Gelatin as It Is: History and Modernity
Abstract
:1. Introduction
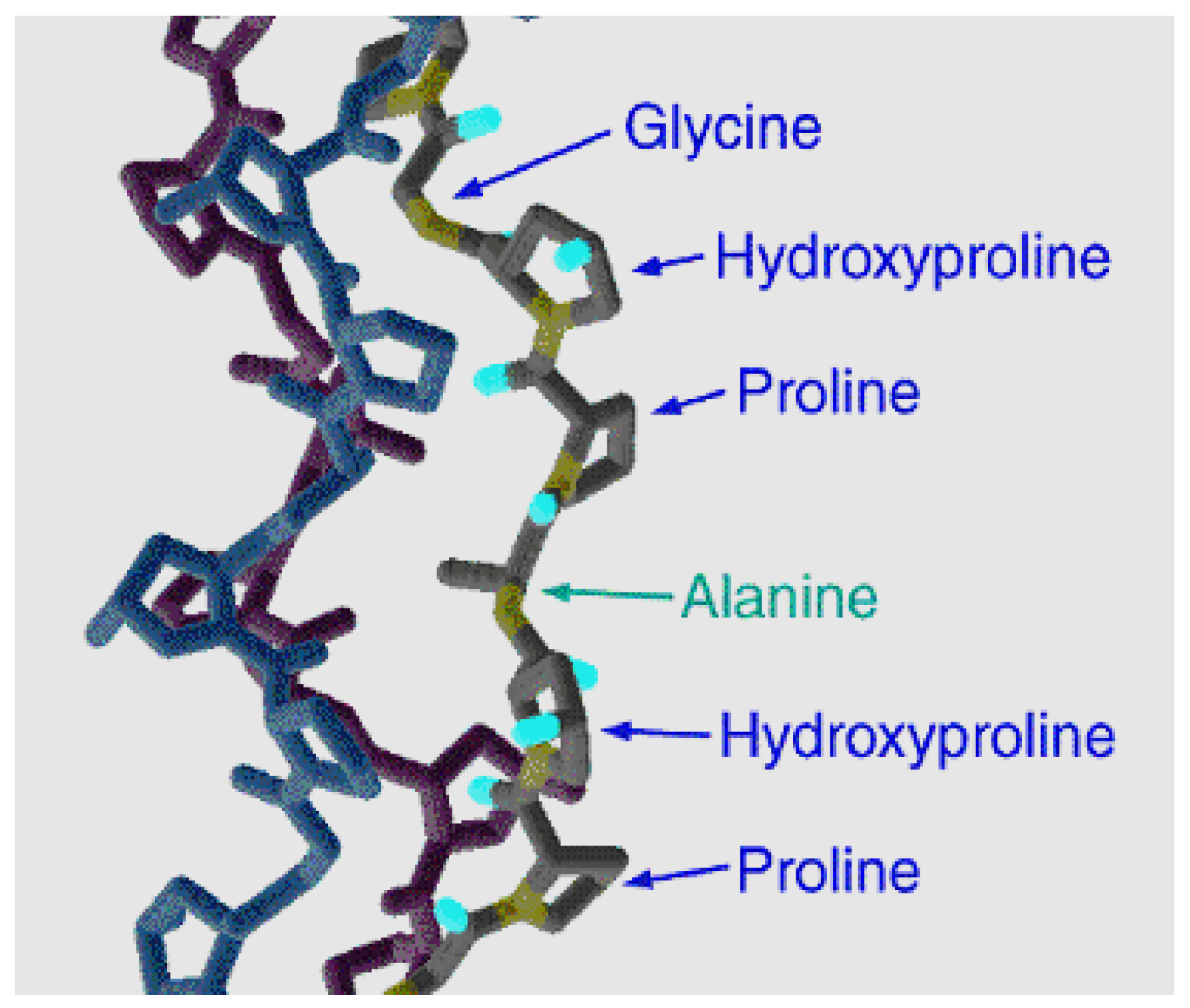
2. Specificity of Gelatin Synthesis
- Treatment with acid or alkali solutions. During this processing, the “ballast” proteins contained in the raw material break down into smaller polypeptides and pass into the processing solution, and only the gelatin precursor, collagen, which does not undergo destruction (although it acquires a looser structure) remains in the processed mass.
- Treatment in a solution of calcium hydroxide (slaked lime). This variant is used for the skins of cattle and provides for the constant renewal of the solution of this reagent since the duration of the processing of raw materials with it is quite long (up to two months or even more). After the completion of this procedure, the above reagent is washed off, neutralized by the action of hydrochloric acid, and the resulting mass is again thoroughly washed with water.
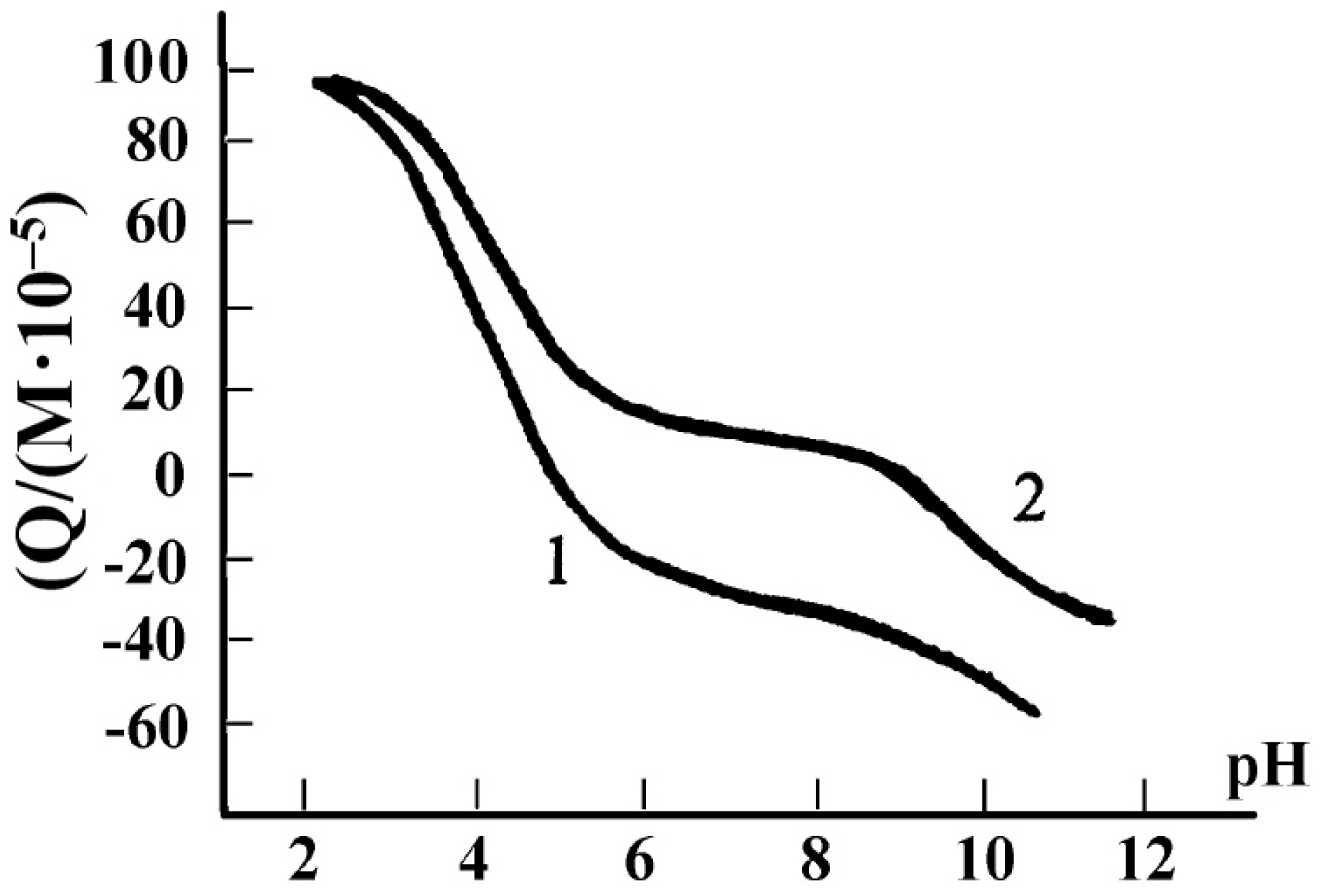
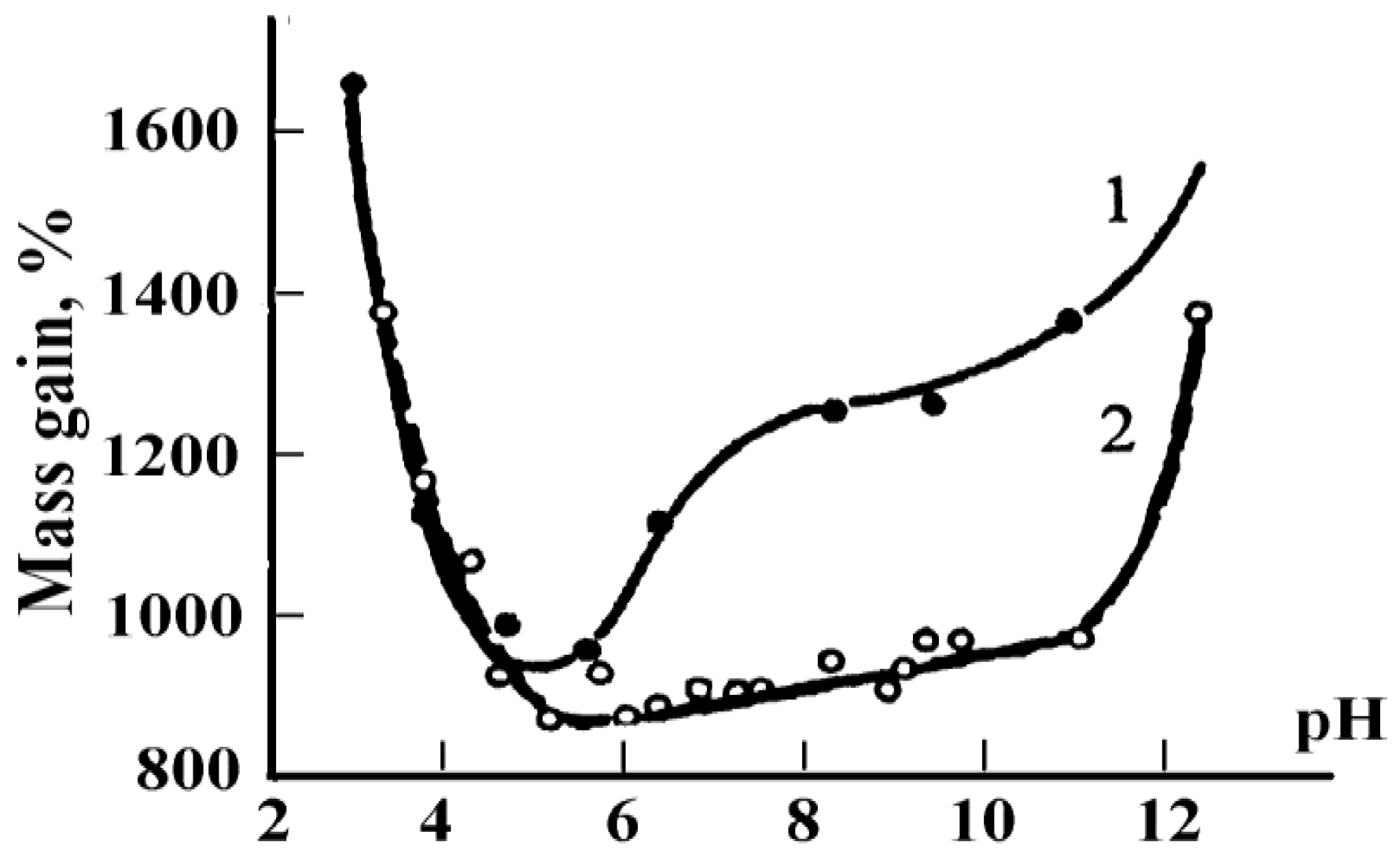
3. Gelatin Physico-Chemistry
- The production of silver halide photographic materials for recording information (using so-called photographic gelatin);
- The creation and immobilization of nanoparticles of various chemical compounds;
- The creation of pharmaceutical dosage forms—as a material for the manufacture of capsules, components of nutrient mixtures and media, as well as a component of plasma-substituting and diagnostic tools;
- The creation of protein-based nanomaterials.
