Taurine and Creatine Transporters as Potential Drug Targets in Cancer Therapy
Abstract
1. Introduction
2. General Overview of Taurine and Creatine Transporters
| Transporter Group | Human Gene Name | Transporter Name | Main Substrates |
|---|---|---|---|
| GABA | SLC6A1 | GAT-1 | GABA |
| SLC6A6 | TauT | Taurine | |
| SLC6A8 | CT-1 | Creatine | |
| SLC6A10 | CT-2 | - | |
| SLC6A11 | GAT-3 | GABA | |
| SLC6A12 | BGT-1 | Betaine, GABA | |
| SLC6A13 | GAT-2 | GABA | |
| Monoamines | SLC6A2 | NET | Norepinephrine, dopamine |
| SLC6A3 | DAT | Dopamine | |
| SLC6A4 | SERT | Serotonin | |
| Neurotransmitter amino acids | SLC6A5 | GLYT-2 | Glycine |
| SLC6A7 | PROT | Proline | |
| SLC6A9 | GLYT-1 | Glycine | |
| SLC6A14 | ATB0,+ | Neutral, cationic amino acids | |
| Nutrient amino acids | SLC6A15 | B0AT2 | Neutral amino acids |
| SLC6A16 | NTT5 | Unknown | |
| SLC6A17 | NTT4 | Neutral amino acids | |
| SLC6A18 | B0AT3 | Neutral amino acids | |
| SLC6A19 | B0AT1 | Neutral amino acids | |
| SLC6A20 | SIT1 | Proline, pipecolate, sarcosine |
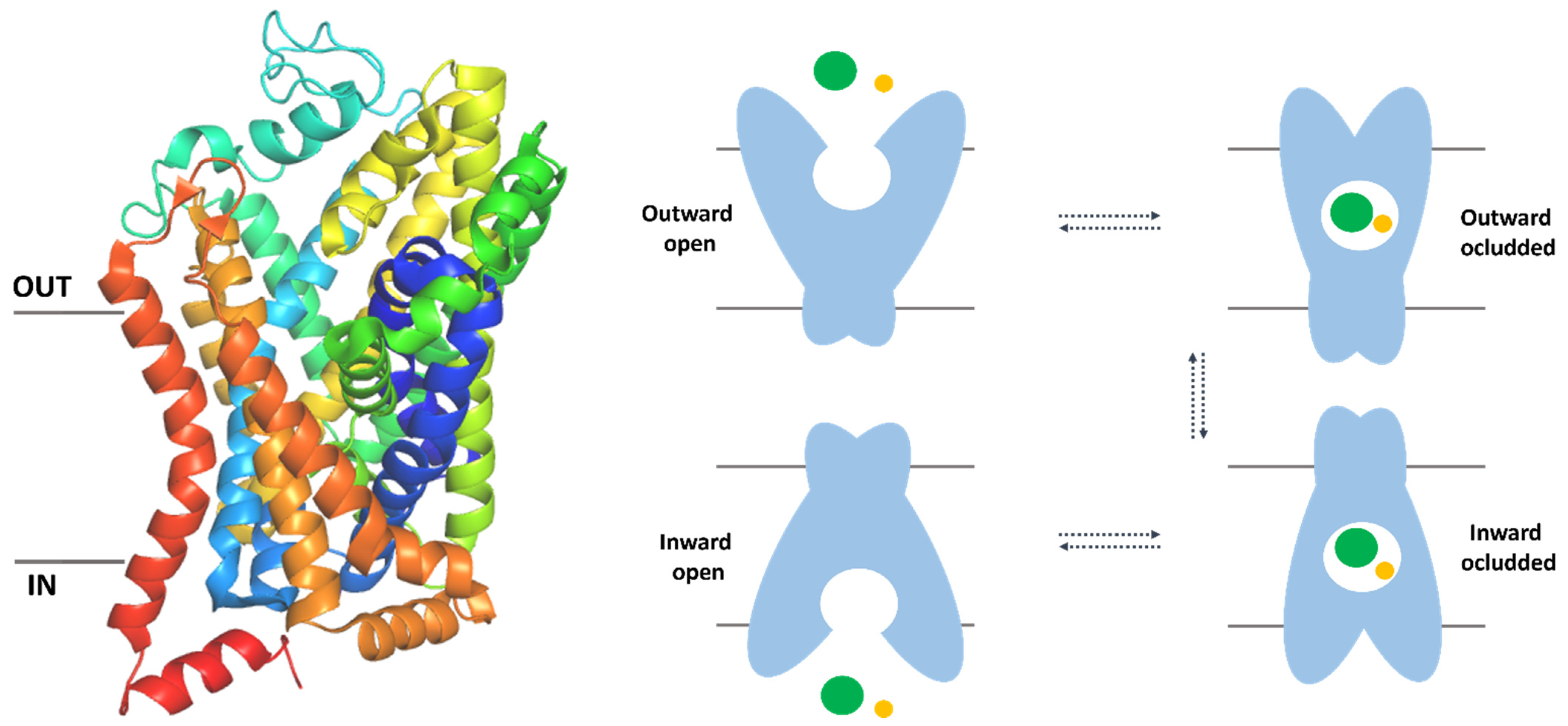
3. Clinical Significance of SLC6A6 and SLC6A8 Transporters
3.1. Taurine Transporter Overexpression in Cancer
3.2. Creatine Transporter Contribution in Cancer
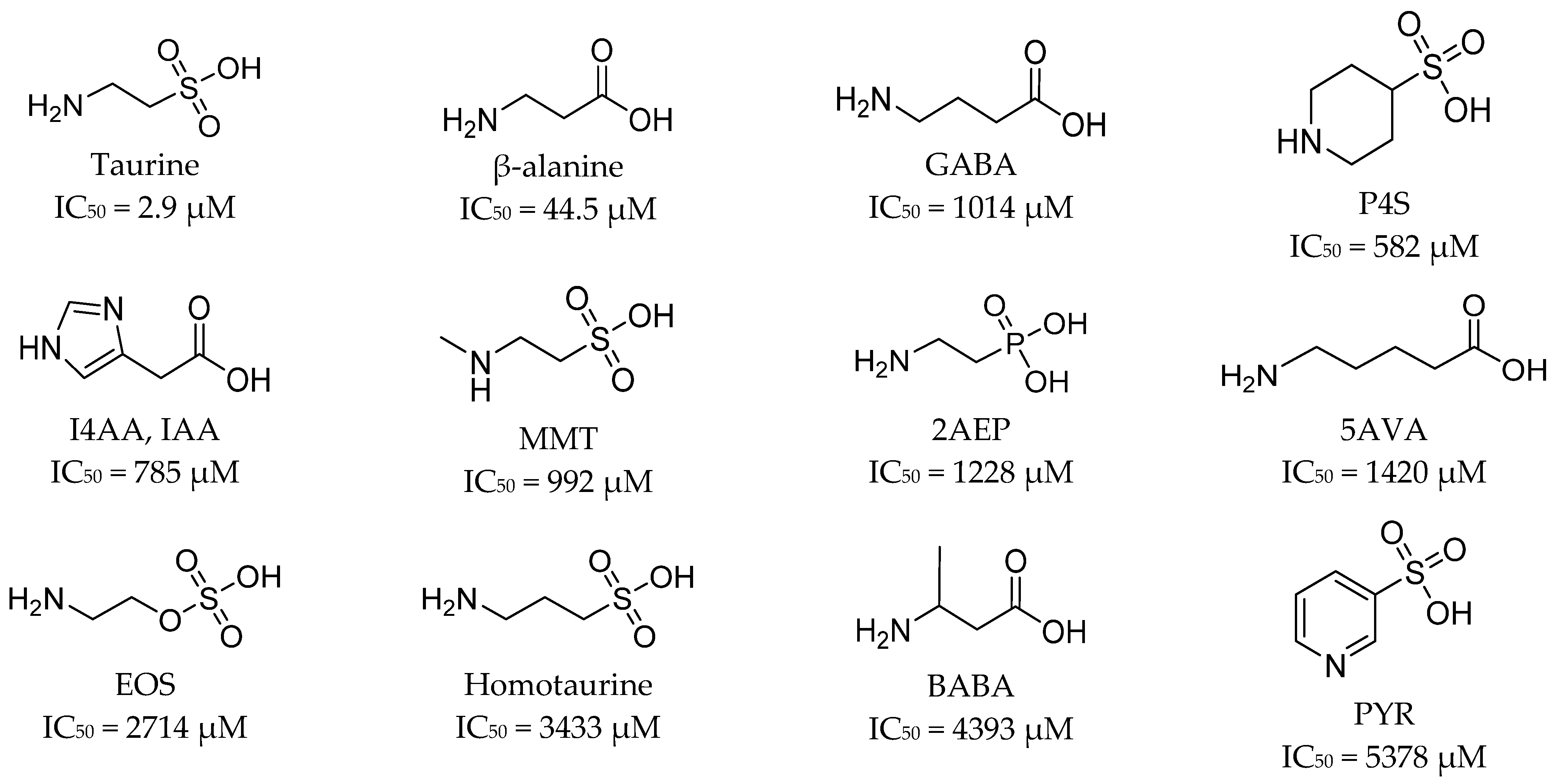
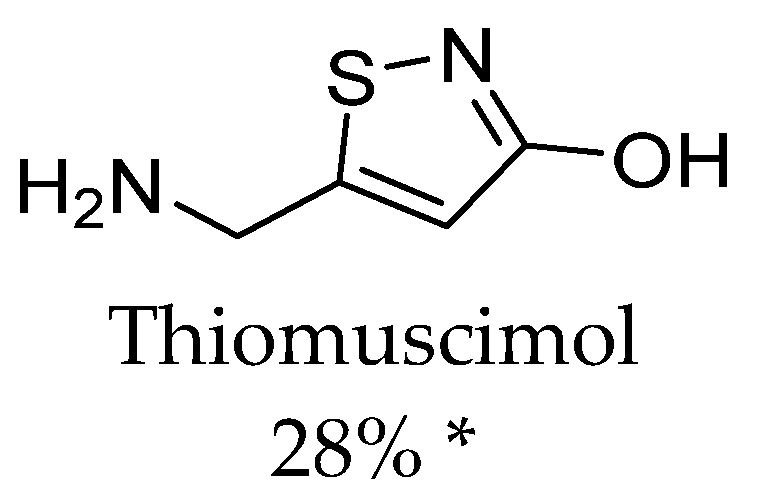
