Paracrine Signals in Calcified Conditioned Media Elicited Differential Responses in Primary Aortic Vascular Smooth Muscle Cells and in Adventitial Fibroblasts
Abstract
:1. Introduction
2. Results
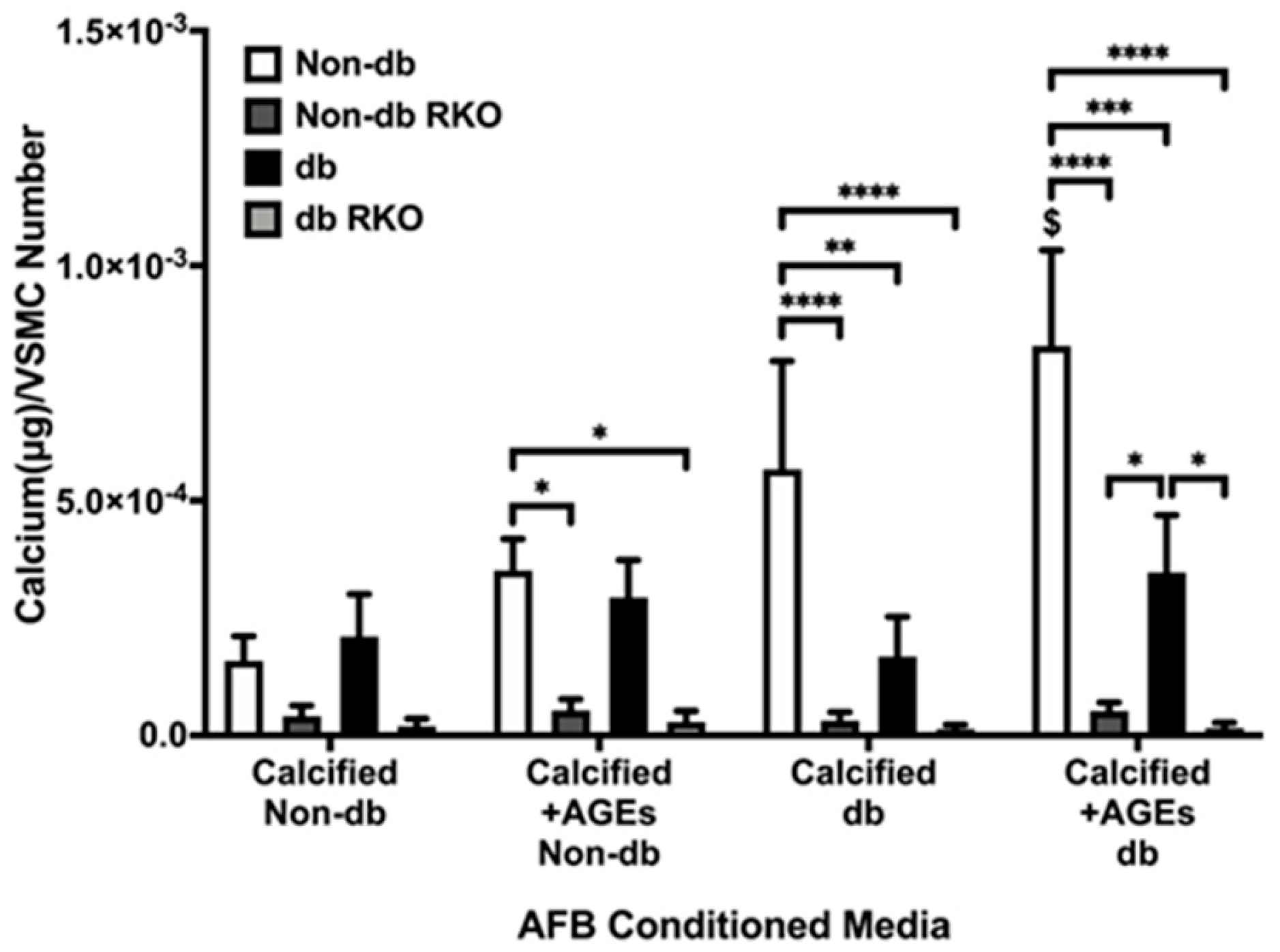
2.1. Conditioned Media from Calcified AFBs Elicited Differential Calcification Responses in VSMCs
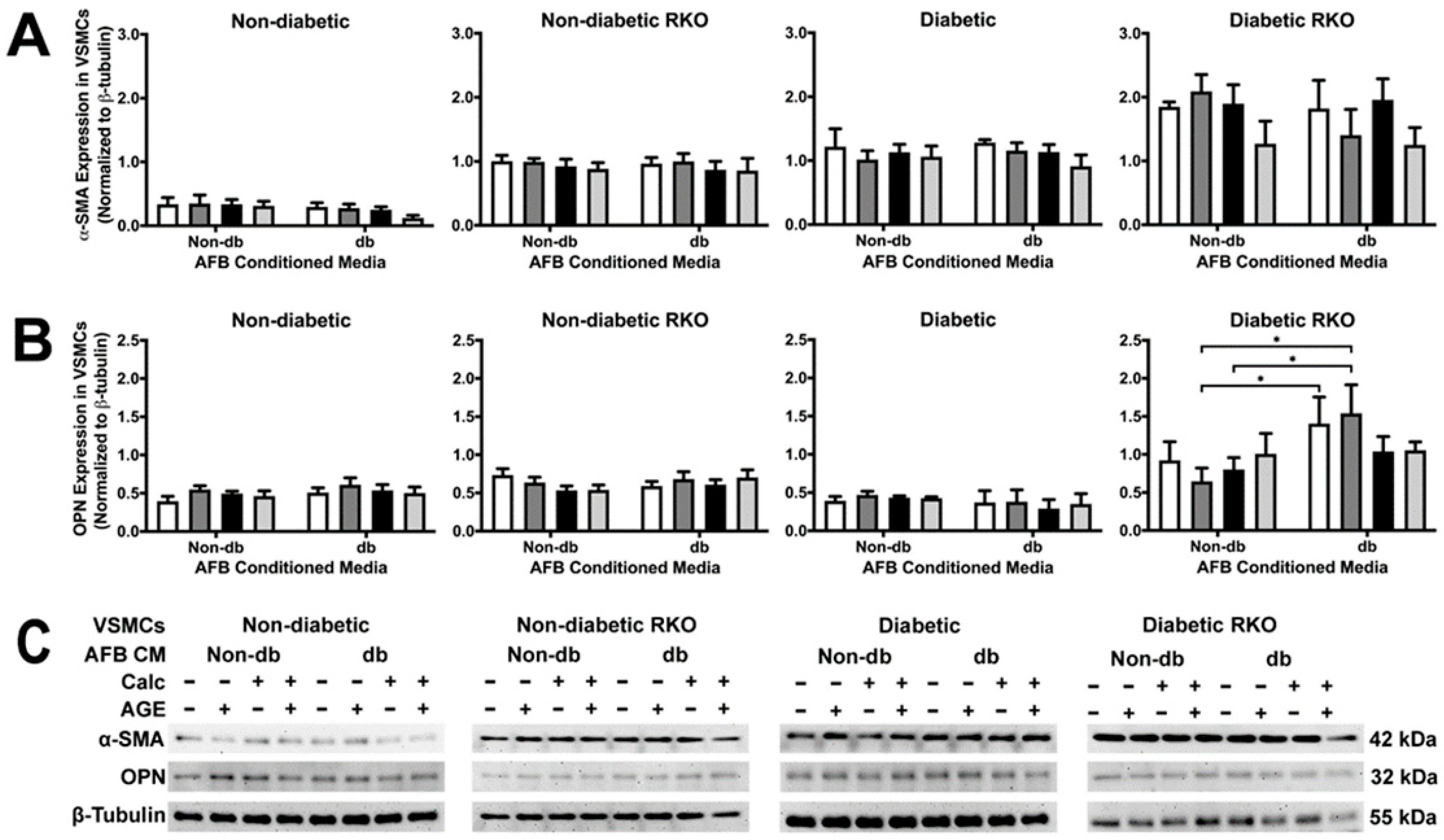
2.2. Conditioned Media from Diabetic AFBs Caused an Increase in OPN Expression in Diabetic RKO VSMCs
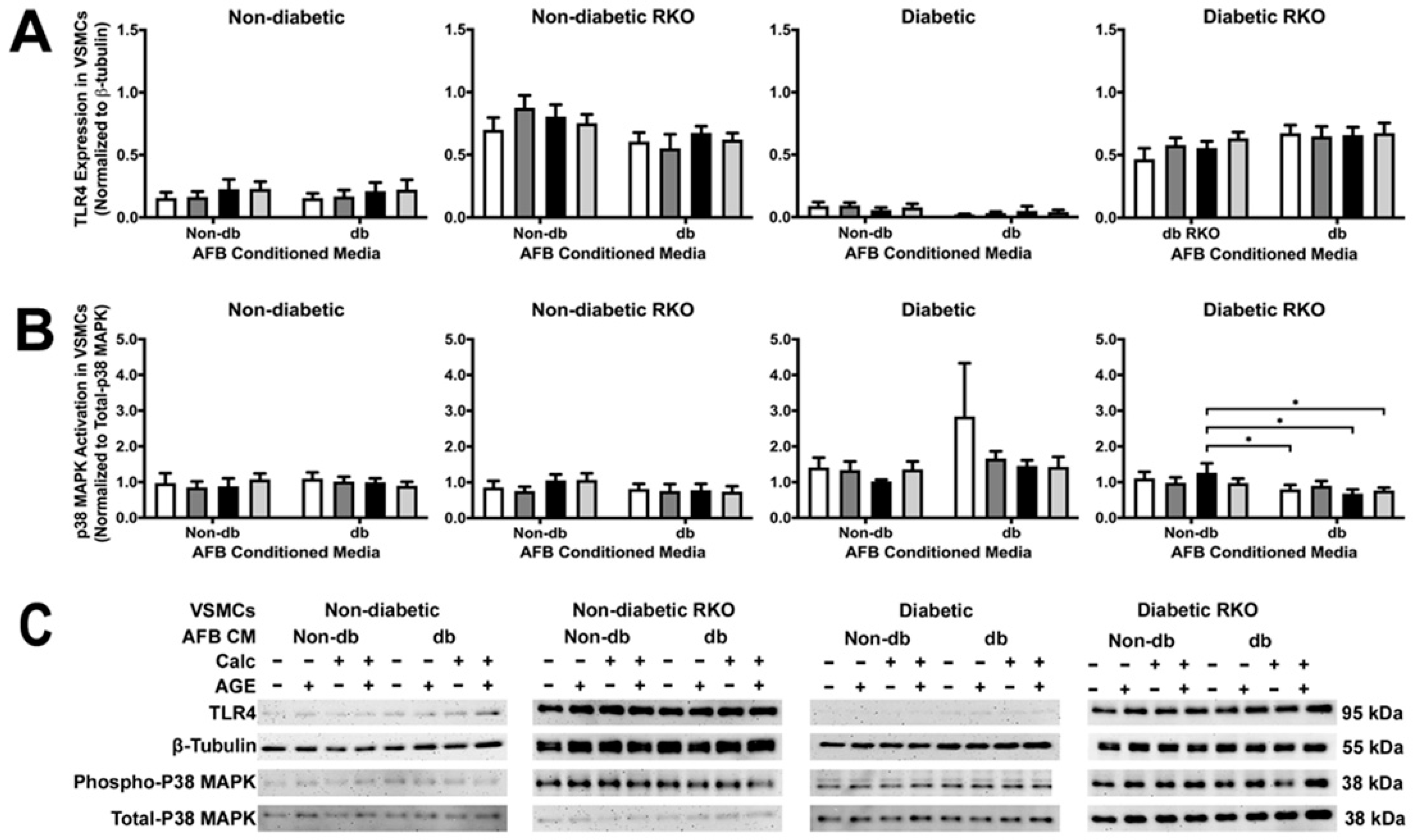
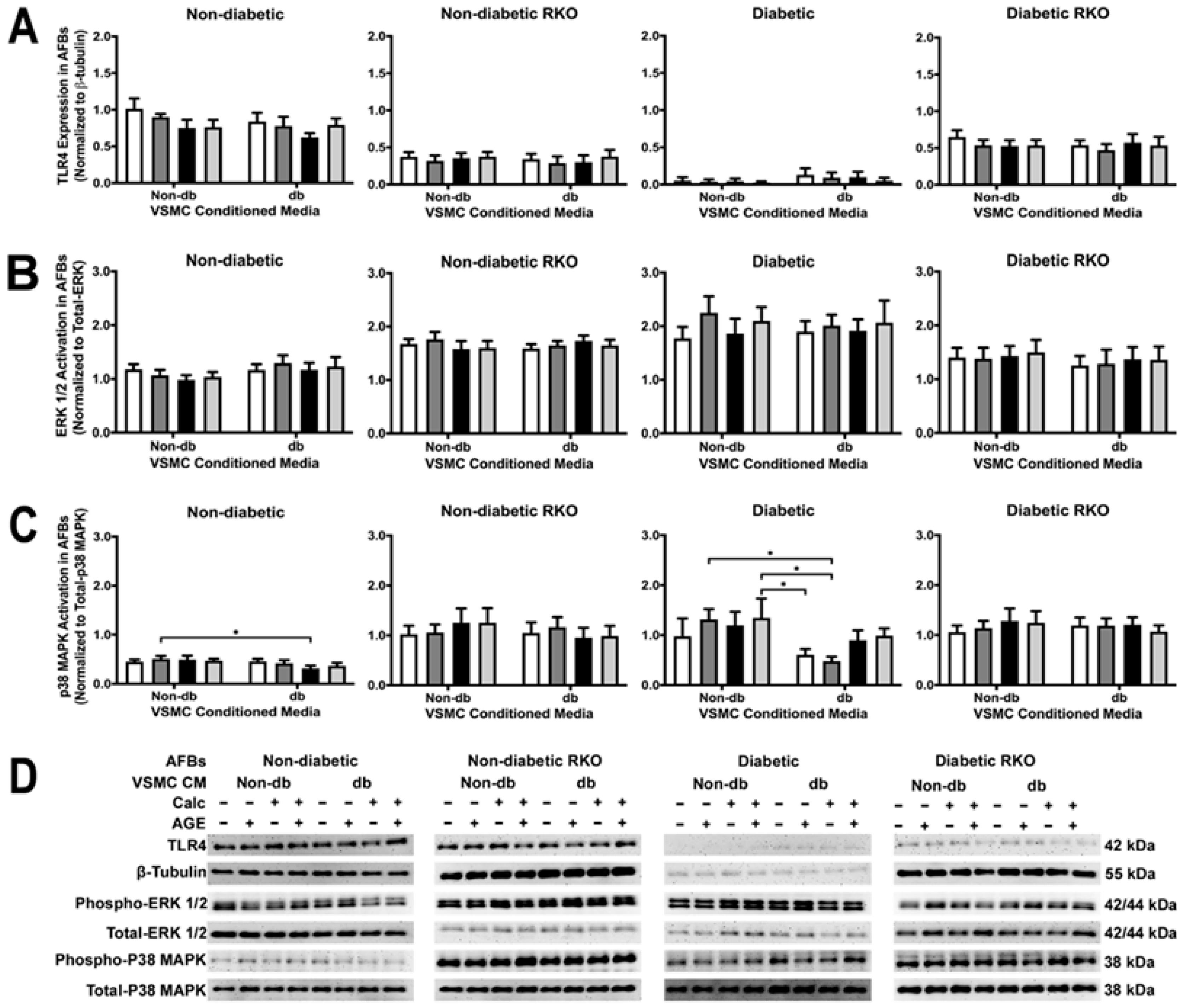
2.3. TLR4 and p38 MAPK Expressions Were Significantly Changed in RKO VSMCs
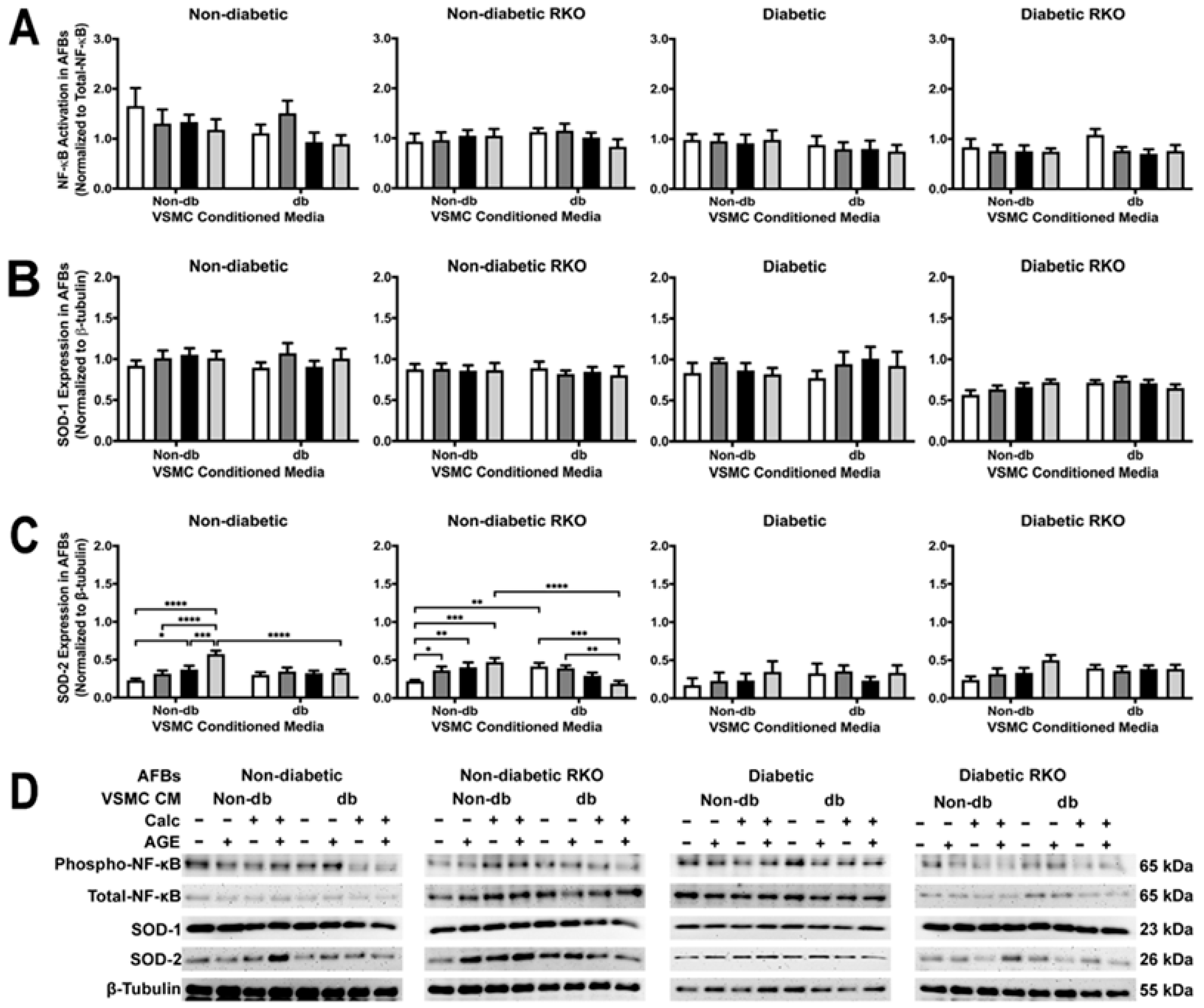
2.4. Conditioned Media from Diabetic AFBs Increased NF-κB Activation and SOD-2 Expression
2.5. Messenger Differences Were Observed between Non-Diabetic and Diabetic AFBs-conditioned media
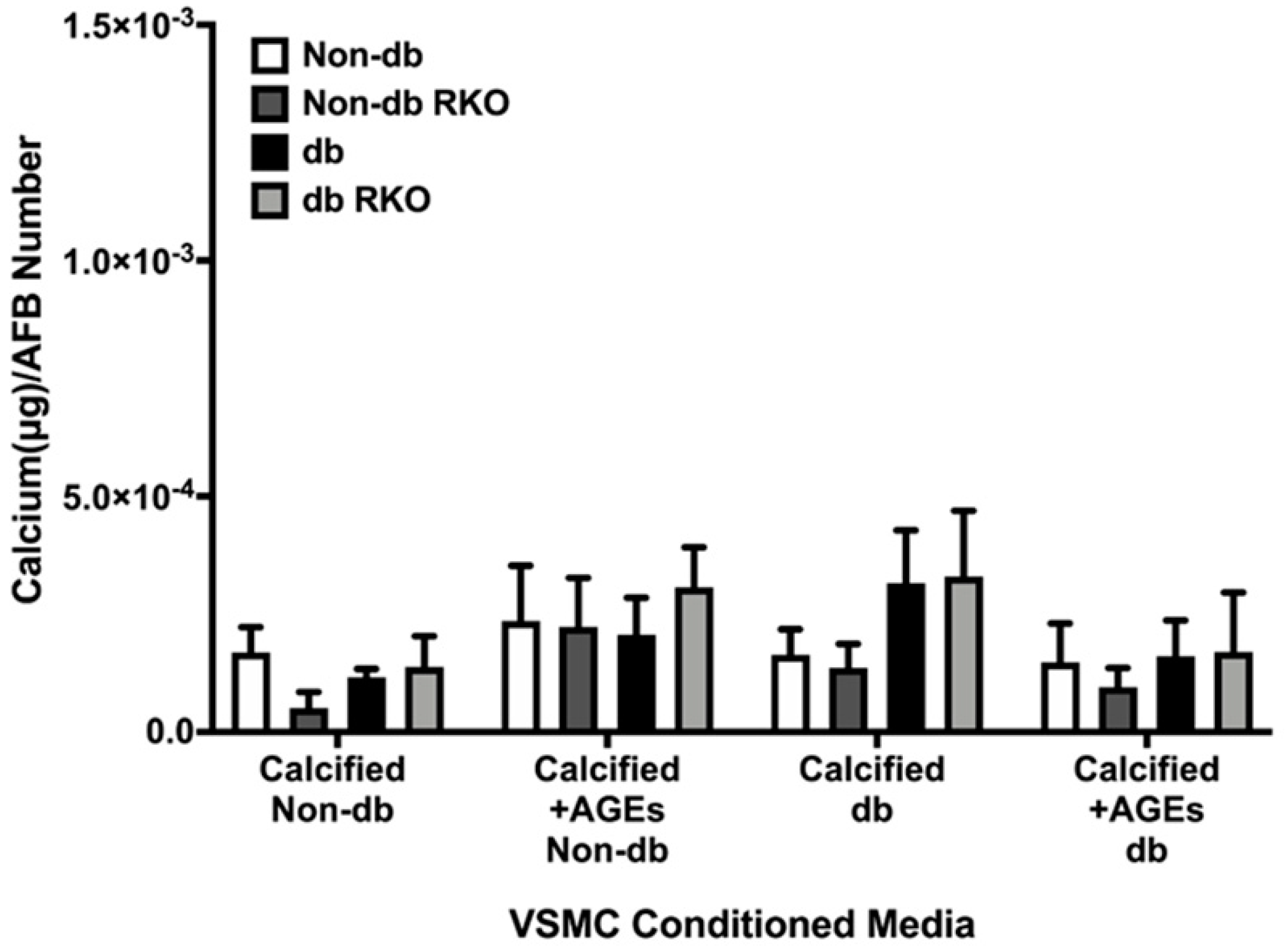
2.6. VSMCs-conditioned media Did Not Elicit a Differential Calcification Response in AFBs
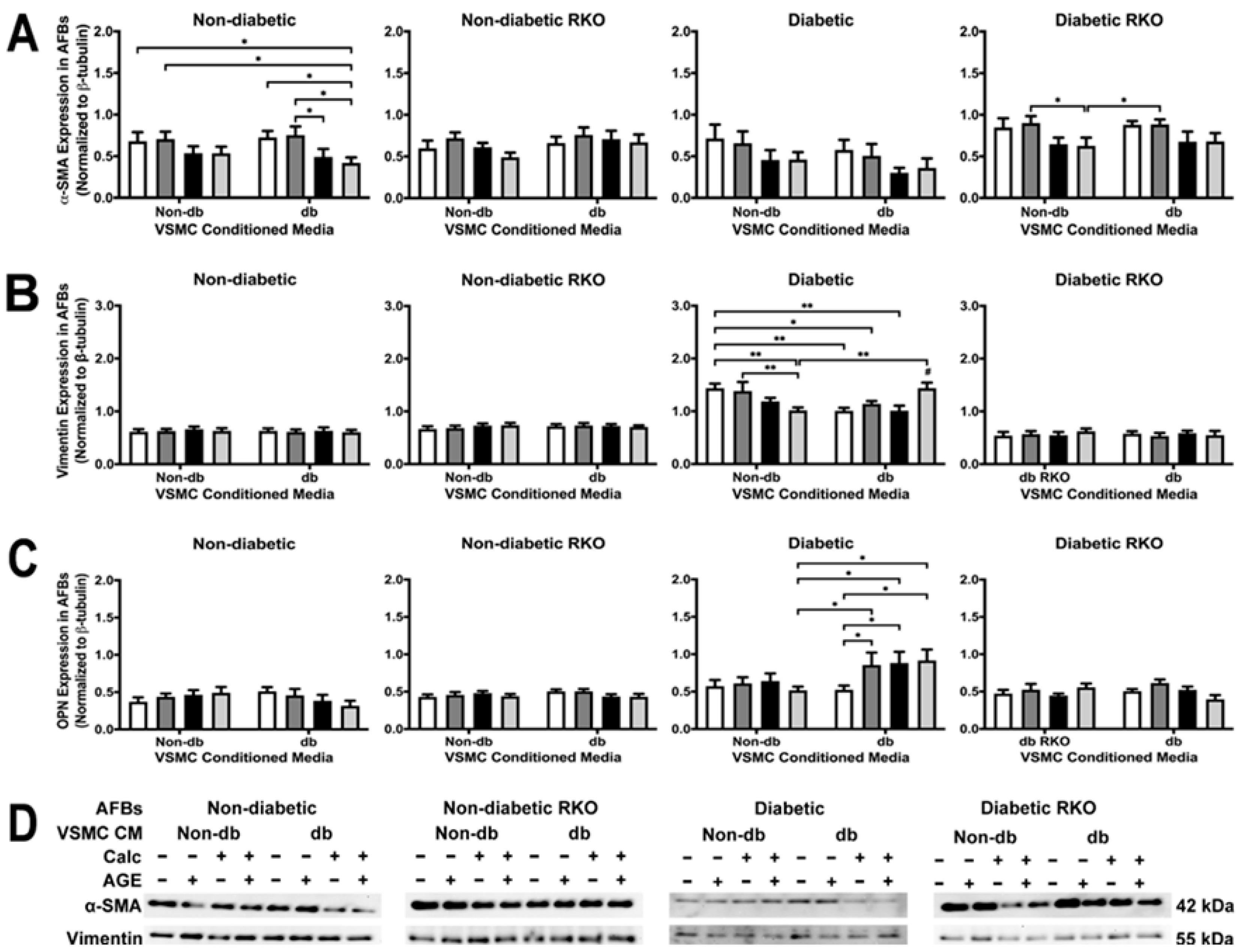
2.7. Phenotypic Changes Were Observed in Diabetic and Non-Diabetic AFBs Due to VSMC-conditioned media Treatment
2.8. VSMC-conditioned media Influenced P38 MAPK Activation
2.9. AFB Non-Diabetic Cell SOD-2 Expression Changed Due to Treatment withVSMC Conditioned Media
2.10. Messenger Differences Were Observed in Calcified VSMCs-conditioned media Depending upon Genotype and AGE Presence
3. Discussion
3.1. Conditioned AFB Media Applied to VSMCs
3.2. VSMC-Conditioned Media Applied to AFBs
4. Materials and Methods
4.1. Animal Model
4.2. Primary Murine Vascular Smooth Muscle Cell (VSMCs) Isolation and Culture
4.3. Primary Murine Adventitial Fibroblast (AFBs) Isolation and Culture
4.4. Conditioned Media Collection and Application
4.5. Calcification Quantification
4.6. DAPI Staining for Cell Number
4.7. Protein Analysis
4.8. Proteome Profiler™ Array
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, C.; Ni, L.; Zhang, C.; Hu, X.; Wu, X. Vascular calcification: New insights into endothelial cells. Microvasc. Res. 2021, 134, 104105. [Google Scholar] [CrossRef]
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef]
- Frismantiene, A.; Philippova, M.; Erne, P.; Resink, T.J. Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity. Cell Signal. 2018, 52, 48–64. [Google Scholar] [CrossRef]
- Majesky, M.W.; Dong, X.R.; Hoglund, V.; Mahoney, W.M.; Daum, G. The adventitia: A dynamic interface containing resident progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, T.; Kobayashi, S. Impact of vascular calcification on cardiovascular mortality in hemodialysis patients: Clinical significance, mechanisms and possible strategies for treatment. Ren. Replace. Ther. 2017, 3, 13. [Google Scholar] [CrossRef]