4. Gelatin as a Binding Agent for Recording Systems in the Silver Halide Photographic Process
5. Gelatin as a Matrix for the Creation and Immobilization of Nanoparticles of Various Chemical Compounds
6. Gelatin as a Matrix for the Creation and Delivery of Pharmaceutical Drug Forms
7. Gelatin as an Object for Creating Protein Nanomaterials
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nasrallah, N. Annals of the Caliphs’ Kitchens: Ibn Sayyār al-Warrāq’s Tenth-Century Baghdadi Cookbook; English Translation with Introduction and Glossary; Brill: Leiden, Germany, 2007; Available online: https://www.cambridge.org/core/journals/review-of-middle-east-studies/article/abs/nawal-nasrallah-annals-of-the-caliphs-kitchens-ibn-sayyar-alwarraqs-tenthcentury-baghdadi-cookbook-english-translation-with-introduction-and-glossary-leiden-brill-2007 (accessed on 10 December 2022).
- Scully, T. The Viandier of Taillevent: An Edition of All Extant Manuscripts; University of Ottawa Press: Ottawa, ON, Canada, 1988; p. 270. ISBN 978-0-7766-0174-8. [Google Scholar]
- Gelatin. Encyclopedia.com. 2016. Available online: https://www.encyclopedia.com/science-and-technology/biochemistry/biochemistry/gelatin (accessed on 10 December 2022).
- Viel, C.; Fournier, J. Histoire des procédés d’extraction de la gélatine et débats des commissions académiques (XIXe siècle). [History of gelatin extraction processes and debates of academic commissions (XIX century)]. Rev. D’Histoire Pharm. 2006, 54, 7–28. Available online: https://www.persee.fr/doc/pharm_0035-2349_2006_num_94_349_5939 (accessed on 10 December 2022). (In French) [CrossRef]
- Davis, J.J. Defining Culinary Authority: The Transformation of Cooking in France, 1650–1830; Louisiana State University Press: Baton Rouge, LA, USA, 2013. [Google Scholar]
- Wyman, C. Jell-o: A Biography: The History and Mystery of America’s Most Famous Dessert; Diane Publishing Company: Collingdale, PA, USA, 2001; ISBN 978-0756788544. [Google Scholar]
- Eastoe, J.E. Treatise on Collagen; Ramachandran, G.N., Ed.; Academic Press: New York, NY, USA, 1976; Chapter 1; pp. 1–72. [Google Scholar]
- Alleavitch, J.; Turner, W.A. Gelatin. In Ullmann’s Encyclopedia of Industrial Chemistry; VCH Publishers: Weinheim, Germany, 1989; Volume A12, Chapter 1; pp. 309–315. [Google Scholar]
- Digenis, G.A.; Gold, T.B.; Shah, V.P. Cross-linking of gelatin capsules and its relevance to their in vitro-in vivo performance. J. Pharm. Sci. 1994, 83, 915–921. [Google Scholar] [CrossRef]
- Cole, G.; Francis, F.J. Gelatin. In Encyclopedia of Food Science and Technology, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2000; Volume 4, pp. 1183–1188. ISBN 978-0-471-19285-5. [Google Scholar]
- Schrieber, R.; Gareis, H. Gelatin Handbook; Wiley VCH: Weinheim, Germany, 2007. [Google Scholar] [CrossRef]
- Gelatin Production in America; GMIA Publishing: Chandler, AZ, USA, 2012; pp. 3–6. Available online: http://www.gelatin-gmia.com/images/GMIA_Gelatin_Manual_2012.pdf (accessed on 10 December 2022).
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Abd Elgadir, M.; Mirghani, M.E.S.; Adam, A. Fish gelatin and its applications in selected pharmaceutical aspects as alternative source to pork gelatin. J. Food Agric. Environ. 2013, 11, 73–79. [Google Scholar] [CrossRef]
- Mahmood, K.; Muhammad, L.; Ariffin, F.; Abd Razak, H.K.B.; Sulaiman, S. Review of Fish Gelatin Extraction, Properties and Packaging Applications. Food Sci. Qual. Manag. 2016, 56, 47–59. [Google Scholar]
- Larry, D.; Vedrines, M. Photographic Gelatin. In Proceedings of the Fourth IAG Conference, Melbourne, Australia, 1983; Ammann-Brass, H., Pouradier, J., Eds.; Internationale Arbeitsgemeinschaft fur Photogelatine: Fribourg, Switzerland, 1985; pp. 35–54. [Google Scholar]
- Chen, X.; Peng, B. Photographic Gelatin. In Proceedings of the Fourth IAG Conference, Melbourne, Australia, 1983; Ammann-Brass, H., Pouradier, J., Eds.; Internationale Arbeitsgemeinschaft fur Photogelatine: Fribourg, Switzerland, 1985; pp. 55–64. [Google Scholar]
- Beutel, J. Photographic Gelatin. In Proceedings of the Fourth IAG Conference, Melbourne, Australia, 1983; Ammann-Brass, H., Pouradier, J., Eds.; Internationale Arbeitsgemeinschaft fur Photogelatine: Fribourg, Switzerland, 1985; pp. 65–78. [Google Scholar]
- Aoyagi, S. Photographic Gelatin. In Proceedings of the Fourth IAG Conference, Melbourne, Australia, 1983; Ammann-Brass, H., Pouradier, J., Eds.; Internationale Arbeitsgemeinschaft fur Photogelatine: Fribourg, Switzerland, 1985; pp. 79–94. [Google Scholar]
- Rich, A.; Crick, F.H.C. The structure of collagen. Nature 1955, 176, 915–916. [Google Scholar] [CrossRef]
- Cowan, P.M.; McGavin, S.; North, A.C.T. The polypeptide chain configuration of collagen. Nature 1955, 176, 1062–1066. [Google Scholar] [CrossRef]
- Smith, C.R. Osmosis and swelling of gelatin. J. Am. Chem. Soc. 1921, 43, 1350–1366. [Google Scholar] [CrossRef]
- Jacobson, R.E. Photographic Gelatin II; Cox, R.J., Ed.; Academic Press: London, UK, 1967; pp. 233–252. [Google Scholar]
- Newman, R.E. The amino acid composition of gelatins, collagens and elastins from different sources. Arch. Biochem. 1949, 24, 289–298. [Google Scholar]
- Eastoe, J.E. The amino acid composition of mammalian collagen and gelatin. Biochem. J. 1955, 61, 589–600. [Google Scholar] [CrossRef]
- Harding, J.J.; Wesley, J.M. The purification and amino acid composition of human uterus collagens, rheumatoid-arthritis-nodule collagen and ox tendon collagen. Biochem. J. 1968, 106, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Pierce, J.A.; Hocott, J.B. Studies on the collagen and elastin content of the human lung. J. Clin. Investig. 1960, 39, 8–14. [Google Scholar] [CrossRef]
- Eastoe, J.E.; Leach, A.A. Recent Advances in Gelatin and Glue Research; Pergamon Press: New York, NY, USA, 1958; pp. 170–175. [Google Scholar]
- Schofield, J.D.; Freeman, I.L.; Jackson, D.S. The isolation, and amino acid and carbohydrate composition, of polymeric collagens prepared from various human tissues. Biochem. J. 1971, 124, 467–473. [Google Scholar] [CrossRef]
- Rose, P.I. Gelatin. In Encyclopedia of Polymer Science and Engineering, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 1987; Volume 7, pp. 500–505. [Google Scholar]
- Piez, K.A. Biochemistry of Collagen; Ramachandran, G.N., Reddi, A.H., Eds.; Plenum Press: New York, NY, USA, 1976; pp. 1–44. [Google Scholar]
- Boedker, H.; Doty, P. A Study of Gelatin Molecules, Aggregates and Gels. J. Phys. Chem. 1954, 58, 968–983. [Google Scholar] [CrossRef]
- Ramachandran, G.N.; Kartha, G. Structure of Collagen. Nature 1955, 176, 593–597. [Google Scholar] [CrossRef]
- Veis, A.; Anesey, J.; Cohen, J. The long range reorganization of gelatin to the collagen structure. Arch. Biochem. Biophys. 1961, 94, 20–31. [Google Scholar] [CrossRef]
- Groome, R.J.; Clegg, F.G. Photographic Gelatin; Focal Press: London, UK, 1965; p. 35. [Google Scholar]
- Elsdale, T.J.; Bard, J. Collagen substrata for studies on cell behavior. J. Cell Biol. 1972, 54, 626–637. [Google Scholar] [CrossRef]
- Hulmes, D.J.S.; Miller, A.; Parry, D.A.D.; Piez, K.A.; Woodhead-Galloway, J. Analysis of the primary structure of collagen for the origins of molecular packing. J. Mol. Biol. 1973, 79, 137–148. [Google Scholar] [CrossRef]
- Miller, E.J. Biochemical characteristics and biological significance of the genetically-distinct collagens. Mol. Cell. Biochem. 1976, 13, 165–192. [Google Scholar] [CrossRef]
- Ramachadran, G.N. Treatise on Collagen; Ramachandran, G.N., Bernard, S., Eds.; Academic Press: New York, NY, USA, 1967; p. 187. [Google Scholar]
- Kronman, J.H.; Goldman, M.; Habib, C.M.; Mengel, L. Electron microscopic evaluation of altered collagen structure induced by N-monochloroglycine (GK-101). J. Dent. Res. 1977, 56, 1539–1545. [Google Scholar] [CrossRef]
- Kurata, H.; Sakaoku, K. The investigation of the structure of collagen in growth process by X-ray analysis and electron microscopy. Biochim. Biophys. Acta 1984, 791, 305–313. [Google Scholar] [CrossRef]
- Chen, J.M.; Kung, C.E.; Feairheller, S.E.; Brown, E.M. An energetic evaluation of a “Smith” collagen microfibril model. J. Protein Chem. 1991, 10, 535–552. [Google Scholar] [CrossRef]
- Fridman, R.; Fuerst, T.R.; Bird, R.E.; Hoyhtya, M.; Oelkuct, M.; Kraus, S.; Komarck, D.; Liotta, L.A.; Berman, M.L.; Stetler-Stevenson, J. Domain structure of human 72-kDa gelatinase/type IV collagenase. Characterization of proteolytic activity and identification of the tissue inhibitor of metalloproteinase-2 (TIMP-2) binding regions. J. Biol. Chem. 1992, 267, 15398–15405. [Google Scholar] [CrossRef]
- Bányai, L.; Tordai, H.; Patthy, L. Structure and Domain-Domain Interactions of the Gelatin-binding Site of Human 72-Kilodalton Type IV Collagenase (Gelatinase A, Matrix Metalloproteinase 2). J. Biol. Chem. 1996, 271, 12003–12008. [Google Scholar] [CrossRef]
- Pickford, A.R.; Potts, J.R.; Bright, J.R.; Han, I.; Campbell, I.D. Solution structure of a type 2 module from fibronectin: Implications for the structure and function of the gelatin-binding domain. Structure 1997, 5, 359–370. [Google Scholar] [CrossRef]
- Tordai, H.; Patthy, L. The gelatin-binding site of the second type-II domain of gelatinase A/MMP-2. Eur. J. Biochem. 1999, 259, 513–518. [Google Scholar] [CrossRef]
- Caldararu, H.; Timmins, G.S.; Gilbert, B.C. The structure of gelatin–water/oil microemulsion sols and gels. An EPR spin-probe and spin-labelling study. Phys. Chem. Chem. Phys. 1999, 1, 5689–5697. [Google Scholar] [CrossRef]
- Phillips, G.O.; Williams, P.A. (Eds.) Handbook of Hydrocolloids; Woodhead Publishing: London, UK, 2000; 450p. [Google Scholar]
- Lin, W.; Yan, L.; Mu, C.; Li, W.; Zhang, M.; Zhu, O. Effect of pH on gelatin self-association investigated by laser light scattering and atomic force microscopy. Polym. Inter. 2002, 51, 233–236. [Google Scholar] [CrossRef]
- Trexler, M.; Briknarova, K.; Gehrmann, M.; Llinas, M.; Patthy, L. Peptide Ligands for the Fibronectin Type II Modules of Matrix Metalloproteinase 2 (MMP-2). J. Biol. Chem. 2003, 278, 12241–12246. [Google Scholar] [CrossRef]
