Effects of 17α-Methyltestosterone on the Transcriptome and Sex Hormones in the Brain of Gobiocypris rarus
Abstract
:1. Introduction
2. Results
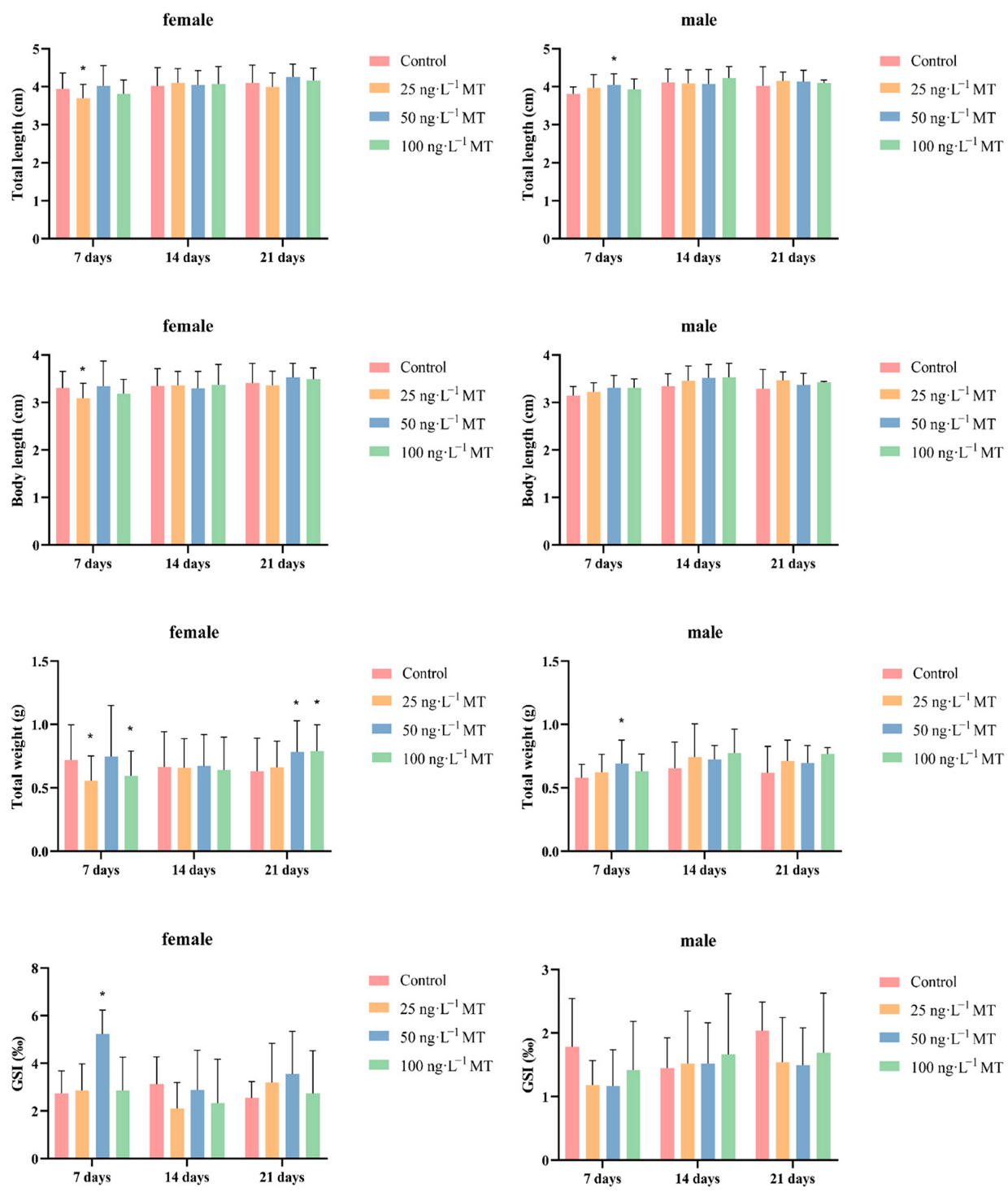
2.1. Effects of MT on Biological Parameters and Gonadal Indices in G. rarus
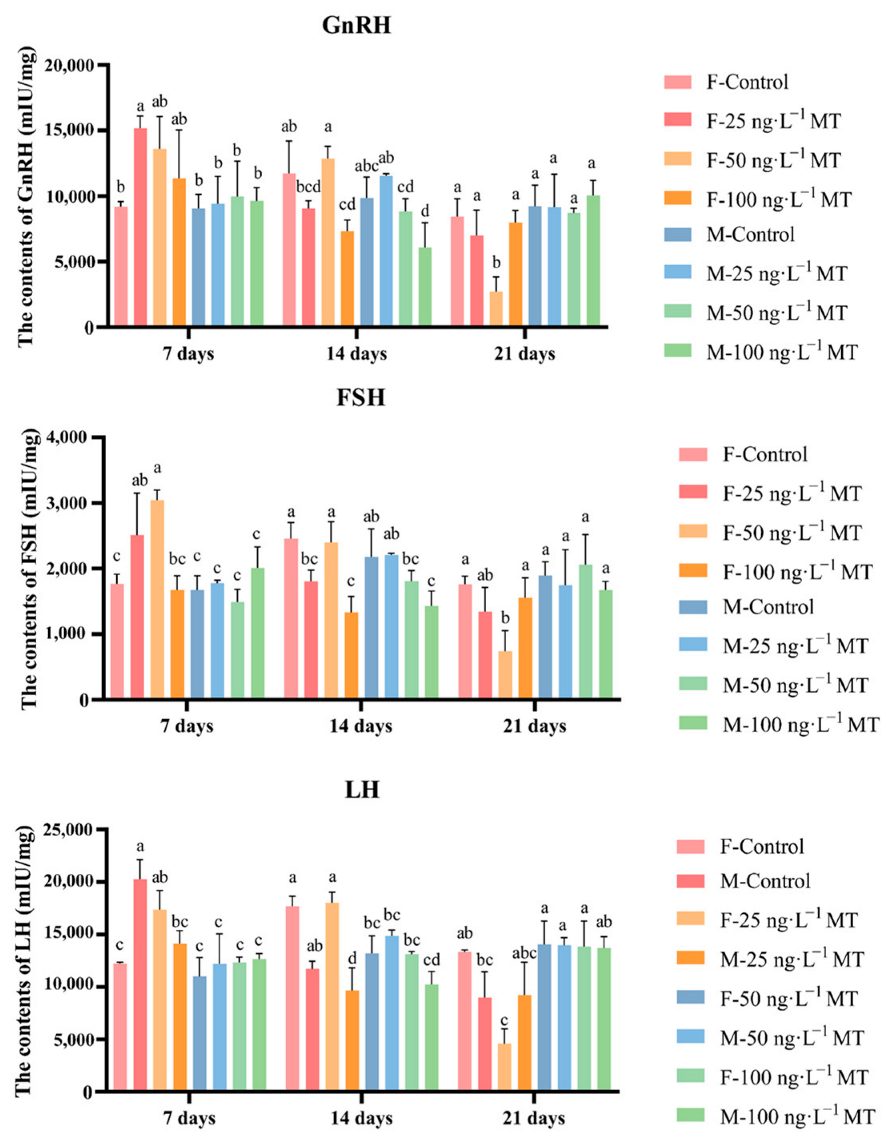
2.2. Effects of MT on Reproduction-Related Hormone Content in the Brain of G. rarus
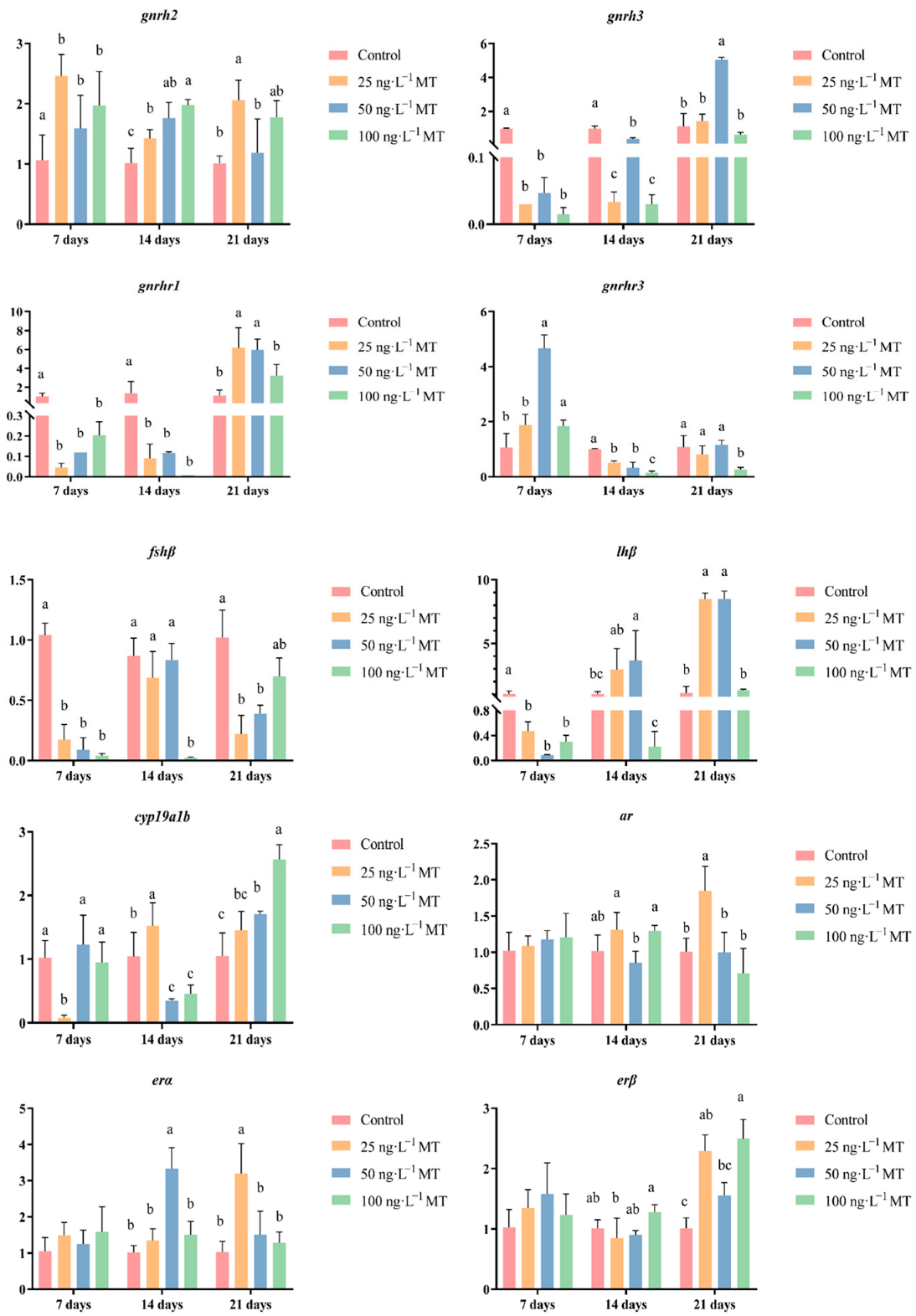
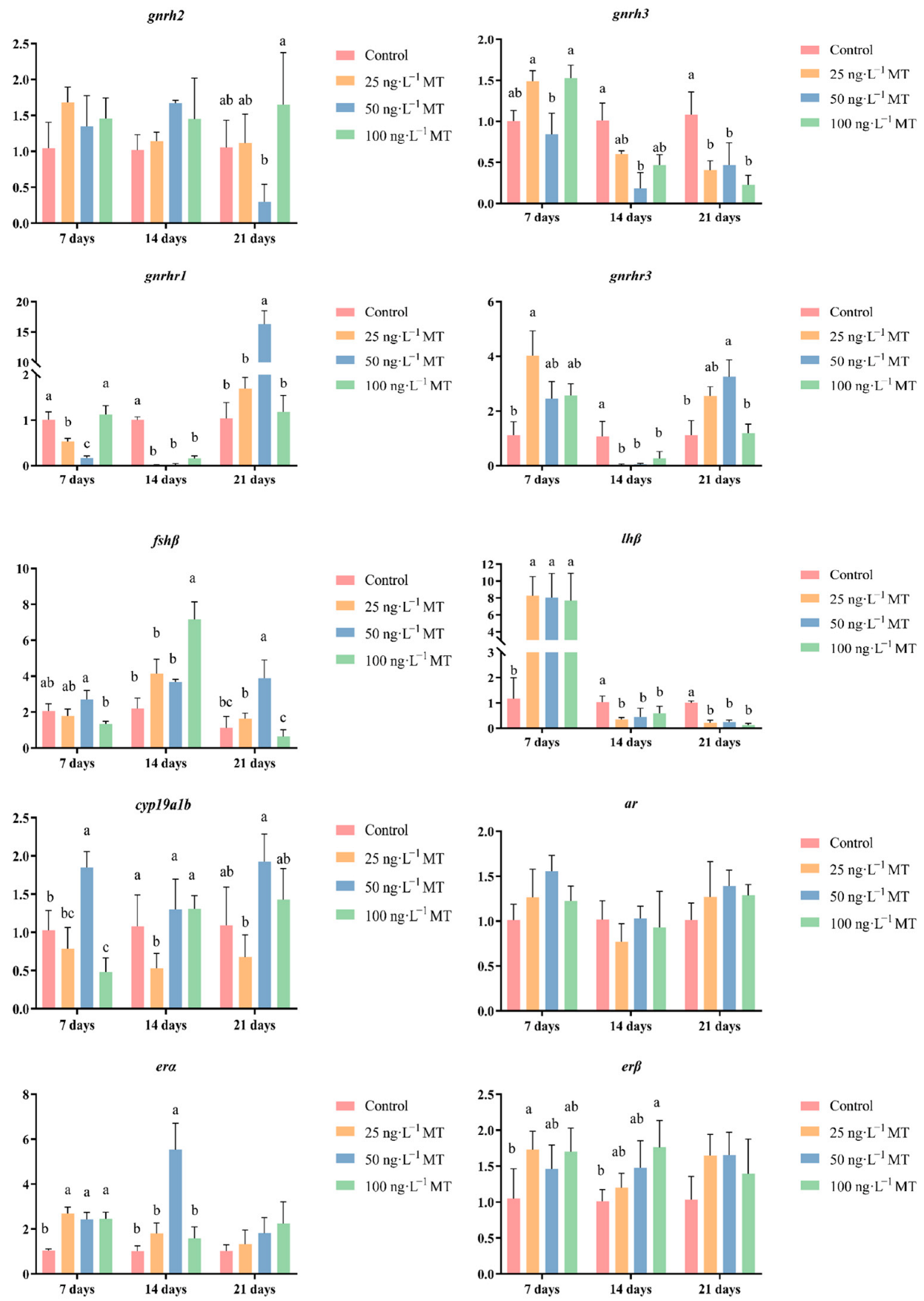
2.3. Effects of MT on the Expressions of Genes Related to Reproduction in G. rarus
2.4. Changes in the Brain Transcriptome following MT Exposure
2.4.1. Sequencing Evaluation
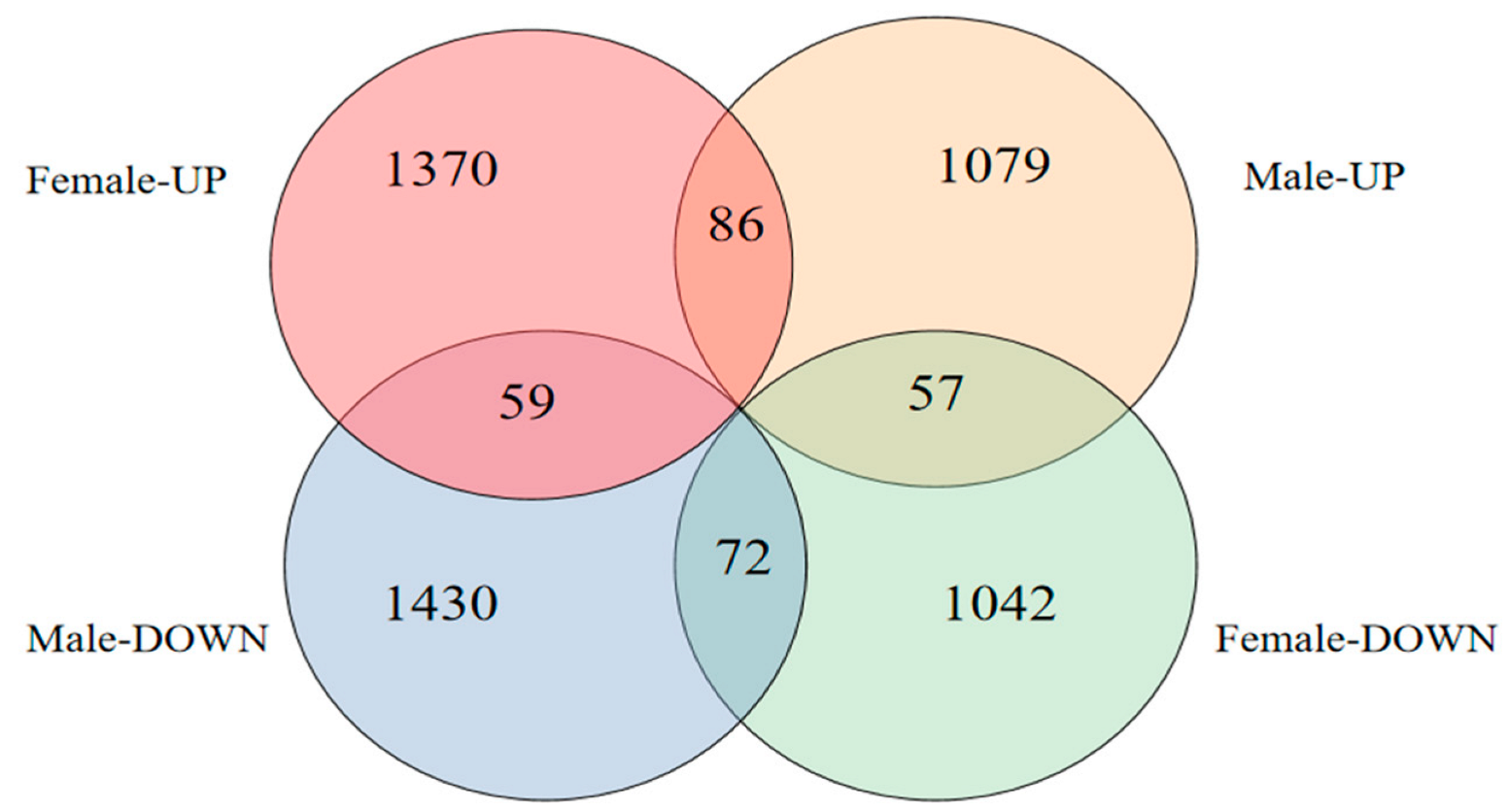
2.4.2. Screening for DEGs
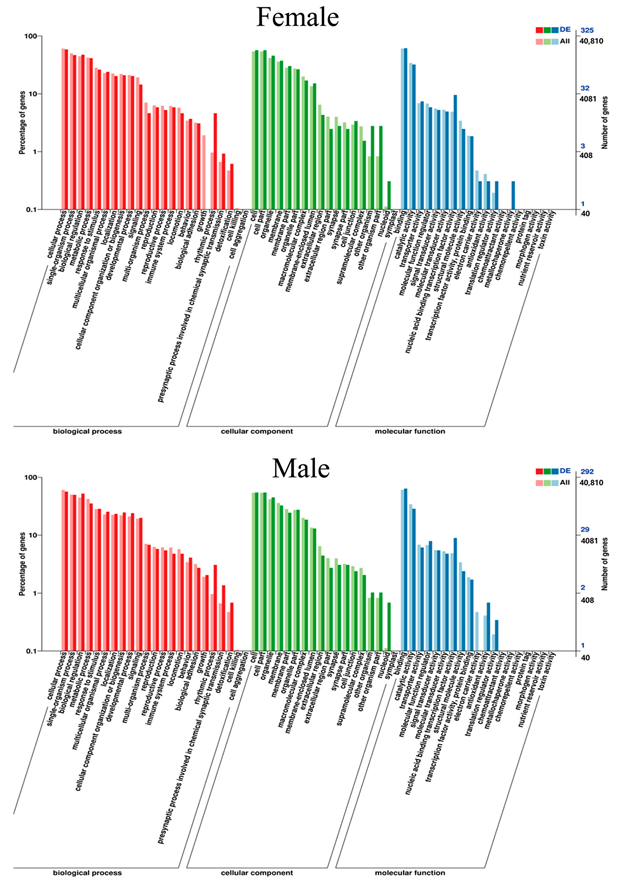
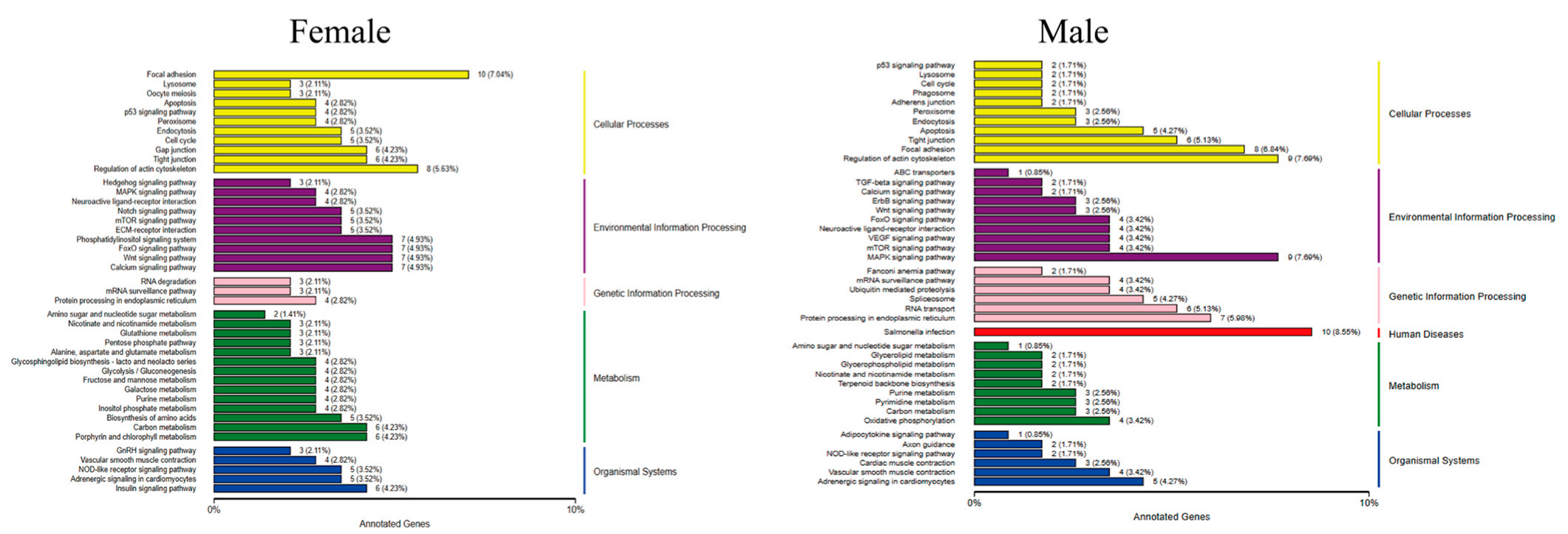
2.4.3. Functional Notes for DEGs
2.4.4. qRT-PCR to Verify the DEGs
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Measurement of Biological Indicators
4.3. ELISA
4.4. RNA Sample Collection and Quality Testing
4.5. qRT-PCR
4.6. Sample Sequencing
4.6.1. RNA Preparation and Sequencing
4.6.2. Bioinformatics Analysis
4.6.3. RT-qPCR Analysis for Validating Expression Profiles of Transcriptome Sequencing
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, Y.; Lian, D.; Su, T.; Wang, Y.Y.L.; Zhang, D.; Wang, Z.; Gimeno, S.; You, J. Species and life-Stage sensitivity of Chinese Rare Minnow (Gobiocypris rarus) to chemical exposure: A critical review. Environ. Toxicol. Chem. 2021, 40, 2680–2692. [Google Scholar] [CrossRef]
- Chen, Q.F.; Zhou, Y.H.; Qiu, Y.L.; Zhu, Z.L.; Zhao, J.F. Research Progress of Fish as Bioindicators for Halogenated Persistent Organic Pollutants. Environ. Sci. Technol. 2016, 39, 99--110,130. [Google Scholar]
- Wu, S.; Zhang, S.; Ji, G.; Liu, J.; Shi, L.; Zhou, B. Research and discussion of Rare minnow as an aquatic model organism. Asian J. Ecotoxicol. 2017, 12, 38–46. [Google Scholar]
- Liang, X.; Zha, J. Toxicogenomic applications of Chinese rare minnow (Gobiocypris rarus) in aquatic toxicology. Comp. Biochem. Physiol. Part D Genom. Proteom. 2016, 19, 174–180. [Google Scholar] [CrossRef]
