Genome-Wide Identification of G Protein-Coupled Receptors in Ciliated Eukaryotes
Abstract
:1. Introduction
2. Results
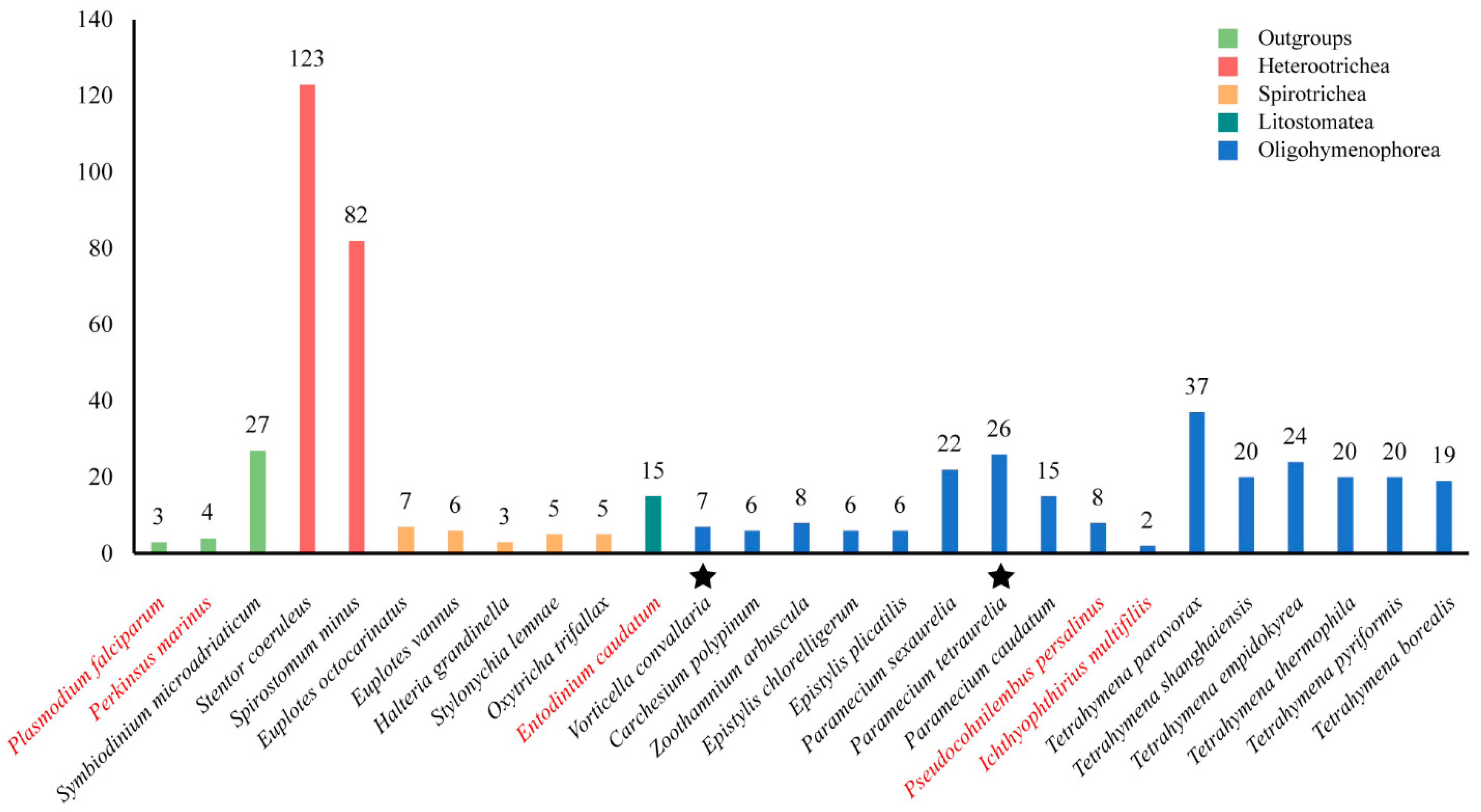
2.1. Identification of GPCRs in Ciliates
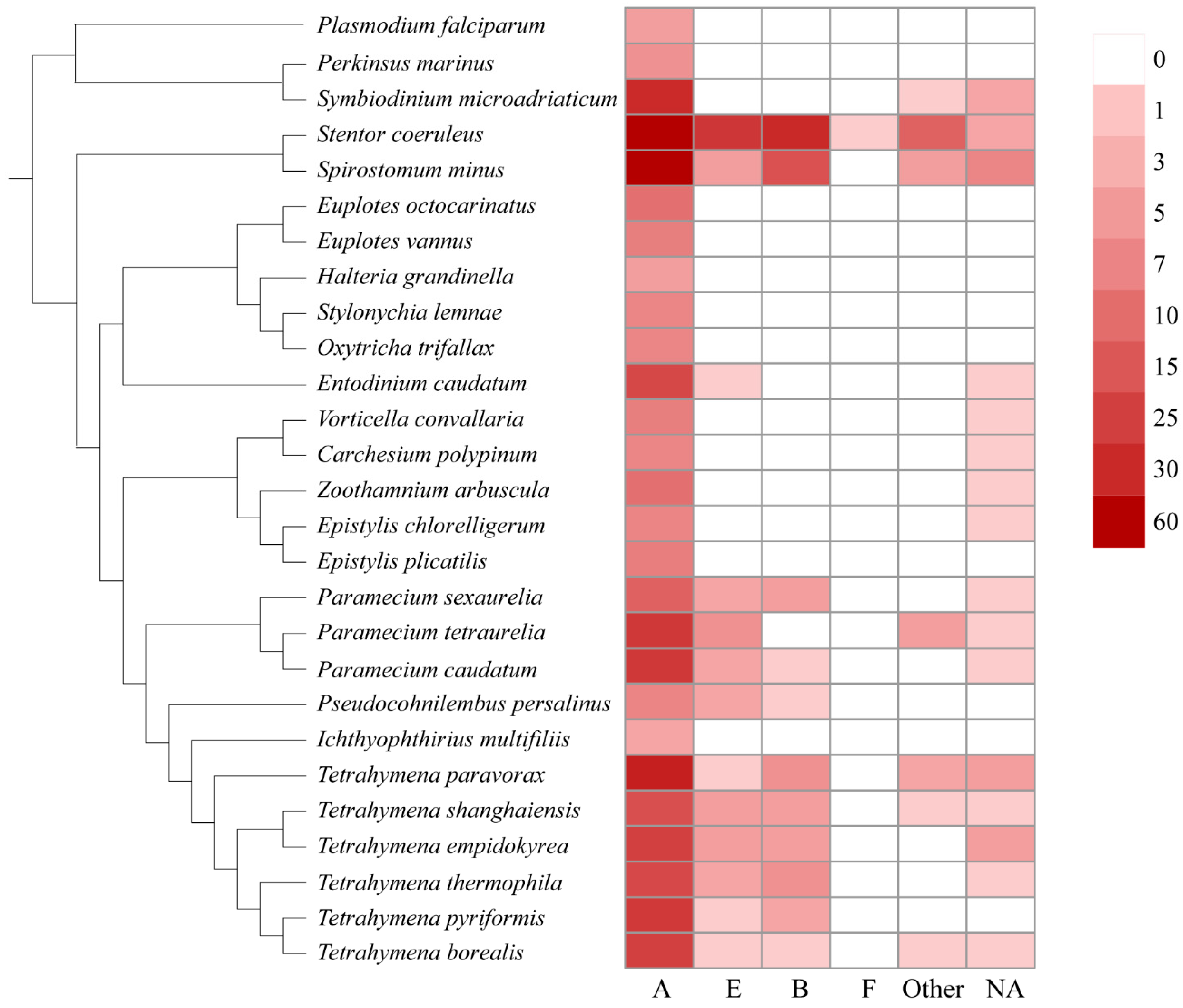
2.2. Distribution GPCR Families in Ciliates
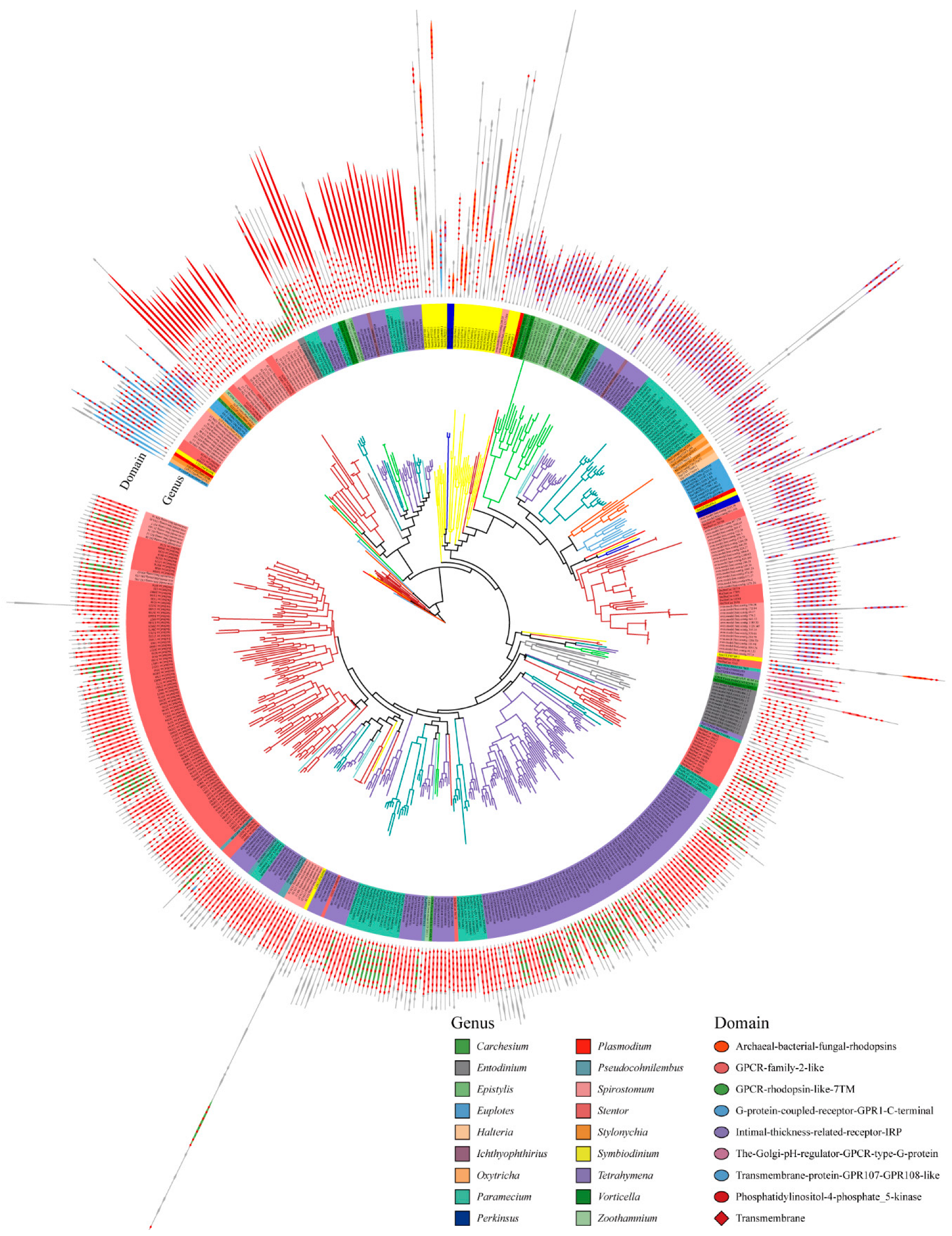
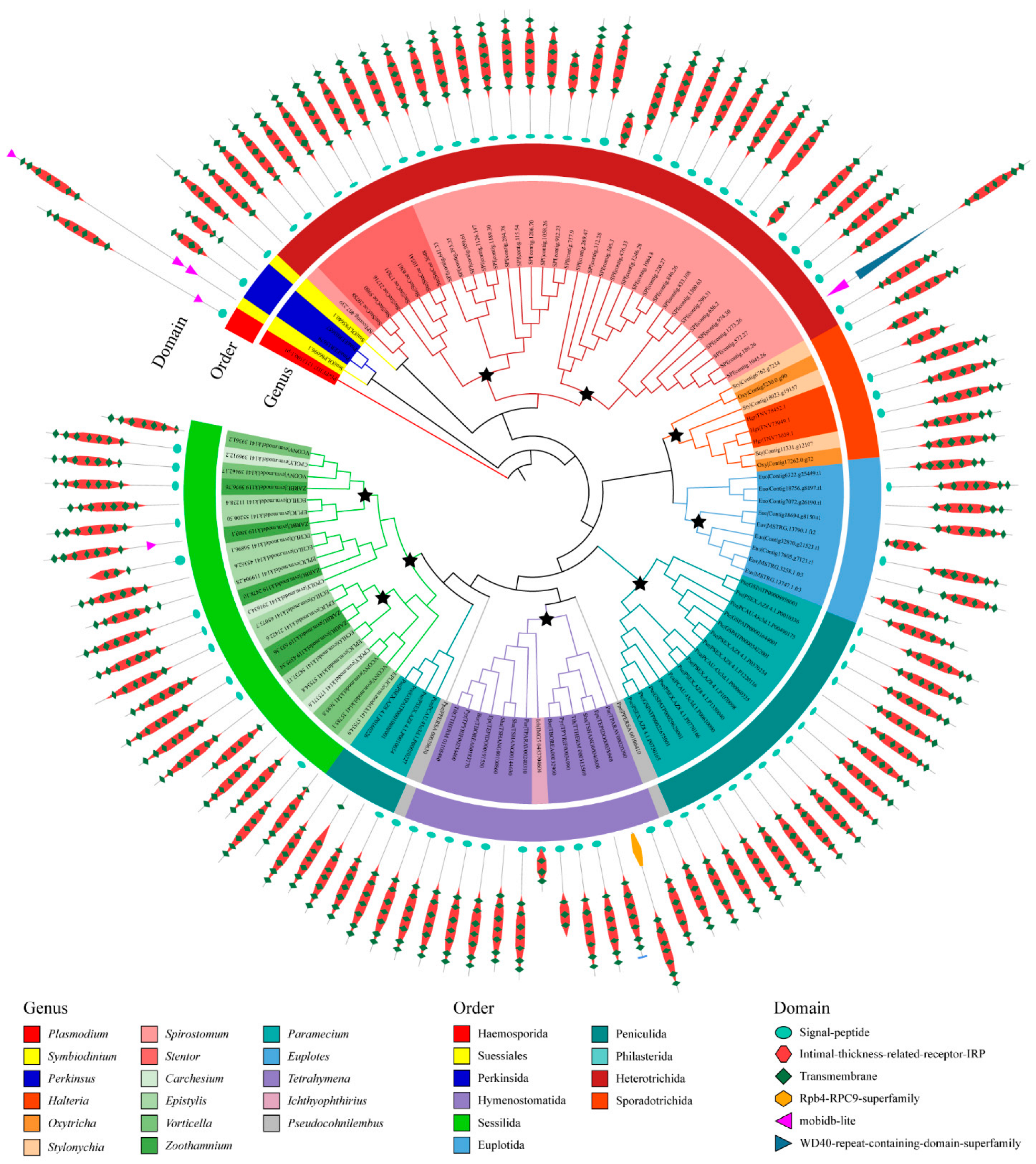
2.3. Protein Domain Architectures of GPCRs in Ciliates
3. Discussions
3.1. High Variation of the Number of GPCR in Ciliates
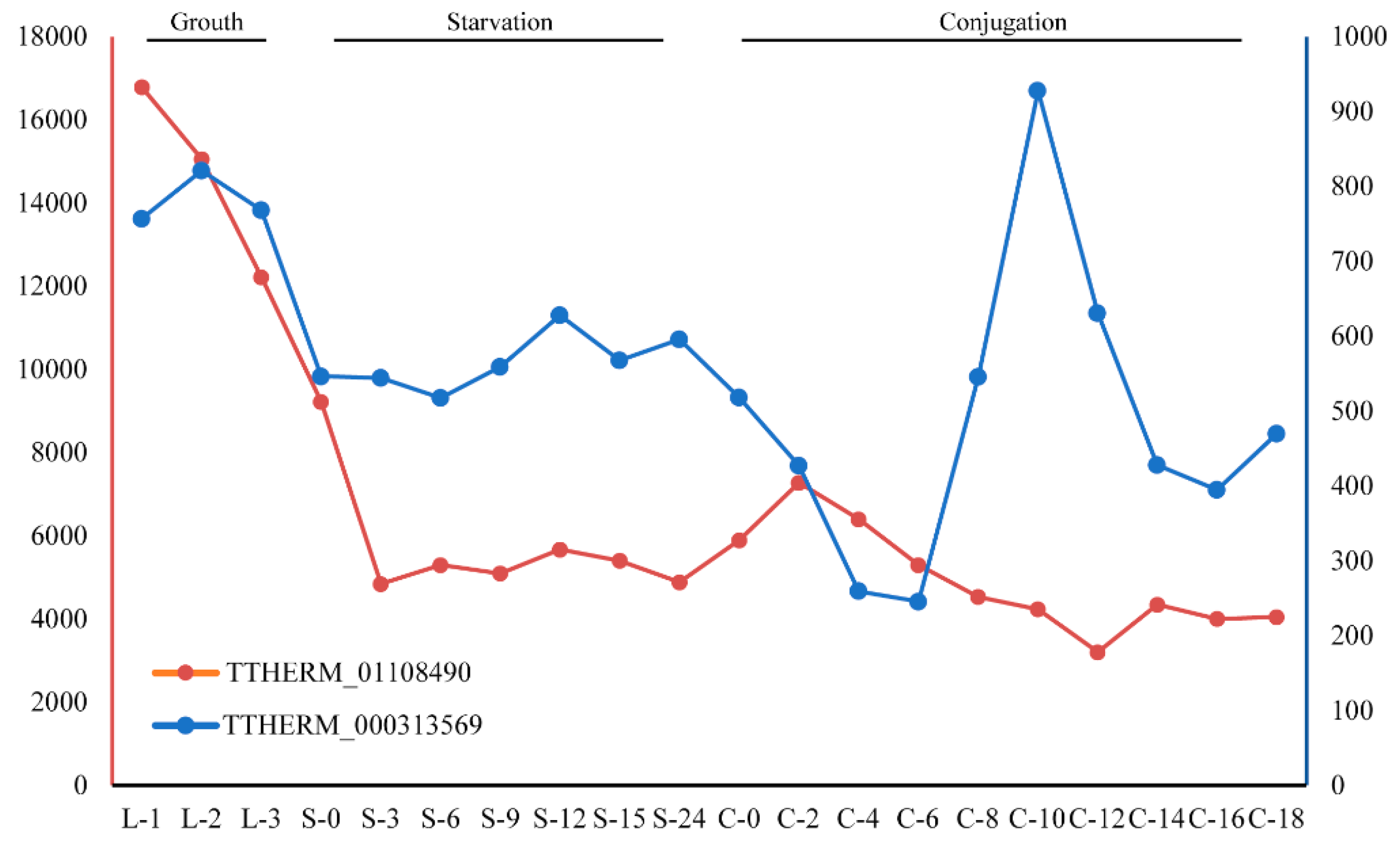
3.2. Clues for the Function of GPCRs in Ciliates
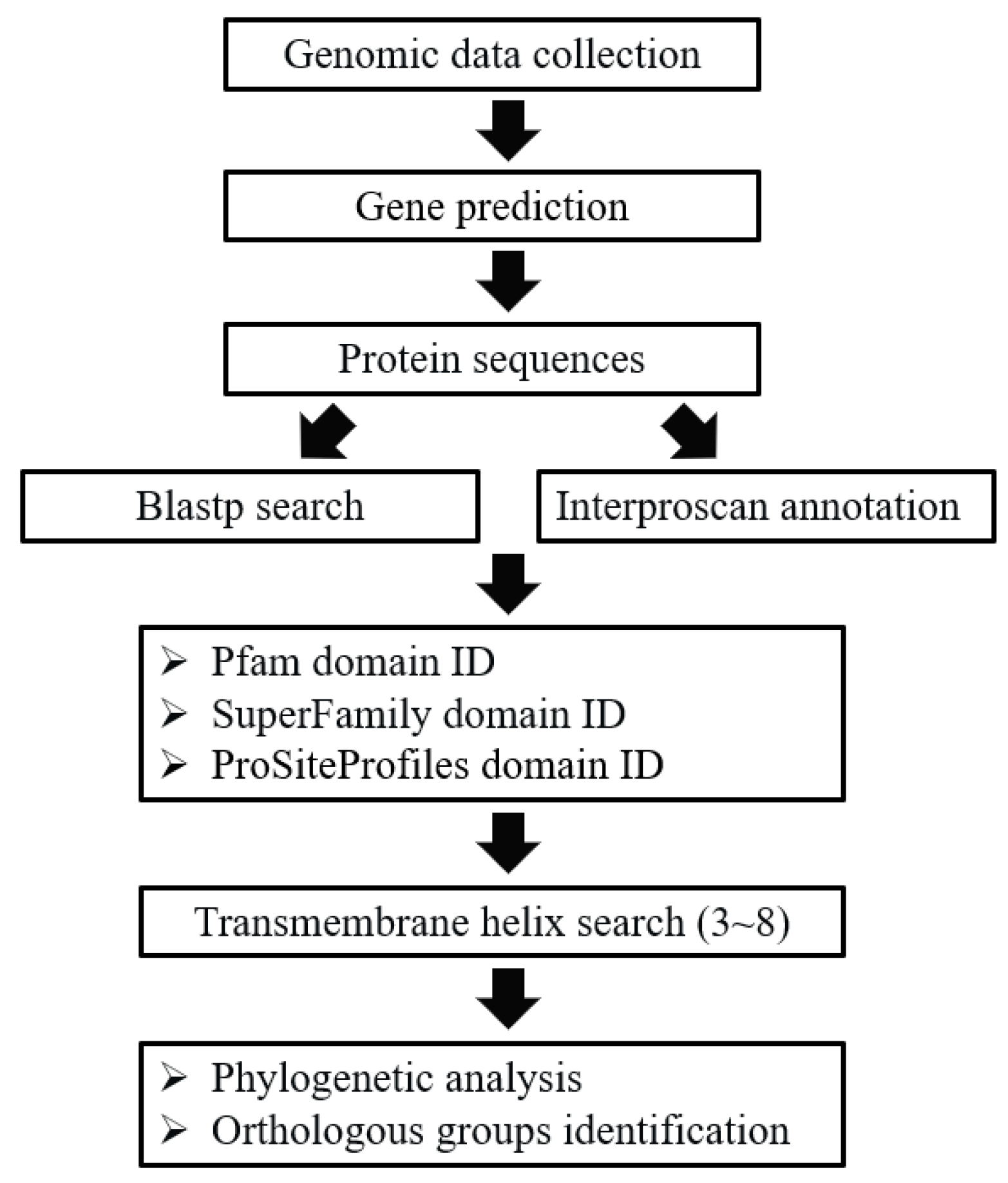
4. Materials and Methods
4.1. Ciliate Genome Data Collection
4.2. Identification of Putative GPCR Genes
4.3. Identification of Ortholog Groups of GPCRs
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorsam, R.T.; Gutkind, J.S. G-protein-coupled receptors and cancer. Nat. Rev. Cancer 2007, 7, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Michelini, S.; Urbanek, M.; Dean, M.; Goldman, D. Polymorphism and genetic mapping of the human oxytocin receptor gene on chromosome 3. Am. J. Med. Genet. 1995, 60, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Kolakowski, L.F., Jr. GCRDb: A G-protein-coupled receptor database. Recept. Channels 1994, 2, 1–7. [Google Scholar] [PubMed]
- Perez, D.M. The evolutionarily triumphant G-protein-coupled receptor. Mol. Pharmacol. 2003, 63, 1202–1205. [Google Scholar] [CrossRef] [Green Version]
- Strotmann, R.; Schröck, K.; Böselt, I.; Stäubert, C.; Russ, A.; Schöneberg, T. Evolution of GPCR: Change and continuity. Mol. Cell. Endocrinol. 2011, 331, 170–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassilatis, D.K.; Hohmann, J.G.; Zeng, H.; Li, F.; Ranchalis, J.E.; Mortrud, M.T.; Brown, A.; Rodriguez, S.S.; Weller, J.R.; Wright, A.C.; et al. The G protein-coupled receptor repertoires of human and mouse. Proc. Natl. Acad. Sci. USA 2003, 100, 4903–4908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagerström, M.C.; Hellström, A.R.; Gloriam, D.E.; Larsson, T.P.; Schiöth, H.B.; Fredriksson, R. The G protein-coupled receptor subset of the chicken genome. PLoS Comput. Biol. 2006, 2, e54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gloriam, D.E.; Fredriksson, R.; Schiöth, H.B. The G protein-coupled receptor subset of the rat genome. BMC Genom. 2007, 8, 338. [Google Scholar] [CrossRef] [Green Version]
