The Role of Cytokines in the Metastasis of Solid Tumors to the Spine: Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Data Items
3. Results and Discussion
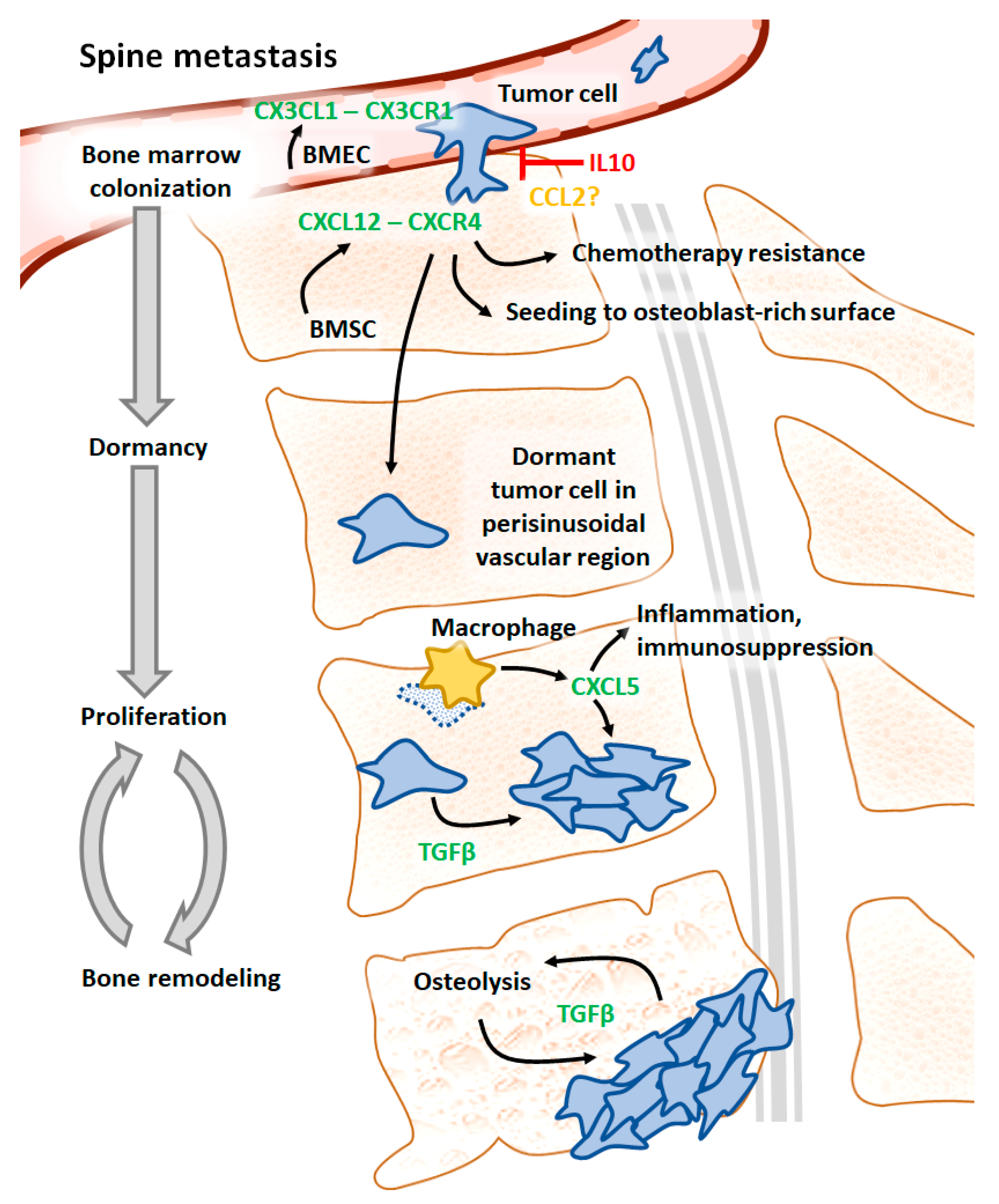
3.1. CX3CL1 and CX3CR1
3.2. IL10
3.3. CCL2
3.4. CXCR6, CXCR4, and CXCL12
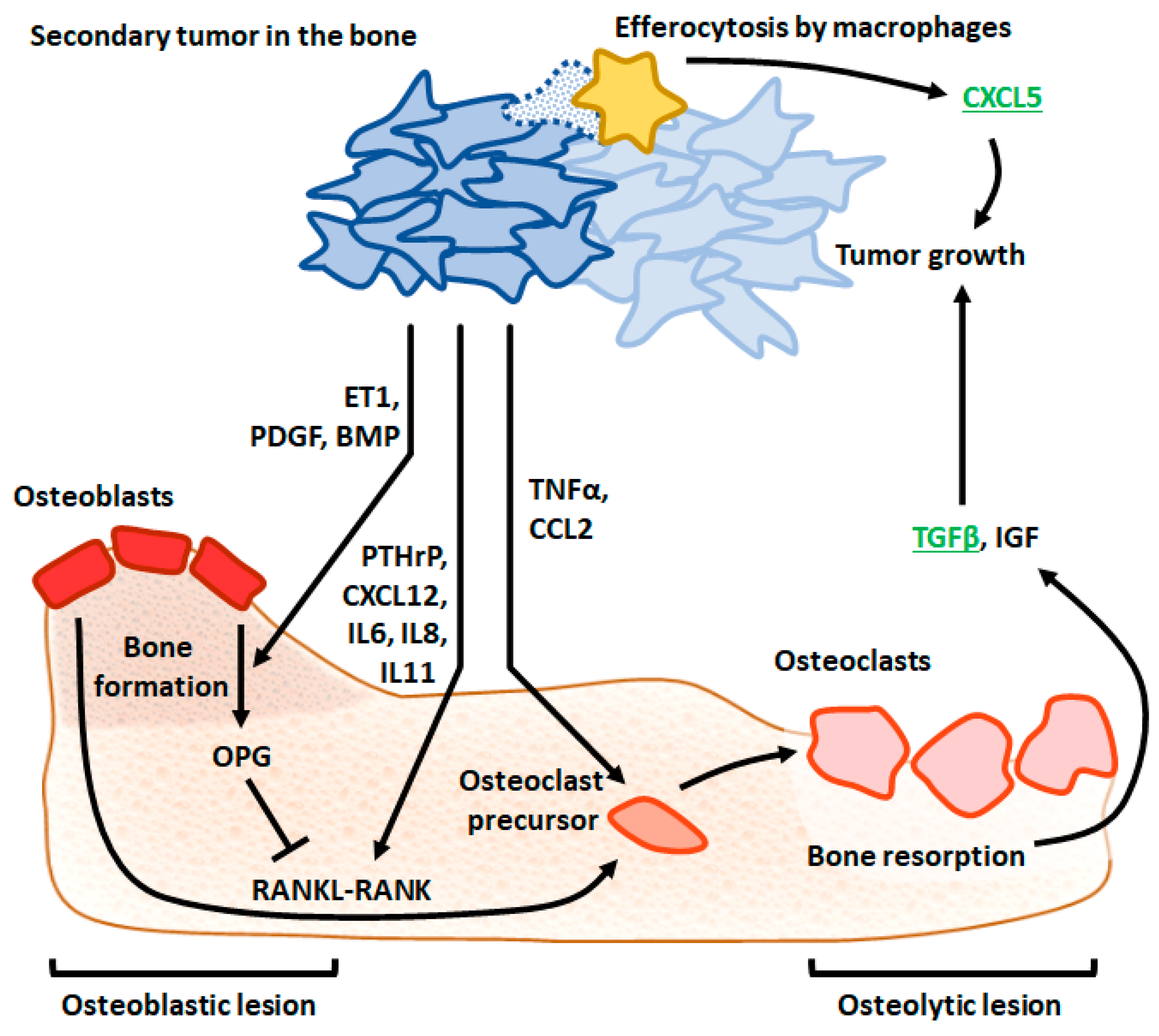
3.5. CXCL5
3.6. TGFβ
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coleman, R.E.; Croucher, P.I.; Padhani, A.R.; Clézardin, P.; Chow, E.; Fallon, M.; Guise, T.; Colangeli, S.; Capanna, R.; Costa, L. Bone metastases. Nat. Rev. Dis. Prim. 2020, 6, 1–28. [Google Scholar] [CrossRef]
- Hong, S.; Youk, T.; Lee, S.J.; Kim, K.M.; Vajdic, C.M. Bone metastasis and skeletal-related events in patients with solid cancer: A Korean nationwide health insurance database study. PLoS ONE 2020, 15, e0234927. [Google Scholar] [CrossRef]
- Kurisunkal, V.; Gulia, A.; Gupta, S. Principles of Management of Spine Metastasis. Indian J. Orthop. 2020, 54, 181–193. [Google Scholar] [CrossRef]
- Kimura, T. Multidisciplinary Approach for Bone Metastasis: A Review. Cancers 2018, 10, 156. [Google Scholar] [CrossRef]
- Litak, J.; Czyżewski, W.; Szymoniuk, M.; Sakwa, L.; Pasierb, B.; Litak, J.; Hoffman, Z.; Kamieniak, P.; Roliński, J. Biological and Clinical Aspects of Metastatic Spinal Tumors. Cancers 2022, 14, 4599. [Google Scholar] [CrossRef]
- Maccauro, G.; Spinelli, M.S.; Mauro, S.; Perisano, C.; Graci, C.; Rosa, M.A. Physiopathology of Spine Metastasis. Int. J. Surg. Oncol. 2011, 2011, 107969. [Google Scholar] [CrossRef]
- Shupp, A.; Kolb, A.; Mukhopadhyay, D.; Bussard, K. Cancer Metastases to Bone: Concepts, Mechanisms, and Interactions with Bone Osteoblasts. Cancers 2018, 10, 182. [Google Scholar] [CrossRef]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Gonçalves, F. Bone metastases: An overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef]
- Paget, S. Stephen Paget’s Paper Reproduced from The Lancet, 1889. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar] [CrossRef]
- Croucher, P.I.; McDonald, M.M.; Martin, T.J. Bone metastasis: The importance of the neighbourhood. Nat. Rev. Cancer 2016, 16, 373–386. [Google Scholar] [CrossRef]
- Sun, Y.-X.; Schneider, A.; Jung, Y.; Wang, J.; Dai, J.; Wang, J.; Cook, K.; Osman, N.I.; Koh-Paige, A.J.; Shim, H.; et al. Skeletal Localization and Neutralization of the SDF-1(CXCL12)/CXCR4 Axis Blocks Prostate Cancer Metastasis and Growth in Osseous Sites In Vivo. J. Bone Miner. Res. 2004, 20, 318–329. [Google Scholar] [CrossRef]