- Gutsmann, T.; Fantner, G.E.; Venturoni, M.; Ekani-Nkodo, A.; Thompson, J.B.; Kindt, J.H.; Morse, D.E.; Fygenson, D.K.; Hansma, P.K. Evidence that collagen fibrils in tendons are inhomogeneously structured in a tubelike manner. Biophys. J. 2003, 84, 2593–2598. [Google Scholar] [CrossRef]
- Gehrmann, M.L.; Douglas, J.T.; Banyai, L.; Tordai, H.; Patthy, L.; Llinas, M. Modular Autonomy, Ligand Specificity, and Functional Cooperativity of the Three In-tandem Fibronectin Type II Repeats from Human Matrix Metalloproteinase 2. J. Biol. Chem. 2004, 279, 46921–46929. [Google Scholar] [CrossRef] [Green Version]
- Brodsky, B.; Persikov, A.V. Molecular structure of the collagen triple helix. Adv. Protein Chem. 2005, 70, 301–339. [Google Scholar] [CrossRef] [PubMed]
- Bella, J.; Liu, J.; Kramer, R.; Brodsky, B.; Berman, H.M. Conformational effects of Gly-X-Gly interruptions in the collagen triple helix. J. Mol. Biol. 2006, 362, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Strasser, S.; Zink, A.; Janko, M.; Heckl, W.M.; Thalhammer, S. Structural investigations on native collagen type I fibrils using AFM. Biochem. Biophys. Res. Commun. 2007, 354, 27–32. [Google Scholar] [CrossRef]
- Brodsky, B.; Thiagarajan, G.; Madhan, B.; Kar, K. Triple-helical peptides: An approach to collagen conformation, stability, and self-association. Biopolymers 2008, 89, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, K. Revisiting the molecular structure of collagen. Connect. Tissue Res. 2008, 49, 299–310. [Google Scholar] [CrossRef]
- Wolf, K.; Alexander, S.; Schacht, V.; Coussens, L.M.; von Andrian, U.H.; van Rheenen, J.; Deryugina, E.; Friedl, P. Collagen-based cell migration models in vitro and in vivo. Semin. Cell Dev. Biol. 2009, 20, 931–941. [Google Scholar] [CrossRef]
- Bella, J. A new method for describing the helical conformation of collagen: Dependence of the triple helical twist on amino acid sequence. J. Struct. Biol. 2010, 170, 377–391. [Google Scholar] [CrossRef]
- Okuyama, K.; Miyama, K.; Mizuno, K.; Bächinger, H.P. Crystal structure of (Gly-Pro-Hyp)(9): Implications for the collagen molecular model. Biopolymers 2012, 97, 607–616. [Google Scholar] [CrossRef]
- Adzhubei, A.A.; Sternberg, M.J.E.; Makarov, A.A. Polyproline-II helix in proteins: Structure and function. J. Mol. Biol. 2013, 425, 2100–2132. [Google Scholar] [CrossRef]
- Walters, B.D.; Stegemann, J.P. Strategies for directing the structure and function of three-dimensional collagen biomaterials across length scales. Acta Biomater. 2014, 10, 1488–1501. [Google Scholar] [CrossRef] [Green Version]
- Bella, J. Collagen structure: New tricks from a very old dog. Biochem. J. 2016, 473, 1001–1025. [Google Scholar] [CrossRef]
- Price, J.C.; Roach, P.; El Haj, A.J. Liquid crystalline ordered collagen substrates for applications in tissue engineering. ACS Biomater. Sci. Eng. 2016, 2, 625–633. [Google Scholar] [CrossRef]
- Bella, J.; Hulmes, D.J.S. Fibrillar collagens. In Fibrous Proteins: Structures and Mechanisms (Subcellular Biochemistry); Parry, D.A.D., Squire, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 82, pp. 457–490. [Google Scholar] [CrossRef]
- Darvish, D.M. Collagen fibril formation in vitro: From origin to opportunities. Mater. Today Bio 2022, 15, 100322. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Sol–gel technology and template synthesis in thin gelatin films. J. Sol-Gel Sci. Technol. 2014, 72, 314–327. [Google Scholar] [CrossRef]
- Engel, J. Investigation of the denaturation and renaturation of soluble collagen by light scattering. Arch. Biochem. Biophys. 1962, 97, 150–156. [Google Scholar] [CrossRef]
- Veis, A.; Drake, M.P. The Introduction of Intramolecular Covalent Cross-linkages into Ichthyocol Tropocollagen with Monofunctional Aldehydes. J. Biol. Chem. 1963, 238, 2003–2011. [Google Scholar] [CrossRef]
- Drake, M.P.; Veis, A. Interchain Interactions in Collagen-Fold Formation. I. The Kinetics of Renaturation of γ-Gelatin. Biochemistry 1964, 3, 135–145. [Google Scholar] [CrossRef]
- Yuan, L.; Veis, A. The self-assembly of collagen molecules. Biopolymers 1973, 12, 1437–1442. [Google Scholar] [CrossRef]
- Olsen, A.K. Evidence of Structure in Gelatin Gels. J. Phys. Chem. 1932, 36, 529–535. [Google Scholar] [CrossRef]
- Hauschka, P.V.; Harrington, W.F. Collagen structure in solution. III. Effect of cross-links on thermal stability and refolding kinetics. Biochemistry 1970, 9, 3734–3745. [Google Scholar] [CrossRef]
- Bianchi, E.; Conio, I.; Ciferri, A.; Puett, D.; Rajagh, L. The Role of pH, Temperature, Salt Type, and Salt Concentration on the Stability of the Crystalline, Helical, and Randomly Coiled Forms of Collagen. J. Biol. Chem. 1967, 242, 1361–1369. [Google Scholar] [CrossRef]
- Brönsted, J.N. Einige Bemerkungen über den Begriff der Säuren und Basen [Some observations about the concept of acids and bases]. Recl. Trav. Chim. Pays-Bas 1923, 42, 718–728. [Google Scholar] [CrossRef]
- Lowry, T.M. The uniqueness of hydrogen. J. Soc. Chem. Ind. 1923, 42, 43–47. [Google Scholar] [CrossRef]
- Paik, S.-H. Understanding the Relationship Among Arrhenius, Brønsted–Lowry, and Lewis Theories. J. Chem. Educ. 2015, 92, 1484–1489. [Google Scholar] [CrossRef]
- Kenchington, A.W.; Ward, A.G. The titration curve of gelatin. Biochem. J. 1954, 58, 202–207. [Google Scholar] [CrossRef]
- Sheppard, S.E.; Houck, R.C.; Dittmar, C. The Sorption of Soluble Dyes by Gelatin. J. Phys. Chem. 1942, 46, 158–176. [Google Scholar] [CrossRef]
- Hofmeister, F. Zur Lehre von der Wirkung der Salze. Arch. Exp. Pathol. Pharmakol. 1888, 24, 247–260. [Google Scholar] [CrossRef]
- Zhang, Y.; Cremer, P.S. Interactions between macromolecules and ions: The Hofmeister series. Curr. Opin. Chem. Biol. 2006, 10, 658–663. [Google Scholar] [CrossRef]
- Gustavson, K.H. The Chemistry and Reactivity of Collagen; Academic Press: Cambridge, MA, USA, 1956; pp. 171–172. [Google Scholar]
- Bianchi, E.; Conio, G.; Ciferri, A. The helix–coil transformation for tropocollagen solutions and its relationship to transformations involving the crystalline form of the protein. Biopolymers 1966, 4, 957–970. [Google Scholar] [CrossRef]
- Green, A.; Levenson, G.I.P. Emulsion Swelling During Washing, Etc. J. Photogr. Sci. 1974, 22, 194–198. [Google Scholar] [CrossRef]
- Mi, S.; Chen, B.; Wright, B.; Connon, C.J. Plastic compression of a collagen gel forms a much improved scaffold for ocular surface tissue engineering over conventional collagen gels. J. Biomed. Mater. Res. 2010, 95, 447–453. [Google Scholar] [CrossRef]
- Micol, L.A.; Ananta, M.; Engelhardt, E.M.; Mudera, V.C.; Brown, R.A.; Hubbell, J.A.; Frey, P. High-density collagen gel tubes as a matrix for primary human bladder smooth muscle cells. Biomaterials 2011, 32, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.G.; Courts, A. (Eds.) The Science and Technology of Gelatin; Academic Press: Cambridge, MA, USA, 1977; 564p. [Google Scholar]
- Maddox, R.L. An Experiment with Gelatino-Bromide. Br. J. Photogr. 1871, 18, 422–423. [Google Scholar]
- Mees, C.E.K.; James, T.H. The Theory of the Photographic Process, 3rd ed.; McMillan Publishing Co: New York, NY, USA; Collier McMillan Publishers: London, UK, 1967; 608p. [Google Scholar]
- James, T.H. The Theory of the Photographic Process, 4th ed.; McMillan Publishing Co: New York, NY, USA; Collier McMillan Publishers: London, UK, 1977; 714p. [Google Scholar]
- Calixto, S.; Ganzherli, N.; Gulyaev, S.; Figueroa-Gerstenmaier, S. Gelatin as a Photosensitive Material. Molecules 2018, 23, 2064. [Google Scholar] [CrossRef] [PubMed]
- Kirillov, N.I. Osnovy Protsessov Obrabotki Kinofotomaterialov (Fundamentals of Processing Film and Photo Materials); Iskusstvo: Moscow, Russia, 1977; 478p. (In Russian) [Google Scholar]
- Yashtold-Govorko, V.A. Fotos"yemka i Obrabotka (Photographic Shooting and Processing); Iskusstvo: Moscow, Russia, 1977; 344p. (In Russian) [Google Scholar]
- Red’ko, A.V. Osnovy Chyorno-Belykh i Tsvetnykh Fotoprotsessov (Fundamentals of Black-White and Color Photoprocesses); Iskusstvo: Moscow, Russia, 1990; 256p. (In Russian) [Google Scholar]
- Evans, R.M. Some Notes on Maxwell’s Colour Photograph. J. Photogr. Sci. 1961, 9, 243–246. [Google Scholar] [CrossRef]
- Vittum, P.W.; Weissberger, A. Recent Advances in the Chemistry of Dye-Forming Development. J. Photogr. Sci. 1958, 6, 157–169. [Google Scholar] [CrossRef]
- Koshofer, G. Farbfotografie (3 Bände). Band 1: Alte Verfahren. Die Zeit der frühen Pioniere. Farbrasterfotografie. Die alten Kopierverfahren und Geräte für Papierbilder und Diapositive. Vom Ausbleichverfahren zum Silberfarbstoff-Bleichverfahren; Laterna Magica: München, Germany, 1981; 189p. (In German) [Google Scholar]
- Koshofer, G. Farbfotografie (3 Bände). Band 2: Moderne Verfahren. Zeitalter der chromogenen Entwicklung. Bilder vom Dia und Negativ. Maskenverfahren. Das farbige Sofortbild; Laterna Magica: München, Germany, 1981; 240p. (In German) [Google Scholar]
- Koshofer, G. Farbfotografie (3 Bände). Band 3: Lexikon der Verfahren, Geräte und Materialien. Das System der Verfahren. Chronik der Farbfotografie; Laterna Magica: München, Germany, 1981; 152p. (In German) [Google Scholar]
- Penichon, S. Twentieth-Century Color Photographs: Identification and Care; Getty Publications: Los Angeles, CA, USA, 2013; 360p, ISBN 978-1-60606-156-5. [Google Scholar]
- Sviridov, V.V.; Kondrat’ev, V.A. Fotograficheskiye protsessy s besserebryanym fizicheskim proyavleniyem (Photographic processes with silver-free physical development). Uspekhi Nauchnoi Fotogr. 1978, 19, 43–64. (In Russian) [Google Scholar]
- Korzun, G.M.; Rakhmanov, S.K.; Belenkov, V.V.; Khvalyuk, V.N.; Vrublevsky, A.V. Enhancement of black-and-white silver image in redox processing II. Zh. Nauch. Priklad. Fotogr. 1991, 36, 366–370. (In Russian) [Google Scholar]
- Rakhmanov, S.K.; Belenkov, V.V.; Korzun, G.M. A photographic process based on the redox dispersion of silver. Zh. Nauch. Priklad. Fotogr. 1999, 44, 44–52. (In Russian) [Google Scholar]
