Abstract
Vitamin D is necessary for the normal functioning of many organs, including the thyroid gland. It is, therefore, not surprising that vitamin D deficiency is considered a risk factor for the development of many thyroid disorders, including autoimmune thyroid diseases and thyroid cancer. However, the interaction between vitamin D and thyroid function is still not fully understood. This review discusses studies involving human subjects that (1) compared vitamin D status (primarily determined by serum calcidiol (25-hydroxyvitamin D [25(OH)D]) levels) with thyroid function assessed by thyroid stimulating hormone (TSH), thyroid hormones, and anti-thyroid antibody levels; and (2) evaluated the effect of vitamin D supplementation on thyroid function. Due to the many inconsistencies in the results between the studies, it is still difficult to draw a definite conclusion on how vitamin D status affects thyroid function. Studies in healthy participants observed either a negative correlation or no association between TSH and 25(OH)D levels, while the results for thyroid hormones showed high variability. Many studies have observed a negative association between anti-thyroid antibodies and 25(OH)D levels, but equally many studies have failed to observe such an association. Regarding the studies that examined the effect of vitamin D supplementation on thyroid function, almost all observed a decrease in anti-thyroid antibody levels after vitamin D supplementation. Factors that could contribute to the high variability between the studies are the use of different assays for the measurement of serum 25(OH)D levels and the confounding effects of sex, age, body-mass index, dietary habits, smoking, and the time of year when the samples were collected. In conclusion, additional studies with larger numbers of participants are needed to fully understand the effect of vitamin D on thyroid function.
1. Introduction
This review aims to investigate the relationship between vitamin D and thyroid function. Since vitamin D has an important role in normal thyroid function, studies that are trying to understand the complex background of vitamin D and thyroid interaction are of utmost importance. In this review, we included studies involving human subjects that (1) compared vitamin D status with thyroid function, and (2) evaluated the effect of vitamin D supplementation on thyroid function. A literature search was performed by two independent researchers and completed on December 31, 2022. It was performed in Medline using the keywords “vitamin D”, “thyroid”, “25-hydroxyvitamin D”, and “25(OH)D” and was not limited by publication date.
2. Vitamin D
Different types of vitamin D are fat-soluble secosteroids (vitamin D1-D5). The most important types of vitamin D for humans are vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol). Most vitamin D is synthesized in the skin after exposure to sunlight (vitamin D3), while only 5–10% is taken from food (vitamin D2 and D3) [1]. Exposure of skin to sunlight causes the transformation of 7-dehydrocholesterol into vitamin D3. Vitamin D3 is then transformed in the liver by the action of vitamin D 25-hydroxylase into 25-hydroxyvitamin D (25(OH)D, also known as calcidiol or calcifediol). The active form of vitamin D3, 1α,25-dihydroxyvitamin D (1,25(OH)2D, also known as 1,25-dihydroxycholecalciferol, 1α,25-dihydroxyvitamin D3 or calcitriol), is produced from the 25(OH)D in the kidneys by the action of the enzyme 1α-hydroxylase encoded by the CYP27B1 gene [2]. Vitamin D status is mainly determined by measuring serum 25(OH)D.
The main role of calcitriol (the active form of vitamin D3) is the regulation of calcium and phosphate concentrations. Calcitriol increases intestinal and renal absorption of calcium and phosphate. It is also crucial for bone mineralization [3]. Additionally, calcitriol is involved in the regulation of cell growth, and immune and neuromuscular functions. Its anticancer and immunosuppressive effects have also been shown [4,5].
Calcitriol binds to the vitamin D receptor (VDR) which belongs to the nuclear receptor superfamily. After calcitriol binding, VDR dimerizes with the retinoid X receptor (RXR), translocates to the nucleus, and binds to vitamin D response elements within DNA [6]. VDR is involved in the regulation of the expression of more than 1000 genes [7,8] and is found in almost all tissues [9].
3. Thyroid Function
The thyroid gland synthesizes thyroid hormones that are crucial for the normal functioning of physiological systems. The hypothalamus-pituitary-thyroid (HPT) axis orchestrates thyroid hormone synthesis by feedback mechanisms. In other words, when the levels of thyroid hormones decrease, the hypothalamus synthesizes thyrotropin-releasing hormone (TRH). TRH stimulates the anterior pituitary, causing an increase in thyroid-stimulating hormone (TSH) secretion. Finally, TSH stimulates thyrocytes which increases the production of thyroid hormones [10]. The synthesis of thyroid hormones requires the active uptake of iodide through sodium/iodide symporter (NIS), production of thyroglobulin (Tg), and iodination of Tg by the enzyme thyroid peroxidase (TPO). When Tg is proteolyzed, thyroid hormones triiodothyronine (T3) and thyroxine (T4) are released. Although the thyroid releases more T4 than T3 (in a ratio of approximately 14:1) [11], the majority of T4 converts to T3 in the tissues [12]. This conversion is mediated by the enzymes type 1 and type 2 iodothyronine deiodinases (Dio1 and Dio2) [12]. When secreted in plasma, thyroid hormones are bound to plasma proteins; only 0.03% of thyroid hormones are in an unbound or free form (fT4 and fT3) that is biologically active [13].
4. Vitamin D in Thyroid Disorders
4.1. Vitamin D in Autoimmune Thyroid Diseases
Autoimmune thyroid diseases are characterized by an immune attack of the thyroid gland. These conditions are the most common autoimmune disorders in general, with a prevalence of approximately 5% [14]. Hashimoto’s thyroiditis, characterized by hypothyroidism, and Graves’ disease, characterized by hyperthyroidism, are the two main types of autoimmune thyroid diseases. Both conditions are T-cell-mediated autoimmune disorders characterized by thyroid lymphocytic infiltration [14].
Vitamin D supplementation has been shown to be beneficial in animal models of Graves’ disease [15] and thyroiditis [16]. To date, many human studies have also been conducted to evaluate the role of vitamin D in autoimmune thyroid diseases. Genetic studies have found that polymorphisms in VDR and other genes involved in vitamin D signaling are associated with an increased risk of autoimmune thyroid diseases [17,18,19]. A recent meta-analysis by Štefanić and Tokić, which included 25 studies (2695 cases with Hashimoto’s thyroiditis and 2263 controls), detected significantly decreased levels of 25(OH)D in patients with Hashimoto’s thyroiditis [20]. Additionally, a meta-analysis by Xu et al., which included 26 studies (1748 cases with Graves’ disease and 1848 controls), noted that patients with Graves’ disease were more likely to be vitamin D deficient [21]. However, a recent meta-analysis by Taheriniya et al., which included 42 studies analyzing patients with autoimmune thyroid diseases (1886 with autoimmune thyroid disease, 372 with hypothyroidism, 1375 with Hashimoto’s thyroiditis, and 604 with Graves’ disease), showed that vitamin D deficiency is associated with the development of autoimmune thyroid diseases, Hashimoto’s thyroiditis, and hypothyroidism. For Graves’ disease, however, association with vitamin D levels was shown only among older subjects [22].
