Non-ROS-Mediated Cytotoxicity of ZnO and CuO in ML-1 and CA77 Thyroid Cancer Cell Lines
Abstract
:1. Introduction
2. Results
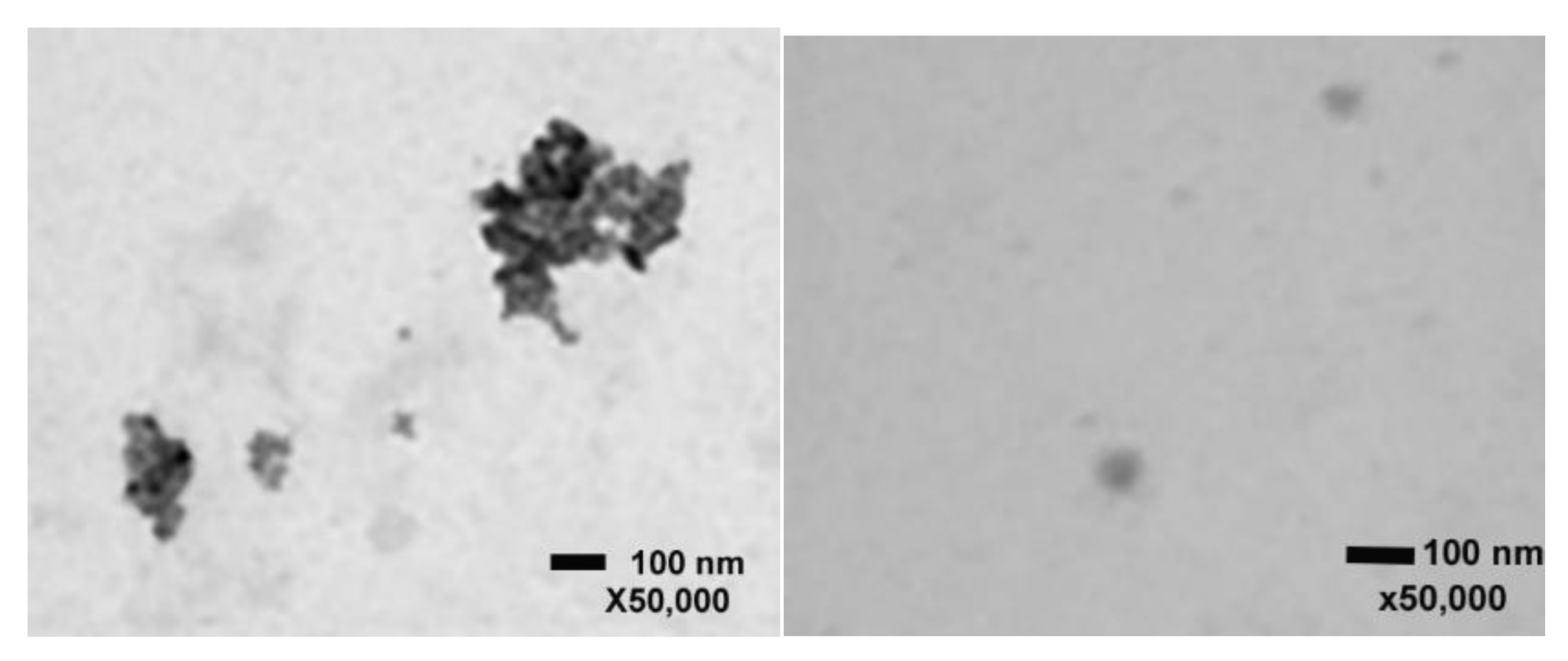
2.1. Scanning Transmission Electron Microscopy with ZnO and CuO NPs
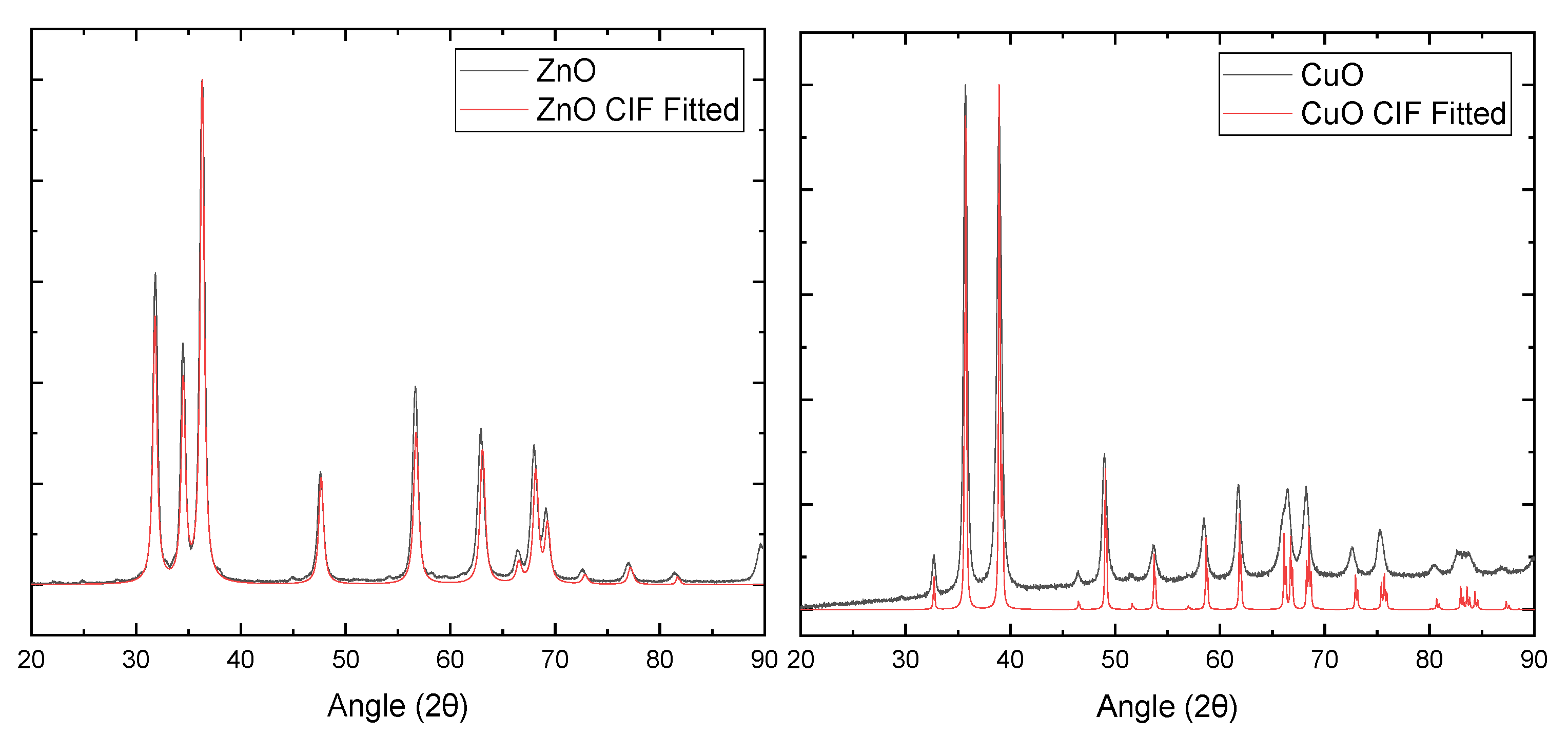
2.2. X-ray Diffraction of ZnO and CuO NPs
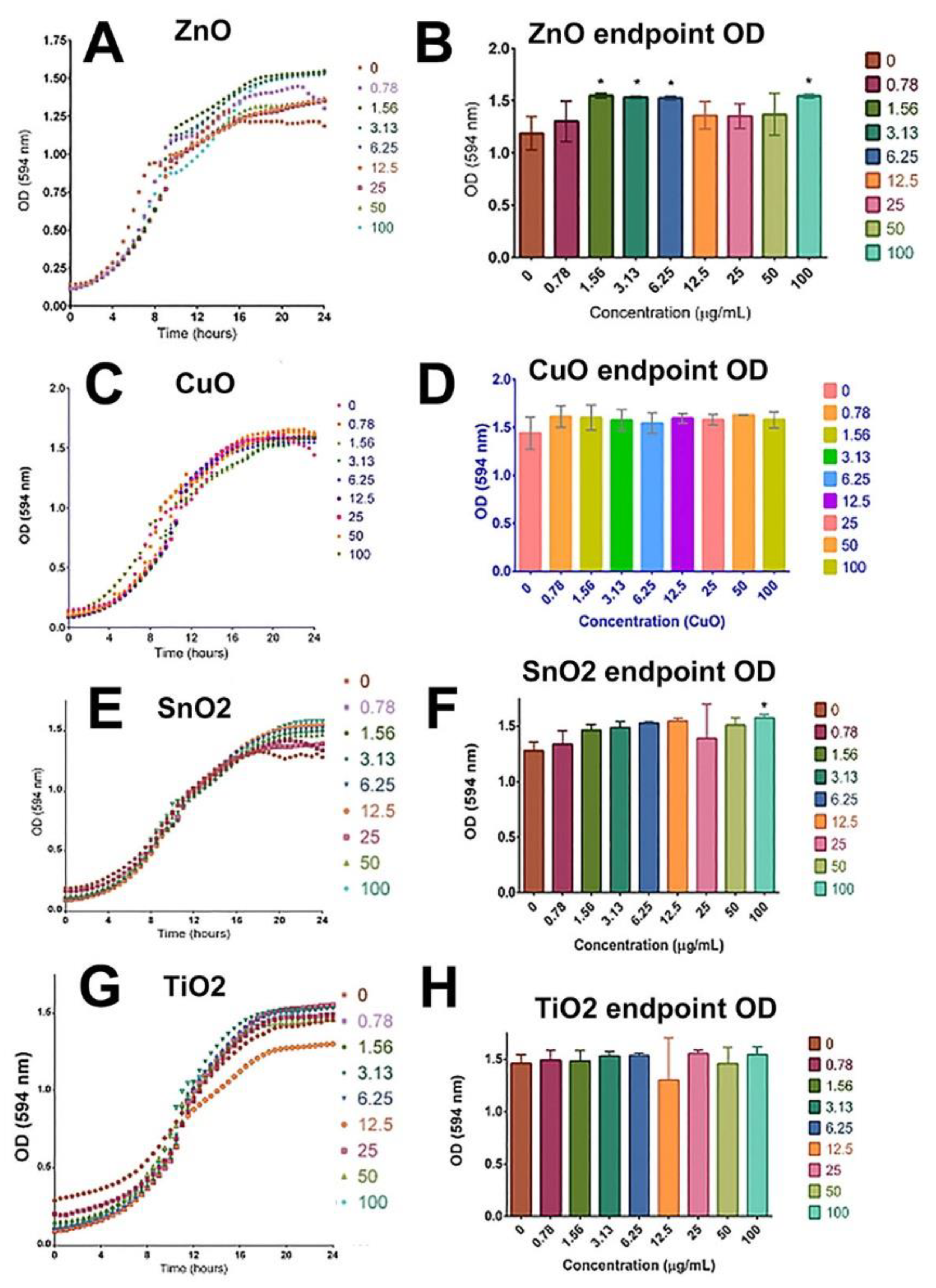
2.3. Yeast Viability Was Not Compromised by the Treatment of ZnO, CuO, TiO2, and SnO2
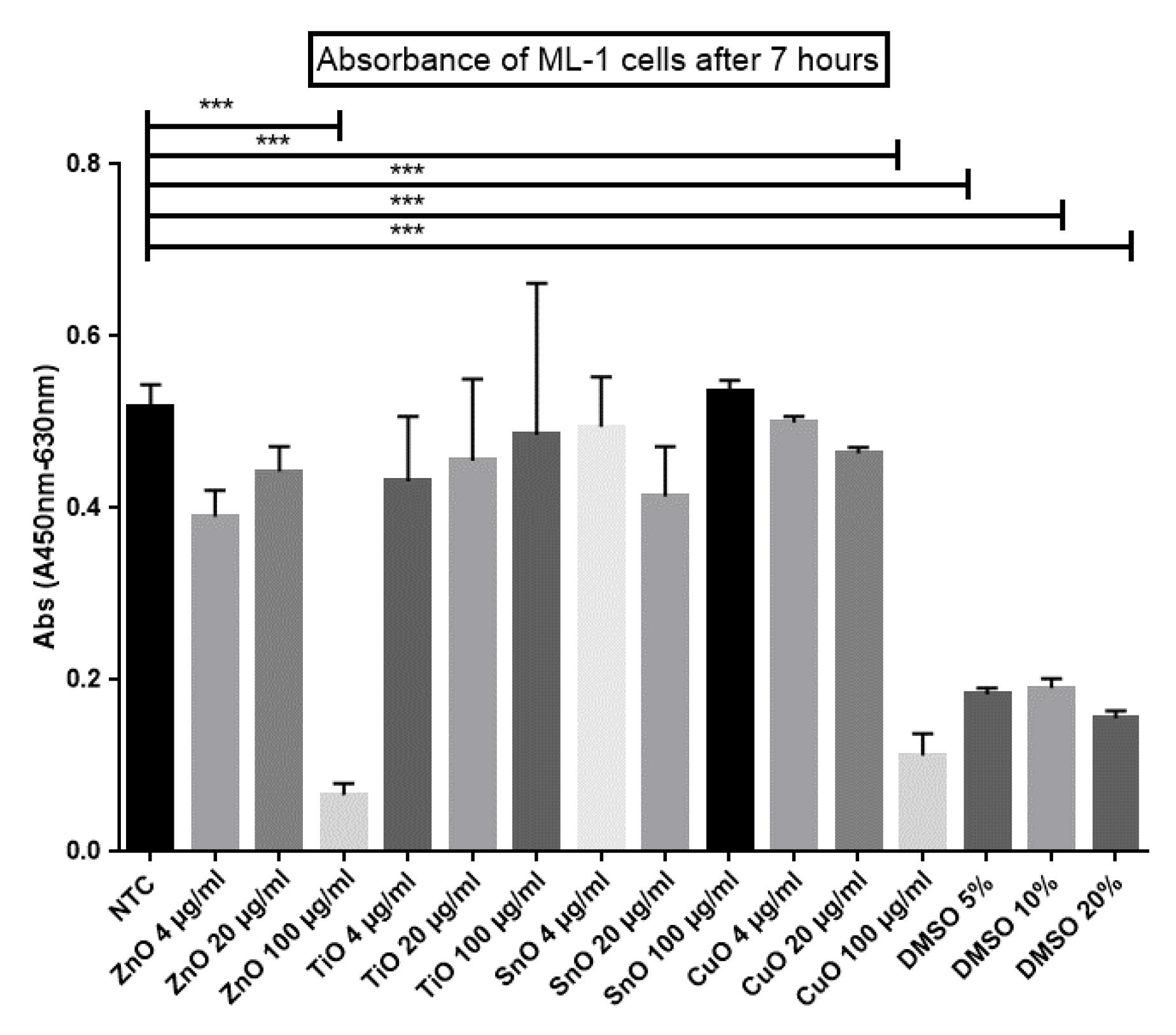
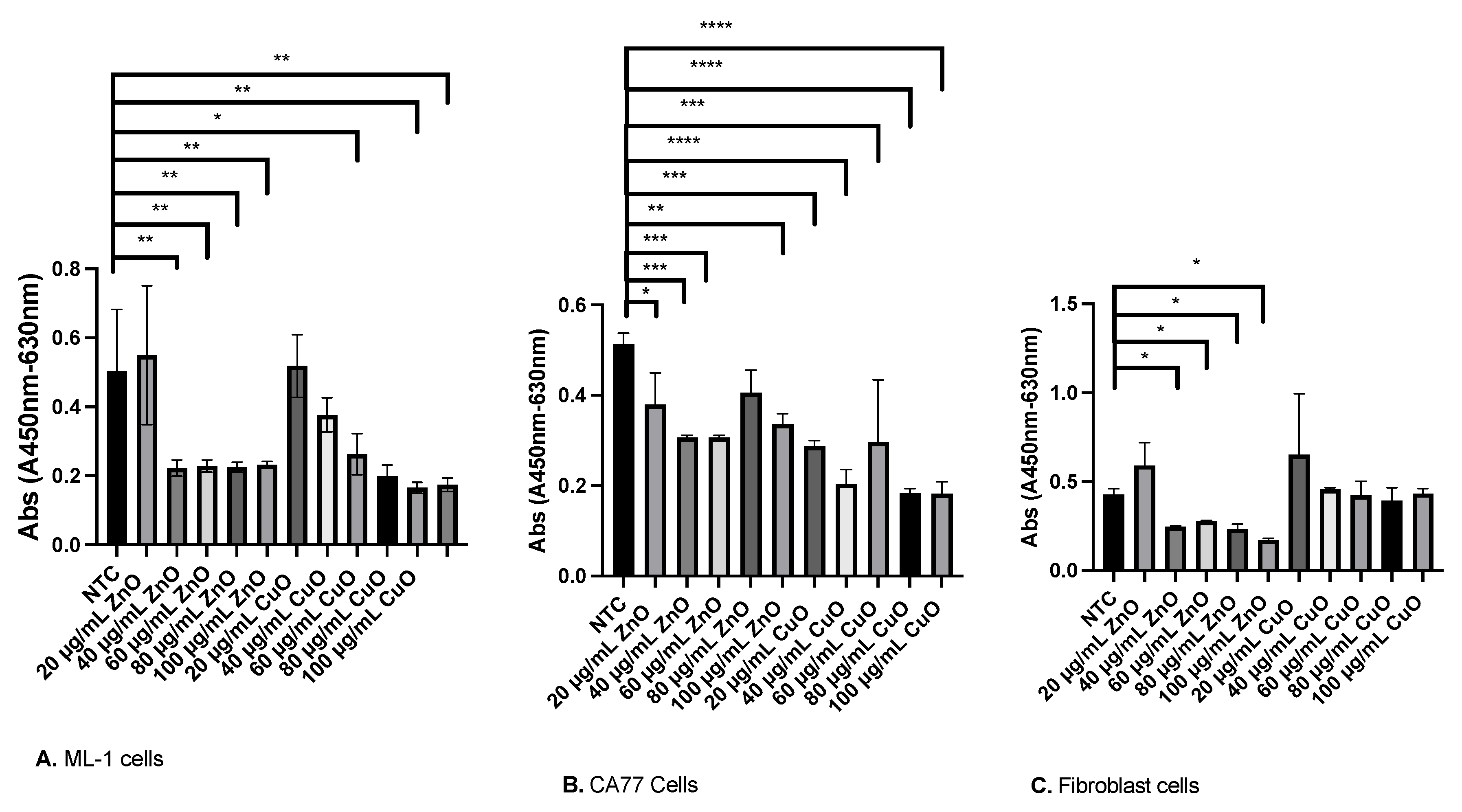
2.4. XTT Cell Viability Assay
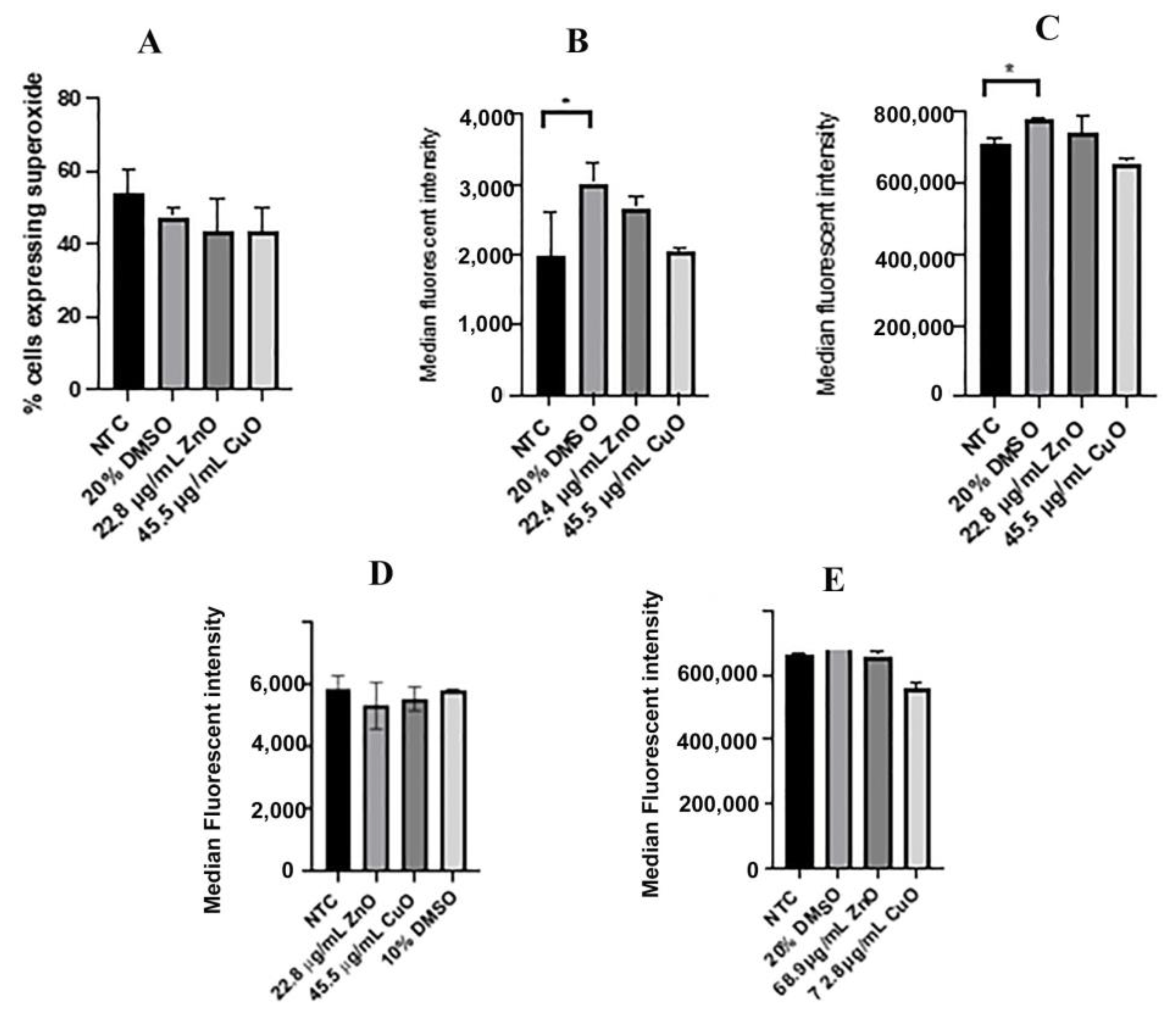
2.5. Reactive Oxygen Species
2.6. ROS Levels Measured with CellROX Green
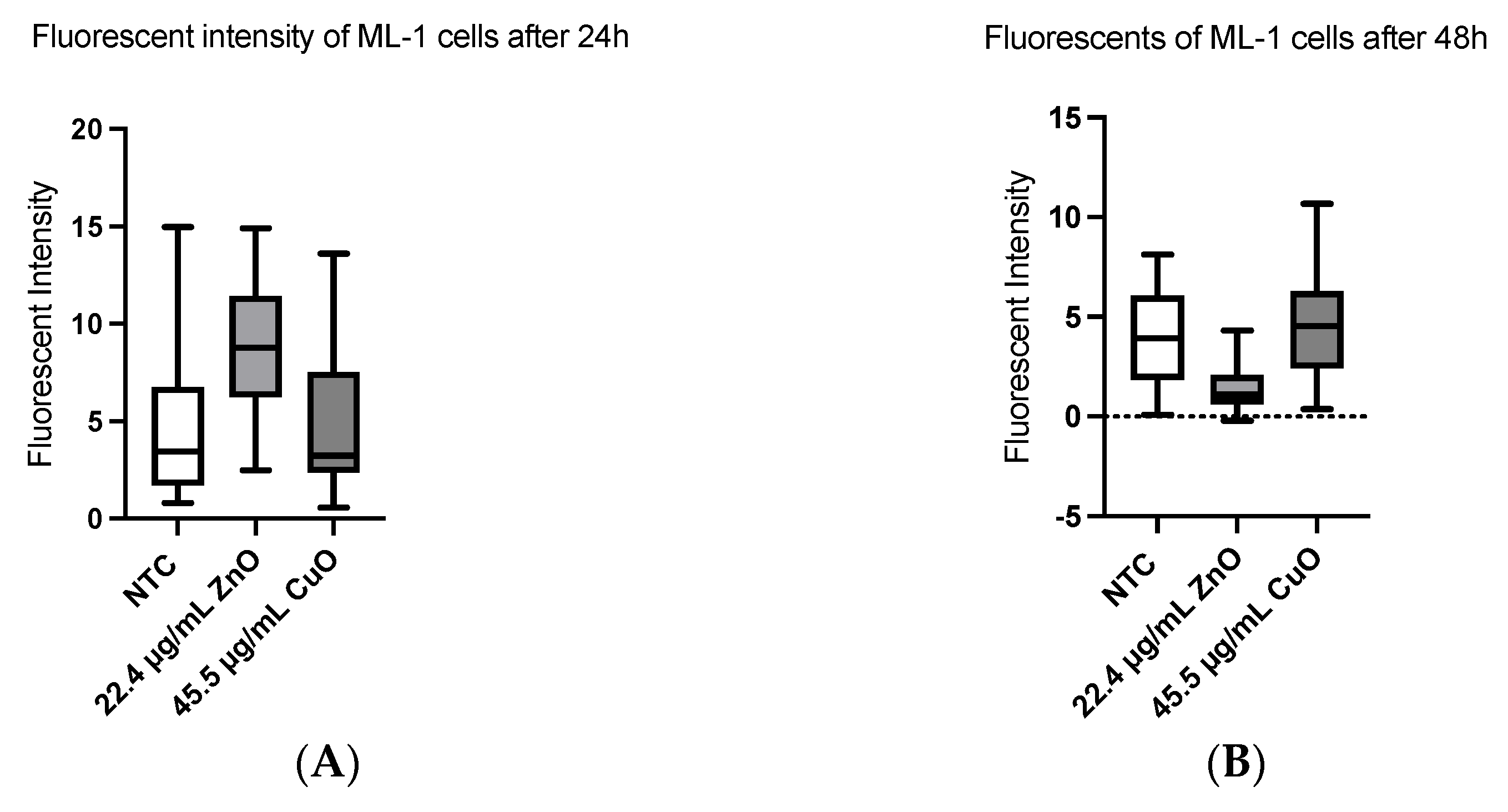
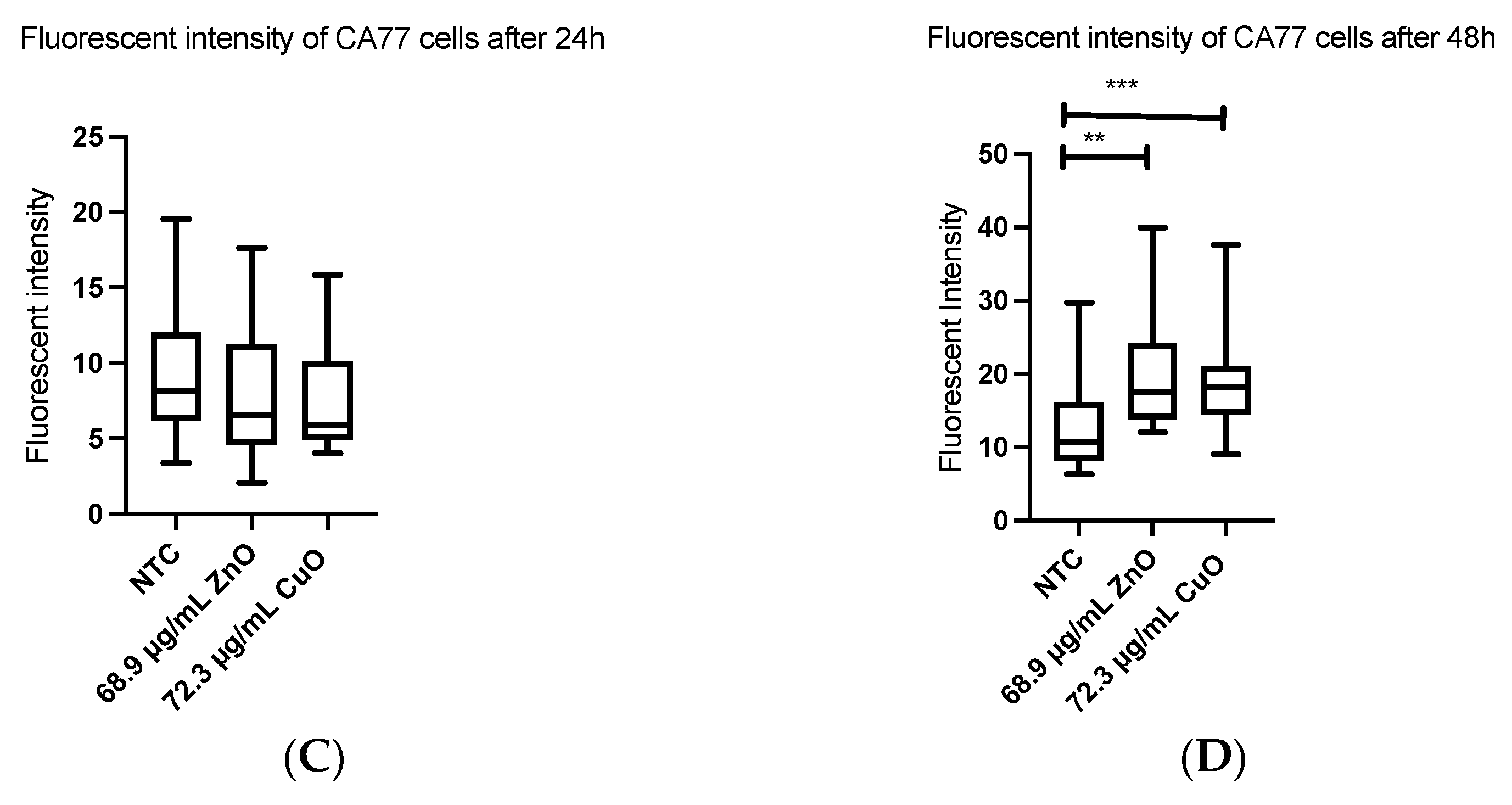
2.7. Apoptosis
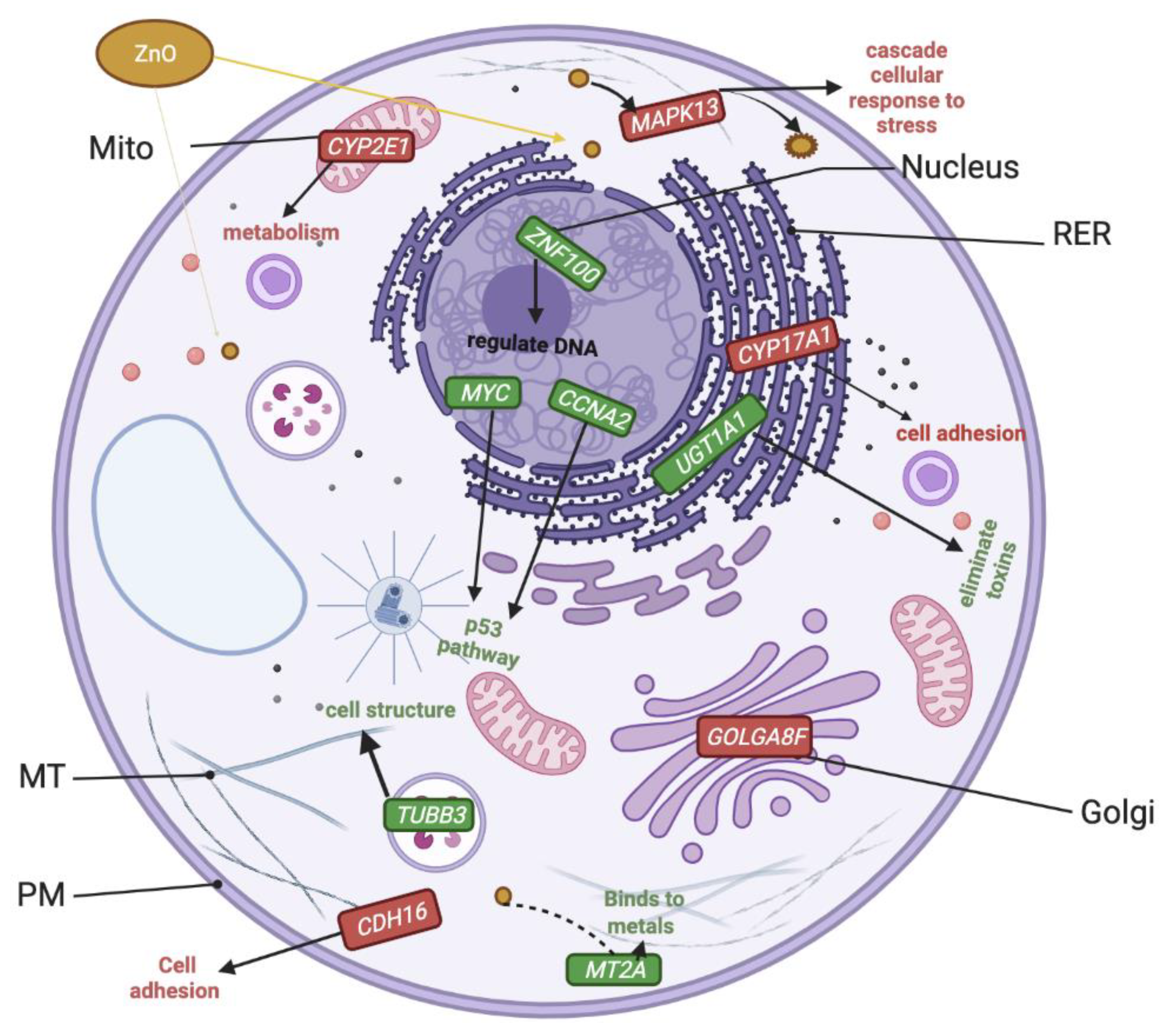
2.8. Altered Genome with ZnO/CuO Treatment upon ML-1 Cells
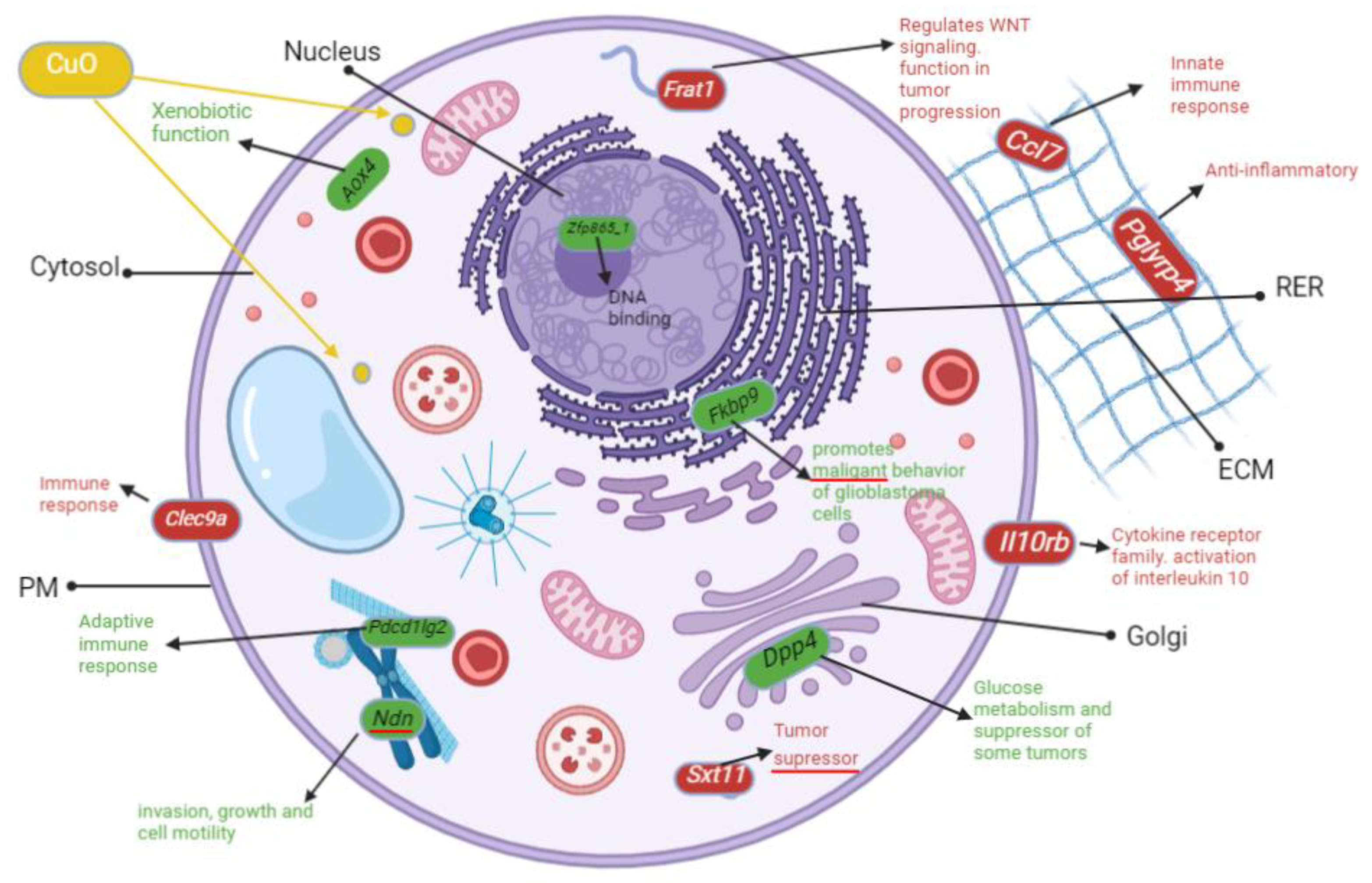
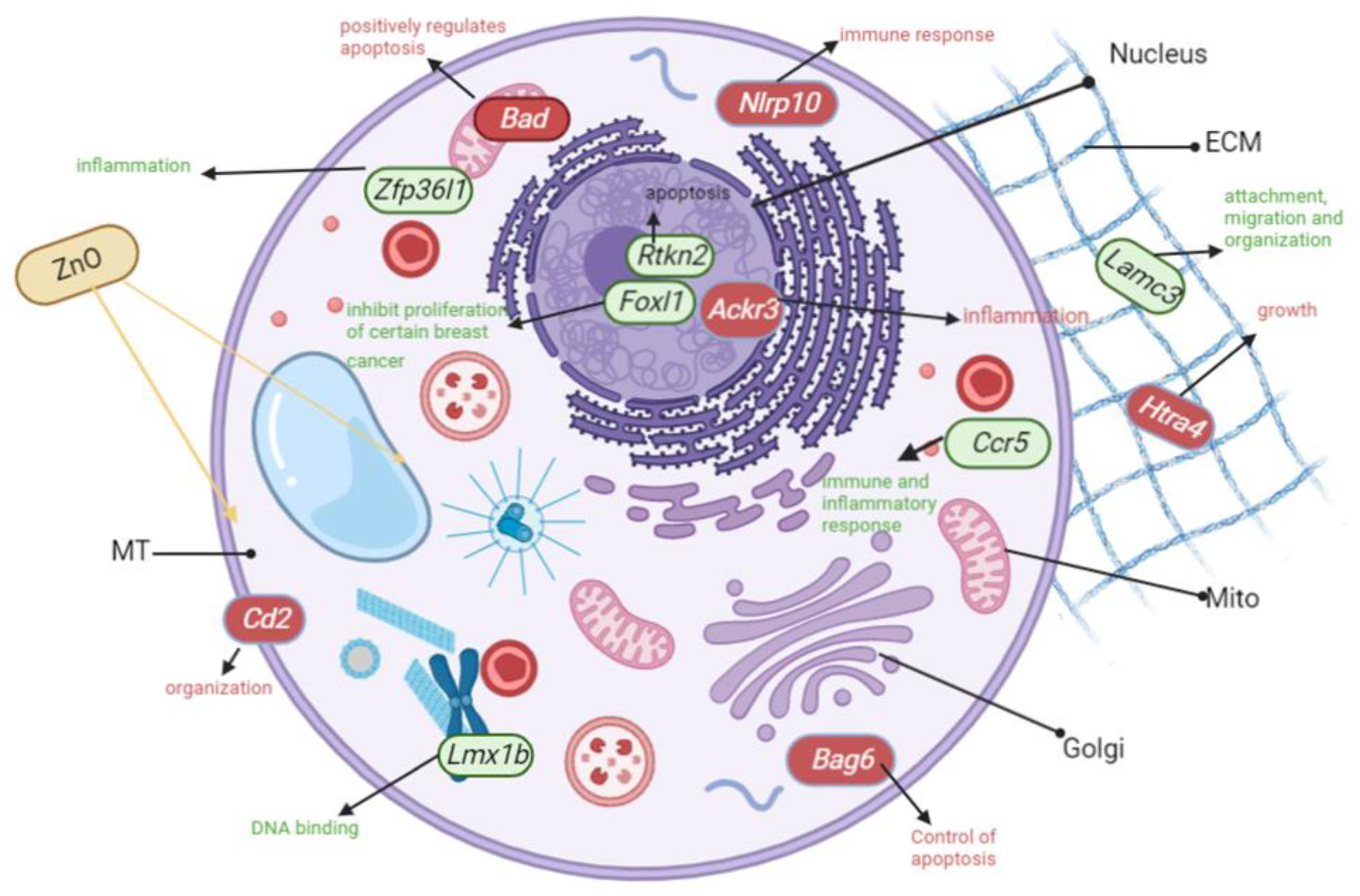
2.9. Altered Genome with ZnO/CuO Treatment on CA77 Cells
3. Discussion
3.1. Both ZnO and CuO Lead to a Reduction in Cell Viability
3.2. Levels of Reactive Oxygen Species Were Not Altered between Treatments with IC50 Values
3.3. Apoptosis Was Induced Both in ML-1 and CA77 Cells with MONPs
3.4. Upregulated Transcripts Implicated in p53 and Wnt Pathways after ZnO Treatment of ML-1 Cells
3.5. Severely Downregulated Genes Include Inflammation and Cadherin Signaling after ZnO Treatment in ML-1 Cells
3.6. Genes Implicated in DNA Repair and Growth Inhibition Were Significantly Upregulated after CuO Treatment to ML-1 Cells
3.7. Genes Implicated in Wnt Signaling and Cadherin Pathways Were Severely Downregulated after CuO Treatment to ML-1 Cells
3.8. Inflammation Is the Most Upregulated Pathway after CuO Treatment to CA77 Cells
3.9. Severely Downregulated Angiogenesis and Cadherin Pathways after CuO-Treatment to CA77 Cells
3.10. Genes Involved in Inflammation Pathways Were Significantly Upregulated after ZnO Treatment to CA77 Cells
3.11. Inflammation and Integrin Genes Were Severely Downregulated after ZnO Treatment to CA77 Cells
4. Materials and Methods
4.1. Materials and Reagents Used in the Study
4.2. ZnO and CuO NP Scanning Transmission Electron Microscopy (STEM)
4.3. X-ray Diffraction of ZnO and CuO NPs
4.4. Yeast Culture and Viability Assay