- Branitskii, G.A.; Stashonok, V.D.; Sergeeva, O.V.; Sviridov, V.V. Photographic images based on the colloidal silver particles. Zh. Nauch. Priklad. Fotogr. 1999, 44, 1–10. (In Russian) [Google Scholar]
- Bokshits, Y.V.; Shevchenko, G.P.; Ponyavina, A.N.; Rakhmanov, S.K. Formation of silver and copper nanoparticles upon the reduction of their poorly soluble precursors in aqueous solution. Colloid J. 2004, 66, 517–522. [Google Scholar] [CrossRef]
- Kaliaha, A.E.; Sergeyeva, O.V.; Stashonok, V.D.; Rakhmanov, S.K. Optical properties of photosensitive layers containing nanosized silver particles. In Physics, Chemistry and Applications of Nanostructures—Reviews and Short Notes to Nanomeeting 2005; World Scientific: Singapore, 2005; pp. 382–385. [Google Scholar]
- Sharabanov, A.A.; Kalent’yev, V.K.; Mikhailov, O.V. «Pereosazhdeniye» elementnogo serebra v Ag-zhelatin-immobilizovannykh matrichnykh implantatakh s ispol’zovaniyem rastvorov, soderzhashchikh anion [BH4]—i kompozitsii N,N’-etilendiamintetraatsetatnykh kompleksov [Cu(II), Ni(II), Sn(II)], [Cu(II), Ni(II), Co(II)] i [Cu(II), Ni(II), Fe(II)]. (“Reprecipitation” of elemental silver in Ag-gelatin-immobilized matrix implants using solutions containing the [BH4]– anion and compositions of N,N′-ethylenediaminetetraacetate complexes [Cu(II), Ni(II), Sn(II)], [Cu(II), Ni(II), Co(II)] and [Cu(II), Ni(II), Fe(II)]). Izv. VUZov Ser. Khimiya I Khimicheskaya Tekhnologiya 2009, 52, 20–23. (In Russian) [Google Scholar]
- Kalent’yev, V.K.; Mikhailov, O.V. Usileniye izobrazheniy na AgHal-radiograficheskikh materialakh «pereosazhdeniyem» elementnogo serebra v rastvore, soderzhashchem kompleks M(II) s N,N’-etilendiamintetraatsetatom (M = Fe, Co) i anion [BH4]−.( Enhancement of images on AgHal radiographic materials by “reprecipitation” of elemental silver in a solution containing a complex of M(II) with N,N′-ethylenediaminetetraacetate (M = Fe, Co) and anion [BH4]−). Izv. VUZov Ser. Khimiya I Khimicheskaya Tekhnologiya 2010, 53, 54–58. (In Russian) [Google Scholar]
- Mikhailov, O.V.; Kondakov, A.V.; Krikunenko, R.I. Image Intensification in Silver Halide Photographic Materials for Detection of High-Energy Radiation by Reprecipitation of Elemental Silver. High Energy Chem. 2005, 39, 324–329. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Budnikov, G.K. 3d-Element Coordination Compounds with Bidentate Sulfur- Containing Ligands as Possible Carriers of Non-Silver Photographic Images. Bull. Chem. Soc. Jpn. 1989, 62, 4016–4020. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Polovnyak, V.K. Photography without Silver: Non- Silver Photographic Images Obtained from Metalorganic Complexes Having Strong Absorption. J. Imaging Sci. 1991, 35, 258–262. [Google Scholar]
- Mikhailov, O.V.; Polovnyak, V.K. Photographic Images Obtained from Ni(II) Complexes with Dithiooxamide and N,N’- Diphenyl-dithiooxamide. 日本写真学会誌 (J. Soc. Photogr. Sci. Technol. Jpn.) 1991, 54, 25–33. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Silverless Photographic Images Consisting of Complexes of Nickel (II) with Dimethylglyoxime. J. Photogr. Sci. 1993, 41, 199–202. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Polovnyak, V.K. Conversion of silver images on silver halide photographic materials into silverless consisting of iron(III) chelates with sulphanylquinoline and some of its derivatives. J. Mater. Chem. 1997, 7, 337–343. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Complexes of d-elements with chelate and macrocyclic ligands as promising components of non-silver photographic systems. Russ. Chem. Revs. 1997, 66, 665–678. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Coordination compounds as components of silverless photographic systems. J. Coord. Chem. 1999, 47, 31–59. [Google Scholar] [CrossRef]
- Pomogailo, A.D. Polimernye Immobilizovannye Metallokompleksnye Katalizatory (Polymer Immobilized Metal Complex Catalysts); Nauka: Moscow, Russia, 1988; pp. 10–20. ISBN 5-02-001390-0. (In Russian) [Google Scholar]
- Pomogailo, A.D. Catalysis by Polymer-Immobilized Metal Complexes; CRC Press: Boca Raton, FL, USA, 2020; pp. 14–24. [Google Scholar] [CrossRef]
- Yermakov, Y.I. Supported Catalysts Obtained by Interaction of Organometallic Compounds of Transition Elements with Oxide Supports. Catal. Rev. Sci. Eng. 1976, 13, 77–120. [Google Scholar] [CrossRef]
- Ballard, D.G.H. Transition metal alkyl compounds as polymerization catalysts. J. Polym. Sci. Polym. Chem. Ed. 1975, 13, 2191–2212. [Google Scholar] [CrossRef]
- Mathur, N.K.; Williams, R.E. Organic Syntheses Using Polymeric Supports, Polymeric Reagents, and Polymeric Catalysts. J. Macromol. Sci. C 1976, 15, 117–142. [Google Scholar] [CrossRef]
- Hartley, E.R. Supported Metal Complexes. A New Generation of Catalysis; Reidel Publishing: Dordrecht, Germany, 1985; 318p. [Google Scholar]
- Mikhailov, O.V. Complex formation processes in 3d-metal hexacyanoferrate(II) gelatin-immobilised matrices. Russ. Chem. Revs. 1995, 64, 657–673. [Google Scholar] [CrossRef]
- Kirschning, A.; Monenschein, H.; Wittenberg, R. Functionalized Polymers-Emerging Versatile Tools for Solution-Phase Chemistry and Automated Parallel Synthesis. Angew. Chem. Int. Ed. 2001, 40, 650–679. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Polovnyak, V.K. The reaction of Ni2[Fe(CN)6] with dithiooxamide in nickel(II)hexacyanoferrate(II) matrices immobilized in thin gelatin layers. Russ. J. Inorg. Chem. 1990, 35, 1169–1173. [Google Scholar]
- Mikhailov, O.V. Complex formation in the Cu2[Fe(CN)6]-dithiooxamide system in copper(II) hexacyanoferrate(II) immobilized in thin gelatin layer. Russ. J. Inorg. Chem. 1992, 37, 172–174. [Google Scholar]
- Mikhailov, O.V. From novel complexing conditions to novel coordination compounds of nickel(II) with dithiooxamide and its bulky analogues. Transit. Met. Chem. 1996, 21, 363–369. [Google Scholar] [CrossRef]
- Mikhailov, O.V. From novel complexing conditions to novel coordination compounds of copper(II) with dithiooxamide and its bulky analogues. Transit. Met. Chem. 1997, 22, 535–540. [Google Scholar] [CrossRef]
- Shigapova, L.S.; Mikhailov, O.V.; Khamitova, A.I. Complex Formation of Dioxouranium(VI) with 8-Hydroxyquinoline and 8-Mercaptoquinoline in Gelatin-immobilized (UO2)2[Fe(CN)6] Matrices. Russ. J. Gen. Chem. 1997, 67, 1935–1936. [Google Scholar]
- Mikhailov, O.V. Complexation of Cobalt(III) with 8- Mercaptoquinoline and its 5-Bromo and 5-Thiomethyl-substituted Derivatives in the KCo[Fe(CN)6]- Gelatin-Immobilized Matrix. Russ. J. Coord. Chem. 1997, 23, 850–855. [Google Scholar]
- Mikhailov, O.V.; Kazymova, M.A. Novel coordination compounds of nickel(II) and copper(II) with N,N’-diphenylthiooxamide; formation of complexed metalhexacyanoferrate(II) gelatin-immobilized matrix systems. Transit. Met. Chem. 1998, 23, 195–199. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Complexation of Nickel(II) with Dioximes in Ni2[Fe(CN)6] Gelatin-Immobilized Matrix Implants. Russ. J. Coord. Chem. 2002, 28, 352–357. [Google Scholar] [CrossRef]
- Tatarintseva, T.B.; Mikhailov, O.V.; Brus’ko, V.V.; Zabirov, N.G. M(II)- N-diisoproxythiophosphorylthiobenzamide complexing processes in M2[Fe(CN)6]-gelatin-immobilized matrices (M = Co, Ni, Cu). Transit. Met. Chem. 2002, 27, 423–428. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Complexation in Binary Pb(II)–Dithiooxamide System in Pb2[Fe(CN)6] Gelatin-Immobilized Matrices. Russ. J. Coord. Chem. 2003, 29, 276–280. [Google Scholar] [CrossRef]
- Khamitova, A.I.; Mikhailov, O.V. Mild Template Synthesis of Co(III)-Dithiooxamide- Glyoxal in Gelatin-immobilized KCoFe(CN)6 Matrix Systems. Russ. J. Gen. Chem. 1997, 67, 1913–1920. [Google Scholar]
- Khamitova, A.I.; Mikhailov, O.V. Mild template synthesis of nickel (II) and copper(II) chelates with an (N,N,S,S)-tetradentate ligand in metal hexacyanoferrate(II) immobilized matrix systems. Mendeleev Commun. 1998, 8, 96–97. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Khamitova, A.I. Low-temperaturic template synthesis of macrocyclic cobalt(III) chelates with (N,N,S,S)-donor atomic ligands in the cobalt(II)-dithiooxamide-formaldehyde and cobalt(II)-dithiooxamide-glyoxal systems in the Co2[Fe(CN)6]-gelatin-immobilized matrices. Transit. Met. Chem. 2000, 25, 26–31. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Soft template synthesis in copper(II)-dithiooxamide-methanal, copper(II)-dithiooxamide-ethanal and copper(II)-dithiooxamide-propanone triple systems in a copper(II)hexacyanoferrate(II) gelatin-immobilized matrix. Transit. Met. Chem. 2000, 25, 552–558. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Khamitova, A.I.; Morozov, V.I. Mild template synthesis of (2,7-dithio-3,6- diazaoctadien-3,5-dithioamide-1,8)nickel(II) and (2,7-dithio-3,6-diazaoctadien-3,5-dithioamide- 1,8)copper(II) in the Ni2[Fe(CN)6]- and Cu2[Fe(CN)6]-gelatin-immobilized matrix systems. Int. J. Inorg. Mater. 2001, 3, 161–167. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Low-temperature template synthesis of (2,8-dithio-3,7-diaza- 5-oxanonandithioamide-1,9)nickel(II), (2,8-dithio-3,7-diaza-4,6-dimethyl-5-oxanonandithioamide- 1,9)nickel(II) and (4,4′,6-trimethyl-2,8-dithio-3,7-diazanonen-6-dithioamide-1,9)nickel(II) in thin films of nanostructured Ni2[Fe(CN)6]-gelatin-immobilized matrix materials. Int. J. Inorg. Mater. 2001, 3, 1053–1061. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Mild template synthesis of a copper(II)-containing macrocyclic compound with 4,4,6-trimethyl-2,3,7,8-tetraazanonen-6-dithiohydrazide-1,9 in a gelatin-immbolized matrix. Transit. Met. Chem. 2003, 28, 665–667. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Kazymova, M.A.; Shumilova, T.A.; Chmutova, G.A.; Solovieva, S.E. Template synthesis in the nickel(II)-thiocarbohydrazide-propanone triple system. Transit. Met. Chem. 2005, 30, 299–304. [Google Scholar] [CrossRef]