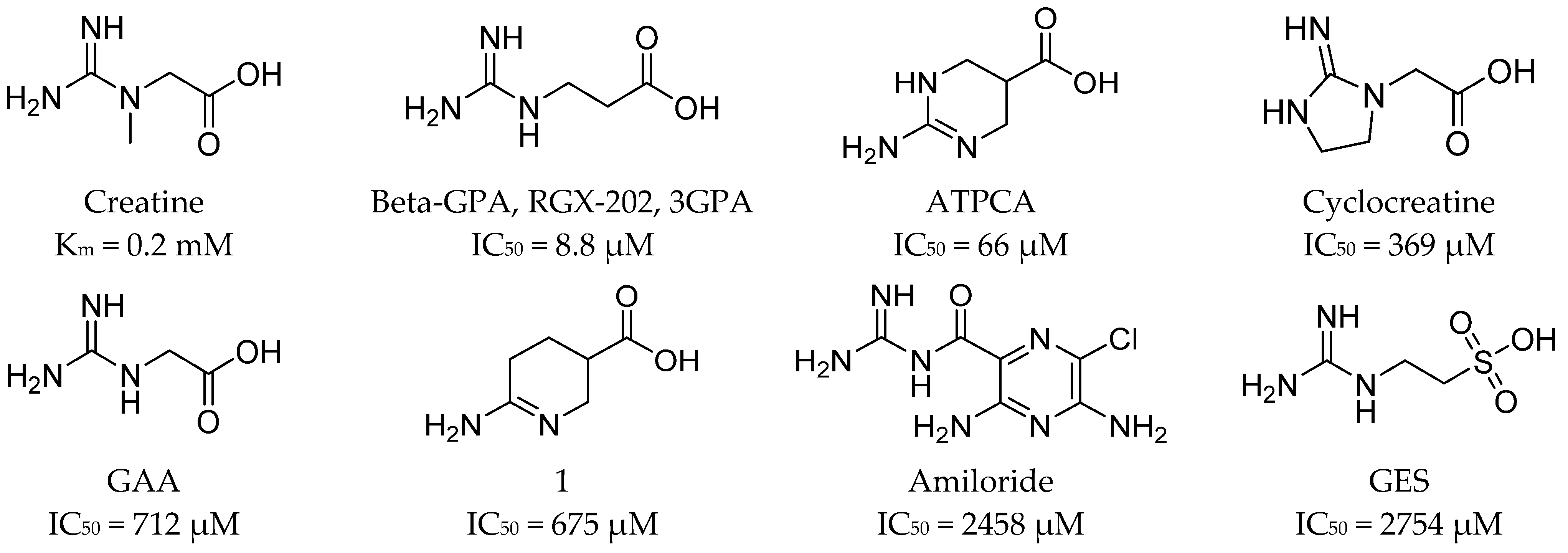
4. Inhibitors of Taurine and Creatine Transporters
4.1. Ligands of Taurine Transporter
4.2. Ligands of the Creatine Transporter
5. Experimental Data in the Development of Novel Ligands
5.1. The Overall Structure of SLC6 Family Transporters
5.2. Mutagenesis Studies on Taurine and Creatine Transporter
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO, International Agency Research on Cancer, Cancer Today. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf (accessed on 10 May 2022).
- Dembic, Z.; Editors, A.; Paveli, K.; Kraljevic Pavelic, S.; Duarte Ciceco, I.F. Antitumor Drugs and Their Targets. Molecules 2020, 25, 5776. [Google Scholar] [CrossRef]
- Enomoto, K.; Hotomi, M. Amino Acid Transporters as Potential Therapeutic Targets in Thyroid Cancer. Endocrinol. Metab. 2020, 35, 227–236. [Google Scholar] [CrossRef]
- Nałęcz, K.A. Amino Acid Transporter SLC6A14 (ATB0,+)—A Target in Combined Anti-cancer Therapy. Front. Cell Dev. Biol. 2020, 8, 594464. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Sheng, J.; Jia, J.; Wang, C.; Zhang, S.; Li, H.; He, F. Aberrant slc6a14 expression promotes proliferation and metastasis of colorectal cancer via enhancing the jak2/stat3 pathway. OncoTargets Ther. 2021, 14, 379–392. [Google Scholar] [CrossRef]
