Unraveling the Role of Scutellaria baicalensis for the Treatment of Breast Cancer Using Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation
Abstract
1. Introduction
2. Results
2.1. Active Compounds in Scutellaria baicalensis and Their Targets
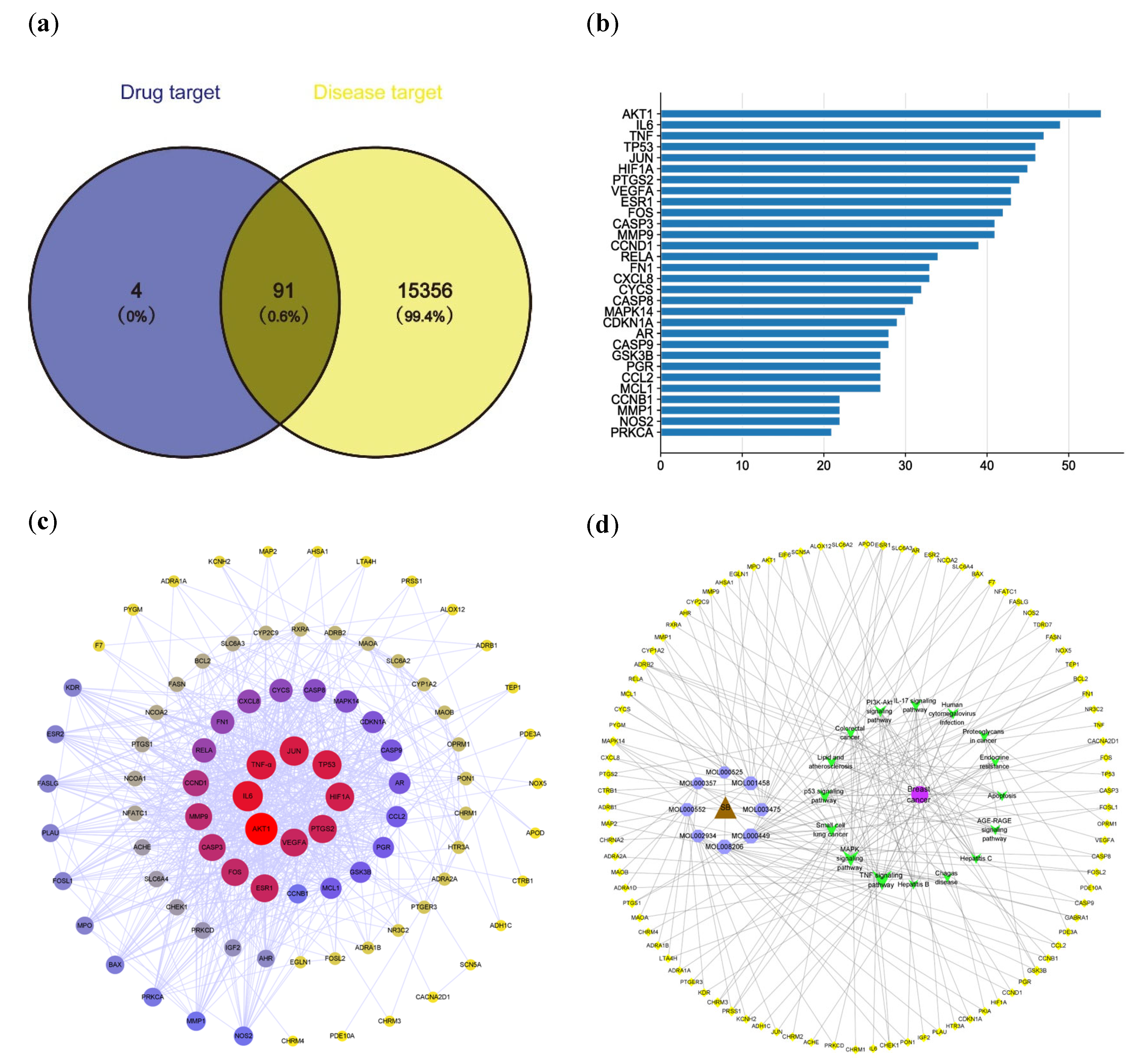
2.2. Effective Targets for Breast Cancer
2.3. PPI Network Analysis
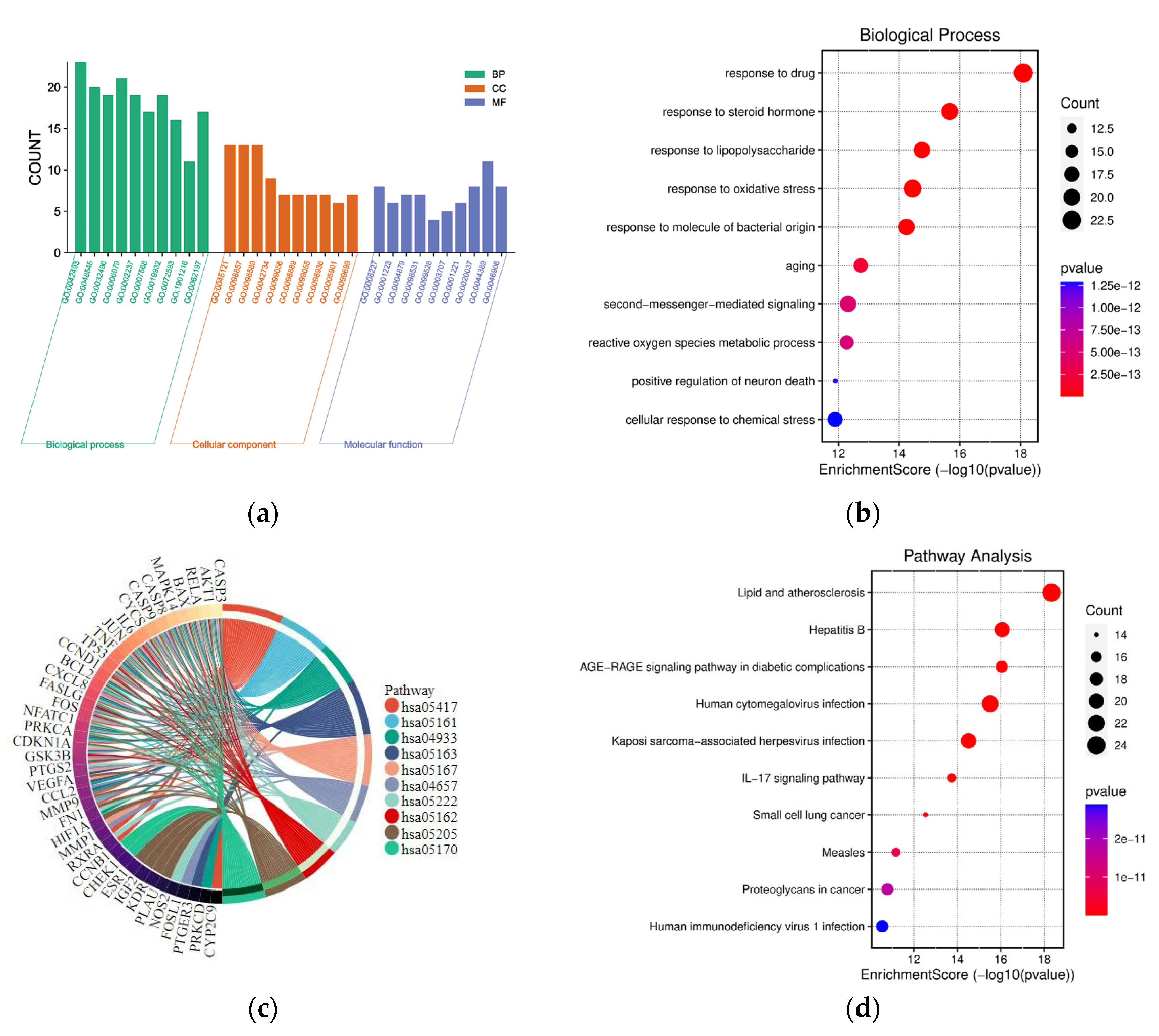
2.4. GO and KEGG Pathway Enrichment Analysis
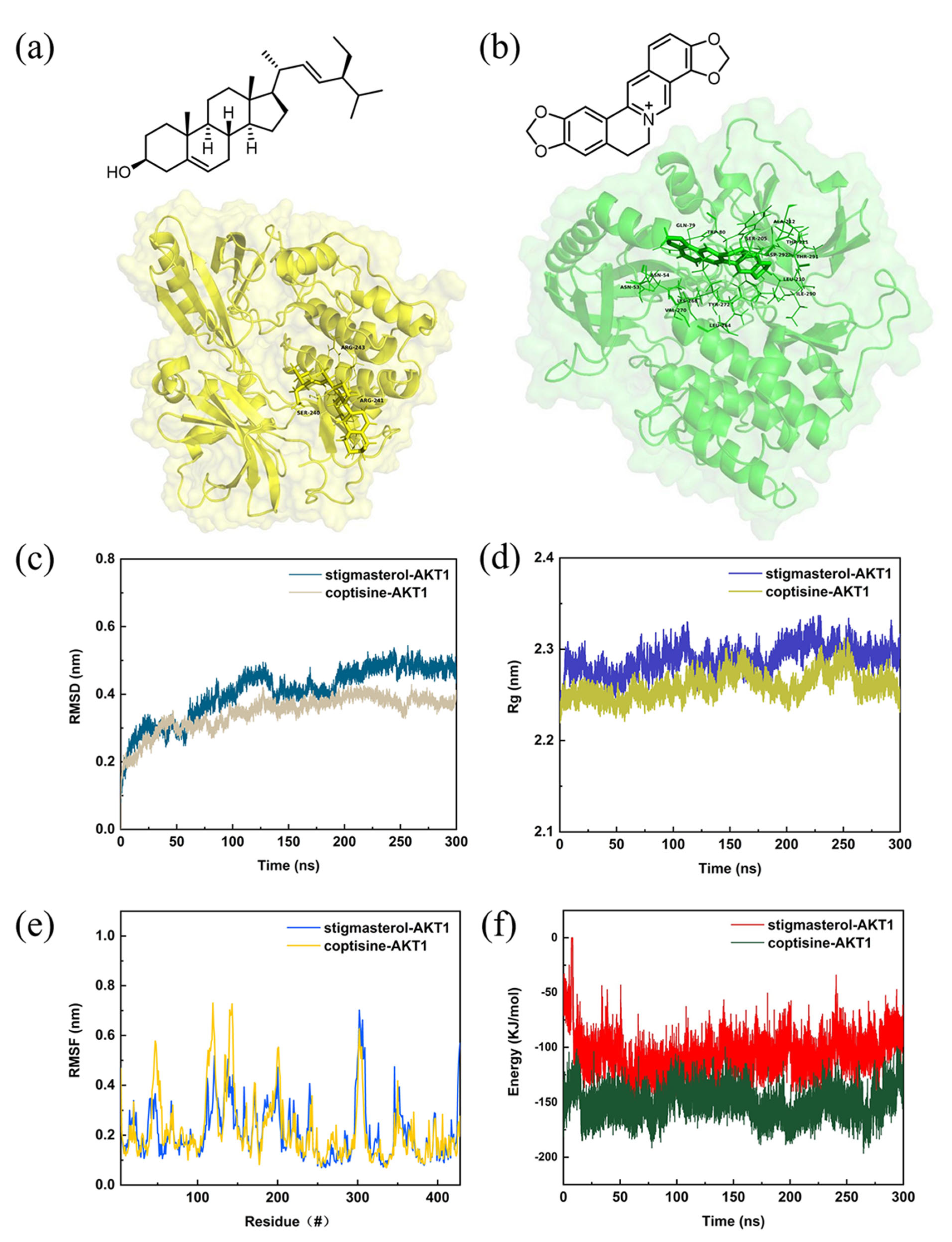
2.5. Molecular Docking of Compound and Target
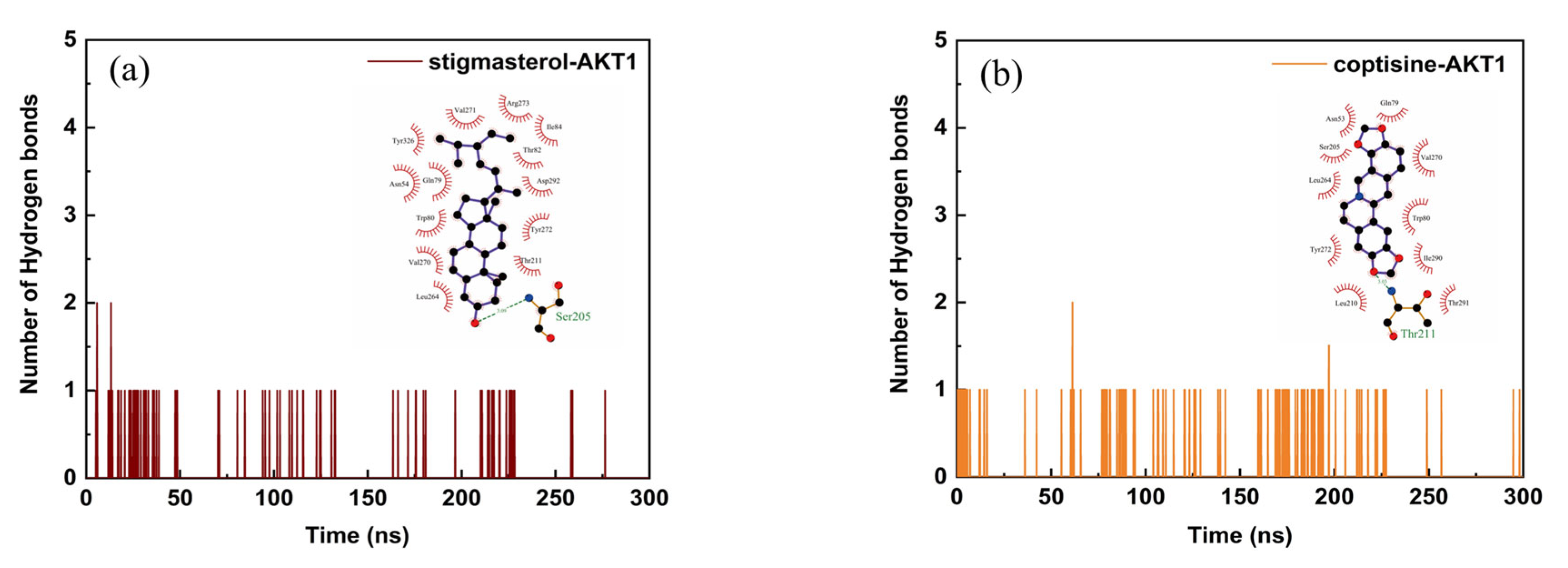
2.6. Structural Stability and Interaction Energy by Molecular Dynamics Simulation
3. Discussion
4. Materials and Methods
4.1. Screening of Active Compounds and Gene Targets of Scutellaria baicalensis
4.2. Constructing a “Compound–Target” Network
4.3. Predicting the Disease Targets
4.4. Acquitting the Intersection Targets
4.5. PPI Network Construction and Cluster Analysis
4.6. GO and KEGG Analysis
4.7. Molecular Docking
4.8. Molecular Dynamics Simulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Youlden, D.R.; Cramb, S.M.; Yip, C.H.; Baade, P.D. Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Biol. Med. 2014, 11, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; McCarron, P.; Parkin, D.M. The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res. BCR 2004, 6, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Testa, U.; Castelli, G.; Pelosi, E. Breast Cancer: A Molecularly Heterogenous Disease Needing Subtype-Specific Treatments. Med. Sci. 2020, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Mohammed, S. Breast cancer metastasis and the lymphatic system. Oncol. Lett. 2015, 10, 1233–1239. [Google Scholar] [CrossRef]
- Abubakar, M.; Chang-Claude, J.; Ali, H.R.; Chatterjee, N.; Coulson, P.; Daley, F.; Blows, F.; Benitez, J.; Milne, R.L.; Brenner, H.; et al. Etiology of hormone receptor positive breast cancer differs by levels of histologic grade and proliferation. Int. J. Cancer 2018, 143, 746–757. [Google Scholar] [CrossRef]
