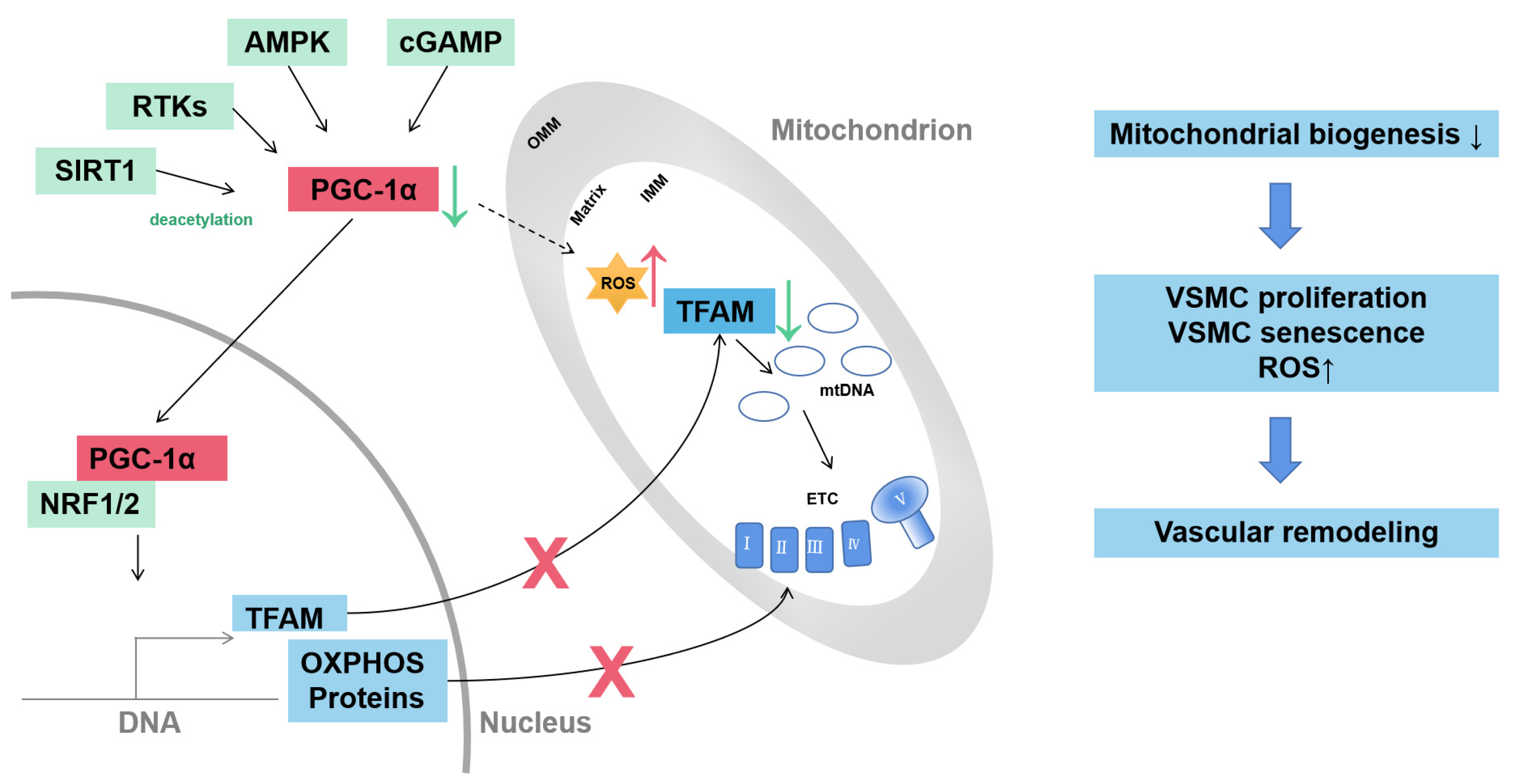
Mitochondrial biogenesis indicates the generation of new mitochondria and is regulated to adapt to cell energy demands. The process is highly regulated by proliferator-activated receptor-γ coactivator 1α (PGC-1α). Diverse pathways are involved in regulating the expression of PGC-1α including receptor tyrosine kinases (RTKs), cyclic guanosine monophosphate (cGMP), AMPK and sirtuin 1 (SIRT1). PGC-1α can regulate the expression of nDNA-encoded OXPHOS components and mitochondrial DNA (mtDNA) by mitochondrial transcription factor A (TFAM) (Figure 1) [29].

Figure 1.
Mitochondrial biogenesis of VSMC in vascular remodeling. Mitochondrial biogenesis is driven by transcriptional coactivator PGC-1α. The expression and activity of PGC-1α are regulated through diverse pathways, including RTKs, cGMP, AMPK and SIRT1. PGC-1α activates transcriptional factors NRF1 and NRF2 to induce transcription of TFAM and nDNA-encoded OXPHOS proteins. TFAM controls the transcription and replication of mtDNA. Decreased PGC-1α contributes to VSMC proliferation, senescence and ROS production and drives vascular remodeling. AMPK, adenosine monophosphate-activated kinase; cGMP, cyclic guanosine monophosphate; ETC, electron transport complex; IMM, inner mitochondrial membrane, mtDNA, mitochondrial DNA; NRF1/2, nuclear respiratory factor-1/2; OMM, outer mitochondrial membrane; OXPHOS, oxidative phosphorylation; RTKs, receptor tyrosine kinases; SIRT1, sirtuin 1; TFAM, mitochondrial transcription factor A; VSMC, vascular smooth muscle cell.
PGC-1α plays a key role in SMC oxidative stress, apoptosis, inflammation and cell proliferation, which are relevant to the progression of vascular disease. Excessive proliferation of SMC contributes to the incidence of atherosclerosis, restenosis and pulmonary hypertension [30]. Serine 570 phosphorylation of PGC-1α stimulated by angiotensin II (Ang II) leads to decreased catalase expression and thus increases ROS levels in VSMCs and subsequent vascular hypertrophy [31].
Mitochondrial dysfunction of VSMCs can drive the aortic aneurysm in Marfan syndrome (MFS). In the aortas of MFS patients, all mitochondrial complex subunit expressions are reduced as are the genes related to mitochondrial function and mitochondrial biogenesis [32]. In non-hereditable aortic aneurysms and dissections induced by atherosclerosis, there is decreased mitochondrial respiration and a decrease in mitochondrial proteins in vascular smooth muscle cells from murine and human aortic aneurysms [33]. Mitochondrial biogenesis is also impaired in synthetic SMCs of abdominal aortic aneurysm patients [34].
3. Mitochondrial Fusion and Fission of VSMC in Vascular Remodeling
Mitochondrial dynamics encompass the processes of fusion, fission and selective degradation [24]. Studies have shown that mitochondrial dynamics play a key role in VSMC migration. Suppressing mitochondrial fission induced by platelet-derived growth factor (PDGF) limits VSMC migration and pathological intimal hyperplasia by altering mitochondrial energetics and ROS levels [35]. In addition to energy supply, mitochondrial morphology also influences cell migration [35]. Mitochondrial fission and fusion control the size, number and shape of mitochondria [24]. Shortened mitochondria promote cell migration without stimulation of PDGF [35], whereas elongated mitochondria correlate with more efficient ATP production [24].
Mitochondrial dynamics also affect the proliferation of VSMCs [36]. In a model of pulmonary hypertension, excessive mitochondrial fission induces the proliferation of pulmonary artery smooth muscle cells (PASMCs) [37]. Mdivi-1, a selective dynamin-related protein 1 (DRP1) inhibitor, can block mitochondrial fission leading to cell cycle arrest in the G2/M phase and reduced PASMC proliferation [27]. Several studies have indicated that chronic hyperglycemia promotes reactive oxygen species (ROS) production and VSMC proliferation, which aggravates vascular remodeling [38,39]. Oxidative stress caused by hyperglycemia stimulates mitochondrial fragmentation by increasing mitochondrial fission and decreasing fusion; activation of DRP1 is involved in this process [40]. Abnormal migration and proliferation of VSMCs are a feature of intimal hyperplasia [41]. Intimal hyperplasia is a chronic structural lesion that develops after vessel wall injury that leads to luminal stenosis and occlusion [41].
VSMC senescence leads to vascular ageing, which is associated with various vascular or metabolism diseases, such as hypertension, atherosclerosis and diabetes [42]. Dysregulation of mitochondrial dynamics is associated with the senescence of smooth muscle cells by producing excessive ROS [43,44]. Abdominal aortic aneurysm (AAA) is induced by mitochondrial reactive oxygen species (mtROS), which can be elevated by mitochondrial fission [45]. Transcriptional factor Kruppel like factor 5 (KLF5) can rescue AAA through the following pathways: (1) binding to the promoter of eukaryotic translation initiation factor 5a (eIF5a) and activating eIF5a transcription, which protects mitochondrial integrity by interacting with mitofusin 1 (MFN1) [44]; and (2) inhibiting mitochondrial fission to reduce mitochondria-derived ROS and prevent VSMC senescence [8]. For mitochondria, chronic stimulation, including angiotensin II (Ang II) and oxidative stress and inflammation, can disrupt the mitochondrial dynamic balance and promote mitochondrial fission and fragmentation, which lead to the accumulation of mtROS and VSMC ageing [46].
