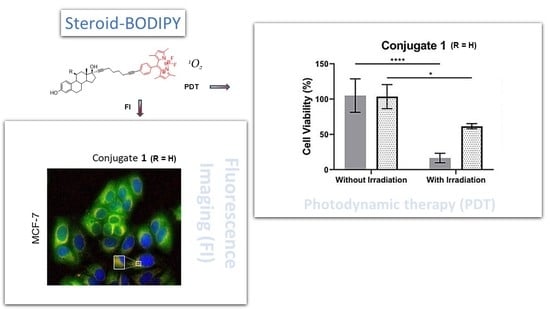
Cell Uptake of Steroid-BODIPY Conjugates and Their Internalization Mechanisms: Cancer Theranostic Dyes
Abstract
1. Introduction
2. Results
2.1. Cell Uptake and Trafficking of Steroid-BODIPY Conjugates
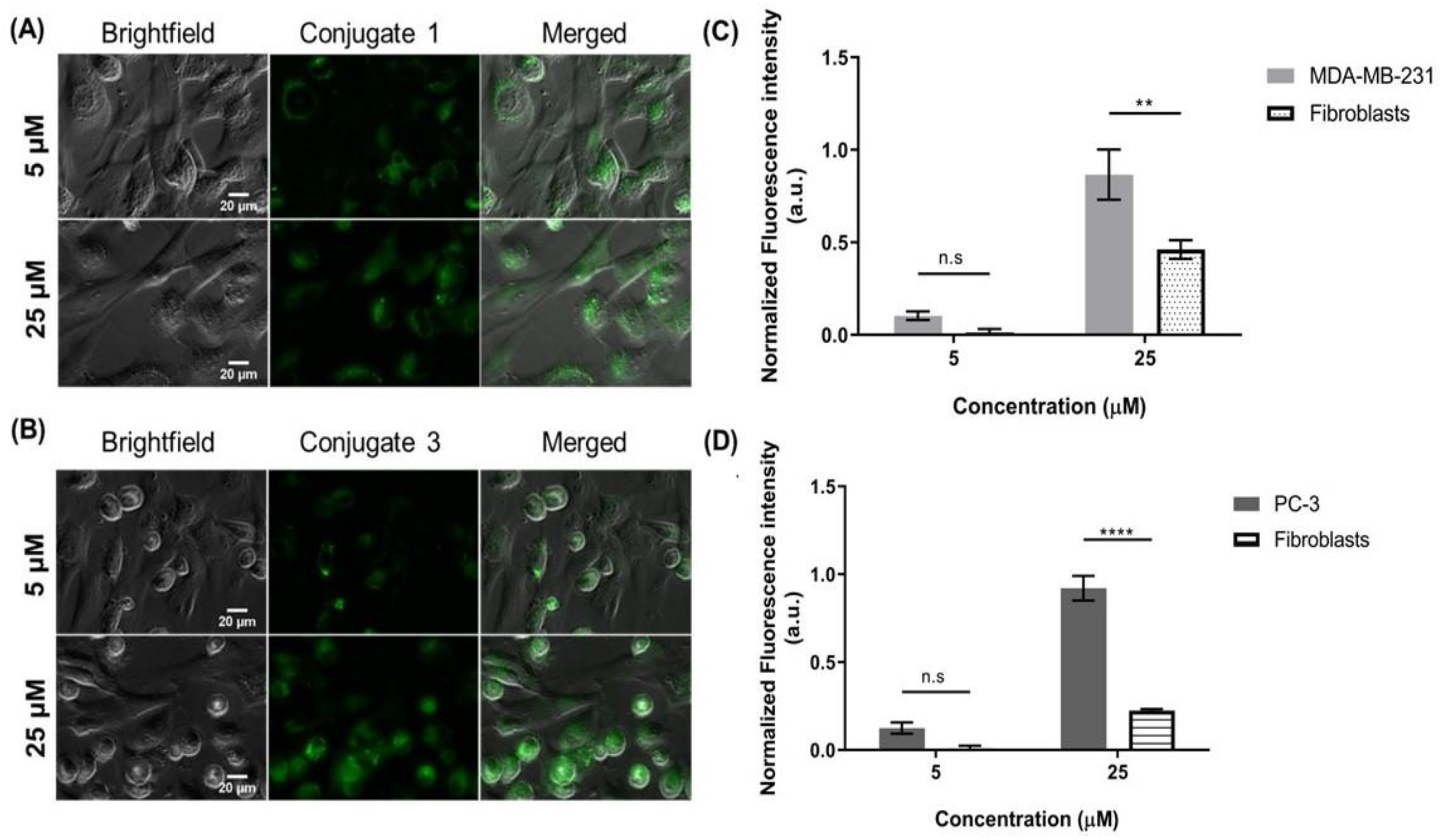
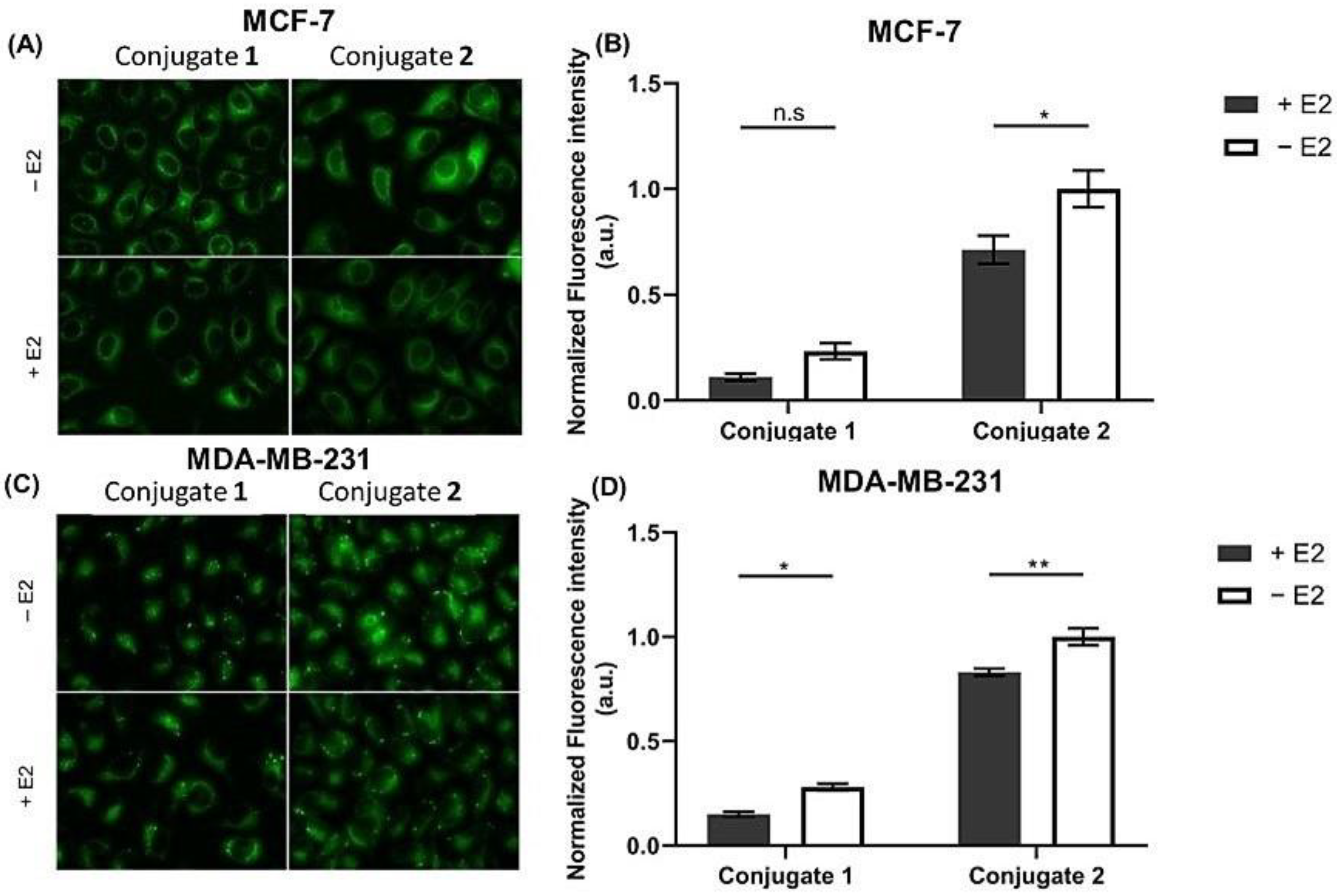
2.1.1. Steroid-BODIPY Conjugates in Breast Cancer Cells
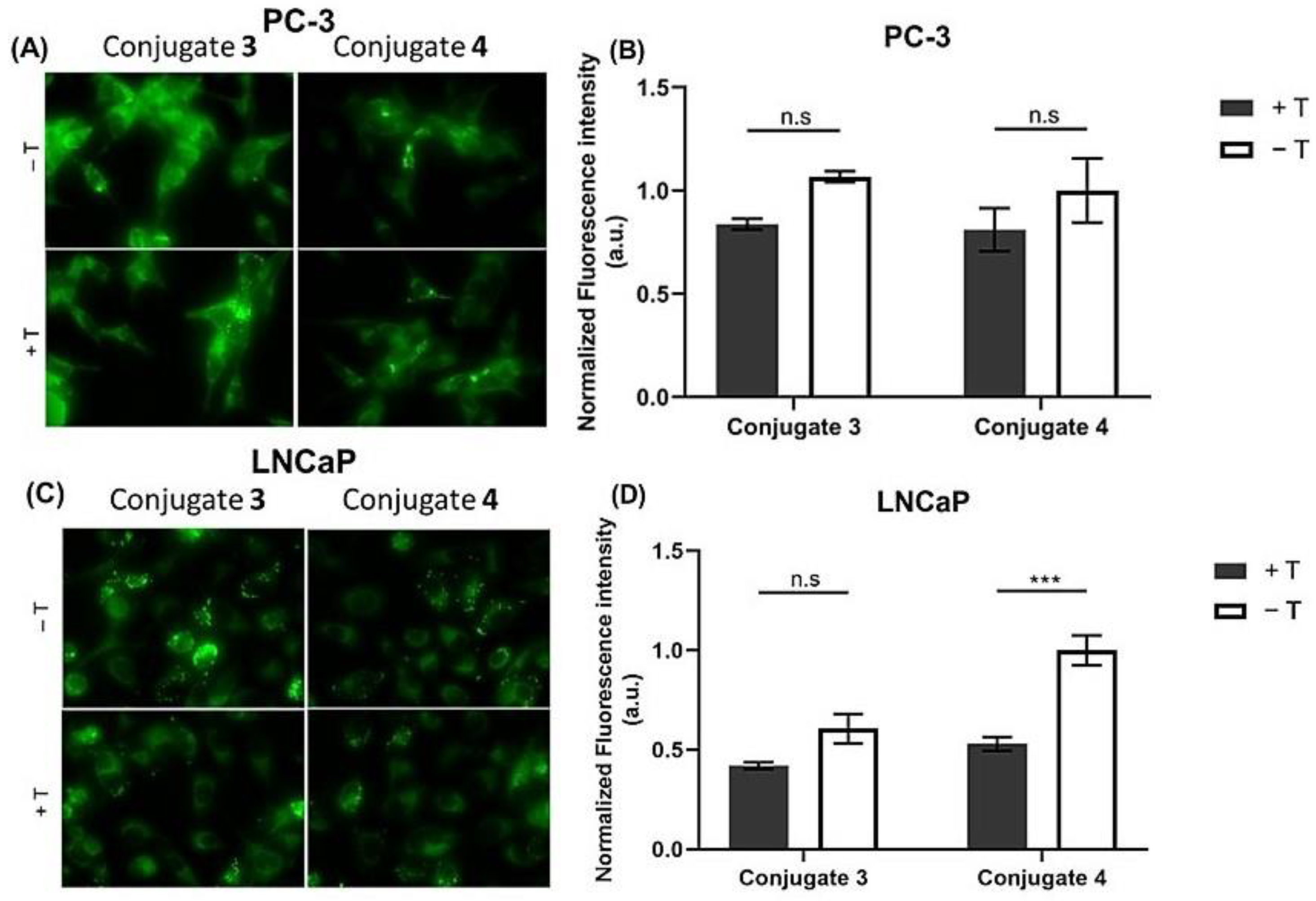
2.1.2. Steroid-BODIPY Conjugates in Prostate Cancer Cells
2.2. Internalization of Steroid-BODIPY Conjugates in 2D Co-Culture
2.3. Internalization of Steroid-BODIPY Conjugates in the Presence of Endocrine Disruptors
2.4. Assessment of Cell Uptake and Trafficking of Steroid-BODIPY Conjugates
2.4.1. Active vs. Passive Transport
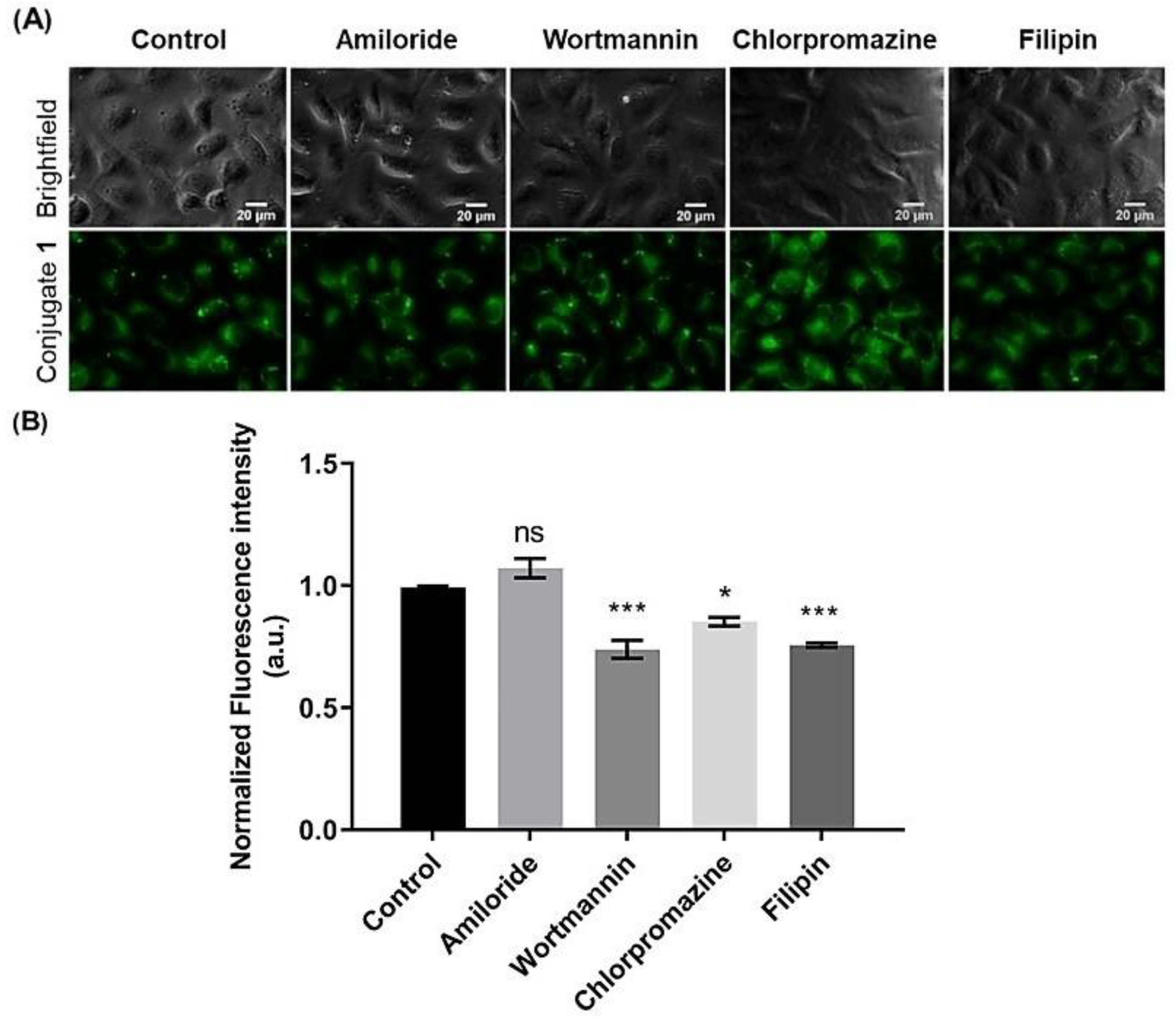
2.4.2. Inhibition of Endocytosis of Steroid-BODIPY Conjugates
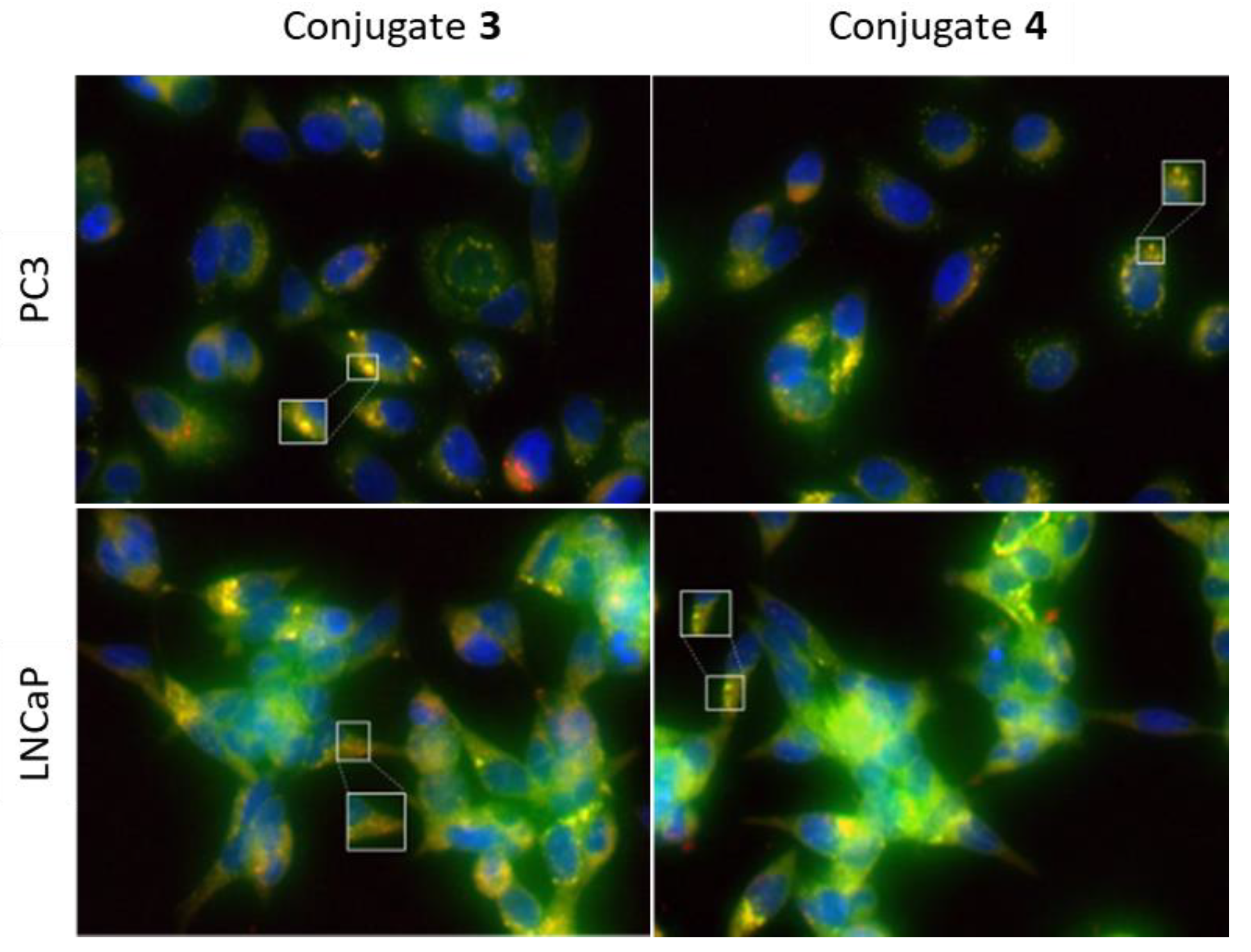
2.4.3. Intracellular Localization of Steroid-BODIPY Conjugates
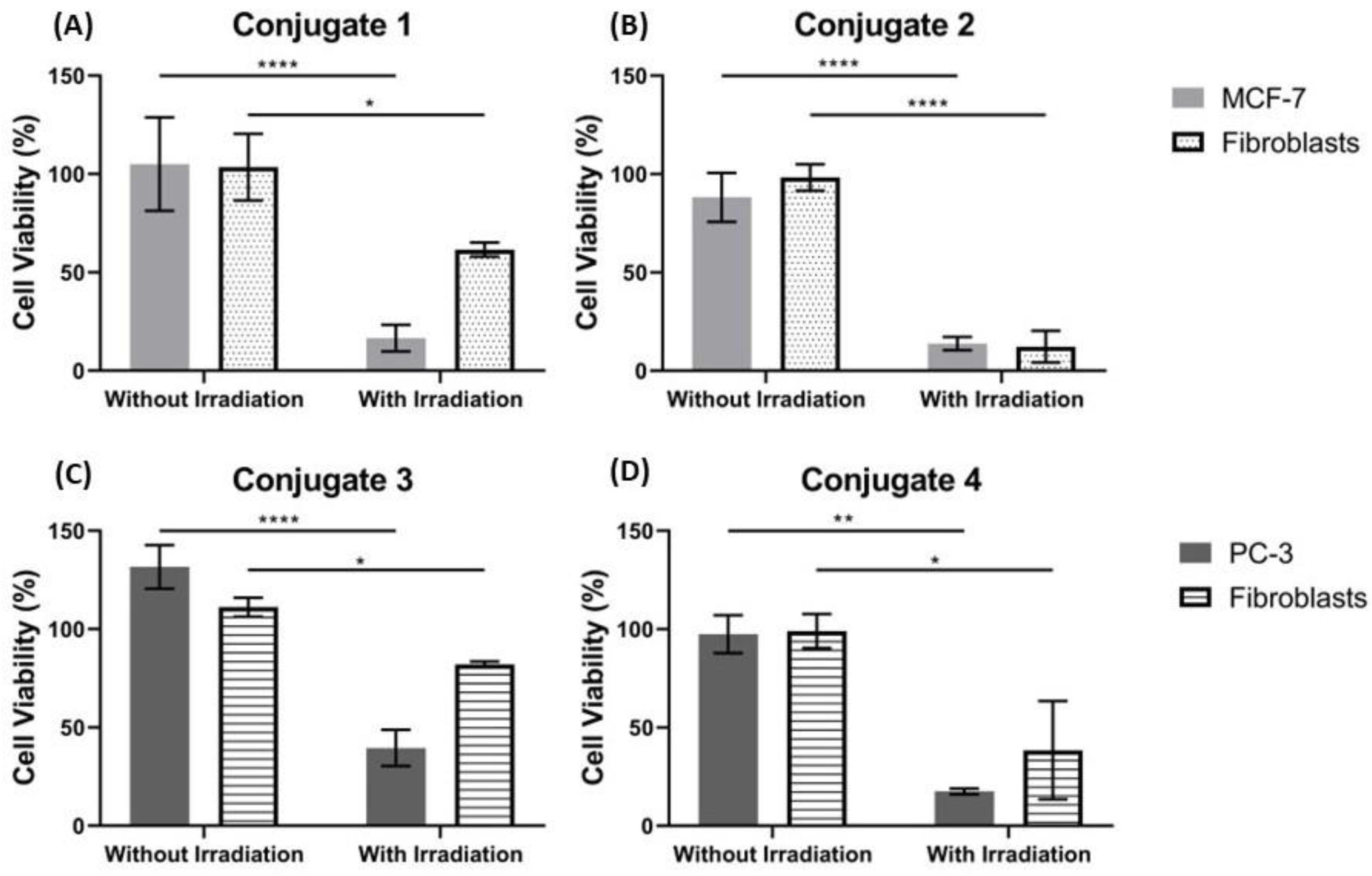
2.5. Visible Light Irradiation
3. Discussion
4. Materials and Methods
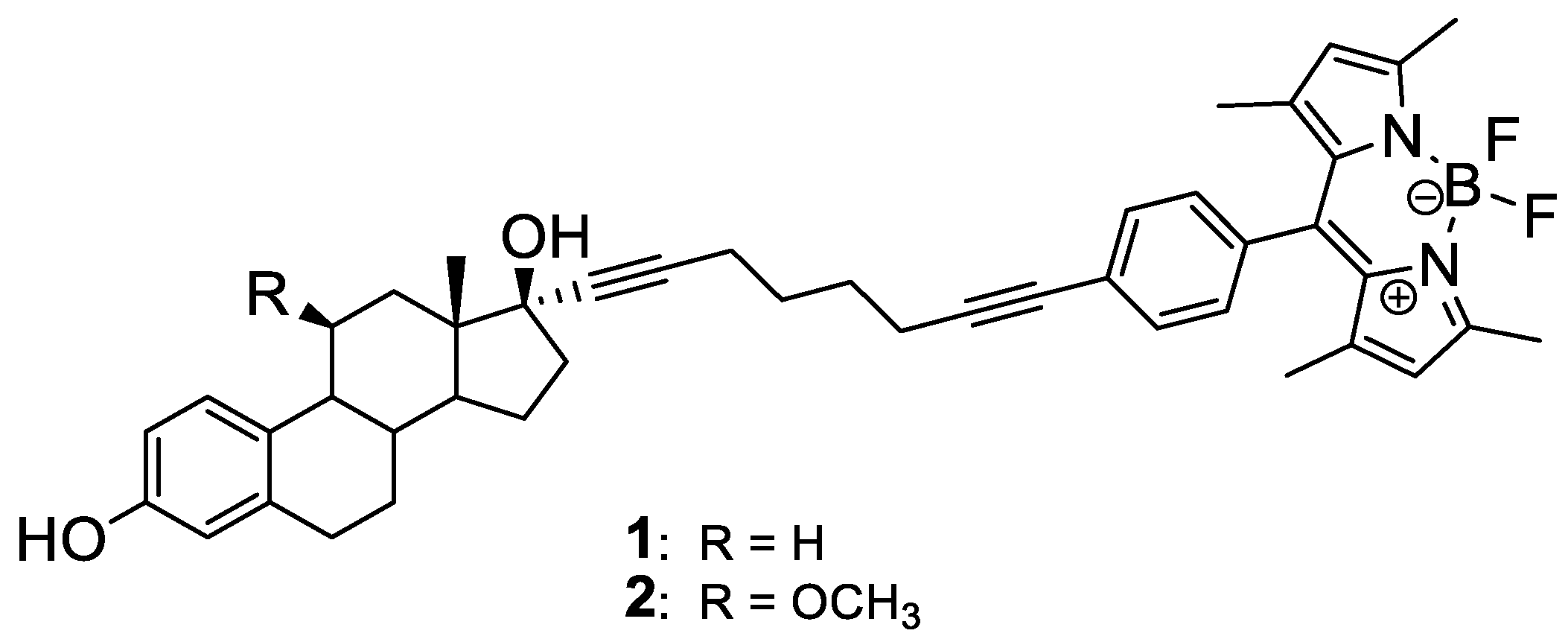
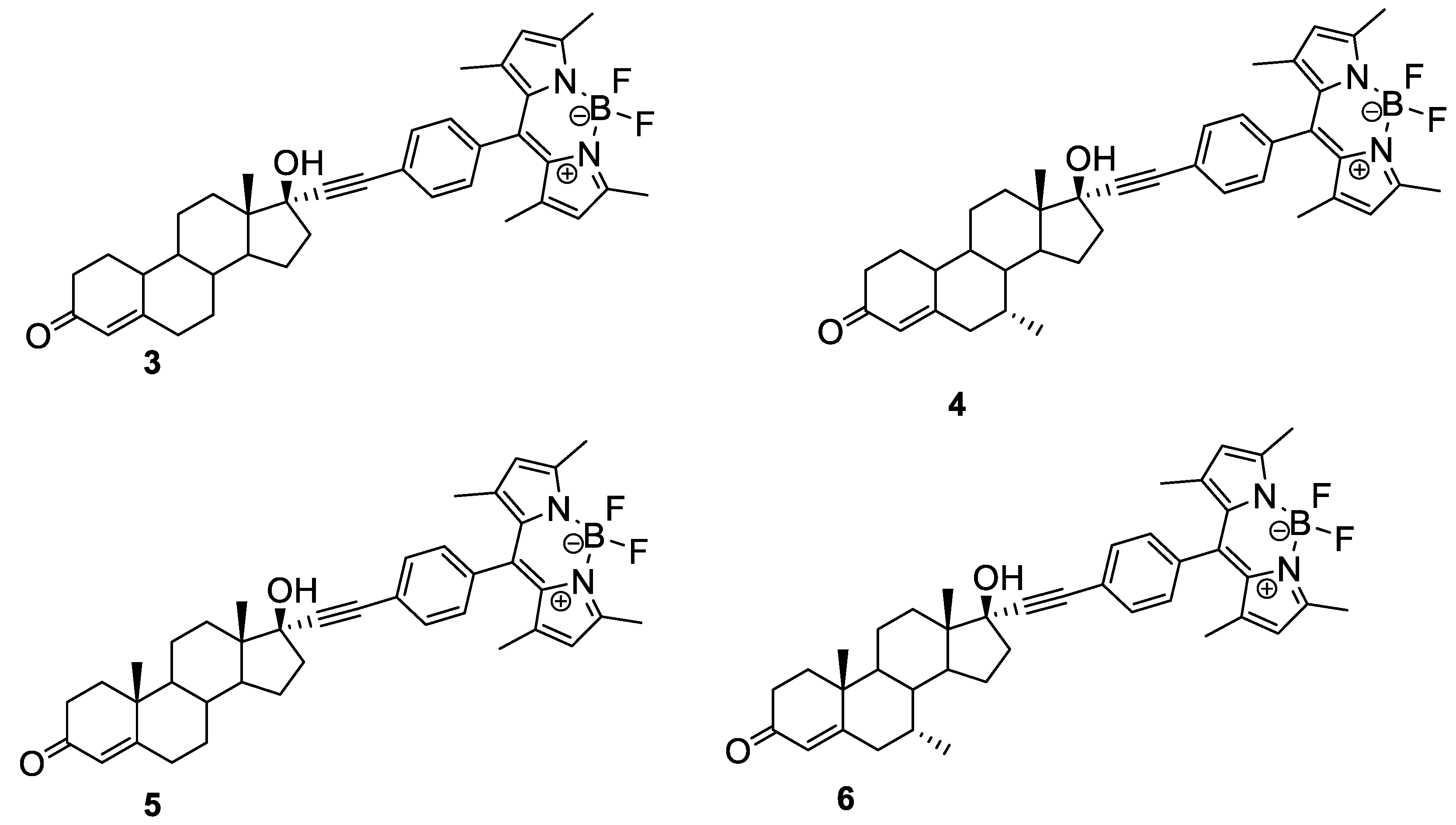
4.1. Steroid-BODIPY Conjugates
4.2. Cell Culture
4.3. Cellular Internalization of Steroid-BODIPY Conjugates
4.3.1. Internalization in 2D Monocultures
4.3.2. Internalization in 2D Co-Cultures
4.3.3. Effects of Endocrine Disruptors on the Internalization of BODIPY-Conjugates in 2D Monolayers
4.3.4. Active vs. Passive Transport
4.3.5. Evaluation of Endocytosis Internalization
4.3.6. Intracellular Tracking
4.4. Cell Viability
4.5. Visible-Light Irradiation
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Antina, E.; Bumagina, N.; Marfin, Y.; Guseva, G.; Nikitina, L.; Sbytov, D.; Telegin, F. BODIPY Conjugates as Functional Compounds for Medical Diagnostics and Treatment. Molecules 2022, 27, 1396. [Google Scholar] [CrossRef] [PubMed]
- Bottari, G.; de La Torre, G.; Guldi, D.M.; Torres, T. Covalent and noncovalent phthalocyanine—Carbon nanostructure systems: Synthesis, photoinduced electron transfer, and application to molecular photovoltaics. Chem. Rev. 2010, 110, 6768–6816. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Mack, J.; Yang, Y.; Shen, Z. Structural modification strategies for the rational design of red/NIR region BODIPYs. Chem. Soc. Rev. 2014, 43, 4778–4823. [Google Scholar] [CrossRef]
- Gemen, J.; Ahrens, J.; Shimon, L.J.W.; Klajn, R. Modulating the Optical Properties of BODIPY Dyes by Noncovalent Dimerization within a Flexible Coordination Cage. J. Am. Chem. Soc. 2020, 142, 17721–17729. [Google Scholar] [CrossRef]
- Choi, H.; Ha Lee, J.; Hwa Jung, J. Fluorometric/colorimetric logic gates based on BODIPY-functionalized mesoporous silica. Analyst 2014, 139, 3866–3870. [Google Scholar] [CrossRef]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef]
- Krämer, J.; Kang, R.; Grimm, L.M.; de Cola, L.; Picchetti, P.; Biedermann, F. Molecular Probes, Chemosensors, and Nanosensors for Optical Detection of Biorelevant Molecules and Ions in Aqueous Media and Biofluids. Chem. Rev. 2022, 122, 3459–3636. [Google Scholar] [CrossRef]
- Kursunlu, A.N.; Bastug, E.; Guler, E. Importance of BODIPY-based Chemosensors for Cations and Anions in Bio-imaging Applications. Curr. Anal. Chem. 2020, 18, 163–175. [Google Scholar] [CrossRef]