- Mohammed, H.; Russell, I.A.; Stark, R.; Rueda, O.M.; Hickey, T.E.; Tarulli, G.A.; Serandour, A.A.; Birrell, S.N.; Bruna, A.; Saadi, A.; et al. Progesterone receptor modulates ERα action in breast cancer. Nature 2015, 523, 313–317. [Google Scholar] [CrossRef]
- Badowska-Kozakiewicz, A.M.; Patera, J.; Sobol, M.; Przybylski, J. The role of oestrogen and progesterone receptors in breast cancer—Immunohistochemical evaluation of oestrogen and progesterone receptor expression in invasive breast cancer in women. Contemp. Oncol. 2015, 19, 220–225. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Desai, A.G.; Qazi, G.N.; Ganju, R.K.; El-Tamer, M.; Singh, J.; Saxena, A.K.; Bedi, Y.S.; Taneja, S.C.; Bhat, H.K. Medicinal plants and cancer chemoprevention. Curr. Drug Metab. 2008, 9, 581–591. [Google Scholar] [CrossRef]
- Marshall, A.C. Traditional Chinese Medicine and Clinical Pharmacology. In Drug Discovery and Evaluation: Methods in Clinical Pharmacology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 455–482. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Zhang, Y.; Fan, H.T.; Lin, H.S. Traditional Chinese medicine and cancer: History, present situation, and development. Thorac. Cancer 2015, 6, 561–569. [Google Scholar] [CrossRef]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, X.Y.; Martin, C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. 2016, 61, 1391–1398. [Google Scholar] [CrossRef]
- Yuan, Q.-J.; Zhang, Z.-Y.; Hu, J.; Guo, L.-P.; Shao, A.-J.; Huang, L.-Q. Impacts of recent cultivation on genetic diversity pattern of a medicinal plant, Scutellaria baicalensis (Lamiaceae). BMC Genet. 2010, 11, 29. [Google Scholar] [CrossRef]
- Huang, S.T.; Lai, H.C.; Lin, Y.C.; Huang, W.T.; Hung, H.H.; Ou, S.C.; Lin, H.J.; Hung, M.C. Principles and treatment strategies for the use of Chinese herbal medicine in patients at different stages of coronavirus infection. Am. J. Cancer Res. 2020, 10, 2010–2031. [Google Scholar]