4.5. Statistical Analysis for Yeast Viability Experiments
4.6. Mammalian Cell Cultures
4.7. XTT Cell Viability Using Mammalian Cells
4.8. Calculation of IC50 Value
4.9. Measuring Levels of Reactive Oxygen Species
4.10. CellRox Green Reactive Oxygen Species
4.11. Apoptosis Measurement
4.12. Total RNA Extraction
4.13. RNA Seq Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nikolova, M.P.; Chavali, M.S. Metal Oxide Nanoparticles as Biomedical Materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Goudoulas, T.B.; Jeevanandam, J.; Tan, K.X.; Chowdhury, S.; Danquah, M.K. Therapeutic Applications of Metal and Metal-Oxide Nanoparticles: Dermato-Cosmetic Perspectives. Front. Bioeng. Biotechnol. 2021, 9, 724499. [Google Scholar] [CrossRef] [PubMed]
- Chai, H.; Yao, J.; Sun, J.; Zhang, C.; Liu, W.; Zhu, M.; Ceccanti, B. The Effect of Metal Oxide Nanoparticles on Functional Bacteria and Metabolic Profiles in Agricultural Soil. Bull. Environ. Contam. Toxicol. 2015, 94, 490–495. [Google Scholar] [CrossRef]
- Niño-Martínez, N.; Salas Orozco, M.F.; Martínez-Castañón, G.-A.; Torres Méndez, F.; Ruiz, F. Molecular Mechanisms of Bacterial Resistance to Metal and Metal Oxide Nanoparticles. Int. J. Mol. Sci. 2019, 20, 2808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and multi metal oxide nanoparticles: Synthesis, antibacterial and cytotoxic properties. J. Nanobiotechnol. 2016, 14, 73. [Google Scholar] [CrossRef] [Green Version]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef] [Green Version]
- Frenk, S.; Ben-Moshe, T.; Dror, I.; Berkowitz, B.; Minz, D. Effect of Metal Oxide Nanoparticles on Microbial Community Structure and Function in Two Different Soil Types. PLoS ONE 2013, 8, e84441. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.W.; Monteiro-Riviere, N.A. Toxicity assessment of six titanium dioxide nanoparticles in human epidermal keratinocytes. Cutan. Ocul. Toxicol. 2019, 38, 66–80. [Google Scholar] [CrossRef]
- Lee, J.H.; Ju, J.E.; Kim, B.I.; Pak, P.J.; Choi, E.-K.; Lee, H.-S.; Chung, N. Rod-shaped iron oxide nanoparticles are more toxic than sphere-shaped nanoparticles to murine macrophage cells. Environ. Toxicol. Chem. 2014, 33, 2759–2766. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, J.; Chen, M.; Liu, X.; Xiong, Y.; Wang, Y.; Feng, T.; Kang, S.; Wang, X. Recent advances in the biotoxicity of metal oxide nanoparticles: Impacts on plants, animals and microorganisms. Chemosphere 2019, 237, 124403. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Nogales, A.; Connolly, M.; Rosenkranz, P.; Fernández-Cruz, M.-L.; Navas, J.M. Negligible cytotoxicity induced by different titanium dioxide nanoparticles in fish cell lines. Ecotoxicol. Environ. Saf. 2017, 138, 309–319. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Khan, M.M.; Alhadlaq, H.A. SnO2-Doped ZnO/Reduced Graphene Oxide Nanocomposites: Synthesis, Characterization, and Improved Anticancer Activity via Oxidative Stress Pathway. Int. J. Nanomed. 2021, 16, 89–104. [Google Scholar] [CrossRef]
- Singh, S. Zinc oxide nanoparticles impacts: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Toxicol. Mech. Methods 2019, 29, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, R.I.; Kadhim, A.A.; Ibraheem, S.; Albukhaty, S.; Mohammed-Salih, H.S.; Abbas, R.H.; Al-Karagoly, H. Biosynthesis of copper oxide nanoparticles mediated Annona muricata as cytotoxic and apoptosis inducer factor in breast cancer cell lines. Sci. Rep. 2022, 12, 16165. [Google Scholar] [CrossRef] [PubMed]
- Vinardell, M.P.; Mitjans, M. Antitumor Activities of Metal Oxide Nanoparticles. Nanomaterials 2015, 5, 1004–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Hedberg, J.; Blomberg, E.; Odnevall, I. Reactive Oxygen Species Formed by Metal and Metal Oxide Nanoparticles in Physiological Media-A Review of Reactions of Importance to Nanotoxicity and Proposal for Categorization. Nanomaterials 2022, 12, 1922. [Google Scholar] [CrossRef]
- Kahlson, M.A.; Dixon, S.J. Copper-induced cell death. Science 2022, 375, 1231–1232. [Google Scholar] [CrossRef]
- Bai, D.-P.; Zhang, X.-F.; Zhang, G.-L.; Huang, Y.-F.; Gurunathan, S. Zinc oxide nanoparticles induce apoptosis and autophagy in human ovarian cancer cells. Int. J. Nanomed. 2017, 12, 6521–6535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, C.T.; Yong, L.Q.; Hande, M.P.; Ong, C.N.; Yu, L.E.; Bay, B.H.; Baeg, G.H. Zinc oxide nanoparticles exhibit cytotoxicity and genotoxicity through oxidative stress responses in human lung fibroblasts and Drosophila melanogaster. Int. J. Nanomed. 2017, 12, 1621–1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namvar, F.; Rahman, H.S.; Mohamad, R.; Azizi, S.; Tahir, P.; Chartrand, M.S.; Yeap, S.K. Cytotoxic Effects of Biosynthesized Zinc Oxide Nanoparticles on Murine Cell Lines. Evidence-Based Complement. Altern. Med. 2015, 2015, 593014. [Google Scholar] [CrossRef] [PubMed]
