Increased Expression of Hepatic Stearoyl-CoA Desaturase (SCD)-1 and Depletion of Eicosapentaenoic Acid (EPA) Content following Cytotoxic Cancer Therapy Are Reversed by Dietary Fish Oil
Abstract
1. Introduction
2. Results
2.1. General Findings
2.2. Liver Triglyceride (TG) and Phospholipid (PL) Fatty Acids
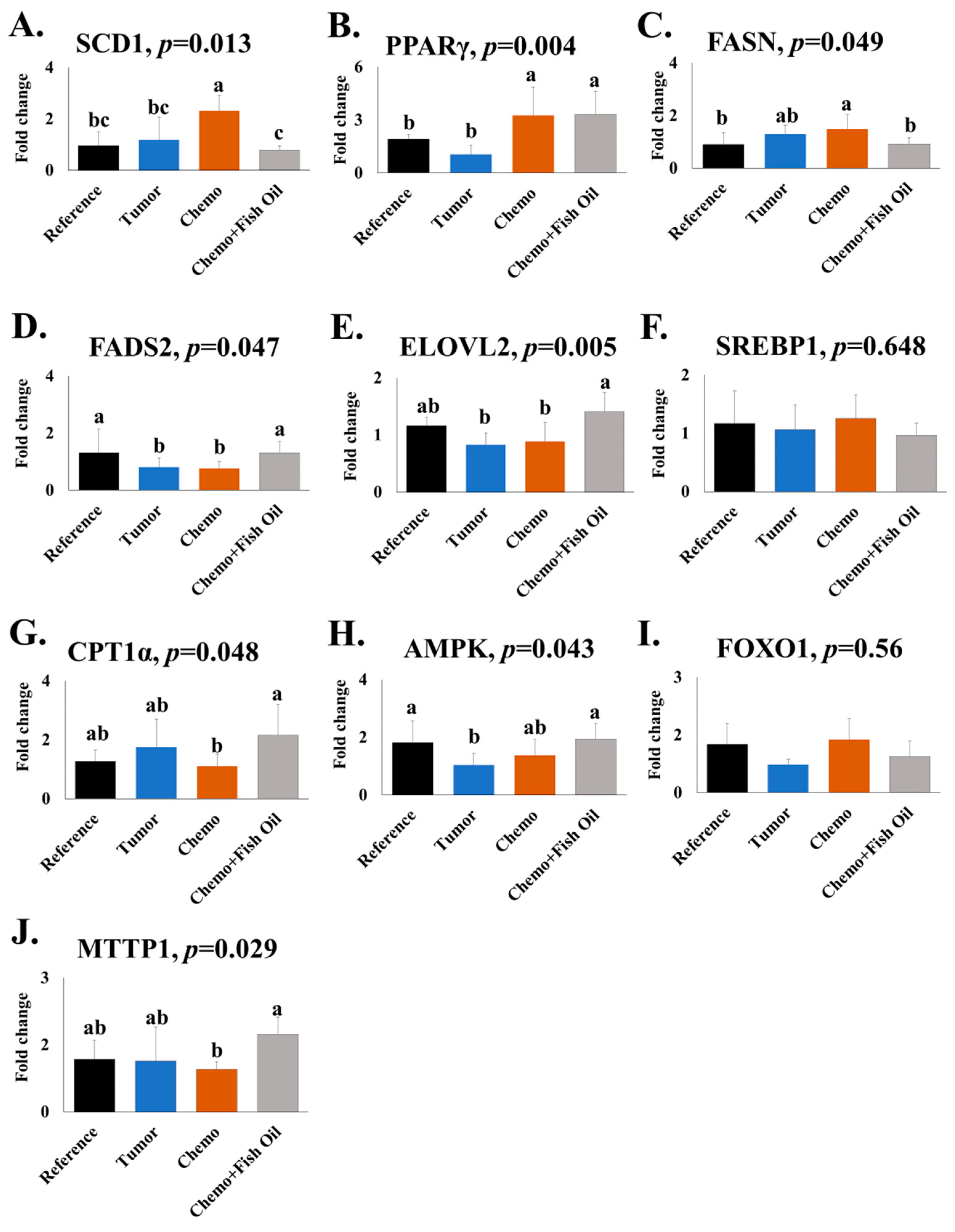
2.3. Gene Expression
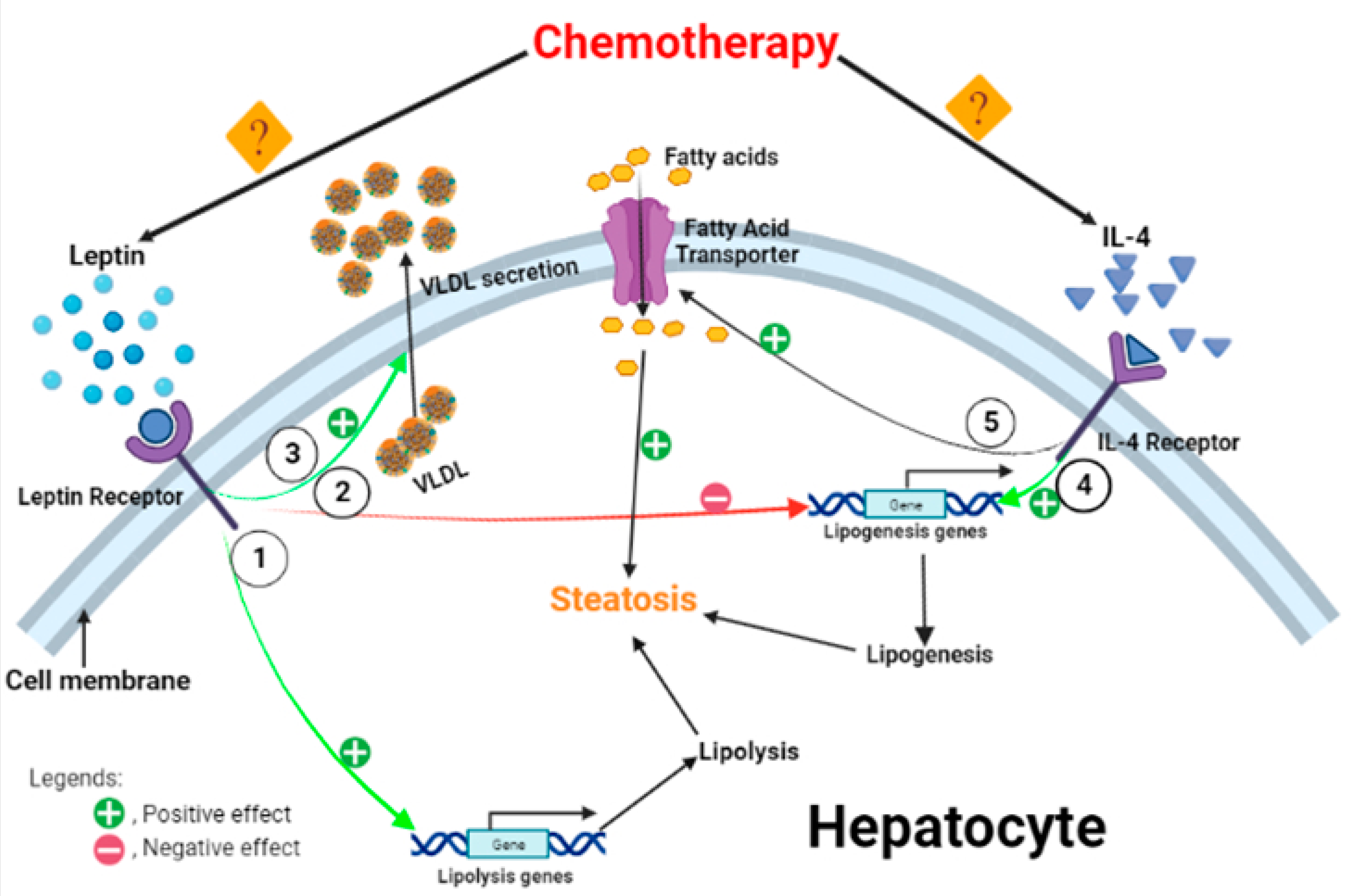
2.4. Hepatic Leptin and Interleukin (IL)-4
3. Discussion
4. Materials and Methods
4.1. Animal Handling, Diet, and Experimental Design
4.2. Fatty Acid Analysis
4.3. RNA Preparation and Gene Expression
4.4. Determination of Leptin and IL-4
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, L.; Bryan, S.; De, P. Canadian Cancer Statistics Advisory Committee; Canadian Cancer Society: Toronto, ON, Canada, 2018. [Google Scholar]
- Fenton, H.M.; Taylor, J.C.; Lodge, J.P.A.; Toogood, G.J.; Finan, P.J.; Young, A.L.; Morris, E.J.A. Variation in the Use of Resection for Colorectal Cancer Liver Metastases. Ann. Surg. 2019, 270, 892–898. [Google Scholar] [CrossRef]
- Ito, K.; Govindarajan, A.; Ito, H.; Fong, Y. Surgical treatment of hepatic colorectal metastasis: Evolving role in the setting of improving systemic therapies and ablative treatments in the 21st century. Cancer J. 2010, 16, 103–110. [Google Scholar] [CrossRef]
- Vauthey, J.-N.; Choti, M.A.; Helton, W.S. AHPBA/SSO/SSAT Consensus Conference on hepatic colorectal metastases: Rationale and overview of the conference. Ann. Surg. Oncol. 2006, 13, 1259–1260. [Google Scholar] [CrossRef] [PubMed]
- Gangi, A.; Lu, S.C. Chemotherapy-associated liver injury in colorectal cancer. Ther. Adv. Gastroenterol. 2020, 13, 1756284820924194. [Google Scholar] [CrossRef]
- Dyson, J.; McPherson, S.; Anstee, Q. Non-alcoholic fatty liver disease: Non-invasive investigation and risk stratification. J. Clin. Pathol. 2013, 66, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 2018, 68, 280–295. [Google Scholar] [CrossRef]
- Mulhall, B.P.; Ong, J.P.; Younossi, Z.M. Non-alcoholic fatty liver disease: An overview. J. Gastroenterol. Hepatol. 2002, 17, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.P.; Younossi, Z.M. Epidemiology and natural history of NAFLD and NASH. Clin. Liver Dis. 2007, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pessayre, D.; Berson, A.; Fromenty, B.; Mansouri, A. Mitochondria in steatohepatitis. In Seminars in Liver Disease; Thieme Medical Publishers, Inc.: Stuttgart, Germany, 2001; pp. 057–070. [Google Scholar]
- Laurent, A.; Nicco, C.; Tran Van Nhieu, J.; Borderie, D.; Chéreau, C.; Conti, F.; Jaffray, P.; Soubrane, O.; Calmus, Y.; Weill, B. Pivotal role of superoxide anion and beneficial effect of antioxidant molecules in murine steatohepatitis. Hepatology 2004, 39, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Veteläinen, R.; van Vliet, A.; Gouma, D.J.; van Gulik, T.M. Steatosis as a risk factor in liver surgery. Ann. Surg. 2007, 245, 20. [Google Scholar] [CrossRef] [PubMed]