- Barba-Bon, A.; Calabuig, L.; Costero, A.M.; Gil, S.; Martínez-Máñez, R.; Sancenón, F. Off-on BODIPY-based chemosensors for selective detection of Al3+ and Cr3+ versus Fe3+ in aqueous media. RSC Adv. 2014, 4, 8962–8965. [Google Scholar] [CrossRef]
- Gao, J.; Chen, X.; Chen, S.; Meng, H.; Wang, Y.; Li, C.; Feng, L. The BODIPY-Based Chemosensor for Fluorometric/Colorimetric Dual Channel Detection of RDX and PA. Anal. Chem. 2019, 91, 13675–13680. [Google Scholar] [CrossRef] [PubMed]
- Maity, A.; Ghosh, U.; Giri, D.; Mukherjee, D.; Maiti, T.K.; Patra, S.K. A water-soluble BODIPY based ‘OFF/ON’ fluorescent probe for the detection of Cd2+ ions with high selectivity and sensitivity. Dalton Trans. 2019, 48, 2108–2117. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Song, W.; Shen, Z. A Highly Selective NIR Fluorescent Turn-on Probe for Hydroxyl Radical and Its Application in Living Cell Images. Front. Chem. 2019, 7, 598. [Google Scholar] [CrossRef] [PubMed]
- Barin, G.; Yilmaz, M.D.; Akkaya, E.U. Boradiazaindacene (Bodipy)-based building blocks for the construction of energy transfer cassettes. Tetrahedron Lett. 2009, 50, 1738–1740. [Google Scholar] [CrossRef]
- Erbas-Cakmak, S.; Akkaya, E.U. Toward singlet oxygen delivery at a measured rate: A self-reporting photosensitizer. Org. Lett. 2014, 16, 2946–2949. [Google Scholar] [CrossRef]
- Singh, S.P.; Gayathri, T. Evolution of BODIPY dyes as potential sensitizers for dye-sensitized solar cells. Eur. J. Org. Chem. 2014, 2014, 4689–4707. [Google Scholar] [CrossRef]
- Madrid-Úsuga, D.; Ortiz, A.; Reina, J.H. Photophysical Properties of BODIPY Derivatives for the Implementation of Organic Solar Cells: A Computational Approach. ACS Omega 2022, 7, 3963–3977. [Google Scholar] [CrossRef]
- Rahman, A.U.; Khan, M.B.; Yaseen, M.; Rahman, G. Rational Design of Broadly Absorbing Boron Dipyrromethene-Carbazole Dyads for Dye-Sensitized Solar Cells: A DFT Study. ACS Omega 2021, 6, 27640–27653. [Google Scholar] [CrossRef]
- Squeo, B.M.; Ganzer, L.; Virgili, T.; Pasini, M. BODIPY-Based Molecules, a Platform for Photonic and Solar Cells. Molecules 2021, 26, 153. [Google Scholar] [CrossRef]
- Zlatić, K.; Antol, I.; Uzelac, L.; Mikecin Dražić, A.M.; Kralj, M.; Bohne, C.; Basarić, N. Labeling of Proteins by BODIPY-Quinone Methides Utilizing Anti-Kasha Photochemistry. ACS Appl. Mater. Interfaces 2020, 12, 347–351. [Google Scholar] [CrossRef]
- Kim, D.; Ma, D.; Kim, M.; Jung, Y.; Kim, N.H.; Lee, C.; Cho, S.W.; Park, S.; Huh, Y.; Jung, J.; et al. Fluorescent Labeling of Protein Using Blue-Emitting 8-Amino-BODIPY Derivatives. J. Fluoresc. 2017, 27, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Kurata, S.; Kanagawa, T.; Yamada, K.; Torimura, M.; Yokomaku, T.; Kamagata, Y.; Kurane, R. Fluorescent quenching-based quantitative detection of specific DNA/RNA using a BODIPY® FL-labeled probe or primer. Nucleic Acids Res. 2021, 29, E34. [Google Scholar] [CrossRef] [PubMed]
- Deore, P.S.; Soldatov, D.V.; Manderville, R.A. A 5′-BODIPY End-label for Monitoring DNA Duplex-Quadruplex Exchange. Sci. Rep. 2018, 8, 16874. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, W.; Li, X.; Ma, H. Recent advances in fluorescent probes for lipid droplets. Chem. Commun. 2022, 58, 2581. [Google Scholar] [CrossRef]