- Stitt, A.W.; Li, Y.M.; Gardiner, T.A.; Bucala, R.; Archer, D.B.; Vlassara, H. Advanced glycation end products (AGEs) co-localize with AGE receptors in the retinal vasculature of diabetic and of AGE-infused rats. Am. J. Pathol. 1997, 150, 523–531. [Google Scholar] [PubMed]
- Stitt, A.W.; Moore, J.E.; Sharkey, J.A.; Murphy, G.; Simpson, D.A.C.; Bucala, R.; Vlassara, H.; Archer, D.B. Advanced glycation end products in vitreous: Structural and functional implications for diabetic vitreopathy. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2517–2523. [Google Scholar]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef]
- Soman, S.; Raju, R.; Sandhya, V.K.; Advani, J.; Khan, A.A.; Harsha, H.C.; Prasad, T.S.K.; Sudhakaran, P.R.; Pandey, A.; Adishesha, P.K. A multicellular signal transduction network of AGE/RAGE signaling. J. Cell Commun. Signal. 2013, 7, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, M.; Murata, H.; Yamamoto, K.; Ono, T.; Sakaguchi, Y.; Motoyama, A.; Hibino, T.; Kataoka, K.; Huh, N. TIRAP, an adaptor protein for TLR2/4, transduces a signal from RAGE phosphorylated upon ligand binding. PLoS ONE 2011, 6, e23132. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, P.; King, G.L. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ. Res. 2010, 106, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, T.; Okada, Y.; Tanikawa, R.; Tanaka, Y. Advanced glycation end products induce calcification of vascular smooth muscle cells through rage/p38 MAPK. J. Vasc. Res. 2009, 46, 572–580. [Google Scholar] [CrossRef]
- Hu, P.; Lai, D.; Lu, P.; Gao, J.; He, H. ERK and Akt signaling pathways are involved in advanced glycation end product-induced autophagy in rat vascular smooth muscle cells. Int. J. Mol. Med. 2012, 29, 613–618. [Google Scholar] [CrossRef]
- El-Asrar, A.M.A.; Missotten, L.; Geboes, K. Expression of high-mobility groups box-1/receptor for advanced glycation end products/osteopontin/early growth response-1 pathway in proliferative vitreoretinal epiretinal membranes. Mol. Vis. 2011, 17, 508–518. [Google Scholar]
- Palanissami, G.; Paul, S.F.D. RAGE and Its Ligands: Molecular Interplay Between Glycation, Inflammation, and Hallmarks of Cancer—A Review. Horm. Cancer 2018, 9, 295–325. [Google Scholar] [CrossRef]
- Kueper, T.; Grune, T.; Prahl, S.; Lenz, H.; Welge, V.; Biernoth, T.; Vogt, Y.; Muhr, G.-M.; Gaemlich, A.; Jung, T.; et al. Vimentin is the specific target in skin glycation. Structural prerequisites, functional consequences, and role in skin aging. J. Biol. Chem. 2007, 282, 23427–23436. [Google Scholar] [CrossRef]
- Han, X.; Wu, A.; Wang, J.; Chang, H.; Mao, Y.Z.Y.Z.Y.; Lou, L.; Gao, Y.; Zhang, D.; Li, T.; Yang, T.; et al. Activation and Migration of Adventitial Fibroblasts Contributes to Vascular Remodeling. Anat. Rec. 2018, 301, 1216–1223. [Google Scholar] [CrossRef]