- Lieffers, J.R.; Mourtzakis, M.; Hall, K.D.; McCargar, L.J.; Prado, C.M.; Baracos, V.E. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: Contributions of organ and tumor mass to whole-body energy demands. Am. J. Clin. Nutr. 2009, 89, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.A.; Wilke, M.S.; Perrine, M.; Pawlowicz, M.; Mourtzakis, M.; Lieffers, J.R.; Maneshgar, M.; Bruera, E.; Clandinin, M.T.; Baracos, V.E.; et al. Loss of adipose tissue and plasma phospholipids: Relationship to survival in advanced cancer patients. Clin. Nutr. 2010, 29, 482–487. [Google Scholar] [CrossRef]
- Xue, H.; Sawyer, M.B.; Field, C.J.; Dieleman, L.A.; Baracos, V.E. Nutritional modulation of antitumor efficacy and diarrhea toxicity related to irinotecan chemotherapy in rats bearing the ward colon tumor. Clin. Cancer Res. 2007, 13, 7146–7154. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, A.S.; Anoveros-Barrera, A.; Dunichand-Hoedl, A.; Martins, K.; Bigam, D.; Khadaroo, R.G.; McMullen, T.; Bathe, O.F.; Putman, C.T.; Clandinin, M.T. Lipid is heterogeneously distributed in muscle and associates with low radiodensity in cancer patients. J. Cachexia Sarcopenia Muscle 2020, 11, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Pratt, V.; Watanabe, S.; Bruera, E.; Mackey, J.; Clandinin, M.; Baracos, V.E.; Field, C. Plasma and neutrophil fatty acid composition in advanced cancer patients and response to fish oil supplementation. Br. J. Cancer 2002, 87, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Monirujjaman, M.; Pant, A.; Nelson, R.; Bathe, O.; Jacobs, R.; Mazurak, V.C. Alterations in hepatic fatty acids reveal depletion of total polyunsaturated fatty acids following irinotecan plus 5-fluorouracil treatment in an animal model of colorectal cancer. Prostaglandins Leukot. Essent. Fat. Acids 2021, 174, 102359. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Leptin in nonalcoholic fatty liver disease: A narrative review. Metabolism 2015, 64, 60–78. [Google Scholar] [CrossRef]
- Lin, T.-C.; Hsiao, M. Leptin and cancer: Updated functional roles in carcinogenesis, therapeutic niches, and developments. Int. J. Mol. Sci. 2021, 22, 2870. [Google Scholar] [CrossRef]
- Yang, C.-P.; Shiau, M.-Y.; Lai, Y.-R.; Ho, K.-T.; Hsiao, C.-W.; Chen, C.-J.; Lo, Y.-L.; Chang, Y.-H. Interleukin-4 Boosts Insulin-Induced Energy Deposits by Enhancing Glucose Uptake and Lipogenesis in Hepatocytes. Oxidative Med. Cell. Longev. 2018, 2018, 6923187. [Google Scholar] [CrossRef]
- Albracht-Schulte, K.; Kalupahana, N.S.; Ramalingam, L.; Wang, S.; Rahman, S.M.; Robert-McComb, J.; Moustaid-Moussa, N. Omega-3 fatty acids in obesity and metabolic syndrome: A mechanistic update. J. Nutr. Biochem. 2018, 58, 1–16. [Google Scholar] [CrossRef]
- Lombardo, Y.B.; Chicco, A.G. Effects of dietary polyunsaturated n-3 fatty acids on dyslipidemia and insulin resistance in rodents and humans. A review. J. Nutr. Biochem. 