Assessment of the Impact of Physical Activity on the Musculoskeletal System in Early Degenerative Knee Joint Lesions in an Animal Model
Abstract
:1. Introduction
2. Results
2.1. Radiological Examination
2.2. Impact of Physical Activity on the Musculoskeletal System—The Whole Body Parameters
2.3. Impact of Physical Activity on the Musculoskeletal System—Limb and Knee Parameters
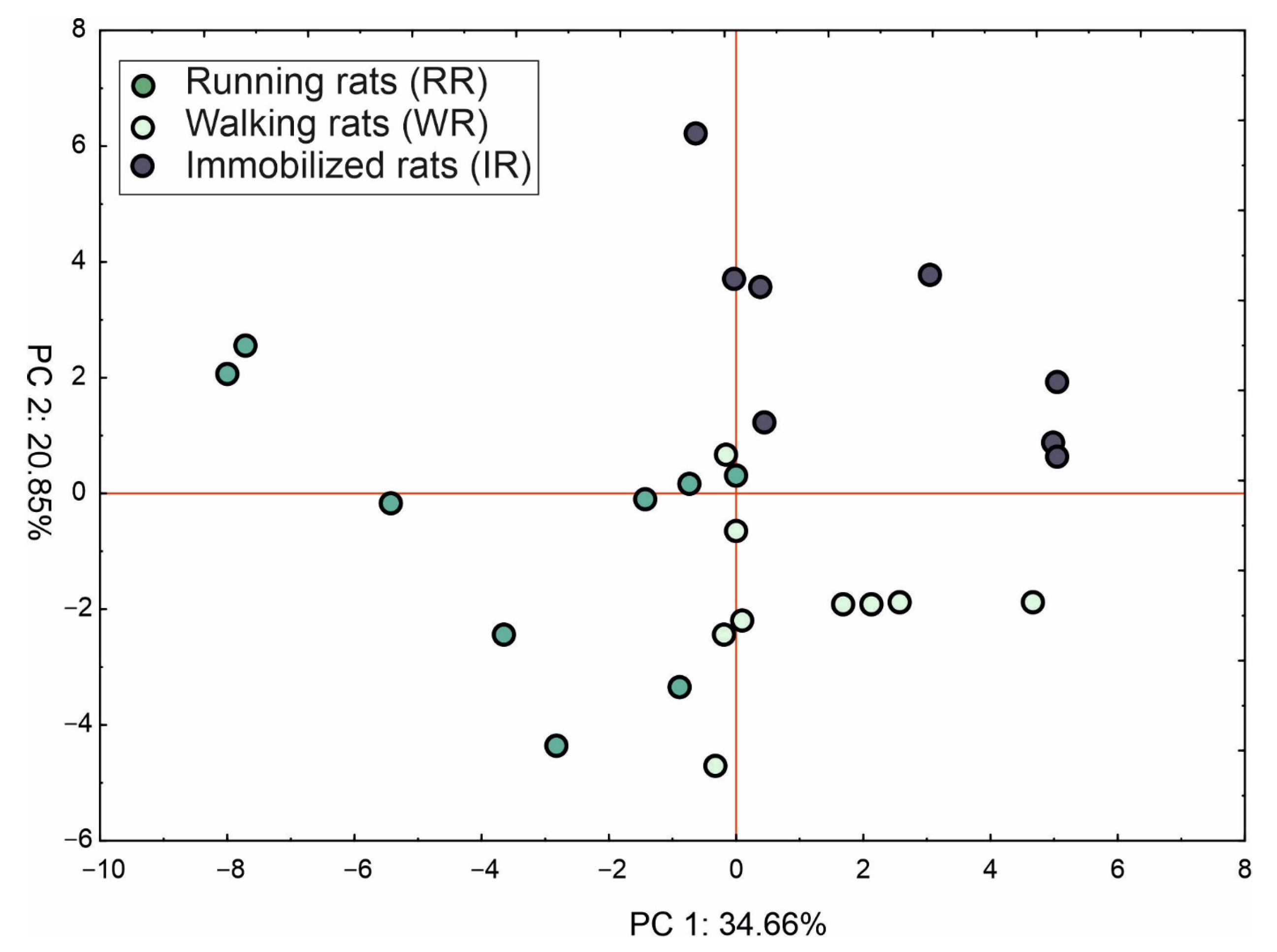
2.4. Principle Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Animal
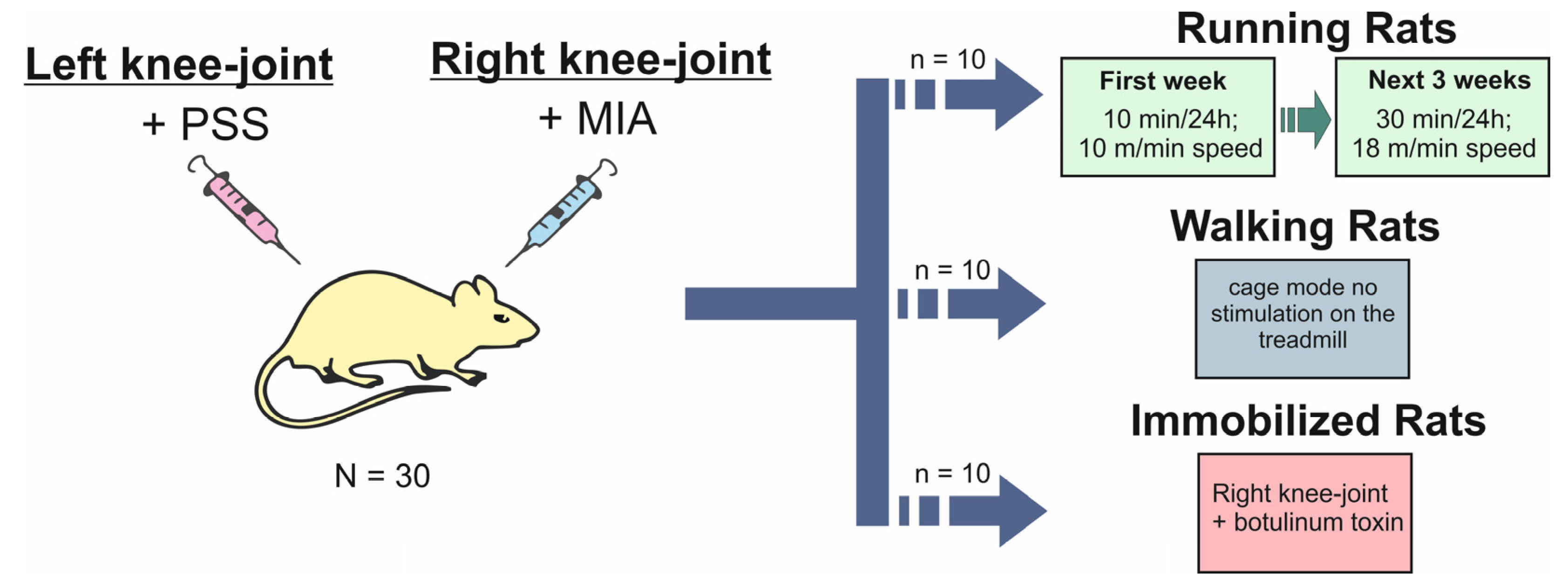
4.2. Experimental Design
4.3. Radiological Examination
4.4. Body Composition Assessment
4.5. Statistical Analysis and Principle Component Analysis (PCA)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jarecki, J.; Małecka-Massalska, T.; Polkowska, I.; Potoczniak, B.; Kosior-Jarecka, E.; Szerb, I.; Tomaszewska, E.; Gutbier, M.; Dobrzyński, M.; Blicharski, T. Level of Adiponectin, Leptin and Selected Matrix Metalloproteinases in Female Overweight Patients with Primary Gonarthrosis. J. Clin. Med. 2021, 10, 1263. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, R. Knee joint osteoarthritis diagnosis based on selected acoustic signal discriminants using machine learning. Appl. Comput. Sci. 2022, 18, 71–85. [Google Scholar] [CrossRef]
- Krakowski, P.; Karpiński, R.; Maciejewski, R.; Jonak, J.; Jurkiewicz, A. Short-Term Effects of Arthroscopic Microfracturation of Knee Chondral Defects in Osteoarthritis. Appl. Sci. 2020, 10, 8312. [Google Scholar] [CrossRef]
- Englund, M.; Guermazi, A.; Lohmander, S.L. The Role of the Meniscus in Knee Osteoarthritis: A Cause or Consequence? Radiol. Clin. N. Am. 2009, 47, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Donell, S. Subchondral Bone Remodelling in Osteoarthritis. EFORT Open Rev. 2019, 4, 221–229. [Google Scholar] [CrossRef]
- Belluzzi, E.; Macchi, V.; Fontanella, C.; Carniel, E.; Olivotto, E.; Filardo, G.; Sarasin, G.; Porzionato, A.; Granzotto, M.; Pozzuoli, A.; et al. Infrapatellar Fat Pad Gene Expression and Protein Production in Patients with and without Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 6016. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Dainese, P.; Wyngaert, K.V.; De Mits, S.; Wittoek, R.; Van Ginckel, A.; Calders, P. Association between Knee Inflammation and Knee Pain in Patients with Knee Osteoarthritis: A Systematic Review. Osteoarthr. Cartil. 2022, 30, 516–534. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, R.; McCullough, D.; Butler, T.; Perez de Heredia, F.; Davies, I.G.; Stewart, C. Sarcopenia during COVID-19 Lockdown Restrictions: Long-Term Health Effects of Short-Term Muscle Loss. GeroScience 2020, 42, 1547–1578. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.C. Osteoporosis in Men: A Review of Endogenous Sex Hormones and Testosterone Replacement Therapy. J. Pharm. Pr. 2011, 24, 307–315. [Google Scholar] [CrossRef]
- Halloran, B.P.; Bikle, D.D.; Harris, J.; Autry, C.P.; Currier, P.A.; Tanner, S.; Patterson-Buckendahl, P.; Morey-Holton, E. Skeletal Unloading Induces Selective Resistance to the Anabolic Actions of Growth Hormone on Bone. J. Bone Miner. Res. 1995, 10, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.R. Proinflammatory Cytokines and Osteoporosis. Curr. Osteoporos. Rep. 2009, 7, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Cruz-Jentoft, A.J.; Maggi, S. Sarcopenia and Fragility Fractures. Eur. J. Phys. Rehabil. Med. 2013, 49, 111–117. [Google Scholar] [PubMed]
- Siddiq, A.; Ansari, M.O.; Mohammad, A.; Mohammad, F.; El-Desoky, G.E. Synergistic Effect of Polyaniline Modified Silica Gel for Highly Efficient Separation of Non Resolvable Amino Acids. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 277–281. [Google Scholar] [CrossRef]
- Jarecki, J.; Sobiech, M.; Turżańska, K.; Tomczyk-Warunek, A.; Jabłoński, M. A Kinesio Taping Method Applied in the Treatment of Postsurgical Knee Swelling after Primary Total Knee Arthroplasty. J. Clin. Med. 2021, 10, 2992. [Google Scholar] [CrossRef] [PubMed]
- Krakowski, P.; Karpiński, R.; Jojczuk, M.; Nogalska, A.; Jonak, J. Knee MRI Underestimates the Grade of Cartilage Lesions. Appl. Sci. 2021, 11, 1552. [Google Scholar] [CrossRef]
- L’Hermette, M.F.; Tourny-Chollet, C.; Polle, G.; Dujardin, F.H. Articular Cartilage, Degenerative Process, and Repair: Current Progress. Int. J. Sports Med. 2006, 27, 738–744. [Google Scholar] [CrossRef]
- Eckstrom, E.; Neukam, S.; Kalin, L.; Wright, J. Physical Activity and Healthy Aging. Clin. Geriatr. Med. 2020, 36, 671–683. [Google Scholar] [CrossRef]
- Messina, O.D.; Vidal Wilman, M.; Vidal Neira, L.F. Nutrition, Osteoarthritis and Cartilage Metabolism. Aging Clin. Exp. Res. 2019, 31, 807–813. [Google Scholar] [CrossRef]
- Daste, C.; Kirren, Q.; Akoum, J.; Lefèvre-Colau, M.-M.; Rannou, F.; Nguyen, C. Physical Activity for Osteoarthritis: Efficiency and Review of Recommandations. Jt. Bone Spine 2021, 88, 105207. [Google Scholar] [CrossRef]