- Karunakaran, S.; Ramachandran, S.; Coothankandaswamy, V.; Elangovan, S.; Babu, E.; Periyasamy-Thandavan, S.; Gurav, A.; Gnanaprakasam, J.P.; Singh, N.; Schoenlein, P.V.; et al. SLC6A14 (ATB 0,+) protein, a highly concentrative and broad specific amino acid transporter, is a novel and effective drug target for treatment of estrogen receptor-positive breast cancer. J. Biol. Chem. 2011, 286, 31830–31838. [Google Scholar] [CrossRef] [PubMed]
- Coothankandaswamy, V.; Cao, S.; Xu, Y.; Prasad, P.D.; Singh, P.K.; Reynolds, C.P.; Yang, S.; Ogura, J.; Ganapathy, V.; Bhutia, Y.D. Amino acid transporter SLC6A14 is a novel and effective drug target for pancreatic cancer. Br. J. Pharmacol. 2016, 173, 3292–3306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bu, P. The two sides of creatine in cancer. Trends Cell Biol. 2022, 32, 380–390. [Google Scholar] [CrossRef]
- Baliou, S.; Kyriakopoulos, A.M.; Spandidos, D.A.; Zoumpourlis, V. Role of taurine, its haloamines and its lncRNA TUG1 in both inflammation and cancer progression. On the road to therapeutics? (Review). Int. J. Oncol. 2020, 57, 631–664. [Google Scholar] [CrossRef]
- A Study of RGX-202-01 as a Single Agent and as Combination Therapy in Patients with Advanced Gastrointestinal Malignancies—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://cancersearch.org/trial/NCT03597581/#.details (accessed on 28 April 2022).