In addition to its association with autoimmune thyroid diseases, vitamin D deficiency has also been observed in other autoimmune diseases such as multiple sclerosis, diabetes mellitus, systemic lupus erythematosus, and others [23,24]. Both VDR and 1α-hydroxylase are expressed in immune cells, T and B lymphocytes, dendritic cells, neutrophils, and monocytes [25,26]. Therefore, these cells can produce calcitriol, the active form of vitamin D3 [27]. Vitamin D can modulate the activity of various immune system cells and is involved in the regulation of the immune system. Vitamin D inhibits the production of proinflammatory cytokines such as IL-6, IL-8, IL-9, IL-12, IFN-γ, and TNF-α. It also enhances the production of anti-inflammatory cytokines such as IL-10, IL-5, and IL-4. The overall effect of vitamin D is considered to be anti-inflammatory [26]. Despite the numerous studies conducted to clarify the role of vitamin D in the development of autoimmune thyroid diseases, it is still unclear whether vitamin D deficiency is an important factor in the pathogenesis or the consequence of autoimmune thyroid diseases [28].
4.2. Vitamin D in Thyroid Cancer
The incidence of thyroid cancer is increasing. In 2017, 255,490 new cases of thyroid cancer were detected worldwide, while only 95,030 new cases were detected in 1990 [29]. In thyroid cancer, both follicular thyroid cells and neuroendocrine cells can be affected. Differentiated thyroid cancer (papillary thyroid cancer, Hurthle cell thyroid cancer, and follicular thyroid cancer), poorly differentiated thyroid cancer, and anaplastic (undifferentiated) thyroid cancer arise from thyroid follicular cells. Medullary thyroid cancer is caused by malignant changes in parafollicular neuroendocrine cells [30]. Differentiated thyroid carcinomas are the most common types of thyroid cancer with 85% of all cases having papillary thyroid cancer [29,30,31].
Both in vitro and in vivo studies have shown a beneficial effect of vitamin D in treating thyroid cancer. In vitro studies have shown that calcitriol and its analogue (MART-10) can inhibit the proliferation [32] and metastatic potential [33] of anaplastic thyroid carcinoma cells, respectively. In addition, the expression levels of VDR and other genes involved in vitamin D signaling are increased in malignant thyroid cells [34,35,36], suggesting a potential antitumor response of vitamin D in cancer [35]. In vivo studies have shown that treatment with calcitriol reduced tumor size in both mouse models of follicular thyroid cancer [37] and metastatic follicular thyroid cancer [38].
As for human studies, in a large randomized clinical trial involving 25,871 participants (including patients with lung, breast, prostate, and colorectal cancer), vitamin D3 supplementation was shown to reduce the risk of developing advanced cancer in individuals without a diagnosis at the beginning of the study [39]. Regarding thyroid cancer, a meta-analysis by Zhao et al. showed that vitamin D deficiency may be a risk factor for thyroid cancer [40]. Some studies, however, have found no association between vitamin D status and risk of developing thyroid cancer [41,42].
5. The Effect of Vitamin D on Secretion of TSH, Thyroid Hormones and Anti-Thyroid Antibodies
5.1. Evidence from Animal/Cell Models
There are several insufficiently understood mechanisms by which vitamin D might alter the levels of TSH and thyroid hormones (reviewed in [43]). Experimental studies have shown that vitamin D has a direct effect on Dio2, the enzyme necessary for the conversion of T4 into T3 in target tissues. Specifically, the administration of vitamin D3 in diabetic rats leads to an increase in Dio2 expression levels in the liver and brain and, consequently, an increase in fT3 levels and a decrease in fT4 levels [44]. However, the thyroid physiology in VDR knockout mice did not show significant changes, and the mice had only a moderate reduction in TSH levels [45]. In vitro studies have shown that calcitriol administration suppressed TSH-stimulated adenylyl cyclase activity [46] and iodide uptake [46,47], while a study in rat pituitary cells has shown that calcitriol administration increases TRH-induced TSH release [48]. These data indicate that vitamin D could have both central and peripheral effects on the release of TSH and thyroid hormones. However, further experimental studies are needed to clarify the underlying mechanisms.
5.2. Evidence from Human Studies
5.2.1. Observational Studies
Many studies have linked 25(OH)D levels with the levels of TSH, thyroid hormones, and anti-thyroid antibodies (Table 1 and Figure 1). Studies in healthy participants mainly observed either a negative correlation [49,50] or no association [51,52] between TSH and 25(OH)D levels, and the same pattern was observed in studies involving patients with thyroid cancer (Table 1). Regarding thyroid hormones, conflicting results were observed in healthy participants with either positive [53], negative [54], or no association with 25(OH)D levels detected [55]. Studies involving patients with autoimmune thyroid diseases have also produced conflicting results. Two-thirds of the studies observed no association, while one-third of the studies detected a negative association between TSH and 25(OH)D levels (summarised in Table 1). On the other hand, most of the studies involving patients with autoimmune thyroid diseases did not observe an association between thyroid hormones and 25(OH)D levels. Regarding anti-thyroid antibodies, most of the studies observed a negative association between anti-thyroid antibody levels (anti-thyroid peroxidase antibody [TPOAb], anti-thyroglobulin antibody [TgAb], or TSH receptor antibody [TSHRAb]) and 25(OH)D levels, but many studies also failed to observe such an association (Table 1).

Table 1.
Correlation of 25(OH)D with TSH, thyroid hormones, thyroglobulin and anti-thyroid antibodies.
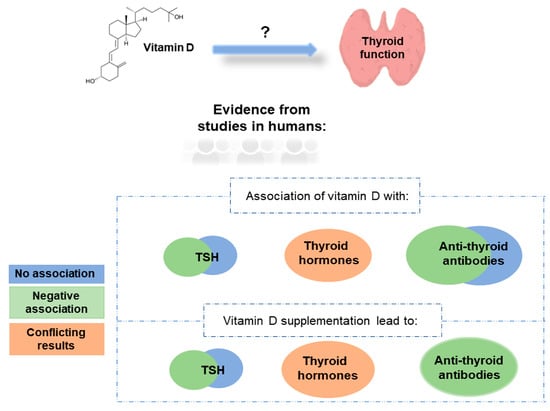
Figure 1.
Association of vitamin D with thyroid function (evidence from studies in humans).
5.2.2. Randomised Controlled Trials
Vitamin D deficiency is widespread, and it is estimated that approximately 40% of Europeans are vitamin D deficient with 13% being severely deficient [106]. Serum 25(OH)D levels below 50 nmol/L (or 20 ng/mL) and 30 nmol/L (or 12 ng/mL) are considered vitamin D deficiency and severe deficiency, respectively [107]. Therefore, many scientists and physicians recommend vitamin D supplementation. An international consensus on the optimal concentration for vitamin D supplementation has not yet been reached. In many countries, a daily vitamin D supplementation ranging from 400 to 2000 IU (10–50 μg) is recommended [107]. However, it is important to take into account that the prevalence of vitamin D deficiency varies in different ethnic groups. A study in Europeans showed that European Caucasians are less vitamin D deficient than non-white individuals [106]. Additionally, a study conducted in the USA showed that White individuals show lower rates of vitamin D deficiency than Black individuals [108]. Vitamin D deficiency is considered a risk factor for various diseases. It can lead to loss of bone density and increase the risk of fractures, osteoporosis, osteomalacia, and rickets in children [109]. In addition to autoimmune diseases, vitamin D deficiency has also been associated with cardiovascular diseases, neuropsychiatric disorders, and cancer [23,24]. Vitamin D deficiency may even contribute to the severity of COVID-19 [110].