- Berenbaum, F.; Wallace, I.J.; Lieberman, D.E.; Felson, D.T. Modern-Day Environmental Factors in the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2018, 14, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Summary Comments. Am. J. Clin. Nutr. 1989, 50, 1231–1233. [Google Scholar] [CrossRef]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical Definition of Sarcopenia. Clin. Cases Miner Bone. Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J. Sarcopenia, the Last Organ Insufficiency. Eur. Geriatr. Med. 2016, 7, 195–196. [Google Scholar] [CrossRef]
- Papalia, R.; Zampogna, B.; Torre, G.; Lanotte, A.; Vasta, S.; Albo, E.; Tecame, A.; Denaro, V. Sarcopenia and Its Relationship with Osteoarthritis: Risk Factor or Direct Consequence? Musculoskelet. Surg. 2014, 98, 9–14. [Google Scholar] [CrossRef]
- Li, C.; Yu, K.; Shyh-Chang, N.; Li, G.; Jiang, L.; Yu, S.; Xu, L.; Liu, R.; Guo, Z.; Xie, H.; et al. Circulating Factors Associated with Sarcopenia during Ageing and after Intensive Lifestyle Intervention. J. Cachexia Sarcopenia Muscle 2019, 10, 586–600. [Google Scholar] [CrossRef]
- Scott, D.; Blizzard, L.; Fell, J.; Jones, G. Prospective Study of Self-Reported Pain, Radiographic Osteoarthritis, Sarcopenia Progression, and Falls Risk in Community-Dwelling Older Adults. Arthritis Care Res. 2012, 64, 30–37. [Google Scholar] [CrossRef]
- Grad, S.; Eglin, D.; Alini, M.; Stoddart, M.J. Physical Stimulation of Chondrogenic Cells In Vitro: A Review. Clin. Orthop. Relat. Res. 2011, 469, 2764–2772. [Google Scholar] [CrossRef]
- Rannou, F.; Lee, T.-S.; Zhou, R.-H.; Chin, J.; Lotz, J.C.; Mayoux-Benhamou, M.-A.; Barbet, J.P.; Chevrot, A.; Shyy, J.Y.-J. Intervertebral Disc Degeneration. Am. J. Pathol. 2004, 164, 915–924. [Google Scholar] [CrossRef]
- Lee, S.; Kim, T.-N.; Kim, S.-H. Sarcopenic Obesity Is More Closely Associated with Knee Osteoarthritis than Is Nonsarcopenic Obesity: A Cross-Sectional Study. Arthritis Rheum. 2012, 64, 3947–3954. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Ro, H.J.; Chung, S.G.; Kang, S.H.; Seo, K.M.; Kim, D.-K. Low Skeletal Muscle Mass in the Lower Limbs Is Independently Associated to Knee Osteoarthritis. PLoS ONE 2016, 11, e0166385. [Google Scholar] [CrossRef] [PubMed]
- Toda, Y.; Segal, N.; Toda, T.; Kato, A.; Toda, F. A Decline in Lower Extremity Lean Body Mass per Body Weight Is Characteristic of Women with Early Phase Osteoarthritis of the Knee. J. Rheumatol. 2000, 27, 2449–2454. [Google Scholar] [PubMed]