- Angelé-Martínez, C.; Nguyen, K.V.T.; Ameer, F.S.; Anker, J.N.; Brumaghim, J.L. Reactive oxygen species generation by copper(II) oxide nanoparticles determined by DNA damage assays and EPR spectroscopy. Nanotoxicology 2017, 11, 278–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kukia, N.R.; Abbasi, A.; Froushani, S.M.A. Copper Oxide Nanoparticles Stimulate Cytotoxicity and Apoptosis in Glial Cancer Cell Line. Dhaka Univ. J. Pharm. Sci. 2018, 17, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.H.; Kim, H.J.; Ryu, H.J.; Ryu, W.I.; Park, Y.-H.; Bae, H.C.; Jang, Y.S.; Son, S.W. ZnO nanoparticles induce TNF-α expression via ROS-ERK-Egr-1 pathway in human keratinocytes. J. Dermatol. Sci. 2013, 72, 263–273. [Google Scholar] [CrossRef]
- Cardozo, T.R.; De Carli, R.F.; Seeber, A.; Flores, W.H.; da Rosa, J.A.; Kotzal, Q.S.; Dihl, R.R. Genotoxicity of zinc oxide nanoparticles: An in vivo and in silico study. Toxicol. Res. 2019, 8, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.W.; Lee, C.H.; Lin, M.S.; Chi, C.W.; Chen, Y.J.; Wang, G.S.; Shen, Y.S. ZnO Nanoparticles Induced Caspase-Dependent Apoptosis in Gingival Squamous Cell Carcinoma through Mitochondrial Dysfunction and p70S6K Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 1612. [Google Scholar] [CrossRef] [Green Version]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef] [Green Version]
- Kao, Y.-Y.; Chen, Y.-C.; Cheng, T.-J.; Chiung, Y.-M.; Liu, P.-S. Zinc Oxide Nanoparticles Interfere With Zinc Ion Homeostasis to Cause Cytotoxicity. Toxicol. Sci. 2012, 125, 462–472. [Google Scholar] [CrossRef] [Green Version]
- Kadhem, H.A.; Ibraheem, S.A.; Jabir, M.S.; Kadhim, A.A.; Taqi, Z.J.; Florin, M.D. Zinc Oxide Nanoparticles Induces Apoptosis in Human Breast Cancer Cells via Caspase-8 and P53 Pathway. Nano Biomed. Eng. 2019, 11, 35–43. [Google Scholar] [CrossRef]