Studies investigating the effect of vitamin D (cholecalciferol) supplementation on thyroid function were mostly conducted in patients with autoimmune thyroid diseases (Table 2). In almost all studies, vitamin D supplementation caused a significant reduction in anti-thyroid antibody (TPOAb and TgAb) levels. Results for TSH and thyroid hormones were conflicting. Some studies have noted a decrease or no change in TSH levels after vitamin D supplementation, while thyroid hormones remained mostly unchanged after vitamin D supplementation (Table 2). However, most of these studies were underpowered, with only four studies including more than 100 individuals [111,112,113,114]. The study with the largest number of participants included 11,017 participants in a wellness program receiving vitamin D supplementation, of whom 2% had hypothyroidism and 22% had subclinical hypothyroidism. Researchers observed a significant decrease in TPOAb, TgAb, TSH, thyroid hormone, and thyroglobulin levels in participants after 12 months of vitamin D supplementation [111]. Moreover, the number of patients with clinical and subclinical hypothyroidism significantly decreased after 12 months of vitamin D supplementation [111].

Table 2.
Changes in the levels of TSH, thyroid hormones, thyroglobulin and anti-thyroid antibodies following vitamin D (cholecalciferol) therapy/supplementation.
5.2.3. Mendelian Randomization
Only two studies until now have used Mendelian randomization (MR) methodology to analyze the association between vitamin D and thyroid function. Ye et al., analyzed the association between serum vitamin D levels with 106 diseases/traits in 326,409 UK Biobank (UKBB) Europeans using MR analysis [128]. Using MR analysis, they did not observe a significant association between genetically predicted serum vitamin D levels and the risk of thyroid cancer, hypothyroidism and hyperthyroidism [128]. In the study that included 10,636 participants from China, Chen et al., using MR analysis, observed a causal relationship between genetically predicted decreased serum vitamin D levels and increased concentration of TPOAb [129]. However, genetically predicted TPOAb levels did not show an association with serum vitamin D levels [129]. Considering the importance of vitamin D for normal thyroid function, additional studies assessing the causal relationship between vitamin D and thyroid function using MR methodology, conducted in different ethnic groups, are of utmost importance.
6. Conclusions
In this review, we provided insight into the relationship between vitamin D status and thyroid function, including studies conducted only in humans. Although considerable progress has been made in elucidating the effect of vitamin D on thyroid function, it is still difficult to draw a definitive conclusion on how vitamin D affects thyroid function due to the high variability between the studies. Even though many studies have correlated 25(OH)D levels with the levels of TSH, thyroid hormones, and anti-thyroid antibodies (Table 1, Figure 1), there is still a large variability in results between studies. Studies in healthy participants and in participants with thyroid cancer observed either a negative correlation or no association between TSH and 25(OH)D levels, while the results for thyroid hormones showed higher variability. Studies comparing anti-thyroid antibodies (TPOAb, TgAb, TSHRAb) with 25(OH)D levels mostly observed a negative association between anti-thyroid antibodies and 25(OH)D levels, but many studies also failed to observe such an association (Table 1). However, in almost all studies investigating the effect of vitamin D supplementation on thyroid function, it was observed that vitamin D supplementation causes a significant decrease in the levels of anti-thyroid antibodies TPOAb and TgAb (Table 2, Figure 1). Several factors could contribute to the large variability between the studies. These include the use of different assays for the measurement of serum 25(OH)D levels between the studies [130], and the possible confounding effects of age, sex, body-mass index, seasonality, smoking, and dietary habits on 25(OH)D levels that were not taken into account in all studies [131,132,133]. Given the important role of vitamin D in normal thyroid function, additional cross-sectional observational studies and randomized controlled trials with long follow-ups are needed to understand the complex background underlying the interaction between vitamin D and thyroid function. Moreover, since only a few studies until today have used MR methodology to assess the causal relationship between vitamin D and thyroid function, additional studies using such methodology are crucial. In fact, MR is revolutionising epidemiological research by removing confounding factors from analyses and establishing the directionality of the inferred associations.
Author Contributions
Conceptualization, T.Z.; validation, T.Z., M.B.L., I.G. and N.P.; resources, T.Z.; writing—original draft preparation, M.B.L., I.J. and I.R.; writing—review and editing, T.Z., M.B.L., I.G. and N.P.; visualization, M.B.L. and T.Z.; supervision, T.Z.; project administration, T.Z.; funding acquisition, T.Z. All authors have read and agreed to the published version of the manuscript.
Funding
Research was funded by the Croatian Science Foundation project “Regulation of Thyroid and Parathyroid Function and Blood Calcium Homeostasis” (No. 2593).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
1,25(OH)2D, 1α, 25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; Dio1, type 1 iodothyronine deiodinase; Dio2, type 2 iodothyronine deiodinase; fT3, free triiodothyronine; fT4, free thyroxine; HPT axis, Hypothalamus-pituitary-thyroid axis; MR, Mendelian randomization; NIS, sodium/iodide symporter; RXR, retinoid X receptor; T3, triiodothyronine; T4, thyroxine; TBII, TSH-binding inhibitory immunoglobulin; Tg, thyroglobulin; TgAb, anti-thyroglobulin antibody; TPO, thyroid peroxidase; TPOAb, anti-thyroid peroxidase antibody; TRH, thyrotropin-releasing hormone; TSAb, thyroid-stimulating antibody; TSH, thyroid stimulating hormone; TSHRAb; TSH receptor antibody; tT3, total T3; tT4, total T4; VDR, vitamin D receptor.
