Dual Role of Interleukin-20 in Different Stages of Osteoclast Differentiation and Its Osteoimmune Regulation during Alveolar Bone Remodeling
Abstract
1. Introduction
2. Results
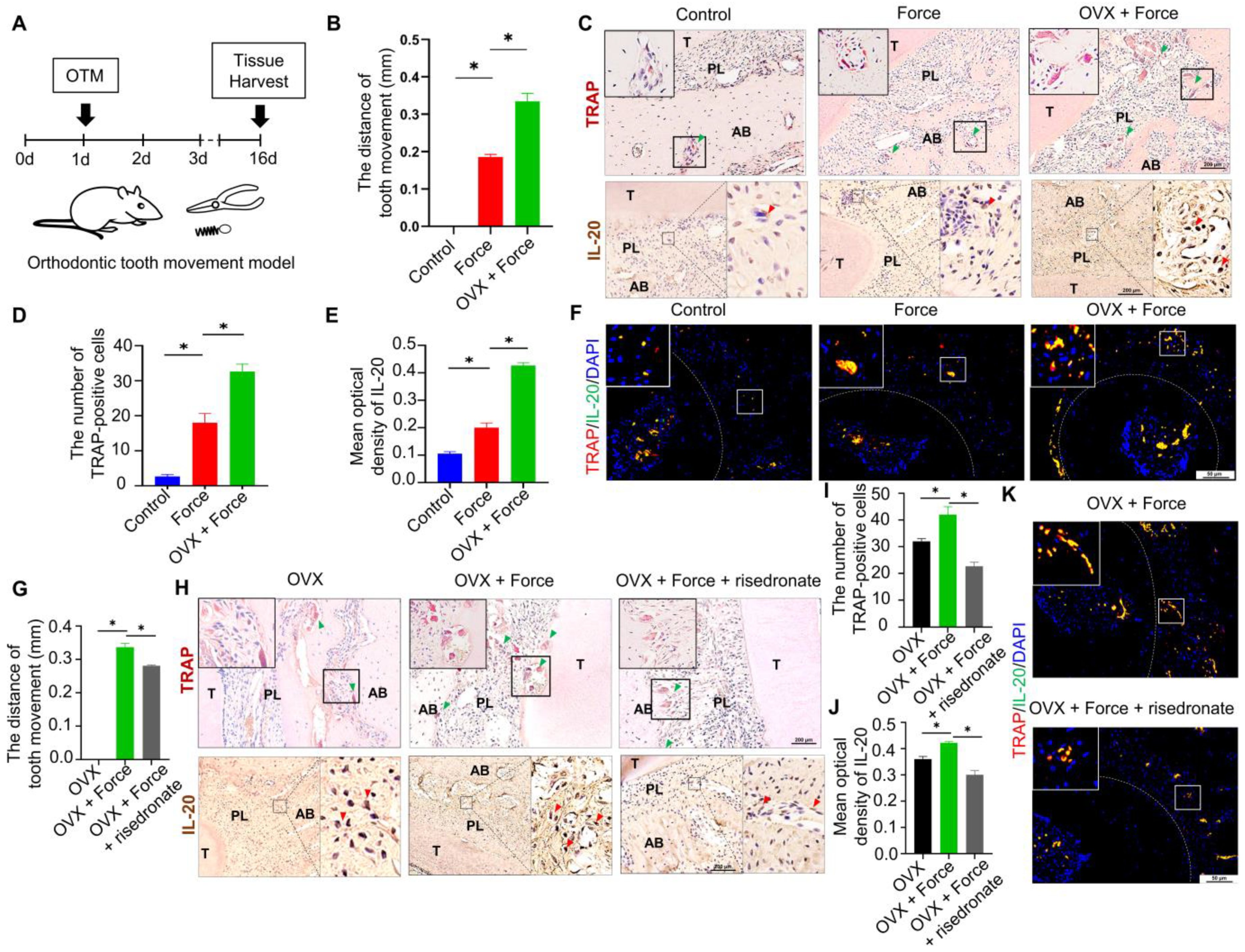
2.1. Osteoclasts and IL-20 Were Synchronously Activated in OTM
2.2. IL-20 Promoted Preosteoclast Proliferation by MAPK Pathways
2.3. IL-20 Had No Effect on Osteoclasts Differentiation and Functions at the Early Stage of Osteoclast Differentiation
2.4. IL-20 Promoted Osteoclasts Differentiation and Functions at the Late Stage of Osteoclast Differentiation
2.5. IL-20 Promoted Osteoclast Differentiation through the NF-κB Pathway
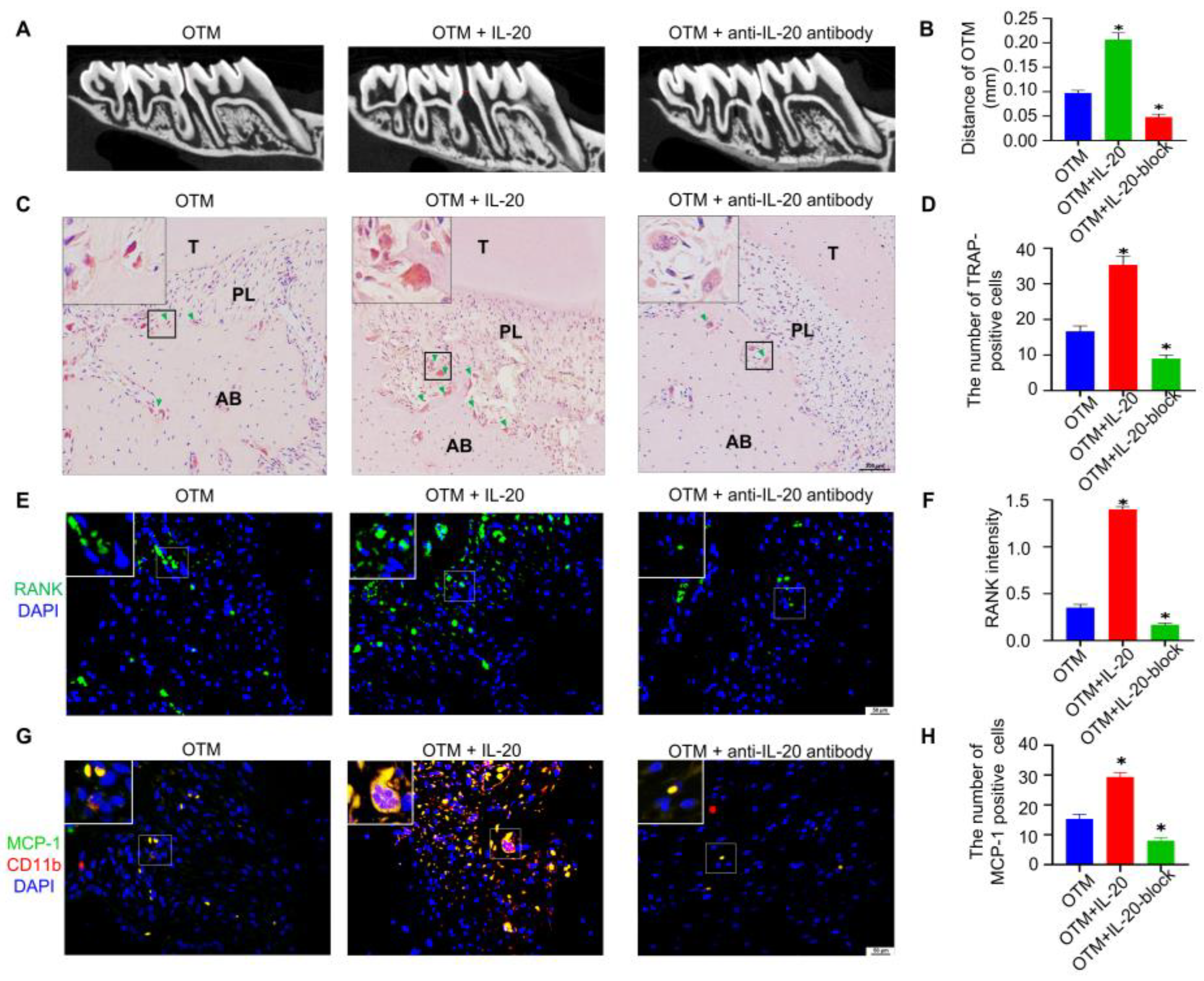
2.6. Exogenous Injection of IL-20 Accelerated Tooth Movement in OTM
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. The Rat Model of Orthodontic Tooth Movement and Ovariectomy
4.3. Immunohistochemistry and TRAP Staining In Vivo
4.4. Osteoclastogenesis Assays In Vitro
4.5. TRAP Staining In Vitro
4.6. Resorption Pit Assay
4.7. Cellular Immunochemistry and Immunofluorescence Staining
4.8. Cell Viability and Cytotoxicity Assay
4.9. Flow Cytometry Detection of Cell Apoptosis
4.10. qRT-PCR Analysis
4.11. Western Blotting Analysis
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okamoto, K.; Nakashima, T.; Shinohara, M.; Negishi-Koga, T.; Komatsu, N.; Terashima, A.; Sawa, S.; Nitta, T.; Takayanagi, H. Osteoimmunology: The Conceptual Framework Unifying the Immune and Skeletal Systems. Physiol. Rev. 2017, 97, 1295–1349. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C.; Jilka, R.L. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N. Engl. J. Med. 1995, 332, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Redlich, K.; Smolen, J.S. Inflammatory bone loss: Pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012, 11, 234–250. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Ofotokun, I. Physiological and pathophysiological bone turnover—Role of the immune system. Nat. Rev. Endocrinol. 2016, 12, 518–532. [Google Scholar] [CrossRef] [PubMed]
- Arron, J.R.; Choi, Y. Bone versus immune system. Nature 2000, 408, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Ogasawara, K.; Hida, S.; Chiba, T.; Murata, S.; Sato, K.; Takaoka, A.; Yokochi, T.; Oda, H.; Tanaka, K.; et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature 2000, 408, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef]