2006, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sangouni, A.A.; Orang, Z.; Mozaffari-Khosravi, H. Effect of omega-3 supplementation on fatty liver and visceral adiposity indices in diabetic patients with non-alcoholic fatty liver disease: A randomized controlled trial. Clin. Nutr. ESPEN 2021, 44, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Scorletti, E.; Byrne, C.D. Omega-3 fatty acids and non-alcoholic fatty liver disease: Evidence of efficacy and mechanism of action. Mol. Asp. Med. 2018, 64, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Orang, Z.; Mohsenpour, M.A.; Mozaffari-Khosravi, H. Effect of Omega-3 fatty acid supplementation on inflammatory markers and insulin resistance indices in patient with type 2 diabetes and nonalcoholic fatty liver: A randomized double-blind clinical trial. Obes. Med. 2020, 19, 100278. [Google Scholar] [CrossRef]
- Alwayn, I.P.; Gura, K.; Nosé, V.; Zausche, B.; Javid, P.; Garza, J.; Verbesey, J.; Voss, S.; Ollero, M.; Andersson, C. Omega-3 fatty acid supplementation prevents hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Pediatr. Res. 2005, 57, 445–452. [Google Scholar] [CrossRef]
- Alwayn, I.P.; Andersson, C.; Zauscher, B.; Gura, K.; Nosé, V.; Puder, M. Omega-3 fatty acids improve hepatic steatosis in a murine model: Potential implications for the marginal steatotic liver donor. Transplantation 2005, 79, 606–608. [Google Scholar] [CrossRef]
- Scorletti, E.; Byrne, C.D. Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annu. Rev. Nutr. 2013, 33, 231–248. [Google Scholar] [CrossRef]
- Pawlowicz, M.C. What Happens to Essential Fatty Acids in Cancer and during Chemotherapy Treatment? Master’s Thesis, University of Edmonton, Edmonton, AB, Canada, 2008. [Google Scholar]
- Lee, M.C.; Kachura, J.J.; Vlachou, P.A.; Dzulynsky, R.; Di Tomaso, A.; Samawi, H.; Baxter, N.; Brezden-Masley, C. Evaluation of Adjuvant Chemotherapy-Associated Steatosis (CAS) in Colorectal Cancer. Curr. Oncol. 2021, 28, 3030–3040. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Popescu, L.A.; Virgolici, B.; Lixandru, D.; Miricescu, D.; Condruţ, E.; Timnea, O.; Ranetti, A.; Militaru, M.; Mohora, M.; Zăgrean, L. Effect of diet and omega-3 fatty acids in NAFLD. Rom. J. Morphol. Embryol. 2013, 54, 785–790. [Google Scholar]
- Bargut, T.C.L.; Frantz, E.D.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Effects of a diet rich in n-3 polyunsaturated fatty acids on hepatic lipogenesis and beta-oxidation in mice. Lipids 2014, 49, 431–444. [Google Scholar] [CrossRef] [PubMed]
- De Castro, G.S.; Cardoso, J.F.R.; Calder, P.C.; Jordão, A.A.; Vannucchi, H. Fish oil decreases hepatic lipogenic genes in rats fasted and refed on a high fructose diet. Nutrients 2015, 7, 1644–1656. [Google Scholar] [CrossRef]
- Janczyk, W.; Lebensztejn, D.; Wierzbicka-Rucińska, A.; Mazur, A.; Neuhoff-Murawska, J.; Matusik, P.; Socha, P. Omega-3 fatty acids therapy in children with nonalcoholic fatty liver disease: A randomized controlled trial. J. Pediatr. 2015, 166, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Šmíd, V.; Dvořák, K.; Šedivý, P.; Kosek, V.; Leníček, M.; Dezortová, M.; Hajšlová, J.; Hájek, M.; Vítek, L.; Bechyňská, K. Effect of Omega-3 Polyunsaturated Fatty Acids on Lipid Metabolism in Patients With Metabolic Syndrome and NAFLD. Hepatol. Commun. 2022, 6, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, L.; Magliocco, O.; Spampinato, D.; Piro, S.; Oliveri, C.; Alagona, C.; Papa, G.; Rabuazzo, A.; Purrello, F. Effects of n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig. Liver Dis. 2008, 40, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S.; Dasarathy, J.; Khiyami, A.; Yerian, L.; Hawkins, C.; Sargent, R.; McCullough, A.J. Double blind randomized placebo controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2015, 49, 137. [Google Scholar] [CrossRef]