References
- Grundmann, M.; von Versen-Höynck, F. Vitamin D-roles in women’s reproductive health? Reprod. Biol. Endocrinol. 2011, 9, 146. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Van Driel, M.; van Leeuwen, J.P.T.M. Vitamin D endocrinology of bone mineralization. Mol. Cell. Endocrinol. 2017, 453, 46–51. [Google Scholar] [CrossRef]
- Chakraborti, C.K. Vitamin D as a promising anticancer agent. Indian J. Pharmacol. 2011, 43, 113–120. [Google Scholar] [CrossRef]
- Arnson, Y.; Amital, H.; Shoenfeld, Y. Vitamin D and autoimmunity: New aetiological and therapeutic considerations. Ann. Rheum. Dis. 2007, 66, 1137–1142. [Google Scholar] [CrossRef]
- Pike, J.W.; Meyer, M.B.; Bishop, K.A. Regulation of target gene expression by the vitamin D receptor—An update on mechanisms. Rev. Endocr. Metab. Disord. 2012, 13, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Nezhad, A.; Spira, A.; Holick, M.F. Influence of vitamin D status and vitamin D3 supplementation on genome wide expression of white blood cells: A randomized double-blind clinical trial. PLoS ONE 2013, 8, e58725. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Vitamin D: A micronutrient regulating genes. Curr. Pharm. Des. 2019, 25, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Stöcklin, E.; Eggersdorfer, M. Vitamin D, an essential nutrient with versatile functions in nearly all organs. Int. J. Vitam. Nutr. Res. 2013, 83, 92–100. [Google Scholar] [CrossRef]
- Fekete, C.; Lechan, R.M. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr. Rev. 2014, 35, 159–194. [Google Scholar] [CrossRef]
- Dayan, C.M.; Panicker, V. Novel insights into thyroid hormones from the study of common genetic variation. Nat. Rev. Endocrinol. 2009, 5, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Kim, B. Deiodinases: Implications of the local control of thyroid hormone action. J. Clin. Invest. 2006, 116, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Hoermann, R.; Midgley, J.E.M.; Larisch, R.; Dietrich, J.W. Relational stability in the expression of normality, variation, and control of thyroid function. Front. Endocrinol. 2016, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Ferrari, S.M.; Corrado, A.; Di Domenicantonio, A.; Fallahi, P. Autoimmune thyroid disorders. Autoimmun. Rev. 2015, 14, 174–180. [Google Scholar] [CrossRef]
- Misharin, A.; Hewison, M.; Chen, C.R.; Lagishetty, V.; Aliesky, H.A.; Mizutori, Y.; Rapoport, B.; McLachlan, S.M. Vitamin D deficiency modulates Graves’ hyperthyroidism induced in BALB/c mice by thyrotropin receptor immunization. Endocrinology 2009, 150, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xiong, F.; Liu, E.; Zhu, M.; Lei, P. Effects of 1,25-dihydroxyvitamin D3 in rats with experimental autoimmune thyroiditis. Nan Fang Yi Ke Da Xue Xue Bao 2010, 30, 1573–1576. [Google Scholar]
- Lopez, E.R.; Zwermann, O.; Segni, M.; Meyer, G.; Reincke, M.; Seissler, J.; Herwig, J.; Usadel, K.H.; Badenhoop, K. A promoter polymorphism of the CYP27B1 gene is associated with Addison’s disease, Hashimoto’s thyroiditis, Graves’ disease and type 1 diabetes mellitus in Germans. Eur. J. Endocrinol. 2004, 151, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; He, S.T.; Jiang, W.J.; Xiao, L.; Li, D.F.; Xu, J.; Shi, X.H.; Zhang, J.A. Genetic susceptibility to autoimmune thyroid diseases in a Chinese Han population: Role of vitamin D receptor gene polymorphisms. Ann. Endocrinol. 2015, 76, 684–689. [Google Scholar] [CrossRef]
- Inoue, N.; Watanabe, M.; Ishido, N.; Katsumata, Y.; Kagawa, T.; Hidaka, Y.; Iwatani, Y. The functional polymorphisms of VDR, GC and CYP2R1 are involved in the pathogenesis of autoimmune thyroid diseases. Clin. Exp. Immunol. 2014, 178, 262–269. [Google Scholar] [CrossRef]
- Štefanić, M.; Tokić, S. Serum 25-hydoxyvitamin D concentrations in relation to Hashimoto’s thyroiditis: A systematic review, meta-analysis and meta-regression of observational studies. Eur. J. Nutr. 2020, 59, 859–872. [Google Scholar] [CrossRef]
- Xu, M.Y.; Cao, B.; Yin, J.; Wang, D.F.; Chen, K.L.; Lu, Q. Bin Vitamin D and Graves’ disease: A meta-analysis update. Nutrients 2015, 7, 3813–3827. [Google Scholar] [CrossRef] [PubMed]
- Taheriniya, S.; Arab, A.; Hadi, A.; Fadel, A.; Askari, G. Vitamin D and thyroid disorders: A systematic review and meta-analysis of observational studies. BMC Endocr. Disord. 2021, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, W.; Li, D.; Yin, X.; Zhang, X.; Olsen, N.; Zheng, S.G. Vitamin D and chronic diseases. Aging Dis. 2017, 8, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Pu, R.; Li, N.; Chen, C.; Li, J.; Dai, W.; Wang, Y.; Hu, J.; Zhu, D.; Yu, Q.; et al. High prevalence of vitamin D deficiency in Asia: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- Bizzaro, G.; Shoenfeld, Y. Vitamin D and thyroid autoimmune diseases: The known and the obscure. Immunol. Res. 2015, 61, 107–109. [Google Scholar] [CrossRef]
- Bui, L.; Zhu, Z.; Hawkins, S.; Cortez-Resendiz, A.; Bellon, A. Vitamin D regulation of the immune system and its implications for COVID-19: A mini review. SAGE Open Med. 2021, 9, 1–8. [Google Scholar] [CrossRef]
- Chun, R.F.; Liu, P.T.; Modlin, R.L.; Adams, J.S.; Hewison, M. Impact of vitamin D on immune function: Lessons learned from genome-wide analysis. Front. Physiol. 2014, 5, 151. [Google Scholar] [CrossRef]
- Nettore, I.C.; Albano, L.; Ungaro, P.; Colao, A.; Macchia, P.E. Sunshine vitamin and thyroid. Rev. Endocr. Metab. Disord. 2017, 18, 347–354. [Google Scholar] [CrossRef]
- Deng, Y.J.; Li, H.T.; Wang, M.; Li, N.; Tian, T.; Wu, Y.; Xu, P.; Yang, S.; Zhai, Z.; Zhou, L.H.; et al. Global burden of thyroid cancer from 1990 to 2017. JAMA Netw. Open 2020, 3, e208759. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Fagin, J.A.; Wells, S.A. Biologic and clinical perspectives on thyroid cancer. N. Engl. J. Med. 2016, 375, 1054–1067. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Wang, K.; Zheng, R.; Derwahl, M. 1,25 dihydroxyvitamin D3 inhibits the proliferation of thyroid cancer stem-like cells via cell cycle arrest. Endocr. Res. 2016, 41, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Kuo, S.F.; Chen, C.H.; Ng, S.; Lin, S.F.; Yeh, C.N.; Chen, L.W.; Takano, M.; Chen, T.C.; Juang, H.H.; et al. MART-10, the vitamin D analog, is a potent drug to inhibit anaplastic thyroid cancer cell metastatic potential. Cancer Lett. 2015, 369, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Khadzkou, K.; Buchwald, P.; Westin, G.; Dralle, H.; Åkerström, G.; Hellman, P. 25-hydroxyvitamin D3 1alpha-hydroxylase and vitamin D receptor expression in papillary thyroid carcinoma. J. Histochem. Cytochem. 2006, 54, 355–361. [Google Scholar] [CrossRef]