- Bjarnadóttir, T.K.; Gloriam, D.E.; Hellstrand, S.H.; Kristiansson, H.; Fredriksson, R.; Schiöth, H.B. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics 2006, 88, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.A.; Fox, A.N.; Pitts, R.J.; Kent, L.B.; Tan, P.L.; Chrystal, M.A.; Cravchik, A.; Collins, F.H.; Robertson, H.M.; Zwiebel, L.J. G protein-coupled receptors in Anopheles gambiae. Science 2002, 298, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Frooninckx, L.; Van Rompay, L.; Temmerman, L.; Van Sinay, E.; Beets, I.; Janssen, T.; Husson, S.J.; Schoofs, L. Neuropeptide GPCRs in C. elegans. Front. Endocrinol. 2012, 3, 167. [Google Scholar] [CrossRef] [Green Version]
- Metpally, R.P.; Sowdhamini, R. Cross genome phylogenetic analysis of human and Drosophila G protein-coupled receptors: Application to functional annotation of orphan receptors. BMC Genom. 2005, 6, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terakita, A. The opsins. Genome Biol. 2005, 6, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baylor, D. How photons start vision. Proc. Natl. Acad. Sci. USA 1996, 93, 560–565. [Google Scholar] [CrossRef] [Green Version]
- Stieb, S.M.; Cortesi, F.; Sueess, L.; Carleton, K.L.; Salzburger, W.; Marshall, N.J. Why UV vision and red vision are important for damselfish (Pomacentridae): Structural and expression variation in opsin genes. Mol. Ecol. 2017, 26, 1323–1342. [Google Scholar] [CrossRef] [PubMed]
- van den Hoogen, J.; Govers, F. GPCR-bigrams: Enigmatic signaling components in oomycetes. PLoS Pathog. 2018, 14, e1007064. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Fenchel, T.; Field, J.G.; Gray, J.S.; Thingstad, T.F. The Ecological Role of Water-Column Microbes in the Sea. Mar. Ecol. Prog. Ser. 1983, 10, 257–263. [Google Scholar] [CrossRef]
- Corliss, J.O. Chapter 4-Phylum Ciliophora: General Description and Overview of the Major Groups. In The Ciliated Protozoa, 2nd ed.; Corliss, J.O., Ed.; Pergamon: Berlin, Germany, 1979; pp. 61–68. [Google Scholar] [CrossRef]
- Elliott, D.T. The Biology and Ecology of Tintinnid Ciliates: Models for Marine Plankton. Estuaries Coasts 2014, 37, 240–241. [Google Scholar] [CrossRef]