- Olesen, P.; Ledet, T.; Rasmussen, L.M. Arterial osteoprotegerin: Increased amounts in diabetes and modifiable synthesis from vascular smooth muscle cells by insulin and TNF-? Diabetologia 2005, 48, 561–568. [Google Scholar] [CrossRef]
- Boström, K.I.; Jumabay, M.; Matveyenko, A.; Nicholas, S.B.; Yao, Y. Activation of vascular bone morphogenetic protein signaling in diabetes mellitus. Circ. Res. 2011, 108, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Greenwell, J.C.; Torres-Gonzalez, E.; Ritzenthaler, J.D.; Roman, J. Fibroblast-derived conditioned media promotes lung cancer progression. Am. J. Med. Sci. 2023, 365, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Oparil, S.; Kelpke, S.S.; Chen, Y.-F.; Thompson, J.A. Fibroblast Growth Factor Receptor-1 Signaling Induces Osteopontin Expression and Vascular Smooth Muscle Cell–Dependent Adventitial Fibroblast Migration In Vitro. Circulation 2002, 106, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Sakuda, H.; Nakashima, Y.; Kuriyama, S.; Sueishi, K. Media conditioned by smooth muscle cells cultured in a variety of hypoxic environments stimulates in vitro angiogenesis. A relationship to transforming growth factor-beta 1. Am. J. Pathol. 1992, 141, 1507–1516. [Google Scholar]
- Liu, J.; Han, Z.; Han, Z.; He, Z. Mesenchymal stem cell-conditioned media suppresses inflammation-associated overproliferation of pulmonary artery smooth muscle cells in a rat model of pulmonary hypertension. Exp. Ther. Med. 2016, 11, 467–475. [Google Scholar] [CrossRef]
- Jha, K.A.; Pentecost, M.; Lenin, R.; Klaic, L.; Elshaer, S.L.; Gentry, J.; Russell, J.M.; Beland, A.; Reiner, A.; Jotterand, V.; et al. Concentrated conditioned media from adipose tissue derived mesenchymal stem cells mitigates visual deficits and retinal inflammation following mild traumatic brain injury. Int. J. Mol. Sci. 2018, 19, 2016. [Google Scholar] [CrossRef]
- Bader, A.M.; Brodarac, A.; Klose, K.; Bieback, K.; Choi, Y.H.; Kurtz, A.; Stamm, C. Mechanisms of paracrine cardioprotection by cord blood mesenchymal stromal cells. Eur. J. Cardio-Thorac. Surg. 2014, 45, 983–992. [Google Scholar] [CrossRef]
- Pati, S.; Gerber, M.H.; Menge, T.D.; Wataha, K.A.; Zhao, Y.; Baumgartner, J.A.; Zhao, J.; Letourneau, P.A.; Huby, M.P.; Baer, L.A.; et al. Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS ONE 2011, 6, e25171. [Google Scholar] [CrossRef]
- Rovai, L.E.; Herschman, H.R.; Smith, J.B. The murine neutrophil-chemoattractant chemokines LIX, KC, and MIP-2 have distinct induction kinetics, tissue distributions, and tissue-specific sensitivities to glucocorticoid regulation in endotoxemia. J. Leukoc. Biol. 1998, 64, 494–502. [Google Scholar] [CrossRef]
- Wang, X.-Z.; Liu, L.-W.; Du, X.-M.; Gu, R.-X.; Sun, Z.-J. CXCL5 is associated with the increased risk of coronary artery disease. Coron. Artery Dis. 2015, 26, 612–619. [Google Scholar] [CrossRef]
