Residual Amino Acid Imbalance in Rats during Recovery from Acute Thioacetamide-Induced Hepatic Encephalopathy Indicates Incomplete Healing
Abstract
1. Introduction
2. Results
2.1. Physiological State of Rats after TAA Intoxication
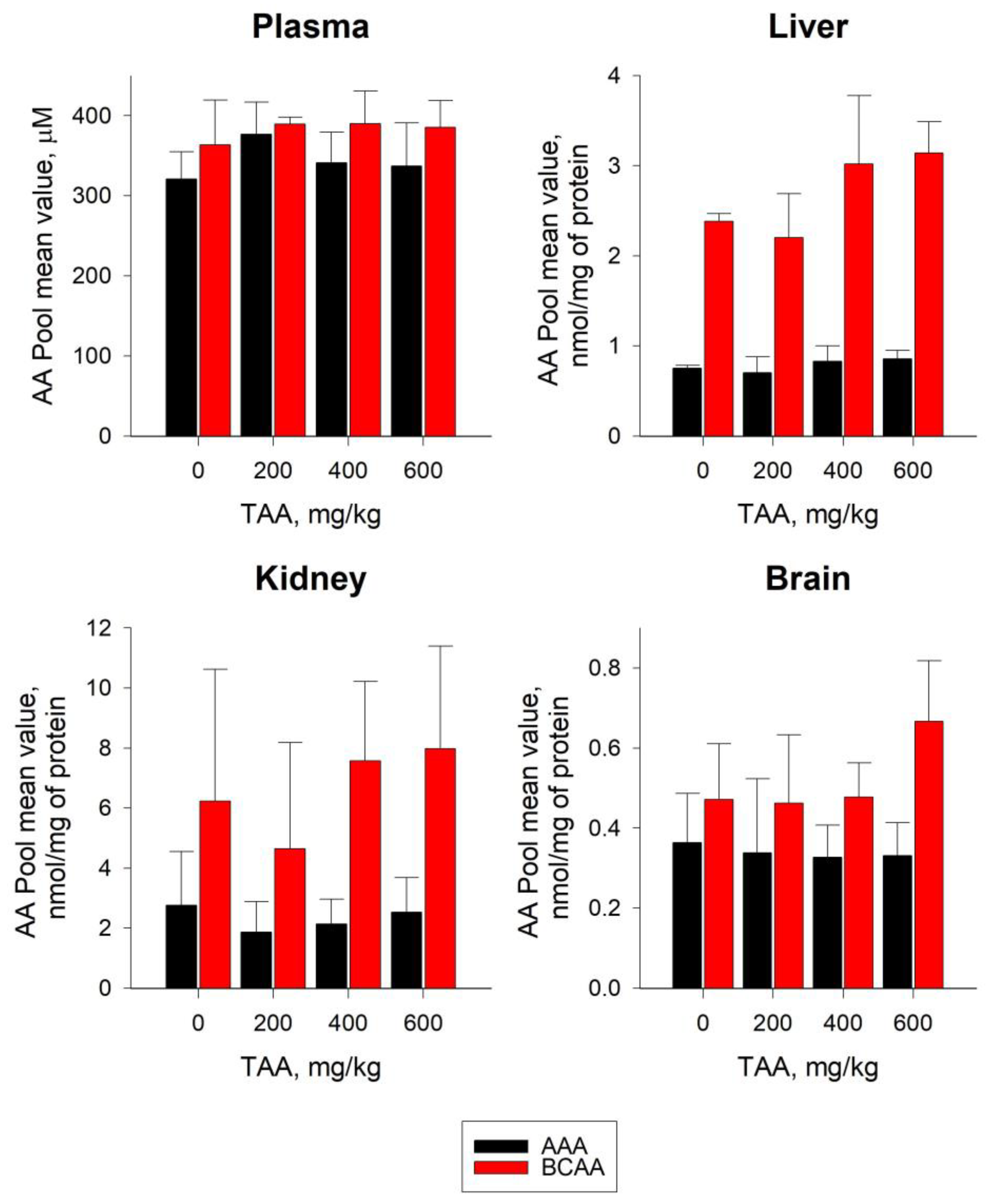
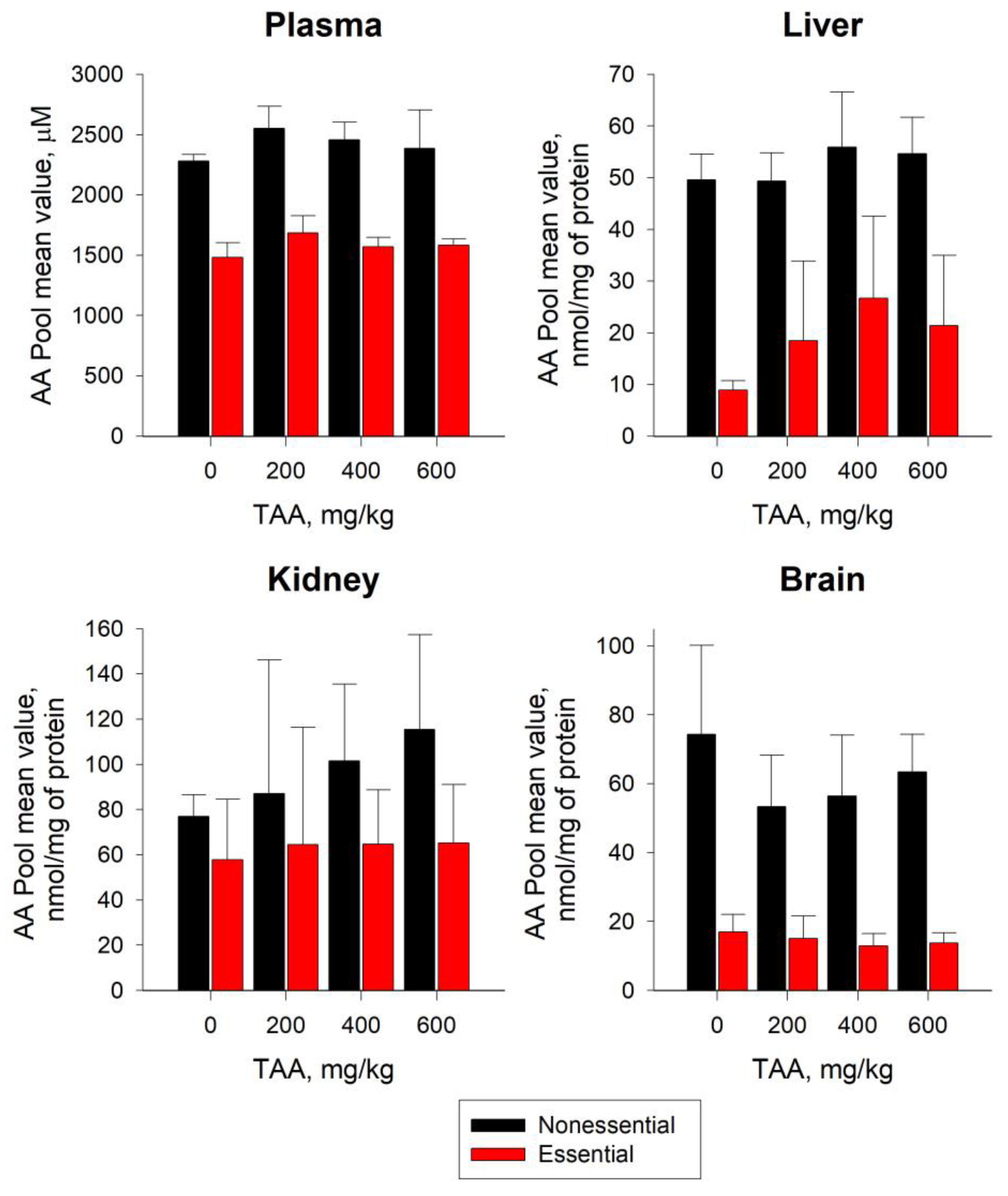
2.2. Distribution of AA in Blood Plasma and Tissues of Rats
2.3. Glutamine and Glutamate in Blood Plasma and Tissues of Rats
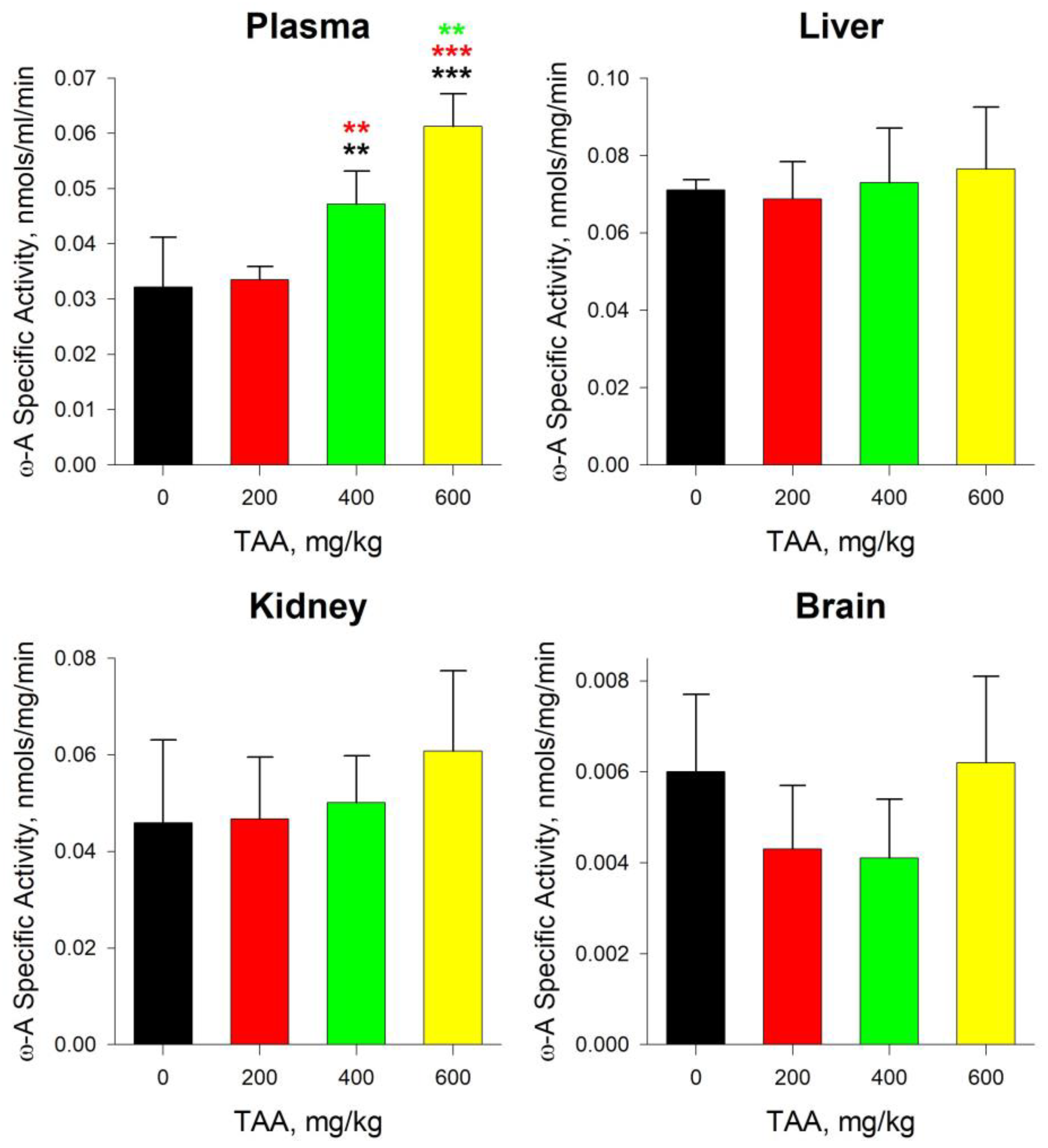
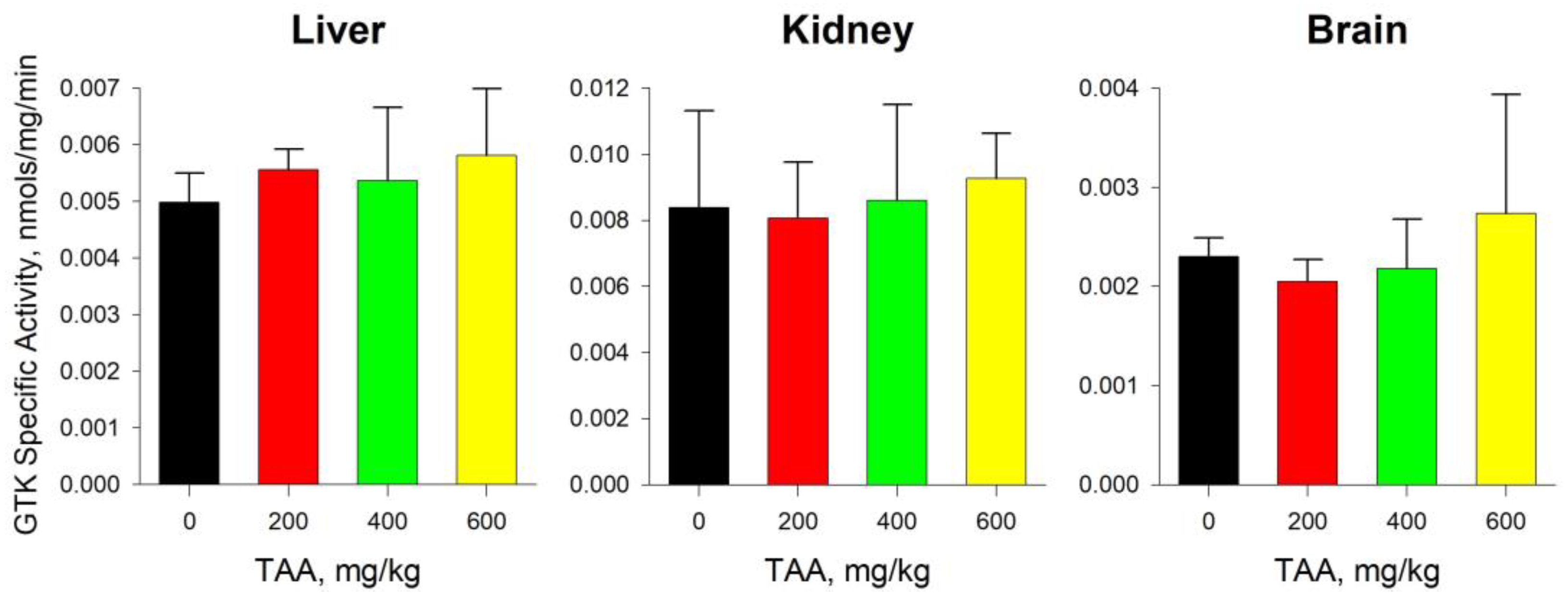
2.4. GTK and ω-Amidase Activity in Blood Plasma and Tissues of Rats
2.5. Statistical Analysis
3. Discussion
3.1. AA Pools in Blood Plasma and Tissues of Rats
3.2. Glu and Gln in Blood Plasma and Tissues of Rats
4. Materials and Methods
4.1. Chemicals
4.2. Rat Model of Liver Failure
4.3. AA Measurements and Sample Preparation
4.4. Tissue Samples Were Prepared as Follows
4.5. Plasma Samples Were Prepared as Follows
4.6. OPA Solution for Sample Derivatization
4.7. Determination of ω-Amidase and GTK Activity
4.7.1. ω-Amidase Endpoint Assay
4.7.2. GTK Endpoint Assay
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferenci, P.; Lockwood, A.; Mullen, K.; Tarter, R.; Weissenborn, K.; Blei, A.T. Hepatic encephalopathy: Definition, nomenclature, diagnosis and quantification. Final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002, 35, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Sanchez, R.; Tamnanloo, F.; Rose, C.F. Hepatic Encephalopathy: From Metabolic to Neurodegenerative. Neurochem. Res. 2021, 46, 2612–2625. [Google Scholar] [CrossRef] [PubMed]
- Rose, C. Increased extracellular brain glutamate in acute liverfailure: Decreased uptake or increased release? Metab. Brain Dis. 2002, 17, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, R.F.; Norenberg, M.D.; Felipo, V.; Ferenci, P.; Albrecht, J.; Blei, A.T. Experimental models of hepatic encephalopathy. Liver Int. 2009, 29, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Shawcross, D.; Jalan, R. The pathophysiologic basis of hepatic encephalopathy: Central role for ammonia and inflammation. Cell Mol. Life Sci. 2005, 62, 2295–2304. [Google Scholar] [CrossRef]
