Severe NAD(P)HX Dehydratase (NAXD) Neurometabolic Syndrome May Present in Adulthood after Mild Head Trauma
Abstract
1. Introduction
2. Case Presentation
2.1. Proband
2.2. Bioinformatics, Next-Generation Sequencing (NGS) and RNAseq Analysis
2.3. Fibroblast Proteomic Analyses in NAXD Patients
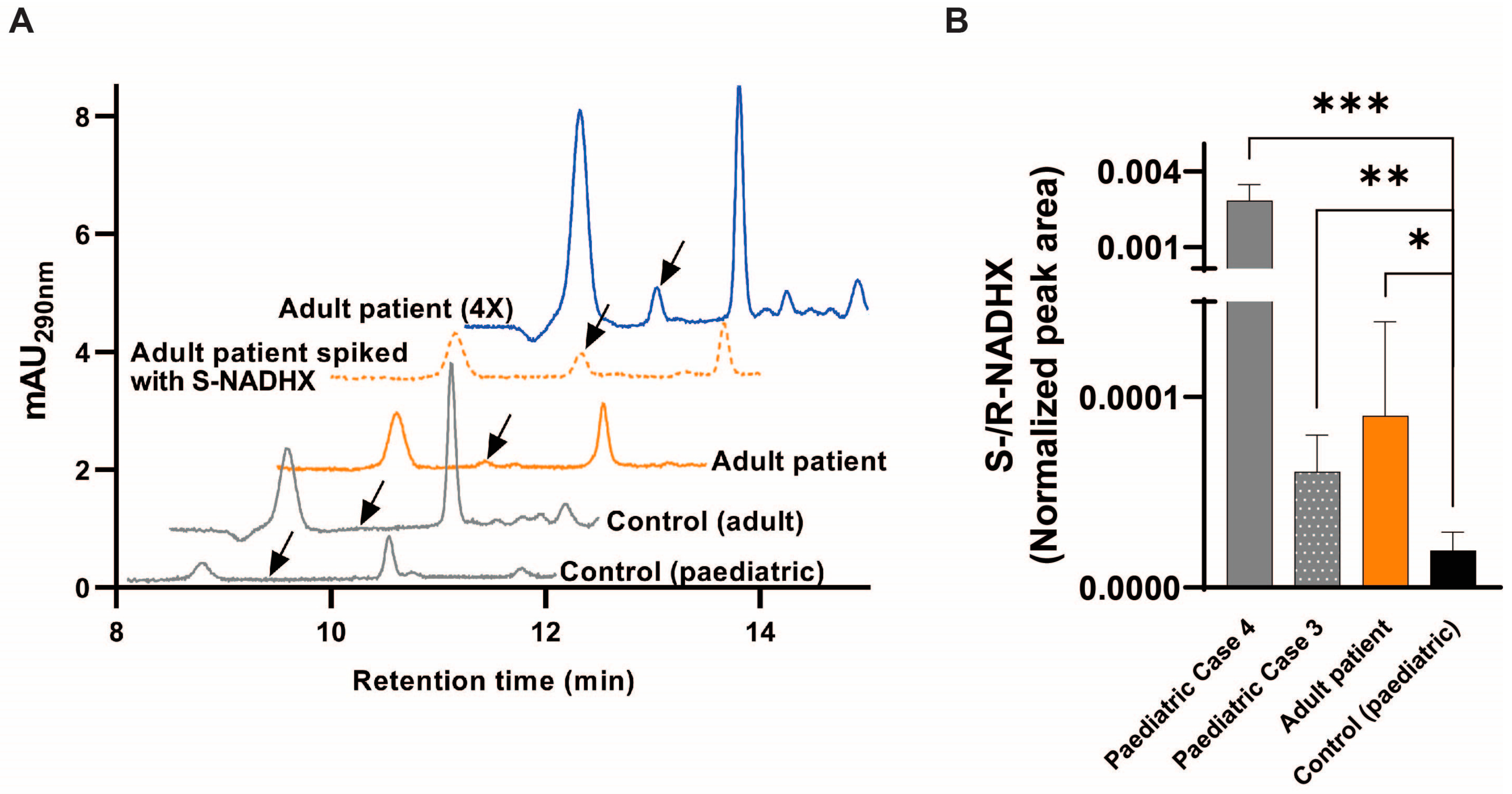
2.4. Fibroblast Metabolic Analyses in NAXD Patients
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Cell Culture
4.3. Whole Genome Sequencing
4.4. RNA Analysis
4.5. Quantitative Mass Spectrometry and Data Analysis
4.6. Metabolic (NADHX) Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Bergen, N.J.; Walvekar, A.S.; Patraskaki, M.; Sikora, T.; Linster, C.L.; Christodoulou, J. Clinical and biochemical distinctions for a metabolite repair disorder caused by NAXD or NAXE deficiency. J. Inherit. Metab. Dis. 2022, 45, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Van Bergen, N.J.; Guo, Y.; Rankin, J.; Paczia, N.; Becker-Kettern, J.; Kremer, L.S.; Pyle, A.; Conrotte, J.F.; Ellaway, C.; Procopis, P.; et al. NAD(P)HX dehydratase (NAXD) deficiency: A novel neurodegenerative disorder exacerbated by febrile illnesses. Brain 2019, 142, 50–58. [Google Scholar] [CrossRef]
- Kremer, L.S.; Danhauser, K.; Herebian, D.; Petkovic Ramadza, D.; Piekutowska-Abramczuk, D.; Seibt, A.; Muller-Felber, W.; Haack, T.B.; Ploski, R.; Lohmeier, K.; et al. NAXE Mutations Disrupt the Cellular NAD(P)HX Repair System and Cause a Lethal Neurometabolic Disorder of Early Childhood. Am. J. Hum. Genet. 2016, 99, 894–902. [Google Scholar] [CrossRef]
- Zhou, J.; Li, J.; Stenton, S.L.; Ren, X.; Gong, S.; Fang, F.; Prokisch, H. NAD(P)HX dehydratase (NAXD) deficiency: A novel neurodegenerative disorder exacerbated by febrile illnesses. Brain 2020, 143, e8. [Google Scholar] [CrossRef]