- Veronese, N.; Stefanac, S.; Koyanagi, A.; Al-Daghri, N.M.; Sabico, S.; Cooper, C.; Rizzoli, R.; Reginster, J.-Y.; Barbagallo, M.; Dominguez, L.J.; et al. Lower Limb Muscle Strength and Muscle Mass Are Associated with Incident Symptomatic Knee Osteoarthritis: A Longitudinal Cohort Study. Front. Endocrinol. 2021, 12, 804560. [Google Scholar] [CrossRef]
- Peng, H.; Zeng, Y. Research progress on the correlation between sarcopenia and osteoarthritis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2022, 36, 1549–1557. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Karpiński, R.; Krakowski, P.; Jonak, J.; Machrowska, A.; Maciejewski, M.; Nogalski, A. Diagnostics of Articular Cartilage Damage Based on Generated Acoustic Signals Using ANN—Part I: Femoral-Tibial Joint. Sensors 2022, 22, 2176. [Google Scholar] [CrossRef]
- Bove, S.E.; Calcaterra, S.L.; Brooker, R.M.; Huber, C.M.; Guzman, R.E.; Juneau, P.L.; Schrier, D.J.; Kilgore, K.S. Weight Bearing as a Measure of Disease Progression and Efficacy of Anti-Inflammatory Compounds in a Model of Monosodium Iodoacetate-Induced Osteoarthritis. Osteoarthr. Cartil. 2003, 11, 821–830. [Google Scholar] [CrossRef]
- Guingamp, C.; Gegout-Pottie, P.; Philippe, L.; Terlain, B.; Netter, P.; Gillet, P. Mono-Iodoacetate-Induced Experimental Osteoarthritis. A Dose-Response Study of Loss of Mobility, Morphology, and Biochemistry. Arthritis Rheum. 1997, 40, 1670–1679. [Google Scholar] [CrossRef]
- Orita, S.; Ishikawa, T.; Miyagi, M.; Ochiai, N.; Inoue, G.; Eguchi, Y.; Kamoda, H.; Arai, G.; Toyone, T.; Aoki, Y.; et al. Pain-Related Sensory Innervation in Monoiodoacetate-Induced Osteoarthritis in Rat Knees That Gradually Develops Neuronal Injury in Addition to Inflammatory Pain. BMC Musculoskelet. Disord. 2011, 12, 134. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Imaizumi, R.; Sumichika, H.; Tanaka, H.; Goda, M.; Fukunari, A.; Komatsu, H. Sodium Iodoacetate-Induced Experimental Osteoarthritis and Associated Pain Model in Rats. J. Vet. Med. Sci. 2003, 65, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Gomes, J.; Adães, S.; Sousa, R.M.; Mendonça, M.; Castro-Lopes, J.M. Dose-Dependent Expression of Neuronal Injury Markers during Experimental Osteoarthritis Induced by Monoiodoacetate in the Rat. Mol. Pain 2012, 8, 1744–8069-8–50. [Google Scholar] [CrossRef]