The phenotypic transition of VSMC from a contractile to synthetic phenotype occurs in vascular remodeling, especially in atherosclerosis and coronary heart disease [47,48,49]. The mitochondrial structure can regulate the phenotype and metabolic characteristics of VSMCs [28]. It has been reported that PDGF is involved in the phenotypic transition of VSMC in a mitochondrial-dynamics-dependent manner [28]. In vascular calcification, AMPK activation promotes mitochondrial fusion by down-regulating DRP1 [50]. During this process, VSMC osteochondrogenic transformation is inhibited and vascular calcification is alleviated. In the pathological state, the phenotypic switch of VSMCs correlated with the fusion and fission of mitochondria [28].
GTP-hydrolyzing enzymes of the dynamin superfamily are the key mediators of mitochondrial fusion and fission. MFN1, mitofusin 2 (MFN2) and optic atrophy protein 1 (OPA1) are crucial for mitochondrial fusion, whereas the center of mitochondrial fission is DRP1, which uses GTP hydrolysis to perform mechanical work on lipid bilayers [50]. Angiotensin II (AngII), inflammation, oxidative stress and other stimuli induce vascular-remodeling-related diseases with increased fission and reduced fusion and result in more mitochondria in the form of short tubules or spheres [14,45]. In addition to affecting mitochondrial morphology, mitochondrial fusion/fission homeostasis is closely related to mitochondrial function, including maintaining the cellular redox state and energy supply [51]. Mitochondrial fragmentation and mitochondrial dysfunction lead to mtROS accumulation and ATP level decline. The pharmacological inhibitor of DRP1 (mdivi-1) can inhibit mitochondrial fission [16] and activate the AMPK pathway to play a protective role in mitochondrial integrity [50]. Elf5a restrains the fragment of mitochondria by interacting with MFN1 [44]. Thus, the mitochondrial dynamics of VSMCs plays a vital role in vascular remodeling by affecting the energy metabolism and phenotypic transition of VSMCs (Figure 2).

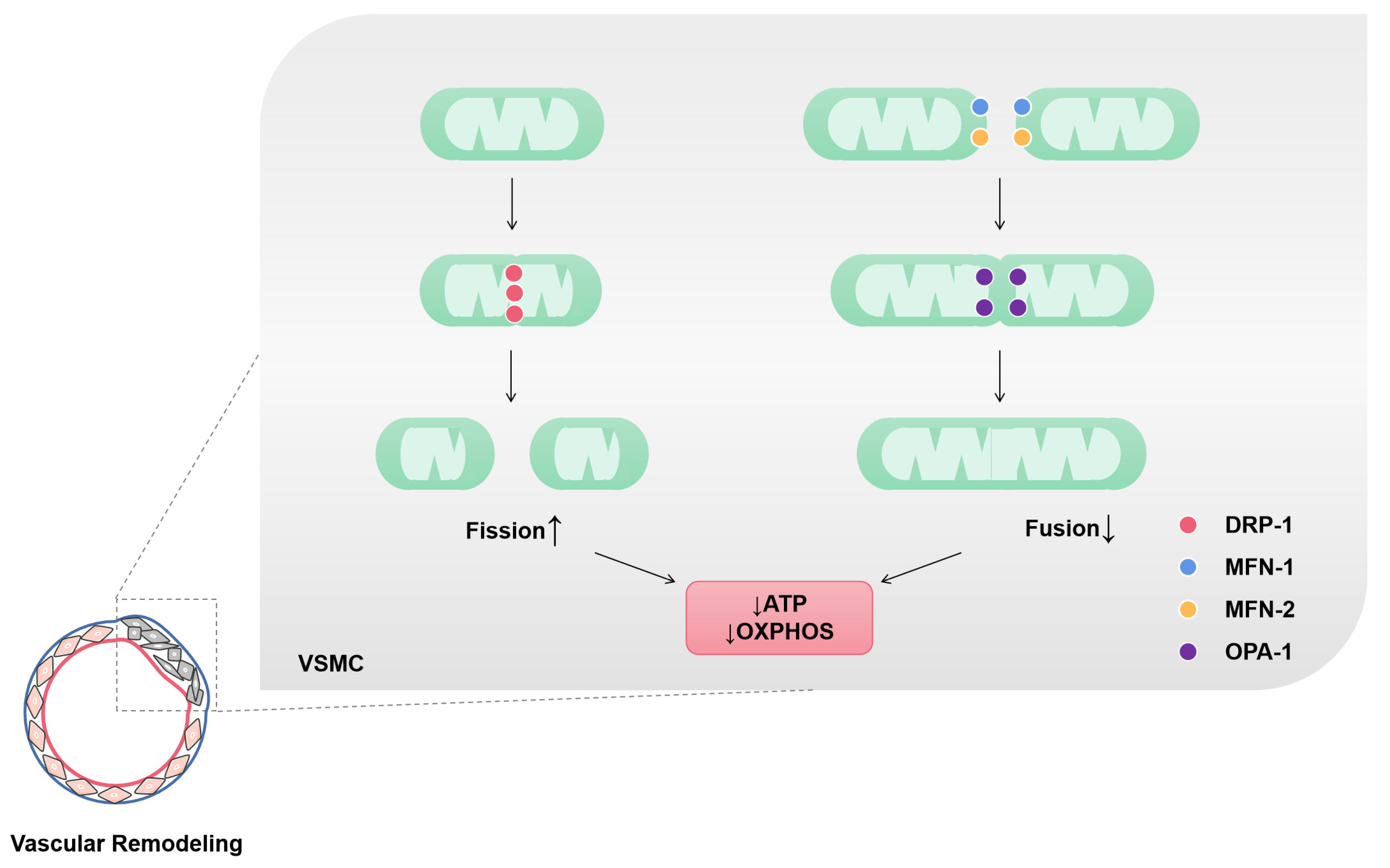
Figure 2.
Mitochondrial fission and fusion of VSMCs in vascular remodeling. Mitochondria in vascular remodeling are characterized by increased fission and reduced fusion. Dynamin-related protein-1 (DRP-1) is a GTP hydrolase (GTPase) that initiates fission. Mitochondrial fission is associated with ATP production and O2 consumption decrease. Mitofusin-1 and 2 (MFN-1 and MFN-2) are GTPases in the outer mitochondrial membrane (OMM). These two enzymes mediate the fusion of OMM adjacent mitochondria. Optic atrophy protein-1 (OPA-1) is a dynamin-related protein localized in the inner mitochondrial membrane (IMM) that participates in the fusion of IMM. Mitochondrial fusion is associated with an increase in ATP production and OXPHOS.
4. Mitophagy of VSMC in Vascular Remodeling
Mitophagy is an autophagic phenomenon targeting damaged or redundant mitochondria to ensure proper mitochondrial quality control [25,52]. Hypoxia, inflammatory stimulation, insulin resistance and oxidative stress lead to mitochondrial damage in VSMCs, and mitophagy can eliminate damaged mitochondria to protect VSMC (Figure 3). PINK1/Parkin-mediated mitophagy obliterates the dysfunctional mitochondria derived from mitochondrial fission [53]. Although mitophagy typically plays a positive role in VSMC homeostasis, its effect on VSMC proliferation remains controversial. VSMC proliferation is a critical process in arteriosclerosis. Several studies have shown that mitophagy promotes VSMC proliferation [54]. Atherosclerosis is characterized by the accumulation of lipids, which evokes Apelin-13 expression [54]. Apelin-13 activates a PINK1/Parkin signal that initiates mitophagy in VSMC. Meanwhile, mitochondria dynamics assists mitophagy to accelerate VSMC proliferation through up-regulated DRP1 and down-regulated MFN1/2 and OPA2 [54]. VSMC proliferation is mainly determined by mitochondrial fission and fusion, wheras mitophagy only plays an auxiliary role. Mitophagy can repair mitochondrial function to inhibit VSMC proliferation. Once the level of damaged mitochondria exceeds the repair capacity of mitophagy, the proliferation of VSMC will increase simultaneously, which may explain the contradictory role of mitophagy in VSMC proliferation.