- Hu, X.; Lin, X.; Song, W. Ciliate Atlas: Species Found in the South China Sea; Springer: Berlin, Germany, 2019. [Google Scholar]
- Leick, V.; Koppelhus, U.; Rosenberg, J. Cilia-mediated oriented chemokinesis in Tetrahymena thermophila. J. Eukaryot. Microbiol. 1994, 41, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.T.; Hennessey, T.M. Chemorepellents in Paramecium and Tetrahymena. J. Eukaryot. Microbiol. 1995, 42, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, T.M. Responses of the ciliates Tetrahymena and Paramecium to external ATP and GTP. Purinergic Signal. 2005, 1, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lampert, T.J.; Coleman, K.D.; Hennessey, T.M. Chemoattraction to lysophosphatidic acid does not require a change in membrane potential in Tetrahymena thermophila. Cell Biol. Int. 2011, 35, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Lagerström, M.C.; Schiöth, H.B. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 2008, 7, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Hsueh, Y.P.; Heitman, J. Magnificent seven: Roles of G protein-coupled receptors in extracellular sensing in fungi. FEMS Microbiol. Rev. 2008, 32, 1010–1032. [Google Scholar] [CrossRef] [Green Version]
- Marino, M.J.; Sherman, T.G.; Wood, D.C. Partial cloning of putative G-proteins modulating mechanotransduction in the ciliate stentor. J. Eukaryot. Microbiol. 2001, 48, 527–536. [Google Scholar] [CrossRef]
- Renaud, F.L.; Colon, I.; Lebron, J.; Ortiz, N.; Rodriguez, F.; Cadilla, C. A novel opioid mechanism seems to modulate phagocytosis in Tetrahymena. J. Eukaryot. Microbiol. 1995, 42, 205–207. [Google Scholar] [CrossRef]
- de Ondarza, J.; Symington, S.B.; Van Houten, J.L.; Clark, J.M. G-protein modulators alter the swimming behavior and calcium influx of Paramecium tetraurelia. J. Eukaryot. Microbiol. 2003, 50, 349–355. [Google Scholar] [CrossRef]
- Sheng, Y.; Duan, L.; Cheng, T.; Qiao, Y.; Stover, N.A.; Gao, S. The completed macronuclear genome of a model ciliate Tetrahymena thermophila and its application in genome scrambling and copy number analyses. Sci. China. Life Sci. 2020, 63, 1534–1542. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, Y.; Gao, F.; Zheng, W.; Krock, T.J.; Stover, N.A.; Lu, C.; Katz, L.A.; Song, W. Genome analyses of the new model protist Euplotes vannus focusing on genome rearrangement and resistance to environmental stressors. Mol. Ecol. Resour. 2019, 19, 1292–1308. [Google Scholar] [CrossRef]