- Yuan, L.; Li, J.; Zha, J.; Wang, Z. Targeting neurotrophic factors and their receptors, but not cholinesterase or neurotransmitter, in the neurotoxicity of TDCPP in Chinese rare minnow adults (Gobiocypris rarus). Environ. Pollut. 2016, 208, 670–677. [Google Scholar] [CrossRef]
- Tian, X.; Yang, W.; Wang, D.; Zhao, Y.; Yao, R.; Ma, L.; Ge, C.; Li, X.; Huang, Z.; He, L.; et al. Chronic brain toxicity response of juvenile Chinese rare minnows (Gobiocypris rarus) to the neonicotinoid insecticides imidacloprid and nitenpyram. Chemosphere 2018, 210, 1006–1012. [Google Scholar] [CrossRef]
- Xiong, X.; Li, H.; Qiu, N.; Su, L.; Huang, Z.; Song, L.; Wang, J. Bioconcentration and depuration of cadmium in the selected tissues of rare minnow (Gobiocypris rarus) and the effect of dietary mulberry leaf supplementation on depuration. Environ. Toxicol. Pharmacol. 2020, 73, 103278. [Google Scholar] [CrossRef]
- Luo, S.; Wu, B.; Xiong, X.; Wang, J. Short-term toxicity of ammonia, nitrite, and nitrate to early life stages of the rare minnow (Gobiocypris rarus). Environ. Toxicol. Chem. 2016, 35, 1422–1427. [Google Scholar] [CrossRef]
- Backe, W.J.; Ort, C.; Brewer, A.J.; Field, J.A. Analysis of androgenic steroids in environmental waters by large-volume injection liquid chromatography tandem mass spectrometry. Anal. Chem. 2011, 83, 2622–2630. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Chu, X.; Lin, J.M. Trace Analysis of Fifteen Androgens in Environmental Waters by LC-ESI-MS-MS Combined with Solid-Phase Disk Extraction Cleanup. Chromatographia 2010, 9, 867–873. [Google Scholar] [CrossRef]
- Borg, B. Androgens in teleost fishes. Comp. Biochem. Physiol. C 1994, 109, 219–245. [Google Scholar] [CrossRef]
- Matsubara, H.; Lokman, P.M.; Kazeto, Y.; Adachi, S.; Yamauchi, K. Serum steroid profiles in artificially maturing female Japanese eel, Anguilla japonica. Aquaculture 2005, 243, 393–402. [Google Scholar] [CrossRef]
- Wang, Y.S.; Lou, S.W. Structural and expression analysis of hepatic vitellogenin gene during ovarian maturation in Anguilla japonica. J. Steroid Biochem. Mol. Biol. 2006, 100, 193–201. [Google Scholar] [CrossRef]