- Helman, G.; Compton, A.G.; Hock, D.H.; Walkiewicz, M.; Brett, G.R.; Pais, L.; Tan, T.Y.; De Paoli-Iseppi, R.; Clark, M.B.; Christodoulou, J.; et al. Multiomic analysis elucidates Complex I deficiency caused by a deep intronic variant in NDUFB10. Hum. Mutat. 2021, 42, 19–24. [Google Scholar] [CrossRef]
- Manor, J.; Calame, D.; Gijavanekar, C.; Tran, A.; Faith, J.; Lalani, S.R.; Mizerik, E.; Parnes, M.; Mehta, V.P.; Lupski, J.R.; et al. Niacin therapy improves clinical outcome with normalization of metabolic abnormalities in a patient with NAXD deficiency. Brain 2022, 145, e36–e40. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef] [PubMed]
- Walsby-Tickle, J.; Gannon, J.; Hvinden, I.; Bardella, C.; Abboud, M.I.; Nazeer, A.; Hauton, D.; Pires, E.; Cadoux-Hudson, T.; Schofield, C.J.; et al. Anion-exchange chromatography mass spectrometry provides extensive coverage of primary metabolic pathways revealing altered metabolism in IDH1 mutant cells. Commun. Biol. 2020, 3, 247. [Google Scholar] [CrossRef]
- Becker-Kettern, J.; Paczia, N.; Conrotte, J.F.; Zhu, C.; Fiehn, O.; Jung, P.P.; Steinmetz, L.M.; Linster, C.L. NAD(P)HX repair deficiency causes central metabolic perturbations in yeast and human cells. FEBS J. 2018, 285, 3376–3401. [Google Scholar] [CrossRef]
- Manor, J.; Calame, D.; Gijavanekar, C.; Fisher, K.; Hunter, J.; Mizerik, E.; Bacino, C.; Scaglia, F.; Elsea, S.H. NAXE deficiency: A neurometabolic disorder of NAD(P)HX repair amenable for metabolic correction. Mol. Genet. Metab. 2022, 136, 101–110. [Google Scholar] [CrossRef]
- Lunke, S.; Eggers, S.; Wilson, M.; Patel, C.; Barnett, C.P.; Pinner, J.; Sandaradura, S.A.; Buckley, M.F.; Krzesinski, E.I.; de Silva, M.G.; et al. Feasibility of Ultra-Rapid Exome Sequencing in Critically Ill Infants and Children With Suspected Monogenic Conditions in the Australian Public Health Care System. JAMA 2020, 323, 2503–2511. [Google Scholar] [CrossRef]