- Pramod, A.B.; Foster, J.; Carvelli, L.; Henry, L.K. SLC6 transporters: Structure, function, regulation, disease association and therapeutics. Mol. Aspects Med. 2013, 34, 197–219. [Google Scholar] [CrossRef]
- Taslimifar, M.; Oparija, L.; Verrey, F.; Kurtcuoglu, V.; Olgac, U.; Makrides, V. Quantifying the relative contributions of different solute carriers to aggregate substrate transport. Sci. Rep. 2017, 7, 40628. [Google Scholar] [CrossRef]
- Rudnick, G.; Krämer, R.; Blakely, R.D.; Murphy, D.L.; Verrey, F. The SLC6 transporters: Perspectives on structure, functions, regulation, and models for transporter dysfunction. Pflugers Arch. Eur. J. Physiol. 2014, 466, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Saks, V.; Kaambre, T.; Guzun, R.; Anmann, T.; Sikk, P.; Schlattner, U.; Wallimann, T.; Aliev, M.; Vendelin, M. Creatine and Creatine Kinase in Health and Disease. Subcell. Biochem. 2007, 46, 27–65. Available online: http://www.researchgate.net/publication/240997256_The_Creatine_Kinase_Phosphotransfer_Network_Thermodynamic_andKinetic_Considerations_the_Impact_of_the_Mitochondrial_OuterMembrane_and_Modelling_Approaches (accessed on 18 May 2021). [PubMed]
- Coleman, J.A.; Green, E.M.; Gouaux, E. X-ray structures and mechanism of the human serotonin transporter. Nature 2016, 532, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Singh, S.K.; Kawate, T.; Jin, Y.; Gouaux, E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature 2005, 437, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, A.S.; Andersen, J.; Jorgensen, T.N.; Sorensen, L.; Eriksen, J.; Loland, C.J.; Stromgaard, K.; Gether, U. SLC6 neurotransmitter transporters: Structure, function, and regulation. Pharmacol. Rev. 2011, 63, 585–640. [Google Scholar] [CrossRef] [PubMed]
- Łątka, K.; Jończyk, J.; Bajda, M. γ-Aminobutyric acid transporters as relevant biological target: Their function, structure, inhibitors and role in the therapy of different diseases. Int. J. Biol. Macromol. 2020, 158, 750–772. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.H.; Reith, M.E.A.; Quick, M.W. Synaptic uptake and beyond: The sodium-and chloride-dependent neurotransmitter transporter family SLC6. Pflugers Arch.-Eur. J. Physiol. 2004, 447, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Ndika, J.D.; Lusink, V.; Beaubrun, C.; Kanhai, W.; Martinez-Munoz, C.; Jakobs, C.; Salomons, G.S. Salomons, Cloning and characterization of the promoter regions from the parent and paralogous creatine transporter genes. Gene 2014, 533, 488–493. [Google Scholar] [CrossRef]
- Wang, K.H.; Penmatsa, A.; Gouaux, E. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature 2015, 521, 322–327. [Google Scholar] [CrossRef]
- OMIM Entry—* 186854—Solute Carrier Family 6 (Neurotransmitter Transporter, Taurine), Member 6; SLC6A6, (n.d.). Available online: https://www.omim.org/entry/186854?search=slc6a6&highlight=slc6a6 (accessed on 19 January 2022).
- OMIM Entry—* 300036—Solute Carrier Family 6 (Neurotransmitter Transporter, Creatine), Member 8; SLC6A8, (n.d.). Available online: https://www.omim.org/entry/300036?search=slc6a8&highlight=slc6a8 (accessed on 19 January 2022).
- SLC6A6—Sodium- and Chloride-Dependent Taurine Transporter—Homo Sapiens (Human)—SLC6A6 Gene & Protein, (n.d.). Available online: https://www.uniprot.org/uniprot/P31641 (accessed on 14 October 2021).
- SLC6A8—Sodium- and Chloride-Dependent Creatine Transporter 1—Homo Sapiens (Human)—SLC6A8 Gene & Protein, (n.d.). Available online: https://www.uniprot.org/uniprot/P48029 (accessed on 18 May 2021).
- Marcinkiewicz, J.; Kontny, E. Taurine and inflammatory diseases. Amino Acids. 2014, 46, 7. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.F. The Continuing Importance of Bile Acids in Liver and Intestinal Disease. Arch. Intern. Med. 1999, 159, 2647–2658. [Google Scholar] [CrossRef]
- Chen, W.Q.; Jin, H.; Nguyen, M.; Carr, J.; Lee, Y.J.; Hsu, C.C.; Faiman, M.D.; Schloss, J.V.; Wu, J.Y. Role of taurine in regulation of intracellular calcium level and neuroprotective function in cultured neurons. J. Neurosci. Res. 2001, 66, 612–619. [Google Scholar] [CrossRef]
- Chen, C.; Xia, S.F.; He, J.; Lu, G.; Xie, Z.; Han, H. Roles of taurine in cognitive function of physiology, pathologies and toxication. Life Sci. 2019, 231, 116584. [Google Scholar] [CrossRef]
- Neuwirth, L.S.; Volpe, N.P.; Corwin, C.; Ng, S.; Madan, N.; Ferraro, A.M.; Furman, Y.; El Idrissi, A. Taurine recovery of learning deficits induced by developmental Pb2+ exposure. Adv. Exp. Med. Biol. 2017, 975, 39–55. [Google Scholar] [CrossRef]
- Baliou, S.; Kyriakopoulos, A.M.; Goulielmaki, M.; Panayiotidis, M.I.; Spandidos, D.A.; Zoumpourlis, V. Significance of taurine transporter (TauT) in homeostasis and its layers of regulation (review). Mol. Med. Rep. 2020, 22, 2163–2173. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef]