- Angus, R. A Short-Term In Vivo Screening System for Endocrine Disruptors Utilizing Mosquitofishes (Gambusia affinis and G. holbrooki); R826130; US Environmental Protection Agency, National Center for Environmental Research Grant: Washington, DC, USA, 2015. [Google Scholar]
- Yamazaki, F. Application of hormones in fish culture. J. Fish. Res. Board Can. 1976, 33, 948–958. [Google Scholar] [CrossRef]
- Zhou, X.H.; Xiang, X.; Wang, J.W. A Preliminary Study on the Effects of Methyltestosterone on Sex Reversal and Body Colour in Sailfin Molly and Red Sword tall. Fish. Sci. Technol. Inf. 2000, 27, 99–137. [Google Scholar]
- Lai, X.J.; Li, Z.Q.; Xie, Y.J.; Chen, S.X.; Wang, Y.L. Androstenedione and 17α-methyltestosterone induce early ovary development of Anguilla japonica. Theriogenology 2018, 120, 16–24. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, J.; Yang, Q.; Wang, W.; Liu, Q.; Liu, W.; Liu, S. Effects of 17 α-methyltestosterone on the transcriptome, gonadal histology and sex steroid hormones in Pseudorasbora parva. Theriogenology 2020, 155, 88–97. [Google Scholar] [CrossRef]
- Seki, M.; Yokota, H.; Matsubara, H.; Maeda, M.; Tadokoro, H.; Kobayashi, K. Fish full life-cycle testing for androgen methyltestosterone on medaka (Oryzias latipes). Environ. Toxicol. Chem. 2004, 23, 774–781. [Google Scholar] [CrossRef]
- Ankley, G.T.; Jensen, K.M.; Kahl, M.D.; Korte, J.J.; Makynen, E.A. Description and evaluation of a short-term reproduction test with the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 2001, 20, 1276–1290. [Google Scholar] [CrossRef]
- Hori, S.H.; Kodama, T.; Tanahashi, K. Induction of vitellogenin synthesis in goldfish by massive doses of androgens. Gen. Comp. Endocrinol. 1979, 37, 306–320. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Qin, F.; Zheng, Y.; Li, M.; Zhang, Y.; Yuan, C.; Wang, Z. Gonadal development and transcript profiling of steroidogenic enzymes in response to 17α-methyltestosterone in the rare minnow Gobiocypris rarus. J. Steroid Biochem. Mol. Biol. 2014, 143, 223–232. [Google Scholar] [CrossRef]
- Jin, Y.; Shu, L.; Huang, F.; Cao, L.; Sun, L.; Fu, Z. Environmental cues influence EDC-mediated endocrine disruption effects in different developmental stages of Japanese medaka (Oryzias latipes). Aquat. Toxicol. 2011, 101, 254–260. [Google Scholar] [CrossRef]