- Zhang, L.; Wang, G.; Hou, W.; Li, P.; Dulin, A.; Bonkovsky, H.L. Contemporary clinical research of traditional Chinese medicines for chronic hepatitis B in China: An analytical review. Hepatol. (Baltim. Md.) 2010, 51, 690–698. [Google Scholar] [CrossRef]
- Song, J.-W.; Long, J.-Y.; Xie, L.; Zhang, L.-L.; Xie, Q.-X.; Chen, H.-J.; Deng, M.; Li, X.-F. Applications, phytochemistry, pharmacological effects, pharmacokinetics, toxicity of Scutellaria baicalensis Georgi. and its probably potential therapeutic effects on COVID-19: A review. Chin. Med. 2020, 15, 102. [Google Scholar] [CrossRef]
- Xin, L.; Gao, J.; Lin, H.; Qu, Y.; Shang, C.; Wang, Y.; Lu, Y.; Cui, X. Regulatory Mechanisms of Baicalin in Cardiovascular Diseases: A Review. Front. Pharmacol. 2020, 11, 583200. [Google Scholar] [CrossRef]
- Yin, B.; Li, W.; Qin, H.; Yun, J.; Sun, X. The Use of Chinese Skullcap (Scutellaria baicalensis) and Its Extracts for Sustainable Animal Production. Anim. Open Access J. MDPI 2021, 11, 1039. [Google Scholar] [CrossRef]
- Kim, Y.O.; Leem, K.; Park, J.; Lee, P.; Ahn, D.K.; Lee, B.C.; Park, H.K.; Suk, K.; Kim, S.Y.; Kim, H. Cytoprotective effect of Scutellaria baicalensis in CA1 hippocampal neurons of rats after global cerebral ischemia. J. Ethnopharmacol. 2001, 77, 183–188. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wang, S.; Kuang, Y.; Hu, Z.M.; Qiao, X.; Ye, M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018, 56, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.O.; Cho, E.J.; Jeong, J.W.; Park, C.; Hong, S.H.; Hwang, H.J.; Moon, S.K.; Son, C.G.; Kim, W.J.; Choi, Y.H. Baicalein Inhibits the Migration and Invasion of B16F10 Mouse Melanoma Cells through Inactivation of the PI3K/Akt Signaling Pathway. Biomol. Ther. 2017, 25, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Li, Z.; Zhu, Z.; Chen, H.; Zhao, L.; Wang, X.; Chen, Y. Baicalein suppresses 17-β-estradiol-induced migration, adhesion and invasion of breast cancer cells via the G protein-coupled receptor 30 signaling pathway. Oncol. Rep. 2015, 33, 2077–2085. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yan, W.; Dai, Z.; Gao, X.; Ma, Y.; Xu, Q.; Jiang, J.; Zhang, S. Baicalein suppresses metastasis of breast cancer cells by inhibiting EMT via downregulation of SATB1 and Wnt/β-catenin pathway. Drug Des. Dev. Ther. 2016, 10, 1419–1441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, X.; Li, Q.; Zhong, B.; Han, Q. Baicalin Induces Apoptosis and Inhibits Migration/ Invasion of Breast Cancer Cell MDA-MB-231 Through Reactive Oxygen Species-Mediated Activation of p38/c-Jun N-Terminal Kinase Signaling Pathway. J. Biomater. Tissue Eng. 2018, 8, 1022–1030. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, H.; Wang, H.; Hu, H.; He, H.; Gu, N.; Han, X.; Guo, Q.; Liu, D.; Cui, S.; et al. Baicalin inhibits breast cancer development via inhibiting IĸB kinase activation in vitro and in vivo. Int. J. Oncol. 2018, 53, 2727–2736. [Google Scholar] [CrossRef]
- Duan, X.; Guo, G.; Pei, X.; Wang, X.; Li, L.; Xiong, Y.; Qiu, X. Baicalin Inhibits Cell Viability, Migration and Invasion in Breast Cancer by Regulating miR-338-3p and MORC4. OncoTargets Ther. 2019, 12, 11183–11193. [Google Scholar] [CrossRef]
- Liu, D.K.; Dong, H.F.; Liu, R.F.; Xiao, X.L. Baicalin inhibits the TGF-β1/p-Smad3 pathway to suppress epithelial-mesenchymal transition-induced metastasis in breast cancer. Oncotarget 2020, 11, 2863–2872. [Google Scholar] [CrossRef]
- Yang, K.; Zeng, L.; Ge, A.; Chen, Z.; Bao, T.; Long, Z.; Ge, J.; Huang, L. Investigating the regulation mechanism of baicalin on triple negative breast cancer’s biological network by a systematic biological strategy. Biomed. Pharmacother. 2019, 118, 109253. [Google Scholar] [CrossRef]
- AmeliMojarad, M.; AmeliMojarad, M.; Pourmahdian, A. The inhibitory role of stigmasterol on tumor growth by inducing apoptosis in Balb/c mouse with spontaneous breast tumor (SMMT). BMC Pharmacol. Toxicol. 2022, 23, 42. [Google Scholar] [CrossRef]