- Gomez-Martinez, I.; Bliton, R.J.; Breau, K.A.; Czerwinski, M.J.; Williamson, I.A.; Wen, J.; Rawls, J.F.; Magness, S.T. A Planar Culture Model of Human Absorptive Enterocytes Reveals Metformin Increases Fatty Acid Oxidation and Export. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 409–434. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Janjanam, J.; Bi, J.; Vegesna, G.; Tiwari, A.; Luo, F.T.; Wei, J.; Liu, H. Highly water-soluble, near-infrared emissive BODIPY polymeric dye bearing RGD peptide residues for cancer imaging. Anal. Chim. Acta 2013, 758, 138–144. [Google Scholar] [CrossRef]
- Mendive-Tapia, L.; Mendive-Tapia, D.; Zhao, C.; Gordon, D.; Benson, S.; Bromley, M.J.; Wang, W.; Wu, J.; Kopp, A.; Ackermann, L.; et al. Rational Design of Phe-BODIPY Amino Acids as Fluorogenic Building Blocks for Peptide-Based Detection of Urinary Tract Candida Infections. Angew. Chem. 2022, 61, e202117218. [Google Scholar] [CrossRef]
- Gai, L.; Sun, W. Recent advances in estrogen receptor-targeted probes conjugated to BODIPY dyes. Steroids 2022, 183, 109031. [Google Scholar] [CrossRef]
- Osati, S.; Ali, H.; van Lier, J.E. BODIPY-steroid conjugates: Syntheses and biological applications. J. Porphyr. Phthalocyanines 2016, 20, 61–75. [Google Scholar] [CrossRef]
- Wüstner, D. Fluorescent sterols as tools in membrane biophysics and cell biology. Chem. Phys. Lipids 2007, 146, 1–25. [Google Scholar] [CrossRef]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY dyes in photodynamic therapy. Chem. Soc. Rev. 2013, 42, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Dong, Y.; Zhao, X.; Imran, M.; Tang, G.; Zhao, J.; Liu, Q. Bodipy Derivatives as Triplet Photosensitizers and the Related Intersystem Crossing Mechanisms. Front. Chem. 2019, 7, 821. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Montero, R.; Prieto-Castañeda, A.; Sola-Llano, R.; Agarrabeitia, A.R.; García-Fresnadillo, D.; López-Arbeloa, I.; Villanueva, A.; Ortiz, M.J.; de la Moya, S.; Martínez-Martínez, V.; et al. Exploring BODIPY Derivatives as Singlet Oxygen Photosensitizers for PDT. Photochem. Photobiol. 2020, 96, 458–477. [Google Scholar] [CrossRef]
- Hendricks, J.A.; Keliher, E.J.; Wan, D.; Hilderbrand, S.A.; Weissleder, R.; Mazitschek, R. Synthesis of [18F]BODIPY: Bifunctional reporter for hybrid optical/positron emission tomography imaging. Angew. Chem. 2012, 51, 4603–4606. [Google Scholar] [CrossRef]
- Kwon, Y.D.; Byun, Y.; Kim, H.K. 18F-labelled BODIPY dye as a dual imaging agent: Radiofluorination and applications in PET and optical imaging. Nucl. Med. Biol. 2021, 93, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Filatov, M.A. Heavy-atom-free BODIPY photosensitizers with intersystem crossing mediated by intramolecular photoinduced electron transfer. Org. Biomol. Chem. 2019, 18, 10–27. [Google Scholar] [CrossRef]
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Meana, Y.; Raymo, F.M. BODIPYs with Photoactivatable Fluorescence. Chem. Eur. J. 2021, 27, 11257–11267. [Google Scholar] [CrossRef]
- Ahmad, N.; Kumar, R. Steroid hormone receptors in cancer development: A target for cancer therapeutics. Cancer Lett. 2011, 300, 1–9. [Google Scholar] [CrossRef]
- Ruibal, A.; Benlloch, J.M.; Olmos, R.V.; Langstrom, B. Molecular imaging in breast cancer. J. Oncol. 2012, 2012, 426260. [Google Scholar] [CrossRef]
- Miladinova, D. Molecular Imaging in Breast Cancer. Nucl. Med. Mol. Imaging 2019, 53, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Wurtz, J.M.; Moras, D. Molecular determinants for agonist and antagonist binding to steroid nuclear receptors. In Ernst Schering Research Foundation Workshop; Springer: New York, NY, USA, 2001; pp. 167–180. [Google Scholar] [CrossRef]