- Gao, J.; Liu, S.; Zhang, Y.; Yang, Y.; Yuan, C.; Chen, S.; Wang, Z. Effects of 17 α-methyltestosterone on transcriptome, gonadal histology and sex steroid hormones in rare minnow Gobiocypris rarus. Comp. Biochem. Physiol. Part D Genom. Proteom. 2015, 15, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, Y.; Zhong, C.; Guo, Y.; Li, S.; Lin, H.; Liu, X. Transcriptome profiling of laser-captured germ cells and functional characterization of zbtb40 during 17alpha-methyltestosterone-induced spermatogenesis in orange-spotted grouper (Epinephelus coioides). BMC Genom. 2020, 21, 73. [Google Scholar] [CrossRef]
- Luckenbach, J.A.; Fairgrieve, W.T. Gonadal sex differentiation and effects of dietary methyltestosterone treatment in sablefish (Anoplopoma fimbria). Fish Physiol. Biochem. 2016, 42, 233–248. [Google Scholar] [CrossRef]
- Park, C.B.; Soyano, K.; Kiros, S.; Kitamura, T.; Minamiyama, M.; Suzuki, Y. Transient effects of methyltestosterone injection on different reproductive parameters of the hermaphrodite fish Kryptolebias marmoratus. Ecotoxicology 2013, 22, 1145–1154. [Google Scholar] [CrossRef]
- Shen, Z.G.; Fan, Q.X.; Yang, W.; Zhang, Y.L.; Wang, H.P. Effects of 17α-Methyltestosterone and Aromatase Inhibitor Letrozole on Sex Reversal, Gonadal Structure, and Growth in Yellow Catfish Pelteobagrus fulvidraco. Biol. Bull. 2015, 228, 108–117. [Google Scholar] [CrossRef]
- Passini, G.; Sterzelecki, F.C.; de Carvalho, C.V.A.; Baloi, M.F.; Naide, V.; Cerqueira, V.R. 17α-Methyltestosterone implants accelerate spermatogenesis in common snook, Centropomus undecimalis, during first sexual maturation. Theriogenology 2018, 106, 134–140. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, M.; Peng, C.; Wang, X.; Xiao, L.; Wang, D.; Chen, J.; Zhao, H.; Zhang, H.; Li, S.; et al. MT-Feeding-Induced Impermanent Sex Reversal in the Orange-Spotted Grouper during Sex Differentiation. Int. J. Mol. Sci. 2018, 19, 2828. [Google Scholar] [CrossRef]
- Costa, D.P.; Sinervo, B. Field physiology: Physiological insights from animals in nature. Annu. Rev. Physiol. 2004, 66, 209–238. [Google Scholar] [CrossRef]