- Sirirat, R.; Tantamango-Bartley, Y.; Heskey, C.; Haddad, E.; Fraser, G.; Mashchak, A.; Jaceldo-Siegl, K. The Association Between Phytosterol Intake and Breast Cancer Incidence in the Adventist Health Study-2 Cohort. Curr. Dev. Nutr. 2020, 4, 352. [Google Scholar] [CrossRef]
- Wu, J.; Luo, Y.; Deng, D.; Su, S.; Li, S.; Xiang, L.; Hu, Y.; Wang, P.; Meng, X. Coptisine from Coptis chinensis exerts diverse beneficial properties: A concise review. J. Cell. Mol. Med. 2019, 23, 7946–7960. [Google Scholar] [CrossRef]
- Li, J.; Qiu, D.M.; Chen, S.H.; Cao, S.P.; Xia, X.L. Suppression of human breast cancer cell metastasis by coptisine in vitro. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 5747–5751. [Google Scholar] [CrossRef]
- Yang, D.; Guo, Q.; Liang, Y.; Zhao, Y.; Tian, X.; Ye, Y.; Tian, J.; Wu, T.; Lu, N. Wogonin induces cellular senescence in breast cancer via suppressing TXNRD2 expression. Arch. Toxicol. 2020, 94, 3433–3447. [Google Scholar] [CrossRef]
- Ishimaru, K.; Nishikawa, K.; Omoto, T.; Asai, I.; Yoshihira, K.; Shimomura, K. Two flavone 2′-glucosides from Scutellaria baicalensis. Phytochemistry 1995, 40, 279–281. [Google Scholar] [CrossRef]
- Awad, A.B.; Chinnam, M.; Fink, C.S.; Bradford, P.G. beta-Sitosterol activates Fas signaling in human breast cancer cells. Phytomedicine Int. J. Phytother. Phytopharm. 2007, 14, 747–754. [Google Scholar] [CrossRef]
- Abd El-Hafeez, A.A.; Khalifa, H.O.; Mahdy, E.A.M.; Sharma, V.; Hosoi, T.; Ghosh, P.; Ozawa, K.; Montano, M.M.; Fujimura, T.; Ibrahim, A.R.N.; et al. Anticancer effect of nor-wogonin (5, 7, 8-trihydroxyflavone) on human triple-negative breast cancer cells via downregulation of TAK1, NF-κB, and STAT3. Pharmacol. Rep. PR 2019, 71, 289–298. [Google Scholar] [CrossRef]
- Chandran, U.; Mehendale, N.; Patil, S.; Chaguturu, R.; Patwardhan, B. Network Pharmacology. In Innovative Approaches in Drug Discovery; Academic Press: Cambridge, MA, USA, 2017; pp. 127–164. [Google Scholar] [CrossRef]