- Song, B.-J.; Moon, K.-H.; Olsson, N.U.; Salem, N., Jr. Prevention of alcoholic fatty liver and mitochondrial dysfunction in the rat by long-chain polyunsaturated fatty acids. J. Hepatol. 2008, 49, 262–273. [Google Scholar] [CrossRef]
- Sekiya, M.; Yahagi, N.; Matsuzaka, T.; Najima, Y.; Nakakuki, M.; Nagai, R.; Ishibashi, S.; Osuga, J.I.; Yamada, N.; Shimano, H. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology 2003, 38, 1529–1539. [Google Scholar] [CrossRef]
- Wada, S.; Yamazaki, T.; Kawano, Y.; Miura, S.; Ezaki, O. Fish oil fed prior to ethanol administration prevents acute ethanol-induced fatty liver in mice. J. Hepatol. 2008, 49, 441–450. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.-J.; Feng, K.; He, C.; Li, P.; Hu, Y.-J.; Su, H.; Wan, J.-B. Dietary α-linolenic acid-rich flaxseed oil prevents against alcoholic hepatic steatosis via ameliorating lipid homeostasis at adipose tissue-liver axis in mice. Sci. Rep. 2016, 6, 26826. [Google Scholar] [CrossRef]
- Huang, W.; Wang, B.; Li, X.; Kang, J.X. Endogenously elevated n-3 polyunsaturated fatty acids alleviate acute ethanol-induced liver steatosis. Biofactors 2015, 41, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-L.; Wan, J.-B.; Wang, B.; He, C.-W.; Ma, H.; Li, T.-W.; Kang, J.X. Suppression of acute ethanol-induced hepatic steatosis by docosahexaenoic acid is associated with downregulation of stearoyl-CoA desaturase 1 and inflammatory cytokines. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Matsusue, K.; Kashireddy, P.; Cao, W.-Q.; Yeldandi, V.; Yeldandi, A.V.; Rao, M.S.; Gonzalez, F.J.; Reddy, J.K. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor γ1 (PPARγ1) overexpression. J. Biol. Chem. 2003, 278, 498–505. [Google Scholar] [CrossRef]
- Larter, C.Z.; Yeh, M.M.; Williams, J.; Bell-Anderson, K.S.; Farrell, G.C. MCD-induced steatohepatitis is associated with hepatic adiponectin resistance and adipogenic transformation of hepatocytes. J. Hepatol. 2008, 49, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-L.; Hernandez-Ono, A.; Siri, P.; Weisberg, S.; Conlon, D.; Graham, M.J.; Crooke, R.M.; Huang, L.-S.; Ginsberg, H.N. Aberrant hepatic expression of PPARγ2 stimulates hepatic lipogenesis in a mouse model of obesity, insulin resistance, dyslipidemia, and hepatic steatosis. J. Biol. Chem. 2006, 281, 37603–37615. [Google Scholar] [CrossRef] [PubMed]
- Skat-Rørdam, J.; Højland Ipsen, D.; Lykkesfeldt, J.; Tveden-Nyborg, P. A role of peroxisome proliferator-activated receptor γ in non-alcoholic fatty liver disease. Basic Clin. Pharmacol. Toxicol. 2019, 124, 528–537. [Google Scholar] [CrossRef]