- Kim, K.-W.; Kim, H.-R.; Park, J.-Y.; Oh, H.-J.; Woo, Y.-J.; Park, M.-K.; Cho, M.-L.; Lee, S.-H. Interleukin-22 promotes osteoclastogenesis in rheumatoid arthritis through induction of RANKL in human synovial fibroblasts. Arthritis Rheum. 2011, 64, 1015–1023. [Google Scholar] [CrossRef]
- Schulze, J.; Bickert, T.; Beil, F.T.; Zaiss, M.M.; Albers, J.; Wintges, K.; Streichert, T.; Klaetschke, K.; Keller, J.; Hissnauer, T.-N.; et al. Interleukin-33 is expressed in differentiated osteoblasts and blocks osteoclast formation from bone marrow precursor cells. J. Bone Miner. Res. 2010, 26, 704–717. [Google Scholar] [CrossRef]
- O’Gradaigh, D.; Ireland, D.; Bord, S.; Compston, J.E. Joint erosion in rheumatoid arthritis: Interactions between tumour necrosis factor alpha, interleukin 1, and receptor activator of nuclear factor kappaB ligand (RANKL) regulate osteoclasts. Ann. Rheum. Dis. 2004, 63, 354–359. [Google Scholar] [CrossRef]
- Rich, B.E.; Kupper, T.S. Cytokines: IL-20—A new effector in skin inflammation. Curr. Biol. 2001, 11, R531–R534. [Google Scholar] [CrossRef]
- Li, H.-H.; Hsu, Y.-H.; Wei, C.-C.; Lee, P.-T.; Chen, W.-C.; Chang, M.-S. Interleukin-20 induced cell death in renal epithelial cells and was associated with acute renal failure. Genes Immun. 2008, 9, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-H.; Wei, C.-C.; Shieh, D.-B.; Chan, C.-H.; Chang, M.-S. Anti-IL-20 Monoclonal Antibody Alleviates Inflammation in Oral Cancer and Suppresses Tumor Growth. Mol. Cancer Res. 2012, 10, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-S.; Wei, C.-C.; Lin, Y.-J.; Hsu, Y.-H.; Chang, M.-S. IL-20 and IL-20R1 antibodies protect against liver fibrosis. Hepatology 2014, 60, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-Y.; Lin, R.-M.; Chen, W.-Y.; Lee, C.-L.; Yan, J.-J.; Chang, M.-S. IL-20 may contribute to the pathogenesis of human intervertebral disc herniation. Spine 2008, 33, 2034–2040. [Google Scholar] [CrossRef]
- Hsu, Y.-H.; Li, H.-H.; Hsieh, M.-Y.; Liu, M.-F.; Huang, K.-Y.; Chin, L.-S.; Chen, P.-C.; Cheng, H.-H.; Chang, M.-S. Function of interleukin-20 as a proinflammatory molecule in rheumatoid and experimental arthritis. Arthritis Rheum. 2006, 54, 2722–2733. [Google Scholar] [CrossRef]