- Meng, X.Y.; Zhang, H.X.; Mezei, M.; Cui, M. Molecular docking: A powerful approach for structure-based drug discovery. Curr. Comput.-Aided Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef]
- Hospital, A.; Goñi, J.R.; Orozco, M.; Gelpí, J.L. Molecular dynamics simulations: Advances and applications. Adv. Appl. Bioinform. Chem. AABC 2015, 8, 37–47. [Google Scholar] [CrossRef]
- Dougan, M.; Li, Y.; Chu, L.; Haile, R.; Whittemore, A.; Han, S.; Moore, S.; Sampson, J.; Andrulis, I.; Amajor, E.; et al. Metabolomic profiles in breast cancer: A pilot case-control study in the breast cancer family registry. BMC Cancer 2018, 18, 532. [Google Scholar] [CrossRef]
- EghbaliFeriz, S.; Taleghani, A.; Tayarani-Najaran, Z. Scutellaria: Debates on the anticancer property. Biomed. Pharmacother. 2018, 105, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Lo Muzio, L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review). Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Lee, D.F.; Xia, W.; Golfman, L.S.; Ou-Yang, F.; Yang, J.Y.; Zou, Y.; Bao, S.; Hanada, N.; Saso, H.; et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 2004, 117, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Soltani, A.; Torki, S.; Ghahfarokhi, M.S.; Jami, M.S.; Ghatrehsamani, M. Targeting the phosphoinositide 3-kinase/AKT pathways by small molecules and natural compounds as a therapeutic approach for breast cancer cells. Mol. Biol. Rep. 2019, 46, 4809–4816. [Google Scholar] [CrossRef] [PubMed]
- Mirzayans, R.; Andrais, B.; Kumar, P.; Murray, D. Significance of Wild-Type p53 Signaling in Suppressing Apoptosis in Response to Chemical Genotoxic Agents: Impact on Chemotherapy Outcome. Int. J. Mol. Sci. 2017, 18, 928. [Google Scholar] [CrossRef]