- Brodeur, M.R.; Bouvet, C.; Bouchard, S.; Moreau, S.; Leblond, J.; DeBlois, D.; Moreau, P. Reduction of advanced-glycation end products levels and inhibition of rage signaling decreases rat vascular calcification induced by diabetes. PLoS ONE 2014, 9, e85922. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, Y.; Liu, X.; Liu, B.; Wang, Z.; Xie, F.; Qiao, W.; Liang, E.; Lu, Q.; Zhang, M. Loss of PARP-1 attenuates diabetic arteriosclerotic calcification via Stat1/Runx2 axis. Cell Death Dis. 2020, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, M.; Yokote, K.; Yamazaki, M.; Ridall, A.L.; Butler, W.T.; Matsumoto, T.; Tamura, K.; Saito, Y.; Mori, S. Enhanced expression of osteopontin by high glucose in cultured rat aortic smooth muscle cells. Biochem. Biophys. Res. Commun. 1999, 258, 722–726. [Google Scholar] [CrossRef]
- Olejarz, W.; Łacheta, D.; Głuszko, A.; Migacz, E.; Kukwa, W.; Szczepański, M.J.; Tomaszewski, P.; Nowicka, G. RAGE and TLRs as Key Targets for Antiatherosclerotic Therapy. BioMed Res. Int. 2018, 2018, 7675286. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Xu, G.; Jay, T.R.; Bhatta, S.; Kim, K.-W.; Jung, S.; Landreth, G.E.; Ransohoff, R.M.; Lamb, B.T. Opposing Effects of Membrane-Anchored CX3CL1 on Amyloid and Tau Pathologies via the p38 MAPK Pathway. J. Neurosci. 2014, 34, 12538–12546. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Morotti, A.; Ponzetto, C. Activation of NF-kappaB is essential for hepatocyte growth factor-mediated proliferation and tubulogenesis. Mol. Cell. Biol. 2002, 22, 1060–1072. [Google Scholar] [CrossRef]
- Rada, B.; Gardina, P.; Myers, T.G.; Leto, T.L. Reactive oxygen species mediate inflammatory cytokine release and EGFR-dependent mucin secretion in airway epithelial cells exposed to Pseudomonas pyocyanin. Mucosal Immunol. 2011, 4, 158–171. [Google Scholar] [CrossRef]
- Yao, D.; Brownlee, M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes 2010, 59, 249–255. [Google Scholar] [CrossRef]
- Patel, H.; Chen, J.; Das, K.C.; Kavdia, M. Hyperglycemia induces differential change in oxidative stress at gene expression and functional levels in HUVEC and HMVEC. Cardiovasc. Diabetol. 2013, 12, 142. [Google Scholar] [CrossRef]
- Cai, L.; Xu, S.; Piao, C.; Qiu, S.; Li, H.; Du, J. Adiponectin induces CXCL1 secretion from cancer cells and promotes tumor angiogenesis by inducing stromal fibroblast senescence. Mol. Carcinog. 2016, 55, 1796–1806. [Google Scholar] [CrossRef]
- Kim, E.K.; Moon, S.; Kim, D.K.; Zhang, X.; Kim, J. CXCL1 induces senescence of cancer-associated fibroblasts via autocrine loops in oral squamous cell carcinoma. PLoS ONE 2018, 13, e0188847. [Google Scholar] [CrossRef] [PubMed]