- Giguère, V.; Tremblay, A.; Tremblay, G.B. Estrogen receptor β: Re-evaluation of estrogen and antiestrogen signaling. Steroids 1998, 63, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, G.; Yasar, P.; Olgun, C.E.; Karakaya, B.; Kars, G.; Razizadeh, N.; Yavuz, K.; Turan, G.; Muyan, M. Dynamic transcriptional events mediated by estrogen receptor alpha. Front. Biosci. 2019, 24, 245–276. [Google Scholar] [CrossRef]
- Acconcia, F.; Fiocchetti, M.; Busonero, C.; Fernandez, V.S.; Montalesi, E.; Cipolletti, M.; Pallottini, V.; Marino, M. The extra-nuclear interactome of the estrogen receptors: Implications for physiological functions. Mol. Cell. Endocrinol. 2021, 538, 111452. [Google Scholar] [CrossRef]
- Cole, K.; Tabernero, M.; Anderson, K.S. Biologic characteristics of premalignant breast disease. Cancer Biomark. 2011, 9, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Grogan Fleege, N.M.; Cobain, E.F. Breast cancer management in 2021: A primer for the obstetrics and gynecology. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, 82, 30–45. [Google Scholar] [CrossRef]
- Xia, S.; Lin, Q. Estrogen Receptor Bio-Activities Determine Clinical Endocrine Treatment Options in Estrogen Receptor-Positive Breast Cancer. Technol. Cancer Res. Treat. 2022, 21, 15330338221090351. [Google Scholar] [CrossRef]
- Abubakar, M.; Mullooly, M.; Nyante, S.; Pfeiffer, R.M.; Aiello Bowles, E.J.; Cora, R.; Bodelon, C.; Butler, E.; Butcher, D.; Sternberg, L.; et al. Mammographic Density Decline, Tamoxifen Response, and Prognosis by Molecular Characteristics of Estrogen Receptor–Positive Breast Cancer. JNCI Cancer Spectr. 2022, 6, pkac028. [Google Scholar] [CrossRef]
- Van de Wiele, C.; de Vos, F.; Slegers, G.; van Belle, S.; Dierckx, R.A. Radiolabeled estradiol derivatives to predict response to hormonal treatment in breast cancer: A review. Eur. J. Nucl. Med. 2000, 27, 1421–1433. [Google Scholar] [CrossRef]
- Katzenellenbogen, J.A. Designing steroid receptor-based radiotracers to image breast and prostate tumors. J. Nucl. Med. 1995, 36, 8S–13S. [Google Scholar]
- Kumar, M.; Salem, K.; Jeffery, J.J.; Fowler, A.M. PET Imaging of Estrogen Receptors Using 18F-Based Radioligands. Methods Mol. Biol. 2022, 2418, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Carlson, K.E.; Coppey, M.; Magdelenat, H.; Katzenellenbogen, J.A. Receptor binding of NBD-labeled fluorescent estrogens and progestins in whole cells and cell-free preparations. J. Steroid Biochem. 1989, 32, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Buschhaus, J.M.; Humphries, B.A.; Eckley, S.S.; Robison, T.H.; Cutter, A.C.; Rajendran, S.; Haley, H.R.; Bevoor, A.S.; Luker, K.E.; Luker, G.D.; et al. Targeting disseminated estrogen-receptor-positive breast cancer cells in bone marrow. Oncogene 2020, 39, 5649–5662. [Google Scholar] [CrossRef] [PubMed]
- Sukerkar, P.A.; MacRenaris, K.W.; Townsend, T.R.; Ahmed, R.A.; Burdette, J.E.; Meade, T.J. Synthesis and biological evaluation of water-soluble progesterone- conjugated probes for magnetic resonance imaging of hormone related cancers. Bioconjugate Chem. 2011, 22, 2304–2316. [Google Scholar] [CrossRef]
- Bhasin, S.; Cunningham, G.R.; Hayes, F.J.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Montori, V.M. Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2010, 95, 2536–2559. [Google Scholar] [CrossRef]