- Méndez-Sánchez, D.; Mayén-Estrada, R.; Luo, X.; Hu, X. A New Subspecies of Oxytricha granulifera (Hypotrichia: Oxytrichidae) from Mexico, with Notes on its Morphogenesis and Phylogenetic Position. J. Eukaryot. Microbiol. 2018, 65, 357–371. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, G.; Cheng, J.; Tian, M.; Pan, X.; Warren, A.; Jiang, C.; Yuan, D.; Miao, W. Genome of the facultative scuticociliatosis pathogen Pseudocohnilembus persalinus provides insight into its virulence through horizontal gene transfer. Sci. Rep. 2015, 5, 15470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnaiz, O.; Meyer, E.; Sperling, L. ParameciumDB 2019: Integrating genomic data across the genus for functional and evolutionary biology. Nucleic Acids Res. 2020, 48, D599–D605. [Google Scholar] [CrossRef]
- Slabodnick, M.M.; Ruby, J.G.; Reiff, S.B.; Swart, E.C.; Gosai, S.; Prabakaran, S.; Witkowska, E.; Larue, G.E.; Fisher, S.; Freeman, R.M., Jr.; et al. The Macronuclear Genome of Stentor coeruleus Reveals Tiny Introns in a Giant Cell. Curr. Biol. CB 2017, 27, 569–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Zhou, Q.; Labroska, V.; Qin, S.; Darbalaei, S.; Wu, Y.; Yuliantie, E.; Xie, L.; Tao, H.; Cheng, J.; et al. G protein-coupled receptors: Structure- and function-based drug discovery. Signal Transduct. Target. Ther. 2021, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Hilger, D.; Masureel, M.; Kobilka, B.K. Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol. 2018, 25, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Edgar, A.J. Human GPR107 and murine Gpr108 are members of the LUSTR family of proteins found in both plants and animals, having similar topology to G-protein coupled receptors. DNA Seq. J. DNA Seq. Mapp. 2007, 18, 235–241. [Google Scholar] [CrossRef]
- Tafesse, F.G.; Guimaraes, C.P.; Maruyama, T.; Carette, J.E.; Lory, S.; Brummelkamp, T.R.; Ploegh, H.L. GPR107, a G-protein-coupled receptor essential for intoxication by Pseudomonas aeruginosa exotoxin A, localizes to the Golgi and is cleaved by furin. J. Biol. Chem. 2014, 289, 24005–24018. [Google Scholar] [CrossRef] [Green Version]