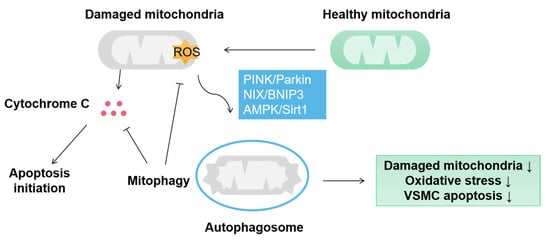
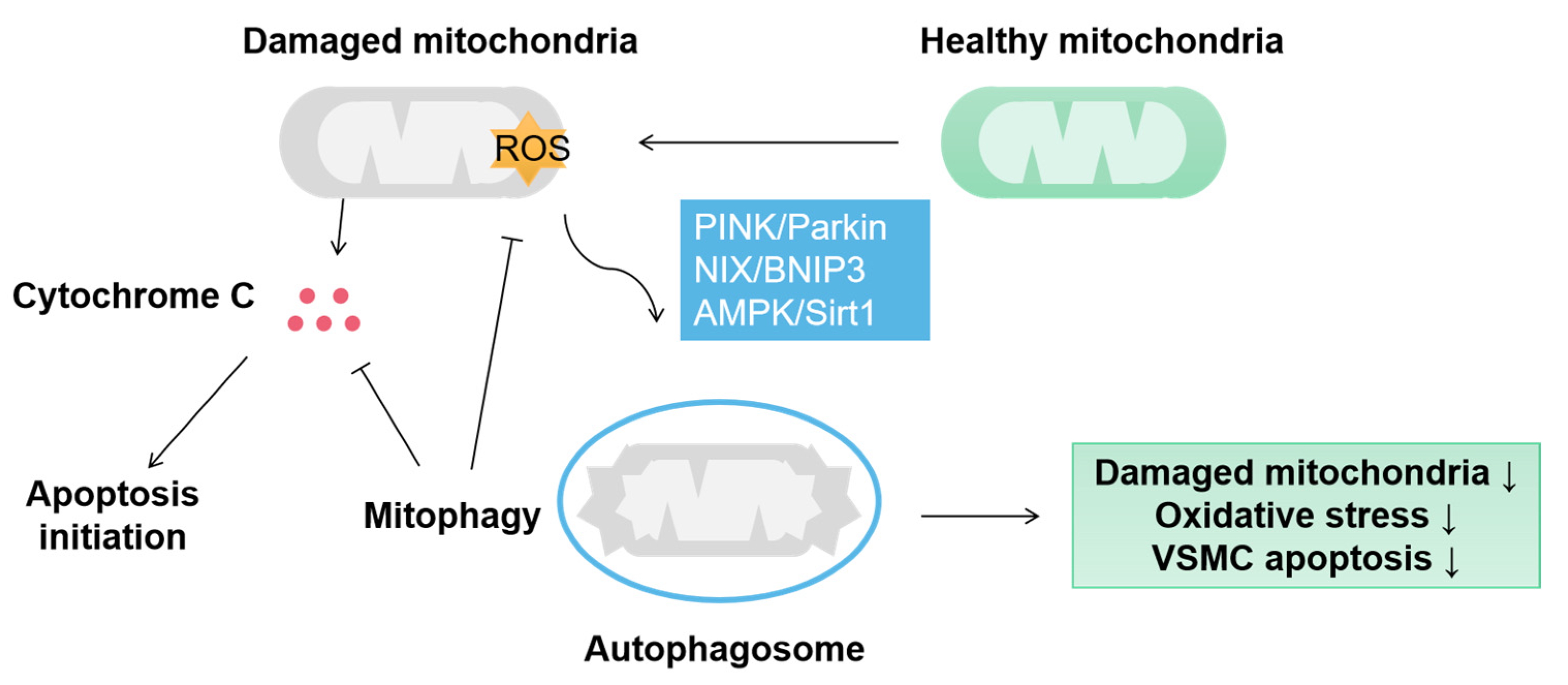
Figure 3.
Mitophagy protects VSMC by eliminating damaged mitochondria. In VSMC, mitophagy is mainly aroused by PINK/Parkin, NIX/BNIP3 and AMPK/Sirt1 pathways and induces sequestration of damaged mitochondria by autophagosomes. Mitophagy protects the VSMC from the damage of oxidative stress and cytochrome C-activated apoptosis.
Mitophagy reduces the release of cytochrome C from dysfunctional mitochondria and other apoptotic factors to activate cell death pathways [55]. Defective mitophagy leads to the accumulation of fragmented mitochondria with reduced bioenergetic efficiency and oxidative stress [56]. Mitophagy protects VSMC against oxidative stress and resists cell apoptosis [56]. Atherosclerosis and vascular calcification are often accompanied by VSMC apoptosis. The metabolism of VSMC is altered in these diseases, with decreased oxidative phosphorylation and increased glycolysis [40,57]. The accumulated lactate induces oxidative stress in VSMC and inhibits BNIP3-mediated mitophagy resulting in VSMA apoptosis [58]. The transcription factor NR4A1 is involved in the damage of mitophagy by lactate. In the pathological state, lactate stimulates mitochondrial fission and damages mitochondrial function. Meanwhile, the self-repair mechanism depending on mitophagy is depressed, facilitating VSMC osteoblastic phenotype transition [58]. NR4A1 interrupts the DNA-PKcs/P53 signaling pathway, which is a cellular self-repairing signal that responds to chronic irritation [58]. These studies indicate that mitophagy restores mitochondrial function by removing damaged mitochondria and protects VSMCs from apoptosis. The inhibitory effect of mitophagy on apoptosis is only limited to the early stage of vascular remodeling. When damaged mitochondria accumulate continuously, insufficient mitophagy will accelerate cell apoptosis [59].
Ageing is an independent factor that induces arterial remodeling even in the absence of cardiovascular diseases or other risk factors [60]. Senescent VSMCs are abundant in atherosclerosis plaques and the vascular wall of the artery disease model [42,61]. Defective mitophagy leads to the accumulation of impaired mitochondria, which is the primary source of mtROS and ultimately contributes to the senescence of VSMCs [62]. P53 can bind to PINK and prevent its transport to mitochondria, which leads to impaired mitophagy mediated by PINK/Parkin [63]. It has been shown that activation of PINK/Parkin-mediated mitophagy can alleviate the senescence of VSMCs [62]. In addition to PINK, an AMPK/SIRT1 signal protects mitophagy by improving bioenergy efficiency [63].
The PINK/Parkin and NIX/BNIP3 pathways play significant regulatory roles in the mitophagy of VSMCs. BNIP3 binds to LC3 linkage to activate mitophagy through phosphorylation at Ser17 and Ser24 [64]. In vascular calcification, P53 expression in VSMCs is up-regulated and BINK-mediated mitophagy restriction accelerates VSMC apoptosis [58]. PINK/Parkin is another important pathway for mitophagy in VSMCs. In atherosclerosis, Apelin13 stimulates PINK/Parkin phosphorylation. In response to this signal, AMPK activation inhibits mTOR to promote mitophagy, which protects VSMCs from abnormal proliferation [54]. As a self-repairing process, mitophagy is closely involved in the senescence and apoptosis of VSMCs during vascular remodeling.
5. Mitochondrial Genes and Vascular Remodeling
mtDNAs are located in the mitochondrial matrix and encode the proteins of the electron transport chain, including subunits of complexes I, III and IV and ATP synthase [26]. Compared with nuclear DNA, mtDNA is more vulnerable to oxidative stress due to the lack of histone protection and mtROS [65]. mtDNA mutations promote cellular ageing through mitochondrial respiratory chain deficiency and increased production of ROS, which, in turn, accelerates mtDNA mutagenesis [66]. VSMC senescence, elevated ROS levels and accumulated mtDNA damage are the main characteristics of VSMCs in atherosclerotic plaques [67].
Accumulated mtDNA mutation induces age-related phenotypes in both rodent and human cases [68]. Although mtDNA encodes critical proteins in ETC, high levels of pathogenic mtDNA mutation can reduce the ATP and increase mtROS. A decreased ATP level in VSMCs leads to the degradation of the extracellular matrix and decreased collagen synthesis, which increase plaque instability in atherosclerosis [69]. In turn, high level of ROS cause damage in mtDNA and accelerate VSMC apoptosis [58]. ROS and oxidized low-density lipoprotein (ox-LDL) induce mtDNA damage and promote mitophagy without compensatory mitochondriogenesis in plaque VSMCs [70]. In fact, a certain degree of ROS can promote the proliferation of VSMC, but excessive ROS will accelerate the apoptosis of VSMC [39]. mtDNA integrity and copy number are associated with vascular remodeling. Overexpression of the mtDNA helicase Twinkle can improve the mitochondrial respiration of VSMCs independent of ROS and results in a decreased necrotic core and increased stability of the atherosclerosis plaque [70,71].
Aberrant methylation of mtDNA in smooth muscle cells inhibits mtDNA expression and causes mitochondrial respiration defects, which leads to the decreased ATP-dependent contractile function of VSMCs; thus, the progression of vascular stenosis occlusive diseases such as atherosclerosis and post-injury restenosis is exacerbated [72]. Platelet-derived growth factor-BB (PDGF-BB) can evoke DNA methyltransferase 1 (DNMT1)-mediated aberrant methylation of mtDNA in VSMC [72]. These studies suggest that mtDNA transcriptional regulation influences the phenotypic transition of VSMCs and participates in the pathological process (Figure 4).