- Wang, M.; Xia, F.; Wei, Y.; Wei, X. Molecular mechanisms and clinical management of cancer bone metastasis. Bone Res. 2020, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Bendre, M.S.; Montague, D.C.; Peery, T.; Akel, N.S.; Gaddy, D.; Suva, L.J. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone 2003, 33, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.-T.; Ridlmaier, N.; Smit, D.; Taipaleenmäki, H. Interleukins as Mediators of the Tumor Cell—Bone Cell Crosstalk during the Initiation of Breast Cancer Bone Metastasis. Int. J. Mol. Sci. 2021, 22, 2898. [Google Scholar] [CrossRef]
- Trivedi, T.; Pagnotti, G.M.; Guise, T.A.; Mohammad, K.S. The Role of TGF-β in Bone Metastases. Biomolecules 2021, 11, 1643. [Google Scholar] [CrossRef]
- Gnant, M.; Pfeiler, G.; Dubsky, P.C.; Hubalek, M.; Greil, R.; Jakesz, R.; Wette, V.; Balic, M.; Haslbauer, F.; Melbinger, E.; et al. Adjuvant denosumab in breast cancer (ABCSG-18): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015, 386, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Egerdie, B.; Toriz, N.H.; Feldman, R.; Tammela, T.L.; Saad, F.; Heracek, J.; Szwedowski, M.; Ke, C.; Kupic, A.; et al. Denosumab in Men Receiving Androgen-Deprivation Therapy for Prostate Cancer. N. Engl. J. Med. 2009, 361, 745–755. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Hu, A.; Wang, S.; Tian, B.; Jiang, L.; Liang, Y.; Wang, H.; Dong, J. ADAM17-regulated CX3CL1 expression produced by bone marrow endothelial cells promotes spinal metastasis from hepatocellular carcinoma. Int. J. Oncol. 2020, 57, 249. [Google Scholar] [CrossRef] [PubMed]
- Roca, H.; Jones, J.D.; Purica, M.C.; Weidner, S.; Koh, A.J.; Kuo, R.; Wilkinson, J.E.; Wang, Y.; Daignault-Newton, S.; Pienta, K.J.; et al. Apoptosis-induced CXCL5 accelerates inflammation and growth of prostate tumor metastases in bone. J. Clin. Investig. 2017, 128, 248–266. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, J.K.; Shiozawa, Y.; Wang, J.; Mishra, A.; Joseph, J.; Berry, J.E.; McGee, S.; Lee, E.; Sun, H.; et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat. Commun. 2013, 4, 1795. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Miyazaki, H.; Furihata, M.; Sakai, H.; Konakahara, T.; Watanabe, M.; Okada, T. Chemokine CCL2/MCP-1 negatively regulates metastasis in a highly bone marrow-metastatic mouse breast cancer model. Clin. Exp. Metastasis 2009, 26, 817–828. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Agyin, J.K.; Wang, L.; Tang, Y.; Lei, X.; Story, B.M.; Cornell, J.E.; Pollock, B.H.; Mundy, G.R.; Sun, L.Z. Inhibition of Pulmonary and Skeletal Metastasis by a Transforming Growth Factor-β Type I Receptor Kinase Inhibitor. Cancer Res. 2006, 66, 6714–6721. [Google Scholar] [CrossRef]