- Clinckspoor, I.; Hauben, E.; Verlinden, L.; van den Bruel, A.; Vanwalleghem, L.; Vander Poorten, V.; Delaere, P.; Mathieu, C.; Verstuyf, A.; Decallonne, B. Altered expression of key players in vitamin D metabolism and signaling in malignant and benign thyroid tumors. J. Histochem. Cytochem. 2012, 60, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Izkhakov, E.; Somjen, D.; Sharon, O.; Knoll, E.; Aizic, A.; Fliss, D.M.; Limor, R.; Stern, N. Vitamin D receptor expression is linked to potential markers of human thyroid papillary carcinoma. J. Steroid Biochem. Mol. Biol. 2016, 159, 26–30. [Google Scholar] [CrossRef]
- Liu, W.; Asa, S.L.; Ezzat, S. 1alpha,25-Dihydroxyvitamin D3 targets PTEN-dependent fibronectin expression to restore thyroid cancer cell adhesiveness. Mol. Endocrinol. 2005, 19, 2349–2357. [Google Scholar] [CrossRef]
- Dackiw, A.P.B.; Ezzat, S.; Huang, P.; Liu, W.; Asa, S.L. Vitamin D3 administration induces nuclear p27 accumulation, restores differentiation, and reduces tumor burden in a mouse model of metastatic follicular thyroid cancer. Endocrinology 2004, 145, 5840–5846. [Google Scholar] [CrossRef]
- Chandler, P.D.; Chen, W.Y.; Ajala, O.N.; Hazra, A.; Cook, N.; Bubes, V.; Lee, I.M.; Giovannucci, E.L.; Willett, W.; Buring, J.E.; et al. Effect of vitamin D3 supplements on development of advanced cancer: A secondary analysis of the VITAL randomized clinical trial. JAMA Netw. Open 2020, 3, e2025850. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, H.; Zhang, Z.; Zhou, X.; Yao, J.; Zhang, R.; Liao, L.; Dong, J. Vitamin D deficiency as a risk factor for thyroid cancer: A meta-analysis of case-control studies. Nutrition 2019, 57, 5–11. [Google Scholar] [CrossRef]
- Danilovic, D.L.S.; Ferraz-de-Souza, B.; Fabri, A.W.; Santana, N.O.; Kulcsar, M.A.; Cernea, C.R.; Marui, S.; Hoff, A.O. 25-Hydroxyvitamin D and TSH as risk factors or prognostic markers in thyroid carcinoma. PLoS ONE 2016, 11, e0164550. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Kim, W.G.; Kim, T.Y.; Bae, S.J.; Kim, H.K.; Jang, E.K.; Jeon, M.J.; Han, J.M.; Shong, Y.K.; Kim, W.B. Serum vitamin D3 levels are not associated with thyroid cancer prevalence in euthyroid subjects without autoimmune thyroid disease. Korean J. Intern. Med. 2017, 32, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Vassalle, C.; Parlanti, A.; Pingitore, A.; Berti, S.; Iervasi, G.; Sabatino, L. Vitamin D, thyroid hormones and cardiovascular risk: Exploring the components of this novel disease triangle. Front. Physiol. 2021, 12, 722912. [Google Scholar] [CrossRef] [PubMed]
- Alrefaie, Z.; Awad, H. Effect of vitamin D3 on thyroid function and de-iodinase 2 expression in diabetic rats. Arch. Physiol. Biochem. 2015, 121, 206–209. [Google Scholar] [CrossRef]
- Clinckspoor, I.; Verlinden, L.; Mathieu, C.; Bouillon, R.; Verstuyf, A.; Decallonne, B. Vitamin D in thyroid tumorigenesis and development. Prog. Histochem. Cytochem. 2013, 48, 65–98. [Google Scholar] [CrossRef]
- Berg, J.P.; Liane, K.M.; Bjørhovde, S.B.; Bjøro, T.; Torjesen, P.A.; Haug, E. Vitamin D receptor binding and biological effects of cholecalciferol analogues in rat thyroid cells. J. Steroid Biochem. Mol. Biol. 1994, 50, 145–150. [Google Scholar] [CrossRef]
- Lamberg-Allardt, C.; Valtonen, E.; Polojärvi, M.; Stewen, P. Characterization of a 1,25-dihydroxy-vitamin D3 receptor in FRTL-5 cells. Evidence for an inhibitory effect of 1,25-dihydroxy-vitamin D3 on thyrotropin-induced iodide uptake. Mol. Cell. Endocrinol. 1991, 81, 25–31. [Google Scholar] [CrossRef]
- D’Emden, M.C.; Wark, J.D. 1,25-Dihydroxyvitamin D3 enhances thyrotropin releasing hormone induced thyrotropin secretion in normal pituitary cells. Endocrinology 1987, 121, 1192–1194. [Google Scholar] [CrossRef]
- Barchetta, I.; Baroni, M.G.; Leonetti, F.; De Bernardinis, M.; Bertoccini, L.; Fontana, M.; Mazzei, E.; Fraioli, A.; Cavallo, M.G. TSH levels are associated with vitamin D status and seasonality in an adult population of euthyroid adults. Clin. Exp. Med. 2015, 15, 389–396. [Google Scholar] [CrossRef]
- Chailurkit, L.O.; Aekplakorn, W.; Ongphiphadhanakul, B. High vitamin D status in younger individuals is associated with low circulating thyrotropin. Thyroid 2013, 23, 25–30. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Mari, D.; Prolo, S.; Fatti, L.M.; Cantone, M.C.; Garagnani, P.; Arosio, B.; Di Somma, C.; Vitale, G. 25 hydroxyvitamin D deficiency and its relationship to autoimmune thyroid disease in the elderly. Int. J. Environ. Res. Public Health 2016, 13, 850. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Song, E.; Oh, H.S.; Park, S.; Kwon, H.; Jeon, M.J.; Kim, W.G.; Kim, W.B.; Shong, Y.K.; Kim, T.Y. Vitamin D deficiency affects thyroid autoimmunity and dysfunction in iodine-replete area: Korea national health and nutrition examination survey. Endocrine 2017, 58, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Verrusio, W.; Magro, V.M.; Renzi, A.; Casciaro, B.; Andreozzi, P.; Cacciafesta, M. Thyroid hormones, metabolic syndrome and Vitamin D in middle-aged and older euthyroid subjects: A preliminary study. Aging Clin. Exp. Res. 2019, 31, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Mansorian, B.; Attari, M.M.A.; Vahabzadeh, D.; Mohebbi, I. Serum vitamin D level and its relation to thyroid hormone, blood sugar and lipid profiles in iranian sedentary work staff. Nutr. Hosp. 2018, 35, 1107–1114. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Z.; Sun, M.; Cao, M.; Zhu, Z.; Fu, Q.; Gao, Y.; Mao, J.; Li, Y.; Shi, Y.; et al. Association of high vitamin D status with low circulating thyroid-stimulating hormone independent of thyroid hormone levels in middle-aged and elderly males. Int. J. Endocrinol. 2014, 2014, 631819. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Chai, Y.; Wei, H.; Wang, K.; Tan, L.; Zhang, W.; Fan, Y.; Li, F.; Shan, Z.; Zhu, M. Vitamin D deficiency is associated with thyroid autoimmunity: Results from an epidemiological survey in Tianjin, China. Endocrine 2021, 73, 447–454. [Google Scholar] [CrossRef]