- Chachkov, D.V.; Mikhailov, O.V. Mild template synthesis in the iron(III)- ethanedithioamide-1,2- formaldehyde triple system on a K[Fe2(CN)6]-gelatin-immobilized matrix. J. Coord. Chem. 2009, 62, 1058–1066. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Chachkov, D.V. Self-assembly of supramolecular Ni(II) and Cu(II) metalmacrocyclic compounds with tetraazamacrocyclic ligand into a gelatin-immobilized matrix. J. Coord. Chem. 2010, 63, 4309–4318. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Kazymova, M.A.; Chachkov, D.V. On Template Synthesis in the Ternary System Ni(II)– Thiosemicarbazide– Diacetyl. Russ. J. Inorg. Chem. 2014, 59, 60–64. [Google Scholar] [CrossRef]
- Chachkov, D.V.; Mikhailov, O.V. Self-assembly of supramolecular complex of Zn(II) and 2,7-dithio-3,6-diazaoctadien-3,5-dithioamide-1,8 in an immobilized Zn2[Fe(CN)6]-gelatin matrix. Eur. Chem. Bull. 2014, 3, 367–371. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Soft template synthesis of aza- and thiazametalmacrocyclic compounds in thin gelatin films. Eur. Chem. Bull. 2014, 3, 976–991. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Nizkotemperaturnyi templatnyi sintez v metallgeksatsianoferrat(II)nykh zhelatin-immobilizovannykh matrichnykh sistemakh (Low-Temperature Template Synthesis in Metal Hexacyanoferrate(II) Gelatin-Immobilized Matrix Systems). Ross. Khim. Zhurn. 2000, 44, 70–79. (In Russian) [Google Scholar]
- Mikhailov, O.V. Substitution reactions and template synthesis in the metal hexacyanoferrate(II) gelatin-immobilized matrix systems. Russ. J. Coord. Chem. 2000, 26, 702–713. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Gelatin-Immobilized Metalcomplexes: Synthesis and Employment. J. Coord. Chem. 2008, 61, 1333–1384. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Self-Assembly of Molecules of Metal Macrocyclic Compounds in Nanoreactors on the Basis of Biopolymer-Immobilized Matrix Systems. Nanotechnol. Russ. 2010, 5, 18–34. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Synthesis of 3d-element metal-macrocyclic chelates into polypeptide biopolymer medium and their molecular structures. Inorg. Chim. Acta 2013, 394, 664–684. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Kazymova, M.A.; Chachkov, D.V. Self-assembly and quantum-chemical design of macrotricyclic and macrotetracyclic metalchelates of 3d-elements formed in the gelatin-immobilized matrix. Russ. Chem. Bull. 2015, 64, 1757–1771. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Molecular structure design and soft template synthesis of aza-, oxaaza- and thiaazamacrocyclic metal chelates in the gelatin matrix. Arab. J. Chem. 2017, 10, 47–67. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Polycyclic 3d-metalchelates formed owing to inner-sphere transmutations in the gelatin matrix: Synthesis and structures. Revs. Inorg. Chem. 2017, 37, 71–94. [Google Scholar] [CrossRef]
- Mikhailov, O.V. The theory of heterogeneous complexation on immobilized matrices of hexacyanoferrates(II) 3d-elements. Sov. J. Coord. Chem. 1992, 18, 1008–1017. [Google Scholar]
- Mikhailov, O.V. Synthesis of Gelatin- Immobilized Hexacyanoferrates of p-, d- and f-Metals. Russ. J. Gen. Chem. 1998, 68, 827–828. [Google Scholar]
- Mikhailov, O.V.; Rozhentsov, R.A. Immobilization of Silver(I) Hexacyanoferrate(II) in Thin Gelatin Layer. Russ. J. Gen. Chem. 2001, 71, 809–810. [Google Scholar] [CrossRef]
- Gafarov, M.P.; Mikhailov, O.V.; Yusupov, R.A. Lead(II) Sulfide. Synthesis in Lead (II) Tetraoxophosphate(V) Gelatin-immobilized Matrix Implantates and Sorption Activity Toward Silver(I) Ions. Russ. J. Gen. Chem. 2003, 73, 1183–1187. [Google Scholar] [CrossRef]
- Gafarov, M.P.; Mikhailov, O.V. Reaction of Gelatin-immobilized Lead(II) Hexacyanoferrate(II) with Dithioxamide. Russ. J. Gen. Chem. 2004, 74, 960–961. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Electron Microscopical Study of Lead(II) Sulfide Implanted into a Glassy Biopolymer Matrix. Glass Phys. Chem. 2017, 43, 191–193. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for the quantitation of microgram quantities on of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Fujita, Y.; Mori, I.; Kitano, S. Color reaction between Pyrogallol Red—Molybdenium (VI) complex and protein. Bunseki Kagaku 1983, 32, 379–386. (In Japanese) [Google Scholar] [CrossRef]
- Temerdashev, Z.A.; Pochinok, T.B.; Tarasova, P.V.; Gosteva, M.A. Issledovaniye immobilizatsii brompirogallolovogo krasnogo v zhelatinovuyu matritsu i otsenka vozmozhnosti sozdaniya na yeye osnove opticheski prozrachnogo sensora dlya opredeleniya metallov (Investigation of the immobilization of brompyrogallol red in a gelatin matrix and evaluation of the possibility of creating an optically transparent sensor based on it for the determination of metals). Anal. Kontrol’ 2012, 16, 39–45. (In Russian) [Google Scholar]
- Reshetnyak, E.A.; Ivchenko, N.V.; Nikitina, N.A. Indikatornyye plenki na osnove otverzhdennogo zhelatinovogo gelya s immobilizovannymi metalloindikatorami (Indicator films based on hardened gelatin gel with immobilized metal indicators). Metod. I Ob"Yekty Khimicheskogo Anal. 2012, 7, 192–201. (In Russian) [Google Scholar]
- Anisimovich, P.V.; Temerdashev, Z.A.; Pochinok, T.B.; Reshetnyak, E.A. Immobilizatsiya pirogallolovogo krasnogo zhelatinovym gelem i ispol’zovaniye kompozita dlya opredeleniya obshchego belka (Immobilization of pyrogallol red gelatin gel and use of the composite for total protein determination). Sorbtsion. Khromatograf Protsessy 2015, 15, 223–233. (In Russian) [Google Scholar]
- Jaiswal, N.; Prakash, O.; Talat, M.; Hasan, S.H.; Pandey, R.K. α-Amylase immobilization on gelatin: Optimization of process variables. J. Gen. Eng. Biotechnol. 2012, 10, 161–167. [Google Scholar] [CrossRef]
- Scardi, V. Immobilization of enzymes and microbial cells in gelatin. Methods Enzymol. 1987, 135, 293–299. [Google Scholar] [CrossRef]
- Naganagouda, K.; Mulimani, V.H. Gelatin blends with alginate: Gel fibers for α-galactosidase immobilization and its application in reduction of non-digestible oligosaccharides in soymilk. Process. Biochem. 2006, 41, 1903–1907. [Google Scholar] [CrossRef]
- Nagatomo, H.; Matsushita, Y.; Sugamoto, K.; Matsui, T. Preparation and Properties of Gelatin-Immobilized β-Glucosidase from Pyrococcus furiosus. Biosci. Biotechnol. Biochem. 2005, 69, 128–136. [Google Scholar] [CrossRef]
- Bayramoglu, Z.; Akbulut, U.; Sungur, S. Immobilization of alfa-amylase into gelatin films with various cross-linkers. Bioorg. Med. Chem. Lett. 1992, 2, 427–432. [Google Scholar] [CrossRef]
- Sunger, S.; Elcin, M.; Akbulut, U. Studies on immobilization of urease in gelatin by cross-linking. Biomaterials 1992, 13, 795–800. [Google Scholar] [CrossRef]
- Fuchsbauer, H.-L.; Gerber, U.; Engelmann, J.; Seeger, T.; Sinks, C.; Hecht, T. Influence of gelatin matrices cross-linked with transglutaminase on the properties of an enclosed bioactive material using -galactosidase as model system. Biomaterials 1996, 17, 1481–1488. [Google Scholar] [CrossRef]
- Labus., K.; Wolanin, K.; Radosinski, Ł. Comparative Study on Enzyme Immobilization Using Natural Hydrogel Matrices—Experimental Studies Supported by Molecular Models Analysis. Catalysts 2020, 10, 489. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Naumkina, N.I.; Mikhailov, O.V.; Kondakov, A.V.; Lygina, T.Z. On a New Phase of Elemental Silver, Appearing on Its “Reprecipitation” in Ag–Gelatin-Immobilized Matrix Systems. Russ. J. Gen. Chem. 2008, 78, 1650–1654. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Naumkina, N.I. Novel modification of elemental silver formed in Ag4[Fe(CN)6]-gelatin-immobilized matrix Implants. Cent. Eur. J. Chem. 2010, 8, 448–452. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Synthesis of Ag nanoparticles under a contact of water solution with silver(I)chloride biopolymer matrix. J. Mol. Liq. 2019, 291, 111354. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Mikhailova, E.O. Elemental Silver Nanoparticles: Biosynthesis and Bio Applications. Materials 2019, 12, 3177. [Google Scholar] [CrossRef]
- Nishimoto, M.; Sakamoto, R.; Mizuta, S.; Yoshinaka, R. Identification and characterization of molecular species of collagen in ordinary muscle and skin of the Japanese flounder (Paralichthys olivaceus). Food Chem. 2005, 90, 151–156. [Google Scholar] [CrossRef]
- Desai, M.P.; Labhasetwar, V.; Amidon, G.L.; Levy, R.J. Gastrointestinal uptake of biodegradable microparticles: Effect of particle size. Pharm. Res. 1996, 13, 1838–1845. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Cortesi, R.; Nastruzzi, C. Gelatin microspheres: Influence of preparation parameters and thermal treatment on chemico-physical and biopharmaceutical properties. Biomaterials 1996, 7, 2009–2020. [Google Scholar] [CrossRef]
- Vandelli, M.; Romagnoli, M.; Monti, A.; Gozzi, M.; Guerra, P.; Rivasi, F.; Forni, F. Microwave treated gelatin microspheres as drug delivery system. J. Control. Release 2004, 96, 67–84. [Google Scholar] [CrossRef]
- Way, D.V.; Nele, M.; Pinto, J.C. Preparation of gelatin beads treated with glucose and glycerol. Polímeros 2018, 28, 468–476. [Google Scholar] [CrossRef]
- Park, K. Biomaterials for Cancer Therapeutics: Diagnosis, Prevention and Therapy; Woodhead Publishing: Sawston, UK, 2013; 542p, ISBN 0857096648. [Google Scholar]
- Park, K. Biomaterials for Cancer Therapeutics: Evolution and Innovation, 2nd ed.; Elsevier: Amsterdam, The Netherlands; Woodhead Publishing: Sawston, UK, 2020; 782p, ISBN 9780081029831. [Google Scholar]
- Magadala, P.; Amiji, M. Epidermal growth factor receptor-targeted gelatin-based engineered nanocarriers for DNA delivery and transfection in human pancreatic cancer cells. AAPS J. 2008, 10, 565–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, C.L.; Wu, S.Y.; Wang, W.H.; Peng, C.L.; Lin, F.H.; Lin, C.C.; Young, T.H.; Shieh, M.J. Targeting efficiency and biodistribution of biotinylated-egf-conjugated gelatin nanoparticles administered via aerosol delivery in nude mice with lung cancer. Biomaterials 2008, 29, 3014–3022. [Google Scholar] [CrossRef]
- Wei, B.; Wei, Y.; Zhang, K.; Wang, J.; Xu, R.; Zhan, S.; Lin, G.; Wang, W.; Liu, M.; Wang, L.; et al. Development of an antisense rna delivery system using conjugates of the ms2 bacteriophage capsids and hiv-1 tat cell-penetrating peptide. Biomed. Pharmacother. 2009, 63, 313–318. [Google Scholar] [CrossRef]