- Fowler, K.J. Storage of skin biopsies at -70 degrees C for future fibroblast culture. J. Clin. Pathol. 1984, 37, 1191–1193. [Google Scholar] [CrossRef]
- Van Bergen, N.J.; Hock, D.H.; Spencer, L.; Massey, S.; Stait, T.; Stark, Z.; Lunke, S.; Roesley, A.; Peters, H.; Lee, J.Y.; et al. Biallelic Variants in PYROXD2 Cause a Severe Infantile Metabolic Disorder Affecting Mitochondrial Function. Int. J. Mol. Sci. 2022, 23, 986. [Google Scholar] [CrossRef]
- Sadedin, S.P.; Dashnow, H.; James, P.A.; Bahlo, M.; Bauer, D.C.; Lonie, A.; Lunke, S.; Macciocca, I.; Ross, J.P.; Siemering, K.R.; et al. Cpipe: A shared variant detection pipeline designed for diagnostic settings. Genome. Med. 2015, 7, 68. [Google Scholar] [CrossRef]
- Sadedin, S.P.; Ellis, J.A.; Masters, S.L.; Oshlack, A. Ximmer: A system for improving accuracy and consistency of CNV calling from exome data. Gigascience 2018, 7, giy112. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Bournazos, A.M.; Riley, L.G.; Bommireddipalli, S.; Ades, L.; Akesson, L.S.; Al-Shinnag, M.; Alexander, S.I.; Archibald, A.D.; Balasubramaniam, S.; Berman, Y.; et al. Standardized practices for RNA diagnostics using clinically accessible specimens reclassifies 75% of putative splicing variants. Genet. Med. 2022, 24, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdottir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Corbett, M.A.; Smith, N.J.C.; Hock, D.H.; Kikhtyak, Z.; Semcesen, L.N.; Morimoto, A.; Lee, S.; Stroud, D.A.; Gleeson, J.G.; et al. Oligonucleotide correction of an intronic TIMMDC1 variant in cells of patients with severe neurodegenerative disorder. NPJ Genom. Med. 2022, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Sharma, R.; Gupta, R.; Ast, T.; Chan, C.; Durham, T.J.; Goodman, R.P.; Grabarek, Z.; Haas, M.E.; Hung, W.H.W.; et al. MitoCarta3.0: An updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 2021, 49, D1541–D1547. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Gnanaprakasam, J.N.R.; Chen, X.; Kang, S.; Xu, X.; Sun, H.; Liu, L.; Rodgers, H.; Miller, E.; Cassel, T.A.; et al. Inosine is an alternative carbon source for CD8(+)-T-cell function under glucose restriction. Nat. Metab. 2020, 2, 635–647. [Google Scholar] [CrossRef]


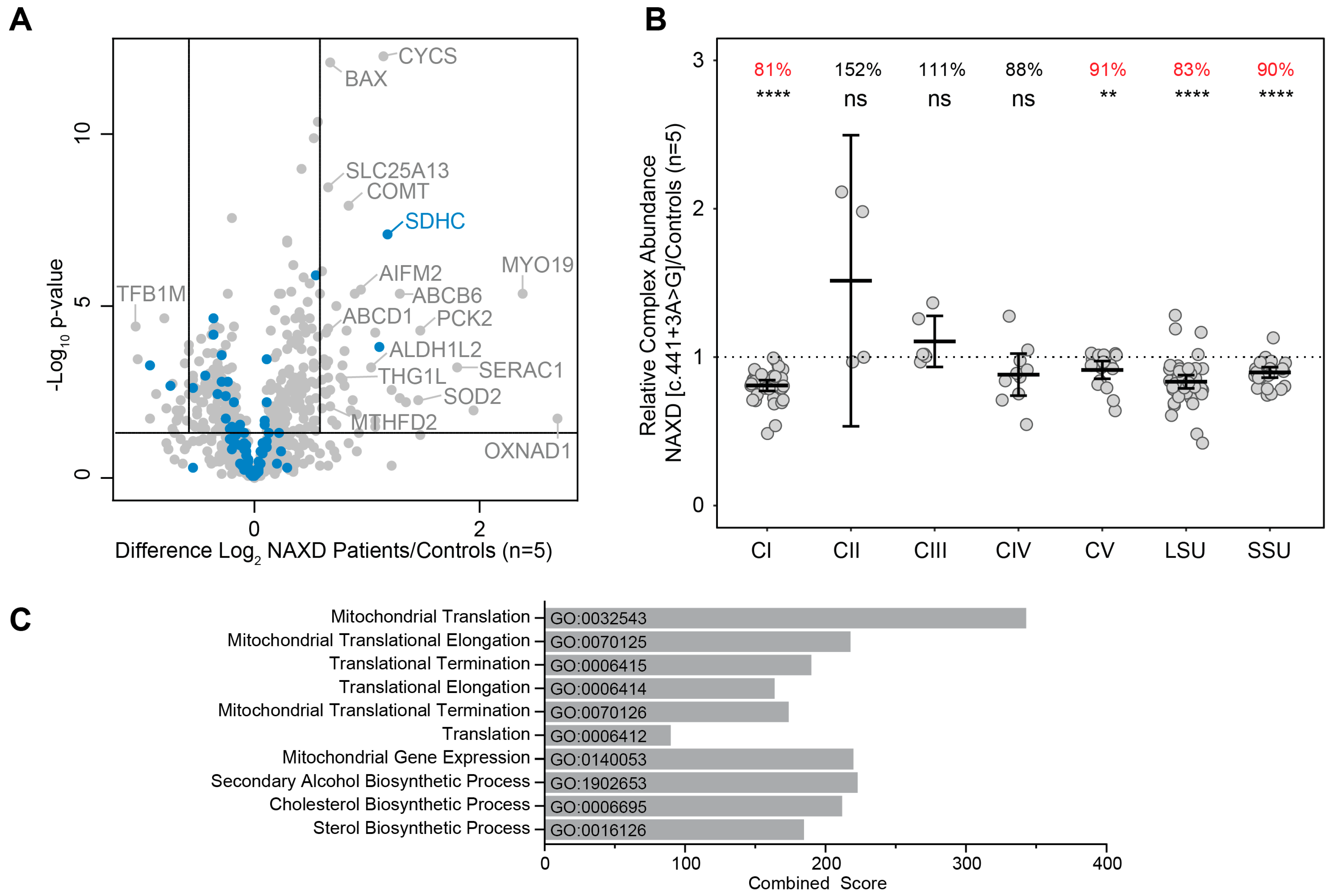
| Age of Onset | NAXD Variant (and In Silico Prediction) | NAXD Peptides Detected | Mitochondrial OXPHOS Respiratory Chain (% of Controls) | Mitoribosome (% of Controls) | |||||
|---|---|---|---|---|---|---|---|---|---|
| CI | CII | CIII | CIV | CV | LSU | SSU | |||
| Paediatric Case 1 # | c.839+1G>T: p(?) and c.922C>T: p.(Arg308Cys) (splicing and missense) | 7 peptides | 79 **** | 164 | 91 | 97 | 91 * | 62 **** | 63 **** |
| Paediatric Case 2 # | c.187G>A: p.(Gly63Ser) and c.948_949insTT: p.(Ala317Leufs*64) (missense and stop loss) | ~1–2 peptides | 81 **** | 150 | 102 | 67 **** | 91 * | 82 **** | 78 **** |
| Paediatric Case 3 # | c.51_54delAGAA: p.(Ala20Phefs*9) (frameshift w/ NMD) | 2 peptides | 93 ** | 124 | 111 | 90 | 86 *** | 85 **** | 87 **** |
| Paediatric Case 4 # | c.308C>T: p.(Pro103Leu) (missense) | 9 peptides | 85 **** | 149 | 102 | 89 | 89 ** | 87 **** | 89 **** |
| Adult (reported here) | c.441+3A>G: p.(?) (residual wild-type, premature truncation and in-frame deletion) | Not detected | 81 **** | 152 | 111 | 91 | 91 ** | 83 **** | 90 **** |
| Control | 9 peptides | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Primer | Sequence |
|---|---|
| Ex1-F | 5ʹ-CAATCCGGGCTTGCAGAC-3ʹ |
| Ex1-F2 | 5ʹ-GTCGTTGTCGCATTGCTCTC-3ʹ |
| Ex2-F | 5ʹ-GAAAGAGCGTTTTCGCTACG-3ʹ |
| Ex5-F | 5ʹ-CCAATGCTGTTCATGAGGTG-3ʹ |
| In5-R | 5ʹ-AGGCACACGGAACACTACAC-3ʹ |