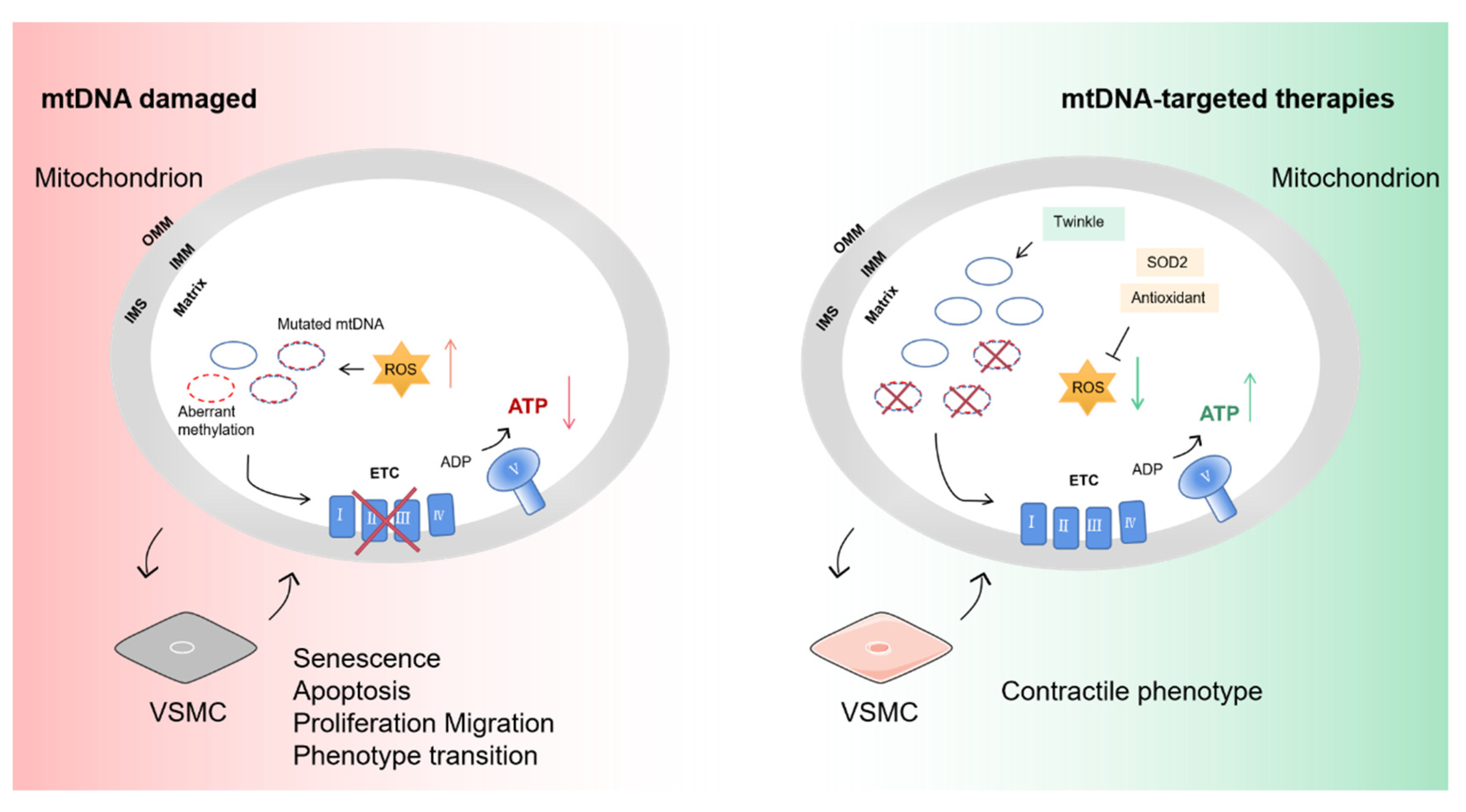
Figure 4.
The influence of mtDNA damage on VSMCs and targeted therapies. mtDNA encodes core subunits of respiratory chain complexes I, III and IV and ATP synthase. Pathogenic mutation and aberrant methylation of mtDNA affect oxidative respiratory function. Reduced ATP and increased mtROS drive the senescence of VSMC and phenotype transition from contractile to non-contractile (synthetic). Overexpression of Twinkle increases the mtDNA copy number to shift the heterogeneity level of mutation. Administration of the SOD2 activator and antioxidants can counteract excess mtROS and restore mitochondrial homeostasis. SOD2, superoxide dismutase 2. Part of the elements in this figure are using resources from Servier Medical Art under a Creative Commons Attribution license.
6. VSMC Mitochondria-Targeted Therapy in Vascular Remodeling
In vascular remodeling, the quality control and function of mitochondria are impaired. The therapies focus on restoring the normal function of mitochondria, including OXPHOS, apoptosis and clearing damaged mitochondria. Mitochondrial-targeted antioxidants can scavenge the ROS and stabilize the mitochondrial redox state. Some molecules are designed to specifically target the core pathways and enzymes to affect mitochondrial homeostasis. Adeno-associated virus-2 (AAV-2), modified by peptides, can enhance the specific transgene expression of SMC [73]. During different stages or types of pathological remodeling, the impaired mitochondrial function of VSMCs is different and requires corresponding treatments.
6.1. Mitochondrial Function Restoration
Existing evidence has suggested that restoring VSMC mitochondrial function can effectively alleviate the pathological process of vascular remodeling [40,44,50], which could be a promising therapy in a variety of cardiovascular diseases. In rats with pulmonary hypertension, transplantation of healthy mitochondria can restore the contractile phenotype of VSMCs in the pulmonary artery, thereby improving the vasoreactivity and right ventricular remodeling [74]. Superoxide dismutase 2 (SOD2) preferentially localizes to the mitochondria and is endowed with the ability of mtROS clearance. Simvastatin can inhibit the expression of NOR-1, a negative regulatory molecule of SOD2. Therefore, statin is considered to be a potential drug for preventing vascular calcification and reducing mtDNA damage (Table 1) [75]. Elevated mitochondrial oxidative stress is involved in a variety of cardiovascular diseases [76]. Thus, mitochondrial antioxidants are potent agents for protecting VSMC by eliminating excess ROS. Mitochondria-targeted antioxidants MitoQ and astaxanthin (ATX) [77] could attenuate vascular remodeling by inhibiting VSMC phenotype transition and improving mitochondrial function [78]. MitoQ10, a mitochondria-targeted ubiquinone, can accumulate within mitochondria and combines with triphenylphosphonium (TPP) to prevent mitochondrial oxidative damage (Table 1) [79]. Another therapeutic target is epigenetic regulation. SOD-mimetic metalloporphyrin Mn (III) tetrakis (4-benzoic acid) porphyrin (MnTBAP) and DNA methyltransferase inhibitor 5-aza-2-deoxycytidine regress experimental pulmonary arterial hypertension. Tissue-specific, epigenetic regulation of SOD2 improves the mitochondrial redox state and reduces the proliferative, apoptosis-resistant SMC obstructing the pulmonary artery [80].
Mitochondria-dependent apoptosis is impaired in vascular remodeling of pulmonary arterial hypertension. Dichloroacetate (DCA) is a mitochondrial enzyme inhibitor that reduces glycolysis and promotes oxidative phosphorylation. DCA-treated pulmonary hypertension rats obtain decreased medial thickness and right ventricular hypertrophy by affecting apoptosis and K channels [81]. The accumulation of HSP90 (heat shock protein 90) in pulmonary artery SMC mitochondria contributes to their proliferation and resistance to apoptosis and promotes vascular remodeling in pulmonary arterial hypertension. Gamitrinib, a mitochondria-targeted HSP90 inhibitor, regulates mitochondrial homeostasis and alleviates vascular remodeling in a rodent PAH model [82].

Table 1.
Therapeutic drugs target mitochondria for vascular remodeling diseases.
Table 1.
Therapeutic drugs target mitochondria for vascular remodeling diseases.
| Therapy | Impact on the VSMC | Related Disease | Target | References |
|---|---|---|---|---|
| Mitochondrial biogenesis | ||||
| Resveratrol | VSMC proliferation | Vascular remodeling and arterial stiffness | SIRT1 | [81,83,84] |
| NR | Mitochondrial respiration of VSMC | Inherited and non-inherited aortic aneurysm | Sirtuins | [32,33] |
| Mitochondrial dynamics | ||||
| Exogenous H2S | VSMC proliferation | TypeII diabetes | ROS | [40] |
| Irish | Phenotypic transformation of VSMC | Chronic kidney disease | AMPK/DRP1 | [50] |
| KLF5 | Mitochondrial integrity of VSMC | Abdominal aortic aneurysm | MFN1 | [44] |
| Mdivi-1 | VSMC proliferation | Pulmonary hypertension | DRP1 | [27,28] |
| Mitophagy | ||||
| Astaxanthin | VSMC proliferation | Hypertension | PINK/Parkin | [77] |
| Astragaloside IV | VSMC senescence | Ageing | PINK/Parkin | [62] |
| KTP | PINK/Parkin | [85] | ||
| MitoQ | Phenotypic transformation of VSMC | Vascular fibrosis | PINK1/Parkin | [78] |
| MtDNA damage | ||||
| MitoQ10 | VSMC respiratory chain | Hypertension | ROS | [79] |
| Statin | VSMC senescence | Vascular calcification | ROS | [75] |
| Mitochondria-dependent apoptosis | ||||
| MnTBAP | VSMC proliferative and apoptosis | Pulmonary hypertension | SOD2 | [80] |
| DCA | VSMC apoptosis | Pulmonary hypertension | Pyruvate dehydrogenase kinase (PDHK) | [86] |
| Gamitrinib | VSMC apoptosis | Pulmonary hypertension | mtHSP90 | [82] |
6.2. Mitochondrial Biogenesis Regulation
Maintaining mitochondrial homeostasis by generating new mitochondria via mitochondrial biogenesis is critical for vascular health. The administration of the SIRT1 activator resveratrol can ameliorate pathological stimuli-induced SMC proliferation, vascular remodeling and arterial stiffness [83]. Resveratrol downregulates the expression of AngII type 1 receptor in VSMCs, serum AngII level and aortic expression of angiotensin converting enzyme (ACE) in a rodent model [79,82]. The regulation is similar in other cell types; resveratrol enhanced mitochondrial biogenesis and ameliorated Ang II-induced cardiac remodeling in transgenic rats harboring human renin–angiotensin genes (Table 1) [87].