- Wang, M.E.; Stearns, M. Antimestatic and Antitumor Activities of Interleukin 10 in Transfected Human Prostate PC-3 ML Clones: Orthotopic Growth in Severe Combined Immunodeficient Mice. Clin. Cancer Res. Am. Assoc. Cancer Res. 1998, 4, 2257–2263. [Google Scholar]
- Jamieson-Gladney, W.L.; Zhang, Y.; Fong, A.M.; Meucci, O.; Fatatis, A. The chemokine receptor CX3CR1 is directly involved in the arrest of breast cancer cells to the skeleton. Breast Cancer Res. 2011, 13, R91. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Bian, C.; Liang, Y.; Jiang, L.; Qian, C.; Dong, J. CX3CL1: A potential chemokine widely involved in the process spinal metastases. Oncotarget 2017, 8, 15213–15219. [Google Scholar] [CrossRef]
- Stearns, M.E.; Fudge, K.; Garcia, F.; Wang, M. IL-10 Inhibition of Human Prostate PC-3 ML Cell Metastases in SCID Mice: IL-10 Stimulation of TIMP-1 and Inhibition of MMP-2/MMP-9 Expression. Invasion Metastasis 1997, 17, 62–74. [Google Scholar] [PubMed]
- Lim, S.Y.; Yuzhalin, A.E.; Gordon-Weeks, A.N.; Muschel, R.J. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget 2016, 7, 28697–28710. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, B.S.; Forwood, M.R.; Morrison, N.A. Monocyte Chemoattractant Protein-1 (MCP-1/CCL2) Drives Activation of Bone Remodelling and Skeletal Metastasis. Curr. Osteoporos. Rep. 2019, 17, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, Q.; Corey, E.; Xie, W.; Fan, J.; Mizokami, A.; Zhang, J. Activation of MCP-1/CCR2 axis promotes prostate cancer growth in bone. Clin. Exp. Metastasis 2008, 26, 161–169. [Google Scholar] [CrossRef]
- Li, X.; Loberg, R.; Liao, J.; Ying, C.; Snyder, L.A.; Pienta, K.J.; McCauley, L.K. A Destructive Cascade Mediated by CCL2 Facilitates Prostate Cancer Growth in Bone. Cancer Res. 2009, 69, 1685–1692. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.; Liu, M.; Du, Y.; Qu, F.; Li, Y.; Zhang, Q.; Xiao, Y.; Zhao, J.; Zeng, F.; Xiao, C. Tumor cell-specific blockade of CXCR4/SDF-1 interactions in prostate cancer cells by hTERT promoter induced CXCR4 knockdown: A possible metastasis preventing and minimizing approach. Cancer Biol. Ther. 2008, 7, 1839–1848. [Google Scholar] [CrossRef]
- Gravina, G.L.; Mancini, A.; Muzi, P.; Ventura, L.; Biordi, L.; Ricevuto, E.; Pompili, S.; Mattei, C.; Di Cesare, E.; Jannini, E.A.; et al. CXCR4 pharmacogical inhibition reduces bone and soft tissue metastatic burden by affecting tumor growth and tumorigenic potential in prostate cancer preclinical models. Prostate 2015, 75, 1227–1246. [Google Scholar] [CrossRef]