- Hsieh, M.-Y.; Chen, W.-Y.; Jiang, M.-J.; Cheng, B.-C.; Huang, T.-Y.; Chang, M.-S. Interleukin-20 promotes angiogenesis in a direct and indirect manner. Genes Immun. 2006, 7, 234–242. [Google Scholar] [CrossRef]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef]
- Ha, H.-L.; Wang, H.; Claudio, E.; Tang, W.; Siebenlist, U. IL-20-Receptor Signaling Delimits IL-17 Production in Psoriatic Inflammation. J. Investig. Dermatol. 2019, 140, 143–151.e3. [Google Scholar] [CrossRef]
- Sabat, R.; Wolk, K. Research in practice: IL-22 and IL-20: Significance for epithelial homeostasis and psoriasis pathogenesis. J. der Dtsch. Dermatol. Ges. 2011, 9, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.R.; Blumenschein, W.; Murphy, E.; Diveu, C.; Wiekowski, M.; Abbondanzo, S.; Lucian, L.; Geissler, R.; Brodie, S.; Kimball, A.B.; et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J. Exp. Med. 2006, 203, 2577–2587. [Google Scholar] [CrossRef]
- Blumberg, H.; Conklin, D.; Xu, W.; Grossmann, A.; Brender, T.; Carollo, S.; Eagan, M.; Foster, D.; Haldeman, B.A.; Hammond, A.; et al. Interleukin 20: Discovery, receptor identification, and role in epidermal function. Cell 2001, 104, 9–19. [Google Scholar] [CrossRef]
- Commins, S.; Steinke, J.; Borish, L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J. Allergy Clin. Immunol. 2008, 121, 1108–1111. [Google Scholar] [CrossRef] [PubMed]
- Myles, I.; Fontecilla, N.M.; Valdez, P.A.; Vithayathil, P.J.; Naik, S.; Belkaid, Y.; Ouyang, W.; Datta, S. Signaling via the IL-20 receptor inhibits cutaneous production of IL-1β and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat. Immunol. 2013, 14, 804–811. [Google Scholar] [CrossRef]
- Tritsaris, K.; Myren, M.; Ditlev, S.B.; Hübschmann, M.V.; van der Blom, I.; Hansen, A.J.; Olsen, U.B.; Cao, R.; Zhang, J.; Jia, T.; et al. IL-20 is an arteriogenic cytokine that remodels collateral networks and improves functions of ischemic hind limbs. Proc. Natl. Acad. Sci. USA 2007, 104, 15364–15369. [Google Scholar] [CrossRef]
- Waszczykowski, M.; Fabiś-Strobin, A.; Bednarski, I.; Narbutt, J.; Fabiś, J. Serum and synovial fluid concentrations of interleukin-18 and interleukin-20 in patients with osteoarthritis of the knee and their correlation with other markers of inflammation and turnover of joint cartilage. AMS 2022, 18, 448–458. [Google Scholar] [CrossRef]