- Williams, A.B.; Schumacher, B. p53 in the DNA-Damage-Repair Process. Cold Spring Harb. Perspect. Med. 2016, 6, a026070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freire, K.; Ordóñez Ramos, F.; Soria, D.B.; Pabón Gelves, E.; Di Virgilio, A.L. Cytotoxicity and DNA damage evaluation of TiO2 and ZnO nanoparticles. Uptake in lung cells in culture. Toxicol. Res. 2021, 10, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, O.; Simakova, O.; Klenerb, P.; Cmejlovaa, J.; Zivnyb, J.; Zavadilc, J.; Stopkaac, T. Inhibition of Smad5 in Human Hematopoietic Progenitors Blocks Erythroid Differentiation Induced by BMP4. Blood Cells Mol. Dis. 2002, 28, 221–233. [Google Scholar] [CrossRef]
- Song, M.S.; Carracedo, A.; Salmena, L.; Song, S.J.; Egia, A.; Malumbres, M.; Pandolfi, P.P. Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell 2011, 144, 187–199. [Google Scholar] [CrossRef] [Green Version]
- Salesa, B.; Serra, R.S.I.; Serrano-Aroca, Á. Zinc Chloride: Time-Dependent Cytotoxicity, Proliferation and Promotion of Glycoprotein Synthesis and Antioxidant Gene Expression in Human Keratinocytes. Biology 2021, 10, 1072. [Google Scholar] [CrossRef]
- Wang, L.; Guo, D.; Wang, Z.; Yin, X.; Wei, H.; Hu, W.; Chen, R.; Chen, C. Zinc oxide nanoparticles induce human tenon fibroblast apoptosis through reactive oxygen species and caspase signaling pathway. Arch. Biochem. Biophys. 2020, 683, 108324. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.-Y.; Huang, M.-S.; Zhong, H.-G.; Ru, H.-M.; Mo, S.-S.; Wei, C.-Y.; Su, Z.-J.; Mo, X.-W.; Yan, L.-H.; Tang, W.-Z. MTUS1 is a promising diagnostic and prognostic biomarker for colorectal cancer. World J. Surg. Oncol. 2022, 20, 257. [Google Scholar] [CrossRef]
- Sebastián, V.P.; Salazar, G.A.; Coronado-Arrázola, I.; Schultz, B.M.; Vallejos, O.P.; Berkowitz, L.; Álvarez-Lobos, M.M.; Riedel, C.A.; Kalergis, A.M.; Bueno, S.M. Heme Oxygenase-1 as a Modulator of Intestinal Inflammation Development and Progression. Front. Immunol. 2018, 9, 1956. [Google Scholar] [CrossRef] [Green Version]
- Kahlert, U.D.; Suwala, A.K.; Koch, K.; Natsumeda, M.; Orr, B.A.; Hayashi, M.; Maciaczyk, J.; Eberhart, C.G. Pharmacologic Wnt Inhibition Reduces Proliferation, Survival, and Clonogenicity of Glioblastoma Cells. J. Neuropathol. Exp. Neurol. 2015, 74, 889–900. [Google Scholar] [CrossRef]
- Baggio, L.L.; Varin, E.M.; Koehler, J.A.; Cao, X.; Lokhnygina, Y.; Stevens, S.R.; Holman, R.R.; Drucker, D.J. Plasma levels of DPP4 activity and sDPP4 are dissociated from inflammation in mice and humans. Nat. Commun. 2020, 11, 3766. [Google Scholar] [CrossRef]