- Shulga, Y.V.; Anderson, R.A.; Topham, M.K.; Epand, R.M. Phosphatidylinositol-4-phosphate 5-kinase isoforms exhibit acyl chain selectivity for both substrate and lipid activator. J. Biol. Chem. 2012, 287, 35953–35963. [Google Scholar] [CrossRef] [Green Version]
- Oesterhelt, D.; Tittor, J. Two pumps, one principle: Light-driven ion transport in halobacteria. Trends Biochem. Sci. 1989, 14, 57–61. [Google Scholar] [CrossRef]
- Blanck, A.; Oesterhelt, D.; Ferrando, E.; Schegk, E.S.; Lottspeich, F. Primary structure of sensory rhodopsin I, a prokaryotic photoreceptor. EMBO J. 1989, 8, 3963–3971. [Google Scholar] [CrossRef]
- Povey, S.; Lovering, R.; Bruford, E.; Wright, M.; Lush, M.; Wain, H. The HUGO Gene Nomenclature Committee (HGNC). Hum. Genet. 2001, 109, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Harmar, A.J. Family-B G-protein-coupled receptors. Genome Biology 2001, 2, reviews3013.3011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, K.; Li, C.; Perrin, M.H.; Blount, A.; Kunitake, K.; Donaldson, C.; Vaughan, J.; Reyes, T.M.; Gulyas, J.; Fischer, W.; et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 7570–7575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamen, F.; Persson, K.; Bertrand, G.; Rodriguez-Henche, N.; Puech, R.; Bockaert, J.; Ahrén, B.; Brabet, P. PAC1 receptor-deficient mice display impaired insulinotropic response to glucose and reduced glucose tolerance. J. Clin. Investig. 2000, 105, 1307–1315. [Google Scholar] [CrossRef] [Green Version]
- Louis, J.M.; Ginsburg, G.T.; Kimmel, A.R. The cAMP receptor CAR4 regulates axial patterning and cellular differentiation during late development of Dictyostelium. Genes Dev. 1994, 8, 2086–2096. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.L.; Saxe, C.L., 3rd; Gollop, R.; Kimmel, A.R.; Devreotes, P.N. Identification and targeted gene disruption of cAR3, a cAMP receptor subtype expressed during multicellular stages of Dictyostelium development. Genes Dev. 1993, 7, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Saxe, C.L., 3rd; Ginsburg, G.T.; Louis, J.M.; Johnson, R.; Devreotes, P.N.; Kimmel, A.R. CAR2, a prestalk cAMP receptor required for normal tip formation and late development of Dictyostelium discoideum. Genes Dev. 1993, 7, 262–272. [Google Scholar] [CrossRef] [Green Version]