- Balestrino, M. Role of Creatine in the Heart: Health and Disease. Nutrients 2021, 13, 1215. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Leibach, F.H.; Mahesh, V.B.; Han, H.; Yang-Feng, T.; Blakely, R.D.; Ganapathy, V. Functional characterization and chromosomal localization of a cloned taurine transporter from human placenta. Biochem. J. 1994, 300, 893–900. [Google Scholar] [CrossRef]
- Schuller-Levis, G.B.; Park, E. Taurine: New implications for an old amino acid. FEMS Microbiol. Lett. 2003, 226, 195–202. [Google Scholar] [CrossRef]
- Janeke, G.; Siefken, W.; Carstensen, S.; Springmann, G.; Bleck, O.; Steinhart, H.; Höger, P.; Wittern, K.P.; Wenck, H.; Stäb, F.; et al. Role of Taurine Accumulation in Keratinocyte Hydration. J. Investig. Dermatol. 2003, 121, 354–361. [Google Scholar] [CrossRef]
- Lowe, M.T.J.; Faull, R.L.M.; Christie, D.L.; Waldvogel, H.J. Distribution of the creatine transporter throughout the human brain reveals a spectrum of creatine transporter immunoreactivity. J. Comp. Neurol. 2015, 523, 699–725. [Google Scholar] [CrossRef]
- Ansar, M.; Ranza, E.; Shetty, M.; Paracha, S.A.; Azam, M.; Kern, I.; Iwaszkiewicz, J.; Farooq, O.; Pournaras, C.J.; Malcles, A.; et al. Taurine treatment of retinal degeneration and cardiomyopathy in a consanguineous family with SLC6A6 taurine transporter deficiency. Hum. Mol. Genet. 2020, 29, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Preising, M.N.; Görg, B.; Friedburg, C.; Qvartskhava, N.; Budde, B.S.; Bonus, M.; Toliat, M.R.; Pfleger, C.; Altmüller, J.; Herebian, D.; et al. Biallelic mutation of human SLC6A6 encoding the taurine transporter TAUT is linked to early retinal degeneration. FASEB J. 2019, 33, 11507–11527. [Google Scholar] [CrossRef]
- Garnier, S.; Harakalova, M.; Weiss, S.; Mokry, M.; Regitz-Zagrosek, V.; Hengstenberg, C.; Cappola, T.P.; Isnard, R.; Arbustini, E.; Cook, S.A.; et al. Genome-wide association analysis in dilated cardiomyopathy reveals two new players in systolic heart failure on chromosomes 3p25.1 and 22q11.23. Eur. Heart J. 2021, 42, 2000–2011. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Oishi, S.; Takai, M.; Kimura, Y.; Uozumi, Y.; Fujio, Y.; Schaffer, S.W.; Azuma, J. Cardiac and skeletal muscle abnormality in taurine transporter-knockout mice. J. Biomed. Sci. 2010, 17, S20. [Google Scholar] [CrossRef] [PubMed]
- Mele, A.; Mantuano, P.; De Bellis, M.; Rana, F.; Sanarica, F.; Conte, E.; Morgese, M.G.; Bove, M.; Rolland, J.F.; Capogrosso, R.F.; et al. A long-term treatment with taurine prevents cardiac dysfunction in mdx mice. Transl. Res. 2019, 204, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Farr, C.V.; El-Kasaby, A.; Freissmuth, M.; Sucic, S. The Creatine Transporter Unfolded: A Knotty Premise in the Cerebral Creatine Deficiency Syndrome. Front. Synaptic Neurosci. 2020, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Ghirardini, E.; Calugi, F.; Sagona, G.; Di Vetta, F.; Palma, M.; Battini, R.; Cioni, G.; Pizzorusso, T.; Baroncelli, L. The Role of Preclinical Models in Creatine Transporter Deficiency: Neurobiological Mechanisms, Biomarkers and Therapeutic Development. Genes 2021, 12, 1123. [Google Scholar] [CrossRef]
- Duran-Trio, L.; Fernandes-Pires, G.; Simicic, D.; Grosse, J.; Roux-Petronelli, C.; Bruce, S.J.; Binz, P.A.; Sandi, C.; Cudalbu, C.; Braissant, O. A new rat model of creatine transporter deficiency reveals behavioral disorder and altered brain metabolism. Sci. Rep. 2021, 11, 1636. [Google Scholar] [CrossRef]
- Uemura, T.; Ito, S.; Masuda, T.; Shimbo, H.; Goto, T.; Osaka, H.; Wada, T.; Couraud, P.O.; Ohtsuki, S. Cyclocreatine Transport by SLC6A8, the Creatine Transporter, in HEK293 Cells, a Human Blood-Brain Barrier Model Cell, and CCDSs Patient-Derived Fibroblasts. Pharm. Res. 2020, 37, 61. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.H.T.; Lee, J.S.; Murphy, E.M.; Gerich, M.E.; Dran, R.; Glover, L.E.; Abdulla, Z.I.; Skelton, M.R.; Colgan, S.P. Creatine Transporter, Reduced in Colon Tissues From Patients With Inflammatory Bowel Diseases, Regulates Energy Balance in Intestinal Epithelial Cells, Epithelial Integrity, and Barrier Function. Gastroenterology 2020, 159, 984–998.e1. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Du, J.; Ren, C.; Zhou, M.; Xia, Z. Elevated SLC6A6 expression drives tumorigenesis and affects clinical outcomes in gastric cancer. Biomark. Med. 2019, 13, 95–104. [Google Scholar] [CrossRef]
- Yasunaga, M.; Matsumura, Y. Role of SLC6A6 in promoting the survival and multidrug resistance of colorectal cancer. Sci. Rep. 2014, 4, 4852. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Guo, X.; Tang, H. SLC6A8 is involved in the progression of non-small cell lung cancer through the Notch signaling pathway. Ann. Transl. Med. 2021, 9, 264. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhou, Y.; Lou, M.; Gao, Z.; Li, X.; Yuan, K. SLC6A8 is a Potential Biomarker for Poor Prognosis in Lung Adenocarcinoma. Front. Genet. 2022, 13, 845373. [Google Scholar] [CrossRef]