- Qin, F.; Wang, L.; Wang, X.; Liu, S.; Xu, P.; Wang, H.; Wu, T.; Zhang, Y.; Zheng, Y.; Li, M.; et al. Bisphenol A affects gene expression of gonadotropin-releasing hormones and type I GnRH receptors in brains of adult rare minnow Gobiocypris rarus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013, 157, 192–202. [Google Scholar] [CrossRef]
- Zohar, Y.; Zmora, N.; Trudeau, V.L.; Muñoz-Cueto, J.A.; Golan, M. A half century of fish gonadotropin-releasing hormones: Breaking paradigms. J. Neuroendocrinol. 2022, 34, e13069. [Google Scholar]
- Kauffman, A.S. Emerging functions of gonadotropin-releasing hormone II in mammalian physiology and behaviour. J. Neuroendocrinol. 2004, 16, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Vosges, M.; Le Page, Y.; Chung, B.C.; Combarnous, Y.; Porcher, J.M.; Kah, O.; Brion, F. 17alpha-ethinylestradiol disrupts the ontogeny of the forebrain GnRH system and the expression of brain aromatase during early development of zebrafish. Aquat. Toxicol. 2010, 99, 479–491. [Google Scholar] [CrossRef]
- Kah, O.; Anglade, I.; Linard, B. Estrogen receptors in the brain-pituitary complex and the neuroendocrine regulation of gonadotropin release in rainbow trout. Fish Physiol. Biochem. 1997, 17, 53–62. [Google Scholar] [CrossRef]
- Qiu, W.; Fang, M.; Liu, J.; Fu, C.; Zheng, C.; Chen, B.; Wang, K.J. In vivo actions of Bisphenol F on the reproductive neuroendocrine system after long-term exposure in zebrafish. Sci. Total Environ. 2019, 665, 995–1002. [Google Scholar] [CrossRef]
- Maqbool, F.; Mostafalou, S.; Bahadar, H.; Abdollahi, M. Review of endocrine disorders associated with environmental toxicants and possible involved mechanisms. Life Sci. 2016, 145, 265–273. [Google Scholar] [CrossRef]
- Steven, C.; Lehnen, N.; Kight, K.; Ijiri, S.; Klenke, U.; Harris, W.A.; Zohar, Y. Molecular characterization of the GnRH system in zebrafish (Danio rerio): Cloning of chicken GnRH-II, adult brain expression patterns and pituitary content of salmon GnRH and chicken GnRH-II. Gen. Comp. Endocrinol. 2003, 133, 27–37. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Q.; Wang, X.Z.; Lv, X.J.; Rong, W.Y.; Li, Y.X.; Liu, Q.; Wang, W.W.; Song, J.; Wang, X.Z.; et al. Effect of KISS/GPR54 System Mediating 17α-methyltestosterone on Gonad Development in Gobiocypris rarus. Asian J. Ecotoxicol. 2022, 17, 150–163. [Google Scholar]