- Wan, K.Y.; Jékely, G. Origins of eukaryotic excitability. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2021, 376, 20190758. [Google Scholar] [CrossRef]
- James, T.Y.; Liou, S.R.; Vilgalys, R. The genetic structure and diversity of the A and B mating-type genes from the tropical oyster mushroom, Pleurotus djamor. Fungal Genet. Biol. FG B 2004, 41, 813–825. [Google Scholar] [CrossRef]
- Bhanot, P.; Brink, M.; Samos, C.H.; Hsieh, J.C.; Wang, Y.; Macke, J.P.; Andrew, D.; Nathans, J.; Nusse, R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 1996, 382, 225–230. [Google Scholar] [CrossRef]
- Chen, Y.; Struhl, G. In vivo evidence that Patched and Smoothened constitute distinct binding and transducing components of a Hedgehog receptor complex. Development 1998, 125, 4943–4948. [Google Scholar] [CrossRef]
- Tanabe, Y.; Masu, M.; Ishii, T.; Shigemoto, R.; Nakanishi, S. A family of metabotropic glutamate receptors. Neuron 1992, 8, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Lampert, T.J.; Coleman, K.D.; Hennessey, T.M. A knockout mutation of a constitutive GPCR in Tetrahymena decreases both G-protein activity and chemoattraction. PLoS ONE 2011, 6, e28022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukada, S.; Iwai, M.; Nishiu, J.; Itoh, M.; Tomoike, H.; Horiuchi, M.; Nakamura, Y.; Tanaka, T. Inhibition of experimental intimal thickening in mice lacking a novel G-protein-coupled receptor. Circulation 2003, 107, 313–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patthy, L. Genome evolution and the evolution of exon-shuffling–a review. Gene 1999, 238, 103–114. [Google Scholar] [CrossRef]
- Coyne, R.S. Genomics: Stentor’s Trumpet Sounds Anew. Curr. Biol. CB 2017, 27, R146–R148. [Google Scholar] [CrossRef] [Green Version]
- Dehal, P.; Satou, Y.; Campbell, R.K.; Chapman, J.; Degnan, B.; De Tomaso, A.; Davidson, B.; Di Gregorio, A.; Gelpke, M.; Goodstein, D.M.; et al. The draft genome of Ciona intestinalis: Insights into chordate and vertebrate origins. Science 2002, 298, 2157–2167. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Gu, X. Evolutionary patterns of gene families generated in the early stage of vertebrates. J. Mol. Evol. 2000, 51, 88–96. [Google Scholar] [CrossRef]
- Holland, P.W. Gene duplication: Past, present and future. Semin. Cell Dev. Biol. 1999, 10, 541–547. [Google Scholar] [CrossRef]