- Lee, J.J.; Lambert, J.E.; Hovhannisyan, Y.; Ramos-Roman, M.A.; Trombold, J.R.; Wagner, D.A.; Parks, E.J. Palmitoleic acid is elevated in fatty liver disease and reflects hepatic lipogenesis. Am. J. Clin. Nutr. 2015, 101, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Jump, D.B.; Lytle, K.A.; Depner, C.M.; Tripathy, S. Omega-3 polyunsaturated fatty acids as a treatment strategy for nonalcoholic fatty liver disease. Pharmacol. Ther. 2018, 181, 108–125. [Google Scholar] [CrossRef]
- Zhu, X.; Bian, H.; Wang, L.; Sun, X.; Xu, X.; Yan, H.; Xia, M.; Chang, X.; Lu, Y.; Li, Y. Berberine attenuates nonalcoholic hepatic steatosis through the AMPK-SREBP-1c-SCD1 pathway. Free Radic. Biol. Med. 2019, 141, 192–204. [Google Scholar] [CrossRef]
- Kotronen, A.; Seppänen-Laakso, T.; Westerbacka, J.; Kiviluoto, T.; Arola, J.; Ruskeepaa, A.-L.; Oresic, M.; Yki-Jarvinen, H. Hepatic stearoyl-CoA desaturase (SCD)-1 activity and diacylglycerol but not ceramide concentrations are increased in the nonalcoholic human fatty liver. Diabetes 2009, 58, 203–208. [Google Scholar] [CrossRef]
- Pettinelli, P.; Videla, L.A. Up-regulation of PPAR-γ mRNA expression in the liver of obese patients: An additional reinforcing lipogenic mechanism to SREBP-1c induction. J. Clin. Endocrinol. Metab. 2011, 96, 1424–1430. [Google Scholar] [CrossRef]
- Cao, B.; Liu, C.; Zhang, Q.; Dong, Y. Maternal high-fat diet leads to non-alcoholic fatty liver disease through upregulating hepatic SCD1 expression in neonate rats. Front. Nutr. 2020, 7, 581723. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, A.; Guiu-Jurado, E.; Porras, J.A.; Auguet, T. Molecular pathways in non-alcoholic fatty liver disease. Clin. Exp. Gastroenterol. 2014, 7, 221. [Google Scholar] [PubMed]
- Wei, Q.; Zhou, B.; Yang, G.; Hu, W.; Zhang, L.; Liu, R.; Li, M.; Wang, K.; Gu, H.F.; Guan, Y. JAZF1 ameliorates age and diet-associated hepatic steatosis through SREBP-1c-dependent mechanism. Cell Death Dis. 2018, 9, 859. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zeng, R.; Cao, G.; Song, Z.; Zhang, Y.; Liu, C. Vibration training triggers brown adipocyte relative protein expression in rat white adipose tissue. BioMed Res. Int. 2015, 2015, 919401. [Google Scholar] [CrossRef]
- Kohjima, M.; Enjoji, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Yada, M.; Yada, R.; Harada, N. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int. J. Mol. Med. 2007, 20, 351–358. [Google Scholar] [CrossRef]
- Smith, B.K.; Marcinko, K.; Desjardins, E.M.; Lally, J.S.; Ford, R.J.; Steinberg, G.R. Treatment of nonalcoholic fatty liver disease: Role of AMPK. Am. J. Physiol.-Endocrinol. Metab. 2016, 311, E730–E740. [Google Scholar] [CrossRef]
- Sharma, A.; Anand, S.K.; Singh, N.; Dwarkanath, A.; Dwivedi, U.N.; Kakkar, P. Berbamine induced activation of the SIRT1/LKB1/AMPK signaling axis attenuates the development of hepatic steatosis in high-fat diet-induced NAFLD rats. Food Funct. 2021, 12, 892–909. [Google Scholar] [CrossRef]
- García-Villafranca, J.; Guillén, A.; Castro, J. Ethanol consumption impairs regulation of fatty acid metabolism by decreasing the activity of AMP-activated protein kinase in rat liver. Biochimie 2008, 90, 460–466. [Google Scholar] [CrossRef]