| Ex5/6-F | 5ʹ-CTTCTCAGAAATGTCCAGGGC-3ʹ |
| Ex7-R | 5ʹ-CTGAACTCCACGTGGTTGG-3ʹ |
| Ex8-R | 5ʹ-GCTGTCATCGCTGTCCATAG-3ʹ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Bergen, N.J.; Gunanayagam, K.; Bournazos, A.M.; Walvekar, A.S.; Warmoes, M.O.; Semcesen, L.N.; Lunke, S.; Bommireddipalli, S.; Sikora, T.; Patraskaki, M.; et al. Severe NAD(P)HX Dehydratase (NAXD) Neurometabolic Syndrome May Present in Adulthood after Mild Head Trauma. Int. J. Mol. Sci. 2023, 24, 3582. https://doi.org/10.3390/ijms24043582
Van Bergen NJ, Gunanayagam K, Bournazos AM, Walvekar AS, Warmoes MO, Semcesen LN, Lunke S, Bommireddipalli S, Sikora T, Patraskaki M, et al. Severe NAD(P)HX Dehydratase (NAXD) Neurometabolic Syndrome May Present in Adulthood after Mild Head Trauma. International Journal of Molecular Sciences. 2023; 24(4):3582. https://doi.org/10.3390/ijms24043582
Chicago/Turabian StyleVan Bergen, Nicole J., Karen Gunanayagam, Adam M. Bournazos, Adhish S. Walvekar, Marc O. Warmoes, Liana N. Semcesen, Sebastian Lunke, Shobhana Bommireddipalli, Tim Sikora, Myrto Patraskaki, and et al. 2023. "Severe NAD(P)HX Dehydratase (NAXD) Neurometabolic Syndrome May Present in Adulthood after Mild Head Trauma" International Journal of Molecular Sciences 24, no. 4: 3582. https://doi.org/10.3390/ijms24043582
APA StyleVan Bergen, N. J., Gunanayagam, K., Bournazos, A. M., Walvekar, A. S., Warmoes, M. O., Semcesen, L. N., Lunke, S., Bommireddipalli, S., Sikora, T., Patraskaki, M., Jones, D. L., Garza, D., Sebire, D., Gooley, S., McLean, C. A., Naidoo, P., Rajasekaran, M., Stroud, D. A., Linster, C. L., ... Christodoulou, J. (2023). Severe NAD(P)HX Dehydratase (NAXD) Neurometabolic Syndrome May Present in Adulthood after Mild Head Trauma. International Journal of Molecular Sciences, 24(4), 3582. https://doi.org/10.3390/ijms24043582