Nicotinamide riboside (NR) (Table 1) is a nicotinamide adenine dinucleotide (NAD) precursor that promotes mitochondrial biogenesis by increasing PGC1α and TFAM expression through Sirtuin1 and other sirtuins [88]. NR raises TFAM levels and increases mitochondrial respiration in murine and human MFS cells (primary SMCs with FBN1 knockdown). In the Marfan mouse model of thoracic aortic aneurysm, NR treatment can normalize aortic function and diameter [32]. In acute aortic aneurysms and lethal ruptures induced by Ang-II, NR treatment has similar effects on aortic ruptures and prevents sudden death [33].
6.3. Mitochondrial Dynamics Regulation
Mitochondrial fusion and fission are involved in the proliferation, migration senescence and phenotypic transformation of VSMCs in vascular remodeling. AngII, high-glucose and oxidative stress facilitate the increase of fission and the decrease of fusion, which is involved in PDGF activation and AMPK depression [35]. GTP-hydrolyzing enzymes, including MFN1/2, OPA1 and DRP1, mediate AMPK and PDGF regulation of mitochondrial dynamics [51]. It is feasible to target these enzymes to maintain the mitochondrial dynamics balance. Mdivi-1 disrupts VSMC proliferation by inhibiting DRP1 expression, which resists the exacerbation in disease models such as intimal hyperplasia and pulmonary hypertension [27,28] (Table 1). Mdivi-1-treated VSMCs showed enhanced morphological integrity and decreased ROS levels. In vascular calcification, Irisin acts on the AMPK/DRP1 pathway to inhibit the transformation of VSMCs to the osteogenic phenotype, thus protecting the function and integrity of mitochondria [50]. Exogenous H2S also prevents arterial hyperplasia by inhibiting mitochondrial fission from disrupting VSMC proliferation (Table 1) [40]. Furthermore, the transcription factor KLF5 has been shown to bind to the elF5a promoter to activate its transcription, which can combine with MFN1 to protect mitochondrial integrity (Table 1) [44].
6.4. Mitophagy Regulation
Mitophagy in VSMCs is mainly mediated through the PINK/Parkin and BNIP3/NIX pathways [52]. In essence, mitophagy is a self-protective action to remove damaged mitochondria that is involved in the proliferation, senescence and apoptosis of VSMCs [54,75,77]. For atherosclerosis, mitophagy and mitochondrial fission participate in VSMC proliferation together, in which mitochondrial fission dominates [25,59]. Mitochondrial antioxidant astaxanthin can reduce mitochondrial fission and activate mitophagy to decrease the excessive proliferation of VSMCs through PINK/Parkin (Table 1) [77]. In fact, the PINK/Parkin pathway can be activated by a variety of drugs to restore a high-quality mitochondrial status. For example, kinetin triphosphate (KTP) is an ATP analogue and has more affinity with PINK than ATP. This feature of KTP enhances the activity of specific PINK kinases and initiates mitophagy (Table 1) [85]. Mitophagy plays a major role in the senescence of smooth muscle cells. In VSMCs, ROS stimulates the upregulation of P53, which binds to Parkin in the cytoplasm and prevents its mitochondrial localization. This causes mitophagy defects and promotes the senescence of VSMCs [63]. Astragaloside IV has been proven to alleviate the senescence of VSMCs through activating Parkin-mediated mitophagy, which protects vessels from the lesion (Table 1) [62]. Therefore, targeting PINK/Parkin-mediated mitophagy is a feasible method to rescue senescence in VSMC. A mitophagy-related gene (ATG7) deficiency leads to mitophagy defects and exacerbates the development of atherosclerosis [56]. Lactate depresses BNIP3/NIX-mediated mitophagy via the NR4A1/DNA-PKcs/P53 pathway to promote VSMC apoptosis that aggravates vascular calcification [58]. For vascular remodeling diseases, targeting the PINK/Parkin and BNIP3/NIX pathway of VSMC is a prospective therapy that recovers mitophagy to maintain mitochondrial homeostasis.
6.5. mtDNA-Targeted Therapy
When the heterogeneity of mutant mtDNA reaches a certain level, abnormal respiratory chain complexes will influence mitochondrial function and cause diseases. Mitochondrial gene therapy is limited by tools. The guide RNA of CRISPR-Cas9 cannot enter the inner mitochondrial membrane to conduct editing. Heteroplasmic shifting is the main method to treat mtDNA damage (Figure 4). Antigenomic therapy decreases mtDNA damage by inhibiting the replication of mutated mtDNA [65]. For instance, peptide nucleic acid (PNA) can specifically bind to a mitochondrial propeptide sequence or TPP, which disrupts the replication of the mutated mtDNA [89,90]. However, it is doubtful whether this method takes effect in vivo; the modified PNAs are not likely to enter the inner mitochondrial membrane and bind to the target position of mtDNA [69]. Using stem cells with fewer mutations to differentiate can also replace cells with mtDNA damage [91]. Overexpressing Twinkle, the mtDNA helicase, increases mtDNA copy number and reduces plaque necrosis, which proves that maintaining mtDNA integrity is a great way to treat vascular disease [71]. mitoTALENs [92] and mtZFNs [93] have been developed to eliminate the mtDNA mutant load and correct single base mutations. However, the copy number depletion and off-target effects of the editing tools still need to be addressed.
7. Conclusions
Phenotypically modified VSMCs play a major role in vascular remodeling. Mitochondrial homeostasis is involved in the phenotypic modulation, proliferation, migration, senescence and apoptosis of VSMCs. Here, we focus on the typical factors affecting mitochondrial homeostasis, including mitochondrial function, mitochondrial fission and fusion, mitophagy and mitochondrial DNA mutation. Mitochondrial dysfunction may be a primary cause of vascular remodeling and is a promising target for new therapies. However, whether these strategies can be used in the clinical treatment of vascular remodeling is worth exploring further.
Author Contributions
Conceptualization, J.L. and Y.L.; investigation, Y.X., P.A. and X.Z.; writing—original draft preparation, Y.X., P.A. and X.Z.; writing—review and editing, J.L. and Y.L.; visualization, Y.X.; supervision, J.L. and Y.L.; project administration, J.L. and Y.L.; funding acquisition, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Beijing Advanced Innovation Center for Food Nutrition and Human Health, the National Natural Science Foundation of China (31970717, 82170429), the Chinese Universities Scientific Fund (2020TC015) and the Beijing Municipal Natural Science Foundation (7222111).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
VSMCs, vascular smooth muscle cells; mtDNA, mitochondrial DNA; ROS, reactive oxygen species; MFN1/2, mitofusin 1/2; OPA1, Optic Atrophy1; DRP1, dynamin-related protein 1; PASMC, pulmonary artery smooth muscle cell; eIF5a, eukaryotic translation initiation factor 5a; PDGF, platelet-derived growth factor; AngII, angiotensin II; AAA, abdominal aortic aneurysm; mtSSB, mitochondrial single-stranded binding protein.