- Tian, L.; Cao, W.; Yue, R.; Yuan, Y.; Guo, X.; Qin, D.; Xing, J.; Wang, X. Pretreatment with Tilianin improves mitochondrial energy metabolism and oxidative stress in rats with myocardial ischemia/reperfusion injury via AMPK/SIRT1/PGC-1 alpha signaling pathway. J. Pharmacol. Sci. 2019, 139, 352–360. [Google Scholar] [CrossRef]
- Berriot-Varoqueaux, N.; Aggerbeck, L.; Samson-Bouma, M.-E.; Wetterau, J. The role of the microsomal triglygeride transfer protein in abetalipoproteinemia. Annu. Rev. Nutr. 2000, 20, 663–697. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Kobayashi, H.; Ashakumary, L.; Rouyer, I.A.; Takahashi, Y.; Aoyama, T.; Hashimoto, T.; Mizugaki, M. Comparative effects of perilla and fish oils on the activity and gene expression of fatty acid oxidation enzymes in rat liver. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2000, 1485, 23–35. [Google Scholar] [CrossRef]
- Dai, J.; Liang, K.; Zhao, S.; Jia, W.; Liu, Y.; Wu, H.; Lv, J.; Cao, C.; Chen, T.; Zhuang, S. Chemoproteomics reveals baicalin activates hepatic CPT1 to ameliorate diet-induced obesity and hepatic steatosis. Proc. Natl. Acad. Sci. USA 2018, 115, E5896–E5905. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Hui, T.Y.; Young, S.G.; Davis, R.A. Blocking microsomal triglyceride transfer protein interferes with apoB secretion without causing retention or stress in the, E.R. J. Lipid Res. 2003, 44, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Björkegren, J.; Beigneux, A.; Bergo, M.O.; Maher, J.J.; Young, S.G. Blocking the secretion of hepatic very low density lipoproteins renders the liver more susceptible to toxin-induced injury. J. Biol. Chem. 2002, 277, 5476–5483. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pereira, I.V.; Stefano, J.T.; Oliveira, C.P. Microsomal triglyceride transfer protein and nonalcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 245–251. [Google Scholar] [CrossRef]
- Moon, H.-S.; Dalamaga, M.; Kim, S.-Y.; Polyzos, S.A.; Hamnvik, O.-P.; Magkos, F.; Paruthi, J.; Mantzoros, C.S. Leptin’s role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr. Rev. 2013, 34, 377–412. [Google Scholar] [CrossRef]
- Denechaud, P.-D.; Dentin, R.; Girard, J.; Postic, C. Role of ChREBP in hepatic steatosis and insulin resistance. FEBS Lett. 2008, 582, 68–73. [Google Scholar] [CrossRef]
- Jiménez-Cortegana, C.; García-Galey, A.; Tami, M.; Del Pino, P.; Carmona, I.; López, S.; Alba, G.; Sánchez-Margalet, V. Role of leptin in non-alcoholic fatty liver disease. Biomedicines 2021, 9, 762. [Google Scholar] [CrossRef]
- Wang, M.Y.; Chen, L.; Clark, G.O.; Lee, Y.; Stevens, R.D.; Ilkayeva, O.R.; Wenner, B.R.; Bain, J.R.; Charron, M.J.; Newgard, C.B. Leptin therapy in insulin-deficient type I diabetes. Proc. Natl. Acad. Sci. USA 2010, 107, 4813–4819. [Google Scholar] [CrossRef]
- Asilmaz, E.; Cohen, P.; Miyazaki, M.; Dobrzyn, P.; Ueki, K.; Fayzikhodjaeva, G.; Soukas, A.A.; Kahn, C.R.; Ntambi, J.M.; Socci, N.D. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J. Clin. Investig. 2004, 113, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.A.; Mourtzakis, M.; Chu, Q.S.; Baracos, V.E.; Reiman, T.; Mazurak, V.C. Nutritional intervention with fish oil provides a benefit over standard of care for weight and skeletal muscle mass in patients with nonsmall cell lung cancer receiving chemotherapy. Cancer 2011, 117, 1775–1782. [Google Scholar] [CrossRef]