- Omil-Lima, D.; Jesse, E.; Gupta, K.; Selke, N.; Muncey, W.; Burrelli, C.; Ghayda, R.; Loeb, A.; Thirumavalavan, N. Testosterone deficiency in male organ transplant recipients. Int. J. Impot. Res. 2022, 34, 679–684. [Google Scholar] [CrossRef]
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef]
- Li, Z.; Wei, H.; Li, S.; Wu, P.; Mao, X. The Role of Progesterone Receptors in Breast Cancer. Drug Des. Dev. Ther. 2022, 16, 305–314. [Google Scholar] [CrossRef]
- Cunha, S.; Gano, L.; Ribeiro Morais, G.; Thiemann, T.; Oliveira, M.C. Progesterone receptor targeting with radiolabelled steroids: An approach in predicting breast cancer response to therapy. J. Steroid Biochem. Mol. Biol. 2013, 137, 223–241. [Google Scholar] [CrossRef]
- Katzenellenbogen, J.A.; Welch, M.J.; Dehdashti, F. The development of estrogen and progestin radiopharmaceuticals for imaging breast cancer. Anticancer Res. 1997, 17, 1573–1576. [Google Scholar]
- Osati, S.; Ali, H.; Marques, F.; Paquette, M.; Beaudoin, S.; Guerin, B.; Leyton, J.V.; van Lier, J.E. BODIPY-17α-ethynylestradiol conjugates: Synthesis, fluorescence properties and receptor binding affinities. Bioorg. Med. Chem. Lett. 2017, 27, 443–446. [Google Scholar] [CrossRef]
- Osati, S.; Ali, H.; Guerin, B.; van Lier, J.E. Synthesis and spectral properties of estrogen- and androgen-BODIPY conjugates. Steroids 2017, 123, 27–36. [Google Scholar] [CrossRef]
- Ali, H.; Rousseau, J.; van Lier, J.E. Synthesis of (17α, 20E/Z)Iodovinyl testosterone and 19-nortestosterone derivatives as potential radioligands for androgen and progesterone receptors. J. Steroid Biochem. Mol. Biol. 1994, 49, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, S.M.; Arnold, J.; Sreekumar, A. Metabolomic profiling of hormone-dependent cancers: A bird’s eye view. Trends Endocrinol. Metab. 2015, 26, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast cancer cell line classification and Its relevance with breast tumor subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef]
- Westaby, D.; Fenor de la Maza, M.L.D.; Paschalis, A.; Jimenez-Vacas, J.M.; Welti, J.; de Bono, J.; Sharp, A. A New Old Target: Androgen Receptor Signaling and Advanced Prostate Cancer. Annu. Rev. Pharmacol. Toxicol. 2021, 62, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.R.; Bernales, S.; Jacobsen, B.M.; Cittelly, D.M.; Howe, E.N.; D’Amato, N.C.; Spoelstra, N.S.; Edgerton, S.M.; Jean, A.; Guerrero, J.; et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014, 16, R7. [Google Scholar] [CrossRef]
- Michmerhuizen, A.R.; Chandler, B.; Olsen, E.; Wilder-Romans, K.; Moubadder, L.; Liu, M.; Pesch, A.M.; Zhang, A.; Ritter, C.; Ward, S.T.; et al. Seviteronel, a Novel CYP17 Lyase Inhibitor and Androgen Receptor Antagonist, Radiosensitizes AR-Positive Triple Negative Breast Cancer Cells. Front. Endocrinol. 2020, 11, 35. [Google Scholar] [CrossRef]
- Powell, E.; Shanle, E.; Brinkman, A.; Li, J.; Keles, S.; Wisinski, K.B.; Huang, W.; Xu, W. Identification of estrogen receptor dimer selective ligands reveals growth-inhibitory effects on cells that co-express ERα and ERβ. PLoS ONE 2012, 7, e30993. [Google Scholar] [CrossRef]