References
- Thompson, A.A.R.; Lawrie, A. Targeting Vascular Remodeling to Treat Pulmonary Arterial Hypertension. Trends Mol. Med. 2017, 23, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Zheng, F.; Wu, N.; Zhu, G.-Q.; Li, X.-Z. Extracellular Vesicles in Vascular Remodeling. Acta Pharmcol. Sin. 2022, 43, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Montero, R.; Pérez-Sáez, J.M.; Cerro-Pardo, I.; Oller, J.; Martinez-Lopez, D.; Nuñez, E.; Maller, S.M.; Gutierrez-Muñoz, C.; Mendez-Barbero, N.; Escola-Gil, J.C.; et al. Galectin-1 Prevents Pathological Vascular Remodeling in Atherosclerosis and Abdominal Aortic Aneurysm. Sci. Adv. 2022, 8, eabm7322. [Google Scholar] [CrossRef]
- Brown, I.A.M.; Diederich, L.; Good, M.E.; DeLalio, L.J.; Murphy, S.A.; Cortese-Krott, M.M.; Hall, J.L.; Le, T.H.; Isakson, B.E. Vascular Smooth Muscle Remodeling in Conductive and Resistance Arteries in Hypertension. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1969–1985. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular Smooth Muscle Contraction in Hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar]
- Quintavalle, M.; Condorelli, G.; Elia, L. Arterial Remodeling and Atherosclerosis: miRNAs Involvement. Vasc. Pharmacol. 2011, 55, 106–110. [Google Scholar] [CrossRef]
- Nemenoff, R.A.; Horita, H.; Ostriker, A.C.; Furgeson, S.B.; Simpson, P.A.; VanPutten, V.; Crossno, J.; Offermanns, S.; Weiser-Evans, M.C.M. SDF-1α Induction in Mature Smooth Muscle Cells by Inactivation of PTEN Is a Critical Mediator of Exacerbated Injury-Induced Neointima Formation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1300–1308. [Google Scholar] [CrossRef]
- Amiri, F.; Virdis, A.; Neves, M.F.; Iglarz, M.; Seidah, N.G.; Touyz, R.M.; Reudelhuber, T.L.; Schiffrin, E.L. Endothelium-Restricted Overexpression of Human Endothelin-1 Causes Vascular Remodeling and Endothelial Dysfunction. Circulation 2004, 110, 2233–2240. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, X.; Sun, Z. Haplodeficiency of Klotho Gene Causes Arterial Stiffening via Upregulation of Scleraxis Expression and Induction of Autophagy. Hypertension 2015, 66, 1006–1013. [Google Scholar]
- Grootaert, M.O.J.; Bennett, M.R. Vascular Smooth Muscle Cells in Atherosclerosis: Time for a Re-Assessment. Cardiovasc. Res. 2021, 117, 2326–2339. [Google Scholar]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and Challenges in Translating the Biology of Atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [PubMed]
- Cui, X.; Pan, G.; Chen, Y.; Guo, X.; Liu, T.; Zhang, J.; Yang, X.; Cheng, M.; Gao, H.; Jiang, F. The P53 Pathway in Vasculature Revisited: A Therapeutic Target for Pathological Vascular Remodeling? Pharmcol. Res. 2021, 169, 105683. [Google Scholar] [CrossRef] [PubMed]
- Allahverdian, S.; Chaabane, C.; Boukais, K.; Francis, G.A.; Bochaton-Piallat, M.-L. Smooth Muscle Cell Fate and Plasticity in Atherosclerosis. Cardiovasc. Res. 2018, 114, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Sherifova, S.; Holzapfel, G.A. Biomechanics of Aortic Wall Failure with a Focus on Dissection and Aneurysm: A Review. Acta Biomater. 2019, 99, 1–17. [Google Scholar]
- Nienaber, C.A.; Clough, R.E.; Sakalihasan, N.; Suzuki, T.; Gibbs, R.; Mussa, F.; Jenkins, M.P.; Thompson, M.M.; Evangelista, A.; Yeh, J.S.M.; et al. Aortic Dissection. Nat. Rev. Dis. Prim. 2016, 2, 16053. [Google Scholar]
- Peterss, S.; Mansour, A.M.; Ross, J.A.; Vaitkeviciute, I.; Charilaou, P.; Dumfarth, J.; Fang, H.; Ziganshin, B.A.; Rizzo, J.A.; Adeniran, A.J.; et al. Changing Pathology of the Thoracic Aorta from Acute to Chronic Dissection. J. Am. Coll. Cardiol. 2016, 68, 1054–1065. [Google Scholar]
- Lacolley, P.; Regnault, V.; Avolio, A.P. Smooth Muscle Cell and Arterial Aging: Basic and Clinical Aspects. Cardiovasc. Res. 2018, 114, 513–528. [Google Scholar] [CrossRef]
- Miano, J.M.; Fisher, E.A.; Majesky, M.W. Fate and State of Vascular Smooth Muscle Cells in Atherosclerosis. Circulation 2021, 143, 2110–2116. [Google Scholar]
- Grootaert, M.O.J.; Moulis, M.; Roth, L.; Martinet, W.; Vindis, C.; Bennett, M.R.; De Meyer, G.R.Y. Vascular Smooth Muscle Cell Death, Autophagy and Senescence in Atherosclerosis. Cardiovasc. Res. 2018, 114, 622–634. [Google Scholar] [CrossRef]
- Melnik, T.; Jordan, O.; Corpataux, J.-M.; Delie, F.; Saucy, F. Pharmacological Prevention of Intimal Hyperplasia: A State-of-the-Art Review. Pharmcol. Ther. 2022, 235, 108157. [Google Scholar]
- Tanaka, L.Y.; Laurindo, F.R.M. Vascular Remodeling: A Redox-Modulated Mechanism of Vessel Caliber Regulation. Free Radic. Biol. Med. 2017, 109, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Atef, M.E.; Anand-Srivastava, M.B. Oxidative Stress Contributes to the Enhanced Expression of Gqα/PLCβ1 Proteins and Hypertrophy of VSMC from SHR: Role of Growth Factor Receptor Transactivation. Am. J. Physiol.-Heart Circ. Physiol. 2016, 310, H608–H618. [Google Scholar]
- Kuznetsov, A.V.; Hermann, M.; Saks, V.; Hengster, P.; Margreiter, R. The Cell-Type Specificity of Mitochondrial Dynamics. Int. J. Biochem. Cell Biol. 2009, 41, 1928–1939. [Google Scholar] [CrossRef]
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 235–259. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Trincado, C.; García-Carvajal, I.; Pennanen, C.; Parra, V.; Hill, J.A.; Rothermel, B.A.; Lavandero, S. Mitochondrial Dynamics, Mitophagy and Cardiovascular Disease. J. Physiol. 2016, 594, 509–525. [Google Scholar] [PubMed]
- Yu, E.; Calvert, P.A.; Mercer, J.R.; Harrison, J.; Baker, L.; Figg, N.L.; Kumar, S.; Wang, J.C.; Hurst, L.A.; Obaid, D.R.; et al. Mitochondrial DNA Damage Can Promote Atherosclerosis Independently of Reactive Oxygen Species Through Effects on Smooth Muscle Cells and Monocytes and Correlates with Higher-Risk Plaques in Humans. Circulation 2013, 128, 702–712. [Google Scholar] [PubMed]
- Marsboom, G.; Toth, P.T.; Ryan, J.J.; Hong, Z.; Wu, X.; Fang, Y.-H.; Thenappan, T.; Piao, L.; Zhang, H.J.; Pogoriler, J.; et al. Dynamin-Related Protein 1-Mediated Mitochondrial Mitotic Fission Permits Hyperproliferation of Vascular Smooth Muscle Cells and Offers a Novel Therapeutic Target in Pulmonary Hypertension. Circ. Res. 2012, 110, 1484–1497. [Google Scholar] [CrossRef] [PubMed]
- Salabei, J.K.; Hill, B.G. Mitochondrial Fission Induced by Platelet-Derived Growth Factor Regulates Vascular Smooth Muscle Cell Bioenergetics and Cell Proliferation. Redox. Biol. 2013, 1, 542–551. [Google Scholar]
- Whitaker, R.M.; Corum, D.; Beeson, C.C.; Schnellmann, R.G. Mitochondrial Biogenesis as a Pharmacological Target: A New Approach to Acute and Chronic Diseases. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 229–249. [Google Scholar]
- Deng, M.; Su, D.; Xu, S.; Little, P.J.; Feng, X.; Tang, L.; Shen, A. Metformin and Vascular Diseases: A Focused Review on Smooth Muscle Cell Function. Front. Pharmacol. 2020, 11, 635. [Google Scholar] [CrossRef]
- Xiong, S.; Salazar, G.; San Martin, A.; Ahmad, M.; Patrushev, N.; Hilenski, L.; Nazarewicz, R.R.; Ma, M.; Ushio-Fukai, M.; Alexander, R.W. PGC-1α Serine 570 Phosphorylation and GCN5-Mediated Acetylation by Angiotensin II Drive Catalase Down-Regulation and Vascular Hypertrophy. J. Biol. Chem. 2010, 285, 2474–2487. [Google Scholar] [PubMed]
- Oller, J.; Gabandé-Rodríguez, E.; Ruiz-Rodríguez, M.J.; Desdín-Micó, G.; Aranda, J.F.; Rodrigues-Diez, R.; Ballesteros-Martínez, C.; Blanco, E.M.; Roldan-Montero, R.; Acuña, P.; et al. Extracellular Tuning of Mitochondrial Respiration Leads to Aortic Aneurysm. Circulation 2021, 143, 2091–2109. [Google Scholar] [PubMed]