- Sun, W.; Jia, Y.; Ding, X.; Dai, L.; Liu, C.; Wang, J.; Zhao, G.; Zhou, H.; Yu, L. Combined effects of pentachlorophenol and its byproduct hexachlorobenzene on endocrine and reproduction in zebrafish. Chemosphere 2019, 220, 216–226. [Google Scholar] [CrossRef]
- Ranganathan, P.; Weaver, K.L.; Capobianco, A.J. Notch signalling in solid tumours: A little bit of everything but not all the time. Nat. Rev. Cancer 2011, 11, 338–351. [Google Scholar] [CrossRef]
- Tsaouli, G.; Barbarulo, A.; Vacca, A.; Screpanti, I.; Felli, M.P. Molecular Mechanisms of Notch Signaling in Lymphoid Cell Lineages Development: NF-κB and Beyond. Adv. Exp. Med. Biol. 2020, 1227, 145–164. [Google Scholar]
- Shang, Y.; Smith, S.; Hu, X. Role of Notch signaling in regulating innate immunity and inflammation in health and disease. Protein Cell 2016, 7, 159–174. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, K. Cellular and molecular regulation of the activation of mammalian primordial follicles: Somatic cells initiate follicle activation in adulthood. Hum. Reprod. Update 2015, 21, 779–786. [Google Scholar] [CrossRef]
- Grosbois, J.; Demeestere, I. Dynamics of PI3K and Hippo signaling pathways during in vitro human follicle activation. Hum. Reprod. 2018, 33, 1705–1714. [Google Scholar] [CrossRef]
- Mabuchi, S.; Kuroda, H.; Takahashi, R.; Sasano, T. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol. Oncol. 2015, 137, 173–179. [Google Scholar] [CrossRef]
- Cheaib, B.; Auguste, A.; Leary, A. The PI3K/Akt/mTOR pathway in ovarian cancer: Therapeutic opportunities and challenges. Chin. J. Cancer 2015, 34, 4–16. [Google Scholar] [CrossRef]
- Dan, Q.H.; Luo, H.Y.; Zhang, Y.T.; Gao, X.B.; Lu, C.L. Mechanism research of effect of pentachloronitrobenzene on follicular development in rats. J. Reprod. Med. 2022, 31, 506–513. [Google Scholar]