- Pietrofesa, R.A.; Park, K.; Mishra, O.P.; Johnson-McDaniel, D.; Myerson, J.W.; Shuvaev, V.V.; Arguiri, E.; Chatterjee, S.; Moorthy, G.S.; Zuppa, A.; et al. Copper Oxide Nanoparticle-Induced Acute Inflammatory Response and Injury in Murine Lung Is Ameliorated by Synthetic Secoisolariciresinol Diglucoside (LGM2605). Int. J. Mol. Sci. 2021, 22, 9477. [Google Scholar] [CrossRef] [PubMed]
- Tulinska, J.; Mikusova, M.L.; Liskova, A.; Busova, M.; Masanova, V.; Uhnakova, I.; Rollerova, E.; Alacova, R.; Krivosikova, Z.; Wsolova, L.; et al. Copper Oxide Nanoparticles Stimulate the Immune Response and Decrease Antioxidant Defense in Mice After Six-Week Inhalation. Front. Immunol. 2022, 13, 874253. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ning, X.; Qin, C.; Wang, J.; Yan, W.; Zhou, X.; Wang, D.; Cao, J.; Feng, Y. Respiratory Exposure to Copper Oxide Particles Causes Multiple Organ Injuries via Oxidative Stress in a Rat Model. Int. J. Nanomed. 2022, ume 17, 4481–4496. [Google Scholar] [CrossRef]
- Jiang, Y.; Huo, Z.; Qi, X.; Zuo, T.; Wu, Z. Copper-induced tumor cell death mechanisms and antitumor theragnostic applications of copper complexes. Nanomedicine 2022, 17, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Amerongen, R.V.; Berns, A. Re-evaluating the role of Frat in Wnt-signal transduction. Cell Cycle 2005, 4, 1065–1072. [Google Scholar]
- Roy, R.; Parashar, V.; Chauhan, L.K.; Shanker, R.; Das, M.; Tripathi, A.; Dwivedi, P.D. Mechanism of uptake of ZnO nanoparticles and inflammatory responses in macrophages require PI3K mediated MAPKs signaling. Toxicol. Vitr. 2014, 28, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Jung, A.; Kim, S.-H.; Yang, J.-Y.; Jeong, J.; Lee, J.K.; Oh, J.-H.; Lee, J.H. Effect of Pulmonary Inflammation by Surface Functionalization of Zinc Oxide Nanoparticles. Toxics 2021, 9, 336. [Google Scholar] [CrossRef]
- Huang, K.-L.; Lee, Y.-H.; Chen, H.-I.; Liao, H.-S.; Chiang, B.-L.; Cheng, T.-J. Zinc oxide nanoparticles induce eosinophilic airway inflammation in mice. J. Hazard. Mater. 2015, 297, 304–312. [Google Scholar] [CrossRef]
- Agarwal, H.; Shanmugam, V. A review on anti-inflammatory activity of green synthesized zinc oxide nanoparticle: Mechanism-based approach. Bioorganic Chem. 2020, 94, 103423. [Google Scholar] [CrossRef]