- Šenolt, L.; Prajzlerová, K.; Hulejová, H.; Šumová, B.; Filková, M.; Veigl, D.; Pavelka, K.; Vencovský, J. Interleukin-20 is triggered by TLR ligands and associates with disease activity in patients with rheumatoid arthritis. Cytokine 2017, 97, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Valentina, M.; Jan, F.; Peder, N.L.; Bo, Z.; Hongjie, D.; Pernille, K. Cytokine detection and simultaneous assessment of rheumatoid factor interference in human serum and synovial fluid using high-sensitivity protein arrays on plasmonic gold chips. BMC Biotechnol. 2015, 15, 73. [Google Scholar] [CrossRef]
- Hsu, Y.-H.; Chen, W.-Y.; Chan, C.-H.; Wu, C.-H.; Sun, Z.-J.; Chang, M.-S. Anti-IL-20 monoclonal antibody inhibits the differentiation of osteoclasts and protects against osteoporotic bone loss. J. Exp. Med. 2011, 208, 1849–1861. [Google Scholar] [CrossRef]
- Hsu, Y.-H.; Hsing, C.-H.; Li, C.-F.; Chan, C.-H.; Chang, M.-C.; Yan, J.-J. Anti-IL-20 monoclonal antibody suppresses breast cancer progression and bone osteolysis in murine models. J. Immunol. 2012, 188, 1981–1991. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Fukasawa, S. Is Inflammation a Friend or Foe for Orthodontic Treatment?: Inflammation in Orthodontically Induced Inflammatory Root Resorption and Accelerating Tooth Movement. Int. J. Mol. Sci. 2021, 22, 2388. [Google Scholar] [CrossRef]
- Antoun, J.S.; Mei, L.; Gibbs, K.; Farella, M. Effect of orthodontic treatment on the periodontal tissues. Periodontology 2000 2017, 74, 140–157. [Google Scholar] [CrossRef]
- Li, Y.; Zhan, Q.; Bao, M.; Yi, J.; Li, Y. Biomechanical and biological responses of periodontium in orthodontic tooth movement: Up-date in a new decade. Int. J. Oral Sci. 2021, 13, 20. [Google Scholar] [CrossRef]
- Meng, B.; Wu, D.; Cheng, Y.; Huang, P.; Liu, Y.; Gan, L.; Liu, C.; Cao, Y. Interleukin-20 differentially regulates bone mesenchymal stem cell activities in RANKL-induced osteoclastogenesis through the OPG/RANKL/RANK axis and the NF-κB, MAPK and AKT signalling pathways. Scand. J. Immunol. 2020, 91, e12874. [Google Scholar] [CrossRef]