- Jordan, C.T.; Guzman, M.L.; Noble, M. Cancer stem cells. N. Engl. J. Med. 2006, 355, 1253–1261. [Google Scholar] [CrossRef]
- Xia, Y.F.; Pei, G.H.; Wang, N.; Che, Y.C.; Yu, F.S.; Yin, F.F.; Liu, H.X.; Luo, B.; Wang, Y.K. miR-3156-3p is downregulated in HPV-positive cervical cancer and performs as a tumor-suppressive miRNA, (n.d.). Virol. J. 2017, 14, 20. [Google Scholar] [CrossRef]
- Satofuka, H.; Kensuke, O.H.S.E.; Mukobata, S.; Akiyama, H.; Ohtsu, M.; Okabe, Y.; Murakami, Y. Therapeutic Pharmaceutical Composition Employing Anti—SLC6A6 Antibody. U.S. Patent 10,214,584, 26 February 2019. [Google Scholar]
- Li, Q.; Liu, M.; Sun, Y.; Jin, T.; Zhu, P.; Wan, X.; Hou, Y.; Tu, G. SLC6A8-mediated intracellular creatine accumulation enhances hypoxic breast cancer cell survival via ameliorating oxidative stress. J. Exp. Clin. Cancer Res. 2021, 40, 168. [Google Scholar] [CrossRef]
- Yuan, L.; Wu, X.J.; Li, W.C.; Zhuo, C.; Xu, Z.; Tan, C.; Ma, R.; Wang, J.; Pu, J. SLC6A8 Knockdown Suppresses the Invasion and Migration of Human Hepatocellular Carcinoma Huh-7 and Hep3B Cells, (n.d.). Technol. Cancer Res. Treat. 2020, 19, 1533033820983029. [Google Scholar] [CrossRef]
- Kurth, I.; Yamaguchi, N.; Andreu-Agullo, C.; Tian, H.S.; Sridhar, S.; Takeda, S.; Gonsalves, F.C.; Loo, J.M.; Barlas, A.; Manova-Todorova, K.; et al. Therapeutic targeting of SLC6A8 creatine transporter suppresses colon cancer progression and modulates human creatine levels. Sci. Adv. 2021, 7, eabi7511. [Google Scholar] [CrossRef]
- Gupta, G.K.; Collier, A.L.; Lee, D.; Hoefer, R.A.; Zheleva, V.; van Reesema, L.L.S.; Tang-Tan, A.M.; Guye, M.L.; Chang, D.Z.; Winston, J.S.; et al. Perspectives on Triple-Negative Breast Cancer: Current Treatment Strategies, Unmet Needs, and Potential Targets for Future Therapies. Cancers 2020, 12, 2392. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Bae, J.S. ROS homeostasis and metabolism: A critical liaison for cancer therapy. Exp. Mol. Med. 2016, 48, e269. [Google Scholar] [CrossRef]
- A Study of RGX-202-01 as Combination Therapy in 2nd Line RAS Mutant Advanced Colorectal Cancer—Full Text View—ClinicalTrials.gov, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT03597581 (accessed on 14 September 2022).
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef]
- Fitch, C.D.; Shields, R.P.; Payne, W.F.; Dacus, J.M. Creatine Metabolism in Skeletal Muscle. J. Biol. Chem. 1966, 243, 2024–2027. [Google Scholar] [CrossRef]
- Oja, S.S.; Kontro, P.; Lähdesmäki, P. Transport of taurine in the central nervous system. Adv. Exp. Med. Biol. 1976, 69, 237–252. [Google Scholar] [CrossRef]
- Huxtable, R.J.; Laird, H.E.; Lippincott, S.E. The transport of taurine in the heart and the rapid depletion of tissue taurine content by guanidinoethyl sulfonate. J. Pharmacol. Exp. Ther. 1979, 211, 465–471. [Google Scholar]
- Quesada, O.; Huxtable, R.J.; Pasantes-Morales, H. Effect of guanidinoethane sulfonate on taurine uptake by rat retina. J. Neurosci. Res. 1984, 11, 179–186. [Google Scholar] [CrossRef]
- Richter, M.; Moroniak, S.J.; Michel, H. Identification of competitive inhibitors of the human taurine transporter TauT in a human kidney cell line. Pharm. Rep. 2019, 71, 121–127. [Google Scholar] [CrossRef]
- Rasmussen, R.N.; Lagunas, C.; Plum, J.; Holm, R.; Nielsen, C.U. Interaction of GABA-mimetics with the taurine transporter (TauT, Slc6a6) in hyperosmotic treated Caco-2, LLC-PK1 and rat renal SKPT cells. Eur. J. Pharm. Sci. 2016, 82, 138–146. [Google Scholar] [CrossRef]
- Valembois, S.; Krall, J.; Frølund, B.; Steffansen, B. Imidazole-4-acetic acid, a new lead structure for interaction with the taurine transporter in outer blood-retinal barrier cells. Eur. J. Pharm. Sci. 2017, 103, 77–84. [Google Scholar] [CrossRef]
- Kubo, Y.; Ishizuka, S.; Ito, T.; Yoneyama, D.; Akanuma, S.I.; Hosoya, K.I. Involvement of TauT/SLC6A6 in Taurine Transport at the Blood–Testis Barrier. Metabolites 2022, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Vinnakota, S.; Qian, X.; Kunze, D.L.; Sarkar, H.K. Molecular characterization of the human CRT-1 creatine transporter expressed in Xenopus oocytes. Arch. Biochem. Biophys. 1999, 361, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.J.; Tavazoie, S.F. Inhibitors of Creatine Transport and Uses Thereof. U.S. Patent 10,308,597, 4 June 2019. [Google Scholar]
- Al-Khawaja, A.; Haugaard, A.S.; Marek, A.; Löffler, R.; Thiesen, L.; Santiveri, M.; Damgaard, M.; Bundgaard, C.; Frølund, B.; Wellendorph, P. Pharmacological Characterization of [3H]ATPCA as a Substrate for Studying the Functional Role of the Betaine/GABA Transporter 1 and the Creatine Transporter. ACS Chem. Neurosci. 2018, 9, 545–554. [Google Scholar] [CrossRef]
- Dodd, J.R.; Birch, N.P.; Waldvogel, H.J.; Christie, D.L. Functional and immunocytochemical characterization of the creatine transporter in rat hippocampal neurons. J. Neurochem. 2010, 115, 684–693. [Google Scholar] [CrossRef]