- Dai, J.; Escara-Wilke, J.; Keller, J.M.; Jung, Y.; Taichman, R.S.; Pienta, K.J.; Keller, E.T. Primary prostate cancer educates bone stroma through exosomal pyruvate kinase M2 to promote bone metastasis. J. Exp. Med. 2019, 216, 2883–2899. [Google Scholar] [CrossRef]
- Conley-LaComb, M.K.; Semaan, L.; Singareddy, R.; Li, Y.; Heath, E.I.; Kim, S.; Cher, M.L.; Chinni, S.R. Pharmacological targeting of CXCL12/CXCR4 signaling in prostate cancer bone metastasis. Mol. Cancer 2016, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Docherty, F.E.; Brown, H.K.; Reeves, K.J.; Fowles, A.C.; Ottewell, P.D.; Dear, T.N.; Holen, I.; Croucher, P.I.; Eaton, C.L. Prostate Cancer Cells Preferentially Home to Osteoblast-rich Areas in the Early Stages of Bone Metastasis: Evidence From In Vivo Models. J. Bone Miner. Res. 2014, 29, 2688–2696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, Y.; Wu, Q.; Xie, L.; Barwick, B.; Fu, C.; Li, X.; Wu, D.; Xia, S.; Chen, J.; et al. Acetylation of KLF5 maintains EMT and tumorigenicity to cause chemoresistant bone metastasis in prostate cancer. Nat. Commun. 2021, 12, 1714. [Google Scholar] [CrossRef]
- Xiang, J.; Hurchla, M.A.; Fontana, F.; Su, X.; Amend, S.R.; Esser, A.K.; Douglas, G.J.; Mudalagiriyappa, C.; Luker, K.E.; Pluard, T.; et al. CXCR4 Protein Epitope Mimetic Antagonist POL5551 Disrupts Metastasis and Enhances Chemotherapy Effect in Triple-Negative Breast Cancer. Mol. Cancer Ther. 2015, 14, 2473–2485. [Google Scholar] [CrossRef]
- Price, T.T.; Burness, M.L.; Sivan, A.; Warner, M.J.; Cheng, R.; Lee, C.H.; Olivere, L.; Comatas, K.; Magnani, J.; Lyerly, H.K.; et al. Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci. Transl. Med. 2016, 8, 340ra73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yeger, H.; Das, B.; Irwin, M.S.; Baruchel, S. Tissue Microenvironment Modulates CXCR4 Expression and Tumor Metastasis in Neuroblastoma. Neoplasia 2007, 9, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Muhlethaler-Mottet, A.; Liberman, J.; Ascencao, K.; Flahaut, M.; Bourloud, K.B.; Yan, P.; Jauquier, N.; Gross, N.; Joseph, J.-M. The CXCR4/CXCR7/CXCL12 Axis Is Involved in a Secondary but Complex Control of Neuroblastoma Metastatic Cell Homing. PLoS ONE 2015, 10, e0125616. [Google Scholar] [CrossRef]
- Ma, N.; Pang, H.; Shen, W.; Zhang, F.; Cui, Z.; Wang, J.; Wang, J.; Liu, L.; Zhang, H. Downregulation of CXCR4 by SDF-KDEL in SBC-5 cells inhibits their migration in vitro and organ metastasis in vivo. Int. J. Mol. Med. 2014, 35, 425–432. [Google Scholar] [CrossRef]