- Micutkova, L.; Diener, T.; Li, C.; Rogowska-Wrzesinska, A.; Mueck, C.; Huetter, E.; Weinberger, B.; Grubeck-Loebenstein, B.; Roepstorff, P.; Zeng, R.; et al. Insulin-like growth factor binding protein-6 delays replicative senescence of human fibroblasts. Mech. Ageing Dev. 2011, 132, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qi, Y.; Wang, R.; Wu, W.; Li, Z.; Wang, M.; Liu, R.; Zhang, C.; Li, W.; Wang, S. IGFBP6 regulates vascular smooth muscle cell proliferation and morphology via cyclin E–CDK2. J. Cell. Physiol. 2020, 235, 9538–9556. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, R.; Crawford, H.C.; Haro, H.; Matrisian, L.M.; Havrda, M.C.; Liaw, L. Osteopontin, a Novel Substrate for Matrix Metalloproteinase-3 (Stromelysin-1) and Matrix Metalloproteinase-7 (Matrilysin). J. Biol. Chem. 2001, 276, 28261–28267. [Google Scholar] [CrossRef]
- Akwii, R.G.; Sajib, M.S.; Zahra, F.T.; Mikelis, C.M. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells 2019, 8, 471. [Google Scholar] [CrossRef]
- Fiedler, U.; Reiss, Y.; Scharpfenecker, M.; Grunow, V.; Koidl, S.; Thurston, G.; Gale, N.W.; Witzenrath, M.; Rosseau, S.; Suttorp, N.; et al. Angiopoietin-2 sensitizes endothelial cells to TNF-α and has a crucial role in the induction of inflammation. Nat. Med. 2006, 12, 235–239. [Google Scholar] [CrossRef]
- Wischhusen, J.; Melero, I.; Fridman, W.H. Growth/Differentiation Factor-15 (GDF-15): From Biomarker to Novel Targetable Immune Checkpoint. Front. Immunol. 2020, 11, 951. [Google Scholar] [CrossRef]
- Frimodt-Møller, M.; von Scholten, B.J.; Reinhard, H.; Jacobsen, P.K.; Hansen, T.W.; Persson, F.I.; Parving, H.-H.; Rossing, P. Growth differentiation factor-15 and fibroblast growth factor-23 are associated with mortality in type 2 diabetes—An observational follow-up study. PLoS ONE 2018, 13, e0196634. [Google Scholar] [CrossRef]
- Giesen, P.L.; Rauch, U.; Bohrmann, B.; Kling, D.; Roqué, M.; Fallon, J.T.; Badimon, J.J.; Himber, J.; Riederer, M.A.; Nemerson, Y. Blood-borne tissue factor: Another view of thrombosis. Proc. Natl. Acad. Sci. USA 1999, 96, 2311–2315. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Venkatesan, B.; Valente, A.J.; Melby, P.C.; Nandish, S.; Reusch, J.E.B.; Clark, R.A.; Chandrasekar, B. WISP1, a Pro-mitogenic, Pro-survival Factor, Mediates Tumor Necrosis Factor-α (TNF-α)-stimulated Cardiac Fibroblast Proliferation but Inhibits TNF-α-induced Cardiomyocyte Death. J. Biol. Chem. 2009, 284, 14414–14427. [Google Scholar] [CrossRef]
- Colston, J.T.; de la Rosa, S.D.; Koehler, M.; Gonzales, K.; Mestril, R.; Freeman, G.L.; Bailey, S.R.; Chandrasekar, B. Wnt-induced secreted protein-1 is a prohypertrophic and profibrotic growth factor. Am. J. Physiol. Circ. Physiol. 2007, 293, H1839–H1846. [Google Scholar] [CrossRef] [PubMed]