- Pereira, F.B.; Leite, A.F.; de Paula, A.P. Relationship between Pre-Sarcopenia, Sarcopenia and Bone Mineral Density in Elderly Men. Arch. Endocrinol. Metab. 2015, 59, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Verschueren, S.; Gielen, E.; O’Neill, T.W.; Pye, S.R.; Adams, J.E.; Ward, K.A.; Wu, F.C.; Szulc, P.; Laurent, M.; Claessens, F.; et al. Sarcopenia and Its Relationship with Bone Mineral Density in Middle-Aged and Elderly European Men. Osteoporos. Int. 2013, 24, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Sarcopenia: Origins and Clinical Relevance. J. Nutr. 1997, 127 (Suppl. S5), 990S–991S. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Fisher, N.M.; White, S.C.; Yack, H.J.; Smolinski, R.J.; Pendergast, D.R. Muscle Function and Gait in Patients with Knee Osteoarthritis before and after Muscle Rehabilitation. Disabil. Rehabil. 1997, 19, 47–55. [Google Scholar] [CrossRef]
- Isaac, C.; Wright, A.; Usas, A.; Li, H.; Tang, Y.; Mu, X.; Greco, N.; Dong, Q.; Vo, N.; Kang, J.; et al. Dystrophin and Utrophin “Double Knockout” Dystrophic Mice Exhibit a Spectrum of Degenerative Musculoskeletal Abnormalities. J. Orthop. Res. 2013, 31, 343–349. [Google Scholar] [CrossRef]
- de Silva, J.M.S.; Alabarse, P.V.G.; Teixeira, V.d.O.N.; Freitas, E.C.; de Oliveira, F.H.; Chakr, R.M.d.S.; Xavier, R.M. Muscle Wasting in Osteoarthritis Model Induced by Anterior Cruciate Ligament Transection. PLoS ONE 2018, 13, e0196682. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Toda, Y.; Kobayashi, T. The Usefulness of Walking for Preventing Sarcopenia in Dieting Postmenopausal Women Complaining of Knee Pain. Ann. N. Y. Acad. Sci. 2000, 904, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Sίrca, A.; Susěc-Michieli, M. Selective Type II Fibre Muscular Atrophy in Patients with Osteoarthritis of the Hip. J. Neurol. Sci. 1980, 44, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low Relative Skeletal Muscle Mass (Sarcopenia) in Older Persons Is Associated with Functional Impairment and Physical Disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Hurley, M.V. The Role of Muscle Weakness in the Pathogenesis of Osteoarthritis. Rheum. Dis. Clin. N. Am. 1999, 25, 283–298. [Google Scholar] [CrossRef]
- Rice, D.A.; McNair, P.J. Quadriceps Arthrogenic Muscle Inhibition: Neural Mechanisms and Treatment Perspectives. Semin. Arthritis Rheum. 2010, 40, 250–266. [Google Scholar] [CrossRef]
- Zachařová, G.; Knotková-Urbancová, H.; Hník, P.; Soukup, T. Nociceptive Atrophy of the Rat Soleus Muscle Induced by Bone Fracture: A Morphometric Study. J. Appl. Physiol. 1997, 82, 552–557. [Google Scholar] [CrossRef]