- Tseng, C.L.; Su, W.Y.; Yen, K.C.; Yang, K.C.; Lin, F.H. The use of biotinylated-egf-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. Biomaterials 2009, 30, 3476–3485. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.H.; Wei, F.; Wang, T.X.; Wang, D.; Wang, J.; Lin, X.N.; Wang, P.; Ren, L. Blood-brain barrier transport of tat peptide and polyethylene glycol decorated gelatin-siloxane nanoparticle. Mater. Lett. 2012, 68, 94–96. [Google Scholar] [CrossRef]
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef]
- Nezhad-Mokhtari, P.; Arsalani, N.; Javanbakht, S.; Shaabani, A. Development of gelatin microsphere encapsulated Cu-based metal-organic framework nanohybrid for the methotrexate delivery. J. Drug Deliv. Sci. Technol. 2019, 50, 174–180. [Google Scholar] [CrossRef]
- Santoro, M.; Tatara, A.M.; Mikos, A.G. Gelatin carriers for drug and cell delivery in tissue engineering. J. Control. Release 2014, 190, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Mariod, A.A.; Adam, H.F. Review: Gelatin, source, extraction and industrial applications. Acta Sci. Pol. Technol. Aliment. 2013, 12, 135–147. [Google Scholar]
- Wheeler, D.L.; Dunn, E.F.; Harari, P.M. Understanding resistance to egfr inhibitors-impact on future treatment strategies. Nat. Rev. Clin. Oncol. 2010, 7, 493–507. [Google Scholar] [CrossRef]
- Sivakumar, M.; Panduranga, R.K. Preparation, characterization and in vitro release of gentamicin from coralline hydroxyapatite-gelatin composite microspheres. Biomaterials 2002, 23, 3175–3181. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, H.E.; Salih, V. Stimulation of Osteoblast Responses to Biomimetic Nanocomposites of Gelatin-Hydroxyapatite for Tissue Engineering Scaffolds. Biomaterials 2005, 26, 5221–5230. [Google Scholar] [CrossRef]
- Kim, H.W.; Knowles, J.C.; Kim, H.E. Porous scaffolds of gelatin-hydroxyapatite nanocomposites obtained by biomimetic approach: Characterization and antibiotic drug release. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 686–698. [Google Scholar] [CrossRef]
- Teng, S.; Chen, L.; Guo, Y.; Shi, J. Formation of nano-hydroxyapatite in gelatin droplets and the resulting porous composite microspheres. J. Inorg. Biochem. 2007, 101, 686–691. [Google Scholar] [CrossRef]
- Landi, E.; Valentini, F.; Tampieri, A. Porous hydroxyapatite/gelatine scaffolds with ice-designed channel-like porosity for biomedical applications. Acta Biomater. 2008, 4, 1620–1626. [Google Scholar] [CrossRef]
- Dressler, M.; Dombrowski, F.; Simon, U.; Börnstein, J.; Hodoroaba, V.; Feigl, M.; Grunow, S.; Gildenhaar, R.; Neumann, M. Influence of gelatin coatings on compressive strength of porous hydroxyapatite ceramics. J. Eur. Ceram. Soc. 2011, 31, 523–529. [Google Scholar] [CrossRef]
- Perez, R.A.; Del Valle, S.; Altankov, G.; Ginebra, M.P. Porous hydroxyapatite and gelatin/hydroxyapatite microspheres obtained by calcium phosphate cement emulsion. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 97, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.P.; Traykova, T.; Planell, J.A. Calcium phosphate cements as bone drug delivery systems: A review. J. Control. Release 2006, 113, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.J.; de Jonge, L.T.; Wolke, J.G.; Yubao, L.; Mikos, A.G.; Jansen, J.A. Introduction of gelatin microspheres into an injectable calcium phosphate cement. J. Biomed. Mater. Res. A. 2008, 87, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.J.; Boerman, O.C.; Wolke, J.G.; Mikos, A.G.; Jansen, J.A. In vitro growth factor release from injectable calcium phosphate cements containing gelatin microspheres. J. Biomed. Mater. Res. A. 2009, 91, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, X.; Ge, B. Calcium phosphate cement with bmp-2-loaded gelatin microspheres enhances bone healing in osteoporosis: A pilot study. Clin. Orthop. Relat. Res. 2010, 468, 1978–1985. [Google Scholar] [CrossRef]
- Liao, H.; Walboomers, X.F.; Habraken, W.J.; Zhang, Z.; Li, Y.; Grijpma, D.W.; Mikos, A.G.; Wolke, J.G.; Jansen, J.A. Injectable calcium phosphate cement with plga, gelatin and ptmc microspheres in a rabbit femoral defect. Acta Biomater. 2011, 7, 1752–1759. [Google Scholar] [CrossRef]
- Thacharodi, D.; Panduranga, R.K. Collagen-chitosan composite membranes for controlled release of propranolol hydrochloride. Int. J. Pharm. 1995, 120, 115–118. [Google Scholar] [CrossRef]
- Zhao, F.; Yin, Y.; Lu, W.W.; Leong, J.C.; Zhang, W.; Zhang, J.; Zhang, M.; Yao, K. Preparation and histological evaluation of biomimetic three-dimensional hydroxyapatite/chitosan-gelatin network composite scaffolds. Biomaterials 2002, 23, 3227–3234. [Google Scholar] [CrossRef]
- Sinha, V.R.; Singla, A.K.; Wadhawan, S.; Kaushik, R.; Kumria, R.; Bansal, K.; Dhawan, S. Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 2004, 274, 1–33. [Google Scholar] [CrossRef]
- Zhao, F.; Grayson, W.L.; Ma, T.; Bunnell, B.; Lu, W.W. Effects of hydroxyapatite in 3-d chitosan-gelatin polymer network on human mesenchymal stem cell construct development. Biomaterials 2006, 27, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, H.J.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials 2006, 27, 6064–6082. [Google Scholar] [CrossRef]
- Liu, H.; Fan, H.; Cui, Y.; Chen, Y.; Yao, K.; Goh, J.C. Effects of the controlled-released basic fibroblast growth factor from chitosan-gelatin microspheres on human fibroblasts cultured on a chitosan-gelatin scaffold. Biomacromolecules 2007, 8, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.B.; Mann, J.K.; Kundu, S.C. Silk fibroin/gelatin multilayered films as a model system for controlled drug release. Eur. J. Pharm. Sci. 2009, 37, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Shubhra, Q.T.H.; Alam, A.K.M.M.; Beg, M.D.H. Mechanical and degradation characteristics of natural silk fiber reinforced gelatin composites. Mater. Lett. 2011, 65, 333–336. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan and its binding proteins, the hyaladherins. Curr. Opin. Cell Biol. 1990, 2, 839–844. [Google Scholar] [CrossRef]
- Luo, Y.; Kirker, K.R.; Prestwich, G.D. Cross-linked hyaluronic acid hydrogel films: New biomaterials for drug delivery. J. Control. Release 2000, 69, 169–184. [Google Scholar] [CrossRef]
- Shu, X.Z.; Liu, Y.C.; Palumbo, F.; Prestwich, G.D. Disulfide-crosslinked hyaluronan-gelatin hydrogel films: A covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials 2003, 24, 3825–3834. [Google Scholar] [CrossRef]
- Allison, D.D.; Grande-Allen, K.J. Review. Hyaluronan: A powerful tissue engineering tool. Tissue Eng. 2006, 12, 2131–2140. [Google Scholar] [CrossRef]
- Li, J.; He, A.; Zheng, J.; Han, C.C. Gelatin and gelatin-hyaluronic acid nanofibrous membranes produced by electrospinning of their aqueous solutions. Biomacromolecules 2006, 7, 2243–2247. [Google Scholar] [CrossRef] [PubMed]
- Peattie, R.A.; Pike, D.B.; Yu, B.; Cai, S.; Shu, X.Z.; Prestwich, G.D.; Firpo, M.A.; Fisher, R.J. Effect of gelatin on heparin regulation of cytokine release from hyaluronan-based hydrogels. Drug Deliv. 2008, 15, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Hosack, L.W.; Firpo, M.A.; Scott, J.A.; Prestwich, G.D.; Peattie, R.A. Microvascular maturity elicited in tissue treated with cytokine-loaded hyaluronan-based hydrogels. Biomaterials 2008, 29, 2336–2347. [Google Scholar] [CrossRef] [PubMed]
- Tracy, M.A.; Ward, K.L.; Firouzabadian, L.; Wang, Y.; Dong, N.; Qian, R.; Zhang, Y. Factors affecting the degradation rate of poly(lactide-co-glycolide) microspheres in vivo and in vitro. Biomaterials 1999, 20, 1057–1062. [Google Scholar] [CrossRef]
- Zeng, J.; Xu, X.; Chen, X.; Liang, Q.; Bian, X.; Yang, L.; Jing, X. Biodegradable electrospun fibers for drug delivery. J. Control. Release 2003, 92, 227–231. [Google Scholar] [CrossRef]
- Persson, G.R.; Salvi, G.E.; Heitz-Mayfield, L.J.; Lang, N.P. Antimicrobial therapy using a local drug delivery system (arestin) in the treatment of peri-implantitis. I: Microbiological outcomes. Clin. Oral Implant. Res. 2006, 17, 386–393. [Google Scholar] [CrossRef]
- Tan, H.; Huang, D.; Lao, L.; Gao, C. Rgd modified plga/gelatin microspheres as microcarriers for chondrocyte delivery. J. Biomed. Mater. Res. B Appl Biomater. 2009, 91, 228–238. [Google Scholar] [CrossRef]
- Meng, Z.; Wang, Y.; Ma, C.; Zheng, W.; Li, L.; Zheng, Y. Electrospinning of plga/gelatin and randomlyoriented and aligned nanofibers as potential scaffold in tissue engineering. Mater. Sci. Eng. C 2010, 30, 1204–1210. [Google Scholar] [CrossRef]
- Meng, Z.X.; Xu, X.X.; Zheng, W.; Zhou, H.M.; Li, L.; Zheng, Y.F.; Lou, X. Preparation and characterization of electrospun plga/gelatin nanofibers as a potential drug delivery system. Coll. Surf. B Biointerfaces 2011, 84, 97–102. [Google Scholar] [CrossRef]
- Holland, T.A.; Tabata, Y.; Mikos, A.G. In vitro release of transforming growth factor-beta 1 from gelatin microparticles encapsulated in biodegradable, injectable oligo(poly(ethylene glycol) fumarate) hydrogels. J. Control. Release 2003, 91, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Holland, T.A.; Tessmar, J.K.; Tabata, Y.; Mikos, A.G. Transforming growth factor-beta 1 release from oligo(poly(ethylene glycol) fumarate) hydrogels in conditions that model the cartilage wound healing environment. J. Control. Release 2004, 94, 101–114. [Google Scholar] [CrossRef]
- Temenoff, J.S.; Park, H.; Jabbari, E.; Conway, D.E.; Sheffield, T.L.; Ambrose, C.G.; Mikos, A.G. Thermally cross-linked oligo(poly(ethylene glycol) fumarate) hydrogels support osteogenic differentiation of encapsulated marrow stromal cells in vitro. Biomacromolecules 2004, 5, 5–10. [Google Scholar] [CrossRef]
- Holland, T.A.; Tabata, Y.; Mikos, A.G. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J. Control. Release 2005, 101, 111–125. [Google Scholar] [CrossRef]