- Yang, B.; Fu, C.; Wu, Y.; Liu, Y.; Zhang, Z.; Chen, X.; Wu, D.; Gan, Z.; Chen, Z.; Cao, Y. γ-Secretase inhibitors suppress IL-20-mediated osteoclastogenesis via Notch signalling and are affected by Notch2 in vitro. Scand. J. Immunol. 2022, 96, e13169. [Google Scholar] [CrossRef]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef]
- Otero, K.; Turnbull, I.; Poliani, P.; Vermi, W.; Cerutti, E.; Aoshi, T.; Tassi, I.; Takai, T.; Stanley, S.; Miller, M.; et al. Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and beta-catenin. Nat. Immunol. 2009, 10, 734–743. [Google Scholar] [CrossRef]
- Yoshida, H.; Hayashi, S.-I.; Kunisada, T.; Ogawa, M.; Nishikawa, S.; Okamura, H.; Sudo, T.; Shultz, L.D.; Nishikawa, S.-I. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 1990, 345, 442–444. [Google Scholar] [CrossRef]
- Zur, Y.; Rosenfeld, L.; Keshelman, C.; Dalal, N.; Guterman-Ram, G.; Orenbuch, A.; Einav, Y.; Levaot, N.; Papo, N. A dual-specific macrophage colony-stimulating factor antagonist of c-FMS and alphavbeta3 integrin for osteoporosis therapy. PLoS Biol. 2018, 16, e2002979. [Google Scholar] [CrossRef]
- Wiktor-Jedrzejczak, W.; Gordon, S. Cytokine regulation of the macrophage (M phi) system studied using the colony stimulating factor-1-deficient op/op mouse. Physiol. Rev. 1996, 76, 927–947. [Google Scholar] [CrossRef]
- Liu, L.; Ding, C.; Zeng, W.; Heuer, J.G.; Tetreault, J.W.; Noblitt, T.W.; Hangoc, G.; Cooper, S.; Brune, K.A.; Sharma, G.; et al. Selective enhancement of multipotential hematopoietic progenitors in vitro and in vivo by IL-20. Blood 2003, 102, 3206–3209. [Google Scholar] [CrossRef] [PubMed]
- Rutz, S.; Wang, X.; Ouyang, W. The IL-20 subfamily of cytokines--from host defence to tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Magadi, S.; Li, Z.; Smith, C.W.; Burns, A.R. IL-20 promotes epithelial healing of the injured mouse cornea. Exp. Eye Res. 2017, 154, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Ikebuchi, Y.; Aoki, S.; Honma, M.; Hayashi, M.; Sugamori, Y.; Khan, M.; Kariya, Y.; Kato, G.; Tabata, Y.; Penninger, J.M.; et al. Coupling of bone resorption and formation by RANKL reverse signalling. Nature 2018, 561, 195–200. [Google Scholar] [CrossRef]
- Coury, F.; Peyruchaud, O.; Machuca-Gayet, I. Osteoimmunology of Bone Loss in Inflammatory Rheumatic Diseases. Front. Immunol. 2019, 10, 679. [Google Scholar] [CrossRef]