- Goswami, R.; Marwaha, R.K.; Gupta, N.; Tandon, N.; Sreenivas, V.; Tomar, N.; Ray, D.; Kanwar, R.; Agarwal, R. Prevalence of vitamin D deficiency and its relationship with thyroid autoimmunity in Asian Indians: A community-based survey. Br. J. Nutr. 2009, 102, 382–386. [Google Scholar] [CrossRef]
- Sayki Arslan, M.; Topaloglu, O.; Ucan, B.; Karakose, M.; Karbek, B.; Tutal, E.; Caliskan, M.; Ginis, Z.; Cakal, E.; Sahin, M.; et al. Isolated vitamin D deficiency is not associated with nonthyroidal illness syndrome, but with thyroid autoimmunity. Sci. World J. 2015, 2015, 239815. [Google Scholar] [CrossRef]
- Zhou, P.; Cai, J.; Markowitz, M. Absence of a relationship between thyroid hormones and vitamin D levels. J. Pediatr. Endocrinol. Metab. 2016, 29, 703–707. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, C.Y.; Deng, Y.H.; Wu, J.L. Associations between serum 25-hydroxyvitamin D levels and thyroid function parameters in previously healthy children aged 6 to 24 months. Risk Manag. Healthc. Policy 2020, 13, 1647–1653. [Google Scholar] [CrossRef]
- Zaidman, V.; Maceiras, M.; Lazzati, J.; Kutasz, E.; D’Isa, G.; Chilleli, C.; Tau, C.; Viterbo, G.; Rivarola, M.A.; Belgorosky, A.; et al. High prevalence of anti-thyroid antibodies associated with a low vitamin D status in a pediatric cohort. Clin. Chem. Lab. Med. 2014, 52, e119-22. [Google Scholar] [CrossRef] [PubMed]
- De Pergola, G.; Triggiani, V.; Bartolomeo, N.; Giagulli, V.A.; Anelli, M.; Masiello, M.; Candita, V.; De Bellis, D.; Silvestris, F. Low 25 hydroxyvitamin D levels are independently associated with autoimmune thyroiditis in a cohort of apparently healthy overweight and obese subjects. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 646–652. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Chen, J.; Huang, Y.; Li, L.; Rampersad, S.; Qu, S. Metabolic characteristics in obese patients complicated by mild thyroid hormone deficiency. Horm. Metab. Res. 2016, 48, 331–337. [Google Scholar] [CrossRef]
- Chao, G.; Zhu, Y.; Fang, L. Correlation between Hashimoto’s thyroiditis-related thyroid hormone levels and 25-hydroxyvitamin D. Front. Endocrinol. 2020, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Cvek, M.; Kaličanin, D.; Barić, A.; Vuletić, M.; Gunjača, I.; Torlak Lovrić, V.; Škrabić, V.; Punda, A.; Boraska Perica, V. Vitamin D and Hashimoto’s thyroiditis: Observations from CROHT biobank. Nutrients 2021, 13, 2793. [Google Scholar] [CrossRef] [PubMed]
- Yasmeh, J.; Farpour, F.; Rizzo, V.; Kheradnam, S.; Sachmechi, I. Hashimoto thyroiditis not associated with vitamin D deficiency. Endocr. Pract. 2016, 22, 809–813. [Google Scholar] [CrossRef]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The relationship between statin action on thyroid autoimmunity and vitamin D status: A pilot study. Exp. Clin. Endocrinol. Diabetes 2019, 127, 23–28. [Google Scholar] [CrossRef]
- Camurdan, O.; Doger, E.; Bideci, A.; Celik, N.; Cinaz, P. Vitamin D status in children with Hashimoto thyroiditis. J. Pediatr. Endocrinol. Metab. 2012, 25, 467–470. [Google Scholar] [CrossRef]
- Hanna, H.W.Z.; Rizzo, C.; Abdel Halim, R.M.; El Haddad, H.E.; Salam, R.; El-Sayed Abou-Youssef, H. Vitamin D status in Hashimoto’s thyroiditis and its association with vitamin D receptor genetic variants. J. Steroid Biochem. Mol. Biol. 2021, 212, 105922. [Google Scholar] [CrossRef]
- Stoica, R.A.; Guja, C.; Pantea-Stoian, A.; van Staden, R.I.S.; Popa-Tudor, I.; Ștefan, S.D.; Ancuceanu, R.; Serafinceanu, C.; Tîrgoviște, C.I. No association between 25-hydroxyvitamin D and insulin resistance or thyroid hormone concentrations in a Romanian observational study. Medicina 2020, 57, 25. [Google Scholar] [CrossRef]
- Koehler, V.F.; Filmann, N.; Mann, W.A. Vitamin D status and thyroid autoantibodies in autoimmune thyroiditis. Horm. Metab. Res. 2019, 51, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The effect of gluten-free diet on thyroid autoimmunity in drug-naïve women with Hashimoto’s thyroiditis: A pilot study. Exp. Clin. Endocrinol. Diabetes 2019, 127, 417–422. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalcze, K.; Okopień, B. The impact of combination therapy with metformin and exogenous vitamin D on hypothalamic-pituitary-thyroid axis activity in women with autoimmune thyroiditis and high-normal thyrotropin levels. J. Clin. Pharm. Ther. 2020, 45, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Lee, Y.J.; Choi, J.H.; Lee, S.Y.; Lee, H.Y.; Jeong, D.H.; Choi, Y.J. The association between low vitamin D status and autoimmune thyroid disease in Korean premenopausal women: The 6th Korea national health and nutrition examination survey, 2013–2014. Korean J. Fam. Med. 2019, 40, 323–328. [Google Scholar] [CrossRef]
- Botelho, I.M.B.; Neto, A.M.; Silva, C.A.; Tambascia, M.A.; Alegre, S.M.; Zantut-Wittmann, D.E. Vitamin D in Hashimoto’s thyroiditis and its relationship with thyroid function and inflammatory status. Endocr. J. 2018, 65, 1029–1037. [Google Scholar] [CrossRef]
- Giovinazzo, S.; Vicchio, T.M.; Certo, R.; Alibrandi, A.; Palmieri, O.; Campennì, A.; Cannavò, S.; Trimarchi, F.; Ruggeri, R.M. Vitamin D receptor gene polymorphisms/haplotypes and serum 25(OH)D3 levels in Hashimoto’s thyroiditis. Endocrine 2017, 55, 599–606. [Google Scholar] [CrossRef]
- MacFarlane, I.A.; Mawer, E.B.; Berry, J.; Hann, J. Vitamin D metabolism in hyperthyroidism. Clin. Endocrinol. 1982, 17, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Sun, T.; Zhang, Y.; He, L.; Wu, Q.; Liu, J.; Zha, B. 25-Hydroxyvitamin D serum level in Hashimoto’s thyroiditis, but not Graves’ disease is relatively deficient. Endocr. J. 2017, 64, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Kim, D. Low vitamin D status is associated with hypothyroid Hashimoto’s thyroiditis. Hormones 2016, 15, 385–393. [Google Scholar] [CrossRef]
- Kmieć, P.; Minkiewicz, I.; Rola, R.; Sworczak, K.; Zmijewski, M.A.; Kowalski, K. Vitamin D status including 3-epi-25(OH)D3 among adult patients with thyroid disorders during summer months. Endokrynol. Pol. 2018, 69, 653–660. [Google Scholar] [CrossRef]