- Murphy, R.A.; Mourtzakis, M.; Chu, Q.S.; Baracos, V.E.; Reiman, T.; Mazurak, V.C. Supplementation with fish oil increases first-line chemotherapy efficacy in patients with advanced nonsmall cell lung cancer. Cancer 2011, 117, 3774–3780. [Google Scholar] [CrossRef]
- Cao, S.; Rustum, Y.M. Synergistic antitumor activity of irinotecan in combination with 5-fluorouracil in rats bearing advanced colorectal cancer: Role of drug sequence and dose. Cancer Res. 2000, 60, 3717–3721. [Google Scholar] [PubMed]
- Xue, H.; Le Roy, S.; Sawyer, M.B.; Field, C.J.; Dieleman, L.A.; Baracos, V.E. Single and combined supplementation of glutamine and n-3 polyunsaturated fatty acids on host tolerance and tumour response to 7-ethyl-10-[4-(1-piperidino)-1-piperidino] carbonyloxy-camptothecin (CPT-11)/5-fluorouracil chemotherapy in rats bearing Ward colon tumour. Br. J. Nutr. 2009, 102, 434–442. [Google Scholar]
- Palmisano, B.T.; Zhu, L.; Eckel, R.H.; Stafford, J.M. Sex differences in lipid and lipoprotein metabolism. Mol. Metab. 2018, 15, 45–55. [Google Scholar] [CrossRef]
- Almasud, A.A.; Giles, K.H.; Miklavcic, J.J.; Martins, K.J.; Baracos, V.E.; Putman, C.T.; Guan, L.L.; Mazurak, V.C. Fish oil mitigates myosteatosis and improves chemotherapy efficacy in a preclinical model of colon cancer. PLoS ONE 2017, 12, e0183576. [Google Scholar] [CrossRef] [PubMed]
- McGlory, C.; Galloway, S.D.; Hamilton, D.L.; McClintock, C.; Breen, L.; Dick, J.R.; Bell, J.G.; Tipton, K.D. Temporal changes in human skeletal muscle and blood lipid composition with fish oil supplementation. Prostaglandins Leukot Essent Fat. Acids 2014, 90, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]


| (a) | |||||
| Fatty Acid (%) | Reference | Tumor | Chemo | Chemo + Fish Oil | p-Value |
|---|---|---|---|---|---|
| C16:0 | 19.6 ± 0.4 b | 22.0 ± 1.4 a | 18.6 ± 1.3 b | 17.6 ± 1.8 b | <0.001 |
| C18:0 | 7.1 ± 0.1 | 6.9 ± 0.5 | 7.9 ± 0.1 | 7.95 ± 1.1 | 0.058 |
| C18:1n-9 | 47.6 ± 1.7 a | 46.7 ± 2.31 a | 49.9 ± 2.9 a | 37.2 ± 1.2 b | 0.001 |
| C18:2n-6 | 16.1 ± 1.0 | 15.0 ± 1.2 | 14.1 ± 2.5 | 13.8 ± 3.0 | 0.270 |
| C18:3n-3 | 1.2 ± 0.4 | 1.0 ± 0.1 | 0.9 ± 0.2 | 0.9 ± 0.1 | 0.060 |
| C20:4n-6 | 2.5 ± 0.7 | 2.5 ± 0.6 | 2.4 ± 0.7 | 2.2 ± 0.3 | 0.873 |
| C20:5n-3 | 0.3 ± 0.1 bc | 0.3 ± 0.1 b | 0.2 ± 0.1 c | 6.0 ± 0.8 a | 0.003 |
| C22:5n-3 | 0.4 ± 0.1 b | 0.4 ± 0.1 b | 0.4 ± 0.1 b | 3.6 ± 0.8 a | 0.008 |
| C22:6n-3 | 1.5 ± 0.5 b | 1.3 ± 0.2 b | 1.1 ± 0.3 bc | 8.1 ± 1.9 a | 0.002 |
| ∑n-6 FA | 18.8 ± 1.8 | 17.7 ± 1.8 | 16.8 ± 3.3 | 16.2 ± 3.4 | 0.248 |
| ∑n-3 FA | 3.6 ± 1.2 b | 3.0 ± 0.5 bc | 2.7 ± 0.8 bcd | 18.8 ± 3.5 a | 0.001 |
| n-6/n-3 | 5.2 ± 1.5 a | 5.8 ± 3.3 a | 6.2 ± 4.0 a | 0.9 ± 0.9 b | 0.002 |
| ∑SFA | 27.0 ± 1.5 b | 29.1 ± 2.0 a | 26.8 ± 1.6 b | 25.7 ± 2.9 b | 0.004 |
| ∑MUFA | 49.8 ± 2.6 a | 48.5 ± 2.6 a | 51.2 ± 3.2 a | 38.4 ± 1.3 b | 0.002 |
| ∑Total FA (μg/g) | 2293.5 ± 909.9 ab | 1683.4 ± 642.7 b | 3243.8 ± 848.0 a | 2624.1 ± 654.5 ab | 0.024 |