- Palomero-Gallagher, N.; Bidmon, H.J.; Cremer, M.; Schleicher, A.; Kircheis, G.; Reifenberger, G.; Kostopoulos, G.; Häussinger, D.; Zilles, K. Neurotransmitter receptor imbalances in motor cortex and basal ganglia in hepatic encephalopathy. Cell Physiol. Biochem. 2009, 24, 291–306. [Google Scholar] [CrossRef]
- Amodio, P. Hepatic encephalopathy: Diagnosis and management. Liver Int. 2018, 38, 966–975. [Google Scholar] [CrossRef]
- Lemberg, A.; Fernández, M.A. Hepatic encephalopathy, ammonia, glutamate, glutamine and oxidative stress. Ann. Hepatol. 2009, 8, 95–102. [Google Scholar] [CrossRef]
- Rama Rao, K.V.; Jayakumar, A.R.; Norenberg, M.D. Glutamine in the pathogenesis of acute hepatic encephalopathy. Neurochem. Int. 2012, 61, 575–580. [Google Scholar] [CrossRef]
- Lai, J.C.; Cooper, A.J.L. Brain alpha-ketoglutarate dehydrogenase complex: Kinetic properties, regional istribution, and effects of inhibitors. J. Neurochem. 1986, 47, 1376–1386. [Google Scholar] [CrossRef]
- Hertz, L.; Kala, G. Energy metabolism in brain cells: Effects of elevated ammonia concentrations. Metab. Brain Dis. 2007, 22, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Moreira, E.; Torres, M.I.; Fernández, M.I.; Ríos, A.; Sánchez de Medina, F.; Gil, A. Serum amino acid changes in rats with thioacetamide-induced liver cirrhosis. Toxicology 1996, 106, 197–206. [Google Scholar] [CrossRef]
- Yamato, M.; Muto, Y.; Yoshida, T.; Kato, M.; Moriwaki, H. Clearance rate of plasma branched-chain amino acids correlates significantly with blood ammonia level in patients with liver cirrhosis. Int. Hepatol. Commun. 1995, 3, 91–96. [Google Scholar] [CrossRef]