- Mansournia, N.; Mansournia, M.A.; Saeedi, S.; Dehghan, J. The association between serum 25OHD levels and hypothyroid Hashimoto’s thyroiditis. J. Endocrinol. Invest. 2014, 37, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Sönmezgöz, E.; Ozer, S.; Yilmaz, R.; Önder, Y.; Bütün, I.; Bilge, S. Hypovitaminosis D in children with Hashimoto’s thyroiditis. Rev. Med. Chil. 2016, 144, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhu, X.Y.; Sun, H.; Xu, X.Q.; Xu, S.A.; Suo, Y.; Cao, L.J.; Zhou, Q.; Yu, H.J.; Cao, W.Z. Low vitamin D levels are associated with cognitive impairment in patients with Hashimoto thyroiditis. BMC Endocr. Disord. 2018, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Evliyaoğlu, O.; Acar, M.; Özcabı, B.; Erginöz, E.; Bucak, F.; Ercan, O.; Kucur, M. Vitamin D deficiency and Hashimoto’s thyroiditis in children and adolescents: A critical vitamin D level for this association? J. Clin. Res. Pediatr. Endocrinol. 2015, 7, 128–133. [Google Scholar] [CrossRef]
- Ma, J.; Wu, D.; Li, C.; Fan, C.; Chao, N.; Liu, J.; Li, Y.; Wang, R.; Miao, W.; Guan, H.; et al. Lower serum 25-hydroxyvitamin D level is associated with 3 types of autoimmune thyroid diseases. Medicine 2015, 94, e1639. [Google Scholar] [CrossRef]
- Shin, D.Y.; Kim, K.J.; Kim, D.; Hwang, S.; Lee, E.J. Low serum vitamin D is associated with anti-thyroid peroxidase antibody in autoimmune thyroiditis. Yonsei Med. J. 2014, 55, 476–481. [Google Scholar] [CrossRef]
- Tau, C.; Garabedian, M.; Farriaux, J.P.; Czemichow, P.; Pomarede, R.; Balsan, S. Hypercalcemia in infants with congenital hypothyroidism and its relation to vitamin D and thyroid hormones. J. Pediatr. 1986, 109, 808–814. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Xin, M.; Zhuang, H. Changes in bone mineral density, 25-hydroxyvitamin D3 and inflammatory factors in patients with hyperthyroidism. Exp. Ther. Med. 2021, 21, 617. [Google Scholar] [CrossRef]
- Ahn, H.Y.; Chung, Y.J.; Cho, B.Y. Serum 25-hydroxyvitamin D might be an independent prognostic factor for Graves disease recurrence. Medicine 2017, 96, e7700. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Lu, Z.; Chen, M.; Tan, J.; Fang, X. Serum 25-hydroxyvitamin D predict prognosis in radioiodine therapy of Graves’ disease. J. Endocrinol. Invest. 2015, 38, 753–759. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, L.; Xie, Z. Low vitamin D status is associated with increased thyrotropin-receptor antibody titer in Graves disease. Endocr. Pract. 2015, 21, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Liu, Y.; Zhao, C.; Fang, J.; Wang, X.; Wei, L. Changes of serum 25(OH) D3 and IGF-1 levels in patients with thyroid nodules. BMC Endocr. Disord. 2019, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Abuduwaili, M.; Xing, Z.; Xia, B.; Fei, Y.; Zhu, J.; Su, A. Correlation between pre-operative 25-hydroxyvitamin D levels and poor prognostic factors for papillary thyroid cancer. J. Investig. Surg. 2022, 35, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Kim, B.H.; Kim, S.M.; Oh, M.Y.; Kim, W.J.; Jeon, Y.K.; Kim, S.S.; Lee, B.J.; Kim, Y.K.; Kim, I.J. Low serum 25 hydroxyvitamin D is associated with poor clinicopathologic characteristics in female patients with papillary thyroid cancer. Thyroid 2014, 24, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.Y.; Chung, Y.J.; Park, K.Y.; Cho, B.Y. Serum 25-hydroxyvitamin D level does not affect the aggressiveness and prognosis of papillary thyroid cancer. Thyroid 2016, 26, 429–433. [Google Scholar] [CrossRef]
- Kuang, J.; Jin, Z.; Chen, L.; Zhao, Q.; Huang, H.; Liu, Z.; Yang, W.; Feng, H.; Yang, Z.; Díez, J.J.; et al. Serum 25-hydroxyvitamin D level is unreliable as a risk factor and prognostic marker in papillary thyroid cancer. Ann. Transl. Med. 2022, 10, 193. [Google Scholar] [CrossRef]
- Zhuo, Y.; Ling, L.; Sun, Z.; Huang, W.; Hong, Z.; Zhang, Y.; Peng, X.; Liu, X.; Yuan, W.; Xu, W.Y.; et al. Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China. Open Life Sci. 2021, 16, 150–159. [Google Scholar] [CrossRef]
- Luciardi, M.C.; Alemán, M.N.; Martinez, D.; Maxzud, M.C.; Soria, A.; Aldonati, M.E.; Luciardi, H.L. Vitamin D levels in a population from Argentina with metabolic disorders. Porto Biomed. J. 2022, 7, e159. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalska, B.; Okopien, B. Serum 25-hydroxyvitamin D and parathyroid hormone levels in non-lactating women with post-partum thyroiditis: The effect of L-thyroxine treatment. Basic Clin. Pharmacol. Toxicol. 2015, 116, 503–507. [Google Scholar] [CrossRef]
- Bozdag, H.; Akdeniz, E. Does severe vitamin D deficiency impact obstetric outcomes in pregnant women with thyroid autoimmunity? J. Matern. Neonatal Med. 2020, 33, 1359–1369. [Google Scholar] [CrossRef]
- Musa, I.R.; Rayis, D.A.; Ahmed, M.A.; Khamis, A.H.; Nasr, A.M.; Adam, I. Thyroid function and 25 (OH) vitamin D level among Sudanese women in early pregnancy. Open Access Maced. J. Med. Sci. 2018, 6, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Miao, W.; Li, C.; Yu, X.; Shan, Z.; Guan, H.; Teng, W. Dynamic changes in serum 25-hydroxyvitamin D during pregnancy and lack of effect on thyroid parameters. PLoS ONE 2014, 9, e90161. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhong, S.; Liu, Q.; Wang, C.-B.; Zhu, W.-H.; Shen, X.-A.; Lu, B.; Shen, L.-W.; Zeng, Y. Investigating the relationship between 25-hydroxyvitamin D and thyroid function in second-trimester pregnant women. Gynecol. Endocrinol. 2018, 34, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, Z.; Li, B.; Guo, S.; Yao, M. Expression and clinical significance of serum 25-OH-D in pregnant women with SCH (Subclinical Hypothyroidism) and GDM (Gestational Diabetes Mellitus). Pakistan J. Med. Sci. 2018, 34, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Palomba, S.; Caggiano, M.; Tafuri, D.; Colao, A.; Orio, F. Low 25 (OH) vitamin D levels are associated with autoimmune thyroid disease in polycystic ovary syndrome. Endocrine 2016, 53, 538–542. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.M.; Solway, J. Association of vitamin D levels, race/ethnicity, and clinical characteristics with COVID-19 test results. JAMA Netw. Open 2021, 4, e214117. [Google Scholar] [CrossRef]