- Lee, A.Y.; Lee, J.-Y.; Chun, J.M. Exploring the Mechanism of Gyejibokryeong-hwan against Atherosclerosis Using Network Pharmacology and Molecular Docking. Plants 2020, 9, 1750. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Z.; Li, W.; Kong, F.; Yi, P.; Huang, J.; Mao, D.; Peng, W.; Zhang, S. Network Pharmacology-Based Prediction and Verification of the Active Ingredients and Potential Targets of Zuojinwan for Treating Colorectal Cancer. Drug Des. Dev. Ther. 2020, 14, 2725–2740. [Google Scholar] [CrossRef]
- Hou, Y.; Pi, C.; Feng, X.; Wang, Y.; Fu, S.; Zhang, X.; Zhao, L.; Wei, Y. Antitumor Activity In Vivo and Vitro of New Chiral Derivatives of Baicalin and Induced Apoptosis via the PI3K/Akt Signaling Pathway. Mol. Ther.—Oncolytics 2020, 19, 67–78. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Yan, J.; Li, X.; Li, J.; Hu, J.; Shang, X.; Yang, H. Deciphering the Efficacy and Mechanism of Astragalus membranaceus on High Altitude Polycythemia by Integrating Network Pharmacology and In Vivo Experiments. Nutrients 2022, 14, 4968. [Google Scholar] [CrossRef]
- Hassan, H.; Shanak, S. GOTrapper: A tool to navigate through branches of gene ontology hierarchy. BMC Bioinform. 2019, 20, 20. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef]
- Ferguson, K.M. Structure-based view of epidermal growth factor receptor regulation. Annu. Rev. Biophys. 2008, 37, 353–373. [Google Scholar] [CrossRef]


| ID | Name | Degree |
|---|---|---|
| MOL000449 | stigmasterol | 12 |
| MOL008206 | moslosooflavone | 11 |
| MOL001458 | coptisine | 10 |
| MOL000552 | 5, 2′-dihydroxy-6, 7, 8-trimethoxyflavone | 9 |
| MOL003475 | 9-cedranone | 9 |
| MOL002934 | neobaicalein | 8 |
| MOL000525 | norwogonin | 7 |
| MOL000357 | sitogluside | 7 |
| MOL002915 | salvigenin | 6 |
| MOL000358 | beta-sitosterol | 6 |
| MOL000073 | ent-epicatechin | 6 |
| MOL002917 | 5, 2′, 6′-trihydroxy-7, 8-dimethoxyflavone | 5 |
| MOL002927 | skullcapflavone II | 5 |
| MOL002897 | epiberberine | 5 |
| MOL002933 | 5, 7, 4′-trihydroxy-8-methoxyflavone | 4 |
| MOL002937 | dihydrooroxylin | 4 |
| MOL000007 | cosmetin | 3 |
| MOL002925 | 5, 7, 2′, 6′-tetrahydroxyflavone | 2 |
| MOL002928 | oroxylin a | 2 |
| MOL002932 | panicolin | 2 |
| MOL000359 | sitosterol | 2 |