- Soeters, P.B.; Fischer, J.E. Insulin, glucagon, aminoacid imbalance, and hepatic encephalopathy. Lancet 1976, 2, 880–882. [Google Scholar] [CrossRef]
- Rosen, H.M.; Yoshimura, N.; Hodgman, J.M.; Fischer, J.E. Plasma amino acid patterns in hepatic encephalopathy of different etiology. Gastrornterology 1977, 72, 483–487. [Google Scholar] [CrossRef]
- Cornelis, H.C.D.; van de Poll, M.C.G.; Soeters, P.B.; Jalan, R.; Olde Damink, S.W.M. Aromatic amino acid metabolism during liver failure. J. Nutr. 2007, 137, 1579S–1585S. [Google Scholar] [CrossRef]
- Avraham, Y.; Grigoriadis, N.C.; Poutahidis, T.; Vorobiev, L.; Magen, I.; Ilan, Y.; Mechoulam, R.; Berry, E.M. Cannabidiol improves brain and liver function in a fulminant hepatic failure-induced model of hepatic encephalopathy in mice. Br. J. Pharmacol. 2011, 162, 1650–1658. [Google Scholar] [CrossRef]
- Shmarakov, I.O.; Borschovetska, V.L.; Marchenko, M.M.; Blaner, W.S. Retinoids modulate thioacetamide-induced acute hepatotoxicity. Toxicol. Sci. 2014, 139, 284–292. [Google Scholar] [CrossRef]
- Mladenović, D.; Krstić, D.; Colović, M.; Radosavljević, T.; Rasić-Marković, A.; Hrncić, D.; Macut, D.; Stanojlović, O. Different sensitivity of various brain structures to thioacetamide-induced lipid peroxidation. Med. Chem. 2012, 8, 52–58. [Google Scholar] [CrossRef]
- Bruck, R.; Weiss, S.; Traister, A.; Zvibel, I.; Aeed, H.; Halpern, Z.; Oren, R. Induced hypothyroidism accelerates the regression of liver fibrosis in rats. J. Gastroenterol. Hepatol. 2007, 12, 2189–2194. [Google Scholar] [CrossRef] [PubMed]
- Kakulavarapu, V.; Rama, R.; Reddy, P.V.B.; Tong, X.; Norenberg, M.D. Brain edema in acute liver failure inhibition by L-Histidine. Am. J. Pathol. 2010, 176, 1400–1408. [Google Scholar] [CrossRef]