- Lange, A.; Katsu, Y.; Ichikawa, R.; Paull, G.C.; Chidgey, L.L.; Coe, T.S.; Iguchi, T.; Tyler, C.R. Altered sexual development in roach (Rutilus rutilus) exposed to environmental concentrations of the pharmaceutical 17alpha-ethinylestradiol and associated expression dynamics of aromatases and estrogen receptors. Toxicol. Sci. 2008, 106, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Wang, X.; Liu, S.; Zheng, Y.; Li, M.; Zhang, Y.; Wang, Z. Gene expression profiling of key genes in hypothalamus-pituitary-gonad axis of rare minnow Gobiocypris rarus in response to EE2. Gene 2014, 552, 8–17. [Google Scholar] [CrossRef]
- Qin, F.; Wang, L.H.; Liu, S.Z.; Wang, Z.Z. Characterization of Reference Genes in Rare Minnow, Gobiocypris Rarus (Actinopterygii: Cypriniformes: Cyprinidae), in Early Postembryonic Development and in Response to Edcs Treatment. Acta Ichthyol. Piscat. 2013, 43, 127–138. [Google Scholar] [CrossRef]

| Sex | Gene | Gene ID | Fold by RNA-Seq | Fold by qRT-PCR |
|---|---|---|---|---|
| Female | cry1 | BMK_Unigene_048464 | 1.74 | 1.27 |
| foxo3 | BMK_Unigene_019235 | 1.67 | 1.05 | |
| ccnd2 | BMK_Unigene_005574 | 1.53 | 1.46 | |
| pms2 | BMK_Unigene_089388 | −2.11 | −1.32 | |
| wtap | BMK_Unigene_183473 | −3.50 | −1.82 | |
| pik3c3 | BMK_Unigene_058799 | −1.70 | −0.13 | |
| Male | tpr | BMK_Unigene_184367 | 4.92 | 0.66 |
| cpd | BMK_Unigene_148720 | 2.54 | 0.41 | |
| arntl | BMK_Unigene_031409 | 1.07 | 0.88 | |
| fundc1 | BMK_Unigene_095253 | −6.40 | −1.96 | |
| rbbp4 | BMK_Unigene_094652 | −1.70 | −1.81 | |
| etv4 | BMK_Unigene_098452 | −1.44 | −0.82 |
| Gene | Forward Primer Sequence | Reverse Primer Sequence | Tm (°C) | Primer Length (bp) |
|---|---|---|---|---|
| cyp19a1b | CTAGGTTCCTGTGGATGGG | CGTCGGAGGACTTGCTGA | 60 | 164 |
| fshβ | CCATTACCGTGGAAAGTGAGGA | CTGTGATGTCCGAGTTACATTTGC | 60 | 252 |
| lhβ | TGAGACTGTTGCTGTGGAAAAAG | AGTATGCGGGGAAATCCTCTC | 60 | 323 |
| gnrh2 | TGGGGATGTTGCTGTGTCTAAG | TCTGTTTGCTGGAAAGGTCGT | 60 | 285 |
| gnrh3 | ATGGAGTGGAAAGGAAGGGTG | TGGAGTGTCCGCAGGAATAGA | 60 | 186 |
| gnrhr1 | GAGCGGAGAGCGAGGACTT | CCAGCAGACCACAAACGACAT | 60 | 284 |
| gnrhr3 | TGCTCGCCTCTCCACAGTTA | TTCCCCACCCTTCCCTTTAC | 60 | 233 |
| ar | CACTGCCAACAAAGGTCAGC | TGGGTGGAGTCGGTATGGAT | 60 | 151 |
| erα | TCACCCATGTACCCCAAGGA | GAGTGGTGTCCTCCGTGATG | 60 | 272 |