- Hurley, M.V.; Scott, D.L.; Rees, J.; Newham, D.J. Sensorimotor Changes and Functional Performance in Patients with Knee Osteoarthritis. Ann. Rheum. Dis. 1997, 56, 641–648. [Google Scholar] [CrossRef]
- Pap, G.; Machner, A.; Awiszus, F. Strength and Voluntary Activation of the Quadriceps Femoris Muscle at Different Severities of Osteoarthritic Knee Joint Damage. J. Orthop. Res. 2004, 22, 96–103. [Google Scholar] [CrossRef]
- Nakamura, T.; Suzuki, K. Muscular Changes in Osteoarthritis of the Hip and Knee. Nihon Seikeigeka Gakkai Zasshi 1992, 66, 467–475. [Google Scholar]
- Wiewiorski, M.; Dopke, K.; Steiger, C.; Valderrabano, V. Muscular Atrophy of the Lower Leg in Unilateral Post Traumatic Osteoarthritis of the Ankle Joint. Int. Orthop. (SICOT) 2012, 36, 2079–2085. [Google Scholar] [CrossRef]
- Braun, S.; Zaucke, F.; Brenneis, M.; Rapp, A.E.; Pollinger, P.; Sohn, R.; Jenei-Lanzl, Z.; Meurer, A. The Corpus Adiposum Infrapatellare (Hoffa’s Fat Pad)—The Role of the Infrapatellar Fat Pad in Osteoarthritis Pathogenesis. Biomedicines 2022, 10, 1071. [Google Scholar] [CrossRef]
- Jarecki, J.; Małecka-Masalska, T.; Kosior-Jarecka, E.; Widuchowski, W.; Krasowski, P.; Gutbier, M.; Dobrzyński, M.; Blicharski, T. Concentration of Selected Metalloproteinases and Osteocalcin in the Serum and Synovial Fluid of Obese Women with Advanced Knee Osteoarthritis. Int. J. Environ. Res. Public Health 2022, 19, 3530. [Google Scholar] [CrossRef] [PubMed]
- Hart, H.F.; Culvenor, A.G.; Patterson, B.E.; Doshi, A.; Vora, A.; Guermazi, A.; Birmingham, T.B.; Crossley, K.M. Infrapatellar Fat Pad Volume and Hoffa-synovitis after ACL Reconstruction: Association with Early Osteoarthritis Features and Pain over 5 Years. J. Orthop. Res. 2022, 40, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Fontanella, C.G.; Belluzzi, E.; Pozzuoli, A.; Scioni, M.; Olivotto, E.; Reale, D.; Ruggieri, P.; De Caro, R.; Ramonda, R.; Carniel, E.L.; et al. Exploring Anatomo-Morphometric Characteristics of Infrapatellar, Suprapatellar Fat Pad, and Knee Ligaments in Osteoarthritis Compared to Post-Traumatic Lesions. Biomedicines 2022, 10, 1369. [Google Scholar] [CrossRef] [PubMed]


| Limb | RR | p | WR | p | IR | p | |
|---|---|---|---|---|---|---|---|
| Area (cm2) | R | 6.23 ± 0.74 | 0.9405 | 6.40 ± 0.41 | 0.9618 | 6.10 ± 0.72 | 0.1259 |
| L | 6.20 ± 0.90 | 6.42 ± 0.68 | 6.57 ± 0.78 | ||||