- Gammal, S.H.; Basile, A.S.; Geller, D.; Skolnick, P.; Jones, E.A. Reversal of the behavioral and electrophysiological abnormalities of an animal model of hepatic encephalopathy by benzodiazepine receptor ligands. Hepatology 1990, 11, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Díez-Fernández, C.; Boscá, L.; Fernández-Simón, L.; Alvarez, A.; Cascales, M. Relationship between genomic DNA ploidy and parameters of liver damage during necrosis and regeneration induced by thioacetamide. Hepatology 1993, 18, 912–918. [Google Scholar] [CrossRef]
- Koblinova, E.; Mrazova, I.; Vernerova, Z.; Ryska, M. Acute Liver Failure Induced by Thioacetamide: Selection of Optimal Dosage in Wistar and Lewis Rats. Physiol. Res. 2014, 63, 491–503. [Google Scholar] [CrossRef]
- Murphy, M.G.; Crocker, J.F.; Lee, S.H.; Acott, P.; Her, H. Sequestration of coenzyme A by the industrial surfactant, Toximul MP8. A possible role in the inhibition of fatty-acid beta-oxidation in a surfactant/influenza B virus mouse model for acute hepatic encephalopathy. Biochim. Biophys. Acta. 1997, 1361, 103–113. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Pistone, G.; Elvira, R.; Leotta, C.; Scarpello, L.; Liborio, R. Effects of L-carnitine in patients with hepatic encephalopathy. World J. Gastroenterol. 2005, 11, 7197–7202. [Google Scholar] [CrossRef]
- Shurubor, Y.I.; Cooper, A.J.L.; Krasnikov, A.B.; Isakova, E.P.; Deryabina, Y.I.; Beal, M.F.; Krasnikov, B.F. Changes of Coenzyme A and Acetyl-Coenzyme A concentrations in rats after a single-dose intraperitoneal injection of hepatotoxic thioacetamide are not consistent with rapid recovery. Int. J. Mol. Sci. 2020, 21, 8918. [Google Scholar] [CrossRef]
- Cooper, A.J.L.; Lai, J.C.K.; Gelbard, A.S. Ammonia in Liver and Extrahepatic Tissues: An Overview of Metabolism and Toxicity in Mammals. Hepatic Enceph. 1997, 22, 27–48. [Google Scholar]
- Limón, I.D.; Angulo-Cruz, I.; Sánchez-Abdon, L.; Patricio-Martínez, A. Disturbance of the Glutamate-Glutamine Cycle, Secondary to Hepatic Damage, Compromises Memory Function. Front. Neurosci. 2021, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kroupina, K.; Bémeur, C.; Rose, C.F. Amino acids, ammonia, and hepatic encephalopathy. Anal. Biochem. 2022, 649, 114696. [Google Scholar] [CrossRef]