- Dominska, K.; Kowalski, A.; Ochedalski, T.; Rebas, E. Effects of testosterone and 17β-estradiol on angiotensin-induced changes in tyrosine kinase activity in the androgen-independent human prostate cancer cell line, DU145. Int. J. Mol. Med. 2017, 40, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Sampson, N.; Neuwirt, H.; Puhr, M.; Klocker, H.; Eder, I.E. In vitro model systems to study androgen receptor signaling in prostate cancer. Endocr. Relat. Cancer 2013, 20, R49–R64. [Google Scholar] [CrossRef]
- Msheik, Z.S.; Nassar, F.J.; Chamandi, G.; Itani, A.R.; Gadaleta, E.; Chalala, C.; Alwan, N.; Nasr, R.R. miR-126 Decreases Proliferation and Mammosphere Formation of MCF-7 and Predicts Prognosis of ER+ Breast Cancer. Diagnostics 2022, 12, 745. [Google Scholar] [CrossRef] [PubMed]
- Awuah, S.G.; You, Y. Boron dipyrromethene (BODIPY)-based photosensitizers for photodynamic therapy. RSC Adv. 2012, 2, 11169–11183. [Google Scholar] [CrossRef]
- Xuan, S.; Zhao, N.; Ke, X.; Zhou, Z.; Fronczek, F.R.; Kadish, K.M.; Smith, K.M.; Vicente, M.G.H. Synthesis and Spectroscopic Investigation of a Series of Push-Pull Boron Dipyrromethenes (BODIPYs). J. Org. Chem. 2017, 82, 2545–2557. [Google Scholar] [CrossRef]
- Kazan, H.H.; Özcan, E.; Eçik, E.T.; Çoşut, B. Novel 17α-Etinylestradiol-Substituted BODIPY Dyes: Synthesis, Photophysical Properties and Fluorescence Imaging Studies in Breast Cancer Cell Lines. ChemistrySelect 2018, 3, 2962–2967. [Google Scholar] [CrossRef]
- Napolitano, E.; Fiaschi, R.; Carlson, K.E.; Katzenellenbogen, J.A. 11β-Substituted Estradiol Derivatives, Potential High-Affinity Carbon-11-Labeled Probes for the Estrogen Receptor: A Structure-Affinity Relationship Study. J. Med. Chem. 1995, 38, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Girgert, R.; Emons, G.; Gründker, C. Estrogen signaling in ERα-negative breast cancer: ERβ and GPER. Front. Endocrinol. 2019, 9, 781. [Google Scholar] [CrossRef]
- Giovannelli, P.; di Donato, M.; Galasso, G.; di Zazzo, E.; Bilancio, A.; Migliaccio, A. The androgen receptor in breast cancer. Front. Endocrinol. 2018, 9, 492. [Google Scholar] [CrossRef] [PubMed]
- Di Leone, A.; Fragomeni, S.M.; Scardina, L.; Ionta, L.; Mulè, A.; Magno, S.; Terribile, D.; Masetti, R.; Franceschini, G. Androgen receptor expression and outcome of neoadjuvant chemotherapy in triple-negative breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1910–1915. [Google Scholar] [CrossRef]
- Lafront, C.; Germain, L.; Weidmann, C.; Audet-Walsh, É. A Systematic Study of the Impact of Estrogens and Selective Estrogen Receptor Modulators on Prostate Cancer Cell Proliferation. Sci. Rep. 2020, 10, 4024. [Google Scholar] [CrossRef]
- Pisolato, R.; Lombardi, A.P.G.; Vicente, C.M.; Lucas, T.F.G.; Lazari, M.F.M.; Porto, C.S. Expression and regulation of the estrogen receptors in PC-3 human prostate cancer cells. Steroids 2016, 107, 74–86. [Google Scholar] [CrossRef]
- Carter, M.; Shieh, J. Chapter 14 Cell culture equipement and reagents. In Guide to Research Techniques in Neuroscience, 2nd ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 295–310. [Google Scholar] [CrossRef]
- Goers, L.; Freemont, P.; Polizzi, K.M. Co-culture systems and technologies: Taking synthetic biology to the next level. J. R. Soc. Interface 2014, 11, 20140065. [Google Scholar] [CrossRef] [PubMed]