| (b) | |||||
| Fatty Acid (%) | Reference | Tumor | Chemo | Chemo + Fish Oil | p-Value |
| C16:0 | 8.1 ± 0.1 | 8.8 ± 0.4 | 9.5 ± 2.3 | 9.4 ± 1.9 | 0.104 |
| C18:0 | 37.1 ± 0.8 ab | 38.3 ± 1.1 a | 35.6 ± 2.2 b | 36.1 ± 1.2 b | 0.017 |
| C18:1n-9 | 4.5 ± 0.5 b | 4.8 ± 0.5 b | 5.6 ± 0.5 a | 5.3 ± 0.5 ab | 0.007 |
| C18:2n-6 | 8.1 ± 0.5 | 8.6 ± 0.4 | 8.7 ± 1.6 | 8.4 ± 1.3 | 0.344 |
| C18:3n-3 | 0.06 ± 0.05 | 0.02 ± 0.03 | 0.03 ± 0.04 | 0.05 ± 0.06 | 0.533 |
| C20:4n-6 | 29.9 ± 0.6 a | 26.8 ± 1.2 bc | 28.1 ± 2.5 b | 20.2 ± 0.9 d | <0.001 |
| C20:5n-3 | 0.5 ± 0.1 c | 0.7 ± 0.1 b | 0.5 ± 0.1 c | 6.7 ± 0.7 a | <0.001 |
| C22:5n-3 | 0.6 ± 0.1 b | 0.6 ± 0.1 b | 0.5 ± 0.3 bc | 1.5 ± 0.1 a | 0.006 |
| C22:6n-3 | 9.3 ± 1.2 b | 9.5 ± 1.1 b | 9.4 ± 0.5 b | 11.6 ± 0.9 a | <0.001 |
| ∑n-6 FA | 39.0 ± 0.8 a | 36.5 ± 1.2 bc | 37.6 ± 1.4 b | 29.3 ± 2.1 d | <0.001 |
| ∑n-3 FA | 10.4 ± 1.4 b | 10.8 ± 1.1 b | 10.4 ± 0.79 b | 19.8 ± 1.3 a | 0.004 |
| n-6/n-3 | 3.8 ± 0.6 a | 3.4 ± 0.4 a | 3.6 ± 0.3 a | 1.5 ± 0.2 b | 0.003 |
| ∑SFA | 45.6 ± 0.7 | 47.2 ± 1.4 | 45.4 ± 1.7 | 45.9 ± 1.3 | 0.124 |
| ∑MUFA | 4.8 ± 0.5 b | 5.2 ± 0.5 ab | 5.9 ± 0.6 a | 5.6 ± 0.5 a | 0.007 |
| ∑Total FA (μg/g) | 27.9 ± 2.9 ab | 24.0 ± 2.2 b | 33.6 ± 4.0 a | 32.0 ± 6.3 a | 0.003 |
| Control Diet | Fish Oil Diet | |
|---|---|---|
| Saturated fatty acids | 58.7 | 59.9 |
| Monounsaturated fatty acids | 17.3 | 14.3 |
| Polyunsaturated fatty acids | 20.6 | 22.5 |
| Total n-6 | 18.6 | 13.6 |
| Total n-3 | 2.00 | 8.90 |
| EPA | Nil | 5.10 |
| DHA | Nil | 2.10 |
| Other fatty acids | 3.40 | 3.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monirujjaman, M.; Renani, L.B.; Isesele, P.; Dunichand-Hoedl, A.R.; Mazurak, V.C. Increased Expression of Hepatic Stearoyl-CoA Desaturase (SCD)-1 and Depletion of Eicosapentaenoic Acid (EPA) Content following Cytotoxic Cancer Therapy Are Reversed by Dietary Fish Oil. Int. J. Mol. Sci. 2023, 24, 3547. https://doi.org/10.3390/ijms24043547
Monirujjaman M, Renani LB, Isesele P, Dunichand-Hoedl AR, Mazurak VC. Increased Expression of Hepatic Stearoyl-CoA Desaturase (SCD)-1 and Depletion of Eicosapentaenoic Acid (EPA) Content following Cytotoxic Cancer Therapy Are Reversed by Dietary Fish Oil. International Journal of Molecular Sciences. 2023; 24(4):3547. https://doi.org/10.3390/ijms24043547
Chicago/Turabian StyleMonirujjaman, Md, Leila Baghersad Renani, Peter Isesele, Abha R. Dunichand-Hoedl, and Vera C. Mazurak. 2023. "Increased Expression of Hepatic Stearoyl-CoA Desaturase (SCD)-1 and Depletion of Eicosapentaenoic Acid (EPA) Content following Cytotoxic Cancer Therapy Are Reversed by Dietary Fish Oil" International Journal of Molecular Sciences 24, no. 4: 3547. https://doi.org/10.3390/ijms24043547
APA StyleMonirujjaman, M., Renani, L. B., Isesele, P., Dunichand-Hoedl, A. R., & Mazurak, V. C. (2023). Increased Expression of Hepatic Stearoyl-CoA Desaturase (SCD)-1 and Depletion of Eicosapentaenoic Acid (EPA) Content following Cytotoxic Cancer Therapy Are Reversed by Dietary Fish Oil. International Journal of Molecular Sciences, 24(4), 3547. https://doi.org/10.3390/ijms24043547