- Ralston, S.; Schett, G. Osteoimmunology. Calcif. Tissue Int. 2018, 102, 501–502. [Google Scholar] [CrossRef]
- Okamoto, K.; Takayanagi, H. Osteoimmunology. Cold Spring Harb. Perspect. Med. 2019, 9, a031245. [Google Scholar] [CrossRef]
- Xiong, J.; Onal, M.; Jilka, R.L.; Weinstein, R.S.; Manolagas, S.C.; O’Brien, C.A. Matrix-embedded cells control osteoclast formation. Nat. Med. 2011, 17, 1235–1241. [Google Scholar] [CrossRef]
- Cao, X. Targeting osteoclast-osteoblast communication. Nat. Med. 2011, 17, 1344–1346. [Google Scholar] [CrossRef]
- Theill, L.E.; Boyle, W.J.; Penninger, J.M. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu. Rev. Immunol. 2022, 20, 795–823. [Google Scholar] [CrossRef]
- Danks, L.; Komatsu, N.; Guerrini, M.M.; Sawa, S.; Armaka, M.; Kollias, G.; Nakashima, T.; Takayanagi, H. RANKL expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation. Ann. Rheum. Dis. 2015, 75, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Lechner, J.; Rudi, T.; von Baehr, V. Osteoimmunology of tumor necrosis factor-alpha, IL-6, and RANTES/CCL5: A review of known and poorly understood inflammatory patterns in osteonecrosis. Clin. Cosmet. Investig. Dent. 2018, 10, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Mansky, K.C.; Sankar, U.; Han, J.; Ostrowski, M.C. Microphthalmia transcription factor is a target of the p38 MAPK pathway in response to receptor activator of NF-kappa B ligand signaling. J. Biol. Chem. 2002, 277, 11077–11083. [Google Scholar] [CrossRef]
- Kim, T.; Yoon, J.; Cho, H.; Lee, W.B.; Kim, J.; Song, Y.H.; Kim, S.N.; Yoon, J.H.; Kim-Ha, J.; Kim, Y.J. Downregulation of lipopolysaccharide response in Drosophila by negative crosstalk between the AP1 and NF-kappaB signaling modules. Nat. Immunol. 2005, 6, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Seeman, E.; Delmas, P.D. Bone quality--the material and structural basis of bone strength and fragility. N. Engl. J. Med. 2006, 354, 2250–2261. [Google Scholar] [CrossRef]
- Lacey, D.L.; Boyle, W.J.; Simonet, W.S.; Kostenuik, P.J.; Dougall, W.C.; Sullivan, J.K.; Martin, J.S.; Dansey, R. Bench to bedside: Elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat. Rev. Drug Discov. 2012, 11, 401–419. [Google Scholar] [CrossRef]