- Christodoulou, S.; Goula, T.; Ververidis, A.; Drosos, G. Vitamin D and Bone Disease. BioMed Res. Int. 2013, 2013, 396541. [Google Scholar] [CrossRef]
- Munshi, R.; Hussein, M.H.; Toraih, E.A.; Elshazli, R.M.; Jardak, C.; Sultana, N.; Youssef, M.R.; Omar, M.; Attia, A.S.; Fawzy, M.S.; et al. Vitamin D insufficiency as a potential culprit in critical COVID-19 patients. J. Med. Virol. 2021, 93, 733–740. [Google Scholar] [CrossRef]
- Mirhosseini, N.; Brunel, L.; Muscogiuri, G.; Kimball, S. Physiological serum 25-hydroxyvitamin D concentrations are associated with improved thyroid function-observations from a community-based program. Endocrine 2017, 58, 563–573. [Google Scholar] [CrossRef]
- Talaei, A.; Ghorbani, F.; Asemi, Z. The effects of Vitamin D supplementation on thyroid function in hypothyroid patients: A randomized, double-blind, placebo-controlled trial. Indian J. Endocrinol. Metab. 2018, 22, 584–588. [Google Scholar] [CrossRef]
- Villa, A.; Corsello, A.; Cintoni, M.; Papi, G.; Pontecorvi, A.; Corsello, S.M.; Paragliola, R.M. Effect of vitamin D supplementation on TSH levels in euthyroid subjects with autoimmune thyroiditis. Endocrine 2020, 70, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chen, X.; Qian, X.; Shao, S. Effects of vitamin D treatment on thyroid function and autoimmunity markers in patients with Hashimoto’s thyroiditis—A meta-analysis of randomized controlled trials. J. Clin. Pharm. Ther. 2022, 47, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Dutta, D.; Kumar, M.; Saha, S.; Mondal, S.; Kumar, A.; Mukhopadhyay, S. Vitamin D supplementation reduces thyroid peroxidase antibody levels in patients with autoimmune thyroid disease: An open-labeled randomized controlled trial. Indian J. Endocrinol. Metab. 2016, 20, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Nodehi, M.; Ajami, A.; Izad, M.; Asgarian Omran, H.; Chahardoli, R.; Amouzegar, A.; Yekaninejad, S.; Hemmatabadi, M.; Azizi, F.; Esfahanian, F.; et al. Effects of vitamin D supplements on frequency of CD4+ T-cell subsets in women with Hashimoto’s thyroiditis: A double-blind placebo-controlled study. Eur. J. Clin. Nutr. 2019, 73, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Kowalcze, K.; Okopień, B. Hyperprolactinaemia attenuates the inhibitory effect of vitamin D/selenomethionine combination therapy on thyroid autoimmunity in euthyroid women with Hashimoto’s thyroiditis: A pilot study. J. Clin. Pharm. Ther. 2020, 45, 1334–1341. [Google Scholar] [CrossRef]
- Krysiak, R.; Szkróbka, W.; Okopień, B. Dehydroepiandrosterone potentiates the effect of vitamin D on thyroid autoimmunity in euthyroid women with autoimmune thyroiditis: A pilot study. Clin. Exp. Pharmacol. Physiol. 2021, 48, 195–202. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalcze, K.; Okopień, B. Gluten-free diet attenuates the impact of exogenous vitamin D on thyroid autoimmunity in young women with autoimmune thyroiditis: A pilot study. Scand. J. Clin. Lab. Invest. 2022, 82, 518–524. [Google Scholar] [CrossRef]
- Vahabi Anaraki, P.; Aminorroaya, A.; Amini, M.; Momeni, F.; Feizi, A.; Iraj, B.; Tabatabaei, A. Effect of vitamin D deficiency treatment on thyroid function and autoimmunity markers in Hashimoto’s thyroiditis: A double-blind randomized placebo-controlled clinical trial. J. Res. Med. Sci. 2017, 22, 103. [Google Scholar] [CrossRef]
- Barsony, J.; Lakatos, P.; Foldes, J.; Feher, T. Effect of vitamin D3 loading and thyroid hormone replacement therapy on the decreased serum 25-hydroxyvitamin D level in patients with hypothyroidism. Acta Endocrinol 1986, 113, 329–334. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalcze, K.; Okopien, B. The effect of vitamin D on thyroid autoimmunity in non-lactating women with postpartum thyroiditis. Eur. J. Clin. Nutr. 2016, 70, 637–639. [Google Scholar] [CrossRef]
- Krysiak, R.; Szkróbka, W.; Okopień, B. Moderate-dose simvastatin therapy potentiates the effect of vitamin D on thyroid autoimmunity in levothyroxine-treated women with Hashimoto’s thyroiditis and vitamin D insufficiency. Pharmacol. Rep. 2018, 70, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The effect of vitamin D on thyroid autoimmunity in levothyroxine-treated women with Hashimoto’s thyroiditis and normal vitamin D status. Exp. Clin. Endocrinol. Diabetes 2017, 125, 229–233. [Google Scholar] [CrossRef]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The effect of vitamin D and selenomethionine on thyroid antibody titers, hypothalamic-pituitary-thyroid axis activity and thyroid function tests in men with Hashimoto’s thyroiditis: A pilot study. Pharmacol. Rep. 2019, 71, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Kowalcze, K.; Okopień, B. The effect of vitamin D on thyroid autoimmunity in euthyroid men with autoimmune thyroiditis and testosterone deficiency. Pharmacol. Rep. 2019, 71, 798–803. [Google Scholar] [CrossRef]
- Chahardoli, R.; Saboor-Yaraghi, A.A.; Amouzegar, A.; Khalili, D.; Vakili, A.Z.; Azizi, F. Can supplementation with vitamin D modify thyroid autoantibodies (Anti-TPO Ab, Anti-Tg Ab) and thyroid profile (T3, T4, TSH) in Hashimoto’s thyroiditis? A double blind, randomized clinical trial. Horm. Metab. Res. 2019, 51, 296–301. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, H.; Wang, Y.; Zhao, H. A comprehensive genetic and epidemiological association analysis of vitamin D with common diseases/traits in the UK Biobank. Genet. Epidemiol. 2020, 45, 24–35. [Google Scholar] [CrossRef]
- Chen, Y.; Han, B.; Zhu, C.; Li, Q.; Chen, C.; Zhai, H.; Wang, N.; Chen, Y.; Lu, Y. Bidirectional mendelian randomization analysis for vitamin D and thyroid peroxidase antibody. Int. J. Endocrinology. 2022, 2022, 2260388. [Google Scholar] [CrossRef]
- Snellman, G.; Melhus, H.; Gedeborg, R.; Byberg, L.; Berglund, L.; Wernroth, L.; Michaëlsson, K. Determining vitamin D status: A comparison between commercially available assays. PLoS ONE 2010, 5, e11555. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Diffey, B.L. Modelling the seasonal variation of vitamin D due to sun exposure. Br. J. Dermatol. 2010, 162, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Pal, M.; De, A. The dependency of vitamin D status on anthropometric data. Malaysian J. Med. Sci. 2014, 21, 54. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).