- Kaiser, A.; Doerig, C. Editorial: Heading Against Parasitic Resistance: A Screen for Next Generation Drugs Against Targets of cAMP- or cGMP-regulated Pathways. Front. Microbiol. 2021, 12, 727978. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Pinto, V.C.; Vieira, F.A.; Clark, M.S.; Power, D.M. Evolution of secretin family GPCR members in the metazoa. BMC Evol. Biol. 2006, 6, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, S.P.; Yong, P. The evolution of gene duplicates. Adv. Genet. 2002, 46, 451–483. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yan, W.; McCorvy, J.D.; Cheng, J. Biased Ligands of G Protein-Coupled Receptors (GPCRs): Structure-Functional Selectivity Relationships (SFSRs) and Therapeutic Potential. J. Med. Chem. 2018, 61, 9841–9878. [Google Scholar] [CrossRef]
- Csaba, G.; Kovács, P. Pheromone and insulin induced chemotaxis in Tetrahymena. Microbios 1993, 76, 35–39. [Google Scholar]
- Chen, X.; Jung, S.; Beh, L.Y.; Eddy, S.R.; Landweber, L.F. Combinatorial DNA Rearrangement Facilitates the Origin of New Genes in Ciliates. Genome Biol. Evol. 2015, 7, 2859–2870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aury, J.M.; Jaillon, O.; Duret, L.; Noel, B.; Jubin, C.; Porcel, B.M.; Ségurens, B.; Daubin, V.; Anthouard, V.; Aiach, N.; et al. Global trends of whole-genome duplications revealed by the ciliate Paramecium tetraurelia. Nature 2006, 444, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.P.; Jiang, X.; Zhu, L.; Zhou, D.; Hong, M.; He, L.; Chen, L.; Yao, S.; Zhao, Y.; Chen, G.; et al. A G-Protein-Coupled Receptor Modulates Gametogenesis via PKG-Mediated Signaling Cascade in Plasmodium berghei. Microbiol. Spectr. 2022, 10, e0015022. [Google Scholar] [CrossRef]
- Fan, Y.; Sun, P.; Wang, Y.; He, X.; Deng, X.; Chen, X.; Zhang, G.; Chen, X.; Zhou, N. The G protein-coupled receptors in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2010, 40, 581–591. [Google Scholar] [CrossRef]
- Wang, C.; Long, Y.; Wang, B.; Zhang, C.; Ma, D.K. GPCR signaling regulates severe stress-induced organismic death in Caenorhabditis elegans. Aging Cell 2022, 22, e13735. [Google Scholar] [CrossRef]