- Hirose, T.; Shimizu, K.; Ogura, H.; Yamano, S.; Ohnishi, M.; Kuwagata, Y.; Shimazu, T. Plasma amino acid levels in acute hepatic failure. Jpn. J. Surg. Metab. Nutr. 2013, 47, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Zieve, L. Amino acids in liver failure. Gastroenterology 1979, 76, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, L.E.; Striver, C.R. Disorders of amino acid metabolism. In Metabolic Control and Disease; Bondy, P.K., Ed.; Saunders: Philadelphia, PA, USA, 1980; pp. 707–710. [Google Scholar]
- Corkey, B.E.; Martin-Requero, A.; Walajtys-Rode, E.; Williams, R.J.; Williamson, J.R. Regulation of the branched-chain-ketoacid pathway in liver. J. Biol. Chem. 1982, 57, 9668–9676. [Google Scholar] [CrossRef]
- O’Keefe, S.J.D.; Abraham, R.; El-Zayadi, A.; Marshall, W.; Davis, M.; Williams, R. Increased plasma tyrosine concentrations in patients with cirrhosis and fulminant hepatic failure associated with increased plasma tyrosine flux and reduced hepatic oxidation capacity. Gastroenterology 1981, 81, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, G.; Stacchiotti, A.; Tedesco, L.; D’Antona, G.; Pasini, E.; Dioguardi, F.S.; Nisoli, E.; Rezzani, R. Essential amino acid supplementation decreases liver damage induced by chronic ethanol consumption in rats. Int. J. Immunopathol. Pharmacol. 2011, 24, 611–619. [Google Scholar] [CrossRef]
- Monirujjaman, M. Metabolic and Physiological Roles of Branched-Chain Amino Acids. Adv. Mol. Biol. 2014, 2014, 364976. [Google Scholar] [CrossRef]
- Vaquero, J.; Butterworth, R.F. The brain glutamate system in liver failure. J. Neurochem. 2006, 98, 661–669. [Google Scholar] [CrossRef]