- Romero-Moreno, R.; Curtis, K.J.; Coughlin, T.R.; Miranda-Vergara, M.C.; Dutta, S.; Natarajan, A.; Facchine, B.A.; Jackson, K.M.; Nystrom, L.; Li, J.; et al. The CXCL5/CXCR2 axis is sufficient to promote breast cancer colonization during bone metastasis. Nat. Commun. 2019, 10, 4404. [Google Scholar] [CrossRef]
- Halpern, J.L.; Kilbarger, A.; Lynch, C.C. Mesenchymal stem cells promote mammary cancer cell migration in vitro via the CXCR2 receptor. Cancer Lett. 2011, 308, 91–99. [Google Scholar] [CrossRef]
- Krzeszinski, J.Y.; Wan, Y. New therapeutic targets for cancer bone metastasis. Trends Pharmacol. Sci. 2015, 36, 360–373. [Google Scholar] [CrossRef]
- Ban, J.; Fock, V.; Aryee, D.N.T.; Kovar, H. Mechanisms, Diagnosis and Treatment of Bone Metastases. Cells 2021, 10, 2944. [Google Scholar] [CrossRef]
- Kolb, A.D.; Bussard, K.M. The Bone Extracellular Matrix as an Ideal Milieu for Cancer Cell Metastases. Cancers 2019, 11, 1020. [Google Scholar] [CrossRef]
- Javelaud, D.; Mohammad, K.S.; McKenna, C.R.; Fournier, P.; Luciani, F.; Niewolna, M.; André, J.; Delmas, V.; Larue, L.; Guise, T.A.; et al. Stable Overexpression of Smad7 in Human Melanoma Cells Impairs Bone Metastasis. Cancer Res. 2007, 67, 2317–2324. [Google Scholar] [CrossRef]
- Futakuchi, M.; Nannuru, K.C.; Varney, M.L.; Sadanandam, A.; Nakao, K.; Asai, K.; Shirai, T.; Sato, S.-Y.; Singh, R.K. Transforming growth factor-β signaling at the tumor-bone interface promotes mammary tumor growth and osteoclast activation. Cancer Sci. 2009, 100, 71–81. [Google Scholar] [CrossRef]
- Ganapathy, V.; Ge, R.; Grazioli, A.; Xie, W.; Banach-Petrosky, W.; Kang, Y.; Lonning, S.; McPherson, J.; Yingling, J.M.; Biswas, S.; et al. Targeting the Transforming Growth Factor-β pathway inhibits human basal-like breast cancer metastasis. Mol. Cancer 2010, 9, 122. [Google Scholar] [CrossRef]
- Biswas, S.; Nyman, J.S.; Alvarez, J.; Chakrabarti, A.; Ayres, A.; Sterling, J.; Edwards, J.; Rana, T.; Johnson, R.; Perrien, D.S.; et al. Anti-Transforming Growth Factor ß Antibody Treatment Rescues Bone Loss and Prevents Breast Cancer Metastasis to Bone. PLoS ONE 2011, 6, e27090. [Google Scholar] [CrossRef]
- Korpal, M.; Yan, J.; Lü, X.; Xu, S.; Lerit, D.A.; Kang, Y. Imaging transforming growth factor-β signaling dynamics and therapeutic response in breast cancer bone metastasis. Nat. Med. 2009, 15, 960–966. [Google Scholar] [CrossRef]