- Nishida, H.; Suzuki, H.; Madokoro, H.; Hayashi, M.; Morimoto, C.; Sakamoto, M.; Yamada, T. Blockade of CD26 signaling inhibits human osteoclast development. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2014, 29, 2439–2455. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, B.H.; Saar, K.; Simpson, C.; Troiano, N.; Dallas, S.L.; Tiede-Lewis, L.M.; Nevius, E.; Pereira, J.P.; Weinstein, R.S.; et al. Deletion of Rac in Mature Osteoclasts Causes Osteopetrosis, an Age-Dependent Change in Osteoclast Number, and a Reduced Number of Osteoblasts In Vivo. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2016, 31, 864–873. [Google Scholar] [CrossRef]
- Su, N.; Li, X.; Tang, Y.; Yang, J.; Wen, X.; Guo, J.; Tang, J.; Du, X.; Chen, L. Deletion of FGFR3 in Osteoclast Lineage Cells Results in Increased Bone Mass in Mice by Inhibiting Osteoclastic Bone Resorption. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2016, 31, 1676–1687. [Google Scholar] [CrossRef]
- Wu, D.; Meng, B.; Cheng, Y.; Gan, L.; Huang, P.; Cao, Y. The effect of risedronate on orthodontic tooth movement in ovariectomized rats. Arch. Oral Biol. 2019, 105, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-H.; Chiu, Y.-S.; Chen, W.-Y.; Huang, K.-Y.; Jou, I.-M.; Wu, P.-T.; Wu, C.-H.; Chang, M.-S. Anti-IL-20 monoclonal antibody promotes bone fracture healing through regulating IL-20-mediated osteoblastogenesis. Sci. Rep. 2016, 6, 24339. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Y.; Meng, B.; Wu, D.; Wu, Y.; Cao, Y. Interleukin-20 inhibits the osteogenic differentiation of MC3T3-E1 cells via the GSK3β/β-catenin signalling pathway. Arch. Oral Biol. 2021, 125, 105111. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Han, Y.; Xie, F.; Ma, X.; Xu, Z.; Liu, X.; Zou, W.; Wang, J. A RANKL-based Osteoclast Culture Assay of Mouse Bone Marrow to Investigate the Role of mTORC1 in Osteoclast Formation. J. Vis. Exp. 2018, 133, e56468. [Google Scholar]
- Marino, S.; Logan, J.G.; Mellis, D.; Capulli, M. Generation and culture of osteoclasts. BoneKEy Rep. 2014, 3, 570. [Google Scholar] [CrossRef]




| Gene | Forward Primer Sequence (5′-3′) (Tm) Reverse Primer Sequence (5′-3′) (Tm) | Product Size |
|---|---|---|
| IL-20 | ACTGCAAACCTACAGGCGATACAA (64.1 °C) AGAACCTCACTAGATGGCGGAGA (63.7 °C) | 163 bp |