- Oller, J.; Gabandé-Rodríguez, E.; Roldan-Montero, R.; Ruiz-Rodríguez, M.J.; Redondo, J.M.; Martín-Ventura, J.L.; Mittelbrunn, M. Rewiring Vascular Metabolism Prevents Sudden Death Due to Aortic Ruptures—Brief Report. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 462–469. [Google Scholar]
- Gabrielson, M.; Vorkapic, E.; Folkesson, M.; Welander, M.; Matussek, A.; Dimberg, J.; Länne, T.; Skogberg, J.; Wågsäter, D. Altered PPARγ Coactivator-1 Alpha Expression in Abdominal Aortic Aneurysm: Possible Effects on Mitochondrial Biogenesis. J. Vasc. Res. 2016, 53, 17–26. [Google Scholar] [CrossRef]
- Wang, L.; Yu, T.; Lee, H.; O’Brien, D.K.; Sesaki, H.; Yoon, Y. Decreasing Mitochondrial Fission Diminishes Vascular Smooth Muscle Cell Migration and Ameliorates Intimal Hyperplasia. Cardiovasc. Res. 2015, 106, 272–283. [Google Scholar]
- Chan, D.C. Mitochondria: Dynamic Organelles in Disease, Aging, and Development. Cell 2006, 125, 1241–1252. [Google Scholar]
- Archer, S.L.; Weir, E.K.; Wilkins, M.R. Basic Science of Pulmonary Arterial Hypertension for Clinicians: New Concepts and Experimental Therapies. Circulation 2010, 121, 2045–2066. [Google Scholar]
- Smith, S.A.; Newby, A.C.; Bond, M. Ending Restenosis: Inhibition of Vascular Smooth Muscle Cell Proliferation by CAMP. Cells 2019, 8, 1447. [Google Scholar] [CrossRef]
- Li, M.; Absher, P.M.; Liang, P.; Russell, J.C.; Sobel, B.E.; Fukagawa, N.K. High Glucose Concentrations Induce Oxidative Damage to Mitochondrial DNA in Explanted Vascular Smooth Muscle Cells. Exp. Biol. Med. 2001, 226, 450–457. [Google Scholar]
- Sun, A.; Wang, Y.; Liu, J.; Yu, X.; Sun, Y.; Yang, F.; Dong, S.; Wu, J.; Zhao, Y.; Xu, C.; et al. Exogenous H2S Modulates Mitochondrial Fusion–Fission to Inhibit Vascular Smooth Muscle Cell Proliferation in a Hyperglycemic State. Cell. Biosci. 2016, 6, 36. [Google Scholar]
- Davies, M.G.; Hagen, P.O. Pathobiology of Intimal Hyperplasia. Br. J. Surg. 1994, 81, 1254–1269. [Google Scholar]
- Chi, C.; Li, D.-J.; Jiang, Y.-J.; Tong, J.; Fu, H.; Wu, Y.-H.; Shen, F.-M. Vascular Smooth Muscle Cell Senescence and Age-Related Diseases: State of the Art. Biochim. Biophys. Acta Mol. Basis. Dis. 2019, 1865, 1810–1821. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.R.; Wardell, S.E.; Cakir, M.; Yip, C.; Ahn, Y.; Ali, M.; Yllanes, A.P.; Chao, C.A.; McDonnell, D.P.; Wood, K.C. Dysregulation of Mitochondrial Dynamics Proteins Are a Targetable Feature of Human Tumors. Nat. Commun. 2018, 9, 1677. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zheng, B.; Liu, H.; Zhao, Y.; Liu, X.; Zhang, X.; Li, Q.; Shi, W.; Suzuki, T.; Wen, J. Klf5 Down-Regulation Induces Vascular Senescence through EIF5a Depletion and Mitochondrial Fission. PLoS Biol. 2020, 18, e3000808. [Google Scholar]
- Cooper, H.A.; Cicalese, S.; Preston, K.J.; Kawai, T.; Okuno, K.; Choi, E.T.; Kasahara, S.; Uchida, H.A.; Otaka, N.; Scalia, R.; et al. Targeting Mitochondrial Fission as a Potential Therapeutic for Abdominal Aortic Aneurysm. Cardiovasc. Res. 2021, 117, 971–982. [Google Scholar]
- Forte, M.; Schirone, L.; Ameri, P.; Basso, C.; Catalucci, D.; Modica, J.; Chimenti, C.; Crotti, L.; Frati, G.; Rubattu, S.; et al. The Role of Mitochondrial Dynamics in Cardiovascular Diseases. Br. J. Pharm. 2021, 178, 2060–2076. [Google Scholar] [CrossRef]
- Shankman, L.S.; Gomez, D.; Cherepanova, O.A.; Salmon, M.; Alencar, G.F.; Haskins, R.M.; Swiatlowska, P.; Newman, A.A.C.; Greene, E.S.; Straub, A.C.; et al. KLF4-Dependent Phenotypic Modulation of Smooth Muscle Cells Has a Key Role in Atherosclerotic Plaque Pathogenesis. Nat. Med. 2015, 21, 628–637. [Google Scholar]
- Pan, H.; Reilly, M.P. A Protective Smooth Muscle Cell Transition in Atherosclerosis. Nat. Med. 2019, 25, 1194–1195. [Google Scholar] [CrossRef]
- Kato, M.; Kyogoku, M. Competence Growth Factors Evoke the Phenotypic Transition of Arterial Smooth Muscle Cells. Ann. N. Y. Acad. Sci. 1990, 598, 232–237. [Google Scholar]
- Wang, P.-W.; Pang, Q.; Zhou, T.; Song, X.-Y.; Pan, Y.-J.; Jia, L.-P.; Zhang, A.-H. Irisin Alleviates Vascular Calcification by Inhibiting VSMC Osteoblastic Transformation and Mitochondria Dysfunction via AMPK/Drp1 Signaling Pathway in Chronic Kidney Disease. Atherosclerosis 2022, 346, 36–45. [Google Scholar] [CrossRef]
- Pernas, L.; Scorrano, L. Mito-Morphosis: Mitochondrial Fusion, Fission, and Cristae Remodeling as Key Mediators of Cellular Function. Annu. Rev. Physiol. 2016, 78, 505–531. [Google Scholar] [PubMed]
- Bravo-San Pedro, J.M.; Kroemer, G.; Galluzzi, L. Autophagy and Mitophagy in Cardiovascular Disease. Circ. Res. 2017, 120, 1812–1824. [Google Scholar] [PubMed]
- Pickles, S.; Vigié, P.; Youle, R.J. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr. Biol. 2018, 28, R170–R185. [Google Scholar] [CrossRef]
- He, L.; Zhou, Q.; Huang, Z.; Xu, J.; Zhou, H.; Lv, D.; Lu, L.; Huang, S.; Tang, M.; Zhong, J.; et al. PINK1/Parkin-mediated Mitophagy Promotes Apelin-13-induced Vascular Smooth Muscle Cell Proliferation by AMPKα and Exacerbates Atherosclerotic Lesions. J. Cell. Physiol. 2019, 234, 8668–8682. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Kepp, O.; Trojel-Hansen, C.; Kroemer, G. Mitochondrial Control of Cellular Life, Stress, and Death. Circ. Res. 2012, 111, 1198–1207. [Google Scholar]
- Nahapetyan, H.; Moulis, M.; Grousset, E.; Faccini, J.; Grazide, M.-H.; Mucher, E.; Elbaz, M.; Martinet, W.; Vindis, C. Altered Mitochondrial Quality Control in Atg7-Deficient VSMCs Promotes Enhanced Apoptosis and Is Linked to Unstable Atherosclerotic Plaque Phenotype. Cell Death Dis. 2019, 10, 119. [Google Scholar]
- Vallée, A.; Vallée, J.-N.; Lecarpentier, Y. Metabolic Reprogramming in Atherosclerosis: Opposed Interplay between the Canonical WNT/β-Catenin Pathway and PPARγ. J. Mol. Cell. Cardiol. 2019, 133, 36–46. [Google Scholar]
- Zhu, Y.; Han, X.-Q.; Sun, X.-J.; Yang, R.; Ma, W.-Q.; Liu, N.-F. Lactate Accelerates Vascular Calcification through NR4A1-Regulated Mitochondrial Fission and BNIP3-Related Mitophagy. Apoptosis 2020, 25, 321–340. [Google Scholar]
- Zhang, X.; Zheng, Y.; Chen, Z. Autophagy and Mitochondrial Encephalomyopathies. Adv. Exp. Med. Biol. 2020, 1207, 103–110. [Google Scholar]
- Katsuumi, G.; Shimizu, I.; Yoshida, Y.; Minamino, T. Vascular Senescence in Cardiovascular and Metabolic Diseases. Front. Cardiovasc. Med. 2018, 5, 18. [Google Scholar]
- Minamino, T.; Miyauchi, H.; Yoshida, T.; Ishida, Y.; Yoshida, H.; Komuro, I. Endothelial Cell Senescence in Human Atherosclerosis: Role of Telomere in Endothelial Dysfunction. Circulation 2002, 105, 1541–1544. [Google Scholar] [PubMed]
- Li, H.; Xu, J.; Zhang, Y.; Hong, L.; He, Z.; Zeng, Z.; Zhang, L. Astragaloside IV Alleviates Senescence of Vascular Smooth Muscle Cells through Activating Parkin-Mediated Mitophagy. Hum. Cell 2022, 35, 1684–1696. [Google Scholar] [CrossRef]
- Abate, M.; Festa, A.; Falco, M.; Lombardi, A.; Luce, A.; Grimaldi, A.; Zappavigna, S.; Sperlongano, P.; Irace, C.; Caraglia, M.; et al. Mitochondria as Playmakers of Apoptosis, Autophagy and Senescence. Semin. Cell Dev. Biol. 2020, 98, 139–153. [Google Scholar] [PubMed]
- Zhu, Y.; Massen, S.; Terenzio, M.; Lang, V.; Chen-Lindner, S.; Eils, R.; Novak, I.; Dikic, I.; Hamacher-Brady, A.; Brady, N.R. Modulation of Serines 17 and 24 in the LC3-Interacting Region of Bnip3 Determines pro-Survival Mitophagy versus Apoptosis. J. Biol. Chem. 2013, 288, 1099–1113. [Google Scholar] [PubMed]