- Yin, J.J.; Selander, K.; Chirgwin, J.M.; Dallas, M.; Grubbs, B.G.; Wieser, R.; Massagué, J.; Mundy, G.R.; Guise, T.A. TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Investig. 1999, 103, 197–206. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, Z.; Gupta, J.; Krimmel, J.D.; Gerseny, H.M.; Berg, A.F.; Robbins, J.S.; Du, H.; Prabhakar, B.; Seth, P. Intravenous administration of adenoviruses targeting transforming growth factor beta signaling inhibits established bone metastases in 4T1 mouse mammary tumor model in an immunocompetent syngeneic host. Cancer Gene Ther. 2012, 19, 630–636. [Google Scholar] [CrossRef]
- Buenrostro, D.; Kwakwa, K.A.; Putnam, N.E.; Merkel, A.; Johnson, J.R.; Cassat, J.E.; Sterling, J.A. Early TGF-β inhibition in mice reduces the incidence of breast cancer induced bone disease in a myeloid dependent manner. Bone 2018, 113, 77–88. [Google Scholar] [CrossRef]
- Kang, Y.; He, W.; Tulley, S.; Gupta, G.P.; Serganova, I.; Chen, C.-R.; Manova-Todorova, K.; Blasberg, R.; Gerald, W.L.; Massagué, J. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc. Natl. Acad. Sci. USA 2005, 102, 13909–13914. [Google Scholar] [CrossRef]
- Deckers, M.; van Dinther, M.; Buijs, J.; Que, I.; Lowik, C.; van der Pluijm, G.; Dijke, P.T. The Tumor Suppressor Smad4 Is Required for Transforming Growth Factor β–Induced Epithelial to Mesenchymal Transition and Bone Metastasis of Breast Cancer Cells. Cancer Res. 2006, 66, 2202–2209. [Google Scholar] [CrossRef]
- Petersen, M.; Pardali, E.; van der Horst, G.; Cheung, H.; Hoogen, C.V.D.; van der Pluijm, G.; Dijke, P.T. Smad2 and Smad3 have opposing roles in breast cancer bone metastasis by differentially affecting tumor angiogenesis. Oncogene 2009, 29, 1351–1361. [Google Scholar] [CrossRef]
- Wilson, T.J.; Nannuru, K.C.; Futakuchi, M.; Singh, R.K. Cathepsin G-mediated enhanced TGF-β signaling promotes angiogenesis via upregulation of VEGF and MCP-1. Cancer Lett. 2010, 288, 162–169. [Google Scholar] [CrossRef]
- Meng, X.; Ark, A.V.; Lee, P.; Hostetter, G.; Bhowmick, N.A.; Matrisian, L.M.; Williams, B.O.; Miranti, C.K.; Li, X. Myeloid-specific TGF-β signaling in bone promotes basic-FGF and breast cancer bone metastasis. Oncogene 2016, 35, 2370–2378. [Google Scholar] [CrossRef]
- Hiraga, T.; Myoui, A.; Choi, M.E.; Yoshikawa, H.; Yoneda, T. Stimulation of Cyclooxygenase-2 Expression by Bone-Derived Transforming Growth Factor-β Enhances Bone Metastases in Breast Cancer. Cancer Res. 2006, 66, 2067–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Käkönen, S.-M.; Selander, K.S.; Chirgwin, J.M.; Yin, J.J.; Burns, S.; Rankin, W.A.; Grubbs, B.G.; Dallas, M.; Cui, Y.; Guise, T.A. Transforming Growth Factor-β Stimulates Parathyroid Hormone-related Protein and Osteolytic Metastases via Smad and Mitogen-activated Protein Kinase Signaling Pathways. J. Biol. Chem. 2002, 277, 24571–24578. [Google Scholar] [CrossRef]