- Bosman, D.K.; Deutz, N.E.; De Graaf, A.A.; Vd Hulst, R.W.; Van Eijk, H.M.; Bovee, W.M.; Maas, M.A.; Jorning, G.G.; Chamuleau, R.A. Changes in brain metabolism during hyperammonemia and acute liver failure: Results of a comparative 1H-NMR spectroscopy and biochemical investigation. Hepatology 1990, 12, 281–290. [Google Scholar] [CrossRef]
- Hilgier, W.; Olson, J.E. Brain ion and amino acid contents during edema development in hepatic encephalopathy. J. Neurochem. 1994, 62, 197–204. [Google Scholar] [CrossRef]
- Butterworth, R.F. Neuroactive amino acids in hepatic encephalopathy. Metab. Brain Dis. 1996, 11, 165–173. [Google Scholar] [CrossRef]
- Shurubor, Y.I.; Rogozhin, A.E.; Isakova, E.P.; Deryabina, Y.I.; Krasnikov, B.F. Tricarboxylic Acid Metabolites Imbalance in Rats with Acute Thioacetamide-Induced Hepatic Encephalopathy Indicates Incomplete Recovery. Int. J. Mol. Sci. 2023, 24, 1384. [Google Scholar] [CrossRef]
- Cooper, A.J.L.; Kuhara, T. α-Ketoglutaramate: An overlooked metabolite of glutamine and a biomarker for hepatic encephalopathy and inborn errors of the urea cycle. Metab. Brain Dis. 2014, 29, 991–1006. [Google Scholar] [CrossRef]
- Cooper, A.J.L. The role of glutamine transaminase K (GTK) in sulfur and α-keto acid metabolism in the brain, and in the possible bioactivation of neurotoxicants. Neurochem. Int. 2004, 44, 557–577. [Google Scholar] [CrossRef] [PubMed]
- Krasnikov, B.F.; Nostramo, R.; Pinto, J.T.; Cooper, A.J.L. Assay and purification of ω-amidase/Nit2, a ubiquitously expressed putative tumor suppressor that catalyzes the deamidation of the α-keto acid analogues of glutamine and asparagine. Anal. Biochem. 2009, 391, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, B.; Lindner, G.; Reiberger, T.; Schneeweiss, B.; Trauner, M.; Zauner, C.; Funk, G.-C. Acid-base disorders in liver disease. J. Hepatol. 2017, 67, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Nissim, I. Newer aspects of glutamine/glutamate metabolism: The role of acute pH changes. Am. J. Physiol. 1999, 277, F493–F497. [Google Scholar] [CrossRef]