- Yakes, F.M.; Van Houten, B. Mitochondrial DNA Damage Is More Extensive and Persists Longer than Nuclear DNA Damage in Human Cells Following Oxidative Stress. Proc. Natl. Acad. Sci. USA 1997, 94, 514–519. [Google Scholar]
- Harman, D. The Biologic Clock: The Mitochondria? J. Am. Geriatr. Soc. 1972, 20, 145–147. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Mercer, J.; Bennett, M. DNA Damage and Repair in atherosclerosis. Atheroscler. Cardiovasc. Res. 2006, 71, 259–268. [Google Scholar]
- Bratic, A.; Larsson, N.-G. The Role of Mitochondria in Aging. J. Clin. Invest. 2013, 123, 951–957. [Google Scholar] [CrossRef]
- Finn, A.V.; Nakano, M.; Narula, J.; Kolodgie, F.D.; Virmani, R. Concept of Vulnerable/Unstable Plaque. Arter. Thromb Vasc. Biol. 2010, 30, 1282–1292. [Google Scholar] [CrossRef]
- Yu, E.P.K.; Reinhold, J.; Yu, H.; Starks, L.; Uryga, A.K.; Foote, K.; Finigan, A.; Figg, N.; Pung, Y.-F.; Logan, A.; et al. Mitochondrial Respiration Is Reduced in Atherosclerosis, Promoting Necrotic Core Formation and Reducing Relative Fibrous Cap Thickness. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2322–2332. [Google Scholar] [CrossRef]
- Tyynismaa, H.; Sembongi, H.; Bokori-Brown, M.; Granycome, C.; Ashley, N.; Poulton, J.; Jalanko, A.; Spelbrink, J.N.; Holt, I.J.; Suomalainen, A. Twinkle Helicase Is Essential for MtDNA Maintenance and Regulates MtDNA Copy Number. Hum. Mol. Genet. 2004, 13, 3219–3227. [Google Scholar] [PubMed]
- Liu, Y.-F.; Zhu, J.-J.; Yu Tian, X.; Liu, H.; Zhang, T.; Zhang, Y.-P.; Xie, S.-A.; Zheng, M.; Kong, W.; Yao, W.-J.; et al. Hypermethylation of Mitochondrial DNA in Vascular Smooth Muscle Cells Impairs Cell Contractility. Cell Death Dis. 2020, 11, 35. [Google Scholar] [CrossRef]
- Work, L.M.; Nicklin, S.A.; Brain, N.J.R.; Dishart, K.L.; Von Seggern, D.J.; Hallek, M.; Büning, H.; Baker, A.H. Development of Efficient Viral Vectors Selective for Vascular Smooth Muscle Cells. Mol. Ther. 2004, 9, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-H.; Roan, J.-N.; Fang, S.-Y.; Chiu, M.-H.; Cheng, T.-T.; Huang, C.-C.; Lin, M.-W.; Lam, C.-F. Transplantation of Viable Mitochondria Improves Right Ventricular Performance and Pulmonary Artery Remodeling in Rats with Pulmonary Arterial Hypertension. J. Thorac. Cardiovasc. Surg. 2022, 163, e361–e373. [Google Scholar] [PubMed]
- Tsai, Y.-T.; Yeh, H.-Y.; Chao, C.-T.; Chiang, C.-K. Superoxide Dismutase 2 (SOD2) in Vascular Calcification: A Focus on Vascular Smooth Muscle Cells, Calcification Pathogenesis, and Therapeutic Strategies. Oxidative Med. Cell. Longev. 2021, 2021, 6675548. [Google Scholar]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Mitochondrial Oxidative Stress and Dysfunction in Myocardial Remodelling. Cardiovasc. Res. 2008, 81, 449–456. [Google Scholar] [PubMed]
- Chen, Y.; Li, S.; Guo, Y.; Yu, H.; Bao, Y.; Xin, X.; Yang, H.; Ni, X.; Wu, N.; Jia, D. Astaxanthin Attenuates Hypertensive Vascular Remodeling by Protecting Vascular Smooth Muscle Cells from Oxidative Stress-Induced Mitochondrial Dysfunction. Oxidative Med. Cell. Longev. 2020, 2020, 4629189. [Google Scholar] [CrossRef]
- Ning, R.; Li, Y.; Du, Z.; Li, T.; Sun, Q.; Lin, L.; Xu, Q.; Duan, J.; Sun, Z. The Mitochondria-Targeted Antioxidant MitoQ Attenuated PM2.5-Induced Vascular Fibrosis via Regulating Mitophagy. Redox Biol. 2021, 46, 102113. [Google Scholar] [CrossRef]
- James, A.M.; Cochemé, H.M.; Smith, R.A.J.; Murphy, M.P. Interactions of Mitochondria-Targeted and Untargeted Ubiquinones with the Mitochondrial Respiratory Chain and Reactive Oxygen Species. Implications for the Use of Exogenous Ubiquinones as Therapies and Experimental Tools. J. Biol. Chem. 2005, 280, 21295–21312. [Google Scholar] [CrossRef]
- Archer, S.L.; Marsboom, G.; Kim, G.H.; Zhang, H.J.; Toth, P.T.; Svensson, E.C.; Dyck, J.R.B.; Gomberg-Maitland, M.; Thébaud, B.; Husain, A.N.; et al. Epigenetic Attenuation of Mitochondrial Superoxide Dismutase 2 in Pulmonary Arterial Hypertension: A Basis for Excessive Cell Proliferation and a New Therapeutic Target. Circulation 2010, 121, 2661–2671. [Google Scholar]
- Miyazaki, R.; Ichiki, T.; Hashimoto, T.; Inanaga, K.; Imayama, I.; Sadoshima, J.; Sunagawa, K. SIRT1, a Longevity Gene, Downregulates Angiotensin II Type 1 Receptor Expression in Vascular Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Boucherat, O.; Peterlini, T.; Bourgeois, A.; Nadeau, V.; Breuils-Bonnet, S.; Boilet-Molez, S.; Potus, F.; Meloche, J.; Chabot, S.; Lambert, C.; et al. Mitochondrial HSP90 Accumulation Promotes Vascular Remodeling in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2018, 198, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, N.; Hasselwander, S.; Daiber, A. Resveratrol and Vascular Function. Int. J. Mol. Sci. 2019, 20, 2155. [Google Scholar]
- Kim, E.N.; Kim, M.Y.; Lim, J.H.; Kim, Y.; Shin, S.J.; Park, C.W.; Kim, Y.-S.; Chang, Y.S.; Yoon, H.E.; Choi, B.S. The Protective Effect of Resveratrol on Vascular Aging by Modulation of the Renin–Angiotensin System. Atherosclerosis 2018, 270, 123–131. [Google Scholar] [CrossRef]
- Hertz, N.T.; Berthet, A.; Sos, M.L.; Thorn, K.S.; Burlingame, A.L.; Nakamura, K.; Shokat, K.M. A Neo-Substrate That Amplifies Catalytic Activity of Parkinson’s-Disease-Related Kinase PINK1. Cell 2013, 154, 737–747. [Google Scholar]
- McMurtry, M.S.; Bonnet, S.; Wu, X.; Dyck, J.R.B.; Haromy, A.; Hashimoto, K.; Michelakis, E.D. Dichloroacetate Prevents and Reverses Pulmonary Hypertension by Inducing Pulmonary Artery Smooth Muscle Cell Apoptosis. Circ. Res. 2004, 95, 830–840. [Google Scholar] [PubMed]
- Biala, A.; Tauriainen, E.; Siltanen, A.; Shi, J.; Merasto, S.; Louhelainen, M.; Martonen, E.; Finckenberg, P.; Muller, D.N.; Mervaala, E. Resveratrol Induces Mitochondrial Biogenesis and Ameliorates Ang II-Induced Cardiac Remodeling in Transgenic Rats Harboring Human Renin and Angiotensinogen Genes. Blood Press. 2010, 19, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, R.; Pirinen, E.; Lamperti, C.; Marchet, S.; Sauve, A.A.; Li, W.; Leoni, V.; Schon, E.A.; Dantzer, F.; Auwerx, J.; et al. NAD+-Dependent Activation of Sirt1 Corrects the Phenotype in a Mouse Model of Mitochondrial Disease. Cell Metab. 2014, 19, 1042–1049. [Google Scholar] [CrossRef]
- Chinnery, P.F.; Taylor, R.W.; Diekert, K.; Lill, R.; Turnbull, D.M.; Lightowlers, R.N. Peptide Nucleic Acid Delivery to Human Mitochondria. Gene Ther. 1999, 6, 1919–1928. [Google Scholar]
- Jean, S.R.; Ahmed, M.; Lei, E.K.; Wisnovsky, S.P.; Kelley, S.O. Peptide-Mediated Delivery of Chemical Probes and Therapeutics to Mitochondria. Acc. Chem. Res. 2016, 49, 1893–1902. [Google Scholar]
- Mercer, J.R. Mitochondrial Bioenergetics and Therapeutic Intervention in Cardiovascular Disease. Pharmacol. Ther. 2014, 141, 13–20. [Google Scholar] [PubMed]
- Mok, B.Y.; de Moraes, M.H.; Zeng, J.; Bosch, D.E.; Kotrys, A.V.; Raguram, A.; Hsu, F.; Radey, M.C.; Peterson, S.B.; Mootha, V.K.; et al. A Bacterial Cytidine Deaminase Toxin Enables CRISPR-Free Mitochondrial Base Editing. Nature 2020, 583, 631–637. [Google Scholar] [PubMed]
- Gammage, P.A.; Rorbach, J.; Vincent, A.I.; Rebar, E.J.; Minczuk, M. Mitochondrially Targeted ZFN s for Selective Degradation of Pathogenic Mitochondrial Genomes Bearing Large-scale Deletions or Point Mutations. EMBO Mol. Med. 2014, 6, 458–466. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).