- Sugano, K.; Kansy, M.; Artursson, P.; Avdeef, A.; Bendels, S.; Di, L.; Ecker, G.F.; Faller, B.; Fischer, H.; Gerebtzoff, G.; et al. Coexistence of passive and carrier-mediated processes in drug transport. Nat. Rev. Drug Discov. 2010, 9, 597–614. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.A.R.; Carvalho, P.H.P.R.; Guido, B.C.; de Oliveira, H.C.B.; Soares, T.A.; Corrêa, J.R.; Neto, B.A.D. Bioimaging, cellular uptake and dynamics in living cells of a lipophilic fluorescent benzothiadiazole at low temperature (4 °C). Chem. Sci. 2014, 5, 3995–4003. [Google Scholar] [CrossRef]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control. Release 2010, 145, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, D.; Zhang, M.; Sun, Y.; Zhang, X.; Guan, G.; Zhao, X.; Qiao, M.; Chen, D.; Hu, H.; et al. The cellular uptake mechanism, intracellular transportation, and exocytosis of polyamidoamine dendrimers in multidrug-resistant breast cancer cells. Int. J. Nanomed. 2016, 11, 3677–3690. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Donaldson, J.G. Search for inhibitors of endocytosis: Intended specificity and unintended consequences. Cell. Logist. 2012, 2, 203–208. [Google Scholar] [CrossRef]
- Ying, S.; Du, S.; Dong, J.; Ng, B.X.; Zhang, C.; Li, L.; Ge, J.; Zhu, Q. Intracellular effects of prodrug-like wortmannin probes. Chin. Chem. Lett. 2019, 30, 67–70. [Google Scholar] [CrossRef]
- Mercer, J.; Helenius, A. Gulping rather than sipping: Macropinocytosis as a way of virus entry. Curr. Opin. Microbiol. 2012, 15, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Cui, L.; Yu, X.; Zhang, Z.; Shen, X.; Hua, X. Porcine sapelovirus enters PK-15 cells via caveolae-dependent endocytosis and requires Rab7 and Rab11. Virology 2019, 529, 160–168. [Google Scholar] [CrossRef]
- Gibbs, J.H.; Zhou, Z.; Kessel, D.; Fronczek, F.R.; Pakhomova, S.; Vicente, M.G.H. Synthesis, spectroscopic, and in vitro investigations of 2,6-diiodo-BODIPYs with PDT and bioimaging applications. J. Photochem. Photobiol. B Biol. 2015, 145, 35–47. [Google Scholar] [CrossRef]
- Osati, S.; Ali, H.; Guérin, B.; van Lier, J.E. Steroid-photosensitizer conjugates: Syntheses and applications. J. Porphyr. Phthalocyanines 2017, 21, 701–730. [Google Scholar] [CrossRef]
- Zhang, R.; Qin, X.; Kong, F.; Chen, P.; Pan, G. Improving cellular uptake of therapeutic entities through interaction with components of cell membrane. Drug Deliv. 2019, 26, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Parsons, T.K.; Pratt, R.N.; Tang, L.; Wu, Y. An active and selective molecular mechanism mediating the uptake of sex steroids by prostate cancer cells. Mol. Cell. Endocrinol. 2018, 477, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Treviño, L.S.; Gorelick, D.A. The interface of nuclear and membrane steroid signaling. Endocrinology 2021, 162, bqab107. [Google Scholar] [CrossRef]
- Levin, E.R.; Hammes, S.R. Nuclear receptors outside the nucleus: Extranuclear signalling by steroid receptors. Vnature Rev. Mol. Cell Biol. 2016, 17, 783–797. [Google Scholar] [CrossRef]
- Lajoie, P.; Nabi, I.R. Lipid rafts, caveolae, and their endocytosis. Int. Rev. Cell Mol. Biol. 2010, 282, 135–163. [Google Scholar] [CrossRef]
- Totta, P.; Pesiri, V.; Marino, M.; Acconcia, F. Lysosomal function is involved in 17β-estradiol-induced estrogen receptor α degradation and cell proliferation. PLoS ONE 2014, 9, e94880. [Google Scholar] [CrossRef]
- Morell, C.; Bort, A.; Vara-Ciruelos, D.; Ramos-Torres, Á.; Altamirano-Dimas, M.; Díaz-Laviada, I.; Rodríguez-Henche, N. Up-regulated expression of LAMP2 and autophagy activity during neuroendocrine differentiation of prostate cancer LNCaP cells. PLoS ONE 2016, 11, e0162977. [Google Scholar] [CrossRef]