- Sowndhar Rajan, B.; Manivasagam, S.; Dhanusu, S.; Chandrasekar, N.; Krishna, K.; Kalaiarasu, L.P.; Babu, A.A.; Vellaichamy, E. Diet with high content of advanced glycation end products induces systemic inflammation and weight gain in experimental mice: Protective role of curcumin and gallic acid. Food Chem. Toxicol. 2018, 114, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.R.; Kasteler, S.D.; Schmitt, R.E.; Hoidal, J.R. Receptor for Advanced Glycation End-Products Signals through Ras during Tobacco Smoke–Induced Pulmonary Inflammation. Am. J. Respir. Cell Mol. Biol. 2011, 45, 411–418. [Google Scholar] [CrossRef]
- McCarthy, A.D.; Etcheverry, S.B.; Cortizo, A.M. Effect of advanced glycation endproducts on the secretion of insulin-like growth factor-I and its binding proteins: Role in osteoblast development. Acta Diabetol. 2001, 38, 113–122. [Google Scholar] [CrossRef]
- Li, N.; Cheng, W.; Huang, T.; Yuan, J.; Wang, X.; Song, M. Vascular adventitia calcification and its underlying mechanism. PLoS ONE 2015, 10, e0132506. [Google Scholar] [CrossRef]
- Simionescu, A.; Simionescu, D.T.; Vyavahare, N.R. Osteogenic responses in fibroblasts activated by elastin degradation products and transforming growth factor-β1: Role of myofibroblasts in vascular calcification. Am. J. Pathol. 2007, 171, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Pillai, I.C.L.; Li, S.; Romay, M.; Lam, L.; Lu, Y.; Huang, J.; Dillard, N.; Zemanova, M.; Rubbi, L.; Wang, Y.; et al. Cardiac Fibroblasts Adopt Osteogenic Fates and Can Be Targeted to Attenuate Pathological Heart Calcification. Cell Stem Cell 2017, 20, 218–232. [Google Scholar] [CrossRef]
- Zorzi, P.; Aplin, A.C.; Smith, K.D.; Nicosia, R.F. Technical Advance: The rat aorta contains resident mononuclear phagocytes with proliferative capacity and proangiogenic properties. J. Leukoc. Biol. 2010, 88, 1051–1059. [Google Scholar] [CrossRef]
- Calvayrac, O.; Rodríguez-Calvo, R.; Alonso, J.; Orbe, J.; Martín-Ventura, J.L.; Guadall, A.; Gentile, M.; Juan-Babot, O.; Egido, J.; Beloqui, O.; et al. CCL20 is increased in hypercholesterolemic subjects and is upregulated by LDL in vascular smooth muscle cells: Role of NF-κB. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2733–2741. [Google Scholar] [CrossRef]
- Hou, K.-Z.; Fu, Z.-Q.; Gong, H. Chemokine ligand 20 enhances progression of hepatocellular carcinoma via epithelial-mesenchymal transition. World J. Gastroenterol. 2015, 21, 475–483. [Google Scholar] [CrossRef]
- Song, T.; Wang, C.; Guo, C.; Liu, Q.; Zheng, X. Pentraxin 3 overexpression accelerated tumor metastasis and indicated poor prognosis in hepatocellular carcinoma via driving epithelial-mesenchymal transition. J. Cancer 2018, 9, 2650–2658. [Google Scholar] [CrossRef]