| BMC (g) | R | 0.95 ± 0.08 | 0.8007 | 0.97 ± 0.08 | 0.4211 | 0.77 ± 0.09 | 0.0231 * |
| L | 0.95 ± 0.11 | 0.95 ± 0.12 | 0.88 ± 0.12 | ||||
| BMD (g/cm2) | R | 0.15 ± 0.02 | 0.9861 | 0.15 ± 0.009 | 0.0534 ^ | 0.12 ± 0.008 | 0.0359 * |
| L | 0.15 ± 0.02 | 0.14 ± 0.01 | 0.13 ± 0.007 | ||||
| Fat mass (g) | R | 4.07 ± 1.45 | 0.4100 | 3.14 ± 0.72 | 0.1376 | 4.16 ± 1.12 | 0.3384 |
| L | 3.88 ± 0.97 | 2.67 ± 0.74 | 4.52 ± 1.6 | ||||
| Fat-free mass (g) | R | 23.7 ± 2.68 | 0.2525 | 23.76 ± 2.84 | 0.1036 | 20.24 ± 3.33 | 0.0120 * |
| L | 22.75 ± 2.27 | 21.77 ± 2.15 | 23.62 ± 4.54 | ||||
| Mass (g) | R | 28.19 ± 4.93 | 0.3600 | 26.86 ± 3.04 | 0.0960 | 24.42 ± 4.08 | 0.0200 * |
| L | 26.94 ± 2.59 | 23.87 ± 3.28 | 28.12 ± 6.01 | ||||
| Fat (%) | R | 14.15 ± 3.25 | 0.4900 | 11.62 ± 2.07 | 0.3740 | 16.91 ± 3.04 | 0.1418 |
| L | 14.37 ± 3.43 | 11.02 ± 1.78 | 15.65 ± 2.8 |
| Knee Joint | RR | p | WR | p | IR | p | |
|---|---|---|---|---|---|---|---|
| Area (cm2) | R | 2.18 ± 0.18 | 0.4443 | 2.12 ± 0.24 | 0.125 | 1.90 ± 0.15 | 0.0073 * |
| L | 2.26 ± 0.28 | 2.31 ± 0.35 | 2.2 ± 0.24 | ||||
| BMC (g) | R | 0.37 ± 0.07 | 0.7950 | 0.32 ± 0.04 | 0.5062 | 0.2 ± 0.02 | 0.0001 * |
| L | 0.39 ± 0.13 | 0.33 ± 0.05 | 0.28 ± 0.04 | ||||
| BMD (g/cm2) | R | 0.17 ± 0.03 | 0.9679 | 0.15 ± 0.01 | 0.3992 | 0.11 ± 0.01 | 0.0008 * |
| L | 0.17 ± 0.05 | 0.15 ± 0.01 | 0.13 ± 0.01 | ||||
| Fat mass (g) | R | 1.11 ± 0.38 | 0.7912 | 0.65 ± 0.22 | 0.0462 * | 0.95 ± 0.29 | 0.0011 * |
| L | 1.23 ± 1.04 | 0.48 ± 0.09 | 0.6 ± 0.2 | ||||
| Fat-free mass (g) | R | 7.07 ± 2.49 | 0.9950 | 5.75 ± 1.33 | 0.2948 | 4.62 ± 0.6 | 0.3397 |
| L | 7.37 ± 3.86 | 5.24 ± 1.08 | 4.28 ± 0.58 | ||||
| Mass (g) | R | 8.25 ± 2.58 | 0.700 | 6.56 ± 1.41 | 0.1100 | 5.48 ± 0.54 | 0.2400 |
| L | 8.31 ± 4.62 | 5.8 ± 1.11 | 5.01 ± 0.73 | ||||
| Fat (%) | R | 13.6 ± 3.72 | 0.4100 | 10.19 ± 2.88 | 0.0430 * | 17.15 ± 5.00 | 0.002 * |
| L | 12.87 ± 3.51 | 8.39 ± 1.51 | 10.95 ± 2.47 |
| RR | WR | IR | p WR vs. RR | p RR vs. IR | p WR vs. IR | ||
|---|---|---|---|---|---|---|---|
| Area (cm2) | Limb | 6.23 ± 0.74 | 6.40 ± 0.41 | 6.10 ± 0.72 | 0.3900 | 0.5300 | 0.5200 |
| Knee joint | 2.18 ± 0.18 | 2.12 ± 0.24 | 1.90 ± 0.15 | 0.3103 | 0.0010 * | 0.0990 | |
| BMC (g) | Limb | 0.95 ± 0.08 | 0.97 ± 0.08 | 0.77 ± 0.09 | 0.7041 | 0.0006 * | 0.0087 * |
| Knee joint | 0.37 ± 0.07 | 0.32 ± 0.04 | 0.20 ± 0.02 | 0.2573 | 0.0006 * | 0.0013 * | |
| BMD (g/cm2) | Limb | 0.15 ± 0.02 | 0.15 ± 0.009 | 0.12 ± 0.008 | 0.6048 | 0.0131 * | 0.0005 * |