- Nolan, T.L.; Geffert, L.M.; Kolber, B.J.; Madura, J.D.; Surratt, C.K. Discovery of Novel-scaffold monoamine transporter ligands via in silico screening with the S1 pocket of the serotonin transporter. ACS Chem. Neurosci. 2014, 5, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Bateman, A. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Beuming, T.; Shi, L.; Javitch, J.A.; Weinstein, H. A comprehensive structure-based alignment of prokaryotic and eukaryotic neurotransmitter/Na+ symporters (NSS) aids in the use of the LeuT structure to probe NSS structure and function. Mol. Pharmacol. 2006, 70, 1630–1642. [Google Scholar] [CrossRef]
- Łątka, K.; Jończyk, J.; Bajda, M. Structure modeling of γ-aminobutyric acid transporters—Molecular basics of ligand selectivity. Int. J. Biol. Macromol. 2020, 158, 1380–1389. [Google Scholar] [CrossRef]
- Colas, C.; Banci, G.; Martini, R.; Ecker, G.F. Studies of structural determinants of substrate binding in the Creatine Transporter (CreaT, SLC6A8) using molecular models. Sci. Rep. 2020, 10, 6241. [Google Scholar] [CrossRef] [PubMed]
- Joseph, D.; Pidathala, S.; Mallela, A.K.; Penmatsa, A. Structure and Gating Dynamics of Na+/Cl− Coupled Neurotransmitter Transporters. Front. Mol. Biosci. 2019, 6, 80. [Google Scholar] [CrossRef]
- Yahara, T.; Tachikawa, M.; Akanuma, S.I.; Kubo, Y.; Hosoya, K.I. Amino acid residues involved in the substrate specificity of TauT/SLC6A6 for taurine and γ-aminobutyric acid. Biol. Pharm. Bull. 2014, 37, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, L.; Paravicini, C.; Laurenzi, T.; Adobati, S.; Saporiti, S.; Guerrini, U.; Gianazza, E.; Indiveri, C.; Anderson, C.M.H.; Thwaites, D.T.; et al. SLC6A14, a Pivotal Actor on Cancer Stage: When Function Meets Structure. SLAS Discov. 2019, 24, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Kantcheva, A.K.; Quick, M.; Shi, L.; Winther, A.M.L.; Stolzenberg, S.; Weinstein, H.; Javitch, J.A.; Nissen, P. Chloride binding site of neurotransmitter sodium symporters. Proc. Natl. Acad. Sci. USA 2013, 110, 8489–8494. [Google Scholar] [CrossRef] [PubMed]
- Kroncke, B.M.; Horanyi, P.S.; Columbus, L. Structural origins of nitroxide side chain dynamics on membrane protein α-helical sites. Biochemistry 2010, 49, 10045–10060. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, C.L.; Gouaux, E. Insights into transport mechanism from LeuT engineered to transport tryptophan. EMBO J. 2012, 31, 228–235. [Google Scholar] [CrossRef]
- Wang, H.; Gouaux, E. Substrate binds in the S1 site of the F253A mutant of LeuT, a neurotransmitter sodium symporter homologue. EMBO Rep. 2012, 13, 861–866. [Google Scholar] [CrossRef]
- Singh, S.K.; Yamashita, A.; Gouaux, E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters, (n.d.). Nature 2007, 448, 952–956. [Google Scholar] [CrossRef]
- Krishnamurthy, H.; Gouaux, E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature 2012, 481, 469–474. [Google Scholar] [CrossRef]
- Wang, H.; Goehring, A.; Wang, K.H.; Penmatsa, A.; Ressler, R.; Gouaux, E. Structural basis for action by diverse antidepressants on biogenic amine transporters. Nature 2013, 503, 141–145. [Google Scholar] [CrossRef]
- Malinauskaite, L.; Said, S.; Sahin, C.; Grouleff, J.; Shahsavar, A.; Bjerregaard, H.; Noer, P.; Severinsen, K.; Boesen, T.; Schiøtt, B.; et al. A conserved leucine occupies the empty substrate site of LeuT in the Na+-free return state. Nat. Commun. 2016, 7, 11673. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhen, J.; Karpowich, N.K.; Law, C.J.; Reith, M.E.A.; Wang, D.N. Antidepressant specificity of serotonin transporter suggested by three LeuT-SSRI structures. Nat. Struct. Mol. Biol. 2009, 16, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Elferich, J.; Gouaux, E. Structures of LeuT in bicelles define conformation and substrate binding in a membrane-like context. Nat. Struct. Mol. Biol. 2012, 19, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Gotfryd, K.; Boesen, T.; Mortensen, J.S.; Khelashvili, G.; Quick, M.; Terry, D.S.; Missel, J.W.; LeVine, M.V.; Gourdon, P.; Blanchard, S.C.; et al. X-ray structure of LeuT in an inward-facing occluded conformation reveals mechanism of substrate release. Nat. Commun. 2020, 11, 1005. [Google Scholar] [CrossRef] [PubMed]
- Quick, M.; Winther, A.M.L.; Shi, L.; Nissen, P.; Weinstein, H.; Javitch, J.A. Binding of an octylglucoside detergent molecule in the second substrate (S2) site of LeuT establishes an inhibitor-bound conformation. Proc. Natl. Acad. Sci. USA 2009, 106, 5563–5568. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.G.; Shekar, A.; Aguilar, J.I.; Peng, D.; Navratna, V.; Yang, D.; Morley, A.N.; Duran, A.M.; Galli, G.; O’grady, B.; et al. Structural, functional, and behavioral insights of dopamine dysfunction revealed by a deletion in SLC6A3. Proc. Natl. Acad. Sci. USA 2019, 116, 3853–3862. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhen, J.; Karpowich, N.K.; Goetz, R.M.; Law, C.J.; Reith, M.E.A.; Wang, D.N. LeuT-desipramine structure reveals how antidepressants block neurotransmitter reuptake. Science 2007, 317, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Piscitelli, C.L.; Yamashita, A.; Gouaux, E. A competitive inhibitor traps LeuT in an open-to-out conformation. Science 2008, 322, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.I.; Cheng, M.H.; Font, J.; Schwartz, A.C.; Ledwitch, K.; Duran, A.; Mabry, S.J.; Belovich, A.N.; Zhu, Y.; Carter, A.M.; et al. Psychomotor impairments and therapeutic implications revealed by a mutation associated with infantile Parkinsonism-Dystonia. Elife 2021, 10, e68039. [Google Scholar] [CrossRef]