- Bachmann, M.; Kukkurainen, S.; Hytönen, V.P.; Wehrle-Haller, B. Cell Adhesion by Integrins. Physiol. Rev. 2019, 99, 1655–1699. [Google Scholar] [CrossRef]
- Fernández-Cruz, M.L.; Lammel, T.; Connolly, M.; Conde, E.; Barrado, A.I.; Derick, S.; Perez, Y.; Fernandez, M.; Furger, C.; Navas, J.M. Comparative cytotoxicity induced by bulk and nanoparticulated ZnO in the fish and human hepatoma cell lines PLHC-1 and Hep G2. Nanotoxicology 2013, 7, 935–952. [Google Scholar] [CrossRef]
- Horstmann, C.; Kim, K. Comparing Transcriptome Profiles of Saccharomyces Cerevisiae Cells Exposed to Cadmium Selenide/Zinc Sulfide and Indium Phosphide/Zinc Sulfide. Genes 2021, 12, 428. [Google Scholar] [CrossRef]
- Horstmann, C.; Kim, D.S.; Campbell, C.; Kim, K. Transcriptome Profile Alteration with Cadmium Selenide/Zinc Sulfide Quantum Dots in Saccharomyces cerevisiae. Biomolecules 2019, 9, 653. [Google Scholar] [CrossRef] [Green Version]
- Horstmann, C.; Campbell, C.; Kim, D.S.; Kim, K. Transcriptome profile with 20 nm silver nanoparticles in yeast. FEMS Yeast Res. 2019, 19, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Davenport, V.; Horstmann, C.; Patel, R.; Wu, Q.; Kim, K. An Assessment of InP/ZnS as Potential Anti-Cancer Therapy: Quantum Dot Treatment Increases Apoptosis in HeLa Cells. J. Nanotheranostics 2021, 2, 16–32. [Google Scholar] [CrossRef]
- Hens, B.; Smothers, J.; Rizvanovic, H.; Patel, R.; Wu, Q.; Kim, K. The Future of Anticancer Drugs: A Cytotoxicity Assessment Study of CdSe/ZnS Quantum Dots. J. Nanotheranostics 2020, 1, 3. [Google Scholar] [CrossRef]
- Kim, S.D.; Zhang, M.; Le, N.; Harris, S.; Kim, K. Effects of Platinum-based Chemotherapeutic Agents on ML-1 Thyroid Cancer Cells. Acta Sci. Microbiol. 2022, 5. [Google Scholar] [CrossRef]


| Pathways with DEG | ML-1 Cells | CA77 Cells | ||||||
|---|---|---|---|---|---|---|---|---|
| Up by ZnO | Down by ZnO | Up by CuO | Down by CuO | Up by ZnO | Down by ZnO | Up by CuO | Down by CuO | |
| Gonadotrophin-releasing hormone receptor | 13 | 5 | 6 | 2 | 1 | 3 | ||
| CCKR signaling | 13 | 3 | 5 | 1 | 1 | |||
| Wnt signaling | 12 | 7 | 6 | 7 | 3 | 2 | ||
| P53 | 12 | 1 | 2 | |||||
| Nicotine degradation/nicotine acetylcholine receptor | 11 | 6 | 2 | 1 | 2 | |||
| Huntington disease | 11 | 2 | 2 | 2 | 2 | |||
| Integrin signaling | 10 | 5 | 4 | 4 | 2 | 4 | ||
| Inflammation | 10 | 7 | 6 | 3 | 4 | 2 | 7 | |