| Knee joint | 0.17 ± 0.03 | 0.15 ± 0.01 | 0.11 ± 0.01 | 0.3849 | 0.0079 * | 0.0003 * | |
| Fat mass (g) | Limb | 4.07 ± 1.45 | 3.14 ± 0.72 | 4.16 ± 1.12 | 0.1200 | 0.8225 | 0.0093 * |
| Knee joint | 1.11 ± 0.38 | 0.65 ± 0.22 | 0.95 ± 0.29 | 0.0284 * | 0.7970 | 0.0228 * | |
| Fat-free mass (g) | Limb | 23.70 ± 2.68 | 23.76 ± 2.84 | 20.24 ± 3.33 | 0.9339 | 0.1591 | 0.1385 |
| Knee joint | 7.07 ± 2.49 | 5.75 ± 1.33 | 4.62 ± 0.6 | 0.3858 | 0.0202 * | 0.0608 ^ | |
| Mass (g) | Limb | 28.19 ± 4.93 | 26.86 ± 3.04 | 24.42 ± 4.08 | 0.4000 | 0.2500 | 0.5800 |
| Knee joint | 8.25 ± 2.58 | 6.56 ± 1.41 | 5.48 ± 0.54 | 0.2800 | 0.0080 * | 0.0700 ^ | |
| Fat (%) | Limb | 14.15 ± 3.25 | 11.62 ± 2.07 | 16.91 ± 3.04 | 0.0875 | 0.0972 | 0.0004 * |
| Knee joint | 13.6 ± 3.72 | 10.19 ± 2.88 | 17.15 ± 5.00 | 0.2849 | 0.0234 * | 0.1531 |
| Grade | Severity of OA |
|---|---|
| Grade 0 | definite absence of X-ray changes of osteoarthritis |
| Grade I | doubtful joint space narrowing and possible osteophytic lipping |
| Grade II | definite osteophytes and possible joint space narrowing |
| Grade III | moderate multiple osteophytes, definite narrowing of joint space and some sclerosis and possible deformity of bone ends |
| Grade IV | large osteophytes, marked narrowing of joint space, severe sclerosis and definite deformity of bone ends |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarecki, J.; Polkowska, I.; Kazimierczak, W.; Wójciak, M.; Sowa, I.; Dresler, S.; Blicharski, T. Assessment of the Impact of Physical Activity on the Musculoskeletal System in Early Degenerative Knee Joint Lesions in an Animal Model. Int. J. Mol. Sci. 2023, 24, 3540. https://doi.org/10.3390/ijms24043540
Jarecki J, Polkowska I, Kazimierczak W, Wójciak M, Sowa I, Dresler S, Blicharski T. Assessment of the Impact of Physical Activity on the Musculoskeletal System in Early Degenerative Knee Joint Lesions in an Animal Model. International Journal of Molecular Sciences. 2023; 24(4):3540. https://doi.org/10.3390/ijms24043540
Chicago/Turabian StyleJarecki, Jaromir, Izabela Polkowska, Waldemar Kazimierczak, Magdalena Wójciak, Ireneusz Sowa, Sławomir Dresler, and Tomasz Blicharski. 2023. "Assessment of the Impact of Physical Activity on the Musculoskeletal System in Early Degenerative Knee Joint Lesions in an Animal Model" International Journal of Molecular Sciences 24, no. 4: 3540. https://doi.org/10.3390/ijms24043540