- Piao, S.; Amaravadi, R.K. Targeting the lysosome in cancer. Ann. N. Y. Acad. Sci. 2016, 1371, 45–54. [Google Scholar] [CrossRef]
- Li, X.; Lee, S.; Yoon, J. Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem. Soc. Rev. 2018, 47, 1174–1188. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Si, W.; Huang, W.; Chen, P.; Shao, J.; Dong, X. Organic Dye Based Nanoparticles for Cancer Phototheranostics. Small 2018, 14, 1704247. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.M.; MacRobert, A.J.; Mosse, C.A.; Periera, B.; Bown, S.G.; Keshtgar, M.R.S. Photodynamic therapy: Inception to application in breast cancer. Breast 2017, 31, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; de Zonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Malta, G.; Peixoto, D.; Ferreira, L.M.; Baptista, P.V.; Fernandes, A.R.; Branco, P.S. Synthesis of new hetero-arylidene-9(10H)-anthrone derivatives and their biological evaluation. Bioorg. Chem. 2020, 99, 103849. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Reigosa-Chamorro, F.; Raposo, L.R.; Munín-Cruz, P.; Pereira, M.T.; Roma-Rodrigues, C.; Baptista, P.V.; Fernandes, A.R.; Vila, J.M. In Vitro and In Vivo Effect of Palladacycles: Targeting A2780 Ovarian Carcinoma Cells and Modulation of Angiogenesis. Inorg. Chem. 2021, 60, 3939–3951. [Google Scholar] [CrossRef]




| Cell Line | ATCC Reference | Origin | AR | ER | Reference |
|---|---|---|---|---|---|
| MCF-7 | HTB-22 | Metastatic breast adenocarcinoma | + | + | [66,68,81] |
| MDA-MB-231 | CRM-HTB-26 | Metastatic breast adenocarcinoma | − | − | [66,69,81] |
| PC-3 | CRL-1435 | Metastatic prostate adenocarcinoma; Grade IV | − | + | [70,71] |
| LNCaP | CRL-1740 | Metastatic prostate carcinoma | + | − | [72] |
| Fibroblasts | PCS-201-010 | Primary normal dermal, neonatal | − | − | This work (Figure S1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amendoeira, A.F.; Luz, A.; Valente, R.; Roma-Rodrigues, C.; Ali, H.; van Lier, J.E.; Marques, F.; Baptista, P.V.; Fernandes, A.R. Cell Uptake of Steroid-BODIPY Conjugates and Their Internalization Mechanisms: Cancer Theranostic Dyes. Int. J. Mol. Sci. 2023, 24, 3600. https://doi.org/10.3390/ijms24043600
Amendoeira AF, Luz A, Valente R, Roma-Rodrigues C, Ali H, van Lier JE, Marques F, Baptista PV, Fernandes AR. Cell Uptake of Steroid-BODIPY Conjugates and Their Internalization Mechanisms: Cancer Theranostic Dyes. International Journal of Molecular Sciences. 2023; 24(4):3600. https://doi.org/10.3390/ijms24043600
Chicago/Turabian StyleAmendoeira, Ana F., André Luz, Ruben Valente, Catarina Roma-Rodrigues, Hasrat Ali, Johan E. van Lier, Fernanda Marques, Pedro V. Baptista, and Alexandra R. Fernandes. 2023. "Cell Uptake of Steroid-BODIPY Conjugates and Their Internalization Mechanisms: Cancer Theranostic Dyes" International Journal of Molecular Sciences 24, no. 4: 3600. https://doi.org/10.3390/ijms24043600
APA StyleAmendoeira, A. F., Luz, A., Valente, R., Roma-Rodrigues, C., Ali, H., van Lier, J. E., Marques, F., Baptista, P. V., & Fernandes, A. R. (2023). Cell Uptake of Steroid-BODIPY Conjugates and Their Internalization Mechanisms: Cancer Theranostic Dyes. International Journal of Molecular Sciences, 24(4), 3600. https://doi.org/10.3390/ijms24043600