| MOL001490 | bis[(2S)-2-ethylhexyl] benzene-1, 2-dicarboxylate | 2 |
| MOL002879 | diop | 2 |
| MOL010415 | 11, 13-eicosadienoic acid, methyl ester | 2 |
| MOL000458 | campesterol | 2 |
| ID | MOL000552 | MOL008206 | MOL000449 | MOL001458 | MOL003475 |
|---|---|---|---|---|---|
| Target | 5, 2′-Dihydroxy-6, 7, 8-trimethoxy | Moslosooflavone | Stigmasterol | Coptisine | 9-Cedranone |
| AKT1 | −9.1 | −9.5 | −11.1 | −11.1 | −7.9 |
| IL6 | −7.8 | −7.1 | −7.5 | −8.4 | −6.6 |
| TNF | −7.7 | −7.8 | −8.9 | −9.3 | −7.4 |
| TP53 | −8 | −8 | −9.7 | −9.1 | −7.5 |
| JUN | −7.1 | −6.9 | −7.9 | −9.6 | −6.2 |
| HIF1A | −6.8 | −6.8 | −7.6 | −7.9 | −6.5 |
| PTGS2 | −8.5 | −9 | −8.6 | −11 | −7.1 |
| VEGFA | −6.7 | −6.7 | −6.9 | −7.8 | −5.8 |
| ESR1 | −8.1 | −8 | −7.5 | −7.7 | −8 |
| FOS | −7.6 | −7.5 | −8 | −8.2 | −6.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Y.; Shi, C.; Sun, Y. Unraveling the Role of Scutellaria baicalensis for the Treatment of Breast Cancer Using Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation. Int. J. Mol. Sci. 2023, 24, 3594. https://doi.org/10.3390/ijms24043594
Jiao Y, Shi C, Sun Y. Unraveling the Role of Scutellaria baicalensis for the Treatment of Breast Cancer Using Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation. International Journal of Molecular Sciences. 2023; 24(4):3594. https://doi.org/10.3390/ijms24043594
Chicago/Turabian StyleJiao, Yanqi, Chengcheng Shi, and Yao Sun. 2023. "Unraveling the Role of Scutellaria baicalensis for the Treatment of Breast Cancer Using Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation" International Journal of Molecular Sciences 24, no. 4: 3594. https://doi.org/10.3390/ijms24043594
APA StyleJiao, Y., Shi, C., & Sun, Y. (2023). Unraveling the Role of Scutellaria baicalensis for the Treatment of Breast Cancer Using Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation. International Journal of Molecular Sciences, 24(4), 3594. https://doi.org/10.3390/ijms24043594