- Biswas, S.; Guix, M.; Rinehart, C.; Dugger, T.C.; Chytil, A.; Moses, H.L.; Freeman, M.L.; Arteaga, C.L. Inhibition of TGF-β with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J. Clin. Investig. 2007, 117, 1305–1313. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Wang, L.; Agyin, J.; Tang, Y.; Lin, S.; Yeh, I.-T.; De, K.; Sun, L.-Z. Doxorubicin in Combination with a Small TGFβ Inhibitor: A Potential Novel Therapy for Metastatic Breast Cancer in Mouse Models. PLoS ONE 2010, 5, e10365. [Google Scholar] [CrossRef] [PubMed]
- Rana, T.; Chakrabarti, A.; Freeman, M.; Biswas, S. Doxorubicin-Mediated Bone Loss in Breast Cancer Bone Metastases Is Driven by an Interplay between Oxidative Stress and Induction of TGFβ. PLoS ONE 2013, 8, e78043. [Google Scholar] [CrossRef]
- Wan, X.; Li, Z.-G.; Yingling, J.M.; Yang, J.; Starbuck, M.W.; Ravoori, M.K.; Kundra, V.; Vazquez, E.; Navone, N.M. Effect of transforming growth factor beta (TGF-β) receptor I kinase inhibitor on prostate cancer bone growth. Bone 2012, 50, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Tang, Y.; Wang, L.; Degraffenried, L.; Yeh, I.-T.; Werner, S.; Troyer, D.; Copland, J.A.; Sun, L.-Z. Blockade of transforming growth factor-beta (TGFβ) signaling inhibits osteoblastic tumorigenesis by a novel human prostate cancer cell line. Prostate 2011, 71, 1441–1454. [Google Scholar] [CrossRef]
- Hu, Z.; Gupta, J.; Zhang, Z.; Gerseny, H.; Berg, A.; Chen, Y.J.; Zhang, Z.; Du, H.; Brendler, C.B.; Xiao, X.; et al. Systemic Delivery of Oncolytic Adenoviruses Targeting Transforming Growth Factor-β Inhibits Established Bone Metastasis in a Prostate Cancer Mouse Model. Hum. Gene Ther. 2012, 23, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Ark, A.V.; Daft, P.; Woodford, E.; Wang, J.; Madaj, Z.; Li, X. Loss of TGF-β signaling in osteoblasts increases basic-FGF and promotes prostate cancer bone metastasis. Cancer Lett. 2018, 418, 109–118. [Google Scholar] [CrossRef]
- Kominsky, S.L.; Doucet, M.; Brady, K.; Weber, K.L. TGF-β Promotes the Establishment of Renal Cell Carcinoma Bone Metastasis. J. Bone Miner. Res. 2006, 22, 37–44. [Google Scholar] [CrossRef]

| Reference | Title | Cytokine, Cytokine Receptor | Primary Tumor | Setting |
|---|---|---|---|---|