- Hung, T.-W.; Tsai, J.-P.; Lin, S.-H.; Lee, C.-H.; Hsieh, Y.-H.; Chang, H.-R. Pentraxin 3 Activates JNK Signaling and Regulates the Epithelial-To-Mesenchymal Transition in Renal Fibrosis. Cell. Physiol. Biochem. 2016, 40, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- De Silva, V.; Okura, T.; Miyoshi, K.; Irita, J.; Kurata, M.; Pei, Z.; Enomoto, D.; Nagao, T.; Jotoku, M.; Higaki, J. Involvement of Osteopontin in High Glucose Induced Type I and Type III Collagen Expression in Renal Fibroblasts. Annu. Res. J. SLSAJ 2012, 12, 50–55. [Google Scholar]
- Zhang, D.; Cui, Y.; Niu, L.; Xu, X.; Tian, K.; Young, C.Y.F.; Lou, H.; Yuan, H. Regulation of SOD2 and β-arrestin1 by interleukin-6 contributes to the increase of IGF-1R expression in docetaxel resistant prostate cancer cells. Eur. J. Cell Biol. 2014, 93, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Taglieri, N.; Koenig, W.; Kaski, J.C. Cystatin C and Cardiovascular Risk. Clin. Chem. 2009, 55, 1932–1943. [Google Scholar] [CrossRef]
- Olesen, P.; Nguyen, K.; Wogensen, L.; Ledet, T.; Rasmussen, L.M. Calcification of human vascular smooth muscle cells: Associations with osteoprotegerin expression and acceleration by high-dose insulin. Am. J. Physiol. Circ. Physiol. 2007, 292, H1058–H1064. [Google Scholar] [CrossRef]
- Cohick, W.S.; Gockerman, A.; Clemmons, D.R. Regulation of insulin-like growth factor (IGF) binding protein-2 synthesis and degradation by platelet-derived growth factor and the I GFs is enhanced by serum deprivation in vascular smooth muscle cells. J. Cell. Physiol. 1995, 164, 187–196. [Google Scholar] [CrossRef]
- Perkins, T.N.; Oczypok, E.A.; Dutz, R.E.; Donnell, M.L.; Myerburg, M.M.; Oury, T.D. The receptor for advanced glycation end products is a critical mediator of type 2 cytokine signaling in the lungs. J. Allergy Clin. Immunol. 2019, 144, 796–808.e12. [Google Scholar] [CrossRef]
- Voloshyna, I.; Gamez-Godoy, J.; Littlefield, M.J.; De Leon, J.; Castro-Magana, M.; Reiss, A.B. Advanced Glycation End Products Promote Pro-Atherogenic Changes in Cholesterol Transport: A Possible Mechanism for Cardiovascular Risk in Diabetes. Intern. Med. 2014, 11, 005. [Google Scholar] [CrossRef]
- Kislinger, T.; Tanji, N.; Wendt, T.; Qu, W.; Lu, Y.; Ferran, L.J.; Taguchi, A.; Olson, K.; Bucciarelli, L.; Goova, M.; et al. Receptor for Advanced Glycation End Products Mediates Inflammation and Enhanced Expression of Tissue Factor in Vasculature of Diabetic Apolipoprotein E–Null Mice. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 905–910. [Google Scholar] [CrossRef]
- Bierhaus, A.; Illmer, T.; Kasper, M.; Luther, T.; Quehenberger, P.; Tritschler, H.; Wahl, P.; Ziegler, R.; Müller, M.; Nawroth, P.P. Advanced Glycation End Product (AGE)–Mediated Induction of Tissue Factor in Cultured Endothelial Cells Is Dependent on RAGE. Circulation 1997, 96, 2262–2271. [Google Scholar] [CrossRef]