- Holland, T.A.; Bodde, E.W.; Baggett, L.S.; Tabata, Y.; Mikos, A.G.; Jansen, J.A. Osteochondral repair in the rabbit model utilizing bilayered, degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds. J. Biomed. Mater. Res. A. 2005, 75, 156–167. [Google Scholar] [CrossRef]
- Kasper, F.K.; Kushibiki, T.; Kimura, Y.; Mikos, A.G.; Tabata, Y. In vivo release of plasmid DNA from composites of oligo(poly(ethylene glycol)fumarate) and cationized gelatin microspheres. J. Control. Release 2005, 107, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Nejadnik, M.R.; Mikos, A.G.; Jansen, J.A.; Leeuwenburgh, S.C. Facilitating the mineralization of oligo(poly(ethylene glycol) fumarate) hydrogel by incorporation of hydroxyapatite nanoparticles. J. Biomed. Mater. Res. A. 2012, 100, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Wang, S.; Fox, B.C.; Ritman, E.L.; Yaszemski, M.J.; Lu, L. Poly(propylene fumarate) bone tissue engineering scaffold fabrication using stereolithography: Effects of resin formulations and laser parameters. Biomacromolecules 2007, 8, 1077–1084. [Google Scholar] [CrossRef]
- Fisher, J.P.; Vehof, J.W.; Dean, D.; van der Waerden, J.P.; Holland, T.A.; Mikos, A.G.; Jansen, J.A. Soft and hard tissue response to photocrosslinked poly(propylene fumarate) scaffolds in a rabbit model. J. Biomed. Mater. Res. 2002, 59, 547–556. [Google Scholar] [CrossRef]
- Dean, D.; Topham, N.S.; Meneghetti, S.C.; Wolfe, M.S.; Jepsen, K.; He, S.; Chen, J.E.; Fisher, J.P.; Cooke, M.; Rimnac, C.; et al. Poly(propylene fumarate) and poly(dl-lactic-co-glycolic acid) as scaffold materials for solid and foam-coated composite tissue-engineered constructs for cranial reconstruction. Tissue Eng. 2003, 9, 495–504. [Google Scholar] [CrossRef]
- Lewandrowski, K.U.; Cattaneo, M.V.; Gresser, J.D.; Wise, D.L.; White, R.L.; Bonassar, L.; Trantolo, D.J. Effect of a poly(propylene fumarate) foaming cement on the healing of bone defects. Tissue Eng. 1999, 5, 305–316. [Google Scholar] [CrossRef]
- Patel, Z.S.; Ueda, H.; Yamamoto, M.; Tabata, Y.; Mikos, A.G. In vitro and in vivo release of vascular endothelial growth factor from gelatin microparticles and biodegradable composite scaffolds. Pharm. Res. 2008, 25, 2370–2378. [Google Scholar] [CrossRef]
- Kempen, D.H.; Lu, L.; Heijink, A.; Hefferan, T.E.; Creemers, L.B.; Maran, A.; Yaszemski, M.J.; Dhert, W.J. Effect of local sequential vegf and bmp-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials 2009, 30, 2816–2825. [Google Scholar] [CrossRef]
- Lewis, P.L.; Green, R.M.; Shah, R.N. 3D-printed gelatin scaffolds of differing pore geometry modulate hepatocyte function and gene expression. Acta Biomater. 2018, 69, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Claaßen, C.; Southan, A.; Grübel, J.; Tovar, G.E.M.; Borchers, K. Interactions of methacryloylated gelatin and heparin modulate physico-chemical properties of hydrogels and release of vascular endothelial growth factor. Biomed. Mater. 2018, 13, 55008. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Li, Q.; Wen, H.; Chen, J.; Liang, M.; Huang, H.; Lan, D.; Dong, H.; Cao, X. Injection and self-assembly of bioinspired stem cell-laden gelatin/hyaluronic acid hybrid microgels promote cartilage repair in vivo. Adv. Funct. Mater. 2019, 29, 1906690. [Google Scholar] [CrossRef]
- Sattary, M.; Rafienia, M.; Kazemi, M.; Salehi, H.; Mahmoudzadeh, M. Promoting effect of nano hydroxyapatite and vitamin D3 on the osteogenic differentiation of human adipose-derived stem cells in polycaprolactone/gelatin scaffold for bone tissue engineering. Mater. Sci. Eng. C 2019, 97, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Distler, T.; McDonald, K.; Heid, S.; Karakaya, E.; Detsch, R.; Boccaccini, A.R. Ionically and enzymatically dual cross-linked oxidized alginate gelatin hydrogels with tunable stiffness and degradation behavior for tissue engineering, ACS Biomater. Sci. Eng. 2020, 6, 3899–3914. [Google Scholar] [CrossRef]
- Distler, T.; Polley, C.; Shi, F.; Schneidereit, D.; Ashton, M.D.; Friedrich, O.; Kolb, J.F.; Hardy, J.G.; Detsch, R.; Seitz, H.; et al. Electrically conductive and 3Dprintable oxidized alginate-gelatin polypyrrole: PSS hydrogels for tissue engineering. Adv. Healthc. Mater. 2021, 10, 2001876. [Google Scholar] [CrossRef]
- Dutta, S.D.; Hexiu, J.; Patel, D.K.; Ganguly, K.; Lim, K.T. 3D-printed bioactive and biodegradable hydrogel scaffolds of alginate/gelatin/cellulose nanocrystals for tissue engineering. Int. J. Biol. Macromol. 2021, 167, 644–658. [Google Scholar] [CrossRef]
- Nelson, M.; Li, S.; Page, S.J.; Shi, X.; Lee, P.D.; Stevens, M.M.; Hanna, J.V.; Jones, J.R. 3D printed silica-gelatin hybrid scaffolds of specific channel sizes promote collagen Type II, Sox9 and Aggrecan production from chondrocytes. Mater. Sci. Eng. C 2021, 123, 111964. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, S.; Zhang, C.; Ikoma, T.; Guo, H.; Zhang, X.; Li, X.; Chen, W. Novel microsphere-packing synthesis, microstructure, formation mechanism and in vitro biocompatibility of porous gelatin/hydroxyapatite microsphere scaffolds. Ceram. Int. 2021, 47, 32187–32194. [Google Scholar] [CrossRef]
- Kara, A.; Distler, T.; Polley, C.; Schneidereit, D.; Seitz, H.; Friedrich, O.; Tihminlioglu, F.; Boccaccini, A.R. 3D printed gelatin/decellularized bone composite scaffolds for bone tissue engineering: Fabrication, characterization and cytocompatibility study. Mater. Today Bio 2022, 15, 100309. [Google Scholar] [CrossRef] [PubMed]
- Gaihre, B.; Liu, X.; Miller, A.L.; Yaszemski, M.; Lu, L. Poly(Caprolactone Fumarate) and Oligo[Poly(Ethylene Glycol) Fumarate]: Two Decades of Exploration in Biomedical Applications. Polym. Revs. 2021, 61, 319–356. [Google Scholar] [CrossRef]
- Anfinsen, C.B. Principles that Govern the Folding of Protein Chains. Science 1973, 181, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I.; Schaefer, V.J.; Wrinch, D.M. Built-up films of proteins and their properties. Science 1937, 2194, 76–80. [Google Scholar] [CrossRef]
- Demeny, M.; Kochwa, S.; Sobotka, H. Aggregation of Ig G globulin in vivo III. Study of Ig G globulin aggregation by unimolecular layer method. J. Coll. Interface Sci. 1966, 22, 144–148. [Google Scholar] [CrossRef]
- Yamashito, T.; Bull, H.B. Spread monolayers of lysozyme. J. Coll. Interface Sci. 1967, 24, 310–316. [Google Scholar] [CrossRef]
- Ter-Minnassian-Saraga, L. Reporting experimental pressure-area data with film balances (Recommendations 1984). Pure Appl. Chem. 1985, 57, 621–632. [Google Scholar] [CrossRef]
- McRitchie, F. Spread monolayers of proteins. Adv. Coll. Interface Sci. 1986, 25, 341–385. [Google Scholar] [CrossRef]
- Holly, F.J.; Dolowy, K.; Yamada, K.M. Comparative surface chemical studies of cellular fibronectin and submaxillary mucin monolayers: Effects of pH, ionic strength, and presence of calcium ions. J. Coll. Interface Sci. 1984, 100, 210–215. [Google Scholar] [CrossRef]
- Nakagawa, T.; Kakimoto, M.; Miwa, T.; Airawa, M. New method for fabricating Langmuir-Blodgett films of water-soluble proteins with retained enzyme activity. Thin Solid Film. 1991, 202, 151–156. [Google Scholar] [CrossRef]
- Said, M.; Rosen, D.; Hasted, J.B. Electric potential developed across Langmuir–Blodgett preparations of proteins. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1989, 85, 99–109. [Google Scholar] [CrossRef]
- Nitsch, W.; Maksymiw, R. Catalase monolayers at the air/water interface I. Reversibilities and irreversibilities. Coll. Polym. Sci. 1990, 268, 452–459. [Google Scholar] [CrossRef]
- Nitsch, W.; Maksimov, R.; Erdmann, H. Lipase monolayers at the air/water interface: Interfacial behavior and enzymatic activity. J. Coll. Interface Sci. 1991, 141, 322–328. [Google Scholar] [CrossRef]
- Kotov, N.A.; Meldrum, F.C.; Wu, C.; Fendler, J.H. Monoparticulate Layer and Langmuir-Blodgett-Type Multiparticulate Layers of Size-Quantized Cadmium Sulfide Clusters: A Colloid-Chemical Approach to Superlattice Construction. J. Phys. Chem. 1994, 98, 2735–2738. [Google Scholar] [CrossRef]
- Biswas, S.; Bhattacharjee, D.; Nath, R.K.; Hussain, S.A. Formation of complex Langmuir and Langmuir–Blodgett films of water soluble rosebengal. J. Coll. Interface Sci. 2007, 311, 361–367. [Google Scholar] [CrossRef]
- Hussain, S.A.; Bhattacharjee, D. Langmuir-Blodgett Films and Molecular Electronics. Mod. Phys. Lett. B 2009, 23, 3437–3451. [Google Scholar] [CrossRef]
- Kovalchuk, M.V.; Boikova, A.S.; Dyakova, Y.A.; Marchenkova, M.A.; Opolchentsev, A.M.; Yu, V.; Pisarevsky, Y.V.; Prosekov, P.A.; Seregin, A.Y. Modification of the Langmuir–Schaefer method for fabrication of ordered protein films. Crystallogr. Rep. 2017, 62, 632–638. [Google Scholar] [CrossRef]
- Hussain, S.A.; Dey, B.; Bhattacharjee, D.; Mehta, N. Unique supramolecular assembly through Langmuir–Blodgett (LB) technique. Heliyon 2018, 4, e01038. [Google Scholar] [CrossRef]
- Chattopadhyay, P.; Wang, L.; Eychmüller, A.; Simmchen, J. An Undergraduate Project on the Assembly of Langmuir–Blodgett Films of Colloidal Particles. J. Chem. Educ. 2022, 99, 952–956. [Google Scholar] [CrossRef]
- Roberts, G.G. An applied science perspective of Langmuir-Blodgett films. Adv. Phys. 1985, 34, 475–512. [Google Scholar] [CrossRef]
- Andrade, J.D. Thin organic films of proteins. Thin Solid Film. 1987, 152, 335–343. [Google Scholar] [CrossRef]
- Reichert, W.M.; Bruckner, C.J.; Joseph, J. Langmuir-Blodgett films and black lipid membranes in biospecific surface-selective sensors. Thin Solid Film. 1987, 152, 345–376. [Google Scholar] [CrossRef]
- Arya, A.; Krull, U.J.; Thompson, M. Langmuir—Blodgett deposition of lipid films on hydrogel as a basis for biosensor development. Analyt. Chim. Acta 1985, 173, 331–336. [Google Scholar] [CrossRef]
- Okahata, Y.; Tsuruta, T.; Ijiro, K.; Ariga, K. Langmuir-Blodgett films of an enzyme-lipid complex for sensor membranes. Langmuir 1988, 4, 1373–1375. [Google Scholar] [CrossRef]
- Syriyudthsak, M.; Yamagishi, H.; Moriizumi, T. Enzyme-immobilized Langmuir-Blodgett film for a biosensor. Thin Solid Film. 1988, 160, 463–469. [Google Scholar] [CrossRef]