- Wu, G.; Meininger, C.J. Analysis of citrulline, arginine, and methylarginines using high-performance liquid chromatography. Methods Enzymol. 2008, 440, 177–189. [Google Scholar] [CrossRef]

| AA | Plasma | Liver | Kidney | Brain | ||||
|---|---|---|---|---|---|---|---|---|
| Control | TAA 200–600 | Control | TAA 200–600 | Control | TAA 200–600 | Control | TAA 200–600 | |
| Aspartate | 37.5 ± 0.76 | 34.6 ± 11.1 | 4.19 ± 0.76 | 3.30 ± 1.39 | 5.74 ± 2.86 | 6.87 ± 3.08 | 9.68 ± 3.58 | 7.40 ± 1.95 |
| Glutamate | 136 ± 22.7 | 123 ± 33.9 | 8.39 ± 2.49 | 8.91 ± 1.74 | 41.0 ± 8.63 | 47.6 ± 19.2 | 40.7 ± 15.0 | 30.1 ± 7.94 |
| Glutamine | 689 ± 70.7 | 816 ± 87.8 | 14.8 ± 2.83 | 22.2 ± 4.53 ** | 9.94 ± 3.68 | 12.4 ± 4.90 | 17.8 ± 5.50 | 14.78 ± 4.31 |
| Glycine | 316 ± 50.0 | 327 ± 44.3 | 7.33 ± 1.77 | 6.43 ± 1.15 | 13.6 ± 6.57 | 26.9 ± 10.6 | 2.05 ± 0.71 | 1.91 ± 0.41 |
| Citrulline | 233 ± 7.62 | 241 ± 31.0 * | 3.61 ± 0.72 | 3.21 ± 0.59 | NA | NA | 1.31 ± 0.45 | 1.11 ± 0.26 |
| Arginine | 272 ± 35.7 | 311 ± 39.6 | 0.62 ± 0.04 | 0.73 ± 0.26 | 2.41 ± 1.87 | 1.40 ± 0.51 | 0.38 ± 0.10 | 0.39 ± 0.11 |
| Taurine | 207 ± 28.2 | 183 ± 25.6 | 3.57 ± 1.57 | 17.0 ± 14.4 | 42.6 ± 16.1 | 49.9 ± 23.4 | 15.4 ± 4.53 | 12.2 ± 3.56 |
| Alanine | 539 ± 82.9 | 532 ± 68.6 | 9.93 ± 1.94 | 8.09 ± 1.78 | 3.55 ± 1.76 | 6.39 ± 2.69 | 2.38 ± 0.71 | 2.14 ± 0.49 |
| Tryptophan | 266 ± 34.6 | 287 ± 42.6 | 0.39 ± 0.02 | 0.37 ± 0.08 | 1.66 ± 0.75 | 1.82 ± 0.82 | 0.24 ± 0.09 | 0.22 ± 0.08 |
| Methionine | 51.1 ± 11.7 | 69.8 ± 7.53 ** | 0.16 ± 0.01 | 0.23 ± 0.07 * | 1.04 ± 1.01 | 0.49 ± 0.19 | 0.10 ± 0.05 | 0.09 ± 0.04 |
| Valine | 135 ± 22.3 | 144 ± 13.2 | 0.69 ± 0.03 | 0.83 ± 0.20 | 0.78 ± 0.28 | 4.08 ± 2.34 | 0.17 ± 0.06 | 0.18 ± 0.05 |
| Phenylalanine | 54.8 ± 2.86 | 60.7 ± 4.67 ** | 0.36 ± 0.02 | 0.44 ± 0.09 | 1.11 ± 1.03 | 0.38 ± 0.15 | 0.12 ± 0.04 | 0.11 ± 0.03 |