| Sun et al. (2020), [19] | ADAM17-regulated CX3CL1 expression produced by bone marrow endothelial cells promotes spinal metastasis from hepatocellular carcinoma. | CX3CL1, CX3CR1 | Liver (human hepatocellular carcinoma cell line MHCC97H) | Mice injected with CX3CR1-overexpressing MHCC97H cancer cells or with CX3CL1-silenced bone marrow endothelial cells |
| Roca et al. (2018), [20] | Apoptosis-induced CXCL5 accelerates inflammation and growth of prostate tumor metastases in bone. | CXCL5 | Prostate (syngeneic murine prostate carcinoma cell line RM1) | Vertebral bodies inoculated with RM1-iC9 cells, subcutaneously implanted into CXCL5−/− mice, followed by apoptosis induction using the FKBP fusion protein systems |
| Jung et al. (2013), [21] | Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. | CXCL12, CXCR4, CXCR6 | Prostate (syngeneic murine prostate carcinoma cell line RM1) | RM1 cells treated with CXCL12 and/or CXCR4 inhibitor AMD3100, injected into CXCR6−/− mice |
| Takahashi et al. (2009), [22] | Chemokine CCL2/MCP-1 negatively regulates metastasis in a highly bone marrow-metastatic mouse breast cancer model. | CCL2 | Breast (murine mammary carcinoma cell line 4T1E) | Mice injected with CCL2-overexpressing or CCL2-silenced 4T1E cells |
| Bandyopadhyay et al. (2006), [23] | Inhibition of pulmonary and skeletal metastasis by a transforming growth factor-beta type I receptor kinase inhibitor. | TGFβ | Skin (human melanoma cell line MDA-MB-435, formerly described as of breast cancer origin) | TGFβ treatment in mice injected with MDA-MB-435-F-L cells |
| Stearns and Wang (1998), [24] | Antimestatic and antitumor activities of interleukin 10 in transfected human prostate PC-3 ML clones: Orthotopic growth in severe combined immunodeficient mice. | IL10 | Prostate (human prostatic adenocarcinoma cell line PC-3) | IL10-overexpressing or IL10-treated PC-3 cells injected into mice |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łabędź, W.; Przybyla, A.; Zimna, A.; Dąbrowski, M.; Kubaszewski, Ł. The Role of Cytokines in the Metastasis of Solid Tumors to the Spine: Systematic Review. Int. J. Mol. Sci. 2023, 24, 3785. https://doi.org/10.3390/ijms24043785
Łabędź W, Przybyla A, Zimna A, Dąbrowski M, Kubaszewski Ł. The Role of Cytokines in the Metastasis of Solid Tumors to the Spine: Systematic Review. International Journal of Molecular Sciences. 2023; 24(4):3785. https://doi.org/10.3390/ijms24043785
Chicago/Turabian StyleŁabędź, Wojciech, Anna Przybyla, Agnieszka Zimna, Mikołaj Dąbrowski, and Łukasz Kubaszewski. 2023. "The Role of Cytokines in the Metastasis of Solid Tumors to the Spine: Systematic Review" International Journal of Molecular Sciences 24, no. 4: 3785. https://doi.org/10.3390/ijms24043785