- Miyasaka, D.T.; Koyama, K.; Itoh, I. Quantum Conversion and Image Detection by a Bacteriorhodopsin-Based Artificial Photoreceptor. Science 1992, 255, 342–344. [Google Scholar] [CrossRef]
- Oesterhelt, D.; Brauchle, C.; Hampp, N. Bacteriorhodopsin: A biological material for information processing. Quaterly Rev. Biophys. 1991, 24, 425–478. [Google Scholar] [CrossRef]
- Sethi, R.S. Transducer aspects of biosensors. Biosens. Bioelectron. 1994, 9, 243–264. [Google Scholar] [CrossRef]
- Caille, A.; Agren, G. Transitions de phase dans une couche monomoléculaire sur un substrat liquide adhésif. Can. J. Phys. 1975, 53, 2369–2374. [Google Scholar] [CrossRef]
- Frenkel, D.; Eppenga, K. Evidence for algebraic orientational order in a two-dimensional hard-core nematic. Phys. Rev. A 1985, 31, 1776–1787. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, F.; Kurokawa, Y. Studies of a monolayer of rod-like molecules by a lattice gas model. Coll. Surf. 1987, 28, 209–217. [Google Scholar] [CrossRef]
- Hukins, D.W.L.; Woodhead-Galloway, J. Liquid-Crystal Model for the Organization of Molecules in Collagen Fibrils. Biochem. Soc. Trans. 1978, 6, 238–239. [Google Scholar] [CrossRef]
- Murthy, N.S. Liquid crystallinity in collagen solutions and magnetic orientation of collagen fibrils. Biopolymers 1984, 23, 1261–1267. [Google Scholar] [CrossRef]
- Guraud-Guille, M.-M. Cholesteric Twist of Collagen In Vivo and In Vitro. Mol. Cryst. Liq. Cryst. 1987, 153, 15–30. [Google Scholar] [CrossRef]
- Guraud-Guille, M.-M. Liquid crystallinity in condensed type I collagen solutions: A clue to the packing of collagen in extracellular matrices. J. Mol. Biol. 1989, 224, 861–873. [Google Scholar] [CrossRef]
- Knight, D.P.; Feng, D.; Stewart, M.; King, E. Changes in macromolecular organization in collagen assemblies during secretion in the nidamental gland and formation of the egg capsule wall in the dogfish Scyliorhinus canicular. Philos. Trans. Roy. Soc. B 1993, 341, 419–436. [Google Scholar] [CrossRef]
- Knight, D.P.; Feng, D. Interaction of collagen with hydrophobic protein granules in the egg capsule of the dogfish scyliorhinus canicular. Tissue Cell 1994, 26, 155–167. [Google Scholar] [CrossRef]
- Feng, D.; Knight, D.P. The effect of PH on fibrillogenesis of collagen in the egg capsule of the dogfish, Scyliorhinus canicular. Tisssue Cell 1994, 26, 649–659. [Google Scholar] [CrossRef]
- Guraud-Guille, M.-M.; Besseau, L.; Herbage, D.; Gounon, P. Optimization of Collagen Liquid Crystalline Assemblies: Influence of Sonic Fragmentation. J. Struct. Biol. 1994, 113, 99–106. [Google Scholar] [CrossRef]
- Guraud-Guille, M.-M. Liquid crystalline order of biopolymers in cuticles and bones. Microscop. Res. Tech. 1994, 27, 420–428. [Google Scholar] [CrossRef]
- Beseau, L.; Guraud-Guille, M.-M. Stabilization of Fluid Cholesteric Phases of Collagen to Ordered Gelated Matrices. J. Mol. Biol. 1995, 251, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Guraud-Guille, M.-M. Twisted Liquid Crystalline Supramolecular Arrangements in Morphogenesis. Int. Rev. Cytol. 1996, 166, 59–101. [Google Scholar] [CrossRef]
- Yoshioka, K.; O’Konski, C.T. Electric properties of macromolecules. IX. Dipole moment, polarizability, and optical anisotropy factor of collagen in solution from electric birefringence. Biopolymers 1966, 4, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Hutschenreiter, J.; Scheuner, G. Double refraction of collagen. Acta Histochem. 1970, 35, 337–342. [Google Scholar] [PubMed]
- Bernengo, J.C.; Roux, B.; Herbage, D. Electrical birefringence study of monodisperse collagen solutions. Biopolymers 1974, 13, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Kahn, L.D.; Witnauer, L.P. The effects of an electric field on soluble collagen. Biochim. Biophys. Acta 1975, 393, 247–252. [Google Scholar] [CrossRef]
- Thomas, J.C.; Fletcher, G.C. Dynamic light scattering from collagen solutions. II. Photon correlation study of the depolarized light. Biopolymers 1979, 18, 1333–1352. [Google Scholar] [CrossRef]
- Knight, D.P.; Nash, L.; Hu, X.W.; Haffegee, J.; Ho, M.-W. In vitro formation by reverse dialysis of collagen gels containing highly oriented arrays of fibrils. J. Biomed. Mater. Res. 1998, 41, 185–191. [Google Scholar] [CrossRef]
- Vandervoort, J.; Ludwig, A. Preparation and evaluation of drugloaded gelatin nanoparticles for topical ophthalmic use. Eur. J. Pharm. Biopharm. 2004, 57, 251–261. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M.; Yarwood, S.J.; Curtis, A.S. Effect of cellular uptake of gelatin nanoparticles on adhesion, morphology and cytoskeleton organisation of human fibroblasts. J. Control. Release 2004, 95, 197–207. [Google Scholar] [CrossRef]
- Balthasar, S.; Michaelis, K.; Dinauer, N.; von Briesen, H.; Kreuter, J.; Langer, K. Preparation and characterisation of antibody modified gelatin nanoparticles as drug carrier system for uptake in lymphocytes. Biomaterials 2005, 26, 2723–2732. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Ethirajan, A.; Schoeller, K.; Musyanovych, A.; Ziener, U.; Landfester, K. Synthesis and Optimization of Gelatin Nanoparticles Using the Miniemulsion Process. Biomacromolecules 2008, 9, 2383–2389. [Google Scholar] [CrossRef]
- Lai, J.Y. The Role of Bloom Index of Gelatin on the Interaction with Retinal Pigment Epithelial Cells. Int. J. Mol. Sci. 2009, 10, 3442–3456. [Google Scholar] [CrossRef]
- Vijaya Kumar Naidu, B.; Paulson, A.T. A new method for the preparation of gelatin nanoparticles: Encapsulation and drug release characteristics. J. Appl. Polym. Sci. 2011, 121, 3495–3500. [Google Scholar] [CrossRef]
- Nahar, M.; Dubey, V.; Mishra, D.; Mishra, P.K.; Dube, A.; Jain, N.K. In vitro evaluation of surface functionalized gelatin nanoparticles for macrophage targeting in the therapy of visceral leishmaniasis. J. Drug Target. 2010, 18, 93–105. [Google Scholar] [CrossRef]
- Lee, E.; Khan, S.; Lim, K.-H. Gelatin nanoparticle preparation by nanoprecipitation. J. Biomater. Sci. Polym. Ed. 2011, 22, 753–771. [Google Scholar] [CrossRef]
- Khan, S.A.; Schneider, M. Improvement of nanoprecipitation technique for preparation of gelatin nanoparticles and potential macromolecular drug loading. Macromol. Biosci. 2013, 13, 455–463. [Google Scholar] [CrossRef]
- Yaari, A.; Posen, Y.; Shoseyov, O. Liquid Crystalline Human Recombinant Collagen: The Challenge and the Opportunity. Tissue Eng. Part A 2013, 19, 1502–1506. [Google Scholar] [CrossRef]
- Li, L.L.; Xu, J.H.; Qi, G.B.; Zhao, X.; Yu, F.; Wang, H. Core–Shell Supramolecular Gelatin Nanoparticles for Adaptive and “On-Demand” Antibiotic Delivery. ACS Nano 2014, 8, 4975–4983. [Google Scholar] [CrossRef]
- Sahoo, N.; Sahoo, R.K.; Biswas, N.; Guha, A.; Kuotsu, K. Recent advancement of gelatin nanoparticles in drug and vaccine delivery. Int. J. Biol. Macromol. 2015, 81, 317–331. [Google Scholar] [CrossRef]
- Khan, S.A.; Ali, H.; Ihsan, A.; Sabir, N. Tuning the size of gelatin nanoparticles produced by nanoprecipitation. Coll. J. 2015, 77, 672–676. [Google Scholar] [CrossRef]
- Wang, X.; Wei, B.; Cheng, X.; Wang, J.; Tang, R. Phenylboronic acid-decorated gelatin nanoparticles for enhanced tumor targeting and penetration. Nanotechnology 2016, 27, 385101. [Google Scholar] [CrossRef]
- Yasmin, R.; Shah, M.; Khan, S.A.; Ali, R. Gelatin nanoparticles: A potential candidate for medical applications. Nanotechnol. Revs. 2017, 6, 191–207. [Google Scholar] [CrossRef]
- Morán, M.C.; Forniés, I.; Ruano, G.; Busquets, M.A.; Vinardell, M.P. Efficient encapsulation and release of RNA molecules from gelatin-based nanoparticles. Coll. Surf. A Physicochem. Eng. Asp. 2017, 516, 226–237. [Google Scholar] [CrossRef]
- Naskar, S.; Sharma, S.; Kuotsu, K. A smart gelatin nanoparticle for delivery of metoprolol succinate: A strategy for enhancing the therapeutic efficacy by improving bioavailability. J. Drug Deliv. Sci. Technol. 2019, 53, 101214. [Google Scholar] [CrossRef]
- Saber, M.M. Strategies for surface modification of gelatin-based nanoparticles, Coll. Surf. B Biointerfaces 2019, 183, 110407. [Google Scholar] [CrossRef]
- Hathout, R.M.; Metwally, A.A. Gelatin Nanoparticles. In Pharmaceutical Nanotechnology; Methods in Molecular Biology; Weissig, V., Elbayoumi, T., Eds.; Humana: New York, NY, USA, 2019; Volume 2000. [Google Scholar] [CrossRef]
- Vinjamuri, B.P.; Papachrisanthou, K.; Haware, R.V.; Chougule, M.B. Gelatin solution pH and incubation time influences the size of the nanoparticles engineered by desolvation. J. Drug Deliv. Sci. Technol. 2021, 63, 102423. [Google Scholar] [CrossRef]
- Deng, F.; Dang, Y.; Tang, L.; Hu, T.; Ding, C.; Hu, X.; Wu, H.; Chen, L.; Huang, L.; Ni, Y.; et al. Tendon-inspired fibers from liquid crystalline collagen as the pre-oriented bioink. Int. J. Biol. Macromol. 2021, 185, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chaieb, S.; Hemar, Y. Gelatin-Based Nanocomposites: A Review. Polym. Revs. 2021, 61, 765–813. [Google Scholar] [CrossRef]
- Andrée, L.; Egberink, R.O.; Dodemont, J.; Besheli, N.H.; Yang, F.; Brock, R.; Leeuwenburgh, S.C.G. Gelatin Nanoparticles for Complexation and Enhanced Cellular Delivery of mRNA. Nanomaterials 2022, 12, 3423. [Google Scholar] [CrossRef] [PubMed]
- Joseph, X.; Akhil, V.; Arathi, A.; Mohanan, P.V. Microfluidic synthesis of gelatin nanoparticles conjugated with nitrogen-doped carbon dots and associated cellular response on A549 cells. Chem.-Biol. Interact. 2022, 351, 109710. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R.K.W.; Ha, B.-Y.; Saeidi, N. Interplay between nematic and cholesteric interactions in self-consistent field theory. Phys. Rev. E 2022, 105, 54501. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailov, O.V. Gelatin as It Is: History and Modernity. Int. J. Mol. Sci. 2023, 24, 3583. https://doi.org/10.3390/ijms24043583
Mikhailov OV. Gelatin as It Is: History and Modernity. International Journal of Molecular Sciences. 2023; 24(4):3583. https://doi.org/10.3390/ijms24043583
Chicago/Turabian StyleMikhailov, Oleg V. 2023. "Gelatin as It Is: History and Modernity" International Journal of Molecular Sciences 24, no. 4: 3583. https://doi.org/10.3390/ijms24043583