| Isoleucine | 89.5 ± 12.1 | 95.3 ± 10.1 | 0.66 ± 0.03 | 0.75 ± 0.17 | 1.42 ± 1.06 | 1.06 ± 0.39 | 0.14 ± 0.04 | 0.14 ± 0.03 |
| Leucine | 140 ± 21.9 | 150 ± 12.8 | 1.03 ± 0.04 | 1.29 ± 0.31 | 4.02 ± 3.58 | 1.88 ± 0.76 | 0.16 ± 0.05 | 0.22 ± 0.07 * |
| Ornithine | 59.8 ± 10.7 | 73.8 ± 9.80 | 0.70 ± 0.07 | 1.16 ± 0.31 ** | 0.83 ± 0.50 | 1.00 ± 0.53 | 0.11 ± 0.01 | 0.12 ± 0.03 |
| Lysine | 539 ± 52.7 | 613 ± 52.2 * | 2.03 ± 0.21 | 2.30 ± 0.57 | 5.23 ± 3.44 | 5.22 ± 2.54 | 0.64 ± 0.24 | 0.58 ± 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shurubor, Y.I.; Rogozhin, A.E.; Isakova, E.P.; Deryabina, Y.I.; Krasnikov, B.F. Residual Amino Acid Imbalance in Rats during Recovery from Acute Thioacetamide-Induced Hepatic Encephalopathy Indicates Incomplete Healing. Int. J. Mol. Sci. 2023, 24, 3647. https://doi.org/10.3390/ijms24043647
Shurubor YI, Rogozhin AE, Isakova EP, Deryabina YI, Krasnikov BF. Residual Amino Acid Imbalance in Rats during Recovery from Acute Thioacetamide-Induced Hepatic Encephalopathy Indicates Incomplete Healing. International Journal of Molecular Sciences. 2023; 24(4):3647. https://doi.org/10.3390/ijms24043647
Chicago/Turabian StyleShurubor, Yevgeniya I., Alexander E. Rogozhin, Elena P. Isakova, Yulia I. Deryabina, and Boris F. Krasnikov. 2023. "Residual Amino Acid Imbalance in Rats during Recovery from Acute Thioacetamide-Induced Hepatic Encephalopathy Indicates Incomplete Healing" International Journal of Molecular Sciences 24, no. 4: 3647. https://doi.org/10.3390/ijms24043647
APA StyleShurubor, Y. I., Rogozhin, A. E., Isakova, E. P., Deryabina, Y. I., & Krasnikov, B. F. (2023). Residual Amino Acid Imbalance in Rats during Recovery from Acute Thioacetamide-Induced Hepatic Encephalopathy Indicates Incomplete Healing. International Journal of Molecular Sciences, 24(4), 3647. https://doi.org/10.3390/ijms24043647