| IL-20RA IL-20RB | GGGTCTACACGGAGTCGAAGTCA (64.9 °C) ACGCTCATAGTCCGAGGTCTCAA (64.5 °C) AGCACTTGATGGGTTAACAGCC (61.1 °C) AAAACAGAGACACAGCCCTCC (60.2 °C) | 139 bp 72 bp |
| IL-22RA1 | CCTACACGTGCCGAGTGAAGA (63.6 °C) AAAGCTCAGGACACGCTGGA (63.4 °C) | 176 bp |
| Cathepsin K | CGGCTATATGACCACTGCCTTC (63.0 °C) TTTGCCGTGGCGTTATACATACA (64.3 °C) | 114 bp |
| TRAP | TGGCAATGTCTCGGCACAA (64.9 °C) AGCATCACGGTGTCCAGCATAA (65.0 °C) | 138 bp |
| MT1-MMP | GAGAACTTCGTGTTGCCTGATGAC (64.5 °C) TTTCTGGGCTTATCTGGGACAGAG (64.9 °C) | 134 bp |
| MMP9 | CATGCGCTGGGCTTAGATCA (64.6 °C) GAGGCCTTGGGTCAGGTTTAGAG (64.5 °C) | 148 bp |
| NFATc1 | CAAGTCTCACCACAGGGCTCACTA (64.0 °C) TCAGCCGTCCCAATGAACAG (62.2 °C) | 144 bp |
| c-Fos | CGTCTTCCTTTGTCTTCACCTACC (64.8 °C) TTGCTGCTGCTGCCCTTT (63.7 °C) | 81 bp |
| GAPDH | GGCACAGTCAAGGCTGAGAATG (64.4 °C) ATGGTGGTGAAGACGCCAGTA (62.8 °C) | 143 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, B.; Yang, B.; Qu, Y.; Liu, Y.; Wu, D.; Fu, C.; He, Y.; Chen, X.; Liu, C.; Kou, X.; et al. Dual Role of Interleukin-20 in Different Stages of Osteoclast Differentiation and Its Osteoimmune Regulation during Alveolar Bone Remodeling. Int. J. Mol. Sci. 2023, 24, 3810. https://doi.org/10.3390/ijms24043810
Meng B, Yang B, Qu Y, Liu Y, Wu D, Fu C, He Y, Chen X, Liu C, Kou X, et al. Dual Role of Interleukin-20 in Different Stages of Osteoclast Differentiation and Its Osteoimmune Regulation during Alveolar Bone Remodeling. International Journal of Molecular Sciences. 2023; 24(4):3810. https://doi.org/10.3390/ijms24043810
Chicago/Turabian StyleMeng, Bowen, Benyi Yang, Yan Qu, Yuanbo Liu, Dongle Wu, Chaoran Fu, Yifan He, Xi Chen, Chufeng Liu, Xiaoxing Kou, and et al. 2023. "Dual Role of Interleukin-20 in Different Stages of Osteoclast Differentiation and Its Osteoimmune Regulation during Alveolar Bone Remodeling" International Journal of Molecular Sciences 24, no. 4: 3810. https://doi.org/10.3390/ijms24043810
APA StyleMeng, B., Yang, B., Qu, Y., Liu, Y., Wu, D., Fu, C., He, Y., Chen, X., Liu, C., Kou, X., & Cao, Y. (2023). Dual Role of Interleukin-20 in Different Stages of Osteoclast Differentiation and Its Osteoimmune Regulation during Alveolar Bone Remodeling. International Journal of Molecular Sciences, 24(4), 3810. https://doi.org/10.3390/ijms24043810