| erβ | AGGGTAGCAGATGCAGAGGA | TCGCCGTAACCCACATTTCA | 60 | 279 |
| cry1 | TTTTCCCACAATGCACCCTG | GGTTTACTCTCCCATCGCCT | 59 | 160 |
| foxo3 | CAAAGCCCCTAATGCGATGC | TGGCCGAAAAGTGGAGTTCT | 60 | 166 |
| ccnd2 | GAGGTGGGTAGGAGGGTCTT | GACAGCCCGCACAAAAGATG | 60 | 219 |
| pms2 | CACGGCACTACCATCACACT | CTTGCCTTGTCCCATCTGGT | 60 | 153 |
| wtap | GACGGGCTCCACTTCGTTTA | GCGAACACTTACCAGACCCA | 60 | 174 |
| pik3c3 | TTTTCCTCGGGTCTTGTCGG | TGCCACTTCCTTCGGGTTTT | 60 | 102 |
| tpr | ATAAAGCCCACACCTCTGGC | ATTGGCTCTTGGCTCTCCAC | 60 | 145 |
| cpd | CATCGCATAAACGTCCGCTG | TTACCCAAAGCTCCCGGTTC | 60 | 107 |
| arntl | TGGTTTCGGGCAGTATGCTT | CTTCCTCGGCTATCATGCGT | 60 | 170 |
| fundc1 | CCTCGAAACTGGAGCTTGGT | AGCAGCAGACGGAGTTTTCA | 60 | 116 |
| rbbp4 | ACCGCTAACAGGGAGGGTTA | CTGGGTCTGCTGAGAATGGG | 60 | 219 |
| etv4 | AGCTCTTCGTTCTGCCCATC | AATGGTTACAGTGCGGGGAG | 60 | 189 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Chen, Y.; Li, T.; Qiao, L.; Yang, Q.; Rong, W.; Liu, Q.; Wang, W.; Song, J.; Wang, X.; et al. Effects of 17α-Methyltestosterone on the Transcriptome and Sex Hormones in the Brain of Gobiocypris rarus. Int. J. Mol. Sci. 2023, 24, 3571. https://doi.org/10.3390/ijms24043571
Liu S, Chen Y, Li T, Qiao L, Yang Q, Rong W, Liu Q, Wang W, Song J, Wang X, et al. Effects of 17α-Methyltestosterone on the Transcriptome and Sex Hormones in the Brain of Gobiocypris rarus. International Journal of Molecular Sciences. 2023; 24(4):3571. https://doi.org/10.3390/ijms24043571
Chicago/Turabian StyleLiu, Shaozhen, Yue Chen, Tongyao Li, Liying Qiao, Qiong Yang, Weiya Rong, Qing Liu, Weiwei Wang, Jing Song, Xianzong Wang, and et al. 2023. "Effects of 17α-Methyltestosterone on the Transcriptome and Sex Hormones in the Brain of Gobiocypris rarus" International Journal of Molecular Sciences 24, no. 4: 3571. https://doi.org/10.3390/ijms24043571
APA StyleLiu, S., Chen, Y., Li, T., Qiao, L., Yang, Q., Rong, W., Liu, Q., Wang, W., Song, J., Wang, X., & Liu, Y. (2023). Effects of 17α-Methyltestosterone on the Transcriptome and Sex Hormones in the Brain of Gobiocypris rarus. International Journal of Molecular Sciences, 24(4), 3571. https://doi.org/10.3390/ijms24043571