- Penmatsa, A.; Wang, K.H.; Gouaux, E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature 2013, 503, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Penmatsa, A.; Wang, K.H.; Gouaux, E. X-ray structures of Drosophila dopamine transporter in complex with nisoxetine and reboxetine. Nat. Struct. Mol. Biol. 2015, 22, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Pidathala, S.; Mallela, A.K.; Joseph, D.; Penmatsa, A. Structural basis of norepinephrine recognition and transport inhibition in neurotransmitter transporters. Nat. Commun. 2021, 12, 2199. [Google Scholar] [CrossRef] [PubMed]
- Plenge, P.; Yang, D.; Salomon, K.; Laursen, L.; Kalenderoglou, I.E.; Newman, A.H.; Gouaux, E.; Coleman, J.A.; Loland, C.J. The antidepressant drug vilazodone is an allosteric inhibitor of the serotonin transporter. Nat. Commun. 2021, 12, 5063. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.A.; Yang, D.; Zhao, Z.; Wen, P.C.; Yoshioka, C.; Tajkhorshid, E.; Gouaux, E. Serotonin transporter–ibogaine complexes illuminate mechanisms of inhibition and transport. Nature 2019, 569, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.A.; Gouaux, E. Structural basis for recognition of diverse antidepressants by the human serotonin transporter. Nat. Struct. Mol. Biol. 2018, 25, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.A.; Navratna, V.; Antermite, D.; Yang, D.; Bull, J.A.; Gouaux, E. Chemical and structural investigation of the paroxetine-human serotonin transporter complex. Elife 2020, 9, e56427. [Google Scholar] [CrossRef] [PubMed]
- Shahsavar, A.; Stohler, P.; Bourenkov, G.; Zimmermann, I.; Siegrist, M.; Guba, W.; Pinard, E.; Sinning, S.; Seeger, M.A.; Schneider, T.R.; et al. Structural insights into the inhibition of glycine reuptake. Nature 2021, 591, 677–681. [Google Scholar] [CrossRef]
- Motiwala, Z.; Aduri, N.G.; Shaye, H.; Han, G.W.; Lam, J.H.; Katritch, V.; Cherezov, V.; Gati, C. Structural basis of GABA reuptake inhibition. Nature 2022, 606, 820–826. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Dodd, J.R.; Christie, D.L. Selective Amino Acid Substitutions Convert the Creatine Transporter to a γ-Aminobutyric Acid Transporter. J. Biol. Chem. 2007, 282, 15528–15533. [Google Scholar] [CrossRef] [PubMed]
- Dodd, J.R.; Christie, D.L. Cysteine 144 in the Third Transmembrane Domain of the Creatine Transporter Is Located Close to a Substrate-binding Site. J. Biol. Chem. 2001, 276, 46983–46988. [Google Scholar] [CrossRef] [PubMed]
- Backwell, L.; Marsh, J.A. Diverse Molecular Mechanisms Underlying Pathogenic Protein Mutations: Beyond the Loss-of-Function Paradigm. Annu. Rev. Genom. Hum. Genet. 2022, 23, 475–498. [Google Scholar] [CrossRef] [PubMed]
- Tomi, M.; Tajima, A.; Tachikawa, M.; Hosoya, K. Function of taurine transporter (Slc6a6/TauT) as a GABA transporting protein and its relevance to GABA transport in rat retinal capillary endothelial cells. Biochim. Biophys. Acta-Biomembr. 2008, 1778, 2138–2142. [Google Scholar] [CrossRef]



| Gene Name | Transporter Name | Type of Cancer | Ref. |
|---|---|---|---|
| SLC6A6 | Taurine transporter | Gastric cancer | [48] |
| Colorectal cancer (CRC) | [49] | ||
| Cervical cancer (CC) | [53] | ||
| SLC6A8 | Creatine transporter | Triple-negative breast cancer (TNBC) | [55] |
| Colorectal cancer (CRC) | [57] | ||
| Non-small cell lung cancer (NSCLC) | [50] | ||
| Lung adenocarcinoma (LUAD) | [51] | ||
| Hepatocellular cancer | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stary, D.; Bajda, M. Taurine and Creatine Transporters as Potential Drug Targets in Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 3788. https://doi.org/10.3390/ijms24043788
Stary D, Bajda M. Taurine and Creatine Transporters as Potential Drug Targets in Cancer Therapy. International Journal of Molecular Sciences. 2023; 24(4):3788. https://doi.org/10.3390/ijms24043788
Chicago/Turabian StyleStary, Dorota, and Marek Bajda. 2023. "Taurine and Creatine Transporters as Potential Drug Targets in Cancer Therapy" International Journal of Molecular Sciences 24, no. 4: 3788. https://doi.org/10.3390/ijms24043788
APA StyleStary, D., & Bajda, M. (2023). Taurine and Creatine Transporters as Potential Drug Targets in Cancer Therapy. International Journal of Molecular Sciences, 24(4), 3788. https://doi.org/10.3390/ijms24043788