- Niiya, Y.; Abumiya, T.; Shichinohe, H.; Kuroda, S.; Kikuchi, S.; Ieko, M.; Yamagishi, S.; Takeuchi, M.; Sato, T.; Iwasaki, Y. Susceptibility of brain microvascular endothelial cells to advanced glycation end products-induced tissue factor upregulation is associated with intracellular reactive oxygen species. Brain Res. 2006, 1108, 179–187. [Google Scholar] [CrossRef]
- Burr, S.D.; Stewart, J.A. Extracellular matrix components isolated from diabetic mice alter cardiac fibroblast function through the AGE/RAGE signaling cascade. Life Sci. 2020, 250, 117569. [Google Scholar] [CrossRef]
- Lee, C.H.; Taketo, T. Characterization of a novel EGFP reporter mouse to monitor Cre recombination as demonstrated by a Tie2Cre mouse line. Genesis 2001, 30, 36–44. [Google Scholar] [CrossRef]
- Liliensiek, B.; Weigand, M.A.; Bierhaus, A.; Nicklas, W.; Kasper, M.; Hofer, S.; Plachky, J.; Gröne, H.J.; Kurschus, F.C.; Schmidt, A.M.; et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J. Clin. Investig. 2004, 113, 1641–1650. [Google Scholar] [CrossRef]
- Schwenk, F.; Baron, U.; Rajewsky, K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995, 23, 5080–5081. [Google Scholar] [CrossRef]
- Kobayashi, K.; Forte, T.M.; Taniguchi, S.; Ishida, B.Y.; Oka, K.; Chan, L. The db/db mouse, a model for diabetic dyslipidemia: Molecular characterization and effects of Western diet feeding. Metabolism 2000, 49, 22–31. [Google Scholar] [CrossRef]
- Gagea-Iurascu, M.; Craig, S. Euthanasia and Necropsy. In The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; Academic Press: Cambridge, MA, USA, 2012; pp. 117–139. [Google Scholar] [CrossRef]
- Clarkson, J.M.; Martin, J.E.; McKeegan, D.E.F. A review of methods used to kill laboratory rodents: Issues and opportunities. Lab. Anim. 2022, 56, 419–436. [Google Scholar] [CrossRef]
- Kennon, A.K.; Stewart, J.A., Jr. In vitro studies demonstrated RAGE signaling was responsible for driving the calcification response, phenotype changes, and inflammatory responses in VSMCs but not AFBs. Front. Physiol. 2021, 12, 676727. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kennon, A.M.; Stewart, J.A., Jr. Paracrine Signals in Calcified Conditioned Media Elicited Differential Responses in Primary Aortic Vascular Smooth Muscle Cells and in Adventitial Fibroblasts. Int. J. Mol. Sci. 2023, 24, 3599. https://doi.org/10.3390/ijms24043599
Kennon AM, Stewart JA Jr. Paracrine Signals in Calcified Conditioned Media Elicited Differential Responses in Primary Aortic Vascular Smooth Muscle Cells and in Adventitial Fibroblasts. International Journal of Molecular Sciences. 2023; 24(4):3599. https://doi.org/10.3390/ijms24043599
Chicago/Turabian StyleKennon, Amber M., and James A. Stewart, Jr. 2023. "Paracrine Signals in Calcified Conditioned Media Elicited Differential Responses in Primary Aortic Vascular Smooth Muscle Cells and in Adventitial Fibroblasts" International Journal of Molecular Sciences 24, no. 4: 3599. https://doi.org/10.3390/ijms24043599