- Josefsson, L.G.; Rask, L. Cloning of a putative G-protein-coupled receptor from Arabidopsis thaliana. Eur. J. Biochem. 1997, 249, 415–420. [Google Scholar] [CrossRef]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef] [PubMed]
- Quevillon, E.; Silventoinen, V.; Pillai, S.; Harte, N.; Mulder, N.; Apweiler, R.; Lopez, R. InterProScan: Protein domains identifier. Nucleic Acids Res. 2005, 33, W116–W120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wistrand, M.; Käll, L.; Sonnhammer, E.L. A general model of G protein-coupled receptor sequences and its application to detect remote homologs. Protein Sci. A Publ. Protein Soc. 2006, 15, 509–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, G. Estimating the Dimension of a Model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [Green Version]
| Domain Id | Database | Database Annotation | GPCR Family | Numbers |
|---|---|---|---|---|
| PF00001 | Pfam | 7 transmembrane receptors (rhodopsin family) | A | 18 |
| PF00002 | Pfam | 7 transmembrane receptors (secretin family) | B | 13 |
| PF05462 | Pfam | Slime mold cyclic AMP receptor | A, B, E, Other | 120 |
| PF06814 | Pfam | Lung seven transmembrane receptor | A | 22 |
| PF10192 | Pfam | Rhodopsin-like GPCRs transmembrane domain | A | 133 |
| PF11710 | Pfam | G protein-coupled glucose receptor regulating Gpa2 | Unknown | 1 |
| PF11970 | Pfam | G protein-coupled glucose receptor regulating Gpa2 C-term | Unknown | 9 |
| PF12430 | Pfam | Abscisic acid G protein-coupled receptor | Unknown | 7 |
| PS50262 | ProSite | G protein-coupled receptors family 1 profile | Unknown | 10 |
| SSF81321 | Superfamily | Family A G protein-coupled receptor-like | A, B, F, Other | 193 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, S.; Zhang, P.; Miao, W.; Xiong, J. Genome-Wide Identification of G Protein-Coupled Receptors in Ciliated Eukaryotes. Int. J. Mol. Sci. 2023, 24, 3869. https://doi.org/10.3390/ijms24043869
Luo S, Zhang P, Miao W, Xiong J. Genome-Wide Identification of G Protein-Coupled Receptors in Ciliated Eukaryotes. International Journal of Molecular Sciences. 2023; 24(4):3869. https://doi.org/10.3390/ijms24043869
Chicago/Turabian StyleLuo, Shuai, Peng Zhang, Wei Miao, and Jie Xiong. 2023. "Genome-Wide Identification of G Protein-Coupled Receptors in Ciliated Eukaryotes" International Journal of Molecular Sciences 24, no. 4: 3869. https://doi.org/10.3390/ijms24043869