| Apoptosis | 9 | 2 | 3 | 2 | 1 | 1 | ||
| PDGF signaling | 8 | 2 | 1 | 1 | 1 | |||
| Heterotrimeric G-protein signaling | 8 | 6 | 10 | 6 | 2 | 3 | ||
| Angiogenesis | 8 | 4 | 3 | 2 | ||||
| Presenilin (Alzheimer) | 8 | 2 | 3 | 1 | 2 | |||
| Parkinson disease | 7 | 1 | ||||||
| TGF-beta signaling | 6 | 2 | 2 | 1 | ||||
| Cadherin signaling | 6 | 7 | 6 | 1 | ||||
| Cytoskeletal regulation by Rho GTPase | 5 | 3 | 2 | |||||
| Glutamate receptor groups I, I, III | 5 | 5 | 2 | 1 | ||||
| Ionotropic glutamate receptor | 4 | |||||||
| FGF signaling | 4 | 2 | 2 | 1 | 1 | 1 | ||
| EGF receptor signaling | 4 | 3 | 1 | 1 | 1 | |||
| DNA replication | 4 | |||||||
| Plasminogen activating cascade | 4 | |||||||
| Blood coagulation | 4 | 3 | 1 | 1 | ||||
| B cell activation | 4 | 2 | 1 | |||||
| Adrenaline and noradrenaline biosynthesis | 3 | |||||||
| Endothelin | 3 | |||||||
| Axon guidance | 3 | |||||||
| PI3K kinase | 3 | 2 | 2 | |||||
| Oxidative stress response | 3 | 2 | ||||||
| Notch Signaling | 3 | 3 | 2 | |||||
| Amyloid secretase | 2 | |||||||
| Interleukin signaling | 2 | 3 | 1 | |||||
| Dopamine receptor | 3 | 1 | 1 | |||||
| Opioid | 4 | 1 | 1 | |||||
| VEGF signaling | 2 | 2 | 3 | |||||
| Thyrotrophin-releasing hormone receptor | 4 | |||||||
| JAK-STAT | 1 | 1 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, A.N.; Weaver, N.A.; Monahan, K.S.; Kim, K. Non-ROS-Mediated Cytotoxicity of ZnO and CuO in ML-1 and CA77 Thyroid Cancer Cell Lines. Int. J. Mol. Sci. 2023, 24, 4055. https://doi.org/10.3390/ijms24044055
Peters AN, Weaver NA, Monahan KS, Kim K. Non-ROS-Mediated Cytotoxicity of ZnO and CuO in ML-1 and CA77 Thyroid Cancer Cell Lines. International Journal of Molecular Sciences. 2023; 24(4):4055. https://doi.org/10.3390/ijms24044055
Chicago/Turabian StylePeters, Alyse N., Nakaja A. Weaver, Kathryn S. Monahan, and Kyoungtae Kim. 2023. "Non-ROS-Mediated Cytotoxicity of ZnO and CuO in ML-1 and CA77 Thyroid Cancer Cell Lines" International Journal of Molecular Sciences 24, no. 4: 4055. https://doi.org/10.3390/ijms24044055
APA StylePeters, A. N., Weaver, N. A., Monahan, K. S., & Kim, K. (2023). Non-ROS-Mediated Cytotoxicity of ZnO and CuO in ML-1 and CA77 Thyroid Cancer Cell Lines. International Journal of Molecular Sciences, 24(4), 4055. https://doi.org/10.3390/ijms24